Abstract
Mitochondrial toxicity has been recently suggested to be the underlying mechanism of long-term linezolid-associated toxicity in patients with 16S rRNA genetic polymorphisms. Here, we report for the first time two cases of lactic acidosis due to long-term linezolid exposure in liver transplant recipients who presented an A2706G mitochondrial DNA polymorphism.
TEXT
Linezolid was the first oxazolidinone to be approved for clinical use by the Food and Drug Administration in 2000. This antimicrobial inhibits bacterial protein synthesis by binding to the 23S rRNA of the 50S subunit. Linezolid is used to treat a variety of Gram-positive infections, including those due to methicillin-resistant Staphylococcus aureus and vancomycin-resistant enterococci. In addition, it has been used as a valid alternative in the management of Nocardia infections, multidrug-resistant tuberculosis, and other mycobacterial infections. Linezolid is generally well tolerated, and most reported adverse effects are mild, transient, and reversible upon the cessation of therapy. Among the most common side reactions are gastrointestinal disturbances, followed by headache and rash. However, long-term use of this antibiotic can lead to severe toxicity, particularly myelosuppression, peripheral or optic neuropathy, serotonin syndrome (1), and lactic acidosis (1–12).
There is evidence that mitochondrial toxicity is the underlying mechanism of lactic acidosis and peripheral and optic neuropathy (7, 10). Soriano et al. (10) reported that mitochondrial respiratory chain complex IV (synthesized by mitochondrial ribosomes) activity was lower in linezolid-treated patients than in nontreated patients, suggesting a relationship between linezolid-related toxicity and mitochondrial protein synthesis inhibition due to potential structural similarities between bacterial and mitochondrial ribosomes. In addition, De Vriese et al. also reported a decreased mitochondrial enzymatic activity in a patient treated with linezolid (6). Moreover, it has been recently described that the presence of A2706G and G3010A mitochondrial 16S rRNA polymorphisms could predispose to the development of linezolid-related lactic acidosis (4, 11). Here, we have studied the presence of 16S rRNA polymorphisms in two liver transplant patients who developed lactic acidosis while receiving linezolid therapy.
Case 1.
A 72-year-old diabetic woman was diagnosed with disseminated nocardiosis (i.e., bone and central nervous system). She had received a liver transplant due to primary biliary cirrhosis 4 years before. Immunosuppression was reduced, and the patient started antimicrobial treatment. First, the patient received treatment with meropenem that was switched to cotrimoxazole when Nocardia asteroides was identified. One month later, the patient was discharged with oral cotrimoxazole (1 double-strength tablet 3 times a day [t.i.d.]). However, 3 months later, even though the isolate was susceptible to cotrimoxazole, there was no change in the size of the brain lesions and the leg fracture was not healing. Antimicrobial therapy was switched to ceftriaxone (2 g twice a day [b.i.d.]) and linezolid (600 mg b.i.d.). Clinical and radiological improvements were observed after 4 weeks of treatment. She was discharged with oral linezolid. Nine weeks later, she was readmitted to our hospital with hypoglycemic coma and lactic acidosis. At the admission date, arterial blood gas data were as follows: pH 7.25; lactate, 3.20 mmol/liter; and HCO3, 15.60 mmol/liter. Other causes of hyperlactatemia were excluded. No evidence of graft dysfunction was observed. Even though the bicarbonate rate improved with fluid solution, the lactate increased to 4.80 mmol/liter, and she continued with diarrhea and vomiting. Linezolid was stopped and doxycycline was started. Thiamine infusion was administered. The patient was discharged without gastrointestinal symptoms and normalization of lactic acid levels. She successfully completed 1 year of treatment with doxycycline (100 mg/day). She has no signs of nocardiosis up to date (i.e., 7 years after nocardiosis diagnosis).
Case 2.
A 43-year-old man was diagnosed with lung tuberculosis. Three years before he had received a liver transplant due to hepatitis C virus-associated cirrhosis. He started treatment with ethambutol (Myambutol; 400 mg t.i.d.), pyrazinamide (20 mg/kg of body weight), isoniazid (300 mg), and levofloxacin (500 mg) per day. He developed severe myalgias, so levofloxacin and pyrazinamide were stopped and linezolid (600 mg b.i.d.) was orally started. The patient was admitted in our hospital 8 weeks after beginning with linezolid because of nausea, asthenia, and diarrhea. The arterial blood gas data were as follows: pH 7.29; lactate, 7.20 mmol/liter; HCO3, 15.60 mmol/liter. He suffered an acute renal failure with a creatinine clearance of 30 ml/min (creatinine of 2.5 mg/dl) and pancytopenia. Linezolid was stopped and fluid resuscitation with bicarbonate was started. Other causes of hyperlactatemia were excluded, including sepsis, liver failure, and exposure to medications generally known to cause it. No evidence of graft dysfunction was observed. Thiamine infusion was administered. The patient improved and lactic acid decreased to normal values. The antituberculous drugs were changed and ethambutol was reinitiated, continuing with isoniazid with adequate tolerance to the treatment. He completed 1 year of treatment and has no signs of tuberculosis up to date (i.e., 1 year after finishing treatment).
Screening for polymorphisms in mitochondrial 16S rRNA was performed as previously reported (7) following both restriction enzyme digestion and sequencing of PCR fragments amplified from blood DNA. A total of 200 ng of genomic DNA isolated from peripheral blood lymphocytes (Lympholate-H protocol; Cedarlane) was used for the PCR. PCR conditions, using the GoTaq Flexi DNA polymerase (Promega), were as follows: (i) 95°C for 2 min; (ii) 30 cycles at 95°C for 30 s, 57°C for 30 s, and 72°C for 40 s; and (iii) 1 cycle at 72°C for 7 min. PCR was amplified in a 2720 Thermal Cycler apparatus (Applied Biosystems), and DNA sequencing of the PCR products was performed using the BigDye Terminator 3.1 kit and the ABI PRISM 3120 XL apparatus (Applied Biosystems). To identify the A2706G polymorphism, we employed the restriction enzyme NlaIII (New England Biolabs), which recognizes the 5′-CATG-3′ sequence and digests a 237-bp PCR product (amplified with the following primers: forward, 5′-ACCGTGCAAAGGTAGCATAA-3′; and reverse, 5′-GCCCCAACCGAAATTTTTA-3′) in 2 bands (121 and 116 bp) for the sequences containing the A2706G polymorphism but did not cut the wild-type fragment. On the other hand, to identify the G3010A polymorphism, we employed the restriction enzyme Bccl (New England BioLabs), which recognizes the 5′-CCATC-3′ sequence and digests a 1,074-bp PCR product (amplified with the following primers: forward, 5′-ACTCCTCACACCCAATTGGA-3′; and reverse, 5′-GGAGGTTGGCCATGGGTATG-3′) in 3 bands (754, 224, and 96 bp) for wild-type sequences and produces 2 bands (978 and 96 bp) for the sequences containing the G3010A polymorphism. The digestion products were run in a 2.5% agarose gel and visualized in a GELDOC EQ PC system (Bio-Rad).
The analysis of mitochondrial 16S rRNA revealed that both patients possessed the A2706G polymorphism. As expected for this polymorphism, the digestion of the PCR product (237 bp) with the restriction enzyme NlaIII produced two fragments of 121 and 116 bp (Fig. 1A), being confirmed by sequencing the undigested fragment (Fig. 1B). On the other hand, both patients were negative for the G3010A polymorphism (data not shown).
FIG 1.
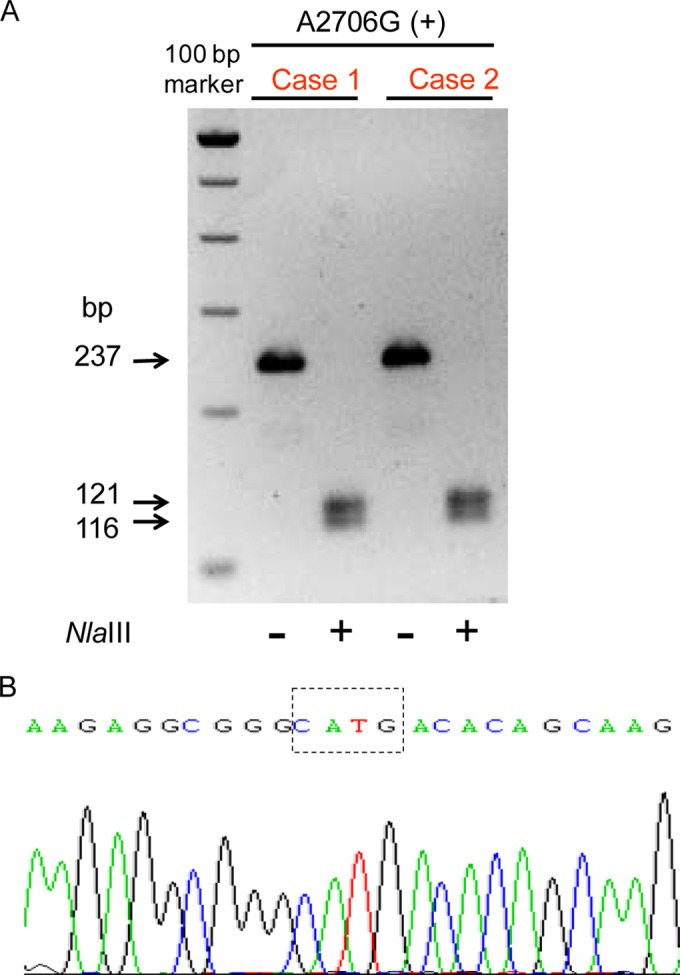
Analysis of mitochondrial 16S rRNA. PCR product (237 bp) digestion with the restriction enzyme NlaIII produced 2 fragments of 121 and 116 bp (A), being the sequence confirmed by sequencing the undigested fragment (B).
To the best of our knowledge, the two cases reported here are the unique case reports of lactic acidosis due to long-term linezolid use in liver transplant recipients. Sequencing of mitochondrial rRNA genes demonstrated that both patients possessed an A2706G mitochondrial DNA polymorphism.
Linezolid-related lactic acidosis was first reported by Apodaca and Rakita (2) in 2003 in a patient who received linezolid for the treatment of pulmonary nocardiosis. Several cases of linezolid-induced lactic acidosis have been described in the literature since then. Nevertheless, not all of the reported cases have occurred after a prolonged course. Thus, Pea et al. (8) hypothesized that early-onset hyperlactatemia would happen with drug overexposure, while late-onset hyperlactatemia would occur after long-lasting drug exposure. In fact, eight of the 22 reported cases (36%) in the literature occurred within the first 3 weeks of linezolid exposure (9). It has been proposed that early-onset hyperlactatemia would happen in relation to a P-glycoprotein inhibition, facilitating the lactic acidosis due to mitochondrial toxicity (9). The interindividual pharmacokinetic variability of linezolid has been reported to be mild, which is consistent with mainly nonrenal, nonenzymatic clearance pathways. However, the case of a liver transplant patient with linezolid plasma levels 4- to 6-fold higher than expected has been reported (8). It is very difficult even to hypothesize about which mechanism may have caused this drug accumulation. Perhaps the critical status of the patient affected drug clearance. Additionally, a drug-drug interaction might have occurred.
Trying to identify a gene susceptibility of certain individuals to linezolid toxic effects, Palenzuela et al. (7) developed a PCR assay to amplify and sequence mitochondrial 12S and 16S rRNA. They described two polymorphism in the mitochondrial 16S rRNA (i.e., A2706G and G3010A) of two patients that could explain a potential susceptibility to linezolid. In other studies (4, 11), the presence of the A2706G polymorphism was linked to linezolid-related lactic acidosis in two patients. Despite these evidences, it is difficult to conclude about the relationship between the presence of these polymorphisms and the susceptibility to linezolid-induced lactic acidosis due to the moderate prevalence of these polymorphisms in the general population (7). The clinical relevance of reported mitochondrial DNA polymorphisms associated with linezolid-induced lactic acidosis is debatable given the small number of patients whose mitochondrial DNA has been analyzed to detect single-nucleotide polymorphisms associated with lactic acidosis. We do not know if the variant was present in the transplanted livers, but we believe that it is not justified to determine liver sequences. As this variant may be common, it is possible that the variant was present in the transplanted livers.
There is no proven therapy to treat linezolid-induced lactic acidosis. Removal of the offending agent and care support are the most important measures. Because the mechanism of lactic acidosis associated with linezolid might be related to mitochondrial toxicity, use of respiratory chain cofactors (e.g., thiamine, riboflavin, l-carnitine, or coenzyme Q10) has been attempted in a few cases as a treatment strategy (4).
In summary, as we report here, the majority of episodes of linezolid-related hyperlactatemia occur in patients receiving prolonged therapy. It could be useful to measure serum lactate levels in liver transplant patients taking linezolid, especially if they are taking other drugs potentially able to induce lactic acidosis or to inhibit the P-glycoprotein or if they have renal insufficiency. In these patients, it could also be useful to perform a genetic study looking for the described polymorphisms.
ACKNOWLEDGMENTS
We have no disclosures to make, and this study did not receive funding.
Footnotes
Published ahead of print 5 May 2014
REFERENCES
- 1.Narita M, Tsuji BT, Yu VL. 2007. Linezolid-associated peripheral and optic neuropathy, lactic acidosis, and serotonin syndrome. Pharmacotherapy 27:1189–1197. 10.1592/phco.27.8.1189 [DOI] [PubMed] [Google Scholar]
- 2.Apodaca AA, Rakita RM. 2003. Linezolid-induced lactic acidosis. N. Engl. J. Med. 348:86–87. 10.1056/NEJM200301023480123 [DOI] [PubMed] [Google Scholar]
- 3.Boutoille D, Grossi O, Depatureaux A, Tattevin P. 2009. Fatal lactic acidosis after prolonged linezolid exposure for treatment of multidrug-resistant tuberculosis. Eur. J. Intern. Med. 20:e134–e135. 10.1016/j.ejim.2008.12.002 [DOI] [PubMed] [Google Scholar]
- 4.Carson J, Cerda J, Chae JH, Hirano M, Maggiore P. 2007. Severe lactic acidosis associated with linezolid use in a patient with the mitochondrial DNA A2706G polymorphism. Pharmacotherapy 27:771–774. 10.1592/phco.27.5.771 [DOI] [PubMed] [Google Scholar]
- 5.Cope TE, McFarland R, Schaefer A. 2011. Rapid-onset, linezolid-induced lactic acidosis in MELAS. Mitochondrion 11:992–993. 10.1016/j.mito.2011.08.010 [DOI] [PubMed] [Google Scholar]
- 6.De Vriese AS, Coster RV, Smet J, Seneca S, Lovering A, Van Haute LL, Vanopdenbosch LJ, Martin JJ, Groote CC, Vandecasteele S, Boelaert JR. 2006. Linezolid-induced inhibition of mitochondrial protein synthesis. Clin. Infect. Dis. 42:1111–1117. 10.1086/501356 [DOI] [PubMed] [Google Scholar]
- 7.Palenzuela L, Hahn NM, Nelson RP, Jr, Arno JN, Schobert C, Bethel R, Ostrowski LA, Sharma MR, Datta PP, Agrawal RK, Schwartz JE, Hirano M. 2005. Does linezolid cause lactic acidosis by inhibiting mitochondrial protein synthesis? Clin. Infect. Dis. 40:e113–e116. 10.1086/430441 [DOI] [PubMed] [Google Scholar]
- 8.Pea F, Scudeller L, Lugano M, Baccarani U, Pavan F, Tavio M, Furlanut M, Rocca GD, Bresadola F, Viale P. 2006. Hyperlactacidemia potentially due to linezolid overexposure in a liver transplant recipient. Clin. Infect. Dis. 42:434–435. 10.1086/499533 [DOI] [PubMed] [Google Scholar]
- 9.Scotton P, Fuser R, Torresan S, Carniato A, Giobbia M, Rossi C, Inojosa WO, Vaglia A. 2008. Early linezolid-associated lactic acidosis in a patient treated for tuberculous spondylodiscitis. Infection 36:387–388. 10.1007/s15010-008-7329-3 [DOI] [PubMed] [Google Scholar]
- 10.Soriano A, Miro O, Mensa J. 2005. Mitochondrial toxicity associated with linezolid. N. Engl. J. Med. 353:2305–2306. 10.1056/NEJM200511243532123 [DOI] [PubMed] [Google Scholar]
- 11.Velez JC, Janech MG. 2010. A case of lactic acidosis induced by linezolid. Nat. Rev. Nephrol. 6:236–242. 10.1038/nrneph.2010.20 [DOI] [PubMed] [Google Scholar]
- 12.Wiener M, Guo Y, Patel G, Fries BC. 2007. Lactic acidosis after treatment with linezolid. Infection 35:278–281. 10.1007/s15010-007-6302-x [DOI] [PubMed] [Google Scholar]


