Abstract
Onychomycosis is a common fungal nail disease that is difficult to treat topically due to the deep location of the infection under the densely keratinized nail plate. Keratin affinity of topical drugs is an important physicochemical property impacting therapeutic efficacy. To be effective, topical drugs must penetrate the nail bed and retain their antifungal activity within the nail matrix, both of which are adversely affected by keratin binding. We investigated these properties for efinaconazole, a new topical antifungal for onychomycosis, compared with those of the existing topical drugs ciclopirox and amorolfine. The efinaconazole free-drug concentration in keratin suspensions was 14.3%, significantly higher than the concentrations of ciclopirox and amorolfine, which were 0.7% and 1.9%, respectively (P < 0.001). Efinaconazole was released from keratin at a higher proportion than in the reference drugs, with about half of the remaining keratin-bound efinaconazole removed after washing. In single-dose in vitro studies, efinaconazole penetrated full-thickness human nails into the receptor phase and also inhibited the growth of Trichophyton rubrum under the nail. In the presence of keratin, efinaconazole exhibited fungicidal activity against Trichophyton mentagrophytes comparable to that of amorolfine and superior to that of ciclopirox. In a guinea pig onychomycosis model with T. mentagrophytes infection, an efinaconazole solution significantly decreased nail fungal burden compared to that of ciclopirox and amorolfine lacquers (P < 0.01). These results suggest that the high nail permeability of efinaconazole and its potent fungicidal activity in the presence of keratin are related to its low keratin affinity, which may contribute to its efficacy in onychomycosis.
INTRODUCTION
Onychomycosis is a chronic superficial mycosis of the nails that is difficult to treat. It has a prevalence of about 10% in Japan (1) and 13.8% in North America (2), and it is more common in older persons, affecting as many as one in three individuals in that population (1, 3). The most common form of the disease is distal lateral subungual onychomycosis (DLSO), and the causative pathogens include Trichophyton rubrum, Trichophyton mentagrophytes, nondermatophyte molds (e.g., Scopulariopsis brevicaulis and Fusarium species), and Candida albicans.
Oral terbinafine and itraconazole are currently the preferred treatments for onychomycosis (4, 5). However, their use is limited by hepatotoxicity and drug-drug interactions (especially with itraconazole), which represent a safety concern, particularly in older persons, in whom underlying disease and polypharmacy are common. Routine liver function testing is recommended for those being treated with oral terbinafine and itraconazole, and their use is contraindicated in patients with abnormal liver function (6). In contrast, topical ciclopirox and amorolfine nail lacquers have a favorable safety profile, but their cure rates are considerably lower (4, 6). Safe and efficacious topical therapies against onychomycosis remain an unmet medical need.
One aspect contributing to the difficulty in developing an effective topical agent against onychomycosis is the location of the fungal infection deep in the nail bed. To be effective, an adequate amount of drug must penetrate the nail bed (7) and remain active within the keratin matrix of the nail and nail bed (8–10). The unique properties of the nail, particularly its thickness and relatively compact construction, make it a formidable barrier to the entry of topically applied agents (11). The upper dorsal layer is only a few cell layers thick but consists of hard keratin, constituting the main barrier for drug diffusion into and through the nail plate (12). Further, many antifungal agents bind strongly to keratin, which not only restricts their penetration through the nail but may also reduce their antifungal activity (8–10). Keratin-bound drugs do not contribute to a concentration gradient, resulting in the accumulation of topically applied drug in the surface nail layers and decreased penetration to the deeper layers and nail bed (8). Thus, low keratin affinity is considered a desirable physicochemical property of drugs for topical treatment of onychomycosis and possibly of other superficial mycoses.
Efinaconazole (Fig. 1) is a new triazole antifungal agent developed as a low-surface-tension 10% (wt/wt) topical solution for the treatment of mild to moderate DLSO. It was originally identified as a potent antifungal with in vitro activity minimally affected by keratin (13). The methylene-piperidine group at the C-4 position of the efinaconazole molecule may be responsible for its relatively low keratin binding. Efinaconazole possesses broader-spectrum antifungal activity than existing antifungals against dermatophyte and nondermatophyte molds and yeasts (14). It has potent in vitro antifungal activity against nondermatophytes, such as Scopulariopsis brevicaulis and Fusarium species, which cause nail infections that respond poorly to oral drugs (15). Thus, efinaconazole may be effective in treating nondermatophyte onychomycosis.
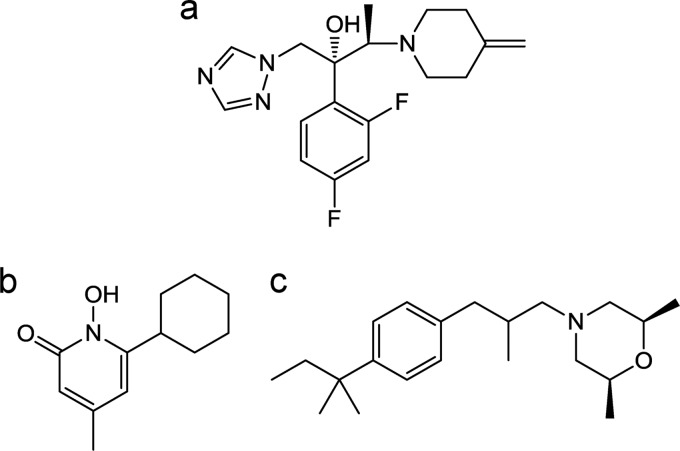
FIG 1.
Chemical structures of test substances. (a) Efinaconazole; (b) ciclopirox; (c) amorolfine.
In a guinea pig onychomycosis model, topically applied efinaconazole was more effective in reducing toenail fungal burden than were amorolfine and terbinafine (10). Further, the therapeutic efficacy of efinaconazole has been established in mild to moderate DLSO patients in two phase III clinical trials (16). Its mycological cure rate is comparable to that reported with oral itraconazole, and the complete cure rate is 2- to 3-fold higher than that of ciclopirox nail lacquer (17, 18).
To further understand the efficacy of efinaconazole in onychomycosis, its keratin affinity was investigated in relation to its nail penetration and antifungal activity in the keratin matrix. In vitro and in vivo models of nail drug delivery and onychomycosis were used to characterize the properties of efinaconazole, which were compared with two topical antifungals currently available in the United States and/or Europe, ciclopirox and amorolfine (Fig. 1).
(This work was presented in part at the 71st Annual Meeting, American Academy of Dermatology, Miami Beach, FL, 1 to 5 March 2013 [abstract P-6546] [19], and International Investigative Dermatology, Edinburgh, Scotland, 8 to 11 May 2013 [abstract 1151] [20].)
MATERIALS AND METHODS
Antifungal agents.
Efinaconazole was synthesized by Kaken Pharmaceutical Co., Ltd. (Tokyo, Japan). Ciclopirox olamine and amorolfine hydrochloride were purchased from Sigma-Aldrich Co. (St. Louis, MO, USA) and Tokyo Chemical Industry Co., Ltd. (Tokyo, Japan), respectively. The efinaconazole solutions (5% and 10% [wt/wt]) were manufactured by Dow Pharmaceutical Sciences, Inc. (Petaluma, CA, USA) and Kaken Pharmaceutical Co., Ltd., respectively. Ciclopirox 8% nail lacquer (Penlac) and amorolfine 5% nail lacquer (Loceryl) were purchased from Sanofi-Aventis (Paris, France) and Galderma Laboratories, Inc. (Lausanne, Switzerland), respectively.
Media.
Sabouraud dextrose agar (SDA) was purchased from Becton, Dickinson and Company (Franklin Lakes, NJ, USA) or Oxoid Ltd. (Basingstoke, United Kingdom). Glucose peptone agar with lecithin and polysorbate 80 (GPLP) was purchased from Wako Pure Chemical Industries, Ltd. (Tokyo, Japan). A modified GPLP medium containing 1% lecithin and antibiotics was used to grow the dermatophytes from guinea pig nails. The antibiotics used were chloramphenicol (Wako Pure Chemical Industries, Ltd.) at 10 μg/ml, cycloheximide (Wako Pure Chemical Industries, Ltd.) at 500 μg/ml, 5-fluorocytosine (Tokyo Chemical Industry Co., Ltd.) at 50 μg/ml, and gentamicin (Merck & Co., Inc., Whitehouse Station, NJ, USA) at 75 μg/ml. The keratin medium was prepared by mixing a 0.2% (wt/vol) K2HPO4, 0.005% (wt/vol) CaCl2, and 0.005% (wt/vol) MgSO4 aqueous solution with 200 mg/ml defatted keratin powder (MP Biomedicals, LLC, Solon, OH, USA).
Test organisms.
T. rubrum and T. mentagrophytes strains were kindly supplied by Cardiff University (Cardiff, United Kingdom) and Niigata University School of Medicine (Niigata, Japan), respectively. Both strains were susceptible to the test drugs used in the experiments (our unpublished data).
Affinity to keratin.
The affinities of the test drugs to keratin were determined as described previously (10), with a slight modification. Briefly, 100 μl of drug solution in dimethyl sulfoxide (DMSO) (50 μg/ml efinaconazole and 100 μg/ml ciclopirox or amorolfine) was mixed with 9.9 ml of 0.2 mol/liter Tris-HCl buffer (pH 7.4) containing 0.5 g of defatted keratin powder. After shaking at 37°C for 1 h (75 rpm), the suspension was centrifuged. The drug concentration in the supernatant was determined by liquid chromatography-tandem mass spectrometry (LC-MS/MS) (TSQ Quantum Ultra; Thermo Fisher Scientific, Inc., Waltham, MA, USA). The lower limit of quantification of the assay was 1 ng/ml for all analytes. The percentage of free drug was calculated for each drug, and the mean values were compared by Tukey's multiple-comparison test (EXSUS version 7.7.1; CAC Exicare Corporation). P values of <0.05 were regarded as significant. The keratin pellet (containing bound drug) was resuspended in 10 ml of 0.2 mol/liter Tris-HCl buffer and washed by shaking for 10 min. The suspension was centrifuged and the washing procedure repeated 5 times. The drug concentration of the supernatant obtained after each wash was measured by LC-MS/MS, and the cumulative percent release of drug was calculated.
In vitro drug penetration in the human nail plate.
Healthy human nails at full thickness were purchased from KAC Co., Ltd. (Kyoto, Japan) and cut in squares of approximately 16 mm2. The nails were fixed between two acrylic plates with a 2-mm-diameter hole and mounted in Franz diffusion cells (Japan Glass Industry Co., Ltd., Tokyo, Japan). The receptor compartment was filled with phosphate-buffered saline (PBS) (pH 7.4) containing 4% (wt/vol) bovine serum albumin (BSA) and 0.01% (wt/vol) sodium azide and placed in an incubator at 32°C. Next, 2 μl of efinaconazole 10% solution, ciclopirox 8% nail lacquer, or amorolfine 5% nail lacquer was singly applied on the dorsal nail surface in the donor compartment. The receptor solution was continuously stirred by a spinning magnetic bar. The receptor fluid was sampled once daily for 14 days, drug concentrations were determined by LC-MS/MS, and the cumulative drug amount permeated per nail unit area (1 cm2) was calculated.
In vitro antifungal activity under the human nail plate.
The drug growth inhibition of T. rubrum under the human nail plate was determined as reported previously (21). Briefly, distal nail clippings of ≥9 mm2 obtained from volunteer toenails were mounted in TurChub cells. SDA containing T. rubrum microconidia (5 × 104 cells) was loaded into the lower chamber of the cells. Ten microliters of efinaconazole 5% solution, ciclopirox 8% nail lacquer, or amorolfine 5% nail lacquer was applied once on the dorsal nail side, dried for 1 h, and incubated for 7 days at 25°C. After incubation, the lengths (cm) of the growth inhibition zones produced in the agar under the nail were measured with a caliper.
Fungicidal activity in the presence of keratin.
Forty microliters of 4-fold serial dilutions of the drugs in DMSO were added into tubes containing 4 ml of keratin medium and T. mentagrophytes microconidia at 2 × 104 cells/ml and incubated at 30°C. The final drug concentrations in the medium were 0 (DMSO), 0.313, 1.25, 5, and 20 μg/ml. After incubation for 2, 4, 7, 10, and 14 days, 0.5 ml of culture suspension was taken from the tube, homogenized with a glass homogenizer, and spread onto GPLP plates. After incubation for 10 days, viable cell counts (CFU/ml) were determined (detection limit, 10 CFU/ml).
Therapeutic efficacy in guinea pig model of onychomycosis.
All experimental procedures were approved by the Institutional Animal Care and Use Committee of Kaken Pharmaceutical Co., Ltd. Arthrospores of T. mentagrophytes were prepared by a previously reported method (22, 23). Six-week-old male Hartley guinea pigs (Japan SLC, Inc., Hamamatsu, Japan) were randomly assigned to one of four treatment groups consisting of six animals each. The nails of guinea pigs were infected with the arthrospores (2 × 107 cells/foot) as previously described (10), with a slight modification. Treatment was initiated on day 29 after fungal inoculation and continued for 4 weeks. Thirty microliters of each drug was individually applied topically to all three nails/foot once daily (efinaconazole 10% solution or ciclopirox 8% nail lacquer) or once weekly (amorolfine 5% nail lacquer). The dosing frequency was based on typical clinical use. Nails treated with ciclopirox nail lacquer or amorolfine nail lacquer were wiped with 70% isopropyl alcohol once a week before applying the drugs to remove any residual material. One group was left untreated as the infected control. The therapeutic efficacy was evaluated by a previously described method (10), with a slight modification. Following a rest period of 7 days after the last treatment, each animal was euthanized and the nails were wiped with 70% ethanol and removed from the feet. The nails from each foot were pooled, minced thoroughly, homogenized with a glass homogenizer in PBS (pH 7.4) containing 0.25% trypsin and 10 mmol/liter FeCl3, and digested at 37°C for about 1 h. The samples were spread onto modified GPLP plates containing 1% lecithin and antibiotics. The plates were incubated for 14 days at 30°C, and the fungal colonies were counted (detection limit, 1.11 log10 CFU/foot). The mean log10 CFU/foot values for all treatments were analyzed by Tukey's multiple-comparison test. P values of <0.05 were regarded as significant.
RESULTS
Affinity to keratin.
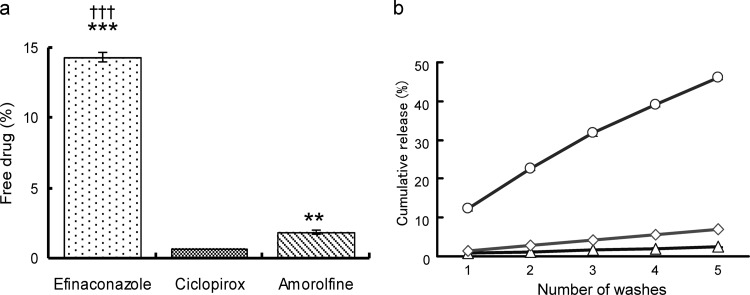
The efinaconazole, ciclopirox, and amorolfine free-drug levels after incubation in keratin suspensions were 14.3% ± 0.4%, 0.7% ± 0.0%, and 1.9% ± 0.2% (mean ± standard deviation [SD]), respectively; the cumulative drug release levels after five washes were 46.0% ± 0.6%, 2.4% ± 0.2%, and 6.9% ± 0.1% (mean ± SD), respectively (Fig. 2). The efinaconazole free-drug and keratin release levels were ≥6-fold higher than those of amorolfine and ciclopirox. The amorolfine levels were about 2-fold higher than those of ciclopirox.
FIG 2.
Affinity to keratin. Efinaconazole (0.5 μg/ml), ciclopirox (1 μg/ml), or amorolfine (1 μg/ml) was incubated in Tris-HCl buffer (pH 7.4) with keratin powder (0.5 g/10 ml). (a) The percentage of free drug in buffer after shaking at 37°C for 1 h. The graphs represent the mean ± SD of three replicates. Tukey multiple-comparison test: **, P < 0.01 versus ciclopirox; ***, P < 0.001 versus ciclopirox; †††, P < 0.001 versus amorolfine. (b) The cumulative release of efinaconazole (○), ciclopirox (△), and amorolfine (◇). Each plot represents the mean value of triplicates.
In vitro drug penetration in the human nail plate.
Drug nail penetration was investigated in Franz diffusion cells after a single topical application of efinaconazole 10% solution, ciclopirox 8% nail lacquer, or amorolfine 5% nail lacquer to human nails for 14 days (n = 6 cells/group). Efinaconazole was detected in the receptor-phase samples as early as day 1, whereas ciclopirox was first detected on day 6 (data not shown). On days 7 and 14, efinaconazole was detected in 4/6 receptor-phase samples, and the cumulative permeated amounts were 2.94 ± 3.91 μg/cm2 and 6.53 ± 8.15 μg/cm2 (mean ± SD), respectively (Table 1). Ciclopirox was detected in 2 to 3 of 6 samples, and the cumulative permeated amounts were 0.326 ± 0.590 μg/cm2 and 4.57 ± 6.89 μg/cm2 (mean ± SD), respectively. On the other hand, amorolfine was not detectable in any receptor-phase sample.
TABLE 1.
Cumulative drug amount permeated after a single application of efinaconazole 10% solution, ciclopirox 8% nail lacquer, and amorolfine 5% nail lacquer to human nailsa
| Time (day) | Cumulative amt permeated (μg/cm2) for: |
||
|---|---|---|---|
| Efinaconazole | Ciclopirox | Amorolfine | |
| 1 | 0.401 ± 0.672 | NCb | NC |
| 2 | 0.742 ± 1.242 | NC | NC |
| 3 | 1.29 ± 1.91 | NC | NC |
| 4 | 1.59 ± 2.37 | NC | NC |
| 5 | 2.16 ± 3.11 | NC | NC |
| 6 | 2.57 ± 3.49 | 0.0998 ± 0.2445 | NC |
| 7 | 2.94 ± 3.91 | 0.326 ± 0.590 | NC |
| 8 | 3.45 ± 4.46 | 0.535 ± 0.908 | NC |
| 9 | 3.98 ± 5.20 | 0.895 ± 1.495 | NC |
| 10 | 4.49 ± 5.70 | 1.32 ± 2.18 | NC |
| 11 | 4.84 ± 6.12 | 2.03 ± 3.32 | NC |
| 12 | 5.42 ± 6.97 | 2.82 ± 4.34 | NC |
| 13 | 5.90 ± 7.39 | 3.56 ± 5.43 | NC |
| 14 | 6.53 ± 8.15 | 4.57 ± 6.89 | NC |
Data represent mean ± SD (n = 6).
NC, not calculated because drug concentration in the receptor phase was below the lower limit of quantification (<1 ng/ml) in all samples.
In vitro antifungal activity under the human nail plate.
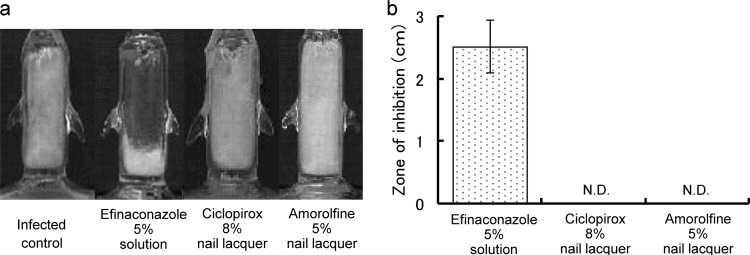
To confirm drug penetration through the nail and further evaluate the antifungal activity of the permeated drug, growth inhibition was studied using TurChub cells (21). A prototype formulation with a lower efinaconazole concentration (5%) was used in the experiment. The efinaconazole 5% solution produced a region of growth inhibition under the nail (mean ± SD, 2.52 ± 0.42 cm), whereas ciclopirox 8% and amorolfine 5% nail lacquers did not (Fig. 3).
FIG 3.
Fungal growth inhibition under the human nail plate. The inhibition of T. rubrum growth under the human nail plate was determined in TurChub cells after 7 days of a single application of efinaconazole 5% solution, ciclopirox 8% nail lacquer, or amorolfine 5% nail lacquer. (a) Representative results of TurChub cells. (b) Mean growth inhibition zone lengths (cm) for 5 or 6 replicates. N.D., not detectable.
Fungicidal activity in the presence of keratin.
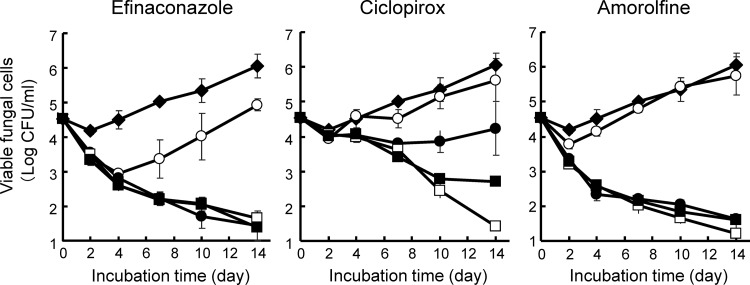
The fungicidal activity of efinaconazole was examined in keratin medium, mimicking the keratin-rich environment of the nail plate and nail bed where the fungal infection resides. In the untreated growth control cultures, viable cell counts were unchanged through day 4 but began increasing on day 7 of incubation, showing that dermatophytes utilize keratin as an energy source (Fig. 4). Both efinaconazole and amorolfine at 1.25, 5, and 20 μg/ml decreased viable cell counts by one log as early as day 2 and produced a near-complete kill by day 14. Exposure to these drugs at 0.313 μg/ml produced an initial transient decrease in viability, which recovered to at least baseline levels by day 14; efinaconazole was slightly more potent than amorolfine at this concentration. Ciclopirox decreased viability from day 7 only at 5 and 20 μg/ml. Thus, the fungicidal activity of efinaconazole was higher than that of ciclopirox and comparable to that of amorolfine in the presence of keratin.
FIG 4.
Fungicidal activity of drugs against T. mentagrophytes in the presence of keratin. The drugs were incubated at 30°C in keratin medium containing T. mentagrophytes at 2 × 104 cells/ml for 14 days. The graphs represent the mean ± SD of three cultures treated with efinaconazole, ciclopirox, and amorolfine (◆, 0 μg/ml; ○, 0.313 μg/ml; ●, 1.25 μg/ml; □, 5 μg/ml; ■, 20 μg/ml).
Therapeutic efficacy in guinea pig model of onychomycosis.
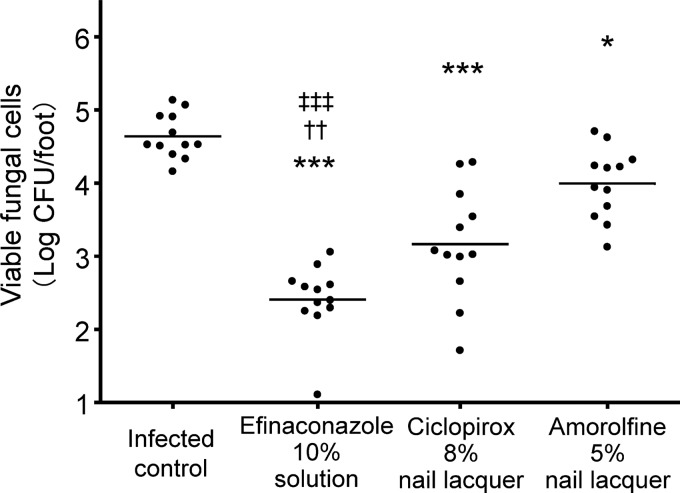
The in vivo efficacy of the efinaconazole 10% solution was investigated in a guinea pig onychomycosis model. After repeated topical treatment, the viable cell counts in the nails in the groups treated with efinaconazole 10% solution, ciclopirox 8% nail lacquer, and 5% amorolfine nail lacquer were 2.41 ± 0.48, 3.17 ± 0.77, and 3.99 ± 0.48 log10 CFU/foot (mean ± SD), respectively (Fig. 5). The viable cell counts in these three groups were significantly lower (P < 0.001, P < 0.05, and P < 0.001, respectively) than those of the infected control group (mean ± SD, 4.64 ± 0.30 log10 CFU/foot). Furthermore, the viable cell counts in the nails treated with the efinaconazole 10% solution were significantly lower than those treated with the ciclopirox 8% and amorolfine 5% nail lacquers (P < 0.01 and P < 0.001, respectively).
FIG 5.
Therapeutic efficacies of topical drugs in a guinea pig onychomycosis model. Viable cell counts in guinea pig nails (log10 CFU/foot) were determined as a measure of drug efficacy after 28 days of treatment with efinaconazole 10% solution (once daily), ciclopirox 8% nail lacquer (once daily), or amorolfine 5% nail lacquer (once weekly). The bars represent the mean viable cell counts from 12 feet (6 animals). The mean values were compared using Tukey's multiple-comparison test (*, P < 0.05 versus infected control; ***, P < 0.001 versus infected control; ††, P < 0.01 versus ciclopirox 8% nail lacquer; ‡‡‡, P < 0.001 versus amorolfine 5% nail lacquer).
DISCUSSION
Topical onychomycosis therapy is challenged by the location of the fungal infection deep within the nail, the unique physicochemical properties of the nail (e.g., thickness and relatively compact construction), and low drug penetration (24). Enhanced drug delivery to the site of action may correlate with greater efficacy; thus, it is expected that drugs targeting the lower nail layers will be more effective in treating onychomycosis. Although the vehicle in which the drug is formulated may aid delivery, the intrinsic properties of the drug molecule are considered to play an important role in its clinical efficacy. Keratin affinity is an important physicochemical property affecting the efficacy of antifungal drugs, many of which are reported to have high affinity to keratin (10, 12, 25).
In the present study, to further understand the properties of efinaconazole in terms of efficacy for onychomycosis and nail permeability, ciclopirox and amorolfine were compared using three unique models of onychomycosis (in vitro and in vivo).
Efinaconazole had significantly lower binding to keratin, with an unbound fraction >7-fold higher and a higher release rate from keratin than those of ciclopirox or amorolfine (Fig. 2). This low keratin affinity of efinaconazole correlated with faster nail penetration and fungicidal activity in the presence of keratin.
When the antifungal activity against T. rubrum was investigated in vitro under healthy human nails at full thickness, growth was inhibited after treatment with the efinaconazole solution but not with the ciclopirox and amorolfine nail lacquers (Fig. 3). These observations are in agreement with the high nail permeability of efinaconazole and its potent antidermatophytic activity (14). These in vitro onychomycosis models used a single application for better differentiation of nail absorption and antifungal activity profiles between the drugs; characterization following repeated application, as intended clinically for these drugs, was not investigated.
Although the fungicidal activities of azole antifungals are generally weaker than those of amorolfine and ciclopirox in RPMI 1640 (9), the fungicidal activity of efinaconazole against T. mentagrophytes in keratin medium was similar to that of amorolfine and higher than that of ciclopirox (Fig. 4). Because keratin medium contains keratin as the sole nutrient source for energy and mimics the nail bed environment, these data more accurately reflect in vivo activity. The high fungicidal activity seen with efinaconazole is reflective of its higher free (unbound) concentration relative to those of the comparator drugs.
Both the efinaconazole 10% solution and the comparator nail lacquer drugs decreased viable dermatophyte cell counts in infected guinea pig nails. However, the in vivo antifungal activity of efinaconazole was significantly higher (Fig. 5), which may be explained by its nail permeability profile and antifungal activity in the presence of keratin.
The limited efficacies of currently marketed topical onychomycosis drugs may be due to keratin binding, which decreases nail drug penetration and antifungal activity. In developing new topical onychomycosis drugs, keratin affinity is an important factor affecting efficacy. For example, oral terbinafine, which is approved for onychomycosis treatment and considered the gold standard, is 98.9% keratin bound under the same in vitro experimental conditions as those in this study (data not shown); however, it has been shown to have limited success in treating the disease when applied topically (26).
The high nail penetration and potent fungicidal activity of efinaconazole in the presence of keratin observed in vitro and in vivo may not necessarily be predictive of clinical outcome. However, the mycological and complete cure rates observed clinically with the efinaconazole 10% solution were 2- to 3-fold greater than those previously reported with the ciclopirox nail lacquer (16, 18). Therefore, our results suggest that these important features of efinaconazole contribute to its clinical efficacy.
ACKNOWLEDGMENTS
We thank Brian Bulley, Jun Nakano, and Hisato Senda for manuscript discussions and review and Marc Brown and Rob Turner (MedPharm Ltd., Guildford, United Kingdom) for performing the antifungal activity study under the human nail plate using TurChub cells.
This study was supported by Kaken Pharmaceutical Co., Ltd., and Valeant Pharmaceuticals North America LLC.
Each of us is an employee and stockholder in Kaken Pharmaceutical Co., Ltd., or Valeant Pharmaceuticals North America LLC.
Footnotes
Published ahead of print 21 April 2014
REFERENCES
- 1.Watanabe S, Harada T, Hiruma M, Iozumi K, Katoh T, Mochizuki T, Naka W, Japan Foot Week Group 2010. Epidemiological survey of foot diseases in Japan: results of 30,000 foot checks by dermatologists. J. Dermatol. 37:397–406. 10.1111/j.1346-8138.2009.00741.x [DOI] [PubMed] [Google Scholar]
- 2.Ghannoum MA, Hajjeh RA, Scher R, Konnikov N, Gupta AK, Summerbell R, Sullivan S, Daniel R, Krusinski P, Fleckman P, Rich P, Odom R, Aly R, Pariser D, Zaiac M, Rebell G, Lesher J, Gerlach B, Ponce-De-Leon GF, Ghannoum A, Warner J, Isham N, Elewski B. 2000. A large-scale North American study of fungal isolates from nails: the frequency of onychomycosis, fungal distribution, and antifungal susceptibility patterns. J. Am. Acad. Dermatol. 43:641–648. 10.1067/mjd.2000.107754 [DOI] [PubMed] [Google Scholar]
- 3.Elewski BE. 2000. Onychomycosis. Treatment, quality of life, and economic issues. Am. J. Clin. Dermatol. 1:19–26 [DOI] [PubMed] [Google Scholar]
- 4.Baker SJ, Hui X, Maibach HI. 2005. Progress on new therapeutics for fungal nail infections. Annu. Rep. Med. Chem. 40:323–335. 10.1016/S0065-7743(05)40021-4 [DOI] [Google Scholar]
- 5.Gupta AK, Konnikov N, Lynde CW. 2001. Single-blind, randomized, prospective study on terbinafine and itraconazole for treatment of dermatophyte toenail onychomycosis in the elderly. J. Am. Acad. Dermatol. 44:479–484. 10.1067/mjd.2001.110874 [DOI] [PubMed] [Google Scholar]
- 6.Niewerth M, Korting HC. 1999. Management of onychomycoses. Drugs 58:283–296. 10.2165/00003495-199958020-00005 [DOI] [PubMed] [Google Scholar]
- 7.Murdan S. 2002. Drug delivery to the nail following topical application. Int. J. Pharm. 236:1–26. 10.1016/S0378-5173(01)00989-9 [DOI] [PubMed] [Google Scholar]
- 8.Narasimha Murthy S, Wiskirchen DE, Bowers CP. 2007. Iontophoretic drug delivery across human nail. J. Pharm. Sci. 96:305–311. 10.1002/jps.20757 [DOI] [PubMed] [Google Scholar]
- 9.Schaller M, Borelli C, Berger U, Walker B, Schmidt S, Weindl G, Jäckel A. 2009. Susceptibility testing of amorolfine, bifonazole and ciclopiroxolamine against Trichophyton rubrum in an in vitro model of dermatophyte nail infection. Med. Mycol. 47:753–758. 10.3109/13693780802577892 [DOI] [PubMed] [Google Scholar]
- 10.Tatsumi Y, Yokoo M, Senda H, Kakehi K. 2002. Therapeutic efficacy of topically applied KP-103 against experimental Tinea unguium in guinea pigs in comparison with amorolfine and terbinafine. Antimicrob. Agents Chemother. 46:3797–3801. 10.1128/AAC.46.12.3797-3801.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hui X, Shainhouse Z, Tanojo H, Anigbogu A, Markus GE, Maibach HI, Wester RC. 2002. Enhanced human nail drug delivery: nail inner drug content assayed by new unique method. J. Pharm. Sci. 91:189–195. 10.1002/jps.10003 [DOI] [PubMed] [Google Scholar]
- 12.Kobayashi Y, Miyamoto M, Sugibayashi K, Morimoto Y. 1999. Drug permeation through the three layers of the human nail plate. J. Pharm. Pharmacol. 51:271–278. 10.1211/0022357991772448 [DOI] [PubMed] [Google Scholar]
- 13.Ogura H, Kobayashi H, Nagai K, Nishida T, Naito T, Tatsumi Y, Yokoo M, Arika T. 1999. Synthesis and antifungal activities of (2R,3R)-2-aryl-1-azolyl-3-(substituted amino)-2-butanol derivatives as topical antifungal agents. Chem. Pharm. Bull. (Tokyo) 47:1417–1425. 10.1248/cpb.47.1417 [DOI] [PubMed] [Google Scholar]
- 14.Jo Siu WJ, Tatsumi Y, Senda H, Pillai R, Nakamura T, Sone D, Fothergill A. 2013. Comparison of in vitro antifungal activities of efinaconazole and currently available antifungal agents against a variety of pathogenic fungi associated with onychomycosis. Antimicrob. Agents Chemother. 57:1610–1606. 10.1128/AAC.02056-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Baudraz-Rosselet F, Ruffieux C, Lurati M, Bontems O, Monod M. 2010. Onychomycosis insensitive to systemic terbinafine and azole treatments reveals non-dermatophyte moulds as infectious agents. Dermatology 220:164–168. 10.1159/000277762 [DOI] [PubMed] [Google Scholar]
- 16.Elewski BE, Rich P, Pollak R, Pariser DM, Watanabe S, Senda H, Ieda C, Smith K, Pillai R, Ramakrishna T, Olin JT. 2013. Efinaconazole 10% solution in the treatment of toenail onychomycosis: two phase III multicenter, randomized, double-blind studies. J. Am. Acad. Dermatol. 68:600–608. 10.1016/j.jaad.2012.10.013 [DOI] [PubMed] [Google Scholar]
- 17.Evans EG, Sigurgeirsson B. 1999. Double blind, randomised study of continuous terbinafine compared with intermittent itraconazole in treatment of toenail onychomycosis. BMJ 318:1031–1035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gupta AK, Fleckman P, Baran R. 2000. Ciclopirox nail lacquer topical solution 8% in the treatment of toenail onychomycosis. J. Am. Acad. Dermatol. 43:S70–S80. 10.1067/mjd.2000.109071 [DOI] [PubMed] [Google Scholar]
- 19.Sugiura K, Hosaka S, Jo W, Tatsumi Y. 2013. Unique properties of efinaconazole 10% solution, a new topical treatment for onychomycosis, abstr. P6546. 71st Ann. Meet., Am. Acad. Dermatol., Miami Beach, FL, 1 to 5 March 2013 [Google Scholar]
- 20.Sugiura K, Hosaka S, Sugimoto N, Tatsumi Y, Jo Siu W. 2013. Efinaconazole's nail penetration and fungicidal activity may contribute to its therapeutic efficacy as a topical treatment for onychomycosis, abstr 1151. Abstr. International Investigative Dermatology, Edinburgh, Scotland, 8 to 11 May, 2013 [Google Scholar]
- 21.Traynor MJ, Turner RB, Evans CR, Khengar RH, Jones SA, Brown MB. 2010. Effect of a novel penetration enhancer on the ungual permeation of two antifungal agents. J. Pharm. Pharmacol. 62:730–737. 10.1211/jpp.62.06.0009 [DOI] [PubMed] [Google Scholar]
- 22.Fujita S, Matsuyama T, Sato Y. 1986. A simple and reliable culturing method for production of arthrospores by dermatophytes. Jpn. J. Med. Mycol. 27:175–181. 10.3314/jjmm1960.27.175 [DOI] [Google Scholar]
- 23.Tatsumi Y, Yokoo M, Arika T, Yamaguchi H. 2001. In vitro antifungal activity of KP-103, a novel triazole derivative, and its therapeutic efficacy against experimental plantar Tinea pedis and cutaneous candidiasis in guinea pigs. Antimicrob. Agents Chemother. 45:1493–1499. 10.1128/AAC.45.5.1493-1499.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hui X, Wester RC, Barbadillo S, Lee C, Patel B, Wortzmman M, Gans EH, Maibach HI. 2004. Ciclopirox delivery into the human nail plate. J. Pharm. Sci. 93:2545–2548. 10.1002/jps.20159 [DOI] [PubMed] [Google Scholar]
- 25.Sobue S, Sekiguchi K, Nabeshima T. 2004. Intracutaneous distributions of fluconazole, itraconazole, and griseofulvin in guinea pigs and binding to human stratum corneum. Antimicrob. Agents Chemother. 48:216–223. 10.1128/AAC.48.1.216-223.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Elewski BE, Ghannoum MA, Mayser P, Gupta AK, Korting HC, Shouey RJ, Baker DR, Rich PA, Ling M, Hugot S, Damaj B, Nyirady J, Thangavelu K, Notter M, Parneix-Spake A, Sigurgeirsson B. 2013. Efficacy, safety and tolerability of topical terbinafine nail solution in patients with mild-to-moderate toenail onychomycosis: results from three randomized studies using double-blind vehicle-controlled and open-label active-controlled designs. J. Eur. Acad. Dermatol. Venereol. 27:287–294. 10.1111/j.1468-3083.2011.04373.x [DOI] [PubMed] [Google Scholar]