Abstract
Piperacillin in combination with tazobactam, a β-lactamase inhibitor, is a commonly used intravenous antibiotic for the empirical treatment of infection in intensive care patients, including burn patients. The purpose of this study was to develop a population pharmacokinetic (PK) model for piperacillin in burn patients and to predict the probability of target attainment (PTA) using MICs and concentrations simulated from the PK model. Fifty burn patients treated with piperacillin-tazobactam were enrolled. Piperacillin-tazobactam was administered via infusion for approximately 30 min at a dose of 4.5 g (4 g piperacillin and 0.5 g tazobactam) every 8 h. Blood samples were collected just prior to and at 1, 2, 3, 4, and 6 h after the end of the infusion at steady state. The population PK model of piperacillin was developed using NONMEM. A two-compartment first-order elimination PK model was finally chosen. The covariates included were creatinine clearance (CLCR), day after burn injury (DAI), and sepsis. The final PK parameters were clearance (liters/h) (equal to 16.6 × [CLCR/132] + DAI × [−0.0874]), central volume (liters) (equal to 25.3 + 14.8 × sepsis [0 for the absence or 1 for the presence of sepsis]), peripheral volume (liters) (equal to 16.1), and intercompartmental clearance (liters/h) (equal to 0.636). The clearance and volume of piperacillin were higher than those reported in patients without burns, and the terminal half-life and PTA decreased with the increased CLCR. Our PK model suggests that higher daily doses or longer durations of infusion of piperacillin should be considered, especially for burn patients with a CLCR of ≥160 ml/min.
INTRODUCTION
Piperacillin-tazobactam (Tabaxin; Penmix Ltd., Jeong-dong, Jung-gu, Seoul, South Korea) is a parenterally administered combination of a β-lactam antibiotic and a β-lactamase inhibitor in a ratio of 8:1 (piperacillin to tazobactam). It shows broad antibacterial activity against Gram-positive and Gram-negative pathogens. This combination has been frequently used for the empirical treatment of infection in intensive care patients, including burn patients (1, 2). In burn patients, Pseudomonas aeruginosa, Acinetobacter spp., and Klebsiella pneumoniae are known to be the most common Gram-negative pathogens, and Staphylococcus aureus is the most common Gram-positive pathogen (3, 4).
Piperacillin, like other β-lactam antibiotics, is an antibiotic that shows time-dependent killing. For these antibiotics, the length of time that the unbound concentrations are maintained above the MIC (fT>MIC) correlates best with antibacterial activity (5, 6). Data on the fT>MIC required for optimal activity of β-lactam antibiotics have been obtained from murine infection models. A target time of 50% fT>MIC is reported to be the goal for near-maximal bacterial killing, and a target time of 30% fT>MIC correlates best with bacteriostasis (5, 6).
Piperacillin, for which the protein binding in human plasma is approximately 30% (2), is mainly eliminated via the kidney by glomerular filtration and tubular secretion. Achieving target concentrations in burn patients remains a challenging issue to clinicians because burn injuries can bring about changes in blood flow, glomerular filtration rate (GFR), and plasma protein levels (7). These physiologic changes may influence the pharmacokinetic (PK) parameters, such as clearance (CL), volume of distribution (V), and protein binding. Additionally, the region or country where the bacteria came from might have an effect on the treatment of the patients. The proportions of piperacillin-tazobactam-resistant (MIC of >128/4 mg/liter) Escherichia coli and K. pneumoniae according to European Committee on Antimicrobial Susceptibility Testing (EUCAST) data were 1.9% and 8.4%, respectively (8). In the United States, the proportions were 3.1% (E. coli) and 11.5% (K. pneumoniae), as reported in the Meropenem Yearly Susceptibility Test Information Collection (MYSTIC) Program in 2008 (9). In Korean hospitals, the resistance rates in 2009, which were 4% and 15%, respectively (10), were slightly higher than those in the EUCAST and MYSTIC data. The differences in the resistance proportions, which are dependent upon the region or country, may result in differences in the probability of treatment success.
There are several population PK studies for piperacillin in patients with cystic fibrosis or renal impairment and in critically ill patients (11–13). However, population PK in burn patients has rarely been reported. The purpose of this study was to characterize the PK of piperacillin in the presence of tazobactam in burn patients via population PK modeling and Monte Carlo simulations. We also sought to predict the probability of target attainment (PTA) by MIC using concentrations simulated from the population PK model.
MATERIALS AND METHODS
Patients.
Fifty patients with burns ranging from 1% to 81% of their total body surface area (TBSA) who were treated with piperacillin-tazobactam were enrolled in this study. They were admitted to the Burn Intensive Care Unit (BICU) of Hangang Sacred Heart Hospital between November 2011 and August 2012. The study protocol was approved by the institutional review board of Hangang Sacred Heart Hospital, and the study was performed in compliance with the principles of the Declaration of Helsinki and the Korean good clinical practice guidelines. All patients or legal representatives (in case the patient could not give consent) gave written informed consent. Patients who were pregnant, breastfeeding, <18 years old, or allergic to penicillin were excluded. The demographic characteristics of the patients are summarized in Table 1.
TABLE 1.
Patient demographics
| Characteristic | Value (mean [range]) |
|---|---|
| No. of patients | 50 |
| Age (yr) | 50.14 (20–83) |
| Sex (no. male/no. female) | 40/10 |
| Weight (kg) | 66.9 (50–90) |
| TBSA (%) | 34.56 (1–81) |
| Day after burn injury (days) | 12.8 (2–68) |
| Albumin (g/dl) | 2.58 (1.6–3.5) |
| No. on CRRT/no. not on CRRT | 5/45 |
| No. with edema/no. without edemaa | 16/34 |
| No. with sepsis/no. without sepsis | 12/38 |
| CLCR (ml/min)b | 132.1 (39–231.4) |
Clinical diagnosis (puffy face and pitting edema in the legs).
CLCR was estimated by the Cockcroft-Gault equation.
Piperacillin administration and blood sampling.
Piperacillin-tazobactam was administered via infusion for approximately 30 min at a dose of 4.5 g every 8 h (q8h). Venous blood samples (5 ml) for the measurement of plasma piperacillin concentrations were collected in heparinized tubes from an indwelling catheter in the central or peripheral vein at 0 h before the initiation of infusion and at 1, 2, 3, 4, and 6 h after the end of the infusion. PK sampling was performed after 5 or more doses so that piperacillin plasma concentrations might reach a steady state. The actual times of administration and blood sampling were recorded. Samples were kept in an ice-water bath until centrifugation at 2,092 × g for 10 min at 4°C. The centrifugation was done within 0.5 h after sampling. Separated plasma samples were transferred into microcentrifuge vials to be stored at −70°C until assayed.
Analytical procedures for plasma piperacillin quantification.
Piperacillin concentrations in plasma were determined by high-performance liquid chromatography (Agilent 1200 series; Agilent Technologies, Santa Clara, CA) coupled with a tandem mass spectrometry (API3200, AB Sciex; Applied Biosystems, Foster City, CA) method (14, 15). Briefly, the assay method was as follows. A volume of 0.05 ml of plasma was mixed with 0.55 ml of internal standard (sulbactam; 8 mg/liter in acetonitrile). After thorough vortexing for 1 min, the samples were centrifuged at 17,311 × g for 10 min at 4°C. A volume of 0.05 ml of the supernatant was mixed with 0.95 ml of 0.1% formic acid, and 5 μl was injected into a liquid chromatography/tandem mass spectrometry (LC-MS/MS) system. The analytes were separated through a Luna C18 column (100 by 2.0 mm; 5 μm) at a flow rate of 0.3 ml/min by a mobile phase consisting of 0.1% formic acid and acetonitrile with 0.1% formic acid (30:70, vol/vol) and detected using the electrospray negative ion mode of tandem mass spectrometry. Mass/charge ratios (m/z) for piperacillin in the multiple-reaction monitoring (MRM) mode were 516.24 to 330.0.
The lower limit of quantification (LLOQ) was 0.5 mg/liter. The coefficients of correlation (r) were >0.9996 in the range of 0.5 to 200 μg/ml for piperacillin by weighted linear regression (1/concentration). Intra- and interday precision values (coefficient of variation [CV%]) and mean accuracies were <5.96% and 100.5 to 104.5%, respectively.
Population PK model development.
The population PK analysis was performed using NONMEM (version 7.2; Icon Development Solutions, Ellicott City, MD) with the GFortran compiler. Based on the first-order elimination, one- and two-compartment open models were tested to estimate the clearance (CL), central volume of distribution (V1), peripheral volume of distribution (V2), and intercompartmental clearance (Q) using the ADVAN subroutines (ADVAN 1, TRANS 2 and ADVAN 3, TRANS 4). The first-order conditional estimation method with interaction was used throughout the model building process.
The interindividual variability (η) of each parameter was applied exponentially. The PK parameters of the jth subject (Pj) were described as Pj = TVP × exp(ηj), where TVP represents the typical population value of PK parameters, such as clearance (CL), volume of distribution (V), and intercompartmental clearance (Q). The interindividual variability, eta (η), for each PK parameter was assumed to follow a Gaussian distribution with a mean of 0 and variance of ω2. Possible correlations between the interindividual variability were also evaluated.
As for the residual error, the additive, proportional, and combined forms were tested. Models were selected based on several criteria, which were based on a decrease in the objective function value (OFV) of >3.84 (P = 0.05, df = 1) and improvement in the diagnostic scatterplots.
Covariate selection.
During the covariate model-building process, stepwise forward selection and backward elimination were applied. The potential covariates were age, sex, body weight, TBSA, day after burn injury, serum albumin, serum creatinine, creatinine clearance (CLCR), abbreviated burn severity index (ABSI), acute physiology and chronic health evaluation II (APACHE II) score, the presence of edema, sepsis, or dehydration, and continuous renal replacement therapy (CRRT). The CLCR was calculated from the Cockcroft-Gault equation (16). Various forms of covariate models were tested, including linear, piecewise, power, and exponential equations for any of the continuous or categorical covariates. The covariate screening process was performed using visual (parameter versus variable scatterplots) and numerical (generalized additive modeling implemented by Xpose (version 4.2.3) approaches. In the forward selection of covariates, variables that decreased the OFV by >3.84 (P < 0.05) and decreased the interindividual variabilities were selected. Covariates that did not increase the OFV by >6.63 (P < 0.01) in backward elimination were removed from the model.
Bootstrapping and visual predictive checks.
The 95% confidence intervals (CIs) for mean population PK parameters were determined by a bootstrap resampling method using Wings for NONMEM, version 720 (http://wfn.sourceforge.net). A total of 1,000 resampled data sets were collected, and the parameters were estimated using the final population PK model. The 95% CIs were described by 2.5th and 97.5th percentiles of the 1,000 bootstrap-estimated PK parameters in a nonparametric manner. The model was also evaluated by visual predictive checks (VPCs) by overlaying observed data points with 5th, 50th, and 95th percentile curves of 10,000 virtual patients simulated from the final model.
Simulation of piperacillin concentration.
The steady-state piperacillin concentrations on the basis of the PK model developed for the currently used dosage regimen (4 g piperacillin and 0.5 g tazobactam as a 30-min infusion every 8 h) were simulated for 1,000 virtual burn patients, considering the covariates. The unbound fraction of piperacillin was assumed to be 0.7. Since protein binding of piperacillin in burn patients has not been reported, its unbound fraction was referenced from the product information (2).
The MIC distribution of Escherichia coli and Klebsiella pneumoniae from EUCAST (8) was used to simulate the fT>MIC. The MIC50/MIC90 ratios against E. coli and K. pneumoniae were 2/8 mg/liter and 2/64 mg/liter, respectively, and the MIC ranges of both species were identical (0.002 to 512 mg/liter). A total of 1,000 virtual patients were simulated to predict the duration of the fT>MIC. The distribution parameters, such as mean, standard deviation (SD), and upper and lower limits of CLCR, were set to be identical to those observed in our patients. Based on the proportions of MICs of E. coli and K. pneumoniae reported from the EUCAST, a MIC was randomly selected and matched with each virtual patient because the MIC histograms did not show smooth curve patterns, which allow a parametric simulation of the distribution. As piperacillin concentrations for each virtual patient were simulated from the final PK model, the patient's own fT>MIC could be calculated using the aforementioned MIC. This procedure was repeated in 1,000 virtual patients, and the distributions of fT>MIC values are shown as histograms. As the result from this step, the probability of target attainment (PTA) was calculated. The PTA was assessed for the presence of sepsis and for the different levels of CLCR.
RESULTS
Final PK model.
The selection of the basic model and covariates was based on the OFV and basic goodness-of-fit plots as well as individual plots. A two-compartment distribution model was chosen over a one-compartment model (the OFV decrease by 70 and better predictive performance). The Michaelis-Menten elimination alone or the parallel first-order and Michaelis-Menten eliminations showed no improvement in the OFV or predictive performance compared with either for the first-order elimination. A two-compartment model with first-order elimination was chosen as a final PK model. The covariates included in the final model were creatinine clearance (CLCR) and day after burn injury (DAI) on piperacillin clearance and sepsis on the central volume of piperacillin. CLCR on CL gave the largest drop in OFV and CV% (ΔOFV = 30.529, ΔCV% = 12.9). DAI on CL (ΔOFV = 9.914, ΔCV% = 3.9), and sepsis on V1 (ΔOFV = 9.844, ΔCV% = 6.4) also significantly decreased the OFV and CV%.
The final structural models were CL = θ1 × (CLCR/132) + DAI × θ5, V1 = θ2 + θ6 × sepsis (1 for the presence of sepsis, 0 for the absence of sepsis), V2 = θ3, and Q = θ4. The interindividual variabilities (CV%) in these parameters were 35.4%, 42.4%, 0% (not estimated), and 90.3%, respectively (Table 2). The correlation between the interindividual variability for CL and V1 (ρCL−V1 was 0.434) improved the predictive performance in the VPCs.
TABLE 2.
Population PK parameters of piperacillin in burn patients
| Parameter | Estimated value | % RSEa | Bootstrap median (95% CIb) |
|---|---|---|---|
| Structural model | |||
| TVCLc = θ1 × (CLCR/132) + (DAI × θ5) | |||
| θ1 | 16.6 liters/h | 5.96 | 16.2 (13.4–18.4) |
| θ5 | −0.0874 liters/h | 21.9 | −0.0862 (−0.122 to −0.0336) |
| TVV1d = θ2 + sepsis × θ6 | |||
| θ2 | 25.3 liters | 7.79 | 24.4 (21.0–29.6) |
| θ6 | 14.8 liters | 28.5 | 13.9 (6.07–24.2) |
| V2 | 16.1 liters | 52.9 | 15.4 (3.72–931) |
| Q | 0.636 liters/h | 22.5 | 0.730 (0.420–2.62) |
| Interindividual variability (CV%) | |||
| ωCL | 35.4% | 26.3 | 34.5 (23.7–45.0) |
| ωV1 | 42.4% | 31.3 | 35.5 (22.9–51.3) |
| ρCL-V1 | 0.434 | 0.589 (0.121–0.832) | |
| ωQ | 90.3% | 38.1 | 79.1 (0.316–122) |
| Residual error | |||
| σadditive | 0.359 mg/liter | 41.4 | 0.348 (0.000–0.590) |
| σproportional | 18.5% | 20.3 | 17.1 (10.3–25.0) |
RSE, relative standard error.
95% CI estimated by applying the final population PK model to 1,000 resampled data sets.
TVCL, typical value of clearance.
TVV1, typical value of central volume of distribution.
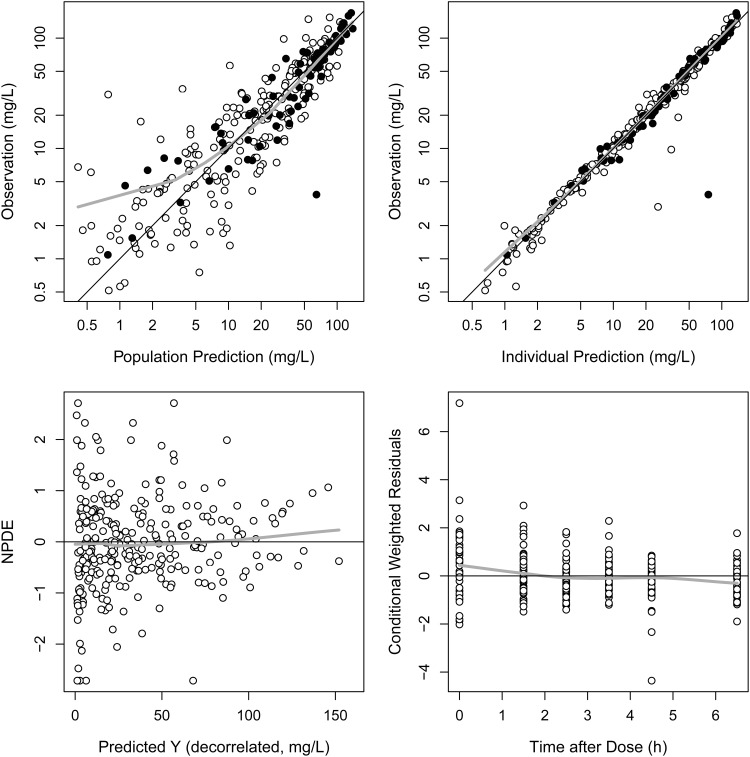
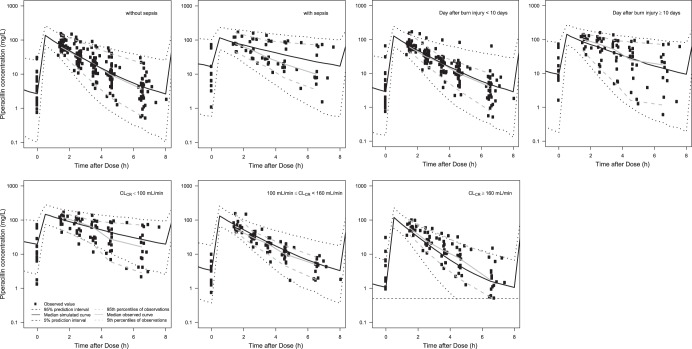
Basic goodness-of-fit plots for the final PK model are presented in Fig. 1 and demonstrate that individual predicted piperacillin concentrations corresponded well to the observations without systemic bias. The median parameter estimates and 95% confidence intervals from 1,000 bootstrap replications are summarized in Table 2. VPCs of the final population PK model, in which the simulated concentrations from PK parameters were stratified by sepsis, day after burn injury, and CLCR (with or without sepsis; DAI of <10 days or DAI of ≥10 days; CLCR of <100 ml/min or 100 ml/min ≤ CLCR < 160 ml/min or ≥ 160 ml/min), are shown (Fig. 2). The lower margins of the predicted intervals in the VPC results were slightly inflated over those of observed concentrations.
FIG 1.
Basic goodness-of-fit plots for the PK model. ●, observations with sepsis; ○, observations without sepsis; black line, line of identity; gray line, loess (locally weighted regression) smoothed line.
FIG 2.
Visual predictive checks of the final PK model classified by sepsis, day after burn injury, and CLCR. Symbols, observed data; solid line, median simulated curve; broken lines, 90% prediction intervals; horizontal dashed line (lower right-most panel), LLOQ (0.5 mg/liter).
In the patients with a CLCR value of ≥160 ml/min in Fig. 2, the gaps between the observed concentrations and the simulated prediction intervals (the 5th percentile curve as the lower margin of the 90% prediction interval) seem to be inflated at low concentrations. This underestimation of the lower margin of the 90% prediction interval was caused by the simulated concentrations below the LLOQ (0.5 mg/liter).
The initial half-lives (the alpha phase half-lives of the two-compartment model), which might influence the PTA, were calculated from PK parameters and classified by covariates (Table 3).
TABLE 3.
Half-lives calculated from PK parameters
| Patient status | Half-life (mean ± SD) (h)a |
|---|---|
| Without sepsis | 1.22 ± 0.70 |
| With sepsis | 2.92 ± 1.99 |
| Days after burn injury | |
| <10 | 1.38 ± 0.99 |
| ≥10 | 2.16 ± 1.81 |
| CLCR < 100 ml/min | 2.78 ± 1.79 |
| 100 ≤ CLCR < 160 ml/min | 1.27 ± 0.59 |
| CLCR ≥ 160 ml/min | 0.89 ± 0.42 |
The initial half-lives (half-lives of alpha phases) are given instead of the terminal half-lives because the former is closer to the effective half-life, the time needed to eliminate 50% of the drug from the body.
Simulated fT>MIC.
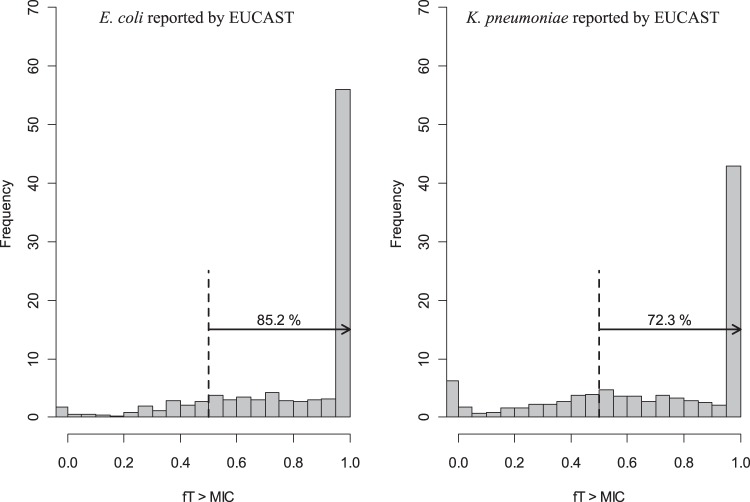
In order to predict the fT>MIC, the simulated steady-state piperacillin concentrations from 1,000 virtual patients with normal renal functions (CLCR, >40 ml/min) were compared with randomly generated MICs according to the distribution described in Materials and Methods (Fig. 3). When 50% fT>MIC was assumed to be the target for clinical effectiveness, 85.2% and 72.3% of the simulated patients were found to be above the targets for E. coli and K. pneumoniae strains, respectively, reported from the EUCAST.
FIG 3.
Frequency distribution of fT>MIC for E. coli and K. pneumoniae from EUCAST data. Dashed black lines indicate 50% of fT>MIC. The percentage values given above the arrows indicate the proportions of patients with fT>MIC of >50%.
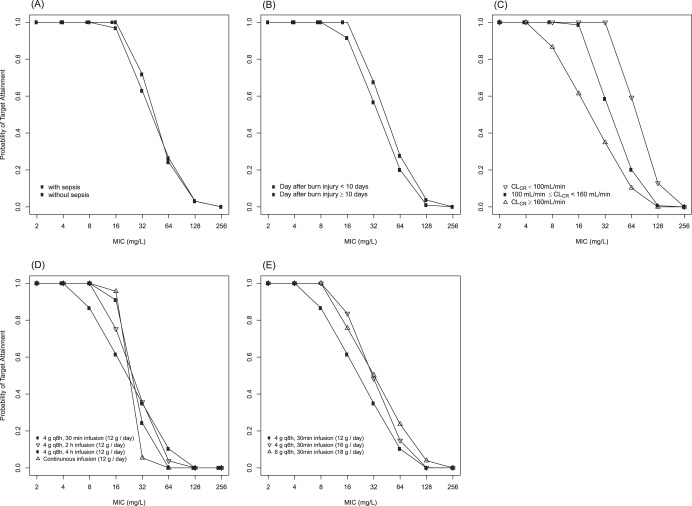
The PTA by the MIC, where the PK/pharmacodynamic (PD) target was also defined as 50% fT>MIC, is shown for sepsis (presence or absence), for day after burn injury, and for three different levels of CLCR in Fig. 4. In contrast to the PTA values showing probabilities of achieving the target for a given MIC, the proportion of patients above the target (fT>MIC) in Fig. 3 is dependent upon the distribution of MICs of the strains isolated from the community, hospital, or any other unit where the patient group is found (EUCAST in this report). Accordingly, the likelihood of successful treatment in patients who are exposed to an environment where highly resistant strains are rather common must be lower than that for those who are not.
FIG 4.
Simulated PTAs by sepsis (A), day after burn injury (B), and different levels of renal function (C) after a 30-min infusion of 4.5 g piperacillin-tazobactam q8h (4 g q8h as piperacillin) at steady state. Panel C contains all patient groups regardless of sepsis status or days after burn injury. Simulated PTAs by different infusion times (D) or daily doses (E) (as piperacillin) for CLCR values of ≥ 160 ml/min. The PK/pharmacodynamic (PD) target value for these PTAs is fT>MIC of at least 50%.
DISCUSSION
There are several population PK reports for piperacillin; however, those in burn patients are rare (17). The aim of this study was to develop a population PK model for piperacillin that considers influential factors in burn patients. The CL values for piperacillin (16.6 liters/h) in our burn patients were higher than those of cystic fibrosis patients and healthy volunteers (11.3 liters/h each) (11). The volumes of distribution at steady state (Vss = V1 + V2) of piperacillin were 41.4 liters (without sepsis) and 56.2 liters (with sepsis) in this study. These were substantially larger than those reported in patients with cystic fibrosis (9.61 liters) (11) or intraabdominal infection (22.3 liters) (18), in critically ill patients with sepsis (25 liters) (13), and in healthy volunteers (10.4 liters) (11).
The average period from burn injury to the initiation of piperacillin therapy in this study was 12.8 days and ranged from 48 h to 10 days in about 70% of the patients, which indicates that they were in the hypermetabolic phase (beyond 48 h after the burn injury). Physiologic changes, such as an increased glomerular filtration rate (GFR) in the hypermetabolic phase, may have increased CL of piperacillin in our patients (7). The noncompartmental CL of piperacillin in burn patients (8.4 liters/h on day 1 and 7.4 liters/h at steady state) reported by Bourget et al. (17) was about half the CL (16.6 liters/h) reported in this study. One possible cause for this discrepancy is the days after burn injury, which were mostly >10 days in the patients of Bourget et al.
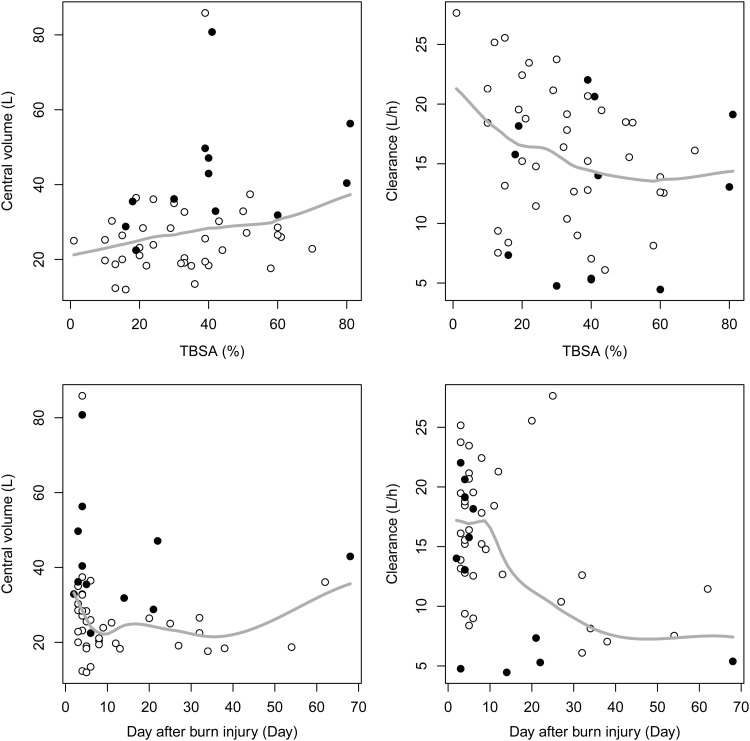
It is also known that hypoalbuminemia caused by leakage to the extravascular space and decreased hepatic production are common in the hypermetabolic phase (19). Hypoalbuminemia (mean serum albumin level of 2.58 g/dl in our patients) and hydration to compensate for the loss of intravascular fluid accompanying hypoalbuminemia may have contributed to the increase in V. PK studies on other antibiotics in burn patients (20–22) also showed increased CL and V, which resulted from pathophysiologic changes and massive hydration in the treatment process in burn patients. V1 was significantly increased in the patients with sepsis, which might be due to capillary leakage and interstitial edema caused by sepsis (23). However, edema was not identified as a covariate influencing V in burn patients, unlike in other reports (20, 21). Body weight was not significant as a covariate for V in this study, and this was consistent with results of a previous report (13). The narrow range of body weight (most of the patients weighed between 60 and 75 kg in this study) might be a possible explanation for this. The scatterplots of piperacillin V1 and CL versus day after burn injury and TBSA are shown in Fig. 5. The V1 and CL at the early stage of the burn injury tended to be greater, and DAI was identified as a covariate for CL. We tried possible covariate models (linear, exponential, and Hill function), and a linear model best described the effect of DAI on CL. Also, the relationship between CL and CLCR was best described in a linear fashion. A power model (e.g., CL = θ × CLCRθ) did not demonstrate statistically significant improvement, and an exponential model (e.g., CL = θ × exp [θ × CLCR]) was not successfully converged by NONMEM.
FIG 5.
Scatterplots of piperacillin V1 and CL versus day after burn injury and TBSA. ●, Observations with sepsis; ○, observations without sepsis; solid line, loess (locally weighted regression) smoothed line.
We selected a two-compartment model as our disposition model over a one-compartment model based on the OFV (1385.743 to 1316.084). Unlike other piperacillin PK studies which also used a two-compartment model (11, 13), the early distribution phase observed within 1 h after the end of infusion was not reflected in our PK model because the blood samples were not collected that early in the distribution phase. Thus, the sum of V1 estimated in our study seems to be relevant to the V1 + V2 in previous reports (11, 13), and the V2 in our study can be regarded as the third slowly distributed compartment that has not been clearly identified so far. In the VPC plots in this report, the median concentration curve of piperacillin seemed to follow a monoexponential decline. This is possibly because the early distribution phase was not included in the PK model and the V2 (16.1 liters), referring to the late distribution phase, was relatively smaller than the V1 (40.1 liters).
The elimination in our final model exhibits linear pharmacokinetics, although there are a few reports on nonlinear elimination of piperacillin (24–26). Thus, elimination models with first-order elimination, Michaelis-Menten elimination, and a mixture of both were tested. When we attempted Michaelis-Menten elimination, the OFV was not improved, nor were the Vmax and Km in acceptable ranges (the two parameters were at least 1,000 times the highest measured concentration). Thus, we concluded that our piperacillin PK data did not support the saturable elimination model. This is probably because of the limitation in our study design of sparse PK sampling without urine collection. The sparse sampling strategy is inevitable in PK studies in critically ill patients, and thus the chances to detect nonlinearity in the elimination process were low due to the lack of concentration data for the maximum concentration of the drug in serum (Cmax).
In this study, the population PK of piperacillin was characterized in burn patients after infusion of piperacillin-tazobactam. The MICs may vary with regions or hospitals because of the differences in resistance rates and in the patients' pathophysiologic conditions. As a result of this study, in burn patients, the current piperacillin dosage regimen might not be changed by sepsis status. However, in the case of a burn patient with a CLCR value (from the Cockcroft-Gault equation) of ≥160 ml/min, an increase in the daily dose of piperacillin should be considered. We performed more Monte Carlo simulations for three different dosing strategies in patients with a CLCR value of ≥160 ml/min to predict the PTA changes: (i) extended infusion time (12 g/day), (ii) shorter dosing interval (4 g q6h), and (iii) increased doses (6 g q8h). Extended infusion, especially a 2-h infusion, seems to give a better PTA profile than a 30-min infusion with the same daily dose, but the shorter dosing interval or increased doses were even better (Fig. 4).
Since we did not measure tazobactam concentrations in this study, the PTA calculation or dose recommendation for piperacillin-tazobactam was performed under the assumption that the β-lactamase remains inhibited throughout the dosing interval without regard to the exponential decay of the tazobactam concentration. Several reports also recommended the dosage regimen of piperacillin-tazobactam based on the piperacillin concentration only (13, 27–29), as in our report. Such recommendations are based upon the report by Strayer et al. (30): despite the tazobactam concentrations at 2 or 3 h after infusion, which were lower than the fixed concentration used in in vitro susceptibility tests (4 mg/liter), the bactericidal effect remained unchanged within the dosing interval because of the post-β-lactamase inhibitor effect. In other words, the exposure to tazobactam can lead to a prolonged susceptibility to piperacillin-induced bactericidal effects, even when concentrations of the beta-lactamase inhibitor are no longer detectable (30, 31). It has also been reported that the fixed ratio (8:1) of piperacillin-tazobactam and the fixed tazobactam concentration (4 mg/liter) showed almost equivalent bactericidal effects in an in vitro susceptibility study (32).
Although there are still questions regarding whether the overly high estimates of the CLCR observed in burn patients in their hypermetabolic phase are reliable, our population PK modeling results (Fig. 2; Table 3) demonstrate that patients with a CLCR value of ≥160 ml/min show a shorter half-life (0.89 h) for increased piperacillin CL than those with a CLCR value in a normal range, 100 to 160 ml/min (1.27 h). Therefore, it is also important that the overly high CLCR values in burn patients should not be truncated at some upper limit (e.g., 120 ml/min) when considering dose adjustment for drugs excreted via the kidney.
ACKNOWLEDGMENT
This study was supported by the Korea Healthcare Technology R&D Project, Ministry for Health and Welfare, Republic of Korea (grant A084589).
Footnotes
Published ahead of print 21 April 2014
REFERENCES
- 1.Perry C, Markham A. 1999. Piperacillin/tazobactam: an updated review of its use in the treatment of bacterial infections. Drugs 57:805. 10.2165/00003495-199957050-00017 [DOI] [PubMed] [Google Scholar]
- 2.Lederle Laboratories. 1999. Piperacillin sodium and tazobactam sodium (Zosyn) product information. Lederle Laboratories, Pearl River, NY [Google Scholar]
- 3.Rezaei E, Safari H, Naderinasab M, Aliakbarian H. 2011. Common pathogens in burn wound and changes in their drug sensitivity. Burns 37:805–807. 10.1016/j.burns.2011.01.019 [DOI] [PubMed] [Google Scholar]
- 4.Church D, Elsayed S, Reid O, Winston B, Lindsay R. 2006. Burn wound infections. Clin. Microbiol. Rev. 19:403–434. 10.1128/CMR.19.2.403-434.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Craig WA. 1998. Pharmacokinetic/pharmacodynamic parameters: rationale for antibacterial dosing of mice and men. Clin. Infect. Dis. 26:1–10. 10.1086/516284 [DOI] [PubMed] [Google Scholar]
- 6.Drusano GL. 2004. Antimicrobial pharmacodynamics: critical interactions of “bug and drug.” Nat. Rev. Microbiol. 2:289–300. 10.1038/nrmicro862 [DOI] [PubMed] [Google Scholar]
- 7.Weinbren M. 1999. Pharmacokinetics of antibiotics in burn patients. J. Antimicrob. Chemother. 44:319–327. 10.1093/jac/44.3.319 [DOI] [PubMed] [Google Scholar]
- 8.European Committee on Antimicrobial Susceptibility Testing. 2010. Piperacillin-tazobactam: rationale for the clinical breakpoints, version 1.0. EUCAST Laboratory for Antimicrobial Susceptibility Testing, Växjö, Sweden: http://www.eucast.org/fileadmin/src/media/PDFs/EUCAST_files/Rationale_documents/Piperacillin-tazobactam_rationale_Nov2010_v_1.0.pdf [Google Scholar]
- 9.Rhomberg PR, Jones RN. 2009. Summary trends for the Meropenem Yearly Susceptibility Test Information Collection Program: a 10-year experience in the United States (1999-2008). Diagn. Microbiol. Infect. Dis. 65:414–426. 10.1016/j.diagmicrobio.2009.08.020 [DOI] [PubMed] [Google Scholar]
- 10.Lee K, Kim MN, Kim JS, Hong HL, Kang JO, Shin JH, Park YJ, Yong D, Jeong SH, Chong Y. 2011. Further increases in carbapenem-, amikacin-, and fluoroquinolone-resistant isolates of Acinetobacter spp. and P. aeruginosa in Korea: KONSAR study 2009. Yonsei Med. J. 52:793–802. 10.3349/ymj.2011.52.5.793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bulitta J, Duffull S, Kinzig-Schippers M, Holzgrabe U, Stephan U, Drusano G, Sörgel F. 2007. Systematic comparison of the population pharmacokinetics and pharmacodynamics of piperacillin in cystic fibrosis patients and healthy volunteers. Antimicrob. Agents Chemother. 51:2497–2507. 10.1128/AAC.01477-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dowell JA, Korth-Bradley J, Milisci M, Tantillo K, Amorusi P, Tse S. 2001. Evaluating possible pharmacokinetic interactions between tobramycin, piperacillin, and a combination of piperacillin and tazobactam in patients with various degrees of renal impairment. J. Clin. Pharmacol. 41:979–986. 10.1177/00912700122010960 [DOI] [PubMed] [Google Scholar]
- 13.Roberts JA, Kirkpatrick CM, Roberts MS, Dalley AJ, Lipman J. 2010. First-dose and steady-state population pharmacokinetics and pharmacodynamics of piperacillin by continuous or intermittent dosing in critically ill patients with sepsis. Int. J. Antimicrob. Agents 35:156–163. 10.1016/j.ijantimicag.2009.10.008 [DOI] [PubMed] [Google Scholar]
- 14.Arzuaga A, Isla A, Gascón A, Maynar J, Martin A, Solinís M, Toral D, Pedraz J. 2005. Quantitation and stability of piperacillin and tazobactam in plasma and ultrafiltrate from patients undergoing continuous venovenous hemofiltration by HPLC. Biomed. Chromatogr. 19:570–578. 10.1002/bmc.482 [DOI] [PubMed] [Google Scholar]
- 15.Al-Nawas B, Kinzig-Schippers M, Soergel F, Shah PM. 2008. Concentrations of piperacillin-tazobactam in human jaw and hip bone. J. Craniomaxillofac. Surg. 36:468–472. 10.1016/j.jcms.2008.06.003 [DOI] [PubMed] [Google Scholar]
- 16.Cockcroft D, Gault M. 1976. Prediction of creatinine clearance from serum creatinine. Nephron 16:31–41. 10.1159/000180580 [DOI] [PubMed] [Google Scholar]
- 17.Bourget P, Lesne-Hulin A, Le Reveille R, Le Bever H, Carsin H. 1996. Clinical pharmacokinetics of piperacillin-tazobactam combination in patients with major burns and signs of infection. Antimicrob. Agents Chemother. 40:139–145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li C, Kuti JL, Nightingale CH, Mansfield DL, Dana A, Nicolau DP. 2005. Population pharmacokinetics and pharmacodynamics of piperacillin/tazobactam in patients with complicated intra-abdominal infection. J. Antimicrob. Chemother. 56:388–395. 10.1093/jac/dki243 [DOI] [PubMed] [Google Scholar]
- 19.Blanchet B, Jullien V, Vinsonneau C, Tod M. 2008. Influence of burns on pharmacokinetics and pharmacodynamics of drugs used in the care of burn patients. Clin. Pharmacokinet. 47:635–654. 10.2165/00003088-200847100-00002 [DOI] [PubMed] [Google Scholar]
- 20.Doh K, Woo H, Hur J, Yim H, Kim J, Chae H, Han S, Yim D-S. 2010. Population pharmacokinetics of meropenem in burn patients. J. Antimicrob. Chemother. 65:2428–2435. 10.1093/jac/dkq317 [DOI] [PubMed] [Google Scholar]
- 21.Han S, Kim J, Yim H, Hur J, Song W, Lee J, Jeon S, Hong T, Woo H, Yim D-S. 2013. Population pharmacokinetic analysis of fluconazole to predict therapeutic outcome in burn patients with Candida infection. Antimicrob. Agents Chemother. 57:1006–1011. 10.1128/AAC.01372-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lee J, Han S, Jeon S, Hong T, Song W, Woo H, Yim D-S. 2013. Population pharmacokinetic analysis of colistin in burn patients. Antimicrob. Agents Chemother. 57:2141–2146. 10.1128/AAC.00271-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.De Paepe P, Belpaire FM, Buylaert WA. 2002. Pharmacokinetic and pharmacodynamic considerations when treating patients with sepsis and septic shock. Clin. Pharmacokinet. 41:1135–1151. 10.2165/00003088-200241140-00002 [DOI] [PubMed] [Google Scholar]
- 24.Vinks AA, den Hollander JG, Overbeek SE, Jelliffe RW, Mouton JW. 2003. Population pharmacokinetic analysis of nonlinear behavior of piperacillin during intermittent or continuous infusion in patients with cystic fibrosis. Antimicrob. Agents Chemother. 47:541–547. 10.1128/AAC.47.2.541-547.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bulitta JB, Kinzig M, Jakob V, Holzgrabe U, Sörgel F, Holford NH. 2010. Nonlinear pharmacokinetics of piperacillin in healthy volunteers—implications for optimal dosage regimens. Br. J. Clin. Pharmacol. 70:682−693. 10.1111/j.1365-2125.2010.03750.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Landersdorfer CB, Bulitta JB, Kirkpatrick CM, Kinzig M, Holzgrabe U, Drusano GL, Stephan U, Sörgel F. 2012. Population pharmacokinetics of piperacillin at two dose levels: influence of nonlinear pharmacokinetics on the pharmacodynamic profile. Antimicrob. Agents Chemother. 56:5715–5723. 10.1128/AAC.00937-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gonzalzles TM, Suseno M, Gaydos JM, Schreckenberger PC, Sincak C, Mehta MS, Peterson LR. 2012. Assessment of susceptibility, pharmacodynamics, and therapeutic response for interpretation of piperacillin-tazobactam in vitro activity in the treatment of Pseudomonas aeruginosa infection. Open J. Med. Microbiol. 2:101. 10.4236/ojmm.2012.23015 [DOI] [Google Scholar]
- 28.Lodise TP, Lomaestro B, Rodvold KA, Danziger LH, Drusano GL. 2004. Pharmacodynamic profiling of piperacillin in the presence of tazobactam in patients through the use of population pharmacokinetic models and Monte Carlo simulation. Antimicrob. Agents Chemother. 48:4718–4724. 10.1128/AAC.48.12.4718-4724.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Johnson CA, Halstenson CE, Kelloway JS, Shapiro BE, Zimmerman SW, Tonelli A, Faulkner R, Dutta A, Haynes J, Greene DS. 1992. Single-dose pharmacokinetics of piperacillin and tazobactam in patients with renal disease. Clin. Pharmacol. Ther. 51:32–41. 10.1038/clpt.1992.5 [DOI] [PubMed] [Google Scholar]
- 30.Strayer AH, Gilbert DH, Pivarnik P, Medeiros AA, Zinner SH, Dudley MN. 1994. Pharmacodynamics of piperacillin alone and in combination with tazobactam against piperacillin-resistant and -susceptible organisms in an in vitro model of infection. Antimicrob. Agents Chemother. 38:2351–2356. 10.1128/AAC.38.10.2351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Okereke C, Dudley MN. 1998. Beta-lactam/beta-lactamase inhibitor combinations: pharmacodynamic considerations and possible role in the management of bacterial infections in the neutropenic host. J. Antimicrob. Chemother. 41(suppl D):43–49. 10.1093/jac/41.suppl_4.43 [DOI] [PubMed] [Google Scholar]
- 32.Pfaller M, Barry A, Fuchs P, Gerlach E, Hardy D, McLaughlin J. 1992. Comparison of fixed concentration and fixed ratio options for testing susceptibility of gram-negative bacilli to piperacillin and piperacillin/tazobactam. Eur. J. Clin. Microbiol. Infect. Dis. 11:728–732. 10.1007/BF01989979 [DOI] [PubMed] [Google Scholar]