Abstract
Tyrocidines are cationic cyclodecapeptides from Bacillus aneurinolyticus that are characterized by potent antibacterial and antimalarial activities. In this study, we show that various tyrocidines have significant activity against planktonic Candida albicans in the low-micromolar range. These tyrocidines also prevented C. albicans biofilm formation in vitro. Studies with the membrane-impermeable dye propidium iodide showed that the tyrocidines disrupt the membrane integrity of mature C. albicans biofilm cells. This membrane activity correlated with the permeabilization and rapid lysis of model fungal membranes containing phosphatidylcholine and ergosterol (70:30 ratio) induced by the tyrocidines. The tyrocidines exhibited pronounced synergistic biofilm-eradicating activity in combination with two key antifungal drugs, amphotericin B and caspofungin. Using a Caenorhabditis elegans infection model, we found that tyrocidine A potentiated the activity of caspofungin. Therefore, tyrocidines are promising candidates for further research as antifungal drugs and as agents for combinatorial treatment.
INTRODUCTION
In recent years, a rise in the frequency and diversity of human fungal infections has been observed (1–3), with growing resistance against conventional antifungal drugs also noted (1, 4–6). Factors that are responsible for the development of fungal resistance include the widespread use of broad-spectrum antibiotics and an increase in the number of immunocompromised individuals. This increase in the number of immunocompromised individuals results, for example, from immunosuppression treatment of patients during transplant surgery, chemotherapy, or radiotherapy and the HIV epidemic correlating to AIDS in HIV-infected individuals (1, 5, 7). Candida species are major causative agents of nosocomial infections in compromised individuals (8, 9). Candida albicans, which predominantly forms biofilms on biotic surfaces (such as vaginal and oral mucosa [10]) and abiotic surfaces (such as indwelling catheters [11]), is one of the most commonly isolated species from patients with nosocomial infections (5, 8, 9, 12). Biofilms are microbial communities embedded in a polymeric matrix. Biofilm formation on biological and/or inert surfaces is usually the culprit behind the instigation of candidiasis, i.e., systemic infection with Candida (8, 13, 14). Furthermore, C. albicans biofilms have been shown to be particularly resistant to antifungal agents commonly used to control fungal infections, including amphotericin B and fluconazole (8, 15–17).
Amphotericin B (AMB) and caspofungin (CAS) are currently two key drugs used for the treatment of fungal infections (18, 19). AMB acts through the binding of its hydrophobic moiety to the fungal sterol ergosterol, resulting in the formation of transmembrane channels and cytoplasmic leakage (18, 20, 21). However, the severe side effects of AMB, such as nausea, vomiting, rigors, fever, and nephrotoxicity, in some cases necessitates premature termination of AMB treatment (18). Furthermore, it is being observed that more and more C. albicans isolates are resistant to AMB treatment (22–24). CAS, a semisynthetic lipopeptide from the echinocandin family, inhibits the synthesis of the major structural component in fungal cell walls, 1,3-β-d-glucan (1, 14, 25). Although CAS has a very good track record regarding side effects, with limited development of microbial resistance, resistance against CAS has been observed (1, 25). Strains of C. albicans with mutations in their (1, 3)-β-d-glucan synthase show resistance against caspofungin in vitro (1). The rise in fungal pathogens that are resistant to conventional treatments has driven the search for novel antifungal compounds and treatments that simultaneously exhibit specificity toward fungal cells and have a low potential for inducing pathogen resistance. Combination drug treatment is a possible solution for curbing the development of resistance against an individual compound. Combining antifungal compounds that act synergistically against a pathogen(s) allows for lower drug dosage, with a concurrent decrease in toxicity (9).
Antimicrobial peptides (AMPs) are the first line of immune defense in most organisms (26, 27) and in recent years have been considered potential alternatives/supplements to traditional antifungal compounds (9). Tyrocidines are a group of cyclic decapeptides that together with the linear gramicidins form the secondary metabolite peptide complex, tyrothricin, produced by Bacillus aneurinolyticus (previously known as Bacillus brevis) (28, 29). The tyrothricin complex was one of the first antibiotic preparations to be used as a topical antibiotic (30) (under the trade names Limex and Tyrosur) and has been illustrated in several studies to have significant antibacterial (28, 31) and antimalarial (32) activities. The primary structures of 28 tyrocidines and their analogues have been determined and illustrated to have a highly conserved sequence (29). The tyrocidines relevant to this study share the pentapeptide repeat of gramicidin S, Val-Orn-Leu-Pro-d-Phe (Table 1). So far, only tyrothricin (the tyrocidine-gramicidin metabolite complex of B. aneurinolyticus) has been observed to have activity against C. albicans (33).
TABLE 1.
Summary of the tyrocidines and analogues used in this study
| Peptide | Abbreviation | Sequenceb | Monoisotopic Mr (theoretical Mr)c |
|---|---|---|---|
| Tyrocidine A | TrcA | cyclo-(VOLfPFfNQY) | 1,269.6515 (1,269.6546) |
| Tyrocidine B | TrcB | cyclo-(VOLfPWfNQY) | 1,308.6610 (1,308.6655) |
| Tyrocidine C | TrcC | cyclo-(VOLfPWwNQY) | 1,347.6713 (1,347.6764) |
| Phenycidine Aa | PhcA | cyclo-(VOLfPFfNQF) | 1,253.6625 (1,253.6597) |
| Tryptocidine C | TpcC | cyclo-(VOLfPWwNQW) | 1,370.6866 (1,370.6924) |
| Gramicidin S | GS | cyclo-(VOLfPVOLfP) | 1,140.7067 (1,140.7059) |
Named by Vosloo et al. (34).
Conventional one-letter abbreviations are used for amino acids, except that O was used for ornithine. Lowercase one-letter abbreviations indicate d-amino acid residues. The sequence data obtained from Tang et al. (29) and the identities were confirmed by our group (34, 35).
Detected monoisotopic Mr of peptides determined from purified peptides using high-resolution time of flight-electrospray mass spectrometry (TOF-ESMS) (refer to Table S1 in the supplemental material).
To our knowledge, this is the first investigation on the activities of the tyrocidines against planktonic and biofilm C. albicans cells. We report the antiyeast, antibiofilm, and membrane activities of the three major tyrocidines (tyrocidine A, B, and C) against C. albicans. With a combinatorial treatment in mind, the tyrocidines were also evaluated for a potential enhancing effect on the biofilm-eradicating activity of AMB and CAS in vitro. A Caenorhabditis elegans infection model was used to assess the in vivo efficacy and toxicity of tyrocidine A in combination with CAS.
MATERIALS AND METHODS
Strains and media.
The pathogenic C. albicans strain SC5314, which is known to form robust biofilms, was used as the target strain for this study. Overnight cultures were grown in YPD (1% yeast extract, 2% peptone, and 2% glucose) or on YPD agar (1% yeast extract, 2% peptone, 2% glucose, and 1.5% agar). Phosphate-buffered saline (PBS) (pH 7.4) was prepared with NaCl (8 g/liter), KCl (0.2 g/liter), Na2HPO4 (1.44 g/liter), and KH2PO4 (0.24 g/liter). C. albicans was grown in RPMI 1640 medium with l-glutamine and without bicarbonate (pH 7.0) purchased from Sigma-Aldrich (St. Louis, MO, USA).
Peptides.
The tyrocidines were purified and analyzed for purity as described previously. De novo sequencing using electrospray mass spectrometry by Vosloo et al. (34) and Spathelf (35) confirmed the identities of the tyrocidines and analogues. All the tyrocidines (TrcA, TrcB, TrcC, TpcC, and PhcA) used in this study had purities of >90% according to ultraperformance liquid chromatography linked to high-resolution electrospray mass spectrometry (see Table S1 in the supplemental material). Gramicidin S (GS) (at 97.5% purity according to the manufacturer and 94% purity according to ultraperformance liquid chromatography-mass spectrometry [UPLC-MS]) (see Table S1 in the supplemental material) was supplied by Sigma (St. Louis, MO, USA).
Antiplanktonic yeast assays.
The antiyeast activity of the Trc mixture (a group of tyrocidines isolated from commercial tyrothricin), selected tyrocidines, tyrocidine analogues, and gramicidin S (GS) was determined using the standard protocol from CLSI document M27-A3 (36).
Biofilm prevention activity assays.
The ability of the tyrocidines and analogous peptides to prevent the formation of C. albicans biofilms was determined using microdilution assays in 96-well microtiter plates (37). C. albicans cells in RPMI 1640 medium (1 × 106 cells/ml) were incubated with 0.78 to 100.0 μM tyrocidines (final ethanol concentration, 1%) for 24 h at 37°C. The control treatment was 1% ethanol in RPMI 1640 medium. The biofilms were washed with PBS, and biofilm formation was determined using CellTiter-Blue (Promega, Madison, WI) staining (38, 39). After 1-h incubation at 37°C, fluorescence was measured with a multimode microplate reader, Synergy Mx (BioTek), at an excitation wavelength of 535 nm and emission wavelength of 590 nm.
In vitro biofilm eradication assay.
C. albicans biofilms were grown in 96-well microtiter plates in RPMI 1640 medium at 37°C (37). Subsequent to a PBS wash step, the biofilms were incubated with tyrocidine (concentration range, 1.6 to 200.0 μM) in RPMI 1640 medium (final ethanol concentration, 1%) for 24 h at 37°C. One percent ethanol in RPMI 1640 medium served as the control treatment. The combined eradication activity (i.e., loss of metabolic activity/viability of cells and/or removal of cells from biofilm) of the tyrocidines and CAS/AMB was determined by incubating 24-h-old biofilms with 0.04 to 5.0 μM either AMB (Sigma-Aldrich, St. Louis, MO, USA) (0.036 to 4.6 μg/ml) or CAS (Merck, United Kingdom) (0.049 to 6.1 μg/ml), in the absence or presence of either 1.8, 3.0, or 6.2 μM TrcA, TrcB, or TrcC (final ethanol concentration, 0.05%; final dimethyl sulfoxide [DMSO] concentration, 0.05%), for 24 h at 37°C. Biofilm survival was measured using CellTiter-Blue assays, and the BEC50 was defined as the concentration that resulted in 50% eradication of 24-h-old C. albicans biofilms compared to the growth control. The combinatorial activities of AMB/CAS and the tyrocidines were assessed by statistically comparing the BEC50 values of AMB/CAS alone and in combination with tyrocidines using Bonferroni's multiple-comparison test, as well as by determining the fractional inhibitory concentration index (FICI) (40). The FICI was calculated using the equation FICI = (CA+B/CA) + (CB+A/CB), where CA and CB are the BEC50s of the antifungal compounds alone, and CA+B and CB+A are the BEC50s of the antifungal compounds in combination. A FICI of ≤0.5 was interpreted as synergistic, a FICI between 0.5 and 4.0 as indifferent, and a FICI of ≥4.0 as antagonistic (40).
Membrane permeability assays.
The membrane-disrupting activities of the tyrocidines on 24-h-old C. albicans biofilms were determined using the membrane-impermeable dye propidium iodide from Sigma-Aldrich (St. Louis, MO, USA). Day-old (24-h) C. albicans biofilms were prepared as described for the biofilm eradication experiments. Subsequent to either a 1-h treatment or 24-h treatment with tyrocidines (0.78 to 100.0 μM) at 37°C, the biofilms were washed with PBS and incubated with 1.5% propidium iodide in the dark for a further 20 min. Propidium iodide fluorescence was measured as described by Bink et al. (40), and the fluorescence values of the samples were corrected with values obtained for untreated biofilms. Triton X-100 (1%) served as a lytic control.
Peptide-induced calcein release from model large unilamellar vesicles (LUVs) resembling fungal membranes were prepared as described previously (41, 42). The LUVs contained 80 mM calcein in 10 mM Tris buffer containing 0.1 mM EDTA (pH 7.4) and consisted of a 70:30 ratio (mol/mol) of 1-palmitoyl-2-oleoyl phosphatidylcholine (POPC) to ergosterol (POPC was from Avanti Polar Lipids, Alabaster, AL, USA, and ergosterol was from Sigma, St. Louis, MO, USA). The LUV suspension (25 μM final concentration) was added to different concentrations of peptide (ranging from 0.3 μM to 100 μM) in buffer (10 mM Tris, 0.1 mM EDTA, 154 mM NaCl [pH 7.4]). The decrease in self-quenching as a function of time was fluorometrically monitored on a Jasco FP-6500 spectrofluorometer (excitation, 490 nm; emission, 520 nm) at 25°C. One hundred percent leakage was induced by adding 100 μl of a 10% (vol/vol) Triton X-100 solution. The percent leakage [F(%)] after 1 min was calculated by the following formula 100(%) (I − I0)/(I100% − I0), where I is the intensity observed in the peptide solution, and I0 and I100% are the fluorescence intensities measured in the absence of peptide and in the presence of Triton X-100, respectively (41).
Assay for determination of endogenous ROS.
The induction of endogenous radical oxygen species (ROS) was determined using the fluorescent dye 2′,7′-dichlorodihydrofluorescein diacetate (H2DCFDA) (Molecular Probes, Eugene, OR, USA). C. albicans biofilms were grown in 96-well microtiter plates in RPMI 1640 medium for 24 h and treated for 24 h with 0.78 to 100.0 μM tyrocidine. The biofilms were washed with PBS and incubated for 1 h with 10.0 μM H2DCFDA and shaking at 37°C. Fluorescence was measured with a multimode microplate reader, Synergy Mx (BioTek) at an excitation wavelength of 492 nm and emission wavelength of 525 nm (7). The relationship between endogenous ROS generation in C. albicans and tyrocidine antifungal activity was investigated using the antioxidant ascorbic acid. The influence of 10.0 mM ascorbic acid on the biofilm eradication and ROS induction activity of 100.0 μM TrcA, TrcB, and TrcC was determined (43, 44). Citric acid served as a pH control.
In vivo studies using a C. elegans model system.
The toxicity of TrcA and its combined effect on the activity of CAS in vivo were evaluated using the C. elegans model system described by Breger et al. (45). The efficacy of CAS alone or in combination with TrcA to increase the survival of C. albicans-infected nematodes were evaluated over a period of 7 days. The temperature-sensitive C. elegans Δglp-4 Δsek-1 mutants were stored on Escherichia coli strain OP50-covered NGM (0.25% Bacto peptone, 0.3% NaCl, 1.7% agar) plates at 16°C. Prior to infection, the nematodes were collected and treated with bleach in order to collect the eggs, which were then incubated at 25°C so that all the nematodes reached the same growth phase (L3 to L4) 4 days later for C. albicans infection and treatment studies. The nematodes were infected for 2 h with C. albicans at 25°C and subsequent to transfer to pathogen-free medium were treated with 0.095 μM (0.12 μg/ml) CAS, 3.0 μM TrcA, and 0.095 μM CAS in combination with 3.0 μM TrcA, or a combination of 0.5% ethanol and 0.5% DMSO as a control. For the toxicity test, the nematodes were treated with 3.0 and 6.0 μM TrcA, and nematode survival was monitored for 7 days. The control group received 0.5% ethanol. For both experiments, survival was calculated relative to the survival on day 0. An uninfected negative control was also included to monitor nematode life span and survival under the experimental conditions. After 5 days, 95% of the uninfected worms survived, showing the nonlethal effects of the experimental conditions.
Data analysis.
The minimum inhibitory concentration (MIC) of planktonic cells and minimum biofilm prevention concentration (BIC) were defined as the lowest concentrations of each that induced visible inhibition (no visible growth) compared to the growth control. For statistical analyses of data, the dose-response assay data were analyzed with GraphPad Prism 4.03 (GraphPad Software, San Diego, CA, USA) using nonlinear regression to generate sigmoidal curves and the data normalization function (range, 0 to 100%), if required (46). The concentrations necessary to cause 50% growth inhibition (MIC50), biofilm eradication (BEC50), and biofilm prevention (BIC50), compared to the growth control, were derived from the whole dose-response curves. The MICmax and BICmax parameters were calculated as the x value at the intercept between the slope and the top plateau of a full dose-response curve and represent the concentrations necessary to cause total growth inhibition and biofilm prevention, respectively, compared to the growth control (46). The parameters calculated from the full dose-response curves, MICmax and BICmax, are therefore distinct from the minimum inhibition concentration values obtained from visual inspection of a dose-response result (MIC and BIC).
GraphPad Prism 4.03 (GraphPad Software, San Diego, CA, USA) was used for statistical analysis of the data. The analysis included 95% confidence levels, the absolute sum of squares, standard error of the mean (SEM), Student t tests, and one-way analysis of variance (ANOVA) using Bonferroni's multiple-comparison test.
RESULTS
Antifungal activities of tyrocidines against C. albicans planktonic cells.
The Trc mixture and the three pure major tyrocidines (TrcA, TrcB, and TrcC) and their analogues (PhcA and TpcC) (Table 1; see also Table S1 in the supplemental material) showed potent activity against planktonic C. albicans in the micromolar range (Table 2). The Trc mixture exhibited antifungal activity against C. albicans, characterized by an MIC of 6.25 μg/ml (Table 2). Purified tyrocidines, TpcC, and the analogous peptide gramicidin S (GS) were characterized by their antifungal activities, with MIC values of 6.25 μM, whereas phenycidine (PhcA) had a higher MIC of 12.5 μM (Table 2). When we consider the inhibition parameters (MIC50 and MICmax) calculated from the full dose-response curve for each peptide (46), PhcA was significantly less active (P < 0.001) than all the other peptides. All the other peptides were found to have statistically similar activities, except for TrcA being more active (P < 0.05) than TrcC (Table 2).
TABLE 2.
Summary of activity parameters for tyrocidines against C. albicansa
| Compound | MIC (MICmax)b | MIC50c | BIC (BICmax)d | BIC50e | BEC50f | % biofilm eradication at 200 μM |
|---|---|---|---|---|---|---|
| Trc mix | 6.25* (5.99 ± 0.26)* | 3.72 ± 0.25* | 12.5* (11.3 ± 0.67)* | 8.30 ± 0.43* | 107 ± 20* | 81 ± 3.3* |
| TrcA | 6.25 (5.21 ± 0.44) | 3.19 ± 0.31 | 12.5 (8.01 ± 1.77) | 5.68 ± 0.71 | 145 ± 23 | 64 ± 4.8 |
| TrcB | 6.25 (6.25 ± 0.00) | 4.31 ± 0.03 | 12.5 (10.6 ± 0.32) | 8.29 ± 0.11 | 164 ± 18 | 55 ± 7.3 |
| TrcC | 6.25 (7.81 ± 0.81) | 4.65 ± 0.05 | 25.0 (24.3 ± 1.44) | 17.4 ± 0.20 | 133 ± 6.3 | 74 ± 3.3 |
| PhcA | 12.5 (12.5 ± 0.00) | 7.93 ± 0.48 | 25.0 (25.6 ± 2.54) | 15.1 ± 2.08 | >200 | 41 ± 3.5 |
| TpcC | 6.25 (5.99 ± 0.26) | 4.16 ± 0.42 | 25.0 (18.4 ± 3.33) | 15.5 ± 0.91 | >200 | 28 ± 5.4 |
| GS | 6.25 (6.77 ± 0.52) | 4.38 ± 0.03 | 12.5 (8.55 ± 0.57) | 5.74 ± 0.29 | 40.9 ± 6.2 | 100 ± 0.04 |
| CAS | 0.06 (0.06 ± 0.00) | 0.04 ± 0.01 | 0.16 (0.10 ± 0.01) | 0.06 ± 0.00 | 0.35 ± 0.03 | NDg |
| AMB | 0.80 (0.79 ± 0.03) | 0.26 ± 0.01 | 1.25 (0.82 ± 0.13) | 0.35 ± 0.05 | 0.56 ± 0.02 | ND |
Each value represents the mean of ≥3 biological repeats, with 2 to 5 technical repeats per assay. The values are given in μM or, where indicated with an asterisk, μg/ml. Values in parentheses are means ± SEM.
The MIC value is the lowest concentration in assay that resulted in visible C. albicans planktonic cell death compared to the growth control. MICmax values were calculated from dose-response curves, according to Rautenbach et al. (46), for statistical analyses.
Lowest concentration in assay that prevents 50% planktonic cell growth, as derived from dose-response curves for statistical analyses (46).
The BIC value is the lowest concentration in assay that prevented visible biofilm formation compared to the growth control. BICmax values were calculated from dose-response curves, according to Rautenbach et al. (46), for statistical analyses.
Lowest concentration in assay that prevents biofilm formation by 50%, as derived from dose-response curves for statistical analyses (46).
Lowest concentration in assay that eradicates 50% of the biofilm cells, as derived from dose-response curves for statistical analyses (46).
ND, not determined.
Activities of tyrocidines against C. albicans biofilm cells.
Apart from their activities against planktonic cells, the tyrocidines and analogues were also able to prevent the formation of C. albicans biofilms in vitro (Table 2). TrcA, TrcB, and GS exhibited biofilm prevention activity, all characterized by a BIC value of 12.5 μM, whereas BIC values of PhcA, TpcC, and TrcC were 2-fold higher (Table 2). When we consider the inhibition parameters (BIC50 and BICmax) calculated from the full dose-response curve for each peptide (46), these differences in activity were found to be significant (P values, <0.05 to <0.001) (Table 2).
The tyrocidines and analogues failed to fully eradicate (remove and/or kill) mature biofilms, and only the analogous GS achieved 100% eradication of 24-h-old C. albicans biofilms (Table 2). Although TrcC exhibited lower fungicidal activity and biofilm preventive activity than those of the other two tyrocidines, it was characterized by the highest biofilm eradication activity; at 200 μM, TrcC resulted in 74% ± 3% eradication of the biofilm, whereas TrcA and TrcB treatment of biofilms resulted in 64% ± 5% and 55% ± 7% eradication, respectively (values represent means ± SEM). Treatment of the biofilms with 200 μM PhcA or TpcC did not result in >50% eradication of the biofilm.
Tyrocidines disrupt membrane integrity of biofilm cells.
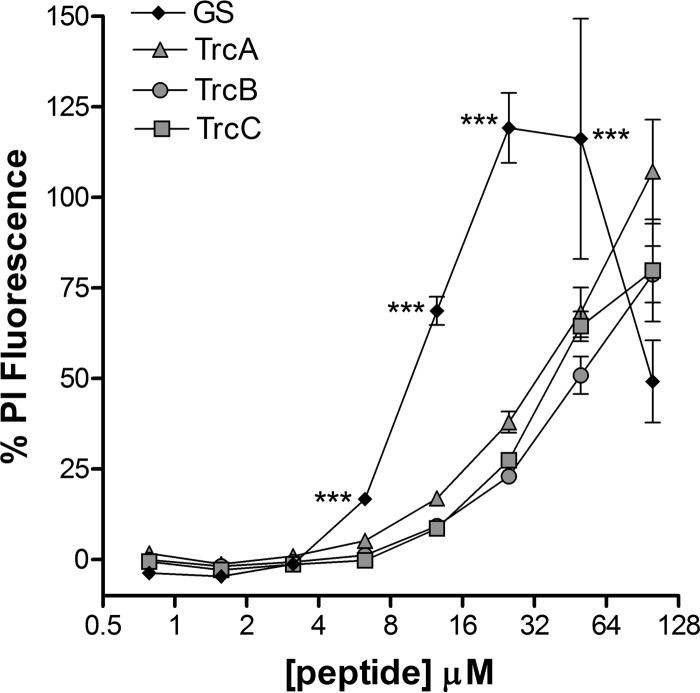
GS is known to disrupt microbial membranes (47, 48), and the tyrocidines have been shown to disrupt the membranes of bacteria (31, 49), erythrocytes (32, 50), and Neurospora organisms (51). We assessed the potential fungal membrane-disrupting activities of TrcA, TrcB and TrcC on in vitro-grown C. albicans biofilm cells using the fluorescent membrane-impermeable dye propidium iodide (Fig. 1). We found that GS induced membrane permeabilization of C. albicans biofilm cells at concentrations starting from ∼6.25 μM, whereas membrane permeabilization is induced by the tyrocidines from a threshold concentration of ∼12.5 μM (Fig. 1). This correlated with their MICs and BICs (Table 1), with a minor difference between the dose-dependent propidium iodide fluorescence increase in established biofilms exposed to the tyrocidines for either 1 h or 24 h (see Fig. S1 in the supplemental material). The higher tyrocidine concentrations required for biofilm eradication (BECs) might indicate a degree of protection of the cells from tyrocidine activity. The decrease in peptide-induced propidium iodide fluorescence at higher GS concentrations corresponds with the biofilm eradication activity of GS (compare Fig. 1 and Table 2).
FIG 1.
Percent propidium iodide (PI) fluorescence induced by TrcA, TrcB, TrcC, and GS on 24-h C. albicans biofilms with 1% Triton X-100 as reference for 100% lysis. The biofilms were incubated for 24 h with peptide prior to PI staining. Fluorescence values were corrected with those obtained from untreated cells. Each data point represents the mean of ≥8 to 10 repeats ± standard error of the mean (SEM). According to one-way ANOVA with Bonferroni's multiple-comparison test, the fluorescence induced by GS is significantly higher than that of TrcA, TrcB, and TrcC from concentrations of ≥6.2 μM (***, P < 0.001). There were no significant differences between the fluorescence values induced by TrcA, TrcB, and TrcC.
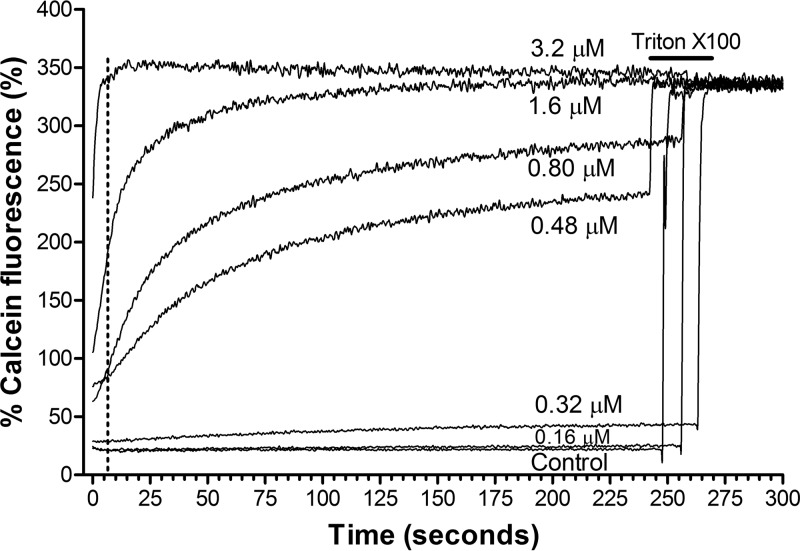
We confirmed the rapid membranolytic activity of the tyrocidines by monitoring the calcein release from lipid systems (LUVs) mimicking fungal membranes (70:30 POPC-to-ergosterol ratio) treated with these peptides. At 0.32 μM, the tyrocidines induced slow calcein leakage (permeabilization), and at 3.2 μM, the peptides led to rapid (within about 5 s) and total lysis of 25 μM POPC-to-ergosterol liposomes (Fig. 2). For more detail on the tyrocidine activity on model membranes, refer to Troskie (52).
FIG 2.
Representative dequenching kinetics of calcein fluorescence via permeabilization induced by tyrocidines in large unilamellar vesicles consisting of POPC and ergosterol (70:30 ratio). The lipid concentration was 25 μM, and the concentration of TrcA ranged from 0.16 μM to 3.2 μM in buffer (10 mM Tris, 0.1 mM EDTA, 154 mM NaCl [pH 7.4]). The vertical dotted line shows the 5-s period needed for total lysis by 3.2 μM TrcA. After approximately 4 min, total dequenching was induced by adding Triton X-100.
These data strongly indicate that fungal membranes are targeted by the tyrocidines, as they compromise the membrane integrity of both model fungal membranes and mature C. albicans biofilm cells.
Induction of endogenous reactive oxygen species by tyrocidines.
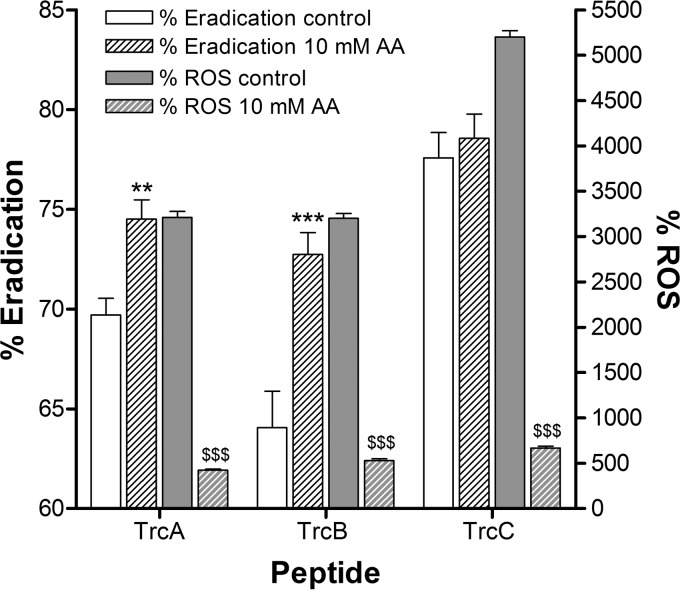
Various antifungal molecules are known to induce the accumulation of ROS in susceptible yeast and fungal species (7, 43, 44). Significant accumulation of endogenous ROS, indicated by the fluorescent dye H2DCFDA, in C. albicans biofilm cells was observed subsequent to 24-h treatment with TrcA, TrcB, and TrcC compared to the untreated cells (Fig. 3). Ascorbic acid at 10 mM significantly decreased the presence of endogenous ROS in C. albicans biofilm cells, as it acts as an ROS scavenger (40), but this did not lead to a decrease in the biofilm-eradicating activities of 100.0 μM TrcA, TrcB, and TrcC (Fig. 3). However, surprisingly, ascorbic acid significantly (P < 0.01) enhanced the activity of the two less-active peptides, TrcA and TrcB. The reason for this is unclear but might point to a subtle difference between TrcC and the less bulky and more hydrophobic TrcA and TrcB.
FIG 3.
ROS induction in mature C. albicans biofilms treated for 24 h with 100 μM TrcA, TrcB, and TrcC prior to staining with H2DCFDA. The influence of 10 mM ascorbic acid (AA) on the ability of the tyrocidines to eradicate biofilms and induce endogenous ROS was determined. According to the Student t test, the ROS induced in the presence of 10 mM ascorbic acid is significantly less ($$$, P < 0.001) than in its absence. There was significant enhancement in the biofilm eradication activity in the presence of ascorbic acid for TrcA (**, P < 0.01) and TrcB (***, P < 0.001). The experiment was performed in quadruplicate (the bars indicate means and SEM), and citric acid served as a pH control.
Potentiating of antibiofilm activity of CAS and AMB by tyrocidines.
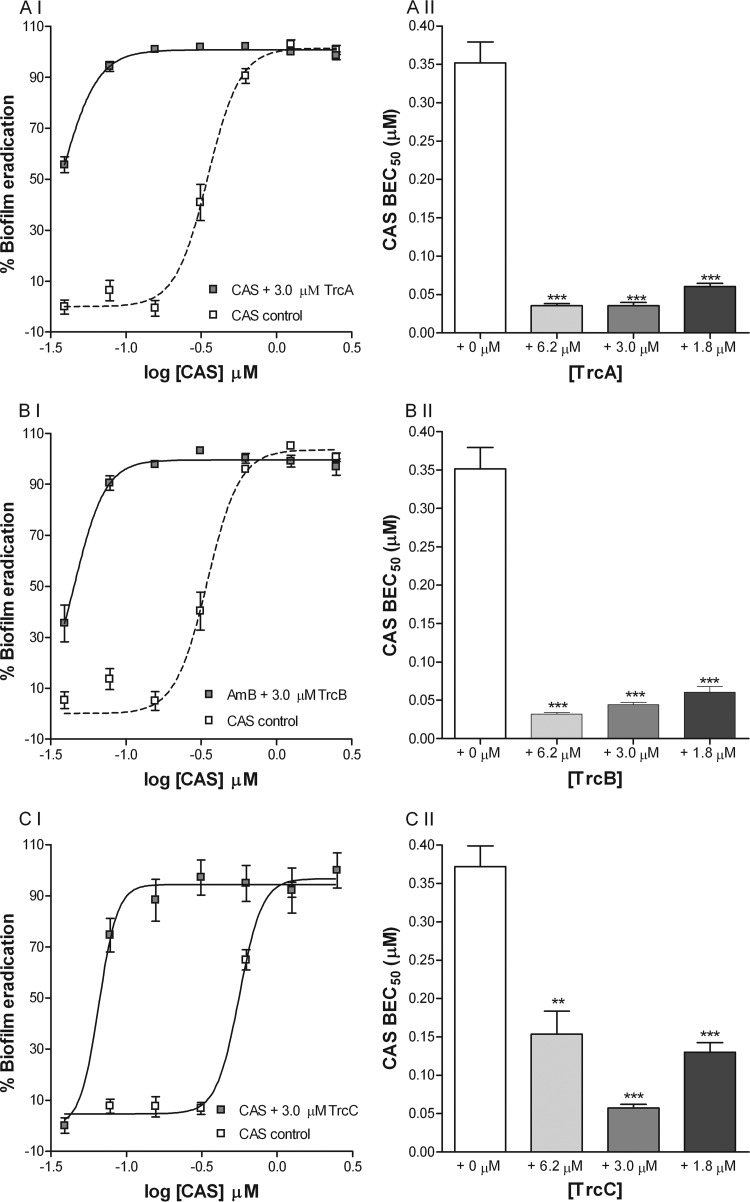
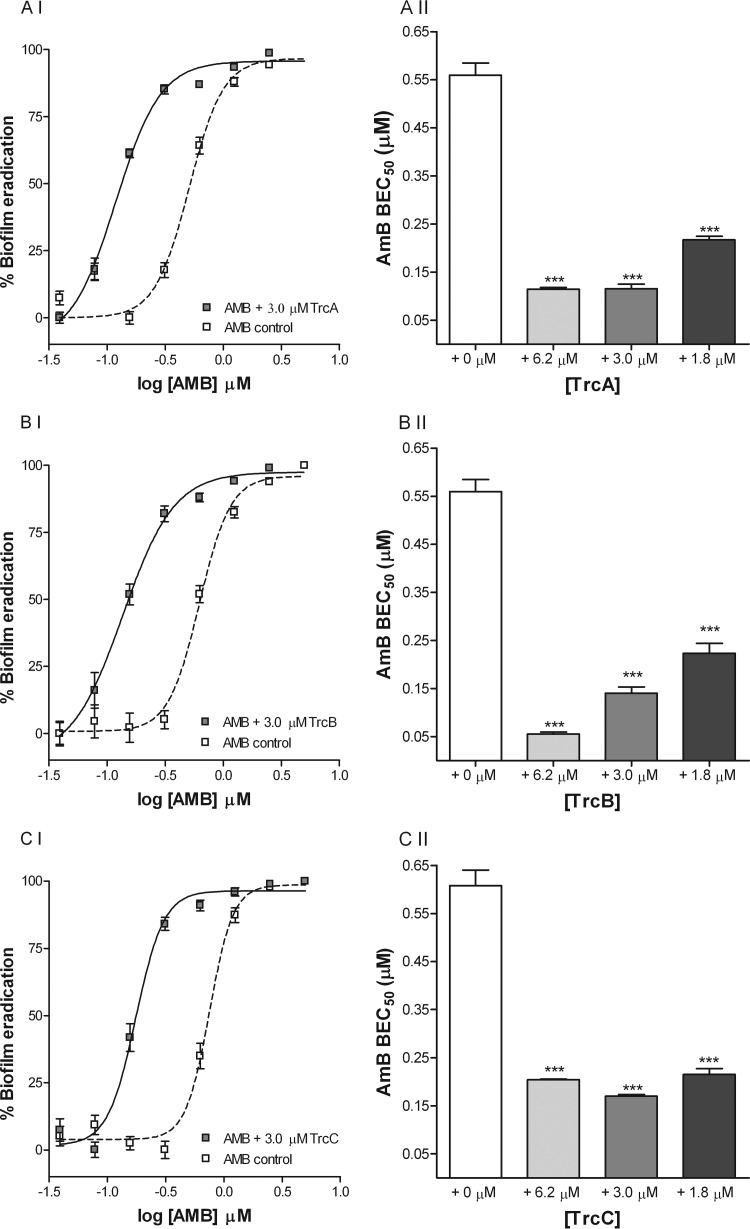
Combination drug therapy might limit resistance; therefore, we assessed combinations of the three major tyrocidines (TrcA, TrcB, and TrcC) with commonly used antimycotics, namely, caspofungin (CAS) and amphotericin B (AMB), in biofilm eradication assays. In combination, TrcA, TrcB, and TrcC significantly increased the biofilm eradication activities of both CAS (Fig. 4) and AMB (Fig. 5). The presence of 1.8 to 6.3 μM TrcA, TrcB, or TrcC decreased the BEC50s (concentration needed to eradicate 50% of biofilm) of CAS (0.35 μM/0.42 μg/ml) and AMB (0.56 μM/0.52 μg/ml) up to 12- and 9-fold, respectively. Furthermore, our results show that although the tyrocidines alone have only moderate biofilm-eradicating activities (BEC50 > 100 μM; Table 2), 1.8 to 6.3 μM TrcA, TrcB, or TrcC in combination with CAS or AMB displayed synergistic activity with regard to the eradication of mature biofilms (Table 3). The calculated FICI values of both AMB and CAS, in combination with the tyrocidines, were well below 0.5, at 0.10 to 0.42, pointing toward pronounced synergistic activity (40) (Table 3). From the FICI values, it was also observed that the tyrocidines have a slightly greater effect on the activity of CAS (FICI, 0.10 to 0.35) than on the activity of AMB (FICI, 0.14 to 0.42) (Table 3). These data indicate that combination therapy using coadministration of the tyrocidines and CAS or AMB might be effective in curing biofilm-associated infections.
FIG 4.
Representative dose-response curves of CAS biofilm eradication activity (I) and comparison of BEC50s of CAS (II) in the absence and presence of TrcA (A), TrcB (B), and TrcC (C). Each data point represents the mean of triplicate biological repeats ± SEM, with triplicate technical repeats per assay. According to one-way ANOVA with Bonferroni's multiple-comparison test, the BEC50s of CAS were significantly (***, P < 0.001; **, P < 0.01) lower in the presence of 1.8, 3.0, and 6.2 μM TrcA, TrcB, and TrcC.
FIG 5.
Representative dose-response curves of AMB biofilm eradication activity (I) and comparison of BEC50s of AMB (II) in the absence and presence of TrcA (A), TrcB (B), and TrcC (C). Each data point represents the mean of triplicate biological repeats ± SEM, with triplicate technical repeats per assay. According to one-way ANOVA with Bonferroni's multiple-comparison test, the BEC50s of AMB were significantly (***, P < 0.001) lower in the presence of 1.8, 3.0, and 6.2 μM TrcA, TrcB, and TrcC.
TABLE 3.
Summary of activity parameters for AMB and CAS in combination with TrcA, TrcB, and TrcC against 24-h-old C. albicans biofilmsa
| Tyrocidine concn (μM) | Plus TrcA |
Plus TrcB |
Plus TrcC |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| AMB |
CAS |
AMB |
CAS |
AMB |
CAS |
|||||||
| BEC50 (μM)b | FICIc | BEC50 (μM) | FICI | BEC50 (μM) | FICI | BEC50 (μM) | FICI | BEC50 (μM) | FICI | BEC50 (μM) | FICI | |
| 6.2 | 0.11 | 0.25 | 0.04 | 0.11 | 0.06 | 0.14 | 0.03 | 0.15 | 0.21 | 0.34 | 0.15 | 0.12 |
| 3.0 | 0.12 | 0.23 | 0.04 | 0.10 | 0.14 | 0.27 | 0.04 | 0.12 | 0.17 | 0.28 | 0.06 | 0.15 |
| 1.8 | 0.22 | 0.41 | 0.06 | 0.18 | 0.22 | 0.42 | 0.06 | 0.19 | 0.22 | 0.35 | 0.13 | 0.35 |
| 0 | 0.56 | 1.00 | 0.35 | 1.00 | 0.56 | 1.00 | 0.35 | 1.00 | 0.56 | 1.00 | 0.35 | 1.00 |
Each value represents the mean of ≥3 biological repeats, with 2 to 5 technical repeats per assay ± standard error of the mean (SEM).
The BEC50 value, derived from a full dose-response curve, represents the concentration AMB or CAS at which 50% eradication was achieved in the presence of a constant tyrocidine concentration. The AMB and CAS dose-response concentration ranges were 0.04 to 5.0 μM and 0.02 to 2.5 μM, respectively.
The fractional inhibitory concentration index (FICI) was calculated using the BEC50 obtained for combinatorial treatment and the BEC50 values for the individual compounds (Table 2).
In vivo activity of TrcA with CAS.
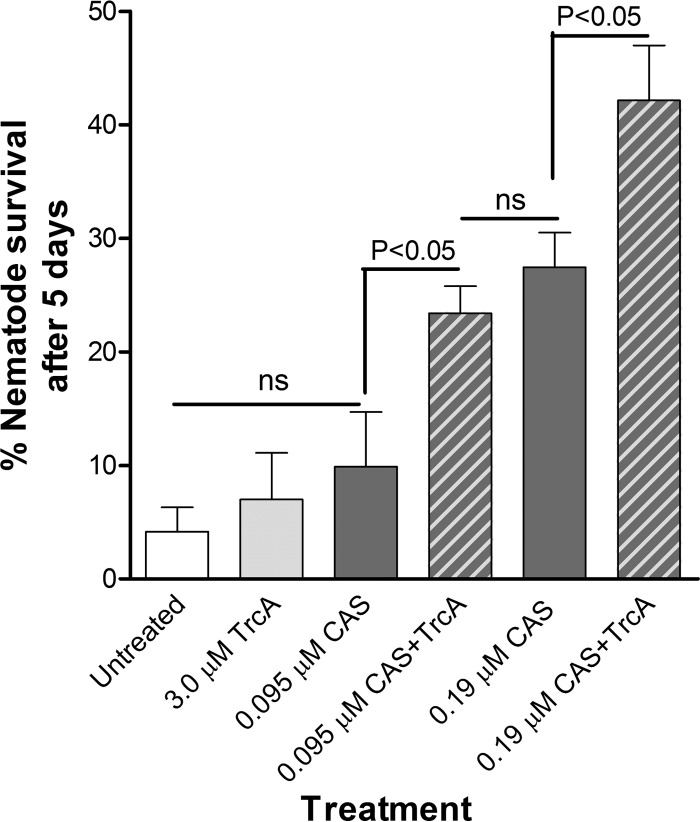
TrcA and CAS were chosen because of their promising FICI values (Table 3) to evaluate their combined antibiofilm activity in vivo using the C. elegans-C. albicans infection model system, as described by Breger et al. (45). We performed dose-response experiments with TrcA and CAS to determine the TrcA and CAS concentrations that were ineffective (<10% survival) in curing C. elegans infected with C. albicans compared to the control treatment (data not shown). Five days after infection with C. albicans, only 4% ± 2% (mean ± SEM) of the C. albicans-infected nematodes survived. The survival rates of nematodes treated with 3.0 μM TrcA (3% ± 1% survival) or 0.095 μM (0.12 μg/ml) CAS (10% ± 5% survival) were similar to the survival rates of the untreated nematodes (Fig. 6). A single treatment of infected nematodes with a combination of 3.0 μM TrcA and 0.095 μM CAS significantly increased the survival of the nematodes to 23% ± 2% at 5 days postinfection and treatment compared to treatment with 3.0 μM TrcA or 0.095 μM CAS alone or control treatment (0.6% DMSO). This combination gave a similar nematode survival rate as treatment with double the CAS dose, namely, 0.19 μM (0.23 μg/ml) CAS, which led to 27% ± 3% survival. Although 0.19 μM CAS led to significant nematode survival, a single treatment with the combination of 3.0 μM TrcA and 0.19 μM CAS again almost doubled the nematode survival rate to 42% ± 3% after 5 days (Fig. 6). These data indicate that TrcA also enhances the in vivo activity of CAS in the C. elegans infection model.
FIG 6.
Comparison of C. albicans-infected nematode survival 5 days posttreatment with either 3 μM TrcA, 0.095 μM/0.19 μM CAS, or a combination of 3 μM TrcA and 0.095 or 0.19 μM μM CAS. Statistical analysis was done using one-way ANOVA with Bonferroni's multiple-comparison test. Each bar represents the average of 3 to 6 repeats ± SEM. There was no toxicity observed for 6.0 μM TrcA 5 days posttreatment, and the EC50 (concentration at which 50% of the nematodes died) was determined to be 25 μM TrcA. ns, not significant.
DISCUSSION
Tyrocidines have a highly conserved sequence, differing in some cases in one amino acid residue only. The major tyrocidines have the basic sequence cyclo-(f1P2X3x4N5Q6Y7V8X9L10), and the relevant tyrocidines vary in only two possible residue positions, Trp3,4 or Phe3,4, in the aromatic dipeptide unit (lowercase letters in the sequence indicate d-amino acid residues [Table 1]). The two tyrocidine analogues in this study, PhcA (a phenycidine) and TpcC (a tryptocidine), have a Phe7 and Trp7, respectively, instead of the Tyr7 found in the major tyrocidines (refer to Table 1).
The Trc mixture and individual tyrocidines relevant to this study exhibited significant antiyeast activity against planktonic C. albicans cells. Except for PhcA, the minor differences in their sequences did not appear to significantly influence their fungicidal activity. Since PhcA has the highest hydrophobicity, the lower activity observed for PhcA might be ascribed to peptide aggregation or alternatively point to the role of a residue with hydrogen-bonding character, such as Tyr or Trp, in residue position 7.
Greater variance in the activities of the individual peptides was observed with regard to their ability to prevent biofilm formation. It seems that in terms of their biofilm prevention activity, a Phe4 instead of a Trp4 as an aromatic amino acid residue is preferred. These aromatic amino acid residues have a substantial influence on the hydrophobicity of tyrocidines and concurrently contribute to the ability of tyrocidines to be involved in membrane partitioning (53, 54). It has been illustrated that Phe has a greater propensity to insert itself more deeply into the membrane (55, 56). However, since the membrane disruptive activities of TrcA and TrcC are similar (Fig. 6), the observed variation in activity may not be membrane related. The presence of a Tyr7 (tyrocidine) instead of, for example, a Phe7 (as in phenycidine A) or Trp7 (as in tryptocidine C) also appears to be advantageous for the biofilm-eradicating activity of the tyrocidines. It has been proposed that a higher-order structure is the active conformation of the tyrocidines (35, 54, 57). Consequently, it might be that the more hydrophobic TrcA and TrcB with Phe4 instead of a Trp4 favors the formation of more active higher-order structures, such as dimers.
In terms of the biofilm-eradicating activity of the major tyrocidines, it would seem as if the Tyr7 amino acid residue is key to higher activity. Sequence-wise, PhcA and TpcC differ from TrcA and TrcC, respectively, in only one residue, namely, a Phe7 or Trp7 instead of a Tyr7 (90% sequence identity). Tyrosine differs from phenylalanine in only an OH group, but this difference makes tyrosine amphipathic, dipolar, and ionizable (pKa = 10.07). Phenylalanine and tryptophan are less-polar amino acids than tyrosine (Tyr > Trp ≫ Phe) (58). The three aromatic amino acids also differ in their hydropathies (Phe, 2.8; Trp, −0.9; Tyr, −1.3) (58). It might be that the Tyr residue has just the right chemical properties for target interaction and activity. As was mentioned above, it has been hypothesized that in order to form amphipathic structures for activity, the tyrocidines require the formation of higher-order structures, such as dimers (35, 54, 57). From our handling of the peptides, we found that PhcA exhibits a high tendency to aggregate in solution, while the tendency of TpcC to aggregate is lower than those of the major tyrocidines, leading to either loss of activity due to solubility or less formation of active amphipathic structures. The significantly higher activity of GS (Table 2) points out the importance of the VOLfP pentapeptide moiety in terms of biofilm-eradicating activity. This pentapeptide moiety, shared by the tyrocidines relevant to this study, has also been linked to an increase in membrane activity (32).
Studies with the membrane-impermeable dye propidium iodide illustrate that the activities of TrcA, TrcB, and TrcC similarly lead to a concentration-dependent loss in the integrity of C. albicans biofilm cells (Fig. 1). This membranolytic activity of the tyrocidines was also illustrated with model fungal membranes as targets (Fig. 2). The loss in C. albicans membrane integrity is in all probability the result of direct peptide-membrane activity resulting in cell death (Fig. 1 and 2; see also Fig. S1 in the supplemental material), as the membranolytic activity of the tyrocidine has been illustrated by a number of investigators (31, 32, 49, 50, 51, 52, 54). The membrane activity of the tyrocidines is not as potent as that of GS (Fig. 1) containing two VOLfP pentapeptide moieties, highlighting the association between the VOLfP sequence and membrane activity. As TrcA, TrcB, and TrcC exhibit membrane activity against C. albicans biofilm cells, this is an indication that membranes remain a major tyrocidine target. It remains to be elucidated if they also target an internal target subsequent to membrane permeabilization, especially at lower concentrations.
Although tyrocidine activity induced ROS formation in a concentration-dependent manner similar to that of propidium iodide fluorescence, the addition of the antioxidant did not influence the activities of the tyrocidines. Therefore, it would seem as if tyrocidine-induced ROS is a secondary result of tyrocidine activity and not essential for their antifungal activity. A clear correlation can be observed between the propidium iodide and ROS fluorescence induced by the tyrocidines (compare Fig. 1 and Fig. S2 in the supplemental material), which points to the possibility that the observed ROS induction is a result of the membrane disruptive activity of the tyrocidines. However, it must also be kept in mind that ascorbic acid is specific to certain ROS species. Although the total ROS is reduced in the presence of 10 mM ascorbic acid, a small percentage of ROS still remains (Fig. 3). It might therefore be that these remaining ROS species are responsible for the activities of the tyrocidines. However, a significant increase (P < 0.01) in the biofilm eradication activities of TrcA and TrcB was found in the presence of ascorbic acid. It is possible that the formulation of the two additional hydrophobic cationic tyrocidines, with the high-concentration ascorbic acid as a possible chaotropic agent, led to less detrimental aggregation and stabilization of more active structures.
The significant decrease in the BEC50 values for CAS and AMB when applied to biofilms in combination with the tyrocidines (Fig. 4 and 5), together with the decrease in FICI values (Table 3), unmistakably points to overt synergistic activity. The results of the propidium iodide studies indicate that the tyrocidines disrupt the membrane integrity of C. albicans biofilm cells. At 6.25 μM, TrcA leads to only about 5% propidium iodide (PI) uptake, which might result in an increased accumulation of the antifungal drug into the cell by the increased membrane permeability. However, the 1.8 and 3.13 μM TrcA and 1.8 to 6.25 μM TrcC and TrcB, which were used to enhance CAS and AMB activities, did not induce overt membrane permeabilization (Fig. 1), indicating that the enhancement of the activity of CAS and AMB against mature C. albicans biofilms may be independent of membrane permeabilization at these concentrations. Since AMB and the tyrocidines both target cell membranes (18, 20, 21, 31, 49, 51, 54), competition for this target might explain why higher synergism was observed for CAS (Fig. 4) than for AMB (Fig. 5). Nevertheless, further studies will need to be conducted to draw definite conclusions regarding the mode of synergism between the tyrocidines and CAS/AMB.
The in vivo study regarding the potentiating effect of TrcA on the in vivo activity of CAS in a C. elegans infection model showed that at a concentration of 3 μM, TrcA significantly enhanced the survival of C. albicans-infected nematodes treated with 0.095 μM μM CAS (Fig. 6). Furthermore, there was no toxicity for the nematodes observed for TrcA up to a concentration of 6 μM. These results reveal the potential of tyrocidines as candidates for further studies as antifungal drugs, especially as potentiating compounds to be used in combination with other antifungal drugs, such as AMB and CAS.
In conclusion, as with the other target cells (31, 49, 51), the tyrocidines probably disturb the integrity of C. albicans cell membranes, limiting resistance potential. Another study showed the broad spectrum and potent activity against filamentous fungi (M. Rautenbach, A. De Beer, A. M. Troskie, and J. A. Vosloo, 2013, PCT patent, WO 2013150394 A1) and indicated that fungi may share a tyrocidine target, which was proposed to be the fungal membrane (52). If there is an alternative/additional target to the membrane target, to further lower resistance potential, it remains to be discovered in future studies. Since this study focused on one clinical C. albicans isolate only, strain SC5314, future investigations of the most active tyrocidines against other Candida isolates will be beneficial to further elucidate strain sensitivity, as to determine the possibility of resistance, and its clinical relevance as an antifungal lead compound.
The tyrocidines have been used as a topical antibiotic since the 1940s (30), and even though hemolytic activity and mammalian cell toxicity have been observed (32, 50), they have been shown to protect mice from Pneumococcus when administered orally (59) and have been used orally for years in throat lozenges (i.e., Tyrozets). The antimalarial activity of intravenous tyrocidine (contained in tyrothricin) was observed to rival that of quinine when tested in chickens infected with Plasmodium gallinaceum (60). These applications showed the potential of tyrocidines for treating bacterial and parasitic infections, but this current study highlights the significant fungicidal and biofilm prevention activities of the tyrocidines against C. albicans, indicating that they have the potential to serve as lead compounds for novel antifungal compounds. The significant synergistic effect of the tyrocidines on the biofilm eradication activity of AMB and CAS in vitro, as well as the potentiating effect of TrcA on the remediating activity of CAS in vivo, represent promising evidence for the potential of combination treatment that will not only decrease the toxicity of the individual compounds but will also decrease the likelihood of resistance developing against the individual compounds. The tyrocidines therefore have the potential to be used clinically as topical and oral antifungal preparations, but modification and/or formulation are necessary if they are to be considered for intravenous and systemic application.
Supplementary Material
ACKNOWLEDGMENTS
A.M.T. and M.R. thank the Erasmus Mundus Action 2 Programme (European Union), National Research Foundation (South Africa), and BIOPEP Peptide Fund for their financial assistance in this study. K.T. and N.D. acknowledge receipt of postdoctoral and predoctoral grants of the Industrial Research Fund, KU Leuven and IWT-Vlaanderen, respectively.
We also thank M. Stander from the LCMS Central Analytical Facility, Stellenbosch University, for electrospray mass spectrometry (ESMS) and UPLC-MS of the peptides used in this study.
Footnotes
Published ahead of print 21 April 2014
Supplemental material for this article may be found at http://dx.doi.org/10.1128/AAC.02381-14.
REFERENCES
- 1.Letscher-Bru V, Herbrecht R. 2003. Caspofungin: the first representative of a new antifungal class. J. Antimicrob. Chemother. 51:513–521. 10.1093/jac/dkg117 [DOI] [PubMed] [Google Scholar]
- 2.Beck-Sagué C, Jarvis WR. 1993. Secular trends in the epidemiology of nosocomial fungal infections in the United States, 1980–1990. National Nosocomial Infections Surveillance System. J. Infect. Dis. 167:1247–1251 [DOI] [PubMed] [Google Scholar]
- 3.Groll AH, Shah PM, Mentzel C, Schneider M, Just-Nuebling G, Huebner K. 1996. Trends in the postmortem epidemiology of invasive fungal infections at a university hospital. J. Infect. 33:23–32. 10.1016/S0163-4453(96)92700-0 [DOI] [PubMed] [Google Scholar]
- 4.Ramage G, Bachmann S, Patterson TF, Wickes BL, López-Ribot JL. 2002. Investigation of multidrug efflux pumps in relation to fluconazole resistance in Candida albicans biofilms. J. Antimicrob. Chemother. 49:973–980. 10.1093/jac/dkf049 [DOI] [PubMed] [Google Scholar]
- 5.Ramage G, Mowat E, Jones B, Williams C, Lopez-Ribot J. 2009. Our current understanding of fungal biofilms. Crit. Rev. Microbiol. 35:340–355. 10.3109/10408410903241436 [DOI] [PubMed] [Google Scholar]
- 6.Tobudic S, Lassnigg A, Kratzer C, Graninger W, Presterl E. 2010. Antifungal activity of amphotericin B, caspofungin and posaconazole on Candida albicans biofilms in intermediate and mature development phases. Mycoses 53:208–214. 10.1111/j.1439-0507.2009.01690.x [DOI] [PubMed] [Google Scholar]
- 7.Bink A, Vandenbosch D, Coenye T, Nelis H, Cammue BPA, Thevissen K. 2011. Superoxide dismutases are involved in Candida albicans biofilm persistence against miconazole. Antimicrob. Agents Chemother. 55:4033–4037. 10.1128/AAC.00280-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ferreira JAG, Carr JH, Starling CEF, de Resende MA, Donlan RM. 2009. Biofilm formation and effect of caspofungin on biofilm structure of Candida species bloodstream isolates. Antimicrob. Agents Chemother. 53:4377–4384. 10.1128/AAC.00316-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bink A, Pellens K, Cammue BPA, Thevissen K. 2011. Anti-biofilm strategies: how to eradicate Candida biofilms? Open Mycol. J. 5:29–38. 10.2174/1874437001105010029 [DOI] [Google Scholar]
- 10.Harriott MM, Lilly EA, Rodriguez TE, Fidel PL, Jr, Noverr MC. 2010. Candida albicans forms biofilms on the vaginal mucosa. Microbiology 156:3635–3644. 10.1099/mic.0.039354-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Blankenship JR, Mitchell AP. 2006. How to build a biofilm: a fungal perspective. Curr. Opin. Microbiol. 9:588–594. 10.1016/j.mib.2006.10.003 [DOI] [PubMed] [Google Scholar]
- 12.Chandra J, Kuhn DM, Mukherjee PK, Hoyer LL, McCormick T, Ghannoum MA. 2001. Biofilm formation by the fungal pathogen Candida albicans: development, architecture, and drug resistance. J. Bacteriol. 183:5385–5394. 10.1128/JB.183.18.5385-5394.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Crump JA, Collignon PJ. 2000. Intravascular catheter-associated infections. Eur. J. Clin. Microbiol. Infect. Dis. 19:1–8. 10.1007/s100960050001 [DOI] [PubMed] [Google Scholar]
- 14.Bachmann SP, VandeWalle K, Ramage G, Patterson TF, Wickes BL, Graybill JR, López-Ribot JL. 2002. In vitro activity of caspofungin against Candida albicans biofilms. Antimicrob. Agents Chemother. 46:3591–3596. 10.1128/AAC.46.11.3591-3596.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hawser SP, Douglas LJ. 1995. Resistance of Candida albicans biofilms to antifungal agents in vitro. Antimicrob. Agents Chemother. 39:2128–2131. 10.1128/AAC.39.9.2128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Baillie GS, Douglas LJ. 1999. Candida biofilms and their susceptibility to antifungal agents. Methods Enzymol. 310:644–656. 10.1016/S0076-6879(99)10050-8 [DOI] [PubMed] [Google Scholar]
- 17.Ramage G, Wickes BL, Lopez-Ribot JL. 2001. Biofilms of Candida albicans and their associated resistance to antifungal agents. Am. Clin. Lab. 20:42–44 [PubMed] [Google Scholar]
- 18.Laniado-Laborín R, Cabrales-Vargas MN. 2009. Amphotericin B: side effects and toxicity. Rev. Iberoam. Micol. 26:223–227. 10.1016/j.riam.2009.06.003 [DOI] [PubMed] [Google Scholar]
- 19.DiDone L, Oga D, Krysan DJ. 2011. A novel assay of biofilm antifungal activity reveals that amphotericin B and caspofungin lyse Candida albicans cells in biofilms. Yeast 28:561–568. 10.1002/yea.1860 [DOI] [PubMed] [Google Scholar]
- 20.Brajtburg J, Bolard J. 1996. Carrier effects on biological activity of amphotericin B. Clin. Microbiol. Rev. 9:512–531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Johnson RH, Einstein HE. 2007. Amphotericin B and coccidioidomycosis. Ann. N. Y. Acad. Sci. 1111:434–441. 10.1196/annals.1406.019 [DOI] [PubMed] [Google Scholar]
- 22.Conly J, Rennie R, Johnson J, Farah S, Hellman L. 1992. Disseminated candidiasis due to amphotericin B-resistant Candida albicans. J. Infect. Dis. 165:761–764. 10.1093/infdis/165.4.761 [DOI] [PubMed] [Google Scholar]
- 23.Kelly SL, Lamb DC, Kelly DE, Manning NJ, Loeffler J, Hebart H, Schumacher U, Einsele H. 1997. Resistance to fluconazole and cross-resistance to amphotericin B in Candida albicans from AIDS patients caused by defective sterol Δ5,6-desaturation. FEBS Lett. 400:80–82. 10.1016/S0014-5793(96)01360-9 [DOI] [PubMed] [Google Scholar]
- 24.Nolte FS, Parkinson T, Falconer DJ, Dix S, Williams J, Gilmore C, Geller R, Wingard JR. 1997. Isolation and characterization of fluconazole- and amphotericin B-resistant Candida albicans from blood of two patients with leukemia. Antimicrob. Agents Chemother. 44:196–199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Deresinski SC, Stevens DA. 2003. Caspofungin. Clin. Infect. Dis. 36:1445–1457. 10.1086/375080 [DOI] [PubMed] [Google Scholar]
- 26.Hancock RE, Scott MG. 2000. The role of antimicrobial peptides in animal defenses. Proc. Natl. Acad. Sci. U. S. A. 97:8856–8861. 10.1073/pnas.97.16.8856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hancock RE, Diamond G. 2000. The role of cationic antimicrobial peptides in innate host defences. Trends Microbiol. 8:402–410. 10.1016/S0966-842X(00)01823-0 [DOI] [PubMed] [Google Scholar]
- 28.Hotchkiss RD, Dubos RJ. 1941. The isolation of bactericidal substances from cultures of Bacillus brevis. J. Biol. Chem. 141:155–162 [Google Scholar]
- 29.Tang XJ, Thibault P, Boyd RK. 1992. Characterisation of the tyrocidine and gramicidin fractions of the tyrothricin complex from Bacillus brevis using liquid chromatography and mass spectrometry. Int. J. Mass Spectrom. Ion Processes 122:153–179. 10.1016/0168-1176(92)87015-7 [DOI] [Google Scholar]
- 30.Rankin LM. 1944. The use of tyrothricin in the treatment of ulcers of the skin. Am. J. Surg. 65:391–392. 10.1016/S0002-9610(44)90351-X [DOI] [Google Scholar]
- 31.Spathelf BM, Rautenbach M. 2009. Anti-listerial activity and structure-activity relationships of the six major tyrocidines, cyclic decapeptides from Bacillus aneurinolyticus. Bioorg. Med. Chem. 17:5541–5548. 10.1016/j.bmc.2009.06.029 [DOI] [PubMed] [Google Scholar]
- 32.Rautenbach M, Vlok NM, Stander M, Hoppe HC. 2007. Inhibition of malaria parasite blood stages by tyrocidines, membrane-active cyclic peptide antibiotics from Bacillus brevis. Biochim. Biophys. Acta 1768:1488–1497. 10.1016/j.bbamem.2007.01.015 [DOI] [PubMed] [Google Scholar]
- 33.Kretschmar M, Nichterlein T, Nebe CT, Hof H, Burger KJ. 1996. Fungicidal effect of tyrothricin on Candida albicans. Mycoses 39:45–50. 10.1111/j.1439-0507.1996.tb00083.x [DOI] [PubMed] [Google Scholar]
- 34.Vosloo JA, Stander M, Leussa AN-N, Spathelf BM, Rautenbach M. 2013. Manipulation of the tyrothricin production profile of Bacillus aneurinolyticus. Microbiology 159:2200–2211. 10.1099/mic.0.068734-0 [DOI] [PubMed] [Google Scholar]
- 35.Spathelf BM. 2010. Qualitative structure-activity relationships of the major tyrocidines, cyclic decapeptides from Bacillus aneurinolyticus. Ph.D. thesis Department of Biochemistry, University of Stellenbosch, Stellenbosch, South Africa: http://scholar.sun.ac.za/handle/10019.1/4001 [Google Scholar]
- 36.CLSI. 2008. Reference method for broth dilution antifungal susceptibility testing of filamentous fungi, 2nd ed. CLSI M38-A2. Clinical and Laboratory Standards Institute, Wayne, PA [Google Scholar]
- 37.Delattin N, De Brucker K, Craik DJ, Cheneval O, Fröhlich M, Veber M, Girandon L, Davis TR, Weeks AE, Kumamoto CA, Cos P, Coenye T, De Coninck B, Cammue BPA, Thevissen K. 2014. The plant-derived decapeptide OSIP108 interferes with Candida albicans biofilm formation without affecting cell viability. Antimicrob. Agents Chemother. 58:2647–2656. 10.1128/AAC.01274-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Peeters E, Nelis HJ, Coenye T. 2007. Comparison of multiple methods for quantification of microbial biofilms grown in microtiter plates. J. Microbiol. Methods 72:157–165. 10.1016/j.mimet.2007.11.010 [DOI] [PubMed] [Google Scholar]
- 39.Van den Driessche F, Rigole P, Brackman G, Coenye T. 2013. Optimization of resazurin-based viability staining for quantification of microbial biofilms. J. Microbiol. Methods 98:31–34. 10.1016/j.mimet.2013.12.011 [DOI] [PubMed] [Google Scholar]
- 40.Bink A, Kucharíková S, Neirinck B, Vleugels J, Van Dijck P, Cammue BP, Thevissen K. 2012. The nonsteroidal anti-inflammatory drug diclofenac potentiates the in vivo activity of caspofungin against Candida albicans biofilms. J. Infect. Dis. 206:1790–1797. 10.1093/infdis/jis594 [DOI] [PubMed] [Google Scholar]
- 41.Dathe M, Schümann M, Wieprecht T, Winkler A, Beyermann M, Krause E, Matsuzaki K, Murase O, Bienert M. 1996. Peptide helicity and membrane surface charge modulate the balance of electrostatic and hydrophobic interactions with lipid bilayers and biological membranes. Biochemistry 35:12612–12622. 10.1021/bi960835f [DOI] [PubMed] [Google Scholar]
- 42.Dathe M, Nikolenko H, Klose J, Bienert M. 2004. Cyclization increases the antimicrobial activity and selectivity of arginine- and tryptophan-containing hexapeptides. Biochemistry 43:9140–9150. 10.1021/bi035948v [DOI] [PubMed] [Google Scholar]
- 43.Aerts AM, François IE, Meert EM, Li QT, Cammue BP, Thevissen K. 2007. The antifungal activity of RsAFP2, a plant defensin from Raphanus sativus, involves the induction of reactive oxygen species in Candida albicans. J. Mol. Microbiol. Biotechnol. 13:243–247. 10.1159/000104753 [DOI] [PubMed] [Google Scholar]
- 44.François IE, Thevissen K, Pellens K, Meert EM, Heeres J, Freyne E, Coesemans E, Viellevoye M, Deroose F, Martinez Gonzalez S, Pastor J, Corens D, Meerpoel L, Borgers M, Ausma J, Dispersyn GD, Cammue BP. 2009. Design and synthesis of a series of piperazine-1-carboxamidine derivatives with antifungal activity resulting from accumulation of endogenous reactive oxygen species. ChemMedChem 4:1714–1721. 10.1002/cmdc.200900249 [DOI] [PubMed] [Google Scholar]
- 45.Breger J, Fuchs BB, Aperis G, Moy TI, Ausubel FM, Mylonakis E. 2007. Antifungal chemical compounds identified using a C. elegans pathogenicity assay. PLoS Pathog. 3:e18. 10.1371/journal.ppat.0030018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rautenbach M, Gerstner GD, Vlok NM, Kulenkampff J, Westerhoff HV. 2006. Analyses of dose-response curves to compare the antimicrobial activity of model cationic α-helical peptides highlights the necessity for a minimum of two activity parameters. Anal. Biochem. 350:81–90. 10.1016/j.ab.2005.11.027 [DOI] [PubMed] [Google Scholar]
- 47.Katsu T, Kobayashi H, Fujita Y. 1986. Mode of action of gramicidin S on Escherichia coli membrane. Biochim. Biophys. Acta 860:608–619. 10.1016/0005-2736(86)90560-2 [DOI] [PubMed] [Google Scholar]
- 48.Jelokhani-Niaraki M, Hodges RS, Meissner JE, Hassenstein UE, Wheaton L. 2008. Interaction of gramicidin S and its aromatic amino-acid analog with phospholipid membranes. Biophys. J. 95:3306–3321. 10.1529/biophysj.108.137471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Aranda FJ, de Kruijff B. 1988. Interrelationships between tyrocidine and gramicidin A′ in their interaction with phospholipids in model membranes. Biochim. Biophys. Acta 937:195–203. 10.1016/0005-2736(88)90241-6 [DOI] [PubMed] [Google Scholar]
- 50.Mann FC, Heilman D, Herrell WE. 1943. Effect of serum on hemolysis by gramicidin and tyrocidine. Exp. Med. Biol. 52:31–33. 10.3181/00379727-52-14011 [DOI] [Google Scholar]
- 51.Mach B, Slayman CW. 1966. Mode of action of tyrocidine on Neurospora. Biochim. Biophys. Acta 124:351–361. 10.1016/0304-4165(66)90198-X [DOI] [PubMed] [Google Scholar]
- 52.Troskie AM. 2014. Tyrocidines, cyclic decapeptides produced by soil bacilli, as potent inhibitors of fungal pathogens. Ph.D. thesis Department of Biochemistry, University of Stellenbosch, Stellenbosch, South Africa [Google Scholar]
- 53.Dubos RJ, Hotchkiss RD. 1941. The production of bactericidal substances by aerobic sporulating bacilli. J. Exp. Med. 73:629–640. 10.1084/jem.73.5.629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Loll PJ, Upton EC, Nahoum V, Economou NJ, Cocklin S. 2014. The high resolution structure of tyrocidine A reveals an amphipathic dimer. Biochim. Biophys. Acta 1838:1199–1207. 10.1016/j.bbamem.2014.01.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Braun P, von Heijne G. 1999. The aromatic residues Trp and Phe have different effects on the positioning of a transmembrane helix in the microsomal membrane. Biochemistry 38:9778–9782. 10.1021/bi990923a [DOI] [PubMed] [Google Scholar]
- 56.Shai Y. 2002. Mode of action of membrane active antimicrobial peptides. Biopolymers 66:236–248. 10.1002/bip.10260 [DOI] [PubMed] [Google Scholar]
- 57.Munyuki G, Jackson GE, Venter GA, Kövér KE, Szilágyi L, Rautenbach M, Spathelf BM, Bhattacharya B, van der Spoel D. 2013. β-Sheet structures and dimer models of two major tyrocidines, antimicrobial peptides from Bacillus aneurinolyticus. Biochemistry 52:7798–7806. 10.1021/bi401363m [DOI] [PubMed] [Google Scholar]
- 58.Frecer V. 2006. QSAR analysis of antimicrobial and haemolytic effects of cyclic cationic antimicrobial peptides derived from protegrin-1. Bioorg. Med. Chem. 14:6065–6074. 10.1016/j.bmc.2006.05.005 [DOI] [PubMed] [Google Scholar]
- 59.Dubos RJ, Cattaneo C. 1939. Studies on a bactericidal agent extracted from a soil bacillus. III. Preparation and activity of a protein-free fraction. J. Exp. Med. 70:249–256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Taliaferro LC, Coulston F, Silverman M. 1944. The antimalarial activity of tyrothricin against Plasmodium gallinaceum. J. Infect. Dis. 75:179–212. 10.1093/infdis/75.3.179 [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.