Abstract
Chagas disease (CD), a neglected tropical disease caused by Trypanosoma cruzi, remains a serious public health problem in several Latin American countries. The available chemotherapies for CD have limited efficacy and exhibit undesirable side effects. Aromatic diamidines and arylimidamides (AIAs) have shown broad-spectrum activity against intracellular parasites, including T. cruzi. Therefore, our aim was to evaluate the biological activity of eight novel AIAs (16DAP002, 16SAB079, 18SAB075, 23SMB022, 23SMB026, 23SMB054, 26SMB070, and 27SMB009) against experimental models of T. cruzi infection in vitro and in vivo. Our data show that none of the compounds induced a loss of cellular viability up to 32 μM. Two AIAs, 18SAB075 and 16DAP002, exhibited good in vitro activity against different parasite strains (Y and Tulahuen) and against the two relevant forms of the parasite for mammalian hosts. Due to the excellent selective indexes of 18SAB075, this AIA was moved to in vivo tests for acute toxicity and parasite efficacy; nontoxic doses (no-observed-adverse-effect level [NOAEL], 50 mg/kg) were employed in the tests for parasite efficacy. In experimental models of acute T. cruzi infection, 18SAB075 reduced parasitemia levels only up to 50% and led to 40% protection against mortality (at 5 mg/kg of body weight), being less effective than the reference drug, benznidazole.
INTRODUCTION
Chagas disease (CD) is a neglected tropical disease caused by the intracellular flagellate protozoan Trypanosoma cruzi, which presents a complex life cycle with distinct morphological stages in its obligatory passage through vertebrate and invertebrate hosts (1). Currently, there are approximately 10 million infected individuals in areas of Latin America where CD is endemic, and many reports also point to the occurrence of CD in geographical areas where it is not endemic, such as the United States and Europe, mainly attributed to migration of infected people (2–6). CD can be transmitted by Triatominae insect feces, blood transfusion, organ transplantation, and laboratory accidents and through oral and congenital routes (7, 8). This pathology has two successive phases, a short, acute phase characterized by a patent parasitemia, followed by a chronic phase in which most of the infected individuals remain asymptomatic (indeterminate form), but about one-third may later manifest cardiac and/or digestive complications, developed progressively for years or decades after infection (9, 10). Benznidazole (Bz) and nifurtimox, introduced into clinical therapy about 40 years ago, are the only available drugs. Both have several shortcomings related to their required long periods of treatment, high toxicity, variable results, and low efficacy during the chronic phase, justifying the identification of novel therapies (11–13). Aromatic diamidines and analogues exhibit broad-spectrum activity against pathogenic microorganisms, including T. cruzi (14). Among the different tested derivatives of amidine compounds, the most effective against T. cruzi have been the bis-arylimidamides (AIAs) like DB766 (15) and DB1831 (16). Thus, in this study, we assayed the biological activities of eight novel AIAs (16DAP002, 16SAB079, 18SAB075, 23SMB022, 23SMB026, 23SMB054, 26SMB070, and 27SMB009) against experimental models of T. cruzi infection in vitro and in vivo, using different parasite strains, and also explored their toxicities in cardiac cell cultures and mouse models of acute toxicity.
MATERIALS AND METHODS
Synthesis of the arylimidamides.
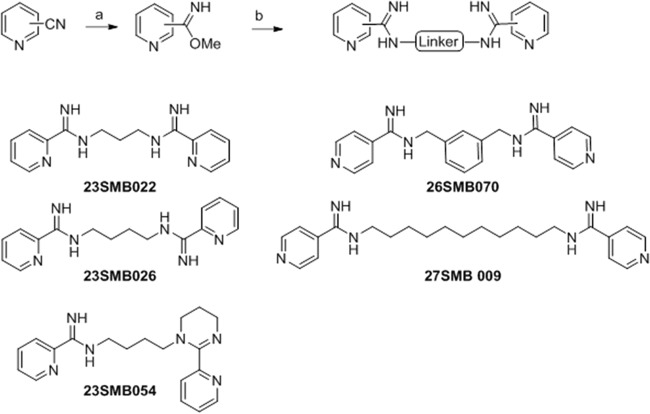
The eight arylimidamides, all isolated as their hydrochloride salts, were prepared by three general methods (see the supplemental material). Briefly, phenylimidamides 16DAP022 and 16SAB079 (Fig. 1) were prepared by the reaction of 2,7-diaminocarbazole (prepared by reduction of 2,7-dinitroocarbazole) (17) or m-xylylenediamine with (2-naphthyl)methyl benzothioimidate hydrobromide (18). The 2-pyridylimidamide 18SAB075 (Fig. 2) was prepared by the reaction of 4,4′-diaminodiphenylacetylene (19, 20) with 2-cyanopyridine by the method of Lange et al. (21) but using lithium (rather than sodium) bis(trimethylsilyl)amide. The reaction of the methyl imidate derivatives of 2- or 4-cyanopyridine with the appropriate α,ω-diamines or spermidine gave 2-pyridylimidamides 23SMB022, 23SMB026, and 23SMB054 and 4-pyridylimidamides 26SBM070 and 27SMB009 (Fig. 3).
FIG 1.
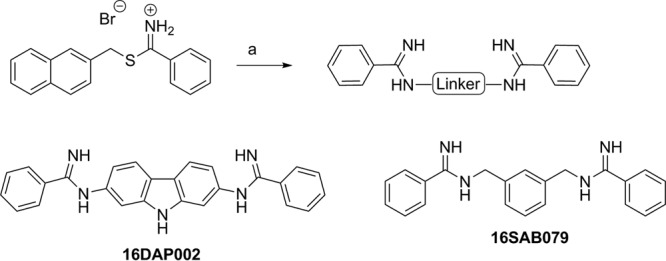
Synthesis of 16DAP002 and 16SAB079. Reagents and conditions: (a) appropriate diamine, ethanol (plus acetonitrile for 16DAP022), 0 to 25°C, overnight (66 to 71%).
FIG 2.
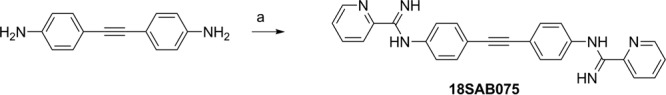
Synthesis of 18SAB075. Reagents and conditions: (a) 2-cyanopyridine, lithium bis(trimethylsilyl)amide, tetrahydrofuran, 25°C, 2 days, (16%).
FIG 3.
Synthesis of 23SMB022, 23SMB026, 23SMB054, 26SMB070, and 27SMB009. Reagents and conditions: (a) sodium methoxide, methanol, 25°C, 1 week; (b) appropriate α,ω-diamine (or spermidine for 23SMB054), 4 M HCl in dioxane, methanol, 25°C (5 to 92%).
Mammalian cell cultures.
For the in vitro analysis of compound toxicity and effects against intracellular parasites (Y strain), primary cultures of embryonic cardiomyocytes (CM) were obtained from Swiss mice as previously reported (22). After purification, the CM were seeded at a density of 0.2 × 106 and 0.05 × 106 cells/well, respectively, in 24- and 96-well microplates containing gelatin-coated coverslips. The cultures were then maintained at 37°C in Dulbecco's modified medium supplemented with 10% horse serum, 5% fetal bovine serum, 2.5 mM CaCl2, 1 mM l-glutamine, and 2% chicken embryo extract (DMEM). For the further analysis of the effect on intracellular parasites of the Tulahuen strain (parasites expressing the Escherichia coli β-galactosidase gene), monolayers of mouse L929 fibroblasts were cultivated (4 × 103 cells/well in 96-well microplates) at 37°C in RPMI 1640 medium (pH 7.2 to 7.4) without phenol red (Gibco BRL) supplemented with 10% fetal bovine serum (FBS) and 2 mM glutamine (RPMIS), as reported previously (23).
Parasites.
Bloodstream trypomastigote (BT) forms of the Y strain were obtained from the blood samples of infected albino Swiss mice at the peak of parasitemia. The purified parasites were resuspended in Eagle's medium modified by Dulbecco's medium (DME) supplemented with 10% FBS (DMES) as reported previously (22). The effect on the intracellular forms was investigated by both the infection of L929 cell lineages with tissue culture-derived trypomastigotes (Tulahuen strain expressing the E. coli β-galactosidase gene) and the infection of CM with bloodstream trypomastigotes (Y strain) using a 10:1 ratio, following previously established protocols (15, 23). Stock solutions were prepared in dimethyl sulfoxide (DMSO) with the final concentrations of the solvent never exceeding 0.6% and 10% for in vitro and in vivo analysis, respectively, which did not exert any toxicity (data not shown). Benznidazole (Bz) (2-nitroimidazole; Laboratório Farmacêutico do Estado de Pernambuco [LAFEPE], Brazil) was used as a reference drug.
Cytotoxicity in vitro tests.
CM were incubated for 24 h at 37°C with different concentrations of each compound (up to 96 μM) diluted in DMEM (without phenol red), their morphology and spontaneous contractibility were evaluated by light microscopy, and then their cellular viability was determined by the alamarBlue assay. For this colorimetric bioassay, 10 μl alamarBlue (Invitrogen) was added to each well, and the plate was further incubated for 24 h, after which the absorbances at 570 and 600 nm were measured. As negative controls, alamarBlue assays were also performed in the absence of cells, using only DMEM and DMEM containing each tested compound (at 96 μM). The results are expressed as the percent differences in the decreases between compound-treated and vehicle-treated cells by following the manufacturer's instructions and the EC50 value corresponds to the concentration that reduces the cellular viability by 50% (23).
Trypanocidal analysis.
Bloodstream trypomastigotes of the Y strain (5 × 106/ml) were incubated for 24 h at 37°C in RPMI medium in the presence or absence of serial dilutions of the compounds (0 to 32 μM). After compound incubation, the parasite death rates were determined by light microscopy through direct quantification of the numbers of live parasites using a Neubauer chamber, and the EC50 (compound concentration that reduces 50% of the number of parasites) was then calculated.
For the assays on intracellular forms, different protocols were performed with the Y and Tulahuen strains. For Tulahuen-infected L929 cells, the cultures were exposed to 1.0 μg/ml diluted in RPMIS, and those compounds that presented ≥50% reductions in the parasite infection index were further screened using increasing concentrations with the aim of determining the EC50s (23). After 96 h of compound incubation at 37°C, chlorophenol red glycoside (500 μM) in 0.5% Nonidet P40 was added to each well, and the plate was incubated for 18 h at 37°C. Next, the absorbance was measured at 570 nm. Uninfected and infected cultures submitted to vehicle and Bz exposure were tested in parallel. The results are expressed as the percentage of T. cruzi growth inhibition in compound-tested cells compared to that in the infected cells and untreated cells (23).
For the analysis of the effect against intracellular amastigotes from the Y strain, after 24 h of parasite-host cell interaction, the infected cultures were washed to remove free parasites and then incubated for another 48 h with increasing concentrations of the test compounds. CM were maintained at 37°C in an atmosphere of 5% CO2 and air, and the medium was replaced every 24 h. Then, untreated and treated infected CM were fixed and stained with Giemsa solution, and the mean numbers of infected host cells and of parasites per infected cell were scored as reported previously (16). Only characteristic T. cruzi nuclei and kinetoplasts were counted as surviving parasites since irregular structures might mean parasites undergoing death. The compound activity was estimated by calculating the infection index (II) (the percentage of infected cells times the average number of intracellular amastigotes per infected host cell) (24). Triplicate assays were run on the same plate, and at least two assays were performed in each analysis.
Mouse acute toxicity.
In order to determine the no-observed-adverse-effect level (NOAEL), each dose of the tested compounds (12.5 to 200 mg/kg of body weight) was injected by the intraperitoneal (i.p.) route individually in Swiss Webster female mice (20 to 23 g). On days 2 and 3, mice were inspected for toxic and subtoxic symptoms according to the Organization for Economic Co-operation and Development (OECD) guidelines. Forty-eight hours after compound injection, the NOAEL values were determined, and plasma biochemical analysis was performed for alanine aminotransferase (ALT), urea, and creatine kinase (CK) as reported previously (16).
Mouse infection and treatment schemes.
Male Swiss mice were obtained from the Fundação Oswaldo Cruz (FIOCRUZ) animal facilities (Rio de Janeiro, Brazil). Mice were housed at a maximum of 6 per cage and kept in a conventional room at 20 to 24°C under a 12 h/12 h light/dark cycle. The animals were provided with sterilized water and chow ad libitum. Infection was performed by i.p. injection of 104 bloodstream trypomastigotes (Y strain). The animals (18 to 21 g) were divided into the following groups: uninfected (noninfected and untreated), untreated (infected with T. cruzi but treated only with vehicle), and treated (infected and treated i.p. with 0.5 to 20 mg/kg/day test compound [up to 0.2 ml] or with 100 mg/kg/day Bz orally [p.o.]). The mouse treatment started at 5 days postinfection (dpi) followed by (i) a 1-s dose at 8 dpi or (ii) five consecutive daily doses. For Bz treatment, infected mice received a 0.2-ml oral dose (gavage) following the same therapeutic schemes as described above (15).
Parasitemia, mortality rates, and ponderal curve analysis.
Parasitemia was individually checked by direct microscopic counting of parasites in 5 μl of blood, as described before (25). Body weight was evaluated weekly, and mortality was checked daily until 30 days posttreatment and expressed as a percentage of cumulative mortality (%CM) (15).
Ethics.
All procedures were carried out in accordance with the guidelines established by the FIOCRUZ Committee of Ethics for the Use of Animals (CEUA LW-16/2013).
RESULTS
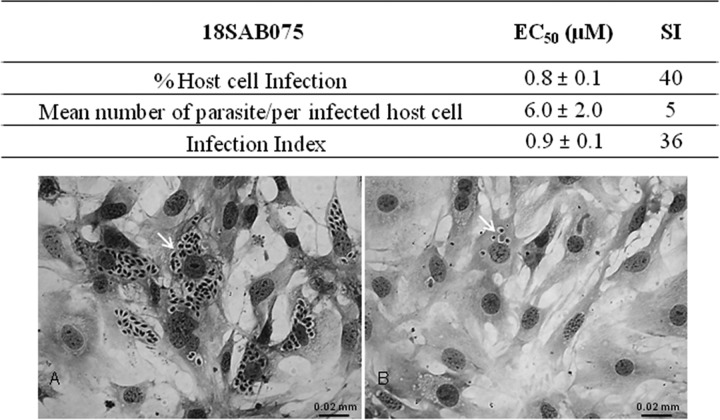
The screening of the eight novel arylimidamides (Fig. 1 to 3) against bloodstream trypomastigotes (Y strain) revealed that after 2 h of incubation at 37°C, 18SAB075 and 16DAP002 showed considerable activity, displaying EC50s of 11 and 14 μM (Table 1). After 24 h of incubation, both were more effective than Bz, displaying EC50s of 0.3 and 3 μM, respectively, with the former molecule exhibiting about 43-fold higher trypanocidal effect than the reference drug Bz (Table 1). In vitro analysis of cytotoxicity on cardiac cell cultures performed by the alamarBlue assay showed that up to 32 μM, none of the studied compounds caused loss of cellular viability (Table 1). An alamarBlue assay performed in the absence of cells, with DMEM containing or not containing each tested compound (at 96 μM), showed no alterations in the absorbance measurements (data not shown). When tested on T. cruzi-infected host cells (the Tulahuen strain transfected with a β-galactosidase gene) under a standardized screening protocol using a fixed concentration of 1 μg/ml (23), again both 16DAP002 and 18SAB075 were effective (Table 2). Next, the two AIAs were screened against Tulahuen-infected host cells in order to determine the EC50s. The findings showed that 18SAB075 was the most active with values of 1.5 μM after 96 h of incubation, comparable to those for Bz and 16DAP002 (Table 3). Further analysis of the effect of 18SAB075 against T. cruzi-infected cardiac cells using another strain (Y strain) confirmed the promising activity (EC50 = 0.9 μM) of this AIA, which was especially notable in the data for the mean number of infected cardiac cells when the infected cultures were incubated with concentrations up to 32 μM without causing loss of host cell viability (Fig. 4A and B). Although no loss of viability was noted up to ≤32 μM as seen by the alamarBlue method, light microscopy analysis demonstrated that 16SAB002 at concentrations >3.5 μM induced cellular vacuolization and impaired the contractibility of the cardiac cells in vitro. Also, no effect on the levels of T. cruzi infection was observed when nontoxic doses (≤3.5 μM) of this AIA were tested (data not shown).
TABLE 1.
Trypanocidal activity (EC50) of the studied arylimidamides against bloodstream trypomastigotes of T. cruzi (Y strain) and their selectivity index related to cardiomyocytes (EC50)
| Compound | EC50 (mean ±SD) (μM) in: |
SI (24 h)a | ||
|---|---|---|---|---|
| Trypomastigotes |
Cardiomyocytes (24 h) | |||
| 2 h | 24 h | |||
| 18SAB075 | 11 ± 1 | 0.3 ± 0 | >32 | >106 |
| 16DAP002 | 14 ± 3 | 3 ± 1 | >32 | >11 |
| 27SMB009 | >100 | 85 ± 21 | >32 | >0.4 |
| 23SMB054 | >100 | >100 | >32 | |
| 23SMB022 | >100 | >100 | >32 | |
| 23SMB026 | >100 | >100 | >32 | |
| 26SMB070 | >100 | >100 | >32 | |
| 16SAB079 | >100 | >100 | >32 | |
| Benznidazole | >100 | 13 ± 2b | 1,000 | 77 |
Treatment for 24 h at 37°C. Selectivity index (SI) = EC50 for cardiomyocytes/EC50 for parasites.
Value from da Silva et al. (16).
TABLE 2.
Activity of arylimidamides (at 1 μg/ml) against intracellular forms of T. cruzi (Tulahuen strain transfected with β-galactosidase) after treatment for 96 h at 37°C
| Compound | Inhibition of the host cell infection (mean ± SD) (%) |
|---|---|
| 16DAP002 | 58 ± 31 |
| 18SAB075 | 50 ± 7 |
| 26SMB070 | 15 ± 8 |
| 16SAB079 | 4 ± 5 |
| 23SMB054 | 3 ± 4 |
| 23SMB022 | 1 ± 1 |
| 27SMB009 | 0 ± 0 |
| 23SMB026 | 0 ± 0 |
| Benznidazole | 82 ± 3 |
TABLE 3.
In vitro effect (EC50s) of arylimidamides against intracellular forms of T. cruzi (Tulahuen strain transfected with β-galactosidase) after treatment for 96 h at 37°C
| Compound | EC50 (mean ± SD) (μM) |
|---|---|
| 16DAP002 | 4.1 ± 1.8 |
| 18SAB075 | 1.5 ± 0.2 |
| Benznidazole | 2.6 ± 0.8 |
FIG 4.
Activity (EC50s) and selective index (SI) of 18SAB075 against intracellular forms of T. cruzi (Y strain) after treatment for 48 h at 37°C. (A) T. cruzi-infected cardiac cells incubated with vehicle. (B) T. cruzi-infected cardiac cells incubated with 32 μM 18SAB075. The arrows point to intracellular parasites. Infection index, host cell infection × number of parasites/infected host cell, as reported in Batista et al. (15).
Next, due to the higher selectivity indexes of 18SAB075 on bloodstream trypomastigotes (>106; Table 1) and on intracellular forms (40; Fig. 4), this compound was moved to in vivo analysis.
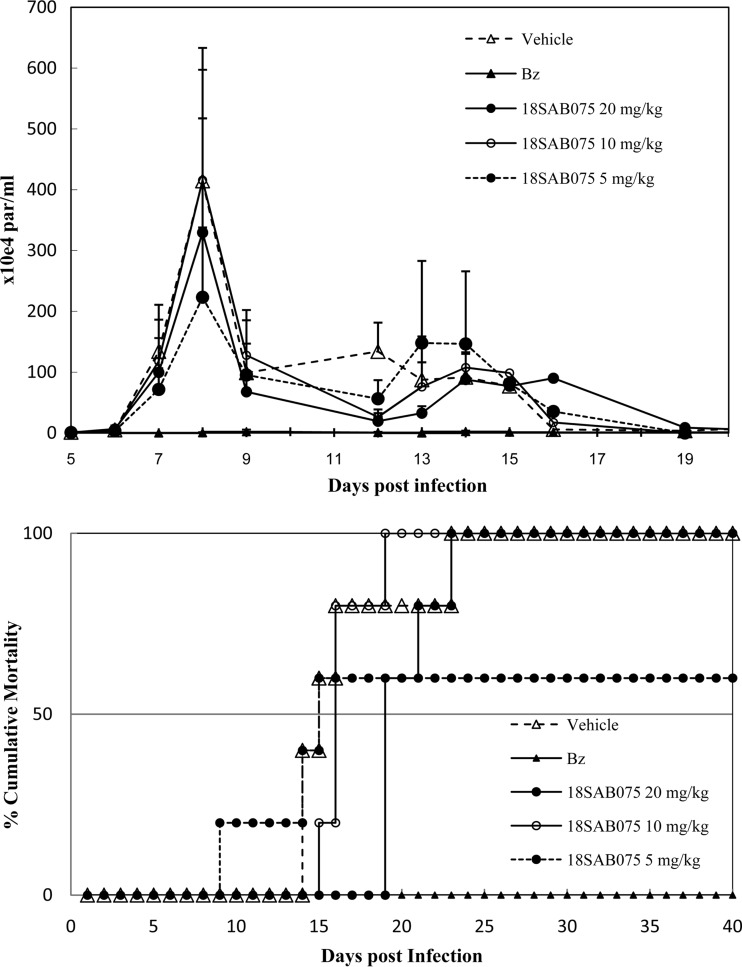
Our first in vivo step was to evaluate aspects of the acute toxicity of 18SAB075, aiming to determine its NOAEL value. The administration of this AIA via the i.p. route to Swiss female mice (12.5 to 200 mg/kg) followed for 48 h showed that none of the doses caused death. Up to 50 mg/kg, no alteration in animal behavior (compared to that of vehicle-treated mice) was found. However, increased doses induced visible neurological disorders like tremors, ataxia, and hyperactivity (data not shown). The gross pathological examination performed at 48 h after drug administration showed that 200 mg/kg induced hemorrhagic hepatic signs (data not shown). This hepatic injury was confirmed by the biochemical plasma analysis of the higher dose as increased levels of aspartate transaminase (AST) and ALT were found compared to those for the animals from the other groups (data not shown). Then, the efficacy of 18SAB075 was tested in Swiss male mice inoculated with 104 bloodstream parasites using a therapeutic scheme employing doses administered once a day, at 5 and 8 dpi (Fig. 5) that correspond to the onset and parasitemia peaks, respectively, in this animal model (15). Only parasitemia-positive mice were used, and Bz treatment was run in parallel using standard protocol (100 mg/kg p.o.), following the same therapeutic scheme as described above (Fig. 5). Although 20 and 10 mg/kg did not reduce or only slightly impaired (20%), respectively, the parasitemia levels, the lower dose (5 mg/kg) resulted in a 50% decrease in the parasitism peak decrease, also leading to 40% animal survival while all the other mice (except for the Bz group) died (Fig. 5).
FIG 5.
In vivo effect of 18SAB075 on T. cruzi infection using male Swiss mice inoculated with 104 bloodstream trypomastigotes (Y strain). The activities of 5, 10, and 20 mg/kg/day 18SAB075 (i.p.) and of benznidazole (100 mg/kg/day by gavage) given at 5 and 8 dpi was evaluated through parasitemia levels (top) and cumulative mortality (bottom).
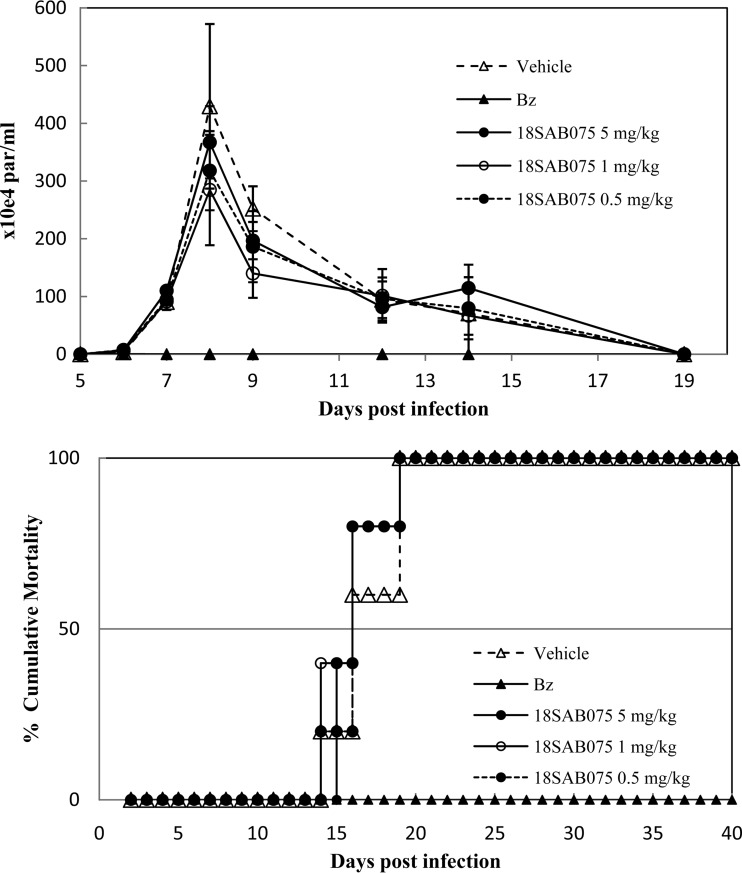
Then, aiming to allow a longer plasma exposure of the compound but taking into consideration the use of maximum nontoxic doses (up to 50 mg/kg), we performed additional studies, providing the AIA for 5 daily consecutive days, starting at 5 dpi (Fig. 6). As expected for this experimental mouse model of T. cruzi acute infection, infected and vehicle-treated animals presented high parasitemia and no animal survival (Fig. 5 and 6). When 18SAB075 was administered via the i.p. route, none of the doses reduced the parasitemia or protected against mortality. As expected, Bz completely suppressed the parasitemia and conferred 100% survival (Fig. 6). Also, none of the doses were able to protect the animals against the body weight loss (data not shown) induced by the T. cruzi infection in this experimental acute model (26).
FIG 6.
In vivo effect of 18SAB075 on T. cruzi infection using male Swiss mice inoculated with 104 bloodstream trypomastigotes (Y strain). The activities of 0.5, 1, and 5 mg/kg/day 18SAB075 (i.p.) and of benznidazole (100 mg/kg/day by gavage) given for 5 consecutive days (5 to 9 dpi) were evaluated through parasitemia levels (top) and cumulative mortality (bottom).
DISCUSSION
Structural variations in the presently available AIAs include seven different linkers between the two AIA moieties and three different outer aromatic rings. Those molecules containing multiple or fused aromatic rings in their linkers were active against the bloodstream and intracellular parasites. The most active compound, 18SAB075, contains a diphenylacetylene linker, which offers extended conjugation. The second most active compound, 16DAP002, contains a carbazole system as its linker, which also offers extended conjugation but a more rigid conformation due to the fused rings. Both compounds displayed AIA nitrogen atoms attached directly to aromatic rings. The xylene derivative 26SMB070, which was less active, bears an aromatic ring in its linker, but the nitrogen atoms are attached to aliphatic carbons. Analogues of the two most active compounds containing the same linkers but different outer rings were not included in this study.
Chemotherapeutic options for treating chagasic patients currently depend on only two nitro derivative drugs, namely, benznidazole (2-nitroimidazole; Laboratório Farmacêutico do Estado de Pernambuco [LAFEPE], Brazil) and nifurtimox (5-nitrofuran, Bayer 2502; Bayer, Germany) (27). Both cause severe side effects, resulting in discontinuation of the treatment and low effectiveness, especially in the later chronic phase of the disease, demanding the identification of novel drugs to treat this devastating chronic pathology. However, there is a lack of interest from most pharmaceutical companies for discovery of new anti-T. cruzi agents mainly due to the long time and high costs associated with the drug pipeline for this and other neglected parasitic illnesses that afflict millions of the poorest people worldwide (28). In this context, our aim was to explore the possibility of finding new amidine derivatives with considerable activity against T. cruzi in vitro and in vivo which possess the potential to act as clinical candidates against this neglected parasitic disease. In this vein, eight novel AIAs were screened against the bloodstream and intracellular forms of the parasite and two of them, 18SAB075 and 16DAP002, demonstrated higher activity, with EC50s of <4 μM. The two compounds were effective against different strains (Y and Tulahuen), and due to its high selectivity, 18SAB075 was further examined in in vivo analyses for preliminary ascertainment of acute toxicities and for efficacy determination using an acute mouse model of T. cruzi infection (male Swiss mice infected with Y strain). The follow-up of acute toxicity signs for 48 h after 18SAB075 administration via the i.p. route showed that although at up to 50 mg/kg, no alterations were noticed, considerable toxic side effects like ataxia, hyperactivity, and tremors were found with doses of 100 and 200 mg/kg. The gross pathological examination confirmed that the higher dose also induced hemorrhagic hepatic signs that were corroborated by increased plasma levels of hepatic markers like AST. Thus, efficacy analysis was further explored using only doses ≤20 mg/kg/day and never exceeding cumulative doses of 50 mg/kg. Bz was included as a reference drug, given at its effective dose of 100 mg/kg/day (23). The i.p. administration of 18SAB075 in all assayed therapeutic schemes (2 and 5 days of treatment) using nontoxic doses failed to demonstrate the activity of this diphenylacetylene AIA in vivo, while Bz not only suppressed the parasitemia but also induced 100% protection against mortality due to the experimental infection as described previously (26). Previous data using bis-AIAs like 2,5-bis[2-(2-propoxy)-4-(2-pyridylimino)aminophenyl]furan (DB 766) (15) and a related analog (DB 1831) (16) showed their high activity in vivo in the acute experimental mouse models of T. cruzi, exhibiting trypanocidal effects similar to that of Bz. In fact, with the same highly stringent experimental in vivo model as presently used for 18SAB075, the two previous studies reported that the presence of pyrimidine and pyridine units of bis-AIAs seemed to be advantageous for T. cruzi activity (14), supporting the continuity of preclinical studies of novel related molecules with the aim of finding new alternatives for treating Chagas disease.
Supplementary Material
ACKNOWLEDGMENTS
The present study was supported by grants from the Fundação Carlos Chagas Filho de Amparo a Pesquisa do Estado do Rio de Janeiro (FAPERJ), the Conselho Nacional Desenvolvimento Científico e Tecnológico (CNPq), and the Fundação Oswaldo Cruz, PDTIS, PROEP/CNPq/Fiocruz, and CAPES. Support was also received from the Bill and Melinda Gates Foundation through a subcontract with the Consortium for Parasitic Drug Development (CPDD).
Footnotes
Published ahead of print 21 April 2014
Supplemental material for this article may be found at http://dx.doi.org/10.1128/AAC.02353-14.
REFERENCES
- 1.De Souza W. 2009. Structural organization of Trypanosoma cruzi. Mem. Inst. Oswaldo Cruz 104:89–100. 10.1590/S0074-02762009000900014 [DOI] [PubMed] [Google Scholar]
- 2.Dias JC. 2007. Southern cone initiative for the elimination of domestic populations of Triatoma infestans and the interruption of transfusion Chagas disease: historical aspects, present situation, and perspectives. Mem. Inst. Oswaldo Cruz 102:11–18. 10.1590/S0074-02762007005000092 [DOI] [PubMed] [Google Scholar]
- 3.Gascón J, Albajar P, Cañas E, Flores M, Gómez i Prat J, Herrera RN, Lafuente CA, Luciardi HL, Moncayo A, Molina L, Muñoz J, Puente S, Sanz G, Treviño B, Sergio-Salles X. 2007. Diagnosis, management and treatment of chronic Chagas' heart disease in areas where Trypanosoma cruzi infection is not endemic. Rev. Esp. Cardiol. 60:285–293. 10.1157/13100280 [DOI] [PubMed] [Google Scholar]
- 4.Rodriguez-Morales AJ, Benitez JA, Tellez I, Franco-Paredes C. 2008. Chagas disease screening among Latin American immigrants in non-endemic settings. Travel Med. Infect. Dis. 6:162–163. 10.1016/j.tmaid.2008.02.009 [DOI] [PubMed] [Google Scholar]
- 5.Guerri-Guttenberg RA, Grana DR, Ambrosio G, Milei J. 2008. Chagas cardiomyopathy: Europe is not spared! Eur. Heart J. 29:2587–2591. 10.1093/eurheartj/ehn424 [DOI] [PubMed] [Google Scholar]
- 6.Milei J, Guerri-Guttenberg RA, Grana DR, Storino R. 2009. Prognostic impact of Chagas disease in the United States. Am. Heart J. 157:22–29. 10.1016/j.ahj.2008.08.024 [DOI] [PubMed] [Google Scholar]
- 7.Nóbrega AA, Garcia MH, Tatto E, Obara MT, Costa E, Sobel J, Araujo WN. 2009. Oral transmission of Chagas disease by consumption of açaí palm fruit, Brazil. Emerg. Infect. Dis. 15:653–655. 10.3201/eid1504.081450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Teixeira AR, Nitz N, Guimaro MC, Gomes C, Santos-Buch CA. 2006. Chagas disease. Postgrad. Med. J. 82:788–798. 10.1136/pgmj.2006.047357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rocha MO, Teixeira MM, Ribeiro AL. 2007. An update on the management of Chagas cardiomyopathy. Expert Rev. Anti Infect. Ther. 5:727–743. 10.1586/14787210.5.4.727 [DOI] [PubMed] [Google Scholar]
- 10.Soeiro MNC, de Castro SL, de Souza EM, Batista DGJ, da Silva CF, Boykin DW. 2008. Diamidines activity upon trypanosomes: The state of the art. Curr. Mol. Pharmacol. 1:151–161. 10.2174/1874467210801020151 [DOI] [PubMed] [Google Scholar]
- 11.Filardi LS, Brener Z. 1987. Susceptibility and natural resistance of Trypanosoma cruzi strains to drugs used clinically in Chagas disease. Trans. R. Soc. Trop. Med. Hyg. 81:755–759. 10.1016/0035-9203(87)90020-4 [DOI] [PubMed] [Google Scholar]
- 12.Rodriques Coura J, de Castro SL. 2002. A critical review on Chagas disease chemotherapy. Mem. Inst. Oswaldo Cruz 97:3–24. 10.1590/S0074-02762002000100001 [DOI] [PubMed] [Google Scholar]
- 13.Soeiro MNC, de Castro SL. 2009. Trypanosoma cruzi targets for new chemotherapeutic approaches. Expert Opin. Ther. Targets 13:105–121. 10.1517/14728220802623881 [DOI] [PubMed] [Google Scholar]
- 14.Soeiro MNC, Werbovetz K, Boykin OW, Wifson WO, Wang MZ, Hemphifl A. 2013. Novel amidines and analogues as promising agents against intracellular parasites: a systematic review. Parasitology 140:929–951. 10.1017/S0031182013000292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Batista DGJ, Batista MM, Oliveira GM, Borges P, Lannes-Vieira J, Britto CC, Junqueira A, Lima MM, Romanha AJ, Sales Junior PA, Stephens CE, Boykin DB, Soeiro MNC. 2010. Arylimidamide DB766: a potential chemotherapeutic candidate for Chagas disease treatment. Antimicrob. Agents Chemother. 54:2940–2952. 10.1128/AAC.01617-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.da Silva CF, Batista DGJ, Oliveira GM, de Souza EM, Hammer ER, da Silva PB, Daliry A, Araujo JS, Britto C, Rodrigues AC, Liu Z, Farahat AA, Kumar A, Boykin DW, Soeiro MNC. 2012. In vitro and in vivo investigation of the efficacy of arylimidamide DB1831 and its mesylated salt form-DB1965-against Trypanosoma cruzi infection. PLoS One 7:e30356. 10.1371/journal.pone.0030356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Leclerc M, Morin JF. August 2002. Conjugated polycarbazole derivatives and process for the preparation thereof. US patent 2002/0103, 332A1
- 18.Stephens CE, Tanious F, Kim S, Wilson WD, Schell WA, Perfect JR, Franzblau SG, Boykin DW. 2001. Diguanidino and “reversed” diamidino 2,5-diarylfurans as antimicrobial agents. J. Med. Chem. 44:1741–1748. 10.1021/jm000413a [DOI] [PubMed] [Google Scholar]
- 19.Chandra R, Oya S, Kung MP, Hou C, Jin LW, Kung HF. 2007. New diphenylacetylenes as probes for positron emission tomographic imaging of amyloid plaques. J. Med. Chem. 50:2415–2423. 10.1021/jm070090j [DOI] [PubMed] [Google Scholar]
- 20.Nishimura D, Oshikiri T, Takashima Y, Hashidzume A, Yamaguchi H, Harada A. 2008. Relative rotational motion between α-cyclodextrin derivatives and a stiff axle molecule. J. Org. Chem. 73:2496–2502. 10.1021/jo702237q [DOI] [PubMed] [Google Scholar]
- 21.Lange JHM, van Stuivenberg HH, Coolen HKAC, Adolfs TJP, McCreary AC, Keizer HG, Wals HC, Veerman W, Borst AJM, de Looff W, Verveer PC, Kruse CG. 2005. Bioisosteric replacements of the pyrazole moiety of rimonabant: synthesis, biological properties, and molecular modeling investigations of thiazoles, triazoles, and imidazoles as potent and selective cb1 cannabinoid receptor antagonists. J. Med. Chem. 48:1823–1838. 10.1021/jm040843r [DOI] [PubMed] [Google Scholar]
- 22.Meirelles MNL, Araujo-Jorge TC, Miranda CF, dE Souza W, Barbosa HS. 1986. Interaction of Trypanosoma cruzi with heart muscle cells: ultrastructural and cytochemical analysis of endocytic vacuole formation and effect upon myogenesis in vitro. Eur. J. Cell Biol. 41:198–206 [PubMed] [Google Scholar]
- 23.Romanha AJ, Castro SL, Soeiro Mde N, Lannes-Vieira J, Ribeiro I, Talvani A, Bourdin B, Blum B, Olivieri B, Zani C, Spadafora C, Chiari E, Chatelain E, Chaves G, Calzada JE, Bustamante JM, Freitas-Junior LH, Romero LI, Bahia MT, Lotrowska M, Soares M, Andrade SG, Armstrong T, Degrave W, Andrade ZA. 2010. In vitro and in vivo experimental models for drug screening and development for Chagas disease. Mem. Inst. Oswaldo Cruz 105:233–238. 10.1590/S0074-02762010000200022 [DOI] [PubMed] [Google Scholar]
- 24.da Silva CF, Batista MM, Mota RA, de Souza EM, Stephens CE, Som P, Boykin DW, Soeiro MNC. 2007. Activity of “reversed” diamidines against Trypanosoma cruzi in vitro. Biochem. Pharmacol. 73:1939–1946. 10.1016/j.bcp.2007.03.020 [DOI] [PubMed] [Google Scholar]
- 25.De Souza EM, Menna-Barreto R, Araújo-Jorge TC, Kumar A, Hu Q, Boykin DW, Soeiro MNC. 2006. Antiparasitic activity of aromatic diamidines is related to apoptosis-like death in Trypanosoma cruzi. Parasitology 133:75–79. 10.1017/S0031182006000084 [DOI] [PubMed] [Google Scholar]
- 26.da Silva CF, Batista DJ, Siciliano JA, Batista MM, Lionel J, de Souza EM, Hammer ER, da Silva PB, de Mieri M, Adams M, Zimmermann S, Hamburger M, Brun R, Schühly W, Soeiro MNC. 2013. Effects of psilostachyin A and cynaropicrin against Trypanosoma cruzi in vitro and in vivo. Antimicrob. Agents Chemother. 57:5307–5314. 10.1128/AAC.00595-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Soeiro MNC, de Castro SL. 2011. Screening of potential anti-Trypanosoma cruzi candidates: in vitro and in vivo studies. Open Med. Chem. J. 5:21–30. 10.2174/1874104501105010021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Soeiro MN, de Souza EM, da Silva CF, Batista DG, Batista MM, Pavão BP, Araújo JS, Aiub CA, da Silva PB, Lionel J, Britto C, Kim K, Sulikowski G, Hargrove TY, Waterman MR, Lepesheva GI. 2013. In vitro and in vivo studies of the antiparasitic activity of sterol 14α-demethylase (CYP51) inhibitor VNI against drug-resistant strains of Trypanosoma cruzi. Antimicrob. Agents Chemother. 57:4151–4163. 10.1128/AAC.00070-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.