Abstract
The purpose of this study was to investigate the pharmacokinetic properties of colistin following intrapulmonary administration of colistin sulfate in rats. Colistin was infused or delivered in nebulized form at a dose of 0.35 mg/kg of body weight in rats, and plasma drug concentrations were measured for 4 h after administration. Bronchoalveolar lavages (BAL) were also conducted at 0.5, 2, and 4 h after intravenous (i.v.) administration and administration via nebulized drug to estimate epithelial lining fluid (ELF) drug concentrations. Unbound colistin plasma concentrations at distribution equilibrium (2 h postdosing) were almost identical after i.v. infusion and nebulized drug inhalation. ELF drug concentrations were undetectable in BAL samples after i.v. administration, but they were about 1,800 times higher than unbound plasma drug levels at 2 h and 4 h after administration of the nebulized drug. Simultaneous pharmacokinetic modeling of plasma and ELF drug concentrations was performed with a model characterized by a fixed physiological volume of ELF (VELF), a passive diffusion clearance (QELF) between plasma and ELF, and a nonlinear influx transfer from ELF to the central compartment, which was assessed by reducing the nebulized dose of colistin by 10-fold (0.035 mg kg−1). The km was estimated to be 133 μg ml−1, and the Vmax, in-to-Km ratio was equal to 2.5 × 10−3 liter h−1 kg−1, which was 37 times higher than the QELF (6.7 × 10−5 liter h−1 kg−1). This study showed that with the higher ELF drug concentrations after administration via nebulized aerosol than after intravenous administration, for antibiotics with low permeability such as colistin, nebulization offers a real potential over intravenous administration for the treatment of pulmonary infections.
INTRODUCTION
Treatment of lung infections by administration of antimicrobial agents using the pulmonary route presents potential clinical use, since it may afford higher drug concentrations at the site of infection with a reduction of systemic exposure (1, 2). Consequently, increased use of inhaled antibiotics, such as tobramycin, aztreonam, or colistin methansulfonate (CMS), has been observed to treat lung infections (3, 4).
CMS is the inactive prodrug of colistin (5), which is a multicomponent cationic polypeptide that belongs to the class of polymyxins, comprised mainly of colistin A and colistin B (6). It is an old antibiotic that was abandoned in the 1970s due to its adverse effects (7). During the last 15 years, it has occasionally been used as “salvage” therapy for the treatment of infections engendered by multidrug-resistant bacteria, due to the lack of new antibiotics (8). When CMS was developed, controlled clinical trials and determination of pharmacokinetic and pharmacodynamic (PK/PD) properties were not required by drug-regulating agencies (6). However, the understanding of colistin pharmacokinetics after CMS intravenous (i.v.) administration to critically ill patients is progressing (9–13). But CMS is also nebulized for the treatment of pulmonary infections in patients with cystic fibrosis (CF) (3, 4, 14) and in patients with ventilator-associated nosocomial pneumonia (VAP) (15, 16) or tracheobronchitis (17) caused by Gram-negative bacteria (4). Pharmacokinetic studies have been conducted in rats (18), in piglets (19), and in human patients (15, 20) after CMS nebulization, and a recent study was conducted after direct administration of a nebulized aerosol of colistin in rats (21).
Administration of nebulized colistin sulfate leads to more severe side effects, such as bronchoconstriction, throat irritation, and cough, than nebulization of CMS (22), which explains why inhaled colistin therapy in patients is almost always conducted after nebulization of CMS instead of colistin sulfate (15–17, 20, 23, 24). However, the data interpretation is then relatively complex, since it is difficult to assess how much of the CMS dose is converted into colistin systemically and presystemically. Therefore, this new study was conducted after direct administration of nebulized colistin in order to better investigate its pharmacokinetic properties after nebulization.
MATERIALS AND METHODS
Chemicals.
Colistin sulfate salt was purchased from Sigma. The colistin solutions were prepared in 0.9% NaCl at concentrations of 0.15 mg ml−1 for i.v. administration and at concentrations of 0.15 mg ml−1 and 1.5 mg ml−1 for administration via nebulized aerosol. The chemicals used in this study were of analytical grade, and the solvents were of high-performance liquid chromatography (HPLC) grade.
Animals.
This work was performed in agreement with the National Research Council's Guide for the Care and Use of Laboratory Animals (25) under license 86.051. Male Sprague-Dawley rats (n = 56) from Janvier Laboratories (Le Genest-St.-Isle, France), with weights of 324 ± 24 g (mean ± standard deviation), were used for the investigations. Before the experiment (5 days), the animals were acclimatized as previously described (18). During the experiments the animals had free access to food (A03; Safe, Villemoisson-sur-Orge, France) and water.
Implantation of femoral vein and artery catheters.
The day before the experiment for the systemic PK study, polyethylene catheters were implanted into the femoral vein and artery in anesthetized rats as previously described (18). For the BAL study, catheters were only inserted into the femoral vein of rats receiving a colistin bolus administration. After surgery, the animals were placed into individual cages.
Colistin administration and collection of samples for the plasma PK study. (i) Intravenous infusion administration of colistin (n = 6).
The i.v. infusion administration of 0.35 mg/kg of body weight [base mean dose]), via the left femoral vein, was performed over 30 min with a flow rate of 2 ml h−1. Arterial blood samples were collected before administration and 0.25, 0.5, 1, 2, 3, and 4 h after administration. Plasma was separated by centrifugation (1,000 × g, 15 min) and frozen at −80°C until analysis.
Intratracheal administration of nebulized colistin (n = 6).
Following a short sedation period with isoflurane (3%; air at 550 ml min−1), a volume of 100 μl of colistin at a dose of 0.35 mg kg−1 (mean colistin base dose) was instilled between the vocal cords by using a nebulizer (Penn Century Inc., Philadelphia, PA) as previously described (18, 26). Arterial blood samples were then collected before administration and 0.25, 0.5, 1, 2, 3, and 4 h after administration. Plasma was treated as described above.
(ii) Colistin administration and collection of samples for determination of local concentrations in BAL fluid (n = 44).
Rats received colistin (0.35 mg kg−1 [base dose]) by intratracheal administration of nebulized drug under anesthesia (isoflurane at 3%, air at 550 ml min−1) or by i.v. infusion (30 min) without anesthesia. BAL fluid collection was performed according to methods described in previous studies (18, 26). Briefly, the animals were anesthetized or reanesthetized by using isoflurane, and a volume of 1 ml of NaCl (0.9%) at 37°C was instilled into the trachea via a catheter at a 50-mm depth. The highest possible volume was collected. BAL fluid collection was carried out at 0.5, 2, and 4 h after administration (4 to 6 rats per group), and colistin and urea concentrations were determined. After collection of BAL fluid, plasma used for colistin and urea concentration measurements was obtained by centrifugation (1,000 × g, 15 min) of blood obtained via heart puncture.
Extra rats (n = 14) received a 10-fold-lower dose of colistin (0.035 mg kg−1) via nebulized drug. BAL fluid and plasma samples were collected from this group as described above.
Analytical assays. (i) Colistin analysis in plasma.
Determination of colistin concentrations in plasma were performed by a liquid chromatography-tandem mass spectrometry (LC-MS/MS) method previously described (27) with slight adjustments. The system consisted of a Waters Alliance 2695 separations module, and a Waters Micromass Quattro micro API tandem mass spectrometer was used. The chromatography of the reversed phase was performed with a Jupiter 300-Å column (5.0 μm, 50 mm by 2.0-mm inner diameter [ID]; Waters, St.-Quentin en Yvelines, France) and a mobile phase consisting of 0.1% (vol/vol) formic acid in acetonitrile–0.1% formic acid in water (25:75, vol/vol). The mass spectrometer was used in the positive/ion mode. The analysis of ions was made by multiple reactions monitoring (MRM), and their transitions were m/z 585.4/101.1 for colistin A, 578.4/101.1 for colistin B, and 602.4/101.1 for polymyxin B1, the compound used as an internal standard. The calibration standard curve was prepared with seven points in rat plasma with drug concentrations ranging between 0.0097 and 10 μg ml−1. The controls were prepared at the following concentrations: 0.156, 0.625, and 3.75 μg ml−1. A volume of 250 μl of calibration standard, control, or sample was mixed into 750 μl of phosphate buffer and 25 μl of internal standard solution (3.12 ng ml−1). The mixture obtained was centrifuged (1,500 × g for 5 min) and loaded onto an Oasis HLB extraction cartridge (30-μm filter; Waters, St.-Quentin en Yvelines, France). Analytes were washed with methanol/water/water (0.5 ml/0.5 ml/0.5 ml) and eluted with 1 ml of 0.5% (vol/vol) formic acid in methanol. A nitrogen stream at 45°C was used to evaporate the eluates, which were redissolved with 200 μl of water containing 0.1% formic acid (vol/vol) after evaporation. The precision and accuracy were less than 15% for the between-day variabilities, which were characterized at the 3 concentration levels.
(ii) Colistin analysis in BAL fluid.
Analysis of colistin in BAL fluid was performed the same as for plasma, with six-point calibration standard curves prepared in NaCl (0.9%) with the same range of concentrations (between 0.0097 and 10 μg ml−1) and three levels of control (0.312, 1.25, and 5 μg ml−1). A volume of 150 μl of NaCl (0.9%) or BAL sample was mixed with 100 μl of plasma, 750 μl of phosphate buffer, and 25 μl of internal standard solution (polymyxin B1; 3.12 ng ml−1). Mixtures were loaded onto an Oasis HLB extraction cartridge (30 μm; Waters, St.-Quentin en Yvelines, France), and sequential washes were made according to the protocol described above. Eluates were then similarly evaporated and harvested in 200 μl of water containing 0.1% (vol/vol) formic acid. The precision and accuracy were less than 15% for the between-day variabilities characterized for the 3 concentrations.
(iii) Urea analysis in BAL fluid and plasma.
The concentrations of urea were determined in BAL fluid by using LC-MS/MS as previously described (18, 26) with one modification, corresponding to the addition of thiourea as an internal standard. The analysis of ions was performed by MRM, and the mass spectrometer was used in the positive/ion mode. The transition of ions was m/z 61 to >44. Reversed-phase chromatography was carried out with a C18 Xterra MS column (5.0 μm, 150 by 4.6-mm ID; Waters, St.-Quentin en Yvelines, France) and a mobile phase of acetonitrile containing formic acid (0.1%, vol/vol) and water containing formic acid (0.1% vol/vol; 10:90; flow rate, 0.25 ml min−1). The standard curves were prepared in NaCl (0.9%) with concentrations ranging between 1.25 and 100 μg ml−1 (eight-point calibration). A volume of 190 μl of internal standard (0.5 μg ml−1of thiourea) was mixed into standard solutions or BAL samples (10 μl) and directly injected (30 μl). Controls were prepared at four levels (75, 25, 2.5, and 1.25 μg ml−1). The precision and accuracy were less than 15% for the between-day variabilities characterized at the 4 concentrations.
The concentrations of urea in plasma were measured by photometric detection by using an modular automatic analyzer (Roche, France).
Calculation of colistin concentrations in ELF.
The volume of ELF (VELF) was estimated by using urea as a marker of dilution, according to equation 1 (28).
| (1) |
where UreaBAL and Ureaplasma correspond to the concentrations of urea determined in BAL fluid and plasma.
Colistin concentrations in ELF (CELF) were derived from measured concentrations in BAL fluid (CBAL) after correction for dilution, according to equation 2.
| (2) |
Simultaneous PK modeling of plasma and ELF concentrations of colistin.
Concentration-versus-time data for colistin in plasma and ELF were simultaneously analyzed by a nonlinear mixed-effects method with the S-ADAPT software (version 1.52) and the MC-PEM (Monte-Carlo parametric expectation maximization) estimation algorithm within the S-ADAPT TRAN translator (29). Whether the colistin PK in plasma was mono- or bicompartmental was determined, while the colistin PK in ELF was assumed to be monocompartmental. Likelihood ratio tests were used to compare models, with a P value of 0.01 required for statistical significance. Only unbound drug in plasma was assumed to distribute in the plasma and lung compartments, and the colistin unbound fraction in plasma was fixed at 45% (30).
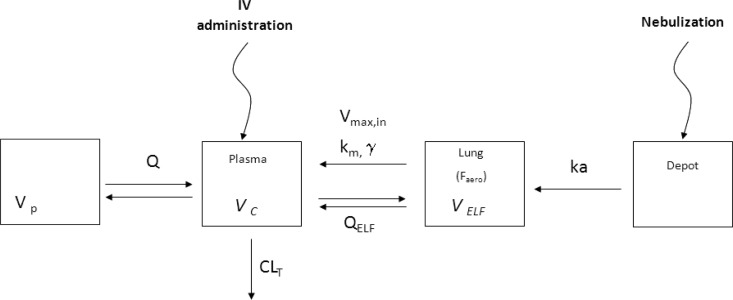
The structural PK model (Fig. 1.) was derived from an initial generic hybrid compartment model, with an ELF compartment characterized by a fixed physiological volume (VELF), estimated at 30 × 10−6 liters kg−1, and connected to a traditional compartment model by a reversible first-order process (26). The residual variability was estimated with a combined additive plus proportional error model in plasma and a proportional error model in ELF. Plasma drug concentrations below the limit of quantification (LOQ) were handled by using the Beal M3 method (31). Typical values and interindividual variabilities of PK parameters are reported along with the precisions of the estimates, expressed as relative standard errors (RSE).
FIG 1.
Simultaneous PK model of plasma and ELF colistin concentrations after i.v. administration or intratracheal administration of nebulized colistin, where Vc is the volume of the central compartment, VELF is the fixed volume of the ELF compartment (30 μl/kg), and Vp is the volume of the peripheral compartment. CLT corresponds to total plasma clearance, Q is the equilibrium distribution clearance between the central and peripheral compartments, and QELF is the diffusion clearance between the central compartment and ELF. Vmax, in is the maximum transfer rate from the ELF to the plasma compartment, km corresponds to the concentration at which the rate is half the maximum rate, and γ is the slope factor. Faero is the systemic bioavailability after aerosol administration.
The final model consisted of a two-compartment model, characterized by a central compartment volume (Vc) connected to a peripheral compartment volume (Vp) with an equilibrium distribution clearance (Q) and to an ELF compartment (fixed VELF) by two-way diffusion clearance (QELF). A nonlinear influx transfer from ELF to the central compartment was implemented with a rate (Vin) characterized by equation 3.
| (3) |
where Vmax, in is the maximum influx transfer rate, km corresponds to the concentration for which Vin = 0.5(Vmax, in), and γ is the slope factor. Elimination from the central compartment was characterized by the total systemic clearance (CLT). A depot compartment and a systemic bioavailability (Faero) were added to the model after nebulization of the drug.
Areas under the unbound drug plasma concentration-time curve (AUCplasma) and ELF drug concentration-time curve (AUCELF) were calculated by using the model for the 0.35 mg · kg−1 i.v. dose (AUCplasma, 0.35), the same high dose that was administered as the nebulized aerosol (AUCELF, 0.35), and the 10-fold-reduced nebulized drug dose (AUCELF, 0.035).
Statistical analysis.
Maximum plasma colistin concentrations observed for the two routes of administration were compared using the Mann-Whitney, nonparametric test (Prism5; GraphPad, La Jolla, CA). Differences were considered significant at a P level of <0.05. Results are expressed as means ± standard deviations (SD).
RESULTS
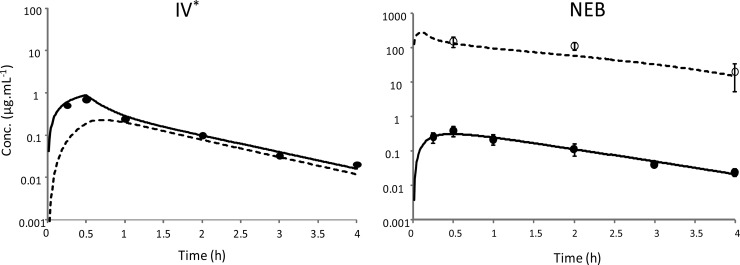
The peak colistin plasma concentration after nebulization occurred early, with a time to peak concentration (tmax) of 0.5 h. The maximum plasma concentration (Cmax) was significantly lower (P < 0.05) after administration of nebulized drug than after i.v. infusion, although the difference was relatively modest (0.20 ± 0.05 μg ml−1 versus 0.31 ± 0.05 μg ml−1) (Table 1). Estimated unbound colistin plasma concentrations at distribution equilibrium (2 h postdosing) were almost identical after i.v. administration and nebulized drug inhalation (0.05 ± 0.01 μg ml−1 and 0.06 ± 0.02 μg ml−1, respectively) (Table 1). ELF drug concentrations were too low to be quantified in BAL samples after i.v. administration, but they were much higher (about 1,700 times higher) than unbound plasma drug concentrations at 2 h and 4 h postadministration of the nebulized drug (Table 1). After pooling data for various doses and routes of administration, the VELF was estimated at 28.4 ± 16.6 × 10−6 1iter kg−1, on average.
TABLE 1.
Colistin concentrations in ELF and in plasma (unbound) after intratracheal or i.v. administrationa
| Time postadministration (h) | i.v. |
NEB |
||||
|---|---|---|---|---|---|---|
| CELF (μg/ml) | C(plasma, unbound) (μg/ml) | CELF/C(plasma, unbound) ratio | CELF (μg/ml) | C(plasma, unbound) (μg/ml) | CELF/C(plasma, unbound) ratio | |
| 0.5 | <LOQ | 0.31 ± 0.05 | NA | 153 ± 49 | 0.20 ± 0.05† | 825 ± 345 |
| 2 | <LOQ | 0.05 ± 0.01 | NA | 112 ± 29 | 0.06 ± 0.02 | 1,794 ± 572 |
| 4 | ND | <LOQ | NA | 20 ± 14 | 0.012 ± 0.004 | 1,770 ± 611 |
Colistin concentrations in ELF and in plasma (unbound) at 0.5, 2, and 4 h after intratracheal administration of the nebulized form (NEB) or i.v. administration (dose, 0.35 mg/kg). Values are means ± standard deviations. C(plasma, unbound) was estimated by multiplying experimental total concentrations by 0.45 (corresponding to the colistin unbound fraction in plasma) (30). †, significantly different from the unbound drug plasma concentration after i.v. administration; LOQ, limit of quantification; ND, not done; NA, not available.
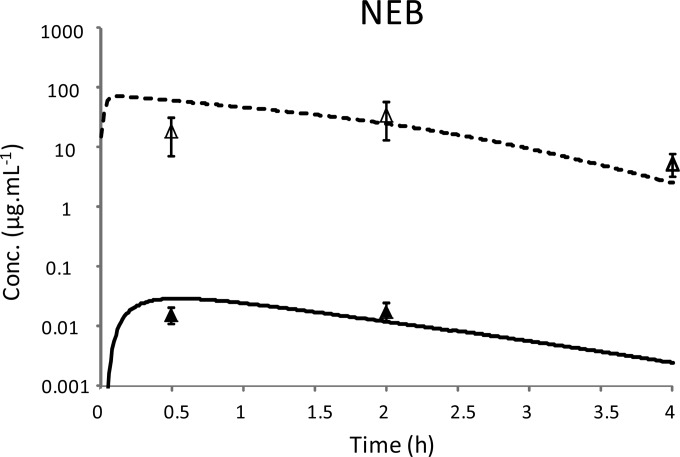
PK modeling using the modified hybrid PK compartment model provided a relatively satisfactory fit of plasma and ELF colistin concentrations after nebulization and of plasma drug concentrations after i.v. administration (Fig. 2 and 3). Estimation of PK parameters after administration of nebulized drug or i.v. administration are presented in Table 2. A nonlinear transfer rate between the ELF compartment and plasma was characterized, with a km estimate at 133 μg ml−1 (Table 2). The Vmax, in-to-Km ratio was equal to 2.5 × 10−3 liter h−1 kg−1, that is, 37 times higher than the QELF (6.7 × 10−5 liters h−1 kg−1). A low residual variability and accurate estimations were obtained for all parameters, and the relative standard error ranged from 1 to 18% (Table 2).
FIG 2.
Predicted concentration-time profiles of colistin (0.35 mg kg−1), administered i.v. and intratracheally in nebulized form, in plasma (solid line) and in ELF (dashed line) from simultaneous PK modeling. Closed and open symbols represent mean ± SD values for experimental concentrations in total plasma and in ELF, respectively. *, BAL drug concentrations after i.v. administration were below the limit of quantification and ELF concentrations could not be estimated.
FIG 3.
Predicted concentration-time profiles of colistin administered intratracheally in nebulized form at a dose of 0.035 mg kg−1 in plasma (solid line) and in ELF (dashed line) from simultaneous PK modeling. Closed and open symbols represent mean ± SD of experimental concentrations in total plasma and in ELF, respectively.
TABLE 2.
Estimated PK parameters after intratracheal (nebulized form) or i.v. administration of colistin at 0.35 mg/kg, based on simultaneous modeling
| Parametera (units) | Typical value (RSEb [%]) | % interindividual variability (RSEb [%]) |
|---|---|---|
| Vc (liters/kg) | 0.19 (7) | 11 (90) |
| Vp (liters/kg) | 0.19 (3) | |
| VELF (liters/kg) | 30 × 10−6 (fixed) | |
| Faero (%) | 69 (5) | 19 (43) |
| CLT (liters/h/kg) | 0.43 (1) | |
| Q (liters/h/kg) | 0.29 (2) | |
| QELF (liters/h/kg) | 6.7 × 10−5 (2) | |
| ka (h−1) | 1.6 (7) | 31 (42) |
| Vmax, in (mg/h/kg) | 0.33 (2) | |
| km (μg/ml) | 133 (18) | 84 (29) |
| γ | 3.9 (1) | |
| Residual errors | ||
| Plasma | ||
| Additive residual error (μg/ml) | 0.003 | |
| Proportional residual error (%) | 15 | |
| ELF | ||
| Proportional residual error (%) | 14 |
Vc, volume of distribution in central compartment; Vp, volume of distribution of peripheral compartment; VELF, volume of distribution in ELF compartment; Faero, systemic bioavailability after nebulization; CLT, total systemic clearance; Q, clearance of distribution between central and peripheral compartments; QELF, diffusion clearance between central compartment and ELF compartment; ka, first-order transfer rate constant from depot compartment toward ELF compartment; Vmax, in, maximal rate of transfer from ELF compartment toward central compartment; km, ELF concentration for which the rate of transfer from the ELF compartment toward the central compartment is Vmax/2; γ, the slope factor.
RSE, relative standard error, expressed as the coefficient of variation.
AUCELF, 0.35-to-AUCplasma, 0.35 ratios after i.v. administration and administration of the nebulized drug were equal to 1 and 1,214, respectively.
DISCUSSION
In clinical practice, colistin is aerosolized as CMS, which implies that this prodrug needs to be converted into the active moiety within the lung to produce antimicrobial activity. This issue was first investigated in rats (18), in which it was estimated that about 2/3 of the dose eventually absorbed reached the systemic circulation directly as CMS, whereas approximately 1/3 (∼39%) was first converted into colistin within lungs before being absorbed. This indicated that in relative terms, CMS was absorbed within the lung two times more rapidly than it was hydrolyzed into colistin. But it was also shown that ELF colistin concentrations were much lower than corresponding CMS concentrations, suggesting that colistin formation was the rate-limiting step for its absorption. These results are in agreement with those of Yapa et al., who also reported higher CMS than colistin concentrations within lungs after administration of nebulized prodrug at a dose of 14 mg kg−1 (21).
In order to facilitate the investigation of colistin bidirectional passage through the blood-alveolar barrier in vivo, this active moiety was administered directly at a dose close to 0.35 mg kg−1, which was selected from preliminary experiments as the highest well-tolerated dose in rats, both intravenously and for the nebulized drug. As expected, the peak colistin concentration in plasma appeared earlier (0.5 h) (Fig. 2) after direct nebulization of this active compound than previously observed after nebulization of CMS (tmax = 2.72 ± 0.68 h [18] or 4 h [21]) under similar experimental conditions. The rapid appearance of colistin in plasma after its nebulization is also consistent with the findings of Yapa et al., who observed a maximum plasma concentration of colistin at 0.5 h after its administration in nebulized form (21). The plasma peak concentration of colistin was about 1/3 lower after administration in the nebulized form than after i.v. infusion, but then at distribution equilibrium (2 h postdosing) plasma drug concentrations were similar after i.v. and nebulized drug exposure (Table 1). Accordingly, the average bioavailability (Faero) was estimated to 69% after exposure to nebulized drug (Table 2), which for unexplained reasons was lower than the almost-complete bioavailability estimated when the Penn Century system was used for nebulization of the drug as CMS and of FQs to rats (18, 26). Noticeably, Yapa et al. estimated an average bioavailability of 50% after administration via nebulized aerosol with the Penn Century system (21).
The most interesting observation was that colistin ELF concentrations were dramatically affected by the route of administration. Indeed, colistin concentrations at distribution equilibrium (2 and 4 h) were 1,700 times higher in ELF than in plasma (unbound concentration) after administration in the nebulized form and were not detectable after i.v. administration (Table 1; Fig. 2). However, using the ELF colistin concentrations simulated by the model based on i.v. administration, it was possible to estimate that the AUCELF, 0.35-to-AUCplasma, 0.35 ratio was 1,214 times higher after administration of the nebulized form versus i.v. administration.
At this stage, it is important to remember that, due to dilution, BAL fluid drug concentrations are much lower (50 times on average, using our experimental setting in rats) than ELF drug concentrations, and therefore in order to detect colistin in BAL fluid (LOQ, 0.0097 μg ml−1), the ELF drug concentrations should be around 0.5 μg ml−1. Accordingly, ELF drug concentrations simulated by the model after intravenous administration of colistin at a dose of 0.35 mg kg−1 remained just below this value, as illustrated in Fig. 2. Li et al. used an intravenous bolus dose of colistin in rats equal to 1 mg/kg (30), which is 3 times higher than our dose, and this could have solved this analytical issue. However, in our hands this higher dose would have been toxic, and this difference in toxicity could possibly be due to the use of different brands of CMS resulting in different ratios of CMS and sulfomethylated CMS derivatives (21).
The PK model used to describe the ELF distribution of colistin was adapted from a previous PK hybrid model used for FQs (26). This simple generic model was developed to compare several antimicrobial agents used, or potential candidates, for nebulization, such as colistin and FQs, and presented the characteristic of the ELF compartment with a fixed volume (VELF) set at 30 × 10−6 liters kg−1 (or 30 μl kg−1). This value was chosen in accordance with the VELF estimated by the correction for the urea dilution factor in the present study (28 ± 17 μl kg−1), but it has also been reported in previous studies (29 ± 23 μl kg−1 [26]) and 24 ± 19 μl kg−1 [18]).
The addition of an influx clearance from the ELF compartment to the central compartment was necessary for satisfactory data fitting, but ELF drug concentrations after nebulization were better fitted when the initial linear transfer between ELF and plasma was replaced by a nonlinear transfer factor (Vin). Interestingly, this nonlinear influx transfer, with a km at 133 μg ml−1, becomes significant after administration of nebulized colistin at the 0.35-mg kg−1 dose, when ELF concentrations reach sufficiently high values (153 ± 49 μg ml−1 at 0.5 h), but not after i.v. administration at the same dose, when ELF drug concentrations are below the LOQ. Accordingly, the unbound colistin AUCs in ELF and plasma are equal after i.v. administration.
The passive QELF for colistin (6.7 × 10−5 liters h−1 kg−1) was approximately 10 and 100 times lower than values previously estimated for CIP (0.634 × 10−3 liters h−1 kg−1) and MXF (6.15 × 10−3 liters h−1 kg−1) (26), suggesting that passive diffusion of colistin across the broncho-alveolar barrier for this large (average molecular mass, 1,166 g mol−1) and positively charged molecule is slower than that of FQs with molecular masses on the order of 350 g mol−1. These in vivo results are in accordance with previous in vitro results obtained with Calu-3 cells as a model of the blood-alveolar barrier (18), leading to an apparent permeability value for colistin (0.042 ± 0.020 cm s−1) that is approximately 20- to 200-fold lower than that of ciprofloxacin and moxifloxacin, which were used as representative FQs (0.8 ± 0.03 cm s−1 and 8.3 ± 0.12 cm s−1, respectively) (32). Therefore, these new data suggest that slow passive diffusion, which can be easily assessed in vitro, may confer an advantage to administration of the nebulized drug form compared with intravenous administration for antibiotic treatment of pulmonary infections.
However, the appearance of colistin in plasma after administration in the nebulized form is a complex process that cannot be simply described by a first-order kinetics. Recently, Yapa et al. used two compartments (BAL fluid 1 and BAL fluid 2) to describe drugs kinetics in BAL fluid (21). Our model includes a depot compartment after administration of nebulized drug to mimic a slow appearance of colistin within ELF, plus a nonlinear transfer rate for the drug from the ELF to plasma with a km value estimated at 133 μg ml−1. This km value is far superior to drug concentrations required for the eradication of sensitive strains in the lung, but it is close to ELF drug concentrations measured during this experiment (Fig. 2) and is also in the same range of ELF drug concentrations estimated in critical care patients under mechanical ventilation and receiving 2 MUI of nebulized CMS over 30 min using the Aeroneb Pro nebulizer (M. Boisson, M. Jacobs, N. Gregoire, S. Marchand, O. Mimoz, and W. Couet, presented at the 1st International Conference on Polymyxins, 2 to 4 May 2013, Prato, Italy [unpublished abstract and poster]). Under these conditions, the Vmax, in-to-km ratio (2.5 × 10−3 liters h−1 kg−1) is 37 times higher than the QELF (6.7 × 10−5 liters h−1 kg−1), suggesting that this nonlinear process plays a major role in colistin transfer between ELF and plasma when administered in the nebulized form.
Reasons for this nonlinearity are unclear. Although passive diffusion was described as the dominant transport mechanism for drug absorption from rat lungs (33, 34), a number of transporters have been described in lung tissue that could interfere with the disposition of inhaled drugs (35). Colistin is probably not a glycoprotein P (P-gp) substrate (36), but it may have an affinity for organic cation transporters (OCTs) and peptide transporters (PEPT) (37). Both transporters, which belong to the superfamily of solute-linked carriers (SLC), have been identified in lung tissues (35). OCTs are capable of transporting organic cations across the plasma membrane in both directions (38). Indeed, in vitro studies across pulmonary cell layers have shown active OCT-mediated transport in both the absorptive and secretory directions (39, 40). PEPT would act by decreasing the drug concentration within ELF by uptake into epithelial cells. Yet, the mechanism describing how drugs leave pulmonary cells has not been characterized (41, 42). Further studies are necessary to characterize the functions of OCTs and PEPT in the transport of colistin at the air-blood barrier level, since this in vivo study could not address this issue.
Because this nonlinearity was not described by Yapa et al. (21), it was important to reproduce our experiment with a different dose in order to challenge our model. Because ELF drug concentrations measured during this study were close to 100 μg ml−1 and therefore close to the km, we decided to assess this nonlinearity by reducing the nebulized dose of colistin by 10 times (0.035 mg kg−1), which would still lead to quantifiable colistin concentrations in BAL fluid. A relatively good fit was obtained (Fig. 3). In accordance with the 10-fold ratio in doses, the AUCplasma, 0.35-to-AUCplasma, 0.035 ratio was estimated as 10.1. However, the corresponding AUC ratio in ELF (AUCELF, 0.35/AUCELF, 0.035) was only close to 3. Accordingly, the ELF-to-unbound plasma AUC ratio was 3.9 times lower after the high dose (1,214 at 0.35 mg kg−1) compared to the ratio after the low dose (4,735 at 0.035 mg kg−1).
In conclusion, the potential advantage of nebulization of antibiotics for the treatment of pulmonary infections is clearly dependent upon their ability to cross biological barriers. For antibiotics with slow diffusion rates, such as colistin, nebulization should provide much higher and prolonged ELF drug concentrations than i.v. administration, offering a real advantage in terms of efficacy. However, in clinical practice, the fraction of the drug reaching the infection site within the lung after nebulization may vary widely depending on the nebulizer and is probably much lower than what could be obtained experimentally with the Penn Century system, and this would have consequences not only in terms of efficacy but also of bioavailability and therefore of systemic toxicity.
ACKNOWLEDGMENT
A. V. L. Gontijo is supported by CAPES (Coordenação de Aperfeiçoamento de Pessoal de Nível Superior), Brazil.
Footnotes
Published ahead of print 5 May 2014
REFERENCES
- 1.Hagerman JK, Hancock KE, Klepser ME. 2006. Aerosolised antibiotics: a critical appraisal of their use. Exp. Opin. Drug Deliv. 3:71–86. 10.1517/17425247.3.1.71 [DOI] [PubMed] [Google Scholar]
- 2.Palmer LB. 2011. Aerosolized antibiotics in the intensive care unit. Clin. Chest Med. 32:559–574. 10.1016/j.ccm.2011.05.012 [DOI] [PubMed] [Google Scholar]
- 3.Michalopoulos A, Papadakis E. 2010. Inhaled anti-infective agents: emphasis on colistin. Infection 38:81–88. 10.1007/s15010-009-9148-6 [DOI] [PubMed] [Google Scholar]
- 4.Michalopoulos AS. 2012. Aerosolized antibiotics: the past, present and future, with a special emphasis on inhaled colistin. Exp. Opin. Drug Deliv. 9:493–495. 10.1517/17425247.2012.676039 [DOI] [PubMed] [Google Scholar]
- 5.Bergen PJ, Li J, Rayner CR, Nation RL. 2006. Colistin methanesulfonate is an inactive prodrug of colistin against Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 50:1953–1958. 10.1128/AAC.00035-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Falagas ME, Kasiakou SK. 2005. Colistin: the revival of polymyxins for the management of multidrug-resistant gram-negative bacterial infections. Clin. Infect. Dis. 40:1333–1341. 10.1086/429323 [DOI] [PubMed] [Google Scholar]
- 7.Nation RL, Li J. 2009. Colistin in the 21st century. Curr. Opin. Infect. Dis. 22:535–543. 10.1097/QCO.0b013e328332e672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li J, Nation RL, Turnidge JD, Milne RW, Coulthard K, Rayner CR, Paterson DL. 2006. Colistin: the re-emerging antibiotic for multidrug-resistant Gram-negative bacterial infections. Lancet Infect. Dis. 6:589–601. 10.1016/S1473-3099(06)70580-1 [DOI] [PubMed] [Google Scholar]
- 9.Couet W, Gregoire N, Gobin P, Saulnier PJ, Frasca D, Marchand S, Mimoz O. 2011. Pharmacokinetics of colistin and colistimethate sodium after a single 80-mg intravenous dose of CMS in young healthy volunteers. Clin. Pharmacol. Ther. 89:875–879. 10.1038/clpt.2011.48 [DOI] [PubMed] [Google Scholar]
- 10.Couet W, Gregoire N, Marchand S, Mimoz O. 2012. Colistin pharmacokinetics: the fog is lifting. Clin. Microbiol. Infect. 18:30–39. 10.1111/j.1469-0691.2011.03667.x [DOI] [PubMed] [Google Scholar]
- 11.Garonzik SM, Li J, Thamlikitkul V, Paterson DL, Shoham S, Jacob J, Silveira FP, Forrest A, Nation RL. 2011. Population pharmacokinetics of colistin methanesulfonate and formed colistin in critically ill patients from a multicenter study provide dosing suggestions for various categories of patients. Antimicrob. Agents Chemother. 55:3284–3294. 10.1128/AAC.01733-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mohamed AF, Karaiskos I, Plachouras D, Karvanen M, Pontikis K, Jansson B, Papadomichelakis E, Antoniadou A, Giamarellou H, Armaganidis A, Cars O, Friberg LE. 2012. Application of a loading dose of colistin methanesulfonate in critically ill patients: population pharmacokinetics, protein binding, and prediction of bacterial kill. Antimicrob. Agents Chemother. 56:4241–4249. 10.1128/AAC.06426-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Plachouras D, Karvanen M, Friberg LE, Papadomichelakis E, Antoniadou A, Tsangaris I, Karaiskos I, Poulakou G, Kontopidou F, Armaganidis A, Cars O, Giamarellou H. 2009. Population pharmacokinetic analysis of colistin methanesulfonate and colistin after intravenous administration in critically ill patients with infections caused by gram-negative bacteria. Antimicrob. Agents Chemother. 53:3430–3436. 10.1128/AAC.01361-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hewer SL. 2012. Inhaled antibiotics in cystic fibrosis: what's new? J. R. Soc. Med. 105(Suppl 2):S19–S24. 10.1258/jrsm.2012.12s004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Athanassa ZE, Markantonis SL, Fousteri MZ, Myrianthefs PM, Boutzouka EG, Tsakris A, Baltopoulos GJ. 2012. Pharmacokinetics of inhaled colistimethate sodium (CMS) in mechanically ventilated critically ill patients. Intensive Care Med. 38:1779–1786. 10.1007/s00134-012-2628-7 [DOI] [PubMed] [Google Scholar]
- 16.Korbila IP, Michalopoulos A, Rafailidis PI, Nikita D, Samonis G, Falagas ME. 2010. Inhaled colistin as adjunctive to intravenous colistin for the treatment of microbiologically documented ventilator-associated pneumonia: a comparative cohort study. Clin. Microbiol. Infect. 16:1230–1236. 10.1111/j.1469-0691.2009.03040.x [DOI] [PubMed] [Google Scholar]
- 17.Athanassa ZE, Myrianthefs PM, Boutzouka EG, Tsakris A, Baltopoulos GJ. 2011. Monotherapy with inhaled colistin for the treatment of patients with ventilator-associated tracheobronchitis due to polymyxin-only-susceptible Gram-negative bacteria. J. Hosp. Infect. 78:335–336. 10.1016/j.jhin.2011.04.004 [DOI] [PubMed] [Google Scholar]
- 18.Marchand S, Gobin P, Brillault J, Baptista S, Adier C, Olivier JC, Mimoz O, Couet W. 2010. Aerosol therapy with colistin methanesulfonate: a biopharmaceutical issue illustrated in rats. Antimicrob. Agents Chemother. 54:3702–3707. 10.1128/AAC.00411-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lu Q, Girardi C, Zhang M, Bouhemad B, Louchahi K, Petitjean O, Wallet F, Becquemin MH, Le Naour G, Marquette CH, Rouby JJ. 2010. Nebulized and intravenous colistin in experimental pneumonia caused by Pseudomonas aeruginosa. Intensive Care Med. 36:1147–1155. 10.1007/s00134-010-1879-4 [DOI] [PubMed] [Google Scholar]
- 20.Ratjen F, Rietschel E, Kasel D, Schwiertz R, Starke K, Beier H, van Koningsbruggen S, Grasemann H. 2006. Pharmacokinetics of inhaled colistin in patients with cystic fibrosis. J. Antimicrob. Chemother. 57:306–311. 10.1093/jac/dki461 [DOI] [PubMed] [Google Scholar]
- 21.Yapa SWS, Li J, Patel K, Wilson JW, Dooley MJ, George J, Clark D, Poole S, Williams E, Porter CJH, Nation RL, McIntosh MP. 2014. Pulmonary and systemic pharmacokinetics of inhaled and intravenous colistin methanesulfonate in cystic fibrosis patients: targeting advantage of inhalational administration. Antimicrob. Agents Chemother. 58:2570–2579. 10.1128/AAC.01705-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Westerman EM, Le Brun PP, Touw DJ, Frijlink HW, Heijerman HG. 2004. Effect of nebulized colistin sulphate and colistin sulphomethate on lung function in patients with cystic fibrosis: a pilot study. J. Cyst. Fibros. 3:23–28. 10.1016/j.jcf.2003.12.005 [DOI] [PubMed] [Google Scholar]
- 23.Michalopoulos A, Kasiakou SK, Mastora Z, Rellos K, Kapaskelis AM, Falagas ME. 2005. Aerosolized colistin for the treatment of nosocomial pneumonia due to multidrug-resistant Gram-negative bacteria in patients without cystic fibrosis. Crit. Care 9:R53–R59. 10.1186/cc3020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Naesens R, Vlieghe E, Verbrugghe W, Jorens P, Ieven M. 2011. A retrospective observational study on the efficacy of colistin by inhalation as compared to parenteral administration for the treatment of nosocomial pneumonia associated with multidrug-resistant Pseudomonas aeruginosa. BMC Infect. Dis. 11:317. 10.1186/1471-2334-11-317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.National Research Council. 2011. Guide for the care and use of laboratory animals, 8th ed. National Academies Press, Washington, DC [Google Scholar]
- 26.Gontijo AVL, Brillault J, Grégoire N, Lamarche I, Gobin P, Couet W, Marchand S. 2014. Biopharmaceutical characterization of nebulized antimicrobial agents in rats: 1. Ciprofloxacin, moxifloxacin, and grepafloxacin. Antimicrob. Agents Chemother. 58:3942–3949. 10.1128/AAC.02818-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gobin P, Lemaitre F, Marchand S, Couet W, Olivier JC. 2010. Assay of colistin and colistin methanesulfonate in plasma and urine by liquid chromatography-tandem mass spectrometry. Antimicrob. Agents Chemother. 54:1941–1948. 10.1128/AAC.01367-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rennard SI, Basset G, Lecossier D, O'Donnell KM, Pinkston P, Martin PG, Crystal RG. 1986. Estimation of volume of epithelial lining fluid recovered by lavage using urea as marker of dilution. J. Appl. Physiol. 60:532–538 [DOI] [PubMed] [Google Scholar]
- 29.Bulitta JB, Bingolbali A, Shin BS, Landersdorfer CB. 2011. Development of a new pre- and post-processing tool (SADAPT-TRAN) for nonlinear mixed-effects modeling in S-ADAPT. AAPS J. 13:201–211. 10.1208/s12248-011-9257-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li J, Milne RW, Nation RL, Turnidge JD, Smeaton TC, Coulthard K. 2003. Use of high-performance liquid chromatography to study the pharmacokinetics of colistin sulfate in rats following intravenous administration. Antimicrob. Agents Chemother. 47:1766–1770. 10.1128/AAC.47.5.1766-1770.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Beal SL. 2001. Ways to fit a PK model with some data below the quantification limit. J. Pharmacokinet. Pharmacodyn. 28:481–504. 10.1023/A:1012299115260 [DOI] [PubMed] [Google Scholar]
- 32.Brillault J, De Castro WV, Harnois T, Kitzis A, Olivier JC, Couet W. 2009. P-glycoprotein-mediated transport of moxifloxacin in a Calu-3 lung epithelial cell model. Antimicrob. Agents Chemother. 53:1457–1462. 10.1128/AAC.01253-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tronde A, Norden B, Jeppsson AB, Brunmark P, Nilsson E, Lennernas H, Bengtsson UH. 2003. Drug absorption from the isolated perfused rat lung: correlations with drug physicochemical properties and epithelial permeability. J. Drug Target. 11:61–74. 10.1080/1061186031000086117 [DOI] [PubMed] [Google Scholar]
- 34.Tronde A, Norden B, Marchner H, Wendel AK, Lennernas H, Bengtsson UH. 2003. Pulmonary absorption rate and bioavailability of drugs in vivo in rats: structure-absorption relationships and physicochemical profiling of inhaled drugs. J. Pharm. Sci. 92:1216–1233. 10.1002/jps.10386 [DOI] [PubMed] [Google Scholar]
- 35.Bosquillon C. 2010. Drug transporters in the lung: do they play a role in the biopharmaceutics of inhaled drugs? J. Pharm. Sci. 99:2240–2255. 10.1002/jps.21995 [DOI] [PubMed] [Google Scholar]
- 36.Jin L, Li J, Nation RL, Nicolazzo JA. 2011. Impact of P-glycoprotein inhibition and lipopolysaccharide administration on blood-brain barrier transport of colistin in mice. Antimicrob. Agents Chemother. 55:502–507. 10.1128/AAC.01273-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ma Z, Wang J, Nation RL, Li J, Turnidge JD, Coulthard K, Milne RW. 2009. Renal disposition of colistin in the isolated perfused rat kidney. Antimicrob. Agents Chemother. 53:2857–2864. 10.1128/AAC.00030-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Koepsell H, Lips K, Volk C. 2007. Polyspecific organic cation transporters: structure, function, physiological roles, and biopharmaceutical implications. Pharm. Res. 24:1227–1251. 10.1007/s11095-007-9254-z [DOI] [PubMed] [Google Scholar]
- 39.Ehrhardt C, Kneuer C, Bies C, Lehr CM, Kim KJ, Bakowsky U. 2005. Salbutamol is actively absorbed across human bronchial epithelial cell layers. Pulm. Pharmacol. Ther. 18:165–170. 10.1016/j.pupt.2004.11.007 [DOI] [PubMed] [Google Scholar]
- 40.Mukherjee M, Pritchard DI, Bosquillon C. 2012. Evaluation of air-interfaced Calu-3 cell layers for investigation of inhaled drug interactions with organic cation transporters in vitro. Int. J. Pharm. 426:7–14. 10.1016/j.ijpharm.2011.12.036 [DOI] [PubMed] [Google Scholar]
- 41.Groneberg DA, Eynott PR, Doring F, Dinh QT, Oates T, Barnes PJ, Chung KF, Daniel H, Fischer A. 2002. Distribution and function of the peptide transporter PEPT2 in normal and cystic fibrosis human lung. Thorax 57:55–60. 10.1136/thorax.57.1.55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Groneberg DA, Fischer A, Chung KF, Daniel H. 2004. Molecular mechanisms of pulmonary peptidomimetic drug and peptide transport. Am. J. Respir. Cell Mol. Biol. 30:251–260. 10.1165/rcmb.2003-0315TR [DOI] [PubMed] [Google Scholar]