Abstract
Fifteen novel arylimidamides (AIAs) (6 bis-amidino and 9 mono-amidino analogues) were assayed against Trypanosoma cruzi in vitro and in vivo. All the bis-AIAs were more effective than the mono-AIAs, and two analogues, DB1967 and DB1989, were further evaluated in vivo. Although both of them reduced parasitemia, protection against mortality was not achieved. Our results show that the number of amidino-terminal units affects the efficacy of arylimidamides against T. cruzi.
TEXT
Chagas disease (CD) is caused by Trypanosoma cruzi and affects more than 8 million people worldwide (1–5). Benznidazole (BZ) and nifurtimox (NF) are used for the treatment of CD, but because of their well-known toxicity and limited efficacy in the later chronic phase of the disease, new drugs are urgently needed (6–9). We have evaluated several classes of natural and synthetic compounds, including arylimidamides (AIAs), aromatic diamidine (AD) derivatives with extraordinary activity against T. cruzi and other trypanosomatids, both in vitro (10–16) and in vivo (17, 18). In AIAs, the imino group is linked via an anilino nitrogen, while in classical amidines, it is directly attached to an aryl ring, yielding reduced pK values (14). Here, we report the results of in vitro and in vivo activity studies and mutagenicity and selectivity assessments of new AIAs (6 bis-amidino analogues, DB1966, DB1967, DB1968, DB1979, DB1989, and DB1995, and 9 mono-amidino analogues, DB1996, DB1997, DB1980, DB2001, DB2002, DB2003, DB2004, DB2006, and DB2007), which provide insight on the relevance of one or two terminal amidino units for biological activity.
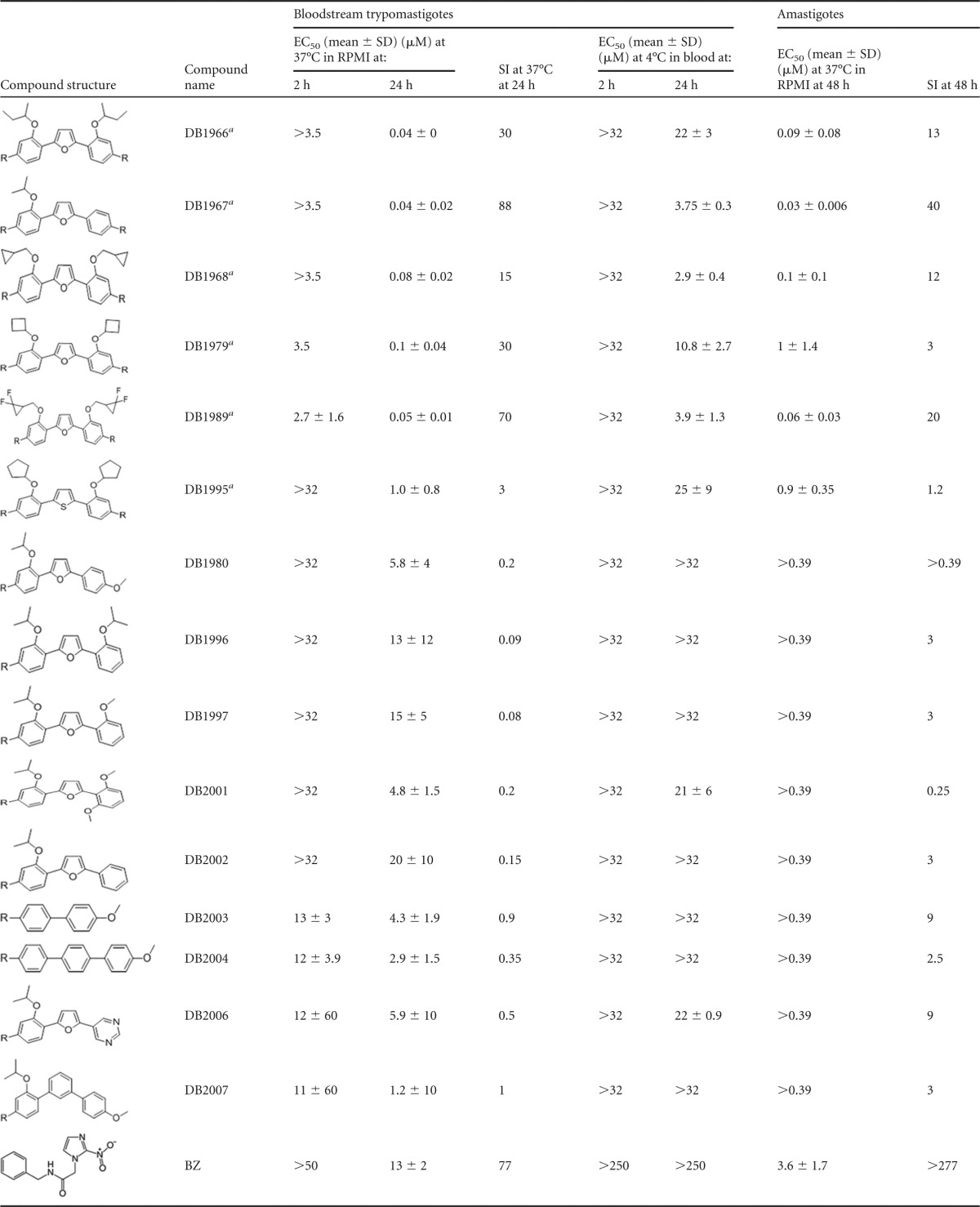
We synthesized the mono- and bis-arylimidamides (see structures in Table 1) as reported (19–21). Benznidazole (BZ) (Laboratório Farmacêutico do Estado de Pernambuco, LAFEPE, Brazil) and gentian violet (Sigma-Aldrich) were used as reference drugs (22). Primary cultures of cardiac cells (CC) were obtained as reported (18, 23). The Y strain of T. cruzi was used, and bloodstream trypomastigotes (BT) and intracellular trypomastigote forms were assayed as described previously (18, 23). Mammalian cell cytotoxicity of AIAs was evaluated on uninfected CC incubated up to 48 h at 37°C with each compound (0 to 32 μM); morphology, spontaneous contractibility, and cell death rates were measured for determination of the 50% effective compound concentrations (EC50s) (24). For trypanocidal analysis, BT were incubated at 37°C for 24 h with nontoxic concentrations of the compounds to determine the EC50 (24). For analysis with intracellular amastigotes, after 24 h of parasite-host cell interaction, increasing nontoxic doses of the compounds were added for 48 h, and drug activity was estimated by calculating the infection index (II) as reported (12, 24). The data shown are the means ± standard deviations from 2 to 4 experiments run in duplicate. A bacterial reverse mutation (Ames) test and a cytotoxicity assay were performed as proposed by Maron and Ames (25) and Organization for Economic Cooperation and Development (OECD) test guideline 471 (26). Statistical analysis was performed by the analysis of variance test (P ≤ 0.05) (22).
TABLE 1.
AIA activity against bloodstream trypomastigotes and intracellular (amastigote) forms of T. cruzi (Y strain) and the corresponding selectivity indexes
Bis-AIA.
Male Swiss Webster mice (18 to 21 g) (Fundação Oswaldo Cruz Animal Facility [CECAL/FIOCRUZ], Brazil) were housed six per cage in a conventional room at 20 to 24°C under a 12/12-h light/dark cycle, with sterilized water and chow provided ad libitum. Infection was achieved by intraperitoneal (i.p.) injection of 104 BT (Y strain), and the mice (6 per group) were uninfected (noninfected and nontreated), untreated (infected and treated with vehicle), or treated with different doses of DB1989 and DB1967 (infected and treated with 0.2-ml i.p. daily doses up to 50 mg/kg of body weight). Infected mice were treated with 100 mg/kg/day BZ orally once a day. Treatment was given at the 5th (parasitemia onset) and 8th day postinfection (dpi) (parasitemia peak). Parasitemia levels, body weights, and percentage of cumulative mortality were checked until 30 days posttreatment, as reported (18). All procedures were carried out in accordance with the guidelines established by the FIOCRUZ Committee of Ethics for the Use of Animals (CEUA 0028/09).
BT incubated for 24 h at 37°C showed that 12 of the 15 compounds (all but DB1996, DB1997, and DB2002) had superior trypanocidal activities (P ≤ 0.05) compared to that of BZ (EC50, 13 μM). Five of the bis-AIAs (DB1966, DB1967, DB1968, DB1979, and DB1989) yielded EC50s of ≤0.1 μM. The bis-AIA DB1989, the fastest-acting trypanocidal compound, provided an EC50 of 2.7 μM after 2 h (Table 1). Bis-AIAs also displayed the best effect under blood bank conditions (in blood at 4°C); DB1967, DB1968, and DB1989 showed EC50s ranging from 2.9 to 3.9 μM, while BZ was ineffective at up to 250 μM (Table 1). The most selective compounds against BT were DB1967 (selectivity index [SI], 88) and DB1989 (SI, 70) (Table 1). The mono-AIAs DB1980, DB2001, and DB2004 were the most toxic against cardiac cell cultures at 48 h. Mono-AIAs were ineffective after 48 h at 37°C on T. cruzi-infected cultures (Table 1). Similar to the effect against BT, four bis-AIAs (DB1966, DB1967, DB1968, and DB1989) were the most effective against intracellular parasites (EC50s of ≤0.1 μM) (Table 1). Mono-AIAs displayed very low selectivities, while the bis-AIAs DB1989 and DB1967 exhibited the highest SI levels (20 and 40, respectively) against the intracellular parasites (Table 1). A bacterial reverse mutation (Ames) test indicated no major mutagenic potential (mutagenic index, <2) with DB1989 (see Table S1 in the supplemental material) or BZ (data not shown). Due to their excellent in vitro activities against the two parasite forms and reasonable selectivities, DB1967 and DB1989 were evaluated in vivo. At 8 dpi (parasitemia peak), DB1989 reduced parasitemia (by 40, 76, and 75% with 12.5, 25, and 50-mg/kg/day doses, respectively), while BZ suppressed parasitemia (Fig. 1A). BZ resulted in 100% survival of the mice, but no dose of DB1989 prevented mortality triggered by the infection (Fig. 1B); the highest dose (50 mg/kg/day) produced higher mortality rates compared to that of the untreated group, possibly due to compound toxicity (a ponderal curve shows higher weight [Fig. 1C]). DB1967 produced dose-response suppression (67 to 87%) of parasitemia but an earlier and higher mortality rate (100% for all DB1967-treated groups, likely due to toxicity; data not shown).
FIG 1.
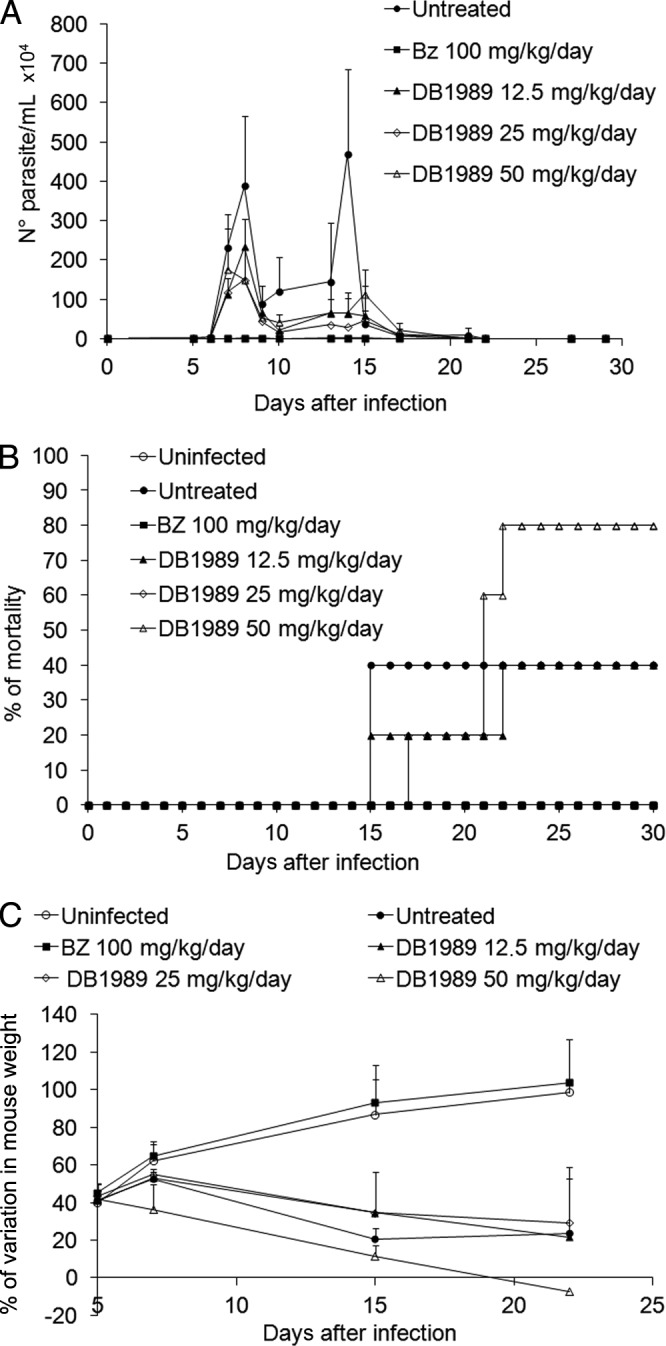
In vivo effect of DB1989 on acute mouse model of infection with the Y strain of T. cruzi. Parasitemia (A), mortality rates (B), and ponderal curve (C) are shown. The effects of DB1989 (i.p.) and BZ (oral) were followed using doses (up to 50 mg/kg/day for DB1989 and 100 mg/kg/day for BZ) administered at the 5th and 8th dpi.
AIAs such as DB766 are effective in vitro and in vivo against intracellular pathogens that cause human and animal pathologies (24, 27–29) and exhibit stronger activity than those of classical diamidines (possibly due to their lower pKa values), better bioavailability, and improved cell membrane permeability (28). Similar to their effect against Leishmania, bis-AIAs are highly active against T. cruzi (12, 15, 22). DB766 showed a selective effect against intracellular amastigotes and upon a large panel of T. cruzi strains, including naturally resistant strains, with a higher efficacy than those of the reference drugs (18).
This work explores the correlation between the trypanocidal activity/selectivity of AIAs with one or two terminal amidino groups. Bis-AIAs were most potent against the two parasite forms relevant to mammalian infection (the bloodstream and intracellular forms), demonstrating that two terminal amidino centers confer a higher parasiticidal effect than those bearing only one. The importance of the second amidino center is seen by comparing the results for DB1967 with those for DB2002 (500-fold activity difference; Table 1), which differ only in the absence of the second amidino group in DB1967. These results corroborate previous findings for classical diamidines, confirming the requirement of a diamidino unit for effectiveness against T. cruzi (15). The bis-AIAs DB1967, DB1968, and DB1989 maintained good trypanocidal activity at 4°C with 96% mouse blood, similar to the activities of other bis-AIAs, including DB766 (18), DB745 (30), and DB1831 (22).
All tested bis-AIAs have alkoxy groups of approximately the same size and with similar in vitro activities (EC50s of ≤0.1 μM). DB1967, with only one 2-propoxy group, has essentially the same antitrypanosomal activity as that of DB766 (18), which has two such groups; yet, DB1967 is more toxic to animals than DB766, suggesting that two moderately sized alkoxy groups reduce animal toxicity. Generally, the activities of the mono-AIAs do not vary significantly with structure (Table 1). Most bis-AIAs were also less toxic toward cardiac cells than were the mono-AIAs. Presently, up to the maximum dose tested, genotoxicity was absent, and only a mild mutagenicity profile was observed when DB1989 was assayed against the Salmonella enterica Typhimurium TA98 strain (see Table S1 in the supplemental material), which is suggestive of a frameshift mutation, probably during the DNA repair or duplication process, adding GC pairs into the genome. Although OECD test guideline 471 recommends using up to 5 mg of a tested compound, the high activity of DB1989 toward the bacterial strains impaired assaying higher AIA concentrations that may mask mutagenic aspects, demanding additional toxicological studies.
DB1989 and DB1967 were moved to T. cruzi in vivo models due to their high in vitro activities and reasonable selectivities. Although parasitemia was reduced, neither DB1967 nor DB1989 protected against mortality. This is in contrast to results with DB766 (18) and DB1965, a mesylate salt form of DB1831 (22) which showed in vivo efficacy comparable to that of BZ. The reduction of parasitemia observed with DB1967 correlates with the in vitro data obtained with bloodstream and intracellular parasites (EC50s of 30 to 40 nM). As low toxicity was observed in vitro, the higher mortality rate of the DB1967-treated mice is likely due to an organ-specific toxicity (e.g., hepatotoxicity) or arose from metabolic products of the bis-AIA.
Our data confirm the importance of two amidino centers for the trypanocidal efficacy of arylimidamides against T. cruzi and demonstrated that mono-AIAs are less effective and selective than bis-AIAs. Although very active in vitro, DB1989 and DB1967 failed to protect against T. cruzi infection in vivo, possibly due to toxicity. Since previous studies demonstrated in vivo efficacies comparable to that of BZ for other bis-AIAs, e.g., DB766 (18) and DB1965 (22), the synthesis of novel AIAs bearing bis-terminal pyrimidines or pyridines merits further investigation as an approach for identifying new anti-T. cruzi agents.
Supplementary Material
ACKNOWLEDGMENTS
This study was supported by Fiocruz and Fundação Carlos Chagas Filho de Amparo a Pesquisa do Estado do Rio de Janeiro (FAPERJ), Conselho Nacional Desenvolvimento Científico e Tecnológico (CNPq), CAPES, PROEP, and the Consortium for Parasitic Drug Development (CPDD). This study was partially supported by The Bill and Melinda Gates Foundation through a subcontract with the CPDD (to D.W.B.).
We thank the Program for Technological Development in Tools for Health-PDTIS-FIOCRUZ and Israel Felzenszwalb for allowing us to use their facilities and laboratories.
Footnotes
Published ahead of print 3 March 2014
Supplemental material for this article may be found at http://dx.doi.org/10.1128/AAC.01403-13.
REFERENCES
- 1.de Soeiro MN, De Castro SL. 2011. Screening of potential anti-Trypanosoma cruzi candidates: in vitro and in vivo studies. Open Med. Chem. J. 5:21–30. 10.2174/1874104501105010021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.World Health Organization. 2013. Sustaining the drive to overcome the global impact of neglected tropical diseases: second WHO report on neglected tropical diseases. World Health Organization, Geneva, Switzerland [Google Scholar]
- 3.Rassi A, Jr, Rassi A, Marcondes de Rezende J. 2012. American trypanosomiasis (Chagas disease). Infect. Dis. Clin. North Am. 26:275–291. 10.1016/j.idc.2012.03.002 [DOI] [PubMed] [Google Scholar]
- 4.Dumonteil E, Bottazzi ME, Zhan B, Heffernan MJ, Jones K, Valenzuela JG, Kamhawi S, Ortega J, Rosales SP, Lee BY, Bacon KM, Fleischer B, Slingsby BT, Cravioto MB, Tapia-Conyer R, Hotez PJ. 2012. Accelerating the development of a therapeutic vaccine for human Chagas disease: rationale and prospects. Expert Rev. Vaccines 11:1043–1055. 10.1586/erv.12.85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rocha MOC, Teixeira MM, Ribeiro AL. 2007. An update on the management of Chagas cardiomyopathy. Expert Rev. Anti Infect. Ther. 5:727–743. 10.1586/14787210.5.4.727 [DOI] [PubMed] [Google Scholar]
- 6.De Castro SL, Batista DG, Batista MM, Batista W, Daliry A, de Souza EM, Menna-Barreto RF, Oliveira GM, Salomão K, Silva CF, Silva PB, Soeiro MN. 2011. Experimental chemotherapy for Chagas disease: a morphological, biochemical, and proteomic overview of potential Trypanosoma cruzi targets of amidines derivatives and naphthoquinones. Mol. Biol. Int. 306928. 10.4061/2011/306928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Soeiro MNC, Daliry A, Silva CF, Batista DGJ, De Souza EM, Oliveira GM, Salomão K, Batista MM, Pacheco M, Silva PB, Santa-Rita RM, Menna-Barreto RFS, Boykin DW, De Castro SL. 2009. Experimental chemotherapy for Chagas disease: 15 years of research contributions through in vivo and in vitro studies. Mem. Inst. Oswaldo Cruz 104:301–310. 10.1590/S0074-02762009000900040 [DOI] [PubMed] [Google Scholar]
- 8.Clayton J. 2010. Chagas disease 101. Nature 465:S4–S5. 10.1038/nature09220 [DOI] [PubMed] [Google Scholar]
- 9.Lima FM, Oliveira P, Mortara RA, Silveira JF, Bahia D. 2010. The challenge of Chagas' disease: has the human pathogen, Trypanosoma cruzi, learned how to modulate signaling events to subvert host cells? N. Biotechnol. 27:837–843. 10.1016/j.nbt.2010.02.003 [DOI] [PubMed] [Google Scholar]
- 10.Wilson WD, Tanious FA, Mathis A, Tevis D, Hall JE, Boykin DW. 2008. Antiparasitic compounds that target DNA. Biochimie 90:999–1014. 10.1016/j.biochi.2008.02.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Soeiro MN, Werbovetz K, Boykin DW, Wilson WD, Wang MZ, Hemphill A. 2013. Novel amidines and analogues as promising agents against intracellular parasites: a systematic review. Parasitology 8:1–23. 10.1017/S0031182013000292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Silva CF, Batista MM, De Souza EM, Meirelles MNL, Stephens CE, Som P, Boykin DW, Soeiro MNC. 2007. Cellular effects of reversed amidines on Trypanosoma cruzi. Antimicrob. Agents Chemother. 51:3803–3809. 10.1128/AAC.00047-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Silva CF, Batista MM, Mota RA, de Souza EM, Stephens CE, Som P, Boykin DW, Soeiro MN. 2007. Activity of “reversed” diamidines against Trypanosoma cruzi in vitro. Biochem. Pharmacol. 73:1939–1946. 10.1016/j.bcp.2007.03.020 [DOI] [PubMed] [Google Scholar]
- 14.Rosypal AC, Werbovetz KA, Salem M, Stephens CE, Kumar A, Boykin DW, Hall JE, Tidwell RR. 2008. Inhibition by dications of in vitro growth of Leishmania major and Leishmania tropica: causative agents of Old World cutaneous leishmaniasis. J. Parasitol. 94:743–749. 10.1645/GE-1387R1.1 [DOI] [PubMed] [Google Scholar]
- 15.Pacheco MGO, Silva CF, De Souza EM, Batista MM, Silva PB, Kumar A, Stephens CE, Boykin DW, Soeiro MNC. 2009. Activity of heterocyclic cationic molecules against Trypanosoma cruzi in vitro. Exp. Parasitol. 123:73–80. 10.1016/j.exppara.2009.06.004 [DOI] [PubMed] [Google Scholar]
- 16.De Souza EM, da Silva PB, Nefertiti AS, Ismail MA, Arafa RK, Tao B, Nixon-Smith CK, Boykin DW, Soeiro MN. 2011. Trypanocidal activity and selectivity in vitro of aromatic amidine compounds upon bloodstream and intracellular forms of Trypanosoma cruzi. Exp. Parasitol. 127:429–435. 10.1016/j.exppara.2010.10.010 [DOI] [PubMed] [Google Scholar]
- 17.Da Silva CF, Batista MM, Batista DG, de Souza EM, da Silva PB, de Oliveira GM, Meuser AS, Shareef AR, Boykin DW, Soeiro MN. 2008. In vitro and in vivo studies of the trypanocidal activity of a diarylthiophene diamidine against Trypanosoma cruzi. Antimicrob. Agents Chemother. 52:3307–3314. 10.1128/AAC.00038-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Batista DG, Batista MM, de Oliveira GM, do Amaral PB, Lannes-Vieira J, Britto CC, Junqueira A, Lima MM, Romanha AJ, Sales Junior PA, Stephens CE, Boykin DW, Soeiro MN. 2010. Arylimidamide DB766, a potential chemotherapeutic candidate for Chagas' disease treatment. Antimicrob. Agents Chemother. 54:2940–2952. 10.1128/AAC.01617-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stephens CE, Tanious F, Kim S, Wilson WD, Schell WA, Perfect JR, Franzblau SG, Boykin DW. 2001. Diguanidino and “reversed” diamidino 2,5-diarylfurans as antimicrobial agents. J. Med. Chem. 44:1741–1748. 10.1021/jm000413a [DOI] [PubMed] [Google Scholar]
- 20.Reid C, Farahat AA, Zhu X, Pandharkar T, Boykin DW, Werbovetz KA. 2012. Antileishmanial bis-arylimidamides: DB766 analogs modified in the linker region and bis-arylimidamide structure-activity relationships. Bioorg. Med. Chem. Lett. 22:6806–6810. 10.1016/j.bmcl.2012.06.037 [DOI] [PubMed] [Google Scholar]
- 21.Banerjee M, Farahat AA, Kumar A, Wenzler T, Brun R, Munde MM, Wilson WD, Zhu X, Werbovetz KA, Boykin DW. 2012. Synthesis, DNA binding and antileishmanial activity of low molecular weight bis-arylimidamides. Eur. J. Med. Chem. 55:449–454. 10.1016/j.ejmech.2012.06.058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Da Silva CF, Batista DG, Oliveira GM, de Souza EM, Hammer ER, da Silva PB, Daliry A, Araujo JS, Britto C, Rodrigues AC, Liu Z, Farahat AA, Kumar A, Boykin DW, Soeiro MN. 2012. In vitro and in vivo investigation of the efficacy of arylimidamide DB1831 and its mesylated salt form—DB1965—against Trypanosoma cruzi infection. PLoS One 7:e30356. 10.1371/journal.pone.0030356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Meirelles MN, Araujo-Jorge TC, Miranda CF, De Souza W, Barbosa HS. 1986. Interaction of Trypanosoma cruzi with heart muscle cells: ultrastructural and cytochemical analysis of endocytic vacuole formation and effect upon myogenesis in vitro. Eur. J. Cell Biol. 41:198–206 [PubMed] [Google Scholar]
- 24.Soeiro MD, de Souza EM, da Silva CF, da Gama Jaen Batista D, Batista MM, Pavão BP, Araújo JS, Aiub CA, da Silva PB, Lionel J, Britto C, Kim K, Sulikowski G, Hargrove TY, Waterman MR, Lepesheva GI. 2013. In vitro and in vivo studies of the antiparasitic activity of sterol 14α-demethylase (CYP51) inhibitor VNI against drug-resistant strains of Trypanosoma cruzi. Antimicrob. Agents Chemother. 57:4151–4163. 10.1128/AAC.00070-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Maron DM, Ames BN. 1983. Revised methods for the Salmonella mutagenicity test. Mutat. Res. 113:173–215. 10.1016/0165-1161(83)90010-9 [DOI] [PubMed] [Google Scholar]
- 26.Organization for Economic Cooperation and Development. 1997. Test guideline 471. Bacterial reverse mutation test. In OECD guideline for testing of chemicals. Organization for Economic Cooperation and Development, Paris, France [Google Scholar]
- 27.Werbovetz K. 2006. Diamidines as antitrypanosomal, antileishmanial and antimalarial agents. Curr. Opin. Investig. Drugs 7:147–157 [PubMed] [Google Scholar]
- 28.Wang MZ, Zhu X, Srivastava A, Liu Q, Sweat JM, Pandharkar T, Stephens CE, Riccio E, Parman T, Munde M, Mandal S, Madhubala R, Tidwell RR, Wilson WD, Boykin DW, Hall JE, Kyle DE, Werbovetz KA. 2010. Novel arylimidamides for treatment of visceral leishmaniasis. Antimicrob. Agents Chemother. 54:2507–2516. 10.1128/AAC.00250-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhu X, Liu Q, Yang S, Parman T, Green CE, Mirsalis JC, de Nazaré Correia Soeiro M, Mello de Souza E, da Silva CF, da Gama Jaen Batista D, Stephens CE, Banerjee M, Farahat AA, Munde M, Wilson WD, Boykin DW, Wang MZ, Werbovetz KA. 2012. Evaluation of arylimidamides DB1955 and DB1960 as candidates against visceral leishmaniasis and Chagas' disease: in vivo efficacy, acute toxicity, pharmacokinetics, and toxicology studies. Antimicrob. Agents Chemother. 56:3690–3699. 10.1128/AAC.06404-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Da Silva CF, Junqueira A, Lima MM, Romanha AJ, Sales Junior PA, Stephens CE, Som P, Boykin DW, Soeiro MN. 2011. In vitro trypanocidal activity of DB745B and other novel arylimidamides against Trypanosoma cruzi. J. Antimicrob. Chemother. 66:1295–1297. 10.1093/jac/dkr140 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


