Abstract
Anthrax is an acute infectious disease caused by the spore-forming bacterium Bacillus anthracis. Timely administration of antibiotics approved for the treatment of anthrax disease may prevent associated morbidity and mortality. However, any delay in initiating antimicrobial therapy may result in increased mortality, as inhalational anthrax progresses rapidly to the toxemic phase of disease. An anthrax antitoxin, AVP-21D9, also known as Thravixa (fully human anthrax monoclonal antibody), is being developed as a therapeutic agent against anthrax toxemia. The efficacy of AVP-21D9 in B. anthracis-infected New Zealand White rabbits and in cynomolgus macaques was evaluated, and its safety and pharmacokinetics were assessed in healthy human volunteers. The estimated mean elimination half-life values of AVP-21D9 in surviving anthrax-challenged rabbits and nonhuman primates (NHPs) ranged from approximately 2 to 4 days and 6 to 11 days, respectively. In healthy humans, the mean elimination half-life was in the range of 20 to 27 days. Dose proportionality was observed for the maximum serum concentration (Cmax) of AVP-21D9 and the area under the concentration-time curve (AUC). In therapeutic efficacy animal models, treatment with AVP-21D9 resulted in survival of up to 92% of the rabbits and up to 67% of the macaques. Single infusions of AVP-21D9 were well tolerated in healthy adult volunteers across all doses evaluated, and no serious adverse events were reported. (This study has been registered at ClinicalTrials.gov under registration no. NCT01202695.)
INTRODUCTION
Bacillus anthracis, the etiologic agent of anthrax disease, is classified by the Centers for Disease Control and Prevention as a category A biological threat agent and poses a risk to national security because of its ease of dissemination and the high mortality rates that would occur within a population in the event of exposure by inhalation. The disease occurs when an individual is exposed to B. anthracis via gastrointestinal, cutaneous, injection, or inhalation routes. Inhalational anthrax is the most lethal form of the disease, and if untreated, it is nearly 100% fatal (1). The morbidity and mortality caused by B. anthracis are predominantly due to three well-characterized virulence factors, which include a polyglutamate capsule and two protein exotoxins. The polyglutamate capsule prevents phagocytosis of the bacterium (2). Three polypeptides, protective antigen (PA), lethal factor (LF), and edema factor (EF), interact to form two exotoxins. PA and LF combine to produce anthrax lethal toxin (LT), and PA and EF combine to produce edema toxin (ET) (2). LT is the predominant cause of severe disease and death following inhalational B. anthracis spore exposure (3, 4).
Mortality may be prevented if antibiotics are administered starting shortly after the exposure to spores (5, 6). However, any delay in initiating antimicrobial therapy may result in toxemia, which accounts for most of the morbidity and mortality associated with progressive inhalational anthrax disease (7, 8). The use of anthrax antitoxins, such as AVP-21D9, has been investigated as a treatment against anthrax toxemia (9–13).
AVP-21D9 is a fully human anti-PA monoclonal IgG1(κ) antibody originally derived from plasma collected from a healthy volunteer who had been immunized with at least four doses of BioThrax (Anthrax Vaccine Adsorbed) and had high levels of anti-PA antibodies (14). AVP-21D9 binds to B. anthracis PA with subnanomolar affinity and neutralizes anthrax toxins (15). Previously, Peterson et al. (15) showed that AVP-21D9 rescued 100% of the rabbits at a dose level as low as 1 mg/kg of body weight when administered at the same time as an intranasal challenge with anthrax spores.
It is not feasible to evaluate the efficacy of medical countermeasures against category A agents in clinical studies, because the incidence of naturally occurring disease is too low, and it is unethical to intentionally expose humans to these pathogens (16). An alternative approach is to perform pharmacokinetic (PK) and efficacy studies in animals and utilize PK parameters, such as maximum and minimum concentrations, area under the concentration-time curve, and elimination half-life, from both naive and B. anthracis-infected animals to correlate the efficacious dose in animals with a comparable dose in humans (17, 18). Rabbits and nonhuman primates (NHPs) are considered acceptable models of inhalational anthrax and are widely used for assessing the efficacy of anthrax countermeasures, such as vaccines and therapeutics (19, 20, 21). Polyclonal and monoclonal antibody-based therapeutics for anthrax (9–13, 22) have been evaluated using these models. Here, we evaluated the therapeutic efficacy of AVP-21D9 in New Zealand White (NZW) rabbits and cynomolgus macaques exposed to aerosolized B. anthracis spores and subsequently treated upon detection of a clinical sign or biomarker of infection. The safety and PK of AVP-21D9 in humans were also assessed in a phase I clinical study (registered at ClinicalTrials.gov under registration no. NCT01202695).
MATERIALS AND METHODS
Test and control articles.
AVP-21D9 was expressed in Chinese hamster ovary (CHO)-K1 cells adapted to growth in serum-free medium in Integra cell culture flasks (Integra Biosciences US, Hudson, NH), and it was produced at a 100-liter scale in a bioreactor, in compliance with the current good manufacturing practices at WuXi AppTec (Philadelphia, PA). AVP-21D9 antibodies were affinity purified on a protein A column to >95% purity as judged by sodium dodecyl sulfate-polyacrylamide gel electrophoresis analysis. The mean (± standard deviation) binding affinity to PA was 0.05 ± 0.03 nM, as determined using a BiaCore 3000 instrument (BiaCore Life Sciences, Piscataway, NJ). Sterile pyrogen-free normal saline for injection was used for the negative-control groups.
Phase I clinical study.
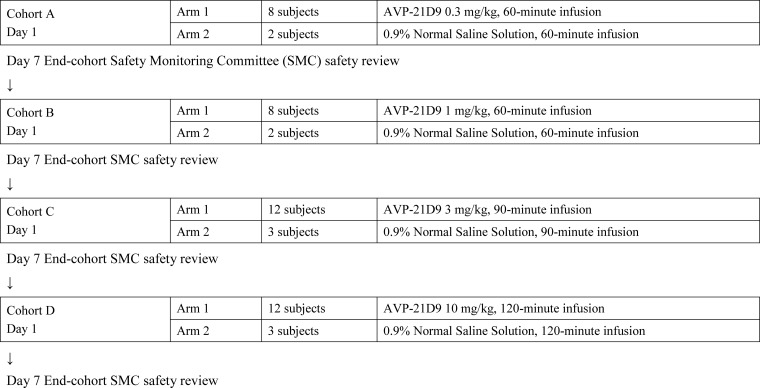
The phase I clinical study was conducted as a double-blind placebo-controlled dose-escalation cohort study at ICON Development Solutions (Austin, TX) with the IntegReview ethics review board as the institutional review board (IRB) (Austin, TX). The study was conducted in accordance with the principles of the International Conference on Harmonization (ICH) E6 Guideline on Good Clinical Practices (GCP) and the principles of the Declaration of Helsinki, and it was approved by the IRB. The main objective was to evaluate the safety and PK of intravenously (i.v.)-infused AVP-21D9 at four dose levels (0.3, 1, 3, and 10 mg/kg) in healthy volunteers between 18 and 45 years of age. The clinical design is shown in Fig. 1. A total of 50 healthy subjects of both sexes were enrolled in the study. The study was conducted in a double-blinded manner; however, the pharmacist and pharmacy technician were unblinded for the purpose of dose preparation. Within the cohorts, each subject was randomized to receive either a single infusion of AVP-21D9 or 0.9% normal saline (placebo) on day 1 in a 250-ml volume. The infusions were delivered over 60 min (cohorts A and B), 90 min (cohort C), or 120 min (cohort D). AVP-21D9 dosing began with the lowest dose (0.3 mg/kg), and sequential dose escalations in subsequent cohorts occurred throughout the study. Safety was assessed through the recording of adverse events and vital signs, physical exams, electrocardiograms, and clinical laboratory testing up to 90 days postinfusion. Pulse oximetry was also performed from the initiation of infusion to 2 h postinfusion. When all subjects in a cohort had completed their day 7 visit, their safety data were summarized for the Safety Monitoring Committee (SMC). The SMC had to recommend dose escalation prior to dosing any subjects at the next highest dose.
FIG 1.
Schematic of dosing cohorts A to D.
Blood samples for determining anti-PA (IgG) concentrations in serum were collected within 1 h preinfusion and at 5 min, 1 h, 1.5 h, 3 h, 6 h, 24 h, and 48 h postinfusion, as well as on days 5, 7, 14, 28, 42, 60, 74, and 90 postinfusion. PK assessment was performed using a Meso Scale Discovery (MSD) electrochemiluminescence (ECL) PA-binding assay (23), described below. The functional activity of AVP-21D9 was assessed by a qualified toxin-neutralizing antibody (TNA) assay (24) on days 2, 28, 60, and 74 postinfusion.
Design of the therapeutic efficacy studies in rabbits and NHPs.
The animal studies were conducted at Battelle Biomedical Research Center (West Jefferson, OH). The work was performed in compliance with the Animal Welfare Act and followed the principles outlined in the National Research Council Guide for the Care and Use of Laboratory Animals. All animal protocols were approved by the Institutional Animal Care and Use Committee prior to the initiation of any studies.
Sixty-two specific-pathogen-free New Zealand White (NZW) rabbits (Oryctolagus cuniculus), approximately 9 months old and weighing between 3.5 and 4.1 kg, were used in the study. Forty-eight cynomolgus macaques (Macaca fascicularis) of Asian origin (Covance Laboratories, Denver, PA, USA), 1.5 to 4.0 years old and weighing between 2.4 and 4.2 kg, were used. Only healthy animals of the specified weight range and that were free of obvious clinical signs of disease or malformations were placed in the studies, and an equal number of male and female animals were assigned to each treatment group. NHPs were confirmed to be seronegative for anti-PA IgG by enzyme-linked immunosorbent assay (ELISA) before being exposed to B. anthracis. The animals were randomized by weight into the treatment groups (Tables 1 and 2). Normal saline was used as a negative control.
TABLE 1.
Rabbit efficacy study design
| Treatment group | Treatment | No. of animals per groupa | AVP-21D9 dose (mg/kg of body weight) |
|---|---|---|---|
| 1 | AVP-21D9 | 12 | 20 |
| 2 | AVP-21D9 | 14 | 10 |
| 3 | AVP-21D9 | 14 | 5 |
| 4 | AVP-21D9 | 14 | 1 |
| 5 | Saline | 8 | 0 |
There were equal numbers of male and female animals per group.
TABLE 2.
Nonhuman primate efficacy study design
| Treatment group | Treatment | No. of animals per groupa | AVP-21D9 dose (mg/kg of body weight) |
|---|---|---|---|
| 1 | AVP-21D9 | 10 | 20 |
| 2 | AVP-21D9 | 10 | 10 |
| 3 | AVP-21D9 | 10 | 5 |
| 4 | AVP-21D9 | 10 | 1 |
| 5 | Saline | 8 | 0 |
There were equal numbers of male and female animals per group.
On study day 0, the animals were individually placed into a plethysmography chamber in a class III biosafety cabinet. The rabbits and NHPs were challenged with a targeted inhaled dose of 200 times the 50% lethal dose (200 LD50) of B. anthracis Ames strain spores aerosolized by a Collison nebulizer (BGI, Waltham, MA, USA) (25, 26). The NHPs were anesthetized with Telazol (1 to 6 mg/kg, administered intramuscularly [i.m.]) prior to being placed into a plethysmography chamber. The rabbits were not anesthetized prior to anthrax exposure. To determine the actual inhaled aerosol concentrations of B. anthracis, effluent aerosol streams were collected directly from an animal exposure port via an in-line impinger (model 7541; Ace Glass Incorporated, Vineland, NJ). Serial dilutions of the impinger samples were plated on Trypticase soy agar plates, and the CFU were enumerated.
Following anthrax challenge, the animals were treated therapeutically on an individual basis upon detection of a specific clinical sign or biomarker of disease. The rabbits were treated as described by Comer et al. (20) upon detection of a significant increase of body temperature (SIBT) or detection of PA in the blood as measured by a qualitative ECL-based assay, whichever biomarker was observed first. The limit of detection (LOD) for the ECL assay in the rabbit model was 1.0 ng PA/ml. A significant increase in body temperature (SIBT) was defined as three consecutive temperature elevations, or two increased temperature readings detected twice in a row. The threshold for an elevated body temperature was set as the average baseline temperature plus two times the standard deviation of a rabbit's baseline temperature (20). Body temperatures were recorded via an implantable programmable temperature transponder (IPTT-300; BMDS, Seaford, DE). The temperatures were recorded twice daily, starting at 7 days prior to challenge, and hourly from approximately 18 to 72 h postchallenge.
The NHPs were challenged on day 0, and blood was collected at baseline (prior to challenge) and at 18, 24, 30, 36, 42, 48, and 54 h postchallenge for bacteremia evaluation and detection of PA by the ECL assay. The treatment was initiated upon detection of PA in the serum. The limit of detection (LOD) for the ECL assay in the NHP model was 2.0 ng PA/ml. The animals were treated within approximately an hour following the detection of PA in the blood, as described by Henning et al. (21). In both the rabbit and NHP studies, only animals confirmed retrospectively to be bacteremic prior to treatment were included in therapeutic efficacy analysis.
AVP-21D9 was administered as an i.v. slow bolus injection (due to a low volume) to the NHPs in groups 2, 3 and 4, which received 10, 5, or 1 mg/kg of AVP-21D9, respectively. The NHPs in group 1 (20 mg/kg of AVP-21D9) and NZW rabbits across all AVP-21D9 treatment groups were administered AVP-21D9 as a slow i.v. infusion via vascular access ports (VAPs) (Instech Laboratories, PA, USA). The infusions were administered at the rate of 0.08 ml/min/kg via a sterile polyurethane infusion line inserted into the VAP and attached to the infusion pump. Normal saline was administered as a slow i.v. infusion to both the rabbits and NHPs. Following the administration of AVP-21D9 or saline, the rabbits and NHPs were monitored for clinical signs of infection for 30 and 60 days, respectively. Blood was collected at the regular intervals to analyze the PK, levels of PA, and bacteremia.
Gross necropsies were performed on all rabbits and NHPs that were found dead or were euthanized prior to scheduled termination. Additionally, all rabbits that survived to the time of study termination (day 30) were subjected to a necropsy assessment. Histopathology was conducted on gross lesions, brain, heart, lungs, spleen, liver, kidney, and mediastinal and bronchial lymph nodes, when deemed necessary, to confirm death from anthrax.
Assessment of bacteremia.
Bacteremia was measured immediately before the infusion (baseline) and at regular intervals after anthrax exposure. Approximately 40 μl of blood (collected in EDTA tubes) was distributed onto blood agar plates and cultured for a minimum of 48 h at 37°C to determine qualitatively the presence or absence of colonies with morphologies consistent with B. anthracis. The LOD of the assay was 25 CFU/ml of whole blood. An animal was considered positive for bacteremia if at least one colony was detected in the agar plate.
Assessment of antigenemia.
Blood was collected in serum separator tubes (SST), processed to serum, and analyzed qualitatively for the presence of PA using the ECL-based assay (21). The thresholds for treatment were 1 ng/ml and 2 ng/ml for the rabbits and NHPs, respectively. The trigger for treatment was based on a positive-control sample that provided a qualitative assessment of the PA in serum. Therefore, if a test sample exhibited an ECL count greater than that of the positive-control sample, it was considered positive, and the animal was treated. Quantitative assessment of PA in serum was performed by ELISA, which had an LOD of 2.0 ng/ml and a lower limit of quantitation (LLOQ) of 4.9 ng/ml.
Toxin-neutralizing antibody assay.
The TNA assay was modified from a previously developed method for detecting toxin-neutralizing activity in human serum (24, 25). This is a high-throughput version of the TNA procedure adopted from the Centers for Disease Control and Prevention (CDC). The TNA assay is designed to measure and quantify the functional ability of serum to neutralize B. anthracis LT activity using an in vitro cytotoxicity assay. The assay colorimetrically determines cell viability using a tetrazolium salt, 3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyl-2H-tetrazolium bromide (MTT), as the reporter, or a signal system. The serum-mediated neutralization of anthrax LT manifests as a suppression of cytotoxicity and hence a preservation of cell viability. The primary assay endpoint of the TNA is the 50% effective dilution (ED50). The ED50 is defined as the reciprocal of the dilution of a serum sample that results in 50% neutralization of anthrax lethal toxin and corresponds to the inflection point (c parameter) of a 4-parameter logistic log fit of the curve. The TNA assay was developed to quantitate AVP-21D9 between 5 and 500 μg/ml.
MSD ECL anti-PA IgG assay.
The PK of AVP-21D9 in rabbits, NHPs, and humans were determined by measuring the anti-PA IgG concentration using an MSD ECL competitive binding assay (23). Briefly, the sample or standard competed with ruthenylated AVP-21D9 for binding to biotinylated recombinant PA. Increasing amounts of AVP-21D9 in the sample generated a dose-dependent decrease in signal that was used to quantify the amount of AVP-21D9 present in the sample. Detection was based upon a chemiluminescent signal generated when voltage was applied by the MSD Sector Imager 2400 system (Meso Scale Discovery, Rockville, MD). A standard curve was calculated from the ECL response generated by the AVP-21D9 standards and used to determine the concentration of AVP-21D9 in the serum samples. The LLOQ of the assay was 1 μg/ml.
RESULTS
Phase I clinical study.
The safety and PK of a single i.v. infusion of AVP-21D9 were evaluated in healthy human subjects. Fifty subjects received a single infusion of AVP-21D9 or saline placebo, as shown in Fig. 1. Forty subjects were infused with AVP-21D9, and 10 subjects received saline. All infusions were well tolerated, and no severe adverse events were reported. The incidences of treatment-emergent adverse events (TEAEs) are summarized in Table 3. For most subjects, TEAEs were mild in severity. No subjects had TEAEs that were assessed to be severe.
TABLE 3.
Number (%) of subjects with treatment-emergent adverse events in the safety population
| TEAEa | Placebo group results (no. [%] of subjects) | Results (no. [%] of subjects) by AVP-21D9 dose (mg/kg) group: |
|||
|---|---|---|---|---|---|
| 0.3 | 1 | 3 | 10 | ||
| No. of subjects | 10 | 8 | 8 | 12 | 12 |
| Any | 4 (40.0) | 3 (37.5) | 5 (62.5) | 5 (41.7) | 7 (58.3) |
| Arthralgia | 0 | 0 | 0 | 0 | 1 (8.3)b |
| Cough | 0 | 0 | 1 (12.5) | 0 | 0 |
| Dermatitis, contact | 0 | 1 (12.5) | 0 | 0 | 0 |
| Diarrhea | 0 | 0 | 1 (12.5) | 1 (8.3) | 0 |
| Erectile dysfunction | 0 | 1 (12.5) | 0 | 0 | 0 |
| Excoriation | 1 (10.0) | 0 | 0 | 0 | 0 |
| Feeling hot | 0 | 0 | 0 | 1 (8.3) | 0 |
| Folliculitis | 0 | 0 | 0 | 0 | 1 (8.3)b |
| Gastroenteritis | 1 (10.0) | 0 | 0 | 0 | 0 |
| Gastroenteritis, viral | 0 | 0 | 0 | 1 (8.3) | 0 |
| Headache | 0 | 0 | 1 (12.5) | 0 | 0 |
| Heat exhaustion | 0 | 1 (12.5)b | 0 | 0 | 0 |
| Infusion site discomfort | 0 | 0 | 1 (12.5) | 0 | 0 |
| Ligament sprain | 0 | 0 | 1 (12.5) | 0 | 0 |
| Limb injury | 0 | 1 (12.5)b | 0 | 0 | 0 |
| Myalgia | 0 | 0 | 0 | 1 (8.3) | 0 |
| Nasal congestion | 1 (10.0) | 0 | 0 | 1 (8.3) | 0 |
| Nausea | 0 | 0 | 0 | 1 (8.3) | 0 |
| Neutropenia | 1 (10.0)b | 0 | 0 | 0 | 0 |
| Oropharyngeal pain | 1 (10.0) | 0 | 1 (12.5) | 0 | 0 |
| Pain in extremity | 0 | 0 | 0 | 0 | 2 (16.7) |
| Pelvic pain | 0 | 0 | 0 | 1 (8.3)b | 0 |
| Pharyngitis | 0 | 0 | 0 | 0 | 1 (8.3)b |
| Rhinitis, allergic | 0 | 0 | 0 | 0 | 2 (16.7) |
| Uterine hemorrhage | 0 | 0 | 1 (12.5) | 0 | 0 |
| Vertigo | 2 (20.0)c | 0 | 0 | 0 | 0 |
| Viral upper respiratory tract infection | 1 (10.0) | 0 | 1 (12.5)b | 3 (25.0) | 1 (8.3)b |
| Vulvovaginitis, trichomonal | 0 | 0 | 0 | 1 (8.3)b | 0 |
TEAE, treatment-emergent adverse event.
This event was moderate in intensity.
One of these 2 events was moderate in intensity.
The mean hematology, chemistry, and urinalysis values on days 2, 5, 7, 14, and 90 were not appreciably different from the mean values at baseline, and no trends were observed with increasing AVP-21D9 doses or with AVP-21D9 versus placebo (data not shown). Oxygen saturation was normal for all subjects and ranged from 92% to 100% at infusion initiation through 2 h after the start of infusion (data not shown).
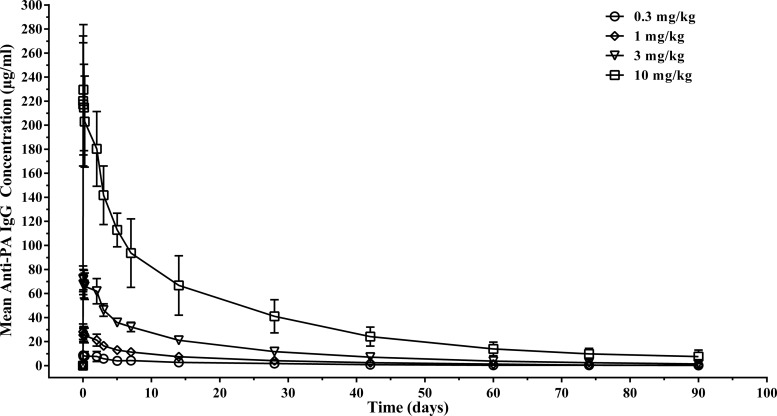
A dose-proportional increase in the serum anti-PA IgG concentration was observed at all dose levels (Fig. 2). Statistical analysis by analysis of variance (ANOVA) indicated no difference in the elimination half-life (t1/2) and total clearance (CL) over doses ranging from 0.3 mg/kg to 10 mg/kg, consistent with linear PK. The mean elimination half-life ranged from 20 to 27 days (Table 4). Functional antibody concentrations, as assessed using the TNA assay and reported as geometric mean concentrations (GMCs) on day 2 (the day following infusion), increased with dose at each time point, ranging from 7.78 ± 1.58 μg/ml (geometric mean ± standard deviation) in the 0.3-mg/kg group to 98.47 ± 35.04 μg/ml in the 10-mg/kg group (data not shown). A regression analysis indicated a very strong linear relationship between the TNA and anti-PA IgG concentrations, with correlation coefficients of 0.875 and 0.949 on a linear and logarithmic scale, respectively (P < 0.001).
FIG 2.
Relationship between the anti-PA IgG concentration and the dose of AVP-21D9 in humans. Anti-PA IgG concentration was measured by an MSD ECL assay. All subjects dosed with AVP-21D9 had measurable anti-PA IgG concentrations from 5 min postdose to day 28. After day 28, some values in some of the dose groups were below the LLOQ. The mean values for the AUC from 0 h to infinity (AUC0-∞) increased in a dose-proportional manner after i.v. infusion of single 0.3-, 1-, 3-, and 10-mg/kg doses of AVP-21D9. The mean t1/2 ranged from 20 to 27 days.
TABLE 4.
Summary of PK parameters for anti-PA IgG concentration in PK population
| PK parametera | Results (mean [SD]) by AVP-21D9 dose (mg/kg) group: |
|||
|---|---|---|---|---|
| 0.3 (n = 8) | 1 (n = 8) | 3 (n = 12) | 10 (n = 12) | |
| AUC0-∞ (days · μg/ml) | 158.8 (122.76) | 376.4 (99.56) | 1,045.0 (154.52) | 3,574.1 (932.91) |
| AUC(0-t) (days · μg/ml) | 115.3 (99.05) | 330.3 (92.32) | 968.8 (139.82) | 3,268.8 (769.18) |
| Cmax (μg/ml) | 9.3 (3.60) | 29.7 (6.25) | 74.8 (10.37) | 256.1 (48.90) |
| t1/2 (days) | 22.2 (11.40) | 21.6 (7.06) | 20.4 (3.53) | 26.9 (10.71) |
| λz (1/days) | 0.037 (0.01) | 0.035 (0.01) | 0.035 (0.01) | 0.030 (0.01) |
| CL (ml/kg · day) | 2.44 (0.88) | 2.81 (0.70) | 2.93 (0.47) | 2.96 (0.71) |
| Vz (ml/kg) | 66.51 (16.59) | 84.24 (21.84) | 84.41 (7.93) | 114.13 (56.99) |
| MRT (days) | 29.80 (14.15) | 26.10 (7.14) | 25.19 (5.10) | 31.10 (9.14) |
| Tmax (h) | 7.35 (10.47) | 1.11 (2.05) | 0.92 (0.97) | 3.36 (6.59) |
AUC0-t, AUC from 0 to t h; λz, elimination constant; CL, clearance; Vz, apparent volume of distribution during terminal phase; MRT, mean residence time; Tmax, time to maximum concentration of drug in serum.
Efficacy of AVP-21D9 in rabbits.
The efficacy of AVP-21D9 was evaluated in the rabbit therapeutic model of anthrax (20), in which the antitoxin was administered upon detection of the signs of disease manifestation. The animals were considered to have signs of anthrax if they exhibited SIBT or if PA was found in the serum. The disease was retrospectively confirmed with bacteremial analysis.
On day 0, the rabbits were exposed to an average (± standard deviation) challenge dose of 187 ± 61 LD50 of B. anthracis spores. The infusion of AVP-21D9 or normal saline was administered between 24 and 38 h after the detection of SIBT or PA in serum by the ECL assay, whichever trigger was first observed. The presence of PA in the serum as measured by the ECL assay was used as a trigger for treatment in 11 of 62 rabbits (18%). The remaining 51 animals were treated based on exhibiting SIBT. The animals exhibited abnormal values for the disease-indicating parameters (temperature elevation, presence of PA in the serum, and bacteremia) between 18 h and 123 h, with the geometric mean times ranging from 24 h to 38 h.
Bacteremia, which is associated with disease progression, was determined retrospectively by blood culture. Animals that were not retrospectively positive for bacteremia at the time of treatment were excluded from the survival analysis. All AVP-21D9-treated rabbits that ultimately succumbed to the disease were bacteremic and antigenemic at the time of death. At study termination (day 30), all of the surviving rabbits were negative for bacteremia and PA in the serum, as measured by ELISA.
Treatment with AVP-21D9 protected 33% to 92% of the animals compared to 0% survival in saline-treated controls (Table 5). The survival rates in the groups treated with 20 mg/kg, 10 mg/kg, or 5 mg/kg of AVP-21D9 were significantly higher than the survival rate observed in the saline control group (Fisher's exact test; P < 0.05). The survival rate observed for the 1-mg/kg treatment group was not significantly different from that for the control group. A correlation between the dose level of AVP-21D9 and survival was not observed, likely because the study was not statistically powered to detect such differences.
TABLE 5.
Rabbit efficacy study survival results
| AVP-21D9 dose (mg/kg) | All animals |
Animals bacteremic prior to treatment |
||
|---|---|---|---|---|
| No. survived/no. dosed | Survival proportion (95% confidence interval) | No. survived/no. dosed | Survival proportion (95% confidence interval) | |
| 20 | 9/12 | 0.75 (0.43, 0.95) | 6/9 | 0.67 (0.30, 0.93) |
| 10 | 13/14 | 0.93 (0.66, 1.00) | 10/11 | 0.91 (0.59, 1.00) |
| 5 | 13/14 | 0.93 (0.66, 1.00) | 12/13 | 0.92 (0.64, 1.00) |
| 1 | 8/14 | 0.57 (0.29, 0.82) | 3/9 | 0.33 (0.07, 0.70) |
| 0 (saline) | 0/8 | 0.00 (0.00, 0.37) | 0/6 | 0.00 (0.00, 0.46) |
Necropsy and histopathological examination confirmed that anthrax was the cause of death for all animals that died (data not shown). Lesions present at gross necropsy included enlarged bronchial lymph nodes, foci in the appendix, and fluid (effusion/edema) in the thoracic cavity and thymus. These lesions corresponded microscopically to acute inflammation, hemorrhage, edema, and necrosis and were typical of anthrax in rabbits. No lesions were found in surviving rabbits on day 30 (at study termination).
Of note, one rabbit treated with 20 mg/kg of AVP-21D9 was found dead 17 days after challenge with B. anthracis, whereas other animals died or became moribund 2 to 8 days following bacterial exposure. This animal had the highest level of PA in the blood at 4 h postexposure (131.3 ng/ml) compared to all other rabbits in the study. This initial high level of PA may have contributed to the late death of this animal despite treatment with 20 mg/kg of AVP-21D9. Microscopic lesions found in this animal were consistent with anthrax, and bacteria consistent in appearance with B. anthracis were noted microscopically in the lungs.
The concentration of AVP-21D9 in serum samples was analyzed by an MSD ECL assay to obtain initial information on the PK profile and parameters of the antibody in rabbits. The PK of AVP-21D9 in surviving rabbits appeared to be dose proportional across the doses of 1, 5, 10, and 20 mg/kg (data not shown). There were no apparent differences between males and females with respect to the serum concentrations of AVP-21D9 postinfusion. The estimated mean elimination half-life ranged between 2 and 4 days (Table 6).
TABLE 6.
Summary of PK parameters in surviving rabbits
| Parametera | Results for cohort (AVP-21D9 dose): |
|||
|---|---|---|---|---|
| 1 (20 mg/kg) | 2 (10 mg/kg) | 3 (5 mg/kg) | 4 (1 mg/kg) | |
| Cmax (μg/ml) | 423 ± 27.2 (9) | 201 ± 29.4 (13) | 121 ± 72.6 (13) | 49.4 ± 53.4 (8) |
| Tmax (h) | 0.70 (9) | 0.40 (13) | 0.30 (13) | 0.18 (8) |
| AUC0-t (h · μg/ml) | 28,179 ± 2,793 (9) | 12,299 ± 1,999 (13) | 6,245 ± 840 (13) | 2,698 ± 1,322 (7) |
| AUC0-∞ (h · μg/ml) | 36,154 ± 11,270 (4) | 13,179 ± 2,084 (11) | 7,263 ± 1,510 (5) | 2,029 (1) |
| λz (h−1) | 0.0157 ± 0.0118 (4) | 0.0166 ± 0.0051 (11) | 0.0128 ± 0.0043 (5) | 0.0067 (1) |
| t1/2 (h) | 85.1 ± 80.6 (4) | 45.3 ± 13.7 (11) | 59.9 ± 21.5 (5) | 103 (1) |
| CL (ml/h/kg) | 0.590 ± 0.162 (4) | 0.777 ± 0.131 (11) | 0.712 ± 0.147 (5) | 0.493 (1) |
| Vz (ml/kg) | 58.8 ± 39.5 (4) | 50.2 ± 13.2 (11) | 58.3 ± 10.0 (5) | 73.1 (1) |
The results are reported as the arithmetic mean ± standard deviation (n) except Tmax, for which the median (n) is reported.
Efficacy of AVP-21D9 in nonhuman primates.
The therapeutic efficacy of AVP-21D9 was also evaluated in the NHP therapeutic model of anthrax (21). On day 0, cynomolgus macaques were exposed to an average (± standard deviation) challenge dose of 186 ± 28 LD50 of B. anthracis spores. The animals were administered AVP-21D9 or normal saline on an individual basis upon detection of PA in the serum by the ECL-based immunoassay. AVP-21D9 or saline was administered between 31 and 49 h depending on the time of detection of PA in the serum. One of the animals in group 3 (5 mg/kg of AVP-21D9) was not bacteremic at the time of treatment, as determined retrospectively, and was therefore excluded from the survival analysis. By study termination (day 60), all NHPs were negative for PA, and all except one animal (treated with 10 mg/kg of AVP-21D9) were negative for bacteremia.
Treatment with AVP-21D9 resulted in the survival of 40% to 67% of the macaques, while all animals in the saline-treated control group succumbed to infection (Table 7). Gross and microscopic pathology findings consistent with anthrax disease were present in all animals that were found dead or euthanized due to moribund condition (data not shown). A statistical analysis using a two-sided Fisher's exact test confirmed that groups treated with 20 mg/kg, 5 mg/kg, or 1 mg/kg of AVP-21D9 had significantly higher survival rates than that of the saline-treated group (P = 0.0385). The survival rate in the group treated with 10 mg/kg was not significantly different from that observed in the saline control group. Similar to the survival results obtained in the rabbit study, a statistical significance between treatment groups with respect to dose response in the NHP study was not noted, likely due to the small group size.
TABLE 7.
NHP efficacy study survival results
| AVP-21D9 dose (mg/kg) | All animals |
Animals bacteremic prior to treatment |
||
|---|---|---|---|---|
| No. survived/No. dosed | Survival proportion (95% confidence interval) | No. survived/no. dosed | Survival proportion (95% confidence interval) | |
| 20 | 6/10 | 0.60 (0.26, 0.88) | 6/10 | 0.60 (0.26, 0.88) |
| 10 | 4/10 | 0.40 (0.12, 0.74) | 4/10 | 0.40 (0.12, 0.74) |
| 5 | 7/10 | 0.70 (0.35, 0.93) | 6/9 | 0.67 (0.30, 0.93) |
| 1 | 6/10 | 0.60 (0.26, 0.88) | 6/10 | 0.60 (0.26, 0.88) |
| 0 (saline) | 0/8 | 0.00 (0.00, 0.37) | 0/8 | 0.0 (0.00, 0.37) |
The concentration of AVP-21D9 in serum was analyzed by the MSD ECL assay to obtain initial information on the PK profile for the antibody in NHPs. The PK profile was dose proportional across dose levels of 1, 5, 10, and 20 mg/kg, and the estimated mean elimination half-life ranged from 6 to 11 days (Table 8).
TABLE 8.
Summary of PK parameters in surviving NHPs
| Parametera | Results for cohort (AVP-21D9 dose): |
|||
|---|---|---|---|---|
| 1 (20 mg/kg) | 2 (10 mg/kg) | 3 (5 mg/kg) | 4 (1 mg/kg) | |
| Cmax (μg/ml) | 344 ± 18.7 (5) | 201 ± 46.2 (4) | 101 ± 9.54 (7) | 20.8 ± 7.25 (6) |
| Tmax (h) | 0.65 (5) | 0.15 (4) | 0.13 (7) | 0.16 (6) |
| AUC0-t (h · μg/ml) | 32,829 ± 2,989 (5) | 19,063 ± 3,251 (4) | 7,462 ± 1,029 (7) | 1,948 ± 1,143 (6) |
| AUC0-∞ (h · μg/ml) | 67,637 ± 29,586 (5) | 30,786 ± 6,103 (3) | 11,377 ± 2,122 (6) | —b |
| λz (h−1) | 0.0031 ± 0.0010 (5) | 0.0036 ± 0.0009 (3) | 0.0047 ± 0.0009 (6) | —b |
| t1/2 (h)c | 263 ± 154 (5) | 205 ± 59.1 (3) | 152 ± 35.0 (6) | —b |
| CL (ml/h/kg)c | 0.329 ± 0.095 (5) | 0.333 ± 0.065 (3) | 0.453 ± 0.091 (6) | —b |
| Vz (ml/kg)c | 108 ± 14.8 (5) | 95.2 ± 11.3 (3) | 96.4 ± 10.7 (6) | —b |
The results are reported as the arithmetic mean ± standard deviation (n) except Tmax, for which the median (n) is reported.
—, parameter could not be calculated for any animals in this group due to the lack of a log-linear decay in serum concentrations.
Wide ranges were observed between cohorts for t1/2, CL, and Vz due to the small number of animals per group.
DISCUSSION
Mortality associated with anthrax may be prevented if treatment with antibiotics is initiated shortly after exposure to spores. However, a delay in initiating antimicrobial therapy may result in toxemia, which predominantly accounts for the morbidity and mortality associated with inhalational anthrax disease (7, 8). While antimicrobials are a critical component of anthrax therapy, adjunct therapies are needed for late-stage disease in order to counteract toxemia. Several anti-PA antibody-based anthrax therapeutics have been shown to increase survival in animal models of inhalational anthrax. These countermeasures act by neutralizing anthrax toxins (9–13, 22). Here, we present data on the safety and efficacy of the anti-PA antibody-based therapeutic, AVP-21D9, which also neutralizes anthrax toxins and therefore might control toxemia and disease progression in the event of delayed treatment following exposure to aerosolized anthrax spores.
Since the evaluation of the efficacy of anthrax therapeutics is not ethical and feasible in humans, an alternative approach is to perform animal therapeutic efficacy studies and use PK assessments in humans to correlate the efficacious dose in animals with a comparable dose in humans (17, 18). Rabbits and NHPs are considered acceptable animal models for evaluating candidate anthrax vaccines and therapeutics because the pathophysiological response to disease in these animals closely resembles the clinical course of anthrax in humans (26–28). SIBT and/or the presence of PA in serum have been generally accepted as the appropriate triggers for treating B. anthracis-infected rabbits and NHPs using antitoxins (20, 21). The literature suggests that disease progression is more rapid in NZW rabbits and cynomolgus macaques than in humans, and that animals succumb to death within 24 to 48 h following the detection of the triggers for treatment (20, 21). As a result, the window of opportunity for successful treatment with antitoxins in the therapeutic animal models is rather limited, as the animal is rapidly overwhelmed with toxin, and the mortality rate increases if antitoxin administration is delayed (20, 21). Indeed, it has been shown that a delay in treatment with 10 mg/kg of AVP-21D9 by 24 and 48 h after intranasal exposure to anthrax spores reduced the survival of rabbits to 80% and 60%, respectively, while treatment administered at 6 and 12 h postexposure resulted in 100% survival (15).
The therapeutic efficacy studies described here indicate that the treatment of anthrax with AVP-21D9 in infected rabbits and NHPs resulted in significant increases in survival compared to with the placebo. Even at a dose level as low as 1 mg/kg of AVP-21D9, 33% of rabbits and 60% of macaques were rescued from anthrax-induced death. Neither study was statistically powered to detect differences in survival among the AVP-21D9-treated groups; thus, a correlation between the dose and associated survival has not yet been established, and the minimum efficacious dose needs to be identified in future studies.
In this phase I clinical study, a single infusion of 0.3, 1, 3, or 10 mg/kg of AVP-21D9 was well tolerated in healthy adults at all dose levels tested. The percentage of subjects with AEs or TEAEs did not increase with AVP-21D9 dose, and for the majority of subjects with TEAEs, the events were mild and unrelated to AVP-21D9.
The animal studies described herein suggest that AVP-21D9 may be a potential treatment for inhalational anthrax and may decrease the morbidity and mortality associated with anthrax disease. Statistically powered studies are warranted to establish a minimum protective dose of AVP-21D9 in B. anthracis-challenged animals, and PK studies in healthy animals will be required to derive the appropriate human dose.
ACKNOWLEDGMENTS
We thank Diane Sweeney and Gregory Stark for statistical support and Weila Wang, Nadia Soukhareva, Christine Valencia, Bryan Fortson, Jeff Smith, Tyler Laudenslager, Kristin Clement, Pierre-Olivier Tremblay, and Janet Lathey for programmatic and technical support. We also thank Sharon Bungo, Earnest Bolin, Renuka Pulitla, and the technical staff at Tandem Labs for bioanalytical assay support.
This work was supported by contract HHSN 272200800040C from the National Institute of Allergy and Infectious Diseases, National Institutes of Health, Department of Health and Human Services.
We are all either current or former employees or contractors of Emergent BioSolutions, the developer of AVP-21D9.
Footnotes
Published ahead of print 14 April 2014
REFERENCES
- 1.Grabenstein JD. 2008. Vaccines: countering anthrax: vaccines and immunoglobulins. Clin. Infect. Dis. 46:129–136. 10.1086/523578 [DOI] [PubMed] [Google Scholar]
- 2.Dixon TC, Meselson M, Guillemin J, Hanna PC. 1999. Anthrax. N. Engl. J. Med. 341:815–826. 10.1056/NEJM199909093411107 [DOI] [PubMed] [Google Scholar]
- 3.Bann JG, Hultgren SJ. 2004. Structural biology: anthrax hijacks host receptor. Nature 430:843–844. 10.1038/430843a [DOI] [PubMed] [Google Scholar]
- 4.Brossier F, Mock M. 2001. Toxins of Bacillus anthracis. Toxicon 39:1747–1755. 10.1016/S0041-0101(01)00161-1 [DOI] [PubMed] [Google Scholar]
- 5.Jernigan DB, Raghunathan PL, Bell BP, Brechner R, Bresnitz EA, Butler JC, Cetron M, Cohen M, Doyle T, Fischer M, Greene C, Griffith KS, Guarner J, Hadler JL, Hayslett JA, Meyer R, Petersen LR, Phillips M, Pinner R, Popovic T, Quinn CP, Reefhuis J, Reissman D, Rosenstein N, Schuchat A, Shieh WJ, Siegal L, Swerdlow DL, Tenover FC, Traeger M, Ward JW, Weisfuse I, Wiersma S, Yeskey K, Zaki S, Ashford DA, Perkins BA, Ostroff S, Hughes J, Fleming D, Koplan JP, Gerberding JL, National Anthrax Epidemiologic Investigation Team 2002. Investigation of bioterrorism-related anthrax, United States, 2001: epidemiologic findings. Emerg. Infect. Dis. 8:1019–1028. 10.3201/eid0810.020353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jernigan JA, Stephens DS, Ashford DA, Omenaca C, Topiel MS, Galbraith M, Tapper M, Fisk TL, Zaki S, Popovic T, Meyer RF, Quinn CP, Harper SA, Fridkin SK, Sejvar JJ, Shepard CW, McConnell M, Guarner J, Shieh WJ, Malecki JM, Gerberding JL, Hughes JM, Perkins BA, Anthrax Bioterrorism Investigation Team 2001. Bioterrorism-related inhalational anthrax: the first 10 cases reported in the United States. Emerg. Infect. Dis. 7:933–944. 10.3201/eid0706.010604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Abramova FA, Grinberg LM, Yampolskaya OV, Walker DH. 1993. Pathology of inhalational anthrax in 42 cases from the Sverdlovsk outbreak of 1979. Proc. Natl. Acad. Sci. U. S. A. 90:2291–2294. 10.1073/pnas.90.6.2291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Inglesby TV, O'Toole T, Henderson DA, Bartlett JG, Ascher MS, Eitzen E, Friedlander AM, Gerberding J, Hauer J, Hughes J, McDade J, Osterholm MT, Parker G, Perl TM, Russell PK, Tonat K, Working Group on Civilian Biodefense 2002. Anthrax as a biological weapon, 2002: updated recommendations for management. JAMA 287:2236–2252. 10.1001/jama.287.17.2236 [DOI] [PubMed] [Google Scholar]
- 9.Migone TS, Subramanian GM, Zhong J, Healey LM, Corey A, Devalaraja M, Lo L, Ullrich S, Zimmerman J, Chen A, Lewis M, Meister G, Gillum K, Sanford D, Mott J, Bolmer SD. 2009. Raxibacumab for the treatment of inhalational anthrax. N. Engl. J. Med. 361:135–144. 10.1056/NEJMoa0810603 [DOI] [PubMed] [Google Scholar]
- 10.Mohamed N, Clagett M, Li J, Jones S, Pincus S, D'Alia G, Nardone L, Babin M, Spitalny G, Casey L. 2005. A high-affinity monoclonal antibody to anthrax protective antigen passively protects rabbits before and after aerosolized Bacillus anthracis spore challenge. Infect. Immun. 73:795–802. 10.1128/IAI.73.2.795-802.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Walsh JJ, Pesik N, Quinn CP, Urdaneta V, Dykewicz CA, Boyer AE, Guarner J, Wilkins P, Norville KJ, Barr JR, Zaki SR, Patel JB, Reagan SP, Pirkle JL, Treadwell TA, Messonnier NR, Rotz LD, Meyer RF, Stephens DS. 2007. A case of naturally acquired inhalation anthrax: clinical care and analyses of anti-protective antigen immunoglobulin G and lethal factor. Clin. Infect. Dis. 44:968–971. 10.1086/512372 [DOI] [PubMed] [Google Scholar]
- 12.Mytle N, Hopkins RJ, Malkevich NV, Basu S, Meister GT, Sanford DC, Comer JE, Van Zandt KE, Al-Ibrahim M, Kramer WG, Howard C, Daczkowski N, Chakrabarti AC, Ionin B, Nabors GS, Skiadopoulos MH. 2013. Evaluation of intravenous anthrax immune globulin for treatment of inhalation anthrax. Antimicrob. Agents Chemother. 57:5684–5692. 10.1128/AAC.00458-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Corey A, Migone TS, Bolmer S, Fiscella M, Ward C, Chen C, Meister G. 2013. Bacillus anthracis protective antigen kinetics in inhalation spore-challenged untreated or levofloxacin/raxibacumab-treated New Zealand white rabbits. Toxins (Basel) 5:120–138. 10.3390/toxins5010120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sawada-Hirai R, Jiang I, Wang F, Sun SM, Nedellec R, Ruther P, Alvarez A, Millis D, Morrow PR, Kang AS. 2004. Human anti-anthrax protective antigen neutralizing monoclonal antibodies derived from donors vaccinated with anthrax vaccine adsorbed. J. Immune Based Ther. Vaccines 2:1–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Peterson JW, Comer JE, Noffsinger DM, Wenglikowski A, Walberg KG, Chatuev BM, Chopra AK, Stanberry LR, Kang AS, Scholz WW, Sircar J. 2006. Human monoclonal anti-protective antigen antibody completely protects rabbits and is synergistic with ciprofloxacin in protecting mice and guinea pigs against inhalation anthrax. Infect. Immun. 74:1016–1024. 10.1128/IAI.74.2.1016-1024.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Goossens PL. 2009. Animal models of human anthrax: the Quest for the Holy Grail. Mol. Aspects Med. 30:467–480. 10.1016/j.mam.2009.07.005 [DOI] [PubMed] [Google Scholar]
- 17.Bergman KL. 2009. The animal rule and emerging infections: the role of clinical pharmacology in determining an effective dose. Clin. Pharmacol. Ther. 86:328–331. 10.1038/clpt.2009.106 [DOI] [PubMed] [Google Scholar]
- 18.FDA. 2009. Guidance for industry: animal models—essential elements to address efficacy under the animal rule. Center for Biologics Evaluation and Research, U.S. Food and Drug Administration, Rockville, MD: http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/ucm078923.pdf [Google Scholar]
- 19.Twenhafel NA. 2010. Pathology of inhalation anthrax in animal models. Vet. Pathol. 47:819–830. 10.1177/0300985810378112 [DOI] [PubMed] [Google Scholar]
- 20.Comer JE, Ray BD, Henning LN, Stark GV, Barnewall RE, Mott JM, Meister GT. 2012. Characterization of a therapeutic model of inhalational anthrax using an increase in body temperature in New Zealand White rabbits as a trigger for treatment. Clin. Vaccine Immunol. 19:1517–1525. 10.1128/CVI.00292-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Henning LN, Comer JE, Stark GV, Ray BD, Tordoff KP, Knostman KA, Meister GT. 2012. Development of an inhalational Bacillus anthracis exposure therapeutic model in cynomolgus macaques. Clin. Vaccine Immunol. 19:1765–1775. 10.1128/CVI.00288-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Peterson JW, Comer JE, Baze WB, Noffsinger DM, Wenglikowski A, Walberg KG, Hardcastle J, Pawlik J, Bush K, Taormina J, Moen S, Thomas J, Chatuev BM, Sower L, Chopra AK, Stanberry LR, Sawada R, Scholz WW, Sircar J. 2007. Human monoclonal antibody AVP-21D9 to protective antigen reduces dissemination of the Bacillus anthracis Ames strain from the lungs in a rabbit model. Infect. Immun. 75:3414–3424. 10.1128/IAI.00352-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Marchese RD, Puchalski D, Miller P, Antonello J, Hammond O, Green T, Rubinstein LJ, Caulfield MJ, Sikkema D. 2009. Optimization and validation of a multiplex, electrochemiluminescence-based detection assay for the quantitation of immunoglobulin G serotype-specific antipneumococcal antibodies in human serum. Clin. Vaccine Immunol. 16:387–396. 10.1128/CVI.00415-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li H, Soroka SD, Taylor TH, Jr, Stamey KL, Stinson KW, Freeman AE, Abramson DR, Desai R, Cronin LX, Oxford JW, Caba J, Pleatman C, Pathak S, Schmidt DS, Semenova VA, Martin SK, Wilkins PP, Quinn CP. 2008. Standardized, mathematical model-based and validated in vitro analysis of anthrax lethal toxin neutralization. J. Immunol. Methods 333:89–106. 10.1016/j.jim.2008.01.007 [DOI] [PubMed] [Google Scholar]
- 25.Omland KS, Brys A, Lansky D, Clement K, Lynn F, Participating Laboratories 2008. Interlaboratory comparison of results of an anthrax lethal toxin neutralization assay for assessment of functional antibodies in multiple species. Clin. Vaccine Immunol. 15:946–953. 10.1128/CVI.00003-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vasconcelos D, Barnewall R, Babin M, Hunt R, Estep J, Nielsen C, Carnes R, Carney J. 2003. Pathology of inhalation anthrax in cynomolgus monkeys (Macaca fascicularis). Lab. Invest. 83:1201–1209. 10.1097/01.LAB.0000080599.43791.01 [DOI] [PubMed] [Google Scholar]
- 27.Zaucha GM, Pitt LM, Estep J, Ivins BE, Friedlander AM. 1998. The pathology of experimental anthrax in rabbits exposed by inhalation and subcutaneous inoculation. Arch. Pathol. Lab. Med. 122:982–992 [PubMed] [Google Scholar]
- 28.Yee SB, Hatkin JM, Dyer DN, Orr SA, Pitt ML. 2010. Aerosolized Bacillus anthracis infection in New Zealand White rabbits: natural history and intravenous levofloxacin treatment. Comp. Med. 60:461–468 [PMC free article] [PubMed] [Google Scholar]