Abstract
Protease inhibitors are largely used for the treatment of HIV infection in combination with other antiretroviral drugs. Their improved pharmacokinetic profiles can be achieved through the concomitant administration of low doses of ritonavir (RTV), a protease inhibitor currently used as a booster, increasing the exposure of companion drugs. Since ritonavir-boosted regimens are associated with long-term adverse events, cobicistat, a CYP3A4 inhibitor without antiviral activity, has been developed. Recently, high intracellular concentrations of ritonavir in lymphocytes and monocytes were reported even when ritonavir was administered at low doses, so we aimed to compare its theoretical antiviral activity with those of the associated protease inhibitors. Intracellular concentrations of ritonavir and different protease inhibitors were determined through the same method. Inhibitory constants were obtained from the literature. The study enrolled 103 patients receiving different boosted protease inhibitors, darunavir-ritonavir 600 and 100 mg twice daily and 800 and 100 mg once daily (n = 22 and 4, respectively), atazanavir-ritonavir 300 and 100 mg once daily (n = 40), lopinavir-ritonavir 400 and 100 mg twice daily (n = 21), or tipranavir-ritonavir 500 and 200 mg twice daily (n = 16). According to the observed concentrations, we calculated the ratios between the intracellular concentrations of ritonavir and those of the companion protease inhibitor and between the theoretical viral protease reaction speeds with each drug, with and without ritonavir. The median ratios were 4.04 and 0.63 for darunavir-ritonavir twice daily, 2.49 and 0.74 for darunavir-ritonavir once daily, 0.42 and 0.74 for atazanavir-ritonavir, 0.57 and 0.95 for lopinavir-ritonavir, and 0.19 and 0.84 for tipranavir-ritonavir, respectively. Therefore, the antiviral effect of ritonavir was less than that of the concomitant protease inhibitors but, importantly, mostly with darunavir. Thus, further in vitro and in vivo studies of the RTV antiviral effect are warranted.
INTRODUCTION
Infection with HIV is a worldwide health problem, with an estimated burden of 34 million infected patients. With the introduction of highly active antiretroviral therapy (HAART), it has been possible to manage infections and prevent the occurrence of AIDS and HIV-related complications (1, 2). HAART is based on the coadministration of drugs that target several important HIV enzymes or cell coreceptors, including reverse transcriptase, integrase, protease, and CCR5. Currently, protease inhibitor (PI)-based regimens are often adopted for HIV treatment (3, 4). Ritonavir (RTV), initially used simply as an active drug, is now used at low dosages (100 mg once [QD] or twice daily [BID]) as a booster in PI-based regimens; this is due to the drug's inhibitory activity on various cytochrome P450 isoenzymes (5). However, the toxicity of this drug (6), which led to its transition from an antiviral drug (high dosage, 600 mg twice daily) to a pharmacoenhancer (low dosage), has led to the introduction of alternative booster molecules, e.g., cobicistat (COBI) (7–9).
To date, the low dosage of RTV when administered as a booster is considered to be completely ineffective in preventing viral replication, while the choice of other CYP3A4-specific inhibitors seems to be a noninferior and safer alternative (8, 9). However, previous studies conducted with RTV have not focused enough on its accumulation rate in peripheral blood mononuclear cells (PBMCs) or on its intrinsic antiviral properties. To date, only a few studies determined intracellular RTV concentrations (10–12). Nevertheless, these studies did not share a unique analytical method, and the calculations of intracellular concentrations were often based on a standard mean cellular volume (MCV) of 400 fl, which was not specific for each PBMC sample (13).
In a previously published work (11), intracellular RTV concentrations were found to be much higher than those from other works, probably due to the adoption of a sample-specific MCV (13), a better validated methodological method (14), and different therapeutic regimens. On this basis, we hypothesized that RTV, when it reaches high intracellular concentrations, exerts an antiviral effect also when used as a booster.
The aim of this work was to investigate the theoretical inhibitory effect of RTV when used as a PI booster, comparing its observed intracellular concentration and its inhibitory constant (Ki) to those of several companion PIs. Moreover, we aimed to compare the theoretical reaction speed of viral protease in the presence of the companion PIs with and without RTV, according to the observed intracellular concentrations, in order to obtain a quantitative estimation of the antiviral role of RTV in each regimen.
MATERIALS AND METHODS
Patients and inclusion criteria.
A total of 103 HIV-monoinfected patients treated with boosted PI regimens with a self-reported adherence rate of at least 95% of the prescribed doses were included in the study. The patients were enrolled at the Amedeo di Savoia Hospital (Turin, Italy) and were treated with atazanavir-ritonavir (ATV-RTV) 300 and 100 mg once daily, darunavir-ritonavir (DRV-RTV) 600 and 100 mg twice daily, DRV-RTV 800 and 100 mg once daily, lopinavir-ritonavir (LPV-RTV) 400 and 100 mg twice daily, and tipranavir-ritonavir (TPV-RTV) 500 and 200 mg twice daily.
Sampling at the end of the dosing interval (trough concentration [Ctrough]) was performed after informed consent was obtained, in accordance with the local ethics committee's indications (Amedeo di Savoia Hospital Ethics Committee).
Patients taking concomitant interacting drugs (i.e., CYP450 inducers or inhibitors) or those with liver or renal impairment were excluded from the study.
Drug quantification in plasma and PBMCs.
Sampling was performed at the end of the dosing interval, at the steady state for each drug, in order to quantify the Ctrough (24 ± 1 h or 12 ± 1 h for drugs given QD or BID, respectively) before the new drug dose intake.
Blood samples were collected in lithium-heparin tubes (7 ml) and then centrifuged at 1,400 × g for 10 min at 4°C to obtain plasma aliquots, which were stored at −20°C until analysis (no more than 1 week). PBMC aliquots were obtained from blood via density gradient separation with Lymphoprep, as previously described (13, 14), and then stored at −80°C in a solution of water-methanol 30:70 (vol/vol) until analysis (about 2 weeks).
Blank plasma was kindly supplied by the blood bank of Maria Vittoria Hospital (Turin, Italy). Blank PBMC aliquots were prepared with the same procedure as was used for the patient samples, using buffy coat provided by the same blood bank.
The count and determination of the MCV for each PBMC sample were concurrently performed with a Beckman Coulter counter, as described by Simiele et al. (13).
Simultaneous quantifications of ritonavir and the companion drugs in plasma and in PBMCs were performed with previously published high-performance liquid chromatography (HPLC)-photodiode array and HPLC mass spectrometry methods, respectively (14, 15).
The intracellular concentrations of ritonavir and other PIs were calculated as previously described (14) using the formula M/N × MCV, where M is the amount of drug in each PBMC aliquot, MCV is the mean cellular volume, and N is the number of PBMCs in the same aliquot. The final plasma and intracellular concentrations were determined in triplicate. Median data were used for the statistical analyses.
Comparison of intra-PBMC concentrations/Ki.
Intracellular concentrations of each drug were expressed in molarity. The ratio between the plasma and intracellular concentrations was calculated for each regimen.
The theoretical inhibitory action of RTV was compared with that of the companion PI for each regimen. For this purpose, the following formula was considered (16):
| (1) |
In the formula, vi is the reaction speed of the viral protease in the presence of a single inhibitor, Vmax is the maximum reaction speed, [S] is the concentration of the Gag-Pol protein, Km is the Michaelis-Menten constant of the reaction, [I] is the inhibitor concentration, and Ki is the inhibitory constant of the inhibitor. Since our aim was to compare the theoretical inhibitory effect of RTV and the concomitant PI in the same environment, Vmax, Km, and [S] were considered constants.
Because of the absence of further explanations about the possible interaction between RTV and the PI at the target level (the viral protease), we chose to primarily consider RTV and PIs singularly in an ideal environment with the same concentration of protease and Gag-Pol protein. Therefore, the only member of the equation which differs between each drug is [I]/Ki, which can be considered the arithmetic expression of the inhibitory activity of the drug. This factor was determined for each drug at the observed concentrations and used for comparison.
The calculation of [I]/Ki was performed with two different Ki values for RTV: the highest Ki reported in the literature (55 pM) (17), as the Ki values for all PIs were evaluated in the same work, and a mean Ki calculated from different works reported in the literature (37.5 pM) (18–20). Moreover, once the observed [I]/Ki values for RTV and for the concomitant PI in each sample were obtained, the ratio between these two values was calculated (RTV 1/concomitant PI 1). This ratio was used to evaluate the difference in antiviral effects between RTV and the PI at the intracellular level; a value of <1 indicates a stronger effect of the PI compared to that of RTV, and, conversely, a value of >1 indicates a stronger activity of RTV compared to that of the concomitant PI.
Changing perspective, if we consider a system in which two mutually exclusive inhibitors (if one inhibitor binds to a protease molecule, the binding of the other one is impossible) interact with the protease, the formula is changed as follows, as described in many articles (21–23):
| (2) |
In this formula, [J] is the concentration of the second inhibitor, and Kj is its inhibition constant.
Combining the two formulas, the ratio between the reaction speed with two inhibitors and that with only one inhibitor can be expressed as
| (3) |
If we assume low concentrations of substrate ([S]), then the ratio is reducible to
| (4) |
To obtain robust data on RTV activity, only the highest Kj (for RTV) was considered in this formula, as the value was retrieved by the same study (17).
Statistical analysis.
The statistical analysis was performed with SPSS statistics 20.0 (IBM). The normality of distributions was determined by the Shapiro-Wilk test; comparisons between nonnormal data were performed with the Mann-Whitney or Kruskal-Wallis test for differences between two or more groups, respectively, or the Wilcoxon test for the comparison of paired cases. P values of <0.05 were considered statistically significant.
RESULTS
Patients.
Patients included in the study were treated with DRV-RTV 600 and 100 mg BID (n = 22 [21.4%]), DRV-RTV 800 and 100 mg QD (n = 4 ([3.9%]), ATV-RTV 300 and 100 mg QD (n = 40 [38.8%]), LPV-RTV 400 and 100 mg BID (n = 21 [20.4%]), and TPV-RTV 500 and 200 mg BID (n = 16 [15.5%]).
Plasma and intra-PBMC concentrations.
The distribution of plasma and intra-PBMC concentrations and the corresponding PBMC/plasma ratio for each PI, used as indices of compartmentalization, are summarized in Table 1.
TABLE 1.
Observed PI dispositions in plasma and PBMCs
| Parameter | Median (interquartile range) for concomitant PI |
||||
|---|---|---|---|---|---|
| ATV | DRV600a | DRV800b | LPV | TPV | |
| No. of samples | 40 | 22 | 4 | 21 | 16 |
| PI intra-PBMC concn (μM) | 2.51 (1.33–4.33) | 0.59 (0.40–1.09) | 0.743 (0.256–2.430) | 3.98 (3.18–6.73) | 6.78 (2.15–9.68) |
| PI plasma concn (μM) | 0.82 (0.47–1.38) | 6.35 (4.92–8.69) | 5.70 (3.35–16.75) | 11.74 (7.43–13.58) | 71.28 (43.33–96.19) |
| PI intra-PBMC/plasma ratio | 2.51 (1.63–5.06) | 0.09 (0.06–0.22) | 0.11 (0.06–0.24) | 0.51 (0.28–1.12) | 0.15 (0.13–0.18) |
DRV600, DRV-RTV 600 and 100 mg BID.
DRV800, DRV-RTV 800 and 100 mg QD.
The PBMC/plasma ratio for RTV was consistently >1, indicating intracellular accumulation. The intracellular accumulation ratios varied significantly among the different PI regimens (P = 0.008), as shown in Table 2.
TABLE 2.
Observed RTV dispositions in combination with each concomitant PI and comparison between RTV and concomitant PI effect on viral protease
| Parameter | Median (interquartile range) for concomitant PI |
||||
|---|---|---|---|---|---|
| ATV | DRV600a | DRV800b | LPV | TPV | |
| No. of samples | 40 | 22 | 4 | 21 | 16 |
| RTV intra-PBMC concn (μM) | 0.90 (0.61–1.40) | 2.96 (1.82–4.95) | 1.45 (1.16–4.47) | 2.60 (1.86–3.76) | 1.65 (0.93–2.30) |
| RTV plasma concn (μM) | 0.09 (0.06–0.19) | 0.48 (0.26–0.55) | 0.17 (0.14–0.61) | 0.39 (0.22–0.65) | 0.38 (0.20–0.70) |
| RTV intra-PBMC/plasma ratio | 9.07 (5.97–12.83) | 7.59 (6.20–9.97) | 7.561 (6.60–11.11) | 7.21 (5.71–10.45) | 5.27 (3.12–6.39) |
| Intracellular [RTV]/[PI] | 0.42 (0.29–0.64) | 4.04 (2.48–8.03) | 2.49 (1.49–5.35) | 0.57 (0.29–0.76) | 0.19 (0.13–0.25) |
| [I]/Ki (RTV)/[I]/Ki (PI) (high RTV Ki) | 0.35 (0.24–0.54) | 0.59 (0.36–1.17) | 0.36 (0.22–0.78) | 0.05 (0.03–0.07) | 0.19 (0.13–0.25) |
| [I]/Ki (RTV)/[I]/Ki (PI) (mean RTV Ki) | 0.49 (0.36–0.80) | 0.91 (0.61–2.24) | 0.53 (0.32–1.14) | 0.07 (0.06–0.10) | 0.29 (0.25–0.37) |
| Reaction speed ratio with/without RTV | 0.74 (0.65–0.80) | 0.63 (0.46–0.74) | 0.74 (0.57–0.82) | 0.95 (0.94–0.96) | 0.84 (0.80–0.88) |
| Fraction of antiviral effect accounted for by RTV (%) | 26 (20–35) | 37 (26–54) | 26 (18–43) | 5 (4–6) | 16 (12–20) |
DRV600, DRV-RTV 600 and 100 mg BID.
DRV800, DRV-RTV 800 and 100 mg QD.
Moreover, statistically significant differences in intra-PBMC RTV concentrations were observed between the different regimens (P < 0.001). In particular, patients treated with DRV-RTV 600 and 100 mg twice daily reported higher intra-PBMC RTV concentrations than patients on the DRV-RTV 800 and 100 mg once daily, ATV, or TPV regimens (P = 0.042 for DRV and P < 0.001 for ATV and TPV). Similarly, the LPV-RTV regimen showed higher intra-PBMC RTV concentrations than did ATV-RTV and TPV-RTV (both P < 0.001).
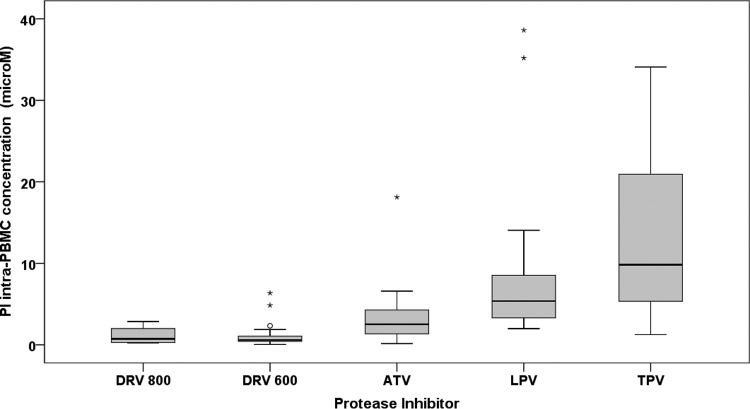
Moreover, the intracellular concentrations among different PIs were considerably different (Fig. 1).
FIG 1.
Distribution of the observed intra-PBMC molar concentrations of the different boosted PIs. DRV800, DRV-RTV 800 and 100 mg once daily; DRV600, DRV-RTV 600 and 100 mg twice daily. Circles and asterisks indicate mild and extreme outliers, respectively.
Intra-PBMC PI concentrations were significantly correlated to the plasma concentrations (P = 0.018, 0.00024, and 0.0001 for ATV, DRV, and TPV, respectively) except for that of LPV. RTV plasma and intra-PBMC concentrations showed a lack of correlation only when administered with LPV.
RTV versus concomitant PI activity.
Intracellular RTV concentrations were comparable to those of concomitant PIs. In particular, RTV intra-PBMC concentrations were higher than those of DRV, especially with DRV-RTV 600 and 100 mg BID (P < 0.001).
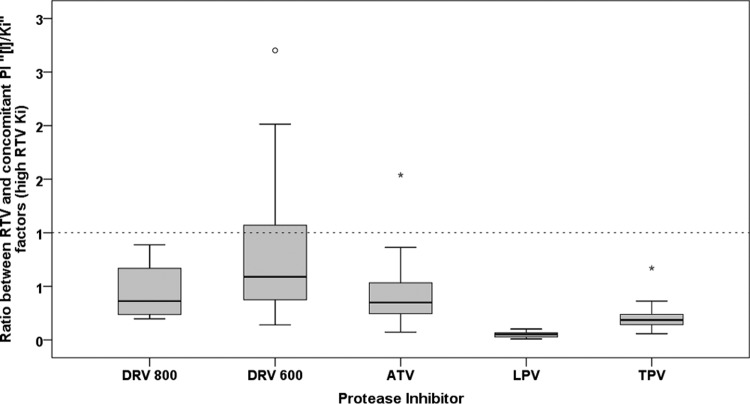
The ratios between [I]/Ki factors of RTV and those of the concomitant PIs were significantly different between each regimen (P < 0.001). The highest ratio was observed in patients treated with DRV-RTV 600 and 100 mg BID, some of whom showed a ratio of >1 (median, 0.59; interquartile range [IQR], 0.36 to 1.17), indicating a higher theoretical activity of RTV compared to that of DRV, as summarized in Fig. 2 and Table 2. By contrast, the lowest ratio was observed with LPV (median, 0.05; IQR, 0.03 to 0.07).
FIG 2.
Distribution of the ratio between the [I]/Ki factor of RTV (considering the highest RTV Ki) and that of the other PIs among the patients. The dashed line marks the cutoff of 1, indicating a higher activity of RTV than that of the concomitant PI. DRV800, DRV-RTV 800 and 100 mg once daily; DRV600, DRV-RTV 600 and 100 mg twice daily. Circles and asterisks indicate mild and extreme outliers, respectively.
In turn, when the mean inhibition constant for RTV was considered in the context of equation 4, these ratios substantially increased for all the regimens. In particular, a ratio of >1 was also observed in three ATV-treated patients (Table 2).
Performing a Wilcoxon test analysis among the patients, the [I]/Ki factor of each PI was significantly higher than that for RTV, indicating that, as expected, the companion PIs exert a higher antiviral effect than does RTV. This evidence was confirmed for all regimens except DRV-RTV 800 and 100 mg QD (P = 0.068).
The absence of significant differences between the I/Ki factor of DRV and that of RTV became increasingly evident when the mean Ki for RTV was considered. In this case, no significant differences were observed between the activities of DRV and RTV in the DRV-RTV 600- and 100-mg BID regimen.
Finally, we obtained insight into how much the presence of RTV affects the reaction speed of viral protease (Table 2) using equation 4. The median ratios between the theoretical reaction speeds of viral protease with and without RTV ranged from 0.63 for DRV-RTV 600 and 100 mg BID (indicating that the presence of RTV accounted for 37% of the antiviral effect) to 0.95 for LPV-RTV 400 and 100 mg BID (indicating that only 5% of the antiviral effect was exerted by RTV).
DISCUSSION
Until now, several methods have been adopted to measure antiretrovirals in PBMCs. However, despite the fact that these methods were fully validated for this measure, all of them determined concentrations without taking into account the physiological differences in the mean cellular volume (MCV) of PBMCs between patients.
A previously published paper (13) addressed this issue and suggested that the frequently used MCV of 400 fl should be revised. For this reason, we chose to determine the number of PBMCs and their MCV values for each sample using an automated counting method (11, 13, 14). Then, with the use of the personalized MCV, a correct and robust normalization of concentration data was achieved. Moreover, the intra-PBMC PI concentrations were simultaneously quantified with the same method (14).
The intracellular penetration was different among the PIs, as previously described (11). In particular, the intracellular concentrations of ATV and RTV were 2.4- and 7-fold higher, respectively, than the plasma concentrations, suggesting a strong intracellular accumulation of these drugs.
Interestingly, intracellular concentrations were correlated with plasma concentrations, the only exception being with LPV. This lack of correlation for LPV may be due to genetic interpatient variability in drug transporters or intracellular metabolism, which affects drug influx and efflux differently in cells and plasma.
The main finding of this study is that RTV intra-PBMC concentrations are comparable or even higher than those of other concomitant PIs, despite the low dosing profile, confirming our previous findings (11). Moreover, RTV penetration into PBMCs was variable among different regimens on the basis of the concomitant PI and the posology.
To evaluate the hypothesis of some contribution of RTV in protease inhibition, we compared the theoretical intracellular inhibitory activity of each PI with that of RTV at the observed concentrations, referring to the general formula of the reaction speed of an enzyme in the presence of a single inhibitor. In this setting, RTV concentrations seemed capable of exerting an antiviral activity slightly lower than or, in some cases, even equal to those of the concomitant PIs. This evidence is particularly robust for DRV-RTV-based regimens.
Equation 3 describes the effect of the addition of a new inhibitor to the system and confirms the validity of the use of the [I]/Ki factor to determine its inhibitory function. Moreover, the vi,j/vi ratio was always <1, which indicates that the presence of a second inhibitor invariably slows down the reaction speed, thus exerting an additive inhibitory effect even though the two inhibitors are considered mutually exclusive (the maximum level of competition). However, this inhibition does not linearly increase with increasing drug concentrations (effect of the natural substrate competition).
Finally, considering the low substrate concentration (Gag-Pol protein), equation 4 was used to compare reaction speeds with and without RTV. Again, the impact of the presence of RTV was increasingly important for DRV- and ATV-based regimens (around 40% and 30%, respectively, of the theoretical inhibition was due to RTV). This finding indicates that the effect of RTV activity when RTV is coadministered with DRV can be very high, and its support might be very important for achieving and maintaining viral suppression in these regimens. Conversely, the low ratio between RTV and LPV activity may indicate that this regimen is less dependent on RTV activity.
The adoption of booster molecules has been fundamental for the achievement of optimal plasmatic exposure to PIs and maintaining an acceptable administration profile. The development of alternative booster molecules, noninferior to RTV in terms of CYP 3A4 inhibition, might allow for the achievement of comparable effectiveness with slightly fewer side effects (8).
The results of this study suggest that RTV, despite being administered at a low dose, can reach intracellular levels sufficient to exert antiviral activity itself.
The observation of high intra-PBMC RTV concentrations leads to some hypothetical questions. Does RTV play a supporting role in the case of low exposure to the companion PI due to suboptimal adherence or low absorption, or might it be helpful in maintaining viral suppression in pharmacological sanctuary sites? To our knowledge, no studies on the intracellular pharmacodynamic interactions of RTV and other PIs have been reported. This issue represents a field of uncertainty that, according to our results, needs to be studied further.
A limitation of our work is the limited knowledge of the real disposition of each drug within the cells. Compartmentalization within cellular organelles may be a source of bias. However, the simultaneous intracellular quantification of each drug is currently the most accurate method for comparing drug pharmacokinetics at active sites. Moreover, the DRV-RTV 800- and 100-mg QD group had a small sample size (n = 4) but was nonetheless representative.
In conclusion, the evidence reported in this paper indicates a need for further in vitro and in vivo studies in order to have a more complete view of the antiviral effect of RTV before switching therapy to other booster molecules.
ACKNOWLEDGMENTS
We have no conflicts of interest to disclose.
This study was supported by internal funding.
Footnotes
Published ahead of print 5 May 2014
REFERENCES
- 1.Hogg RS, Heath KV, Yip B, Craib KJ, O'Shaughnessy MV, Schechter MT, Montaner JS. 1998. Improved survival among HIV-infected individuals following initiation of antiretroviral therapy. JAMA 279:450–454. 10.1001/jama.279.6.450 [DOI] [PubMed] [Google Scholar]
- 2.Palella FJ, Jr, Delaney KM, Moorman AC, Loveless MO, Fuhrer J, Satten GA, Aschman DJ, Holmberg SD. 1998. Declining morbidity and mortality among patients with advanced human immunodeficiency virus infection: HIV Outpatient Study Investigators. N. Engl. J. Med. 338:853–860. 10.1056/NEJM199803263381301 [DOI] [PubMed] [Google Scholar]
- 3.Antinori A, Marcotullio S, Ammassari A, Andreoni M, Angarano G, Carosi G, Cinque P, d'Arminio Monforte A, Di Perri G, Ensoli B, Ferrazzi E, Galli M, Mastroianni C, Matteelli A, Mazzotta F, Moroni M, Palu G, Puoti M, Puro V, Rizzardini G, Sagnelli E, Suter F, Vella S, Lazzarin A. 2011. Italian guidelines for the use of antiretroviral agents and the diagnostic-clinical management of HIV-1 infected persons. New Microbiol. 34:109–146 [PubMed] [Google Scholar]
- 4.Bucher HC, Wolbers M, Porter K. 2010. 2010 guidelines for antiretroviral treatment of HIV from the International AIDS Society-USA Panel. JAMA 304:1897 (Letter.) (Reply, 304:1897–1898.) 10.1001/jama.2010.1561 [DOI] [PubMed] [Google Scholar]
- 5.Hsu A, Granneman GR, Bertz RJ. 1998. Ritonavir: clinical pharmacokinetics and interactions with other anti-HIV agents. Clin. Pharmacokinet. 35:275–291. 10.2165/00003088-199835040-00002 [DOI] [PubMed] [Google Scholar]
- 6.Canta F, Marrone R, Bonora S, D'Avolio A, Sciandra M, Sinicco A, De Rosa FG, Di Perri G. 2005. Pharmacokinetics and hepatotoxicity of lopinavir/ritonavir in non-cirrhotic HIV and hepatitis C virus (HCV) co-infected patients. J. Antimicrob. Chemother. 55:280–281. 10.1093/jac/dkh516 [DOI] [PubMed] [Google Scholar]
- 7.Temesgen Z. 2013. Cobicistat, a pharmacoenhancer for HIV treatments. Drugs Today (Barc) 49:233–237. 10.1358/dot.2013.49.4.1947288 [DOI] [PubMed] [Google Scholar]
- 8.Zolopa A, Sax PE, Dejesus E, Mills A, Cohen C, Wohl D, Gallant JE, Liu HC, Plummer A, White KL, Cheng AK, Rhee MS, Szwarcberg J. 2013. A randomized double-blind comparison of coformulated elvitegravir/cobicistat/emtricitabine/tenofovir disoproxil fumarate versus efavirenz/emtricitabine/tenofovir disoproxil fumarate for initial treatment of HIV-1 infection: analysis of week 96 results. J. Acquir. Immune Defic. Syndr. 63:96–100. 10.1097/QAI.0b013e318289545c [DOI] [PubMed] [Google Scholar]
- 9.Gallant JE, Koenig E, Andrade-Villanueva J, Chetchotisakd P, Dejesus E, Antunes F, Arasteh K, Moyle G, Rizzardini G, Fehr J, Liu Y, Zhong L, Callebaut C, Szwarcberg J, Rhee MS, Cheng AK. 2013. Cobicistat versus ritonavir as a pharmacoenhancer of atazanavir plus emtricitabine/tenofovir disoproxil fumarate in treatment-naive HIV type 1-infected patients: week 48 results. J. Infect. Dis. 208:32–39. 10.1093/infdis/jit122 [DOI] [PubMed] [Google Scholar]
- 10.Ter Heine R, Mulder JW, van Gorp EC, Wagenaar JF, Beijnen JH, Huitema AD. 2010. Intracellular and plasma steady-state pharmacokinetics of raltegravir, darunavir, etravirine and ritonavir in heavily pre-treated HIV-infected patients. Br. J. Clin. Pharmacol. 69:475–483. 10.1111/j.1365-2125.2010.03634.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.D'Avolio A, Simiele M, Calcagno A, Siccardi M, Larovere G, Agati S, Baietto L, Cusato J, Tettoni M, Sciandra M, Trentini L, Di Perri G, Bonora S. 2013. Intracellular accumulation of ritonavir combined with different protease inhibitors and correlations between concentrations in plasma and peripheral blood mononuclear cells. J. Antimicrob. Chemother. 68:907–910. 10.1093/jac/dks484 [DOI] [PubMed] [Google Scholar]
- 12.Jackson A, Watson V, Back D, Khoo S, Liptrott N, Egan D, Gedela K, Higgs C, Abbas R, Gazzard B, Boffito M. 2011. Plasma and intracellular pharmacokinetics of darunavir/ritonavir once daily and raltegravir once and twice daily in HIV-infected individuals. J. Acquir. Immune Defic. Syndr. 58:450–457. 10.1097/QAI.0b013e3182364c67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Simiele M, D'Avolio A, Baietto L, Siccardi M, Sciandra M, Agati S, Cusato J, Bonora S, Di Perri G. 2011. Evaluation of the mean corpuscular volume of peripheral blood mononuclear cells of HIV patients by a Coulter Counter to determine intracellular drug concentrations. Antimicrob. Agents Chemother. 55:2976–2978. 10.1128/AAC.01236-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.D'Avolio A, Simiele M, Siccardi M, Baietto L, Sciandra M, Oddone V, Stefani FR, Agati S, Cusato J, Bonora S, Di Perri G. 2011. A HPLC-MS method for the simultaneous quantification of fourteen antiretroviral agents in peripheral blood mononuclear cell of HIV infected patients optimized using medium corpuscular volume evaluation. J. Pharm. Biomed. Anal. 54:779–788. 10.1016/j.jpba.2010.10.011 [DOI] [PubMed] [Google Scholar]
- 15.D'Avolio A, Baietto L, Siccardi M, Sciandra M, Simiele M, Oddone V, Bonora S, Di Perri G. 2008. An HPLC-PDA method for the simultaneous quantification of the HIV integrase inhibitor raltegravir, the new nonnucleoside reverse transcriptase inhibitor etravirine, and 11 other antiretroviral agents in the plasma of HIV-infected patients. Ther. Drug Monit. 30:662–669. 10.1097/FTD.0b013e318189596d [DOI] [PubMed] [Google Scholar]
- 16.Kakkar T, Boxenbaum H, Mayersohn M. 1999. Estimation of Ki in a competitive enzyme-inhibition model: comparisons among three methods of data analysis. Drug Metab. Dispos. 27:756–762 [PubMed] [Google Scholar]
- 17.Altman MD, Ali A, Reddy GS, Nalam MN, Anjum SG, Cao H, Chellappan S, Kairys V, Fernandes MX, Gilson MK, Schiffer CA, Rana TM, Tidor B. 2008. HIV-1 protease inhibitors from inverse design in the substrate envelope exhibit subnanomolar binding to drug-resistant variants. J. Am. Chem. Soc. 130:6099–6113. 10.1021/ja076558p [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dandache S, Sevigny G, Yelle J, Stranix BR, Parkin N, Schapiro JM, Wainberg MA, Wu JJ. 2007. In vitro antiviral activity and cross-resistance profile of PL-100, a novel protease inhibitor of human immunodeficiency virus type 1. Antimicrob. Agents Chemother. 51:4036–4043. 10.1128/AAC.00149-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shuman CF, Vrang L, Danielson UH. 2004. Improved structure-activity relationship analysis of HIV-1 protease inhibitors using interaction kinetic data. J. Med. Chem. 47:5953–5961. 10.1021/jm0499110 [DOI] [PubMed] [Google Scholar]
- 20.Spaltenstein A, Almond MR, Bock WJ, Cleary DG, Furfine ES, Hazen RJ, Kazmierski WM, Salituro FG, Tung RD, Wright LL. 2000. Novel inhibitors of HIV protease: design, synthesis and biological evaluation of picomolar inhibitors containing cyclic P1/P2 scaffolds. Bioorg. Med. Chem. Lett. 10:1159–1162. 10.1016/S0960-894X(00)00163-3 [DOI] [PubMed] [Google Scholar]
- 21.Asante-Appiah E, Chan WW. 1996. Analysis of the interactions between an enzyme and multiple inhibitors using combination plots. Biochem. J. 320:17–26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chou TC, Talaly P. 1977. A simple generalized equation for the analysis of multiple inhibitions of Michaelis-Menten kinetic systems. J. Biol. Chem. 252:6438–6442 [PubMed] [Google Scholar]
- 23.Martinez-Irujo JJ, Villahermosa ML, Mercapide J, Cabodevilla JF, Santiago E. 1998. Analysis of the combined effect of two linear inhibitors on a single enzyme. Biochem. J. 329:689–698 [DOI] [PMC free article] [PubMed] [Google Scholar]