Abstract
There is a strong need for new broadly active antifungal agents for the treatment of oral candidiasis that not only are active against many species of Candida, including drug-resistant strains, but also evade microbial countermeasures which may lead to resistance. Host defense peptides (HDPs) can provide a foundation for the development of such agents. Toward this end, we have developed fully synthetic, small-molecule, nonpeptide mimetics of the HDPs that improve safety and other pharmaceutical properties. Here we describe the identification of several HDP mimetics that are broadly active against C. albicans and other species of Candida, rapidly fungicidal, and active against yeast and hyphal cultures and that exhibit low cytotoxicity for mammalian cells. Importantly, specificity for Candida over commensal bacteria was also evident, thereby minimizing potential damage to the endogenous microbiome which otherwise could favor fungal overgrowth. Three compounds were tested as topical agents in two different mouse models of oral candidiasis and were found to be highly active. Following single-dose administrations, total Candida burdens in tongues of infected animals were reduced up to three logs. These studies highlight the potential of HDP mimetics as a new tool in the antifungal arsenal for the treatment of oral candidiasis.
INTRODUCTION
Oral infections due to Candida albicans represent an increasing problem in human health. In immunocompromised individuals, especially those suffering from AIDS, candidiasis can result in both localized, painful lesions in the oral cavity and life-threatening systemic infections. Even in intact hosts, Candida can cause persistent infections in the oral cavity, such as stomatitis in individuals wearing dentures (1). Furthermore, due to the use of standard antifungal treatments, an increasing number of infections result from non-albicans Candida (NAC) species (reviewed in reference 2). Oral infections with Candida, i.e., oropharyngeal candidiasis (OPC), were observed in 90% of patients undergoing chemotherapy for acute leukemia (3) and in 95% of patients with HIV/AIDS (4). Although the introduction of highly active antiretroviral therapy has reduced these numbers in HIV/AIDS patients, the occurrence is still very high. One of the most common forms of OPC is pseudomembranous candidiasis, which is characterized by white patches on the surfaces of the labial and buccal mucosa, palate, and tongue and other oral mucosal surfaces. If untreated, the symptoms can result in poor nutrition and other complications. In addition to being a major cause of morbidity in immunocompromised patients (5), OPC can predispose these patients to esophageal candidiasis (6, 7), an invasive form of infection with significant morbidity and higher risk for fatal, disseminated infection (8, 9). While OPC is predominantly due to colonization by C. albicans, other species have been identified in OPC, including C. glabrata, C. tropicalis, C. krusei, and C. dubliniensis, among others. OPC is treated either with topical antifungal agents such as nystatin or with systemic agents (10). These include azoles, such as fluconazole or itraconazole, or echinocandins, such as caspofungin. With the recurrence of OPC in HIV/AIDS patients, long-term treatments have led to a significant rise in antifungal-resistant organisms (for a review, see reference 11). It is thus critical to develop new therapies that can treat both C. albicans infections and those due to NAC.
Host defense peptides (HDPs) are naturally occurring, broad-spectrum antimicrobial agents that have been examined recently for their utility as therapeutic antibiotics and antifungals (12). These agents are particularly strong therapeutic candidates due to infrequent development of resistance by microbes. Unfortunately, they are expensive to produce and are often sensitive to protease digestion (13). To address these problems, we have developed a series of inexpensive nonpeptidic oligomers and compounds that mimic HDPs in both structure and activity (14, 15). We reasoned that small synthetic oligomers that adopt amphiphilic secondary structures and exhibit potent and selective antimicrobial activity would be less expensive to produce, have better tissue distribution, and be much more amenable to structural fine-tuning to improve activity and minimize toxicity (16). This effort has led to the identification of a clinical lead compound, brilacidin (PMX30063), which has successfully completed a phase 2 clinical study for the treatment of acute bacterial skin and skin structure infections (ABSSSI) caused by drug-susceptible and -resistant Staphylococcus aureus (17).
We recently demonstrated that HDP mimetics exhibit potent in vitro activity against C. albicans as well as NAC in both planktonic and biofilm forms (18). The activity was rapid and fungicidal against both blastoconidia and hyphal forms. In addition, long-term growth at sub-MICs did not lead to resistance, suggesting that they are attractive candidates for anti-Candida drugs. In this study, we have identified additional HDP mimetics which demonstrate potent activity against Candida both in vitro and in vivo.
MATERIALS AND METHODS
Yeast and bacterial strains.
A clinical isolate of C. albicans (GDH2346) was used for compound screening. C. dubliniensis (NCPF3949), C. glabrata (ATCC 90030), C. krusei (ATCC 6258), C. parapsilosis (ATCC 22019) and C. tropicalis (ATCC 750) (obtained from the laboratory of David Perlin, PHRI/Rutgers), were used for all assays and were cultured on YPD (1% yeast extract, 2% peptone, 2% dextrose, pH 5.7) agar at 37°C. For liquid assays, single colonies were dispersed in RPMI 1640 (Mediatech, Inc.) with morpholinepropanesulfonic acid (MOPS), pH 7.0 at a concentration of 2.5 × 106 CFU/ml. Escherichia coli 25922, S. aureus 27660, Pseudomonas aeruginosa 10145, Enterococcus faecalis 29212, and Klebsiella pneumoniae 13883 were obtained from ATCC and cultured in cation-adjusted Mueller-Hinton II broth. Streptococcus salivarius and Actinomyces viscosus were obtained from the oral cavities of healthy volunteers and identified by growth on selective medium and microscopic evaluation. They were grown in brain heart infusion (BHI) broth under aerobic conditions at 37°C. MIC assays were carried out using standard CLSI methods as we have previously described (19).
Clinical strains of Candida were obtained under consent, with Institutional Review Board approval, from 60 adult HIV-positive patients with or without evidence of oral candidiasis presenting to oral medicine clinics for care irrespective of current antifungal therapy status. Ten patients exhibited clinical presentation of candidiasis (white lesions over inflamed tissue); 50 had no clinical presentation of candidiasis. Sterile swabs were used to collect specimens from three sites in the patients' mouths (the palate, the dorsal surface of the tongue, and the buccal mucosa), and the specimens were dispersed in sterile phosphate-buffered saline (PBS). Samples were streaked on YPD plates supplemented with ampicillin (50 μg/ml) and chloramphenicol (70 μg/ml) to inhibit bacterial colonization. Parallel swabs were streaked onto ChromAgar Candida (Becton Dickinson) to distinguish between C. albicans and non-albicans Candida species, based on the manufacturer's instructions. All colonies of suspected non-albicans Candida species were restreaked on chromogenic agar medium to confirm their color. All clinical isolates were subjected to MIC/minimal fungicidal concentration (MFC) assays as described above.
HDP mimetic compounds.
All HDP mimetic compounds were dissolved in dimethyl sulfoxide (DMSO) (Sigma) at the stock concentration of 20 mg/ml and stored at −20°C. For animal studies, the stocks were diluted in deionized water.
High-throughput screening and IC50 assay.
A collection of approximately 900 compounds from our in-house chemical library were tested at a single concentration of 10 μM against a clinical isolate of C. albicans (GDH2346) in 96-well plates using a modification of the CLSI method (19, 20). The remaining 400 compounds were tested directly to obtain 50% inhibitory concentrations (IC50s) using 11 serial 2-fold dilutions. Yeasts were diluted 1:1,000 from a measured optical density at 600 nm (OD600) of 1.0 in RPMI-MOPS medium supplemented with 20 μM fluorescein-d-glucopyranoside (FDGlu). FDGlu is a substrate for the yeast enzyme exoglucanase (Exg1p), a secreted enzyme which is expressed proportionally to cell growth (21). Exg1p converts FDGlu to fluorescein, providing a quantitative measure of cell growth without the requirement to lyse cells. This has been used in Saccharomyces cerevisiae in conjunction with growth readouts such as FUS1-H1S3 (22). The fluorescent readout for cell growth was used in addition to the traditional optical density measure of growth and was found to correlate well with the OD600 readings. For the IC50 assays, 50 μl of diluted yeast was added to 50 μl of compound diluted in the same medium. Activity was measured using both OD600 and fluorescence (excitation, 485 nm; emission, 530 nm) at 24 and 48 h. An average of all 4 values was compared to the value for control, untreated cells to calculate percent inhibition. IC50s were calculated from 11 2-fold serial dilutions using Prism GraphPad software (nonlinear fit).
Hyphal cultures and IC50 determinations.
Yeasts were grown in RPMI-MOPS–0.4% sucrose (pH 7.4) medium supplemented with 10% fetal bovine serum in tissue culture-treated flat-bottom 96-well plates for 48 h. The filamentous yeast cultures were then vigorously washed to remove any nonfilamentous, nonattached yeast cells. The remaining attached filamentous biofilm yeast cells were incubated in saline containing serially diluted compounds for 24 h. The cultures were aspirated to remove compound, rinsed, and overlaid with RPMI-MOPS–0.4% sucrose (pH 7.4) medium. Biofilm viability was measured using a 3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium (MTS) cell proliferation assay (CellTiter 96 aqueous kit from Promega) as described previously (23). The tetrazolium compound MTS is combined with an electron-coupling reagent (phenazine methosulfate [PMS]) and added to the growth medium. MTS is bioreduced by dehydrogenase enzymes found in metabolically active cells into a formazan product which can be measured directly by OD490 from 96-well assay plates without additional processing. IC50s were determined using Prism GraphPad software (nonlinear fit).
Yeast MIC assay.
Assays were carried out in 96-well plates using the CLSI method as previously described (19). Mimetic compounds were diluted in 50 μl RPMI/MOPS in a 96-well plate (tissue culture treated; Falcon). Suspensions (50 μl) of Candida were added to each well, and the plate was then incubated at 37°C in a humidified chamber for a period of 24 h. Pooled, clarified human saliva was added to 2× RPMI-MOPS to a concentration of 50% to determine whether components of saliva could inhibit activity of the compounds. In this modified CLSI method, the MIC was determined as the concentration in the first well without visibility of turbidity in the broth for the mimetic compounds or in the first well without an increase of OD600 for fluconazole. In order to determine the minimal fungicidal concentration (MFC), a sample (25 μl) from the well defined as having the MIC and the wells with three higher concentrations were plated onto YPD agar. Colonies were counted after 24 h. The MFC is defined as the lowest concentration at which no colonies are observed (24). All MIC/MFC assays were performed in duplicate.
Cytotoxicity assays.
Cytotoxicity (50% effective concentration [EC50]) was determined against mouse 3T3 fibroblasts (ATCC CRL-1658), OKF6/TERT cells (oral keratinocytes) (25), and human transformed liver HepG2 cells (ATCC HB-8065), using an MTS viability assay from Promega. Growth medium was replaced with medium without serum, and eight 2-fold dilutions of compound were added. Following incubation for 1 h at 37°C, compounds were removed and medium containing serum was returned. Viability was determined using an MTS viability assay (CellTiter 96 aqueous nonradioactive cell proliferation assay) from Promega. The EC50 was calculated using GraphPad Prism software (nonlinear fit).
Fungicidal kinetics.
Assays were carried out as previously described (18). Fresh cultures of Candida were diluted 1:1,000 from a measured OD600 of 1.0 in RPMI-MOPS as in the IC50 assay. Samples were incubated in the presence of the mimetic at 37°C, and aliquots were removed at the indicated time points, diluted in YPD, and plated on YPD agar for colony counts after 24 h growth at 37°C. To visualize kinetics of hyphal killing, yeasts were grown in RPMI-MOPS–10% fetal calf serum (FCS) for 3 days to obtain hyphae. After treatment with the compounds, cultures were stained with FungaLight Live-Dead stain (Invitrogen) and observed under fluorescence microscopy (magnification, ×100).
Elution assays.
To test whether the compounds would elute from the delivery gel and maintain activity, compounds 2, 4, and 5 were dissolved at 10 mg/ml in a 20-mg/ml (wt/vol in water) solution of the hydroxyethylcellulose gel (Natrosol; Ashland Aqualon Inc., Parlin, NJ, USA). This corresponds to concentrations of 11 and 15 mM for compounds 2 and 4 (HCl salts), respectively, and 11.7 mM for compound 5 (trifluoroacetic acid salt). The gels were placed in the wells of a 96-well plate, and suspensions of C. albicans GDH2346 were applied to the surface. Yeasts were removed at increasing times and plated to quantify viable cells.
Mouse models of oral candidiasis. (i) Immunocompromised mouse model.
All mouse experiments were approved by the Rutgers University Institutional Animal Care and Use Committee. Six- to 8-week-old male C57BL/6 mice (n = 5 per group) were injected intraperitoneally (i.p.) with 225 mg/kg cortisone acetate (Sigma) in phosphate-buffered saline (PBS)–0.05% Tween 20 in 0.2 ml on day −1, day +1, and day +3 relative to infection. On day 0, mice were anesthetized with an i.p. injection of ketamine (50 mg/ml)-xylazine (20 mg/ml), and tongues were inoculated with calcium alginate swabs soaked in a suspension of 1 × 105 CFU C. albicans GDH2346 for 75 min, as described previously (26).
(ii) Steroid-free model.
Six- to 8-week-old male mice (n = 5 per group) with a mouse β-defensin-1 gene deletion (mBD-1-KO) (27) were treated with tetracycline in the drinking water (2.5g/liter) for 5 days prior to infection. Mice were anesthetized on day 0 with an i.p. injection of a cocktail of ketamine (50 mg/ml)-xylazine (20 mg/ml)-acepromazine (10 mg/ml), and the dorsal surface of the tongue was scratched with four superficial cuts (no bleeding) with a scalpel. A sterile cotton ball was placed in the mouth against the scored tongue, and 50 μl of PBS was pipetted onto it and remained inside for 2 h to keep the mouth moist and allow for healing. This cotton ball was then removed, and a new sterile cotton ball was inserted. Fifty microliters of C. albicans GDH2346 (5 × 107 CFU/ml) was then pipetted onto the cotton ball and left in place. The second cotton ball was removed after 2 hours more. The mice were kept on tetracycline treatment throughout the experiment, as described previously (28).
In both models, on day 3 after infection, mice were anesthetized i.p. with ketamine-xylazine for 2 h, during which time they were treated orally with a 50-μl bolus of 20-mg/ml hydroxyethylcellulose gel (Natrosol) dissolved in distilled water at 37°C, using a syringe (no needle) to insert the gel in the mouth. The gel contained either water alone, 10 mg/ml peptide mimetic (compound 2, 4, or 5) in water, or a 10-mg/ml suspension of nystatin in water. The gel was applied into one side of the mouth, into the cheek, in order to prevent it from being swallowed immediately. After the 2-h sedation, the mice were returned to their cages and allowed to drink. After 24 h, mice were sacrificed and the tongues were surgically removed by excising the whole tongue with a scalpel from the base. Tongues were homogenized in 5 ml PBS using a IKA Ultra Turrax blender. Kidneys were also excised to assess any dissemination of the Candida during the infection. After homogenization, dilutions were plated onto YPD agar in triplicate, and colonies were enumerated at each 10-fold dilution in triplicate after 48 h. Initial experiments demonstrated that tongues had a mean weight of 0.12 g ± 0.02 g. Since there was little variability between the individual tongues, results were expressed as CFU/tongue rather than per gram of tongue tissue. The Candida inoculum was also plated and counted to verify the inoculum concentration originally placed on the cotton ball.
RESULTS
In our initial studies, two compounds, mPE and PMX519, were identified as anti-Candida compounds from a very limited screen of an HDP mimetic compound library (19). To help ensure that chemical optimization efforts were being focused appropriately, an HDP mimetic collection, consisting of approximately 1,300 compounds, was screened to assess anti-Candida activity. We observed that 109 compounds had IC50s of <5 μg/ml for inhibition of C. albicans growth, giving a hit rate of 8.4% (see Materials and Methods). Importantly, all of the 109 active compounds were found to be cidal (reductions in CFU/ml of >2 log10) at 10 μg/ml following 24-h incubations with the compound. We chose 6 compounds with the lowest IC50s for further testing. The results for these 6 compounds (Fig. 1), in addition to those for the previously described compounds mPE (compound 1) and PMX519 (compound 2), are shown in Tables 1 to 3. The IC50s and MICs for all of the selected compounds against C. albicans GDH2346 ranged from 1.03 to 4.93 μg/ml and 2 to 8 μg/ml, respectively (Table 1). All of the compounds except compound 1 showed low cytotoxicity against the mouse NIH 3T3 fibroblasts and human liver HepG2 cells as well as the target-relevant human OKF6/TERT oral keratinocytes and had selectivity ratios (EC50/MIC) ranging from 54 to 452 across all three cell types, where a ratio of greater than 100 would suggest strong selectivity for antimicrobial activity compared with cytotoxicity (29). Broad activity also extended to 2-day hyphal cultures of C. albicans GDH2346 for all compounds except mPE, where IC50s were comparable to those versus yeast cultures (Table 1). Most compounds (2, 3, 4, 5, and 6) lost little to no activity in the presence of 50% human saliva. The results in Table 2 show that broad activity against NAC was evident in these compounds. Compounds 5, 6, 7, and 8 had MICs of ≤4 μg/ml against all 5 NAC species tested, and compound 4 had MICs of ≤4 μg/ml against 4 of the 5 NAC species. Lastly, screens against common Gram-positive and Gram-negative bacteria, including 2 commensal species, Streptococcus salivarius and Actinomyces viscosus, were conducted (Table 3). Compound 2 was highly active against the commensal and other bacterial strains. Compound 1 was poorly active against the commensal strains but was robustly active against most of the other bacterial species. Compounds 7 and 8 demonstrated poor activity against the commensals and the other bacterial strains (except S. aureus), but compound 4 exhibited the most robust selectivity, with little to no activity against any of the bacterial strains tested. Based on the combined results of these screens, 3 compounds were selected for further study: compounds 2, 4, and 5. While compound 4 met all screening criteria, compound 2 was not as broadly active, with more moderate activity against the NAC species, and compounds 5 was not as selective, having moderate activity against the commensal bacterial strains.
FIG 1.
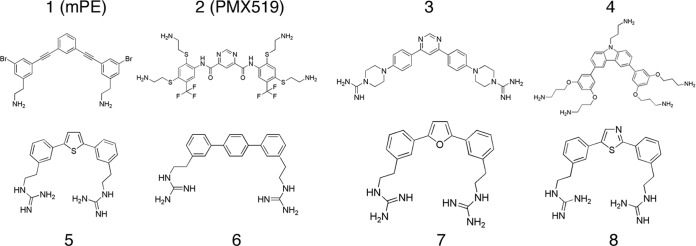
Compound structures.
TABLE 1.
In vitro activities against C. albicans and cytoxicities of selected HDP mimetics
| Compound |
C. albicans GDH2346 |
Cytotoxicity (EC50 [μg/ml], EC50/MIC) |
|||||
|---|---|---|---|---|---|---|---|
| IC50 (μg/ml) |
MIC (μg/ml) |
||||||
| Yeast cultures | Hyphal cultures | Without saliva | With saliva | NIH 3T3 cells | HepG2 cells | OKF6/TERT cells | |
| 1 (mPE) | 4.88 | 11.04 | 4–8 | 32 | 52, 6.8 | 31, 3.9 | 68, 8.5 |
| 2 (519) | 4.93 | 4.9 | 4–8 | 4–8 | 439, 55 | >1,000, >125 | >1,000, >125 |
| 3 | 4.24 | 0.71 | 4 | 4 | 311, 78 | 453, 113 | 466, 116 |
| 4 | 1.44 | 2.68 | 4 | 2 | 436, 109 | 885, 221 | 766, 192 |
| 5 | 1.09 | 1 | 2 | 4 | 108, 54 | 310, 155 | 371, 186 |
| 6 | 1.03 | 1.4 | 2 | 4 | 149, 75 | 288, 144 | 502, 251 |
| 7 | 2.2 | NDa | 2 | 16 | 461, 231 | 904, 452 | ND, ND |
| 8 | 2.08 | 2.22 | 2 | 16 | 523, 262 | 723, 362 | 718, 359 |
ND, not determined.
TABLE 3.
In vitro activities of selected HDP mimetics against Gram-positive and Gram-negative bacteria
| Compound | MIC (μg/ml) against: |
||||||
|---|---|---|---|---|---|---|---|
| E. coli 25922 | S. aureus 27660 | E. faecalis 29212 | P. aeruginosa 10145 | K. pneumoniae 13883 | S. salivarius | A. viscosus | |
| 1 (mPE) | 3.13 | 1.56 | 3.13 | 25 | 3.13 | 16 | 32 |
| 2 (519) | 1.56 | 0.39 | 0.39 | 1.56 | 1.56 | 2 | 4 |
| 3 | 50 | 0.2 | 6.25 | 50 | 50 | 16 | 4 |
| 4 | 6.25 | 25 | 25 | >50 | 12.5 | >64 | >64 |
| 5 | 12.5 | 0.78 | 6.25 | 50 | 25 | 8 | 4 |
| 6 | 12.5 | 0.78 | 12.5 | 50 | >50 | 8 | 4 |
| 7 | 50 | 1.56 | 25 | >50 | >50 | 32 | 8 |
| 8 | >50 | 3.13 | >50 | >50 | >50 | 64 | 16 |
TABLE 2.
In vitro activities of selected HDP mimetics against NAC
| Compound | MIC (μg/ml) against: |
|||||
|---|---|---|---|---|---|---|
| C. albicans GDH2346 | C. tropicalis ATCC 750 | C. parapsilosis ATCC 22019 | C. dubliniensis NCPF3949 | C. glabrata ATCC 90030 | C. krusei ATCC 6258 | |
| 1 (mPE) | 4–8 | NDa | 8 | 8 | ND | ND |
| 2 (519) | 4–8 | 4 | 8 | 8 | 16 | 8 |
| 3 | 4 | 4–8 | 4–8 | 8 | 4 | 32 |
| 4 | 4 | 2–4 | 2 | 4 | 2 | 16 |
| 5 | 2 | 0.5 | 2 | 2 | 2 | 2 |
| 6 | 2 | 0.5 | 2 | 2 | 2 | 2 |
| 7 | 2 | 0.5 | 4 | 4 | 4 | 4 |
| 8 | 2 | 0.5 | 4 | 4 | 4 | 4 |
ND, not determined.
We quantified the antifungal activities of these three compounds against clinical isolates of Candida spp. obtained from 60 HIV-positive patients. Of these 60 patients, 50 had no evidence of oral candidiasis; however, yeasts were isolated from mouths of all but 17 patients. Fifteen demonstrated C. albicans alone, 8 had C. krusei alone (based on the color of the colonies produced on chromogenic medium), and 10 had both C. albicans and C. krusei. Of the remaining 10 patients presenting with oral candidiasis, six had C. albicans, two had C. krusei, and two had both (with the caveat that identification of C. krusei was based solely on growth on chromogenic medium). The 55 clinical isolates obtained from these patients were tested for sensitivity to compounds 2, 4, and 5. All three compounds exhibited MIC values of 4 to 8 μg/ml against all isolates, with MFC values of 8 to 32 μg/ml.
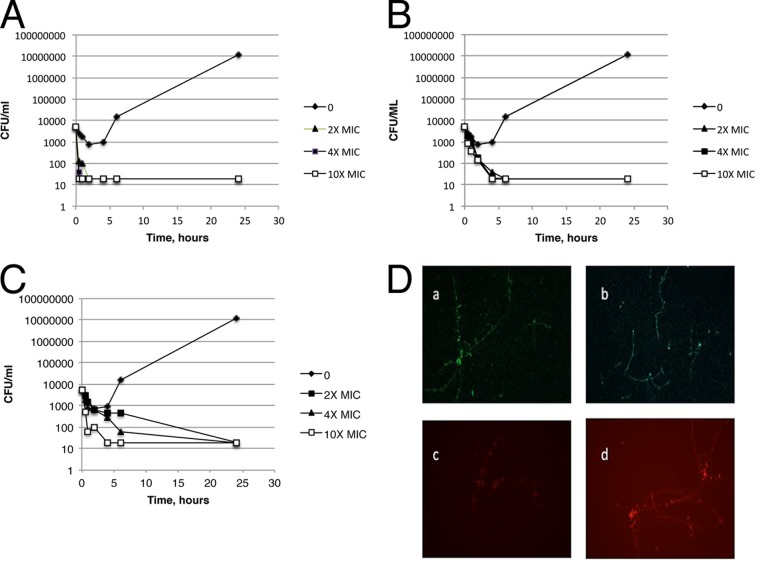
Killing kinetic studies with yeast cultures of C. albicans GDH2346 showed rapid cidal activity by all three compounds (Fig. 2A to C), with complete killing at 2× the MIC within 24 h or less. Furthermore, >2 log10 reductions in CFU/ml were evident with compounds 2 and 4 at 2× their MICs by 4 h after compound treatment, and 2 log10 reductions were found with compound 5 by 6 h at 4× its MIC. To confirm that C. albicans in the hyphal form was also killed, hyphae were treated with compound 2 at 8 μg/ml for increasing times, followed by live-dead staining and visualization by fluorescence microscopy. (Fig. 2D).
FIG 2.
Killing kinetics of HDP mimetics versus C. albicans GDH2346. (A to C) Kinetics of killing against the yeast form. Compounds were diluted in RPMI-MOPS and added to C. albicans as in IC50 assays. Samples were removed at the indicated time points, serially diluted in YPD medium, and then plated on YPD to determine viable CFU. Each line represents increasing concentrations of the drug as a multiple of the MIC. (A) Compound 2 (519); (B) compound 4; (C) Compound 5. (D) Killing of the hyphal form. C. albicans (GDH2346) was grown in 10% FCS for 3 days to achieve hyphae. Hyphae were treated with compound 2 (8 μg/ml) for 0 min (a), 15 min (b), 30 min (c), or 60 min (d). Cultures were stained with FungaLight Live-Dead stain (Invitrogen) and observed under fluorescence microscopy (magnification, ×100). Green, intact cell membrane; red, damaged membrane.
In order to examine the potential of these compounds as a treatment for oral candidiasis, an optimal delivery system was developed. A hydroxyethylcellulose gel (Natrosol) was selected due to its neutral charge and its current use in many oral applications. Compounds 2, 4, and 5 were dissolved in Natrosol and overlaid with a suspension of C. albicans GDH2346. The results shown in Fig. 3 indicate a rapid killing of Candida in the medium after exposure to the gel. A sampling of the medium applied to the gel indicated a rapid elution of the compounds into the medium (data not shown). This suggests that the observed killing is due to the interaction of the compound with the Candida in the liquid medium, rather than to a direct interaction with the gel. These results indicate that the Natrosol hydrogel represents an efficient method to deliver the antifungal compounds into the saliva for substantive treatment.
FIG 3.
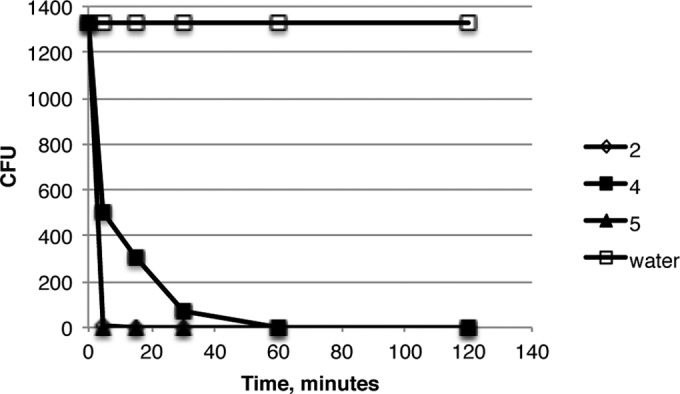
Activities of HDP mimetics in a hydrogel. Candida was incubated in water applied to the surface of hydrogels containing one of three compounds for increasing times, followed by plating for viable colonies.
The in vivo activities of the three compounds were determined in two mouse models of oral candidiasis. In the first model (Fig. 4A), an infection with C. albicans GDH2346 was established on the tongues of mice that were immunosuppressed by cortisol treatment. On day 3 postinfection, a single administration of test agent (10 mg/ml) or water in the Natrosol hydrogel (0.05-ml volume) was applied directly to the infected tongues. At 24 h posttreatment, the tongues were harvested for measurement of fungal burden. Treatments with compounds 2 and 5 resulted in 0.74 and 1.12 log10 reductions in CFU/tongue of C. albicans GDH2346 relative to that in vehicle-treated animals (P = 0.016 and 0.015, respectively), and efficacy was comparable to that achieved with nystatin (1.06 log10 reduction), a commonly used topical antifungal. Compound 4 had a much stronger effect, showing a 2.32 log10 reduction in fungal burden relative to that in vehicle-treated animals (P = 0.023), and the efficacy with compound 4 was statistically more significant (P < 0.05) than the effects observed with compound 2 (P = 0.004) or nystatin (P = 0.03) but not significantly different from that with compound 5.
FIG 4.
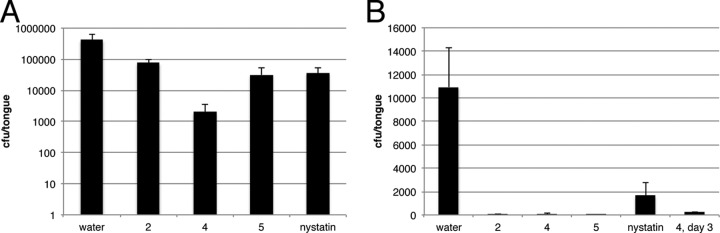
In vivo activities of HDP mimetics in mouse models of oral candidiasis. (A) Immunosuppressed mouse model. Mice (n = 5) were immunosuppressed with cortisol acetate, followed by oral infection with C. albicans GDH2346. (B) Steroid-free mouse model. Mice (n = 5) were infected with C. albicans GDH2346 after scoring the tongues as described in Materials and Methods. In both cases, the infection was allowed to proceed for 3 days, followed by a single treatment of 50 μl of each compound in the hydrogel. After 24 h, the mice were sacrificed and the tongues were homogenized. The homogenates were diluted and plated for viable colonies. Kidneys revealed no colonies of Candida albicans with or without treatment. The data are representative of two (A) or three (B) independent experiments. For panel B, a separate group of mice were treated with compound 4 and the tongues were harvested after 72 h (labeled 4, day 3).
To examine the effect of the treatment on mice that were not immunosuppressed by cortisol, we used a modified mouse model of oral candidiasis in which colonization of the tongue is enhanced by scratching the surface prior to infection (28). The infection and treatment regimens were identical to those described above for the cortisol-injected model except that the Candida inoculation was increased to 5 × 107 CFU/ml and the host mouse strain is an mBD-1-KO strain that lacks the mouse β-defensin-1 gene (27). As expected, the overall infection burden was lower in this model, likely due to a more active immune response. Nevertheless, robust effects were clearly evident with all 3 HDP mimetic compounds, and the reductions in Candida burden were significant over that in vehicle-treated animals, showing 2.30, 2.17, and 3.42 log10 reductions for compounds 2, 4, and 5, respectively (P = 0.03, P = 0.001, and P = 0.001) (Fig. 4B). No significant difference was observed between the compounds. A second group of infected mice were treated with compound 4 and harvested after 72 h. The results showed little regrowth of the Candida in these mice.
DISCUSSION
Numerous studies have suggested that naturally occurring host defense peptides (30–34) and synthetic derivatives of these peptides (35–37) could be useful drugs to treat fungal infections, including those caused by Candida pathogens. Since Candida spp. have demonstrated an innate immune evasion strategy of proteolytically cleaving host defense peptides (38), utilizing nonpeptidic HDP mimetics as therapeutic agents would circumvent this evasion strategy.
We have developed an extensive library of fully synthetic, nonpeptidic mimetics of the host defense proteins. Several chemical scaffolds have been utilized while maintaining common features of the mimetics, including cationic charges of various type and charge density, hydrophobic side groups or backbones, and an amphiphilic structure stabilized by internal hydrogen bonding and steric or ring constraints. The compounds are mostly symmetrical and typically have molecular masses in the range of 600 to 1,200 Da. We initially screened for compounds with potent antifungal activity (IC50s of less than 5 μM and MICs of below 8 μg/ml) which is comparable to that of standard antifungal agents (39). Subsequent screening addressing endpoints important for an oral candidiasis indication identified six compounds, in addition to one previously characterized anti-Candida compound (compound 2), that were potently active against numerous strains of C. albicans and NAC species and showed >50-fold selectivity over 3 mammalian cell types (18). Human saliva had little impact on their activity, and all of the selected compounds were highly active against hyphal cultures. Importantly, several compounds had little to no activity against commensal bacteria, thereby minimizing the potential for fungal overgrowth when treating oral candidiasis.
It is very interesting that we have been able to identify HDP mimetic compounds that are active specifically for Candida over bacteria and mammalian cell types. Studies on the mechanism of action have shown that the antibacterial HDP mimetic compounds disrupt the bacterial cell membrane (14), and we have observed a similar effect on Candida (unpublished data). Compounds that inhibit anti-Candida activity but lack significant antibacterial activity can provide important tools for investigating differences in membrane structure and susceptibility to membrane-disrupting agents. Initial structure-activity relationships implicate charge type, charge density, and hydrophobic/charge balance as important factors in the specificity, but considerable work is needed to define the structural features required for potent and selective anti-Candida activity.
Three compounds that met all (compound 4) or most (compounds 2 and 5) screening criteria were selected for further studies. One important feature of these compounds that distinguishes them from other antifungal agents (40, 41) is their rapid fungicidal activity, where >2 log10 reductions in viable C. albicans GDH2346 were obtained at 2× MICs within 4 h of treatment with compound 2 or 4. This rapid killing activity may enable shorter and more effective treatment regimens in the clinic. While we did not test the kinetics against other strains, our previously published kinetics studies with independent strains (18) and our results here that demonstrate similar MIC values against 55 clinical isolates suggest that the kinetics will be similar.
To investigate activity in vivo, we decided to use a delivery system involving a neutral hydroxyethylcellulose gel (Natrosol) that provided a straightforward, single-component system. The hydrogel mixture was relatively easy to manipulate at room temperature and at 37°C, and it coated the tissue well, allowing the drug good tissue access. While compound 4 demonstrated slightly slower release kinetics, it appears to be sufficient for successful activity in vivo. Future studies to develop these compounds as therapeutic agents will involve a more comprehensive analysis of delivery systems that will be optimized for release and delivery.
Numerous mouse models exist for oral candidiasis (42, 43). The vast majority of these rely on immunosuppression, usually with a steroid. Since these models use a continuous treatment with the steroid, we felt it was important to also demonstrate the activity of the compounds using a second model without the potential interference of exogenous agents. The modification of this published model substituted mice which had an mBD-1 deletion, which in our hands provided a more consistent Candida infection that occurred sooner after inoculation (data not shown). The initial description of this mouse strain reported no change in the differential count of white blood cells compared with that of wild-type mice (27). Our results clearly demonstrate that a single topical treatment with either of three antifungal peptide mimetics in mice with oral candidiasis led to a significant ablation of the infection, in either the presence or absence of an immunosuppressive agent.
While our data in Fig. 3 clearly demonstrate a rapid elution from the gel and killing of Candida in vitro, for this proof-of-concept study we kept the mice sedated for 2 h after delivery of the drug. Our results suggest that not only do these compounds act in vivo, but they may be effective with much shorter applications, such as would be found with a lozenge or other slow-release delivery system. Future experiments will determine the minimal time and doses necessary for optimal efficacy.
Interestingly, the overall responses to nystatin were similar in both models and compared similarly to the reduction observed in other recent studies using oral administration of nystatin in mouse models (44, 45); however, the HDP mimetic response appeared to be greater in the steroid-free model. This increased response could be due to improved activity of the mimetics at lower tissue burdens, not evident with nystatin, or to potential activation of the immune system by the HDP mimetic that helped clear the infection. Immune modulatory effects have been reported for a variety of host defense proteins (reviewed in reference 12), and similar activities have also been reported for other HDP mimetics (18, 46, 47). The potential influence of these anti-Candida HDP mimetics on immune function is an area of active interest.
These studies have shown the value of HDP mimetics as potential antifungal agents for the treatment of oral candidiasis. Compound 4 is a particularly promising compound that possesses numerous positive attributes for an oral candidiasis indication: potent and selective activity against C. albicans and NAC species, comparable activity in the presence or absence of human saliva, activity against hyphal cultures, rapid fungicidal activity, and robust efficacy in two mouse models of oral candidiasis. Fungal infections are an area of immense medical need, and the HDP mimetics offer a promising opportunity for the identification of new and effective agents for treatment of these difficult infections.
ACKNOWLEDGMENTS
This work was supported by Public Health Service grant 2R44 DE018371 to G.D. and R.W.S.
We thank Aviva Azar, Jamie (Ju-Ah) Chung, Stephen Kondorossy, and Max Wasserman for technical assistance. We also thank Meagan Corrigan for bacterial MIC assays and Michael J. Costanzo, Haizhong Tang, and Yongjiang Xu for compound synthesis. We also thank Isaac Rodriguez-Chavez, Director, NIDCR AIDS and Immunosuppression Program, for his scientific input to this paper.
Footnotes
Published ahead of print 21 April 2014
REFERENCES
- 1.Altarawneh S, Bencharit S, Mendoza L, Curran A, Barrow D, Barros S, Preisser J, Loewy ZG, Gendreau L, Offenbacher S. 2013. Clinical and histological findings of denture stomatitis as related to intraoral colonization patterns of Candida albicans, salivary flow, and dry mouth. J. Prosthodont. 22:13–22. 10.1111/j.1532-849X.2012.00906.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Akpan A, Morgan R. 2002. Oral candidiasis. Postgrad. Med. J. 78:455–459. 10.1136/pmj.78.922.455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rodu B, Carpenter JT, Jones MR. 1988. The pathogenesis and clinical significance of cytologically detectable oral Candida in acute leukemia. Cancer 62:2042–2046. [DOI] [PubMed] [Google Scholar]
- 4.Dupont B, Graybill JR, Armstrong D, Laroche R, Touze JE, Wheat LJ. 1992. Fungal infections in AIDS patients. J. Med. Vet. Mycol. 30(Suppl 1):19–28. 10.1080/02681219280000731 [DOI] [PubMed] [Google Scholar]
- 5.Dongari-Bagtzoglou A, Fidel PL., Jr 2005. The host cytokine responses and protective immunity in oropharyngeal candidiasis. J. Dent. Res. 84:966–977. 10.1177/154405910508401101 [DOI] [PubMed] [Google Scholar]
- 6.Gupta KL, Ghosh AK, Kochhar R, Jha V, Chakrabarti A, Sakhuja V. 1994. Esophageal candidiasis after renal transplantation: comparative study in patients on different immunosuppressive protocols. Am. J. Gastroenterol. 89:1062–1065 [PubMed] [Google Scholar]
- 7.Walsh TJ, Gonzalez CE, Piscitelli S, Bacher JD, Peter J, Torres R, Shetti D, Katsov V, Kligys K, Lyman CA. 2000. Correlation between in vitro and in vivo antifungal activities in experimental fluconazole-resistant oropharyngeal and esophageal candidiasis. J. Clin. Microbiol. 38:2369–2373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sheft DJ, Shrago G. 1970. Esophageal moniliasis. The spectrum of the disease. JAMA 213:1859–1862 [DOI] [PubMed] [Google Scholar]
- 9.Thom K, Forrest G. 2006. Gastrointestinal infections in immunocompromised hosts. Curr. Opin. Gastroenterol. 22:18–23 [DOI] [PubMed] [Google Scholar]
- 10.Lortholary O, Petrikkos G, Akova M, Arendrup MC, Arikan-Akdagli S, Bassetti M, Bille J, Calandra T, Castagnola E, Cornely OA, Cuenca-Estrella M, Donnelly JP, Garbino J, Groll AH, Herbrecht R, Hope WW, Jensen HE, Kullberg BJ, Lass-Florl C, Meersseman W, Richardson MD, Roilides E, Verweij PE, Viscoli C, Ullmann AJ, ESMID Fungal Infection Study Group 2012. ESCMID* guideline for the diagnosis and management of Candida diseases 2012: patients with HIV infection or AIDS. Clin. Microbiol. Infect. 18(Suppl 7):68–77. 10.1111/1469-0691.12042 [DOI] [PubMed] [Google Scholar]
- 11.Ghannoum MA, Rice LB. 1999. Antifungal agents: mode of action, mechanisms of resistance, and correlation of these mechanisms with bacterial resistance. Clin. Microbiol. Rev. 12:501–517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Diamond G, Beckloff N, Weinberg A, Kisich KO. 2009. The roles of antimicrobial peptides in innate host defense. Curr. Pharm. Des. 15:2377–2392. 10.2174/138161209788682325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Strom MB, Haug BE, Skar ML, Stensen W, Stiberg T, Svendsen JS. 2003. The pharmacophore of short cationic antibacterial peptides. J. Med. Chem. 46:1567–1570. 10.1021/jm0340039 [DOI] [PubMed] [Google Scholar]
- 14.Mensa B, Kim YH, Choi S, Scott R, Caputo GA, DeGrado WF. 2011. Antibacterial mechanism of action of arylamide foldamers. Antimicrob. Agents Chemother. 55:5043–5053. 10.1128/AAC.05009-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tew GN, Scott RW, Klein ML, DeGrado WF. 2010. De novo design of antimicrobial polymers, foldamers and small molecules: from discovery to practical applications. Acc. Chem. Res. 43:30–39. 10.1021/ar900036b [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Choi S, Isaacs A, Clements D, Liu D, Kim H, Scott RW, Winkler JD, DeGrado WF. 2009. De novo design and in vivo activity of conformationally restrained antimicrobial arylamide foldamers. Proc. Natl. Acad. Sci. U. S. A. 106:6968–6973. 10.1073/pnas.0811818106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jorgensen D, Scott RW, Korczak B. 2012. A phase 2 randomized double-blinded dose-ranging active controlled efficacy and safety evaluation of PMX-30063 for the treatment of acute skin and skin structure infections (ABSSSI) caused by Staphylococcus aureus (SA). Abstr. 52nd Intersci. Conf. Antimicrob. Agents Chemother, p L1–1662 [Google Scholar]
- 18.Hua J, Yamarthy R, Felsenstein S, Scott RW, Markowitz K, Diamond G. 2010. Activity of antimicrobial peptide mimetics in the oral cavity. I. Activity against biofilms of Candida albicans. Mol. Oral Microbiol. 25:418–425. 10.1111/j.2041-1014.2010.00590.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Beckloff N, Laube D, Castro T, Furgang D, Park S, Perlin D, Clements D, Tang H, Scott RW, Tew GN, Diamond G. 2007. Activity of an antimicrobial peptide mimetic against planktonic and biofilm cultures of oral pathogens. Antimicrob. Agents Chemother. 51:4125–4132. 10.1128/AAC.00208-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.National Committee for Clinical Laboratory Standards. 2002. Reference method for broth dilution antifungal susceptibility testing of yeasts; approved standard. National Committee for Clinical Laboratory Standards, Wayne, PA [Google Scholar]
- 21.Chambers RS, Sullivan PA. 1993. Expression of the exoglucanase gene in yeast and hyphal forms of Candida albicans. FEMS Microbiol. Lett. 111:63–67. 10.1111/j.1574-6968.1993.tb06362.x [DOI] [PubMed] [Google Scholar]
- 22.Dowell SJ, Brown AJ. 2009. Yeast assays for G protein-coupled receptors. Methods Mol. Biol. 552:213–229. 10.1007/978-1-60327-317-6_15 [DOI] [PubMed] [Google Scholar]
- 23.Kuhn DM, Balkis M, Chandra J, Mukherjee PK, Ghannoum MA. 2003. Uses and limitations of the XTT assay in studies of Candida growth and metabolism. J. Clin. Microbiol. 41:506–508. 10.1128/JCM.41.1.506-508.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Espinel-Ingroff A, Canton E. 2007. Antifungal susceptibility testing of filamentous fungi, p 209–242 In Schwalbe R, Steele-Moore L, Goodwin AC. (ed), Antimicrobial susceptibility testing protocols. CRC Press, Boca Raton, FL [Google Scholar]
- 25.McMahon L, Schwartz K, Yilmaz O, Brown E, Ryan LK, Diamond G. 2011. Vitamin D-mediated induction of innate immunity in gingival epithelial cells. Infect. Immun. 79:2250–2256. 10.1128/IAI.00099-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Solis NV, Filler SG. 2012. Mouse model of oropharyngeal candidiasis. Nat. Protoc. 7:637–642. 10.1038/nprot.2012.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Moser C, Weiner DJ, Lysenko E, Bals R, Weiser JN, Wilson JM. 2002. beta-Defensin 1 contributes to pulmonary innate immunity in mice. Infect. Immun. 70:3068–3072. 10.1128/IAI.70.6.3068-3072.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hise AG, Tomalka J, Ganesan S, Patel K, Hall BA, Brown GD, Fitzgerald KA. 2009. An essential role for the NLRP3 inflammasome in host defense against the human fungal pathogen Candida albicans. Cell Host Microbe 5:487–497. 10.1016/j.chom.2009.05.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Avery CW, Som A, Xu Y, Tew GN, Chen Z. 2009. Dependence of antimicrobial selectivity and potency on oligomer structure investigated using substrate supported lipid bilayers and sum frequency generation vibrational spectroscopy. Anal. Chem. 81:8365–8372. 10.1021/ac901271f [DOI] [PubMed] [Google Scholar]
- 30.Benincasa M, Scocchi M, Pacor S, Tossi A, Nobili D, Basaglia G, Busetti M, Gennaro R. 2006. Fungicidal activity of five cathelicidin peptides against clinically isolated yeasts. J. Antimicrob. Chemother. 58:950–959. 10.1093/jac/dkl382 [DOI] [PubMed] [Google Scholar]
- 31.Oppenheim FG, Xu T, McMillian FM, Levitz SM, Diamond RD, Offner GD, Troxler RF. 1988. Histatins, a novel family of histidine-rich proteins in human parotid secretion. Isolation, characterization, primary structure, and fungistatic effects on Candida albicans. J. Biol. Chem. 263:7472–7477 [PubMed] [Google Scholar]
- 32.Vylkova S, Jang WS, Li W, Nayyar N, Edgerton M. 2007. Histatin 5 initiates osmotic stress response in Candida albicans via activation of the Hog1 mitogen-activated protein kinase pathway. Eukaryot. Cell 6:1876–1888. 10.1128/EC.00039-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wakabayashi H, Abe S, Okutomi T, Tansho S, Kawase K, Yamaguchi H. 1996. Cooperative anti-Candida effects of lactoferrin or its peptides in combination with azole antifungal agents. Microbiol. Immunol. 40:821–825. 10.1111/j.1348-0421.1996.tb01147.x [DOI] [PubMed] [Google Scholar]
- 34.Zasloff M. 1987. Magainins, a class of antimicrobial peptides from Xenopus skin: isolation, characterization of two active forms, and partial cDNA sequence of a precursor. Proc. Natl. Acad. Sci. U. S. A. 84:5449–5453. 10.1073/pnas.84.15.5449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Burrows LL, Stark M, Chan C, Glukhov E, Sinnadurai S, Deber CM. 2006. Activity of novel non-amphipathic cationic antimicrobial peptides against Candida species. J. Antimicrob. Chemother. 57:899–907. 10.1093/jac/dkl056 [DOI] [PubMed] [Google Scholar]
- 36.Nikawa H, Fukushima H, Makihira S, Hamada T, Samaranayake LP. 2004. Fungicidal effect of three new synthetic cationic peptides against Candida albicans. Oral Dis. 10:221–228. 10.1111/j.1601-0825.2004.01010.x [DOI] [PubMed] [Google Scholar]
- 37.Takakura N, Wakabayashi H, Ishibashi H, Teraguchi S, Tamura Y, Yamaguchi H, Abe S. 2003. Oral lactoferrin treatment of experimental oral candidiasis in mice. Antimicrob. Agents Chemother. 47:2619–2623. 10.1128/AAC.47.8.2619-2623.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Meiller TF, Hube B, Schild L, Shirtliff ME, Scheper MA, Winkler R, Ton A, Jabra-Rizk MA. 2009. A novel immune evasion strategy of candida albicans: proteolytic cleavage of a salivary antimicrobial peptide. PLoS One 4:e5039. 10.1371/journal.pone.0005039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Espinel-Ingroff A, Pfaller MA, Bustamante B, Canton E, Fothergill A, Fuller J, Gonzalez GM, Lass-Florl C, Lockhart SR, Martin-Mazuelos E, Meis JF, Melhem MS, Ostrosky-Zeichner L, Pelaez T, Szeszs MW, St-Germain G, Bonfietti LX, Guarro J, Turnidge J. 2014. Multilaboratory study of epidemiological cutoff values for detection of resistance in eight Candida species to fluconazole, posaconazole, and voriconazole. Antimicrob. Agents Chemother. 58:2006–2012. 10.1128/AAC.02615-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Canton E, Peman J, Gobernado M, Viudes A, Espinel-Ingroff A. 2004. Patterns of amphotericin B killing kinetics against seven Candida species. Antimicrob. Agents Chemother. 48:2477–2482. 10.1128/AAC.48.7.2477-2482.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Venisse N, Gregoire N, Marliat M, Couet W. 2008. Mechanism-based pharmacokinetic-pharmacodynamic models of in vitro fungistatic and fungicidal effects against Candida albicans. Antimicrob. Agents Chemother. 52:937–943. 10.1128/AAC.01030-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Costa AC, Pereira CA, Junqueira JC, Jorge AO. 2013. Recent mouse and rat methods for the study of experimental oral candidiasis. Virulence 4:391–399. 10.4161/viru.25199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Samaranayake YH, Samaranayake LP. 2001. Experimental oral candidiasis in animal models. Clin. Microbiol. Rev. 14:398–429. 10.1128/CMR.14.2.398-429.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Matsubara VH, Silva EG, Paula CR, Ishikawa KH, Nakamae AE. 2012. Treatment with probiotics in experimental oral colonization by Candida albicans in murine model (DBA/2). Oral Dis. 18:260–264. 10.1111/j.1601-0825.2011.01868.x [DOI] [PubMed] [Google Scholar]
- 45.Wong SS, Kao RY, Yuen KY, Wang Y, Yang D, Samaranayake LP, Seneviratne CJ. 2014. In vitro and in vivo activity of a novel antifungal small molecule against Candida infections. PLoS One 9:e85836. 10.1371/journal.pone.0085836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Som A, Navasa N, Percher A, Scott RW, Tew GN, Anguita J. 2012. Identification of synthetic host defense peptide mimics that exert dual antimicrobial and anti-inflammatory activities. Clin. Vaccine Immunol. 19:1784–1791. 10.1128/CVI.00291-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Thaker HD, Som A, Ayaz F, Lui D, Pan W, Scott RW, Anguita J, Tew GN. 2012. Synthetic mimics of antimicrobial peptides with immunomodulatory responses. J. Am. Chem. Soc. 134:11088–11091. 10.1021/ja303304j [DOI] [PMC free article] [PubMed] [Google Scholar]