Abstract
The repurposing of existing drugs is being pursued as a means by which to accelerate the development of novel regimens for the treatment of drug-susceptible and drug-resistant tuberculosis (TB). In the current study, we assessed the activity of the antipsychotic drug thioridazine (TRZ) in combination with the standard regimen in a well-validated murine TB model. Single-dose and steady-state pharmacokinetic studies were performed in BALB/c mice to establish human-equivalent doses of TRZ. To determine the bactericidal activity of TRZ against TB in BALB/c mice, three separate studies were performed, including a dose-ranging study of TRZ monotherapy and efficacy studies of human-equivalent doses of TRZ with and without isoniazid (INH) or rifampin (RIF). Therapeutic efficacy was assessed by the change in mycobacterial load in the lung. The human-equivalent dose of thioridazine was determined to be 25 mg/kg of body weight, which was well tolerated in mice. TRZ was found to accumulate at high concentrations in lung tissue relative to serum levels. We observed modest synergy during coadministration of TRZ with INH, and the addition of TRZ reduced the emergence of INH-resistant mutants in mouse lungs. In conclusion, this study further illustrates the opportunity to reevaluate the contribution of TRZ to the sterilizing activity of combination regimens to prevent the emergence of drug-resistant M. tuberculosis.
INTRODUCTION
Multidrug-resistant (MDR) and extensively drug-resistant (XDR) tuberculosis (TB) are becoming increasingly prevalent in many parts of the world, requiring the urgent development of novel strategies for its control (1). One potential solution is the repurposing of nonantibiotic compounds with antibacterial properties (2, 3), such as enhancement of antibiotic activity (4, 5) reversal of antibiotic resistance (6–8), and inhibition of drug efflux pumps (9–12).
The new use of the old antipsychotic phenothiazine thioridazine (TRZ) for therapy of MDR and XDR TB is now being seriously considered (5, 13–15). The potential advantages of TRZ include its multiple bacterial targets, greatly reducing the risk of drug resistance (16–19), its low cost and the availability of generic formulations, and its documented efficacy against drug-resistant strains both in vitro (20, 21) and in vivo (22–24). TRZ exhibits synergy with rifampin (RIF) and streptomycin in vitro (20) and appears to target both replicating and nonreplicating bacilli (25).
TRZ is known to have significantly higher intracellular than extracellular accumulation, as its MIC against Mycobacterium tuberculosis within macrophages is 100-fold lower than that against extracellular bacilli (8, 26). We recently reported that TRZ lacks bactericidal activity in a guinea pig model of predominantly extracellular TB, perhaps due to limited drug penetration into the necrotic cores of guinea pig granulomas as a result of the relatively high protein binding of the drug (95% in humans) (27–30). We hypothesized that the efficacy of TRZ would be greater in the murine model of TB, in which the bacilli are almost exclusively located in the intracellular compartment (31).
In the current study, we conducted single-dose and steady-state pharmacokinetic studies in BALB/c mice to establish human-equivalent doses of TRZ. Next, we performed three separate studies to determine the bactericidal activity of TRZ in BALB/c mice with chronic TB, including a dose-ranging study of TRZ monotherapy, and efficacy studies of human-equivalent doses of TRZ with and without isoniazid (INH) and rifampin (RIF).
MATERIALS AND METHODS
Pharmacokinetics studies.
TRZ was purchased from Sigma (St. Louis, MO). Separate groups of three mice were given a single dose of TRZ at 12.5 mg/kg, 25 mg/kg, 37.5 mg/kg, or 50 mg/kg. Mice were anesthetized with isoflurane and exsanguinated by cardiac puncture at 1, 3, 5, 7, 9, and 24 h after TRZ dosing. To generate the steady-state data, mice were treated once daily for 14 successive days, and samples were collected before the 14th dose (24 h after the 13th dose; 0-h time point) followed by collections at 1 h, 3 h, 6 h, 9 h, 12 h, 15 h, 18 h, 21 h, 24 h, 29 h, 32 h, and 48 h. Blood was collected in microcentrifuge tubes, held for 30 min at room temperature, and centrifuged at 6,500 rpm for 10 min to obtain serum. Whole lungs and sera at each time point were frozen at −20°C. Serum and lung homogenate concentrations of TRZ were measured using liquid chromatography-mass spectrometry and liquid chromatography-tandem mass spectrometry, and data were entered into a WinNonlin worksheet (WinNonlin version 4.0; Pharsight, Mountain View, CA) and analyzed using standard noncompartmental techniques in order to determine the relevant pharmacokinetic parameters as described earlier (27).
Mycobacterium tuberculosis strains.
An M. tuberculosis H37Rv strain, which has been passaged twice in mice (H37Rv-JHU), was used for this study (27). Prior to aerosol infection, cultures were grown to log phase (optical density at 600 nm [OD600], ∼0.6 and ∼1.0 for chronic and active infections, respectively) in Erlenmeyer flasks containing Middlebrook 7H9 broth (Difco Laboratories) supplemented with 10% OADC (Becton, Dickinson), 0.05% Tween, and 0.1% glycerol on a shaker at 37°C.
Animals.
All animals were maintained under pathogen-free conditions and fed water and chow ad libitum. Protocols/procedures approved by the Institutional Animal Care and Use Committee at the Johns Hopkins University School of Medicine were followed. Female BALB/c mice aged 4 to 6 weeks were purchased from Charles River Labs (Wilmington, MA).
Aerosol infections and study endpoints.
The basic experimental scheme is shown in Table 1. Briefly, we examined activity of TRZ against M. tuberculosis in a low-dose, chronic-infection model, and in a high-dose, acute-infection model. A total of 180 mice were aerosol infected with M. tuberculosis H37Rv using an inhalation exposure system (Glas-Col, Terre Haute, IN) calibrated to deliver ∼102 CFU/mouse lung for the chronic-infection studies (experiments 1 and 2) and ∼104 CFU/mouse lung for the acute-infection studies (experiment 3). After aerosol infection, mice were randomized into different treatment groups as outlined in Table 1. Five mice per group were sacrificed on the day after infection, on the day of treatment initiation, and at the indicated time points after treatment in order to determine the numbers of CFU implanted in the lungs, the pretreatment baseline CFU counts, and bactericidal activity of each regimen, respectively (Table 1). For assessing efficacy of treatment groups, animals were sacrificed 3 days after the last anti-TB drug dose, to prevent possible carryover of anti-TB drugs onto culture plates. Animal body weights were recorded on a weekly basis, and lung and spleen weights were recorded at the time of sacrifice. The lungs of sacrificed animals were examined grossly for visible lesions, and small, randomly selected sections were formalin fixed for histopathology. The remainder of each lung was homogenized in 2.5 ml PBS. Lung homogenates were plated on 7H11 plates containing cycloheximide (50 μg/ml), carbenicillin (100 μg/ml), polymyxin B (200 U/ml), and trimethoprim (20 μg/ml) for CFU enumeration after 4 weeks of incubation at 37°C.
TABLE 1.
Basic experimental schemea
| Expt, group, and dose (mg/kg) | No of mice per time point |
||||
|---|---|---|---|---|---|
| Day 28 | Day 0 | 1 mo | 2 mo | Total | |
| 1 | |||||
| Untreated | 5 | 5 | 5 | 15 | |
| INH, 10 | 5 | 5 | |||
| TRZ, 12.5 | 5 | 5 | |||
| TRZ, 25 | 5 | 5 | |||
| TRZ, 50 | 5 | 5 | |||
| TRZ, 100 | 5 | 5 | |||
| TRZ, 200 | 5 | 5 | |||
| Total | 5 | 5 | 35 | 45 | |
| 2 | |||||
| Untreated | 5 | 5 | 5 | 5 | 20 |
| INH, 10 | 5 | 5 | 10 | ||
| TRZ, 25 | 5 | 5 | 10 | ||
| INH, 10, + TRZ, 25 | 5 | 5 | 10 | ||
| RIF, 10 | 5 | 5 | 10 | ||
| RIF, 10, + TRZ, 25 | 5 | 5 | 10 | ||
| Total | 5 | 5 | 30 | 30 | 70 |
| 3 | |||||
| Untreated | 5 | 5 | 5 | 15 | |
| INH, 10 | 5 | 5 | 10 | ||
| TRZ, 25 | 5 | 5 | 10 | ||
| INH, 10, + TRZ, 25 | 5 | 5 | 10 | ||
| RIF, 10 | 5 | 5 | 10 | ||
| RIF, 10, + TRZ, 25 | 5 | 5 | 10 | ||
| Total | 5 | 5 | 30 | 25 | 65 |
Doses of each drug were determined to be equivalent to human exposures based on area under the serum concentration-time curve (AUC) and were given daily (5/7) by gavage.
In addition, undiluted and diluted lung homogenates were plated on 7H11 antibiotic-containing plates plus 4× MIC of TRZ, INH, and RIF in order to quantify the number of drug-resistant colonies. The MICs used for INH, RIF, and TRZ were 0.06 μg/ml, 0.25 μg/ml, and 10 μg/ml, respectively. The proportion of resistant mutants was calculated as the ratio of the CFU count obtained on antibiotic-containing medium to that obtained on antibiotic-free plates.
Antibiotic therapy.
INH, RIF, and TRZ were purchased from Sigma and were either dissolved or suspended in distilled water before oral gavage (single dose given once daily, 5 times weekly). Stock solutions were prepared weekly and stored at 4°C. The following doses were used: INH, 10 mg/kg (33); RIF, 10 mg/kg (33); and TRZ, 12.5 mg/kg, 25 mg/kg, 50 mg/kg, 100 mg/kg, and 200 mg/kg.
Statistical analysis.
CFU data were derived from five mice per group. Log-transformed CFU were used to calculate means and standard deviations. Comparisons of data among experimental groups were performed by t test. P values of <0.05 were considered to be statistically significant.
RESULTS
Identification of human-equivalent exposures of thioridazine in mice.
Prior to undertaking drug efficacy studies, we sought to determine the human-equivalent dose of TRZ in mice (Table 2) (34). After single-dose oral administration of TRZ at 25 mg/kg, 37.5 mg/kg, and 50 mg/kg, the mean peak serum concentrations (Cmax) were 79.2 ng/ml, 89.3 ng/ml, and 100.9 ng/ml, respectively. The values for the area under the concentration-time curve from 0 to infinity (AUC0→∞) for serum were 931.5 ng · h/liter, 1,693.1 ng · h/liter, and 1,306.1 ng · h/liter following TRZ doses of 12.5 mg/kg, 25 mg/kg, and 50 mg/kg, respectively. At steady state, 25 mg/kg TRZ yielded a Cmax of 86.2 ng/ml and an AUC0→∞ of 852 ng · h/ml, closely approximating the corresponding values in humans (Cmax, 110 ng/ml, and AUC0→∞, 554 ng · h/ml) following daily dosing with 25 mg TRZ. The steady-state Cmax and AUC0→∞ in the lungs of mice following daily dosing with 25 mg/kg TRZ were 3,326 ng/ml and 26,112 ng · h/ml, respectively, indicating that the drug concentrates more than 30-fold in the lungs relative to the serum (Table 2 and Fig. 1).
TABLE 2.
Pharmacokinetics of thioridazine in micea
| Test species | Drug doseb | Cmax (ng/ml) | Tmax (h) | t1/2 (h) | AUC0→∞ (ng · h/ml) |
|---|---|---|---|---|---|
| Mouse (serum) | 25 mg/kg (SD) | 79.2 | 9.0 | 3.4 | 931.5 |
| 37.5 mg/kg (SD) | 89.3 | 9.0 | 1,693.1 | ||
| 50 mg/kg (SD) | 100.9 | 9.0 | 1,306.1 | ||
| 25 mg/kg(SS) | 86.2 | 1 | 5.43 | 852 | |
| Mouse (lung) | 25 mg/kg (SS) | 3,326 | 1 | 2.37 | 26,112 |
| Guinea pigc | 5 mg/kg (SD) | 404 ± 154.86 | 1.50 ± 0.71 | 3.19 ± 1.44 | 2,187.95 ± 9.98 |
| Humand | 25 mg (SD) | 110.83 ± 51.07 | 1.77 ± 0.81 | 6.82 ± 1.67 | 554.86 ± 276.04 |
| Humand | 50 mg (SD) | 197.13 ± 101.86 | 1.36 ± 0.39 | 8.24 ± 1.53 | 1,084.30 ± 582.75 |
| Humand | 100 mg (SD) | 371.80 ± 137.10 | 1.48 ± 0.38 | 9.25 ± 1.91 | 2,639.30 ± 859.20 |
FIG 1.
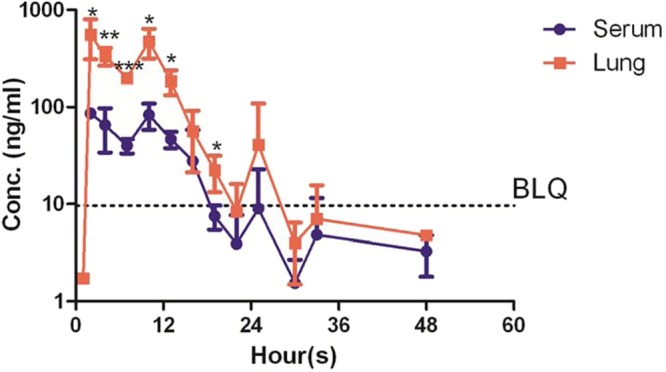
Serum and lung homogenate concentration profile following repeated dosing of 25 mg/kg thioridazine in mice (3 to 4 per time point). The data are medians ± standard deviations. Statistically significant differences were noted between serum and lung concentrations (*, P < 0.01; **, P < 0.001; ***, P < 0.0001). BLQ, below the limit of quantitation.
Morbidity and mortality during treatment.
We found that 25 mg/kg TRZ was well tolerated in mice, and normalized mean body weights showed increasing trends over time (data not shown). In the chronic-TB model, all mice treated with 200 mg/kg TRZ died within 3 days and all mice treated with 100 mg/kg TRZ died within 1 week of the initiation of treatment. All remaining mice survived throughout the course of the experiment. Although the cause of death was not determined in the high-dose treatment groups, we hypothesize that it may have been related to the well-known cardiac toxicity of TRZ, which is dose related (15). In the acute-infection model, untreated mice became moribund by 3 weeks after aerosol infection and were euthanized in accordance with animal care regulations, while treated groups of mice survived until the predetermined time points, except for TRZ monotherapy-treated groups.
Therapeutic efficacy.
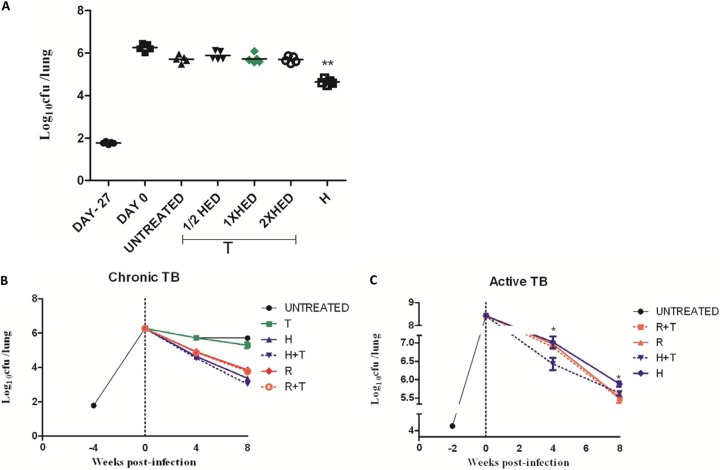
In the chronic-infection model, a total of 1.77 ± 0.04 log10 bacilli were implanted in mouse lungs on day 27, and the organisms multiplied to a peak lung burden of 6.32 ± 0.13 log10 CFU on day 0 (treatment initiation). Bacillary growth was controlled in the lungs of untreated mice, which maintained a relatively stable plateau of ∼6 log10 CFU until the end of the study (Fig. 2A). Positive-control mice received 10 mg/kg INH, which showed significant (P = 0.001) killing activity against M. tuberculosis. However, TRZ given daily at doses ranging from 12.5 mg/kg to 50 mg/kg for a total of 1 month showed minimal bactericidal activity.
FIG 2.
Antitubercular activity of thioridazine in infected mice. Animals were infected via aerosol with ∼102 (A and B) and ∼104 (C) CFU of M. tuberculosis H37Rv and were either left untreated or treated with drugs daily (5 days/week, once daily) beginning 4 or 2 weeks after infection, respectively. R, rifampin at 10 mg/kg; H, isoniazid at 10 mg/kg; T, thioridazine; HED, human-equivalent dose (25 mg/kg). **, P < 0.001 between untreated and INH groups; *, P < 0.05 between INH and INH+TRZ groups.
In the second experiment using the above model, 2 months of treatment with 10 mg/kg INH or 10 mg/kg RIF reduced the lung bacillary burden by 2.3 log10 and 1.4 log10 CFU, respectively, compared to the mean lung CFU at the end of the experiment in the untreated control group (Fig. 2B). Treatment with 25 mg/kg TRZ for 2 months reduced lung CFU by 0.41 log10. However, TRZ in combination with INH or RIF did not show any additive effect (Fig. 2B).
In the third experiment designed to evaluate the activity of TRZ in an acute-TB model, the mean lung CFU count on the day after aerosol infection was 4.37 ± 0.09 log10, and 13 days later, at the initiation of treatment (day 0), the mean lung CFU count was 8.41 ± 0.08 log10 (Fig. 2C). All mice treated with INH or RIF survived, but all mice treated with TRZ alone died in the same time period as untreated mice. Both INH and RIF showed significant bactericidal activity. Coadministration of TRZ with RIF had no additive effect on lung bacillary killing; however, TRZ given in combination with INH showed very modest synergistic activity and a significantly reduced bacterial burden compared to INH alone after 1 month (7.0 ± 0.3 and 6.4 ± 0.4, respectively; Δlog10 = 0.59; P = 0.03) and 2 months (5.8 ± 0.1 and 5.6 ± 0.1, respectively; Δlog10 = 0.24; P = 0.04) of treatment.
In untreated mice, the total frequency of INH-resistant mutants was 1.3 × 10−6, as previously reported (35). After 2 months of treatment, INH-resistant mutants were recovered from mice treated with INH alone at a frequency of 3.4 × 10−4, whereas the addition of TRZ reduced the frequency of INH-resistant mutants 7-fold (2.1 × 10−5).
DISCUSSION
Our study is the first to explore the pharmacokinetics of the phenothiazine TRZ in BALB/c mice, the validated mouse model used for preclinical TB drug screening. We found that TRZ is lethal in mice at concentrations above 50 mg/kg but that the human-equivalent dose of 25 mg/kg is well tolerated. We also found that TRZ accumulates in murine lung tissue relative to serum. However, TRZ monotherapy did not exhibit greater activity during acute or chronic infection in the murine model, which is characterized by intracellular infection, than in the guinea pig model, in which the majority of organisms are located in the extracellular compartment (28, 33, 36). Indeed, monotherapy with human-equivalent doses of TRZ had very limited activity against chronic murine TB infection, and dose response activity was not observed. On the other hand, an important finding of this study is that, when given in combination with INH, TRZ displayed modest synergy and prevented the selection of INH-resistant mutants.
Our findings expand upon prior studies reporting efficacy of TRZ in vivo. Martins et al. showed a >0.7 log10 reduction in lung CFU when TRZ at 0.5 mg daily was initiated 30 days after intraperitoneal infection of BALB/c mice with 106 CFU of M. tuberculosis (22). van Soolingen et al. showed that monotherapy with TRZ at 32 mg/kg and TRZ at 70 mg/kg reduced the lung bacillary burden by 0.2 to 0.4 log10 compared with untreated controls in mice infected with drug-susceptible M. tuberculosis. In mice infected with MDR M. tuberculosis, treatment with TRZ alone at daily doses of 32 and 70 mg/kg reduced CFU counts by 0.1 to 0.2 log10 (23).
Interestingly, Viveiros et al. reported that TRZ enhances the activity of RIF and streptomycin when used in combinations at concentrations that are minimally effective when employed separately against clinical strains of M. tuberculosis resistant to two or more antibiotics (poly-drug-resistant M. tuberculosis) (20). The phenothiazines had no effect on the activity of INH against poly-drug-resistant bacilli. The discrepancy between our findings and those of Viveiros et al. may be due to the different model systems (in vivo and in vitro, respectively), the use of poly-drug-resistant M. tuberculosis strains, or the significantly lower concentrations of INH employed in their studies (20).
Phenothiazines inhibit the activity of calcium-dependent ATPase, contributing to the process of acidification of the phagolysosome and the subsequent activation of its hydrolases, thereby inhibiting the replication of the bacterium (10). TRZ also has been reported to act as a drug efflux pump inhibitor in M. tuberculosis (37), perhaps via the emrE-encoded efflux pump (38). Interestingly, the expression of the Rv3065 gene, which encodes the multidrug-transport integral membrane protein EmrE, and another putative efflux pump gene, Rv1634, is induced following TRZ treatment. In Mycobacterium smegmatis, deletion of the Rv3065 homolog causes increased susceptibility to a number of drugs, including ethidium bromide, acriflavine, and fluoroquinolones.
Several mycobacterial efflux pumps and their regulators are induced during macrophage infection (39). Bacterial efflux pumps that are required for intracellular growth mediate this macrophage-induced tolerance (11). Machado and colleagues have demonstrated that overexpression of such efflux pumps favors accumulation of mutations in INH targets and that INH resistance can be reduced by means of the efflux pumps inhibitors TRZ, chlorpromazine, and verapamil in M. tuberculosis (18). Efflux pump inhibitors are thought to act by enhancing the killing of intracellular M. tuberculosis by nonkilling macrophages, inhibiting the expression of M. tuberculosis efflux pumps responsible for extruding antibiotics prior to reaching their intended targets, and by inhibiting the activity of existing efflux pumps that contribute to the MDR phenotype of M. tuberculosis. Therefore, this class of drug should be seriously considered in the development of new therapeutic strategies for preventing the emergence of MDR-TB during treatment (18).
Accumulating evidence suggests that TRZ has the potential to be used as an adjunct to standard therapy that might improve the treatment costs and outcomes of active, latent, and drug-resistant TB. Future studies should focus on whether TRZ can improve the sterilizing activity of combination regimens against persistent bacilli in vivo, with the goal of shortening the duration of treatment for drug-susceptible and MDR-TB. Although long-term administration of the phenothiazines is limited by their toxicity, the synthesis of less toxic congeners appears promising (40). Structural modification of the phenothiazine core is possible in a manner that does not affect the ability of the phenothiazine derivatives to inhibit M. tuberculosis but abolishes undesirable dopamine and serotonin receptor binding (41).
ACKNOWLEDGMENTS
We thank Michelle A. Rudek and the Analytical Pharmacology Core Laboratory at The SKCCC at Johns Hopkins for their technical assistance with the pharmacokinetics studies.
Research reported in this publication was supported by the National Heart, Lung, and Blood Institute and the National Institute of Allergy and Infectious Diseases of the National Institutes of Health under award numbers R01 HL106786 and R01 AI083125, respectively, to P.C.K.. The funding sources had no role in the study design, data collection, data analysis, data interpretation, or writing of the report.
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health
We have no conflicts of interest to report.
Footnotes
Published ahead of print 5 May 2014
REFERENCES
- 1.Abubakar I, Zignol M, Falzon D, Raviglione M, Ditiu L, Masham S, Adetifa I, Ford N, Cox H, Lawn SD, Marais BJ, McHugh TD, Mwaba P, Bates M, Lipman M, Zijenah L, Logan S, McNerney R, Zumla A, Sarda K, Nahid P, Hoelscher M, Pletschette M, Memish ZA, Kim P, Hafner R, Cole S, Migliori GB, Maeurer M, Schito M. 2013. Drug-resistant tuberculosis: time for visionary political leadership. Lancet Infect. Dis. 13:529–539. 10.1016/S1473-3099(13)70030-6 [DOI] [PubMed] [Google Scholar]
- 2.Mazumdar K, Dastidar SG, Park JH, Dutta NK. 2009. The anti-inflammatory non-antibiotic helper compound diclofenac: an antibacterial drug target. Eur. J. Clin. Microbiol. Infect. Dis. 28:881–891. 10.1007/s10096-009-0739-z [DOI] [PubMed] [Google Scholar]
- 3.Dutta NK, Karakousis PC. 2012. Tuberculosis chemotherapy: present situation, possible solutions, and progress towards a TB-free world. Indian J. Med. Microbiol. 30:261–263. 10.4103/0255-0857.99481 [DOI] [PubMed] [Google Scholar]
- 4.Louw GE, Warren RM, Gey van Pittius NC, Leon R, Jimenez A, Hernandez-Pando R, McEvoy CR, Grobbelaar M, Murray M, van Helden PD, Victor TC. 2011. Rifampicin reduces susceptibility to ofloxacin in rifampicin-resistant Mycobacterium tuberculosis through efflux. Am. J. Respir. Crit. Care Med. 184:269–276. 10.1164/rccm.201011-1924OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Amaral L, Udwadia Z, Abbate E, van Soolingen D. 2012. The added effect of thioridazine in the treatment of drug-resistant tuberculosis. Int. J. Tuberc. Lung Dis. 16:1706–1709. 10.5588/ijtld.12.0616 [DOI] [PubMed] [Google Scholar]
- 6.Choudhuri BS, Bhakta S, Barik R, Basu J, Kundu M, Chakrabarti P. 2002. Overexpression and functional characterization of an ABC (ATP-binding cassette) transporter encoded by the genes drrA and drrB of Mycobacterium tuberculosis. Biochem. J. 367:279–285. 10.1042/BJ20020615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pasca MR, Guglierame P, Arcesi F, Bellinzoni M, De Rossi E, Riccardi G. 2004. Rv2686c-Rv2687c-Rv2688c, an ABC fluoroquinolone efflux pump in Mycobacterium tuberculosis. Antimicrob. Agents Chemother. 48:3175–3178. 10.1128/AAC.48.8.3175-3178.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kristiansen JE, Thomsen VF, Martins A, Viveiros M, Amaral L. 2010. Non-antibiotics reverse resistance of bacteria to antibiotics. In Vivo 24:751–754 http://iv.iiarjournals.org/content/24/5/751.long [PubMed] [Google Scholar]
- 9.Martins M, Viveiros M, Amaral L. 2008. Inhibitors of Ca2+ and K+ transport enhance intracellular killing of M. tuberculosis by non-killing macrophages. In Vivo 22:69–75 http://iv.iiarjournals.org/content/22/1/69.full.pdf+html [PubMed] [Google Scholar]
- 10.Amaral L, Martins M, Viveiros M. 2007. Enhanced killing of intracellular multidrug-resistant Mycobacterium tuberculosis by compounds that affect the activity of efflux pumps. J. Antimicrob. Chemother. 59:1237–1246. 10.1093/jac/dkl500 [DOI] [PubMed] [Google Scholar]
- 11.Adams KN, Takaki K, Connolly LE, Wiedenhoft H, Winglee K, Humbert O, Edelstein PH, Cosma CL, Ramakrishnan L. 2011. Drug tolerance in replicating mycobacteria mediated by a macrophage-induced efflux mechanism. Cell 145:39–53. 10.1016/j.cell.2011.02.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Black PA, Warren RM, Louw GE, van Helden PD, Victor TC, Kana BD. 2014. Energy metabolism and drug efflux in Mycobacterium tuberculosis. Antimicrob. Agents Chemother. 58:2491–2503. 10.1128/AAC.02293-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Boeree MJ. 2011. Global clinical trials for the treatment of TB with thioridazine. Recent Pat. Antiinfect. Drug Discov. 6:99–103. 10.2174/157489111796064533 [DOI] [PubMed] [Google Scholar]
- 14.Amaral L, Boeree MJ, Gillespie SH, Udwadia ZF, van Soolingen D. 2010. Thioridazine cures extensively drug-resistant tuberculosis (XDR-TB) and the need for global trials is now! Int. J. Antimicrob. Agents 35:524–526. 10.1016/j.ijantimicag.2009.12.019 [DOI] [PubMed] [Google Scholar]
- 15.Thanacoody RH. 2011. Thioridazine: the good and the bad. Recent Pat. Antiinfect. Drug Discov. 6:92–98. 10.2174/157489111796064588 [DOI] [PubMed] [Google Scholar]
- 16.Dutta NK, Mazumdar K, Dastidar SG, Karakousis PC, Amaral L. 2011. New patentable use of an old neuroleptic compound thioridazine to combat tuberculosis: a gene regulation perspective. Recent Pat. Antiinfect. Drug Discov. 6:128–138. 10.2174/157489111796064597 [DOI] [PubMed] [Google Scholar]
- 17.Dutta NK, Mehra S, Kaushal D. 2010. A Mycobacterium tuberculosis sigma factor network responds to cell-envelope damage by the promising anti-mycobacterial thioridazine. PLoS One 5:e10069. 10.1371/journal.pone.0010069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Machado D, Couto I, Perdigao J, Rodrigues L, Portugal I, Baptista P, Veigas B, Amaral L, Viveiros M. 2012. Contribution of efflux to the emergence of isoniazid and multidrug resistance in Mycobacterium tuberculosis. PLoS One 7:e34538. 10.1371/journal.pone.0034538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yano T, Li LS, Weinstein E, Teh JS, Rubin H. 2006. Steady-state kinetics and inhibitory action of antitubercular phenothiazines on mycobacterium tuberculosis type-II NADH-menaquinone oxidoreductase (NDH-2). J. Biol. Chem. 281:11456–11463. 10.1074/jbc.M508844200 [DOI] [PubMed] [Google Scholar]
- 20.Viveiros M, Amaral L. 2001. Enhancement of antibiotic activity against poly-drug resistant Mycobacterium tuberculosis by phenothiazines. Int. J. Antimicrob. Agents 17:225–228. 10.1016/S0924-8579(00)00343-5 [DOI] [PubMed] [Google Scholar]
- 21.van Ingen J, van der Laan T, Amaral L, Dekhuijzen R, Boeree MJ, van Soolingen D. 2009. In vitro activity of thioridazine against mycobacteria. Int. J. Antimicrob. Agents 34:190–191. 10.1016/j.ijantimicag.2009.02.015 [DOI] [PubMed] [Google Scholar]
- 22.Martins M, Viveiros M, Kristiansen JE, Molnar J, Amaral L. 2007. The curative activity of thioridazine on mice infected with Mycobacterium tuberculosis. In Vivo 21:771–775 http://iv.iiarjournals.org/content/21/5/771.full.pdf+html [PubMed] [Google Scholar]
- 23.van Soolingen D, Hernandez-Pando R, Orozco H, Aguilar D, Magis-Escurra C, Amaral L, van Ingen J, Boeree MJ. 2010. The antipsychotic thioridazine shows promising therapeutic activity in a mouse model of multidrug-resistant tuberculosis. PLoS One 5:e12640. 10.1371/journal.pone.0012640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Abbate E, Vescovo M, Natiello M, Cufre M, Garcia A, Gonzalez Montaner P, Ambroggi M, Ritacco V, van Soolingen D. 2012. Successful alternative treatment of extensively drug-resistant tuberculosis in Argentina with a combination of linezolid, moxifloxacin and thioridazine. J. Antimicrob. Chemother. 67:473–477. 10.1093/jac/dkr500 [DOI] [PubMed] [Google Scholar]
- 25.Sohaskey C. 2011. Latent tuberculosis: is there a role for thioridazine? Recent Pat. Antiinfect. Drug Discov. 6:139–146. 10.2174/157489111796064551 [DOI] [PubMed] [Google Scholar]
- 26.Ordway D, Viveiros M, Leandro C, Bettencourt R, Almeida J, Martins M, Kristiansen JE, Molnar J, Amaral L. 2003. Clinical concentrations of thioridazine kill intracellular multidrug-resistant Mycobacterium tuberculosis. Antimicrob. Agents Chemother. 47:917–922. 10.1128/AAC.47.3.917-922.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dutta NK, Pinn ML, Zhao M, Rudek MA, Karakousis PC. 2013. Thioridazine lacks bactericidal activity in an animal model of extracellular tuberculosis. J. Antimicrob. Chemother. 68:1327–1330. 10.1093/jac/dkt037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dutta NK, Illei PB, Peloquin CA, Pinn ML, Mdluli KE, Nuermberger EL, Grosset JH, Karakousis PC. 2012. Rifapentine is not more active than rifampin against chronic tuberculosis in guinea pigs. Antimicrob. Agents Chemother. 56:3726–3731. 10.1128/AAC.00500-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dutta NK, Alsultan A, Peloquin CA, Karakousis PC. 2013. Preliminary pharmacokinetic study of repeated doses of rifampin and rifapentine in guinea pigs. Antimicrob. Agents Chemother. 57:1535–1537. 10.1128/AAC.01933-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nyberg G, Martensson E. 1982. Binding of thioridazine and thioridazine metabolites to serum proteins. An in vitro study. Naunyn Schmiedebergs Arch. Pharmacol. 319:189–196. 10.1007/BF00495864 [DOI] [PubMed] [Google Scholar]
- 31.Ahmad Z, Fraig MM, Bisson GP, Nuermberger EL, Grosset JH, Karakousis PC. 2011. Dose-dependent activity of pyrazinamide in animal models of intracellular and extracellular tuberculosis infections. Antimicrob. Agents Chemother. 55:1527–1532. 10.1128/AAC.01524-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Reference deleted.
- 33.Rosenthal IM, Tasneen R, Peloquin CA, Zhang M, Almeida D, Mdluli KE, Karakousis PC, Grosset JH, Nuermberger EL. 2012. Dose-ranging comparison of rifampin and rifapentine in two pathologically distinct murine models of tuberculosis. Antimicrob. Agents Chemother. 56:4331–4340. 10.1128/AAC.00912-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chakraborty BS, Midha KK, McKay G, Hawes EM, Hubbard JW, Korchinski ED, Choc MG, Robinson WT. 1989. Single dose kinetics of thioridazine and its two psychoactive metabolites in healthy humans: a dose proportionality study. J. Pharm. Sci. 78:796–801. 10.1002/jps.2600781003 [DOI] [PubMed] [Google Scholar]
- 35.Stover CK, Warrener P, VanDevanter DR, Sherman DR, Arain TM, Langhorne MH, Anderson SW, Towell JA, Yuan Y, McMurray DN, Kreiswirth BN, Barry CE, Baker WR. 2000. A small-molecule nitroimidazopyran drug candidate for the treatment of tuberculosis. Nature 405:962–966. 10.1038/35016103 [DOI] [PubMed] [Google Scholar]
- 36.Ahmad Z, Nuermberger EL, Tasneen R, Pinn ML, Williams KN, Peloquin CA, Grosset JH, Karakousis PC. 2010. Comparison of the ‘Denver regimen' against acute tuberculosis in the mouse and guinea pig. J. Antimicrob. Chemother. 65:729–734. 10.1093/jac/dkq007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rodrigues L, Wagner D, Viveiros M, Sampaio D, Couto I, Vavra M, Kern WV, Amaral L. 2008. Thioridazine and chlorpromazine inhibition of ethidium bromide efflux in Mycobacterium avium and Mycobacterium smegmatis. J. Antimicrob. Chemother. 61:1076–1082. 10.1093/jac/dkn070 [DOI] [PubMed] [Google Scholar]
- 38.Li XZ, Zhang L, Nikaido H. 2004. Efflux pump-mediated intrinsic drug resistance in Mycobacterium smegmatis. Antimicrob. Agents Chemother. 48:2415–2423. 10.1128/AAC.48.7.2415-2423.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Viveiros M, Portugal I, Bettencourt R, Victor TC, Jordaan AM, Leandro C, Ordway D, Amaral L. 2002. Isoniazid-induced transient high-level resistance in Mycobacterium tuberculosis. Antimicrob. Agents Chemother. 46:2804–2810. 10.1128/AAC.46.9.2804-2810.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Musuka S, Srivastava S, Siyambalapitiyage Dona CW, Meek C, Leff R, Pasipanodya J, Gumbo T. 2013. Thioridazine pharmacokinetic-pharmacodynamic parameters “Wobble” during treatment of tuberculosis: a theoretical basis for shorter-duration curative monotherapy with congeners. Antimicrob. Agents Chemother. 57:5870–5877. 10.1128/AAC.00829-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Salie S, Hsu NJ, Semenya D, Jardine A, Jacobs M. 25 February 2014. Novel non-neuroleptic phenothiazines inhibit Mycobacterium tuberculosis replication. J. Antimicrob. Chemother. 10.1093/jac/dku036 [DOI] [PubMed] [Google Scholar]