Abstract
The role of Acinetobacter nosocomialis and Acinetobacter pittii, which belong to the A. calcoaceticus-A. baumannii complex, in hospital-acquired infections is increasingly recognized. Here we describe a retrospective cohort study of hospital-acquired A. calcoaceticus-A. baumannii complex infections at a university hospital in Thailand. A total of 222 unique cases were identified between January 2010 and December 2011. The genomospecies of the A. calcoaceticus-A. baumannii complex isolates were classified as follows: A. baumannii, 197 (89%); A. nosocomialis, 18 (8%); and A. pittii, 7 (3%). All A. nosocomialis and A. pittii isolates were susceptible to imipenem and meropenem. The patients infected with A. nosocomialis and A. pittii had lower 30-day mortality than those infected with carbapenem-susceptible A. baumannii (P = 0.025) and carbapenem-resistant A. baumannii (P = 0.013). The factors influencing 30-day mortality were infection with non-baumannii A. calcoaceticus-A. baumannii complex (hazard ratio [HR], 0.12; 95% confidence interval [CI], 0.03 to 0.51; P = 0.004), infection with carbapenem-resistant A. baumannii (HR, 1.57; 95% CI, 0.89 to 2.79; P = 0.105), appropriate empirical antimicrobial therapy (HR, 0.38; 95% CI, 0.23 to 0.61; P < 0.001), and higher acute physiology and chronic health evaluation II (APACHE II) score (HR, 1.15; 95% CI, 1.10 to 1.19; P < 0.001). In Galleria mellonella assays, the survival rates were significantly higher for the larvae infected with A. nosocomialis or A. pittii than for those infected with either carbapenem-susceptible A. baumannii or carbapenem-resistant A. baumannii, but no differences in survival rates were observed between carbapenem-susceptible A. baumannii and carbapenem-resistant A. baumannii. These findings suggest intrinsic differences in virulence between non-baumannii A. calcoaceticus-A. baumannii complex species and A. baumannii but not between carbapenem-susceptible and resistant A. baumannii.
INTRODUCTION
Acinetobacter species have emerged as one of the high-priority hospital-acquired pathogens causing substantial mortality and economic burdens (1). Among the Acinetobacter genomospecies described to date, those belonging to the Acinetobacter calcoaceticus-Acinetobacter baumannii complex are the Acinetobacter genomospecies most commonly found in clinical specimens (2). The A. calcoaceticus-A. baumannii complex is comprised primarily of A. baumannii (genomospecies 2), Acinetobacter nosocomialis (genomospecies 13TU), A. calcoaceticus (genomospecies 1), and Acinetobacter pittii (genomospecies 3) (2, 3). Within the A. calcoaceticus-A. baumannii complex, A. baumannii is considered the species most clinically relevant and most frequently resistant to multiple classes of antimicrobial agents, whereas A. nosocomialis and A. pittii are also regarded as clinically relevant species with a geographically dependent incidence that is lower than that of A. baumannii but is likely increasing (4–6). However, the genomospecies within the A. calcoaceticus-A. baumannii complex cannot be identified by routine biochemical methods (7–9). Epidemiological and clinical studies of Acinetobacter spp. therefore often investigate the A. calcoaceticus-A. baumannii complex as a single entity, which is practical but limits the ability to differentiate the clinical features of infection due to A. baumannii and non-baumannii A. calcoaceticus-A. baumannii complex isolates (3). Nonetheless, recent reports are starting to reveal the distinct clinical characteristics and outcomes of patients infected with A. baumannii and non-baumannii A. calcoaceticus-A. baumannii complex species, primarily, A. nosocomialis and A. pittii (5, 6, 10, 11).
Carbapenems are widely used to treat infections caused by multidrug-resistant (MDR) A. baumannii, and several studies have reported significant associations between the carbapenem susceptibility of the infecting isolates and clinical outcomes, length of hospital stay, and hospital cost (12, 13). In general, non-baumannii A. calcoaceticus-A. baumannii complex isolates are more susceptible than A. baumannii isolates to carbapenems as well as to other classes of agents, including fluoroquinolones, aminoglycosides, and ampicillin-sulbactam (14). Carbapenem-resistant non-baumannii A. calcoaceticus-A. baumannii infections have been reported in intensive care units and tertiary care hospitals but appear to be relatively uncommon in comparison with carbapenem-resistant A. baumannii infections (5, 6, 10, 11).
Patients in hospitals in Thailand have suffered from a high incidence of MDR A. baumannii infections (15). However, data regarding the epidemiological and clinical role of non-baumannii A. calcoaceticus-A. baumannii complex species in this region of high endemicity have not been fully elucidated. To address this issue, we conducted a retrospective study to compare the clinical characteristics of non-baumannii A. calcoaceticus-A. baumannii complex infections and A. baumannii infections and to determine the impact of genomospecies on the clinical outcome with reference to carbapenem susceptibility, empirical antimicrobial selection, and severity of infection. We then aimed to corroborate the clinical outcomes using a Galleria mellonella infection model of A. calcoaceticus-A. baumannii complex species.
MATERIALS AND METHODS
Ethics.
This study was approved by the Institutional Review Board (IRB) of the Faculty of Medicine, Prince of Songkla University (EC: 54-080-14-1-2). The authorized researchers were granted the right to extract the data from the database with waiver of consent because of the retrospective nature of the study. The laboratory work was conducted under an IRB approval from the University of Pittsburgh (PRO12060302).
Patients.
The study was conducted in Songklanagarind Hospital, an 800-bed university hospital located in southern Thailand. Adult (age ≥ 18 years) patients who were admitted between 1 January 2010 and 31 December 2011 and had an infection with an A. calcoaceticus-A. baumannii complex isolate were included in the study. The infection status was identified from the hospital microbiology database and confirmed to be hospital acquired in nature using the diagnostic criteria of the Centers for Disease Control and Prevention (CDC) (16). Pneumonia was diagnosed with the following criteria: new or progressive infiltration on chest radiographic examination and microbiological criteria (positive quantitative cultures of airway specimens yielding ≥105 CFU). Those patients deemed to be colonized without infection were excluded. For urinary tract infection, at least two clinical parameters (fever ≥ 38°C, urgency or frequency of urination, dysuria, or suprapubic tenderness) had to be present, with urine culture yielding ≥105 CFU/ml of A. calcoaceticus-A. baumannii complex species with no more than two species of microorganisms. Patients with skin- and soft-tissue infection, including surgical-site infection, were defined with clinical criteria (purulent drainages or abscess with at least one of the following symptoms: pain or tenderness, localized swelling, redness, or heat at the affected site) and positive culture from aseptically obtained fluid or tissue. Patients with intra-abdominal infection were defined by the presence of purulent drainage or abscess, or evidence of infection found at the time of surgical or radiological intervention, with A. calcoaceticus-A. baumannii complex species isolated from aseptically obtained culture or fluid from the affected organ or peritoneal space. Only the first episodes of infection were included in the analysis to avoid case duplication.
Data collection.
Demographic and clinical data were directly retrieved from the surveillance database of the infection control unit of Songklanagarind Hospital. Additional clinical parameters were extracted from the electronic medical record as needed. The demographic variables included age, sex, indication for admission, comorbidities, previous antimicrobial therapy, and admitted wards. We defined admission from emergency rooms without appointment as an emergency indication for admission. The comorbidities included diabetes mellitus, cardiovascular diseases, cerebrovascular diseases, chronic kidney diseases, HIV infection, and other immunocompromised status such as immunosuppressive therapy and neutropenia. Immunosuppressive therapy was defined as receiving cytotoxic agents within 6 weeks or corticosteroids at a dosage equivalent to or higher than 10 mg of prednisolone daily for more than 5 days within 4 weeks prior to the onset of CRAB infection. Neutropenia was defined as an absolute neutrophil count < 0.5 × 109 neutrophils/liter. The extracted clinical variables included site(s) of infection, severity of infection, and antimicrobial treatment given. We used the acute physiology and chronic health evaluation II (APACHE II) scores determined within 24 h of the onset of infection to determine the severity of illness. The treatment data collected included appropriateness of empirical antimicrobial selection prior to the report of antimicrobial susceptibility and nonantimicrobial interventions, including the use of mechanical ventilation, retention of intravascular/thoracic/intra-abdominal devices, and urinary catheterization. The outcomes included in-hospital mortality, 14-day and 30-day mortality, length of hospital stay, and hospital costs. Data regarding the durations from identification of infection to hospital disposition (discharged or expired) were also collected. The lengths of hospital stay were divided into the total lengths of stay and lengths of stay after identification of infection. Hospital costs were divided into antimicrobial pharmacy costs and the remaining costs.
Identification of A. calcoaceticus-A. baumannii complex species.
Acinetobacter species were presumptively identified as Gram-negative, oxidase-negative, nonmotile, nonfermenting coccobacilli and were identified as A. calcoaceticus-A. baumannii complex on the basis of standard biochemical reactions. Following presumptive identification, the isolates were subjected to PCR for detection of blaOXA-51-like genes. PCR for detection of blaOXA-51-like genes was performed using primers F_oxa51_001 (5′-TAA TGC TTT GAT CGG CCT TG-3′) and R_oxa51_001 (5′-TGG ATT GCA CTT CAT CTT GG-3′) (17). The isolates with a positive result for blaOXA-51-like genes were assigned as A. baumannii. The isolates with a negative result for blaOXA-51-like genes underwent rpoB gene sequencing as reported previously (18), using primers rpoB-F (5′-TAY CGY AAA GAY TTG AAA GAA G-3′) and rpoB-R (5′-CMA CAC CYT TGT TMC CRT GA-3′). Nucleotide sequence homology searches of the rpoB gene sequences were performed using BLAST (http://www.ncbi.nlm.nih.gov/BLAST). Sequences were aligned and compared with the published rpoB sequences of the Acinetobacter type strains (2).
Antimicrobial susceptibility testing.
MICs of 11 agents, including imipenem, meropenem, colistin, ciprofloxacin, amikacin, gentamicin, ceftriaxone, cefotaxime, ceftazidime, piperacillin-tazobactam, and ampicillin-sulbactam, were tested using the broth microdilution method and interpreted according to the CLSI guidelines (19). Cefoperazone-sulbactam susceptibility was determined with the disk diffusion method according to previously described criteria (20). Tigecycline susceptibility was determined with the disk diffusion method and interpreted using the U.S. Food and Drug Administration (FDA) breakpoints for Enterobacteriaceae (21).
Galleria mellonella survival assay.
The G. mellonella larvae infection model for A. baumannii was adapted from the method described by Peleg et al. (22). A. calcoaceticus-A. baumannii complex clinical isolates representing carbapenem-susceptible A. nosocomialis, carbapenem-susceptible A. pittii, carbapenem-susceptible A. baumannii, and carbapenem-nonsusceptible A. baumannii from patients with and without in-hospital mortality were included. Therefore, a total of 8 clinical isolates were used for this assay. The larvae were purchased from Grubco (Fairfield, OH). The bacterial suspension was prepared in 10 mM MgSO4 at approximately 1.5 × 107 CFU/ml, and 10 μl of this suspension (approximately 1.5 × 105 CFU) was injected through the last proleg of each larva. The control larvae were injected with 10 μl of 10 mM MgSO4 without bacteria. The larvae were incubated in petri dishes at 37°C for 6 days and observed for survival every 24 h. The larvae were considered dead when they were unresponsive to touch. Fifteen larvae were used for each experiment in triplicate for a total of 45 larvae per isolate tested.
Statistical analysis.
Clinical characteristics and outcomes of non-baumannii A. calcoaceticus-A. baumannii complex and A. baumannii infection were compared by tabulation, followed by chi-square test or Fisher's exact test as appropriate for categorical variables and Student's t test for continuous variables. The differences of levels of variables were expressed with odds ratio (OR) and 95% confidence interval (CI). The OR for 30-day mortality was first identified by tabulation followed by chi-square test or Fisher's exact test as appropriate. The variables with P values < 0.2 were included in a multivariate logistic regression model. These models were fitted to assess the effect of each characteristic, expressed as adjusted ORs. All independent variables were included in the final model. The significance level was set at 0.05. The association of each variable with the outcome was expressed with adjusted OR and 95% CI.
Survival analysis with Cox proportional hazard regression was used to assess the differences in the durations of survival after developing non-baumannii A. calcoaceticus-A. baumannii complex and A. baumannii infection. The latter was further divided into carbapenem-susceptible and nonsusceptible groups. The time started was defined as the day infection was identified. The time ended was defined as the date that the patient outcome was documented. The influences of Acinetobacter genomospecies, carbapenem susceptibility, APACHE II scores, and appropriate empirical antimicrobial therapy were expressed with hazard ratio (HR) and 95% CI. Student's t test was used to determine the differences in the number of days of survival of the larvae.
RESULTS
Genomospecies distribution.
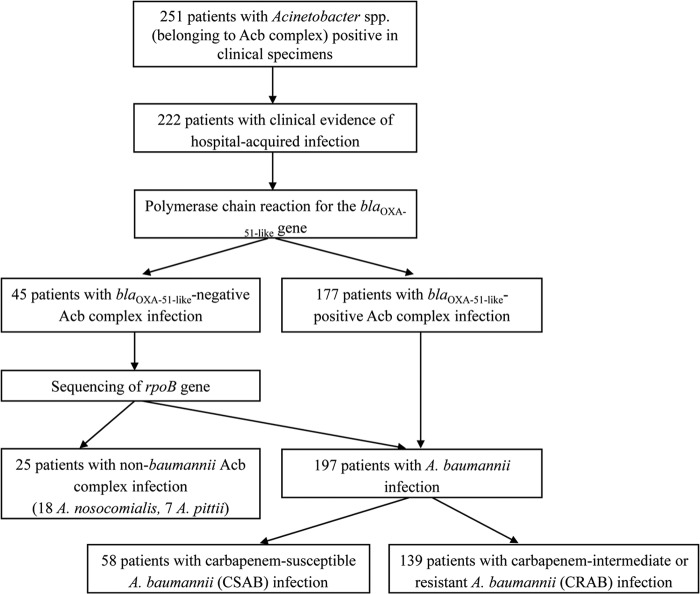
Between 1 January 2010 and 31 December 2011, 251 unique A. calcoaceticus-A. baumannii complex isolates were identified. Of these, 222 isolates were collected from the patients who met the criteria of hospital-acquired infection described above. The genomospecies were as follows: A. baumannii, 197 (89%); A. nosocomialis, 18 (8%); and A. pittii, 7 (3%). Of 197 isolates of A. baumannii, 58 isolates were carbapenem-susceptible A. baumannii (CSAB), 10 isolates were carbapenem-intermediate A. baumannii, and 129 isolates were carbapenem-resistant A. baumannii (CRAB). As carbapenem-intermediate A. baumannii isolates are usually considered resistant to carbapenems in clinical practice, they are included in the CRAB group here. The non-baumannii A. calcoaceticus-A. baumannii complex group consisted exclusively of A. nosocomialis and A. pittii, and all of the isolates were susceptible to imipenem and meropenem. Figure 1 shows the patient enrollment and species identification data.
FIG 1.
Flowchart of the study enrollment. A total of 25, 58, and 139 patients were included in the non-baumannii Acinetobacter calcoaceticus-Acinetobacter baumannii (Acb) complex group, carbapenem-susceptible A. baumannii (CSAB) group, and carbapenem-intermediate or -resistant A. baumannii (CRAB) group.
Antimicrobial susceptibility of non-baumannii A. calcoaceticus-A. baumannii complex and A. baumannii isolates.
The antimicrobial susceptibility data for the three groups are shown in Table 1. All non-baumannii A. calcoaceticus-A. baumannii complex isolates were susceptible to imipenem and meropenem. They also had rates of susceptibility to all other agents tested that were significantly higher than those seen with the CRAB isolates and higher rates of susceptibility to amikacin, ciprofloxacin, ceftriaxone, cefotaxime, ceftazidime, and ampicillin-sulbactam than the CSAB isolates.
TABLE 1.
Comparisons of antimicrobial susceptibilities of non-baumannii A. calcoaceticus-A. baumannii complex and A. baumanniic
| Antimicrobial agent(s) | No. (%) of susceptible isolates |
P valuea | No. (%) of susceptible carbapenem-intermediate or -resistant A. baumannii(CRAB; n = 139) isolates | P valueb | |
|---|---|---|---|---|---|
| Non-baumannii A. calcoaceticus-A. baumannii complex (n = 25) | Carbapenem-susceptible A. baumannii (CSAB; n = 58) | ||||
| Imipenem | 25 (100) | 58 (100) | 0.99 | 0 (0) | <0.001 |
| Meropenem | 25 (100) | 58 (100) | 0.99 | 0 (0) | <0.001 |
| Ampicillin-sulbactam | 19 (76) | 19 (33) | <0.001 | 19 (13) | <0.001 |
| Cefoperazone-sulbactam | 23 (92) | 43 (74) | 0.12 | 16 (12) | <0.001 |
| Piperacillin-tazobactam | 20 (80) | 35 (60) | 0.14 | 9 (7) | <0.001 |
| Gentamicin | 20 (80) | 32 (55) | 0.06 | 9 (7) | <0.001 |
| Amikacin | 20 (80) | 26 (45) | 0.007 | 11 (8) | <0.001 |
| Ciprofloxacin | 21 (84) | 31 (53) | 0.017 | 5 (4) | <0.001 |
| Trimethoprim-sulfamethoxazole | 11 (44) | 28 (48) | 0.91 | 4 (3) | <0.001 |
| Ceftriaxone | 19 (76) | 13 (22) | <0.001 | 3 (2) | <0.001 |
| Cefotaxime | 19 (76) | 13 (22) | <0.001 | 3 (2) | <0.001 |
| Ceftazidime | 22 (88) | 26 (45) | <0.001 | 5 (4) | <0.001 |
| Colistin | 25 (100) | 58 (100) | 0.68 | 139 (100) | 0.40 |
| Tigecycline | 25 (100) | 58 (100) | 0.68 | 139 (100) | 0.40 |
Non-baumannii A. calcoaceticus-A. baumannii complex versus carbapenem-susceptible A. baumannii (CSAB).
Non-baumannii A. calcoaceticus-A. baumannii complex versus carbapenem-intermediate or -resistant A. baumannii (CRAB).
Boldface entries indicate values that reached the significance level set at 0.05.
Clinical features of patients infected with non-baumannii A. calcoaceticus-A. baumannii complex and A. baumannii.
Comparisons of the clinical features of patients infected with non-baumannii A. calcoaceticus-A. baumannii complex (A. nosocomialis or A. pittii) with those of patients infected with CSAB and CRAB are shown in Table 2. Previous therapy with fluoroquinolone, cephalosporin, and carbapenem was significantly less frequent among the patients infected with non-baumannii A. calcoaceticus-A. baumannii complex species than among those infected with CRAB, but no difference from those infected with CSAB was observed. In addition, the duration from admission to infection with non-baumannii A. calcoaceticus-A. baumannii complex was significantly shorter than the duration to infection with CRAB whereas it was significantly longer than the duration to infection with CSAB. We also found that the proportion of patients who were exposed to invasive procedures among those infected with non-baumannii A. calcoaceticus-A. baumannii complex was higher than the proportion of those infected with CSAB whereas it was not different from the proportion of those infected with CRAB.
TABLE 2.
Comparisons of clinical features of the patients infected with non-baumannii A. calcoaceticus-A. baumannii complex and A. baumanniie
| Parameter | Value(s) for patients infected witha: |
P valueb | Value(s) for patients infected with carbapenem-intermediate or -resistant A. baumannii (CRAB; n = 139)a | P valuec | |
|---|---|---|---|---|---|
| Non-baumannii A. calcoaceticus-A. baumannii complex (n = 25) | Carbapenem-susceptible A. baumannii (CSAB; n = 58) | ||||
| Demographics | |||||
| Age (yrs), median (IQRd) | 59 (46–73) | 64 (48–74) | 0.87 | 60 (45–74) | 0.92 |
| Male sex | 17 (68) | 42 (72) | 0.87 | 78 (56) | 0.37 |
| Comorbidities | 8 (32) | 12 (23) | 0.55 | 47 (34) | 1 |
| Clinical characteristics | |||||
| Emergency indication of admission | 19 (76) | 32 (55) | 0.12 | 107 (77) | 1 |
| Initial admission (not mutually exclusive) | |||||
| Intensive care unit | 8 (32) | 16 (28) | 0.87 | 54 (39) | 0.67 |
| Medical ward | 10 (40) | 30 (52) | 0.34 | 64 (46) | 0.81 |
| Duration (days) from admission to infection, median (IQR) | 12 (6–15) | 4 (2–7) | 0.001 | 15 (9–23) | 0.025 |
| Site(s) of infection | 0.29 | 0.70 | |||
| Bloodstream | 1 (4) | 2 (3) | 15 (11) | ||
| Respiratory tract | 16 (64) | 40 (69) | 76 (55) | ||
| Urinary tract | 3 (12) | 6 (10) | 17 (12) | ||
| Skin and soft tissue | 2 (8) | 9 (15) | 20 (14) | ||
| Intra-abdominal | 2 (8) | 0 (0) | 5 (4) | ||
| Two or more sites | 1 (4) | 1 (2) | 6 (4) | ||
| APACHE II score, median (IQR) | 14 (12–17) | 15 (12–20) | 0.79 | 17 (12–22) | 0.12 |
| Invasive procedures | 15 (60) | 8 (15) | <0.001 | 107 (77) | 0.12 |
| Retention of medical devices | 22 (88) | 43 (81) | 0.17 | 96 (69) | 0.09 |
| Previous antimicrobial therapy | |||||
| Penicillin(s) | 4 (16) | 5 (9) | 0.42 | 21 (15) | 1 |
| Aminoglycoside(s) | 8 (32) | 19 (33) | 1 | 49 (35) | 0.93 |
| Cephalosporin(s) | 5 (20) | 12 (21) | 1 | 93 (67) | <0.001 |
| Fluoroquinolone(s) | 7 (28) | 17 (29) | 1 | 98 (71) | <0.001 |
| Carbapenem(s) | 2 (8) | 2 (3) | 0.58 | 83 (60) | <0.001 |
| Appropriate empirical antimicrobial therapy | 16 (64) | 32 (56) | 0.67 | 79 (57) | 0.65 |
Values represent number (percent) of patients unless otherwise indicated.
Non-baumannii A. calcoaceticus-A. baumannii complex versus carbapenem-susceptible A. baumannii (CSAB).
Non-baumannii A. calcoaceticus-A. baumannii complex versus carbapenem-intermediate or -resistant A. baumannii (CRAB).
IQR, interquartile range.
Boldface entries indicate values that reached the significance level set at 0.05.
Clinical outcomes of the patients infected with non-baumannii A. calcoaceticus-A. baumannii complex and A. baumannii.
Comparisons of the clinical outcomes are shown in Table 3. The patients infected with non-baumannii A. calcoaceticus-A. baumannii complex had more favorable outcomes, including mortality, hospital costs, and the length of stay after the onset of infection, than those infected with CRAB. Compared with CSAB-infected patients, those infected with non-baumannii A. calcoaceticus-A. baumannii complex species had nominally lower in-hospital and 14-day mortality and significantly lower 30-day mortality, though these differences were smaller than those seen with CARB-infected patients. The hospital costs were also nominally lower for non-baumannii A. calcoaceticus-A. baumannii complex infection cases than for CSAB infection cases, but the difference did not reach statistical significance.
TABLE 3.
Comparisons of outcomes for the patients infected with non-baumannii A. calcoaceticus-A. baumannii complex and A. baumanniid
| Outcome | Values for patients infected with non-baumannii A. calcoaceticus-A. baumannii complex (n = 25) | Values for patients infected with carbapenem-susceptible A. baumannii (CSAB; n = 58) | P valuea | Values for patients infected with carbapenem-intermediate or -resistant A. baumannii (CRAB; n = 139) | P valueb |
|---|---|---|---|---|---|
| Mortality, no. (%) of patients | |||||
| In-hospital | 3 (12) | 20 (35) | 0.067 | 79 (57) | <0.001 |
| 14 day | 1 (4) | 10 (17) | 0.160 | 42 (30) | <0.001 |
| 30 day | 2 (8) | 20 (35) | 0.025 | 78 (56) | 0.013 |
| Length of hospital stay after infection (days), median (IQR) | 9 (3–14) | 4 (1–9) | 0.368 | 23 (12–52) | <0.001 |
| Cost (bahtc), median (IQR) | |||||
| Total hospital | 88,443 (33,235-100,216) | 38,845 (32,418-66,757) | 0.055 | 123,552 (98,633-170,926) | <0.001 |
| Antimicrobial | 12,006 (7,902-23,902) | 10,034 (9,000–12,987) | 0.154 | 33,456 (22,997-43,757) | <0.001 |
| Nonantimicrobial | 45,456 (23,435-89,654) | 32,334 (22,723-54,672) | 0.104 | 89,312 (70,539-124,521) | <0.001 |
Non-baumannii A. calcoaceticus-A. baumannii complex versus carbapenem-susceptible A. baumannii (CSAB).
Non-baumannii A. calcoaceticus-A. baumannii complex versus carbapenem-intermediate or -resistant A. baumannii (CRAB).
1 U.S. dollar = 32.38 baht (as of 20 March 2014).
Boldface entries indicate values that reached the significance level set at 0.05.
Factors influencing 30-day mortality among patients infected with non-baumannii A. calcoaceticus-A. baumannii complex and A. baumannii.
The demographic and clinical factors predictive of 30-day mortality are shown in Table 4. High APACHE II scores and infection with CRAB were significantly associated with 30-day mortality, whereas infection with non-baumannii A. calcoaceticus-A. baumannii complex and appropriate empirical antimicrobial therapy were significant protective factors against 30-day mortality. Kaplan-Meier survival curves for the 30 days following infection caused by non-baumannii A. calcoaceticus-A. baumannii complex, CSAB, and CRAB are shown in Fig. 2. The survival rates of patients among these three groups were significantly different, with non-baumannii A. calcoaceticus-A. baumannii complex infection and CRAB infection associated with the lowest and highest mortality, respectively (P < 0.001, log-rank test). The survival analysis with the Cox proportional hazard model showed that the factors influencing 30-day mortality were infection with non-baumannii A. calcoaceticus-A. baumannii complex (HR, 0.12; 95% CI, 0.03 to 0.51; P = 0.004), appropriate empirical antimicrobial therapy (HR, 0.38; 95% CI, 0.23 to 0.61; P < 0.001), infection with carbapenem-resistant isolates (HR, 1.57; 95% CI, 0.89 to 2.79; P = 0.105), and higher APACHE II score (HR, 1.15; 95% CI, 1.10 to 1.19; P < 0.001).
TABLE 4.
Factors influencing 30-day mortality among the 222 patients infected with A. calcoaceticus-A. baumannii complex
| Parameter | Valuesa |
Crude OR (95% CI) | Adjusted OR (95% CI) | P valued | |
|---|---|---|---|---|---|
| Survivor (n = 122) | Nonsurvivor (n = 100) | ||||
| Age (yrs), median (IQR)b | 58 (45–71) | 65 (48–76) | 1.00 (0.98–1.00) | 1.02 (0.96–1.04) | 0.07 |
| Male sex | 82 (67.2) | 55 (55.0) | 0.64 (0.36–1.12) | ||
| Comorbidities | 31 (25.4) | 36 (36.0) | 1.47 (0.81–2.63) | ||
| Emergency indication for admission | 34 (27.9) | 30 (30.0) | 1.09 (0.59–2.00) | ||
| APACHE II score, median (IQR)b | 13 (11–15) | 22 (17–25) | 1.33 (1.23–1.43) | 1.32 (1.20–1.43) | <0.001 |
| Initial ICUc admission | 40 (32.8) | 38 (38.0) | 1.25 (0.71–2.22) | ||
| Retention of medical devices | 90 (73.8) | 71 (71.0) | 0.84 (0.45–1.56) | ||
| Bacteremia | 13 (10.7) | 13 (13.0) | 1.61 (0.68–3.85) | ||
| Pneumonia | 77 (63.1) | 63 (63.0) | 1.03 (0.58–1.82) | ||
| Appropriate empirical antimicrobial therapy | 88 (72.1) | 39 (39.0) | 0.22 (0.12–0.39) | 0.28 (0.13–0.63) | 0.002 |
| Infection with non-baumannii A. calcoaceticus-A. baumannii species | 23 (18.9) | 2 (2.0) | 0.09 (0.02–0.41) | 0.08 (0.01–0.63) | 0.005 |
| Infection with CRAB | 61 (50.0) | 78 (78.0) | 3.57 (1.89–6.67) | 2.50 (1.03–6.25) | 0.029 |
Values represent number (percent) of patients unless otherwise indicated.
Continuous data.
ICU, intensive care unit.
Boldface entries indicate values that reached the significance level set at 0.05.
FIG 2.
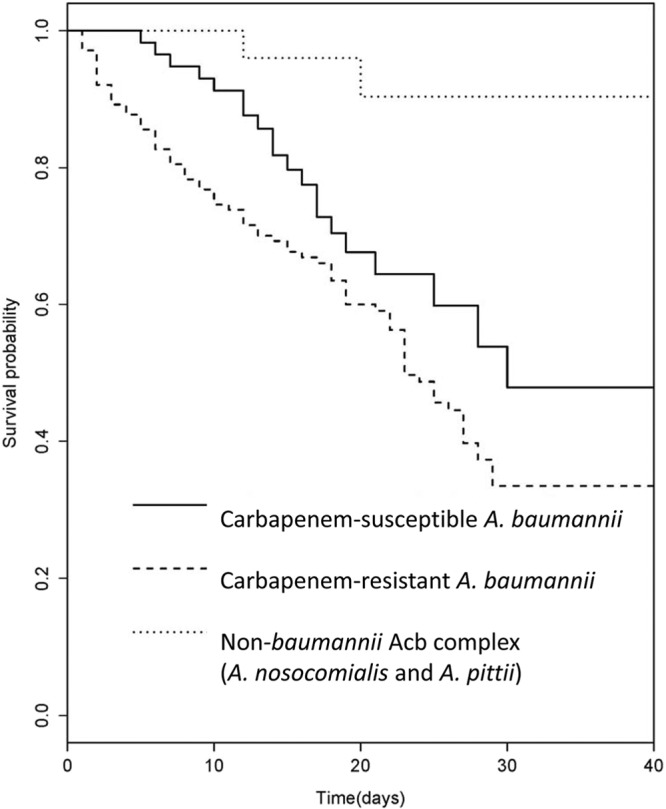
Kaplan-Meier survival curves of the patients in the non-baumannii A. calcoaceticus-A. baumannii complex group (A. nosocomialis or A. pittii), carbapenem-susceptible A. baumannii (CSAB) group, and carbapenem-intermediate or -resistant A. baumannii (CRAB) group. Levels of survival among these three groups were significantly different, with non-baumannii A. calcoaceticus-A. baumannii complex infection and CRAB infection associated with the lowest and highest mortality levels, respectively (P < 0.001, log-rank test).
Galleria mellonella assays.
To corroborate the findings from the clinical data discussed above, we used a G. mellonella infection model to determine survival of larvae infected with non-baumannii A. calcoaceticus-A. baumannii complex isolates, CSAB isolates, or CRAB isolates. G. mellonella survival data are shown in Fig. 3. Survival rates were significantly higher for the larvae infected with non-baumannii A. calcoaceticus-A. baumannii complex species than for those infected with A. baumannii, either CSAB or CRAB, in every observation between day 1 and day 6 (P < 0.01 by Student's t test). There was no significant difference in survival rates between the larvae injected with A. nosocomialis and those injected with A. pittii (data not shown). Furthermore, there was no significant difference in survival rates between the larvae infected with CSAB and those injected with CRAB with any of the observations (Fig. 3).
FIG 3.
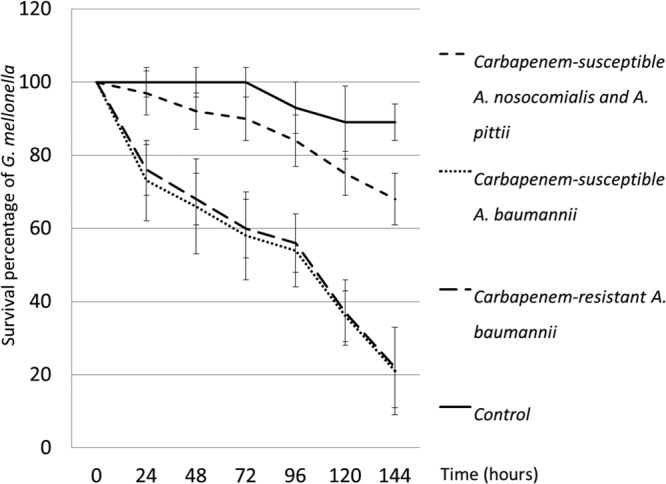
Survival of G. mellonella. The larvae were infected with 2 A. nosocomialis, 2 A. pittii, 2 carbapenem-susceptible A. baumannii (CSAB), and 2 carbapenem-resistant A. baumannii (CRAB) isolates and observed daily for 6 days. Survival rates were significantly higher for the larvae infected with non-baumannii A. calcoaceticus-A. baumannii complex species than for those infected with A. baumannii, either CSAB or CRAB (P < 0.05, Student's t test), whereas there was no significant difference in the levels of survival between the larvae infected with CSAB and those infected with CRAB.
DISCUSSION
Non-baumannii species of the A. calcoaceticus-A. baumannii complex, in particular, A. nosocomialis and A. pittii, are increasingly recognized as significant causes of hospital-acquired infections (2, 5, 9). Observational clinical studies have demonstrated that the mortality rate for patients with infection due to non-baumannii A. calcoaceticus-A. baumannii complex species is lower than the mortality rate for those infected with A. baumannii (4, 5, 23, 24). However, A. baumannii is consistently less susceptible to various antimicrobials than the non-baumannii A. calcoaceticus-A. baumannii complex species; thus, the mortality data are often affected by confounding influences from the differences in the appropriateness of therapy, especially for the empirical phase of treatment. In practice, resistance to carbapenem is the most significant factor affecting the appropriateness of therapy, because it is the class of choice when hospital-acquired infection from Acinetobacter spp. is suspected, and the vast majority of carbapenem-resistant isolates across studies have been A. baumannii (4, 5, 23, 24). We therefore aimed to address this limitation by (i) comparing the clinical characteristics and outcomes of non-baumannii A. calcoaceticus-A. baumannii complex infection with those of carbapenem-susceptible and carbapenem-resistant A. baumannii infections separately and (ii) comparing rates of survival of infections by these three groups using a G. mellonella waxworm model, where no therapy is given and, thus, appropriateness of therapy does not affect the outcome.
Our study yielded several interesting observations. First, severity of illness, infection with CRAB, and inappropriate empirical therapy were independently associated with 30-day mortality, whereas infection with non-baumannii A. calcoaceticus-A. baumannii complex species was protective against mortality, an observation which was comparable with those of previous reports (4, 5, 23, 24). Accordingly, in-hospital, 14-day, and 30-day mortality rates were significantly higher for A. baumannii infection than for non-baumannii A. calcoaceticus-A. baumannii complex infection. However, when only the CSAB cases were included for the comparison, the overall mortality and 14-day mortality were no longer significantly higher for A. baumannii infections than for non-baumannii A. calcoaceticus-A. baumannii complex infections. This finding suggested that at least part of the excess mortality from A. baumannii infection was caused by antimicrobial resistance and the resulting inappropriateness of therapy. This would likely be prominent in the empirical-therapy setting, but, given the somewhat uncertain clinical efficacy of regimens (e.g., colistin and tigecycline) used for carbapenem-resistant cases, it may also negatively affect the therapeutic benefit from definitive, or pathogen-specific, therapy, thus contributing to higher mortality.
Second, survival of G. mellonella larvae was significantly better when they were infected with non-baumannii A. calcoaceticus-A. baumannii complex species (A. nosocomialis or A. pittii) than when they were infected with A. baumannii. On the other hand, there was no difference in the survival rates of the larvae that were infected with CSAB or CRAB isolates. While this is a relatively crude in vivo model of infection, the findings appear to complement the findings from the clinical study and support the notions that (i) A. baumannii is intrinsically more virulent and causes higher mortality than the non-baumannii A. calcoaceticus-A. baumannii complex species and (ii) the difference in the mortality rates observed clinically between patients infected with CSAB and CRAB may at least partly be accounted for by the difference in the appropriateness and efficacy of the antimicrobial therapies given.
Although the proportions of patients who received appropriate empirical therapy upon infection with non-baumannii A. calcoaceticus-A. baumannii complex species were not different from the proportions of those infected with CSAB and CRAB, the 30-day morality rates among these 3 groups were significantly different. This finding also suggests the impact of genomospecies for clinical outcomes. However, there are several potential caveats as we interpret these data. Appropriateness of therapy is defined based on in vitro activity data. In addition, agents considered appropriate for CRAB include colistin and tigecycline, which may have their own limitations such as low serum and epithelial lining fluid concentrations (21, 25).
There were several limitations in this study that must be acknowledged. First, the retrospective study design did not allow us to investigate factors influencing the physicians in selecting antimicrobial as well as overall treatment approaches. Second, this study was conducted in a single tertiary teaching hospital; thus, the findings may not be generalizable to other settings. Third, because of the relatively small number of patients who were infected with non-baumannii A. calcoaceticus-A. baumannii complex species, we could not analyze the characteristics and outcomes of patients infected with A. nosocomialis and A. pittii separately. Fourth, the screening test for A. baumannii with blaOXA-51-like genes may be affected by the emergence of carbapenem-resistant non-baumannii A. calcoaceticus-A. baumannii complex species harboring this gene, which appears to be a rare event (26). Finally, the observations in G. mellonella assays would require confirmation in vertebrate animal models.
In conclusion, the patients infected with non-baumannii A. calcoaceticus-A. baumannii complex species had more favorable outcomes than those infected with either carbapenem-susceptible or carbapenem-resistant A. baumannii. This difference appeared to stem primarily from the intrinsic virulence of the organism in addition to the appropriateness of therapy given. Our findings call for further investigation of virulence and pathogenesis among different genomospecies within the A. calcoaceticus-A. baumannii complex.
ACKNOWLEDGMENTS
The study was supported by the Royal Golden Jubilee Ph.D. Program and the Research Chair grant from the National Science and Technology Development Agency of Thailand awarded to V.C. The study was also supported in part by research grants from the National Institutes of Health to Y.D. (R01AI104895 and R21AI107302).
Footnotes
Published ahead of print 12 May 2014
REFERENCES
- 1.Peleg AY, Seifert H, Paterson DL. 2008. Acinetobacter baumannii: emergence of a successful pathogen. Clin. Microbiol. Rev. 21:538–582. 10.1128/CMR.00058-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nemec A, Krizova L, Maixnerova M, van der Reijden TJ, Deschaght P, Passet V, Vaneechoutte M, Brisse S, Dijkshoorn L. 2011. Genotypic and phenotypic characterization of the Acinetobacter calcoaceticus-Acinetobacter baumannii complex with the proposal of Acinetobacter pittii sp. nov. (formerly Acinetobacter genomic species 3) and Acinetobacter nosocomialis sp. nov. (formerly Acinetobacter genomic species 13TU). Res. Microbiol. 162:393–404. 10.1016/j.resmic.2011.02.006 [DOI] [PubMed] [Google Scholar]
- 3.Gerner-Smidt P, Tjernberg I, Ursing J. 1991. Reliability of phenotypic tests for identification of Acinetobacter species. J. Clin. Microbiol. 29:277–282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Karah N, Haldorsen B, Hegstad K, Simonsen GS, Sundsfjord A, Samuelsen O. 2011. Species identification and molecular characterization of Acinetobacter spp. blood culture isolates from Norway. J. Antimicrob. Chemother. 66:738–744. 10.1093/jac/dkq521 [DOI] [PubMed] [Google Scholar]
- 5.Lee YC, Huang YT, Tan CK, Kuo YW, Liao CH, Lee PI, Hsueh PR. 2011. Acinetobacter baumannii and Acinetobacter genospecies 13TU and 3 bacteraemia: comparison of clinical features, prognostic factors and outcomes. J. Antimicrob. Chemother. 66:1839–1846. 10.1093/jac/dkr200 [DOI] [PubMed] [Google Scholar]
- 6.Chuang YC, Sheng WH, Li SY, Lin YC, Wang JT, Chen YC, Chang SC. 2011. Influence of genospecies of Acinetobacter baumannii complex on clinical outcomes of patients with acinetobacter bacteremia. Clin. Infect. Dis. 52:352–360. 10.1093/cid/ciq154 [DOI] [PubMed] [Google Scholar]
- 7.Paterson DL. 2006. The epidemiological profile of infections with multidrug-resistant Pseudomonas aeruginosa and Acinetobacter species. Clin. Infect. Dis. 43(Suppl 2):S43–S48. 10.1086/504476 [DOI] [PubMed] [Google Scholar]
- 8.Maragakis LL, Perl TM. 2008. Acinetobacter baumannii: epidemiology, antimicrobial resistance, and treatment options. Clin. Infect. Dis. 46:1254–1263. 10.1086/529198 [DOI] [PubMed] [Google Scholar]
- 9.Munoz-Price LS, Weinstein RA. 2008. Acinetobacter infection. N. Engl. J. Med. 358:1271–1281. 10.1056/NEJMra070741 [DOI] [PubMed] [Google Scholar]
- 10.Park YK, Jung SI, Park KH, Kim SH, Ko KS. 2012. Characteristics of carbapenem-resistant Acinetobacter spp. other than Acinetobacter baumannii in South Korea. Int. J. Antimicrob. Agents 39:81–85. 10.1016/j.ijantimicag.2011.08.006 [DOI] [PubMed] [Google Scholar]
- 11.Park KH, Shin JH, Lee SY, Kim SH, Jang MO, Kang SJ, Jung SI, Chung EK, Ko KS, Jang HC. 2013. The clinical characteristics, carbapenem resistance, and outcome of Acinetobacter bacteremia according to genospecies. PLoS One 8:e65026. 10.1371/journal.pone.0065026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Huang ST, Chiang MC, Kuo SC, Lee YT, Chiang TH, Yang SP, Ti Y, Chen TL, Fung CP. 2012. Risk factors and clinical outcomes of patients with carbapenem-resistant Acinetobacter baumannii bacteremia. J. Microbiol. Immunol. Infect. 45:356–362. 10.1016/j.jmii.2011.12.009 [DOI] [PubMed] [Google Scholar]
- 13.Zheng YL, Wan YF, Zhou LY, Ye ML, Liu S, Xu CQ, He YQ, Chen JH. 2013. Risk factors and mortality of patients with nosocomial carbapenem-resistant Acinetobacter baumannii pneumonia. Am. J. Infect. Control 41:e59–e63. 10.1016/j.ajic.2013.01.006 [DOI] [PubMed] [Google Scholar]
- 14.Espinal P, Roca I, Vila J. 2011. Clinical impact and molecular basis of antimicrobial resistance in non-baumannii Acinetobacter. Future Microbiol. 6:495–511. 10.2217/fmb.11.30 [DOI] [PubMed] [Google Scholar]
- 15.Rongrungruang Y, Sawanpanyalert N, Chomdacha P, Surasarang K, Wiruchkul N, Kachintorn K, Tantilipikara P, Danchaivijitr S. 2013. Health-care associated infections in Thailand 2011. J. Med. Assoc. Thai. 96(Suppl 2):S117–S123 [PubMed] [Google Scholar]
- 16.Garner JS, Jarvis WR, Emori TG, Horan TC, Hughes JM. 1988. CDC definitions for nosocomial infections, 1988. Am. J. Infect. Control 16:128–140. 10.1016/0196-6553(88)90053-3 [DOI] [PubMed] [Google Scholar]
- 17.Woodford N, Ellington MJ, Coelho JM, Turton JF, Ward ME, Brown S, Amyes SG, Livermore DM. 2006. Multiplex PCR for genes encoding prevalent OXA carbapenemases in Acinetobacter spp. Int. J. Antimicrob. Agents 27:351–353. 10.1016/j.ijantimicag.2006.01.004 [DOI] [PubMed] [Google Scholar]
- 18.La Scola B, Gundi VA, Khamis A, Raoult D. 2006. Sequencing of the rpoB gene and flanking spacers for molecular identification of Acinetobacter species. J. Clin. Microbiol. 44:827–832. 10.1128/JCM.44.3.827-832.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Clinical and Laboratory Standards Institute. 2013. Performance standards for antimicrobial susceptibility testing: 23rd informational supplement. Clinical and Laboratory Standards Institute, Wayne, PA [Google Scholar]
- 20.Barry AL, Jones RN. 1988. Criteria for disk susceptibility tests and quality control guidelines for the cefoperazone-sulbactam combination. J. Clin. Microbiol. 26:13–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.United States Food and Drug Administration. 2013. Highlights of prescribing information (Tygacil). United States Food and Drug Administration, Silver Spring, MD [Google Scholar]
- 22.Peleg AY, Jara S, Monga D, Eliopoulos GM, Moellering RC, Jr, Mylonakis E. 2009. Galleria mellonella as a model system to study Acinetobacter baumannii pathogenesis and therapeutics. Antimicrob. Agents Chemother. 53:2605–2609. 10.1128/AAC.01533-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wisplinghoff H, Paulus T, Lugenheim M, Stefanik D, Higgins PG, Edmond MB, Wenzel RP, Seifert H. 2012. Nosocomial bloodstream infections due to Acinetobacter baumannii, Acinetobacter pittii and Acinetobacter nosocomialis in the United States. J. Infect. 64:282–290. 10.1016/j.jinf.2011.12.008 [DOI] [PubMed] [Google Scholar]
- 24.Schleicher X, Higgins PG, Wisplinghoff H, Korber-Irrgang B, Kresken M, Seifert H. 2013. Molecular epidemiology of Acinetobacter baumannii and Acinetobacter nosocomialis in Germany over a 5-year period (2005–2009). Clin. Microbiol. Infect. 19:737–742. 10.1111/1469-0691.12026 [DOI] [PubMed] [Google Scholar]
- 25.Yapa SWS, Li J, Patel K, Wilson JW, Dooley MJ, George J, Clark D, Poole S, Williams E, Porter CJ, Nation RL, McIntosh MP. 2014. Pulmonary and systemic pharmacokinetics of inhaled and intravenous colistin methanesulfonate in cystic fibrosis patients: targeting advantage of inhalational administration. Antimicrob. Agents Chemother. 58:2570–2579. 10.1128/AAC.01705-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lee YT, Kuo SC, Chiang MC, Yang SP, Chen CP, Chen TL, Fung CP. 2012. Emergence of carbapenem-resistant non-baumannii species of Acinetobacter harboring a blaOXA-51-like gene that is intrinsic to A. baumannii. Antimicrob. Agents Chemother. 56:1124–1127. 10.1128/AAC.00622-11 [DOI] [PMC free article] [PubMed] [Google Scholar]