Abstract
Surotomycin (CB-183,315) is an orally administered, minimally absorbed, selective bactericidal cyclic lipopeptide in phase 3 development for the treatment of Clostridium difficile-associated diarrhea. The aim of this study was to evaluate the emergence of resistance in C. difficile (ATCC 700057 and three recent clinical isolates from the restriction endonuclease analysis groups BI, BK, and K), vancomycin-susceptible (VS) Enterococcus faecalis (ATCC 49452), vancomycin-resistant (VR) E. faecalis (ATCC 700802), VS Enterococcus faecium (ATCC 6569), and VR E. faecium (ATCC 51559) under anaerobic conditions. The rate of spontaneous resistance was below the limit of detection (<10−8 to <10−9) for surotomycin at 16 and 32× the MIC for all isolates tested. Under selective pressure by serial passage, C. difficile grew in a maximum of 4 μg/ml surotomycin (final MICs of 2 to 8 μg/ml [4- to 16-fold higher than those of the naive control]) at day 15, with the exception of the C. difficile BK strain, which grew in 16 to 32 μg/ml (final MICs of 8 to 32 μg/ml [16- to 64-fold higher than those of the naive control]). Enterococci remained relatively unchanged over 15 days, growing in a maximum of 8 μg/ml surotomycin (final MICs of 2 to 16 μg/ml [8- to 64-fold higher than those of the naive control]). Of the isolates tested, no cross-resistance to vancomycin, rifampin, ampicillin, metronidazole, or moxifloxacin was observed. Surotomycin at 20× MIC demonstrated equally rapid bactericidal activity (≥3-log-unit reduction in CFU/ml in ≤8 h) against naive and reduced-susceptibility isolates of C. difficile, VS Enterococcus (VSE), and VR Enterococcus (VRE), except for C. difficile BK (2.6-log-unit reductions for both). These results suggest that emergence of resistance to surotomycin against C. difficile, E. faecalis, and E. faecium is likely to be rare.
INTRODUCTION
First reported in the 1970s, Clostridium difficile infection is the most common cause of health care-related infectious diarrhea in developed countries, accounting for 20 to 30% of cases of antibiotic-associated diarrhea and nearly all cases of antibiotic-associated colitis (1, 2). C. difficile-associated diarrhea (CDAD) represents a significant clinical and economic burden and is associated with longer length of hospital stay, increased medical costs, and high rates of morbidity and mortality, especially among the elderly (3, 4). The frequency and severity of the disease have increased over the past decade, along with the emergence of particular epidemic strains (e.g., BI/NAP1/027) (5).
Vancomycin and metronidazole are the treatment options for the first and second episodes of CDAD. Each agent is equally effective among patients with mild disease, but vancomycin appears superior to metronidazole for the treatment of patients with severe CDAD (6). However, the greatest unmet need in patients with CDAD is disease recurrence, which may occur in up to 50% of treated patients (6–8). Fidaxomicin, a recently approved macrocyclic antibiotic, demonstrated improved recurrence rates in phase 3 studies (9), but even with the approval of fidaxomicin, additional therapies are required to address the increased prevalence of recurrent CDAD.
Surotomycin (CB-183,315) is an orally administered, minimally absorbed, selective bactericidal cyclic lipopeptide in phase 3 development for the treatment of CDAD (10). Potent activity against C. difficile (MIC90 = 0.5 μg/ml) and other Gram-positive bacteria, with minimal impact on the Gram-negative organisms of the intestinal microbiota, has been demonstrated in vitro (11, 12). In a recent phase 2 clinical trial (NCT01085591), surotomycin doses of 125 mg and 250 mg twice daily (b.i.d.), compared with oral vancomycin, were safe and well tolerated in patients with CDAD (13).
The aim of this study was to evaluate the emergence of spontaneous resistance and multistep resistance under selective pressure of recent clinical isolates of C. difficile (restriction endonuclease analysis groups BI, BK, and K), vancomycin-susceptible and vancomycin-resistant Enterococcus faecalis (VSEfs and VREfs, respectively), and vancomycin-susceptible and vancomycin-resistant Enterococcus faecium (VSEfm and VREfm, respectively), under anaerobic conditions. The activity of surotomycin against naive and reduced-susceptibility isolates was also evaluated, as was cross-resistance to various classes of antibiotics.
(Portions of these data were previously presented at the 4th International Clostridium difficile Symposium [14] and at the 53rd Interscience Conference on Antimicrobial Agents and Chemotherapy [15].)
MATERIALS AND METHODS
Strains, media, and antibiotics.
The study was performed using four Clostridium difficile isolates (ATCC 700057 [quality control {QC}] and three recent clinical isolates from the restriction endonuclease analysis groups BI, BK, and K, collected during the LCD-DR-09-03 phase 2 study), VSEfs (ATCC 49452), VREfs (ATCC 700802), VSEfm (ATCC 6569), and VREfm (ATCC 51559). The starting MICs for surotomycin (Cubist Pharmaceuticals, Lexington, MA, USA), vancomycin, and rifampin against each organism tested are listed in Table 1. The MICs were determined according to methods described in Clinical and Laboratory Standards Institute approved standard M11-A7 (16), as previously reported (17). Besides surotomycin, all antibiotics were purchased from Sigma (St. Louis, MO, USA).
TABLE 1.
C. difficile, E. faecalis, and E. faecium strains and MICs used in this study
| Organism | Strain | ATCC strain no. | MIC (μg/ml) |
||
|---|---|---|---|---|---|
| Surotomycin | Vancomycin | Rifampin | |||
| C. difficile | QC | 700057 | 1 | 1 | <0.001 |
| BI | 0.5 | 0.5 | <0.001 | ||
| BK | 0.5 | 0.5 | <0.001 | ||
| K | 0.5 | 2 | <0.001 | ||
| E. faecalis | VSEfs | 49452 | 0.5 | 1 | 0.125 |
| VREfs | 700802 | 0.25 | 64 | 0.5 | |
| E. faecium | VSEfm | 6569 | 0.5 | 0.5 | 16 |
| VREfm | 51559 | 1 | >64 | 4 | |
All work was performed in a Bactron I anaerobic chamber (Sheldon Manufacturing, Cornelius, OR, USA) infused with anaerobic mixed gas (5% H2, 10% CO2, 85% N2; Airgas, Salem, NH, USA) using reduced media. For Enterococcus studies, Mueller-Hinton broth and Mueller-Hinton agar (18 g/liter of BD Bacto agar added to Mueller-Hinton broth) were used (BD Diagnostic Systems, Sparks, MD, USA). For C. difficile studies, BBL brucella broth and BBL brucella agar from BD Diagnostic Systems (Sparks, MD, USA) were used. All assay media were prepared and sterilized according to the manufacturer's specifications and then supplemented to a final concentration of 50 mg/liter calcium. Additionally, brucella broth and agar were supplemented with hemin (5 μg/ml) and vitamin K1 (1 μg/ml). In order to grow frozen cultures, prepared brucella agar plates supplemented with 5% sheep blood, hemin, and vitamin K1 (BBHK) (BD Diagnostic Systems) and tryptic soy agar fortified with 5% sheep blood (TSAB) (bioMérieux, Durham, NC, USA) were used.
Resistance incidence by direct plating.
Resistance incidence (RI) of C. difficile was determined in supplemented brucella broth and agar, while RIs of E. faecalis and E. faecium were determined in supplemented Mueller-Hinton broth and Mueller-Hinton agar. Cultures suspended to ∼1 McFarland (McF) and incubated at 37°C for ∼4 h were used as inocula (0.5 ml; between 3 to 4 McF) on selection plates containing surotomycin, vancomycin, or rifampin at 8, 16, or 32× the MIC, in independent, duplicate assays. Rifampin was employed as an assay control given its known high RI. Plates were incubated at 37°C for 48 h. Viable CFU/ml of each inoculum was determined, and the RI was calculated as the colony frequency (equal to the number of colonies on drug-containing plates divided by the total number of colonies plated). Colonies recovered on drug-containing plates were all evaluated by broth MIC after three transfers on drug-free media to confirm elevated MIC values compared with the MICs of naive controls.
Selection for resistance by serial passage.
Serial passages of C. difficile, E. faecalis, and E. faecium were performed in the presence of increasing concentrations of surotomycin, vancomycin, or rifampin, with the exception of VREfs and VREfm which were passaged against only surotomycin, as previously described (18). The C. difficile serial passages were performed in supplemented brucella broth, while the E. faecalis and E. faecium serial passages were performed in calcium-supplemented Mueller-Hinton broth. Cultures suspended in media to equal ∼1 McF, incubated at 37°C for ∼3 h, were inoculated into media containing various concentrations of drug above and below the MIC and grown overnight at 37°C for 20 to 24 h. Each day, wells with the highest concentration of drug (in micrograms per milliliter) permitting growth equal to ∼1 McF (equal to 107 CFU/ml) were used to inoculate the next day's drug series; this process was repeated for 15 days with each drug/bacterium combination tested in duplicate. Colonies recovered from day 15 were all evaluated by broth MIC after three drug-free transfers to confirm elevated MIC values compared with those of naive controls.
Time-kill analysis.
Individual C. difficile, E. faecalis, and E. faecium colonies were isolated by streaking onto rich, nonselective media (BBHK agar for C. difficile, TSAB for enterococci) with anaerobic incubation at 35 to 37°C for 48 h. On the day of testing, cultures were scraped off plates, suspended in reduced media (>1 McF), and incubated for ∼2 h. At the start of the assay, cultures were diluted to equal ∼1 McF (107 CFU/ml), either left untreated (no-drug control) or treated separately with 20× the MIC of surotomycin, vancomycin, or metronidazole, and incubated at 35 to 37°C. The concentrations of vancomycin, metronidazole and surotomycin in fecal samples in CDAD patients have all been reported to be ≥20× the MIC of the isolates used in this study (vancomycin [VAN] [19], metronidazole [MET] [20], and surotomycin [SUR] [data on file at Cubist Pharmaceuticals, Lexington, MA, USA]). Cell viability was determined at 0, 2, 4, 8, and 24 h by serial dilution and plating for quantification of CFU/ml.
RESULTS
Spontaneous resistance.
The spontaneous resistance incidence (RI) to surotomycin, vancomycin, and rifampin was determined under anaerobic conditions at 8, 16, and 32× the MIC. The observed RI values of surotomycin, vancomycin, and rifampin against C. difficile strains are shown in Table 2. The rate of spontaneous resistance was below the limit of detection (<10−8 to <10−9) for surotomycin at 16 and 32× the MIC for all isolates tested. A single reduced-susceptibility isolate was identified for strains BI (final MIC of 1 μg/ml; 2-fold higher than that of the naive control) and BK (final MIC of 2 μg/ml; 4-fold higher than that of the naive control) at 8× the MIC. Vancomycin RI was below the limit of detection at 8, 16, and 32× the MIC for all isolates tested. Rifampin, used as a positive assay control, had RI frequencies of 10−7 to 10−9 against C. difficile. Resistant isolates had MICs 30- to 1,000-fold higher than those of naive controls.
TABLE 2.
Resistance incidence of C. difficile BI, BK, K, and QC strains against surotomycin, vancomycin, and rifampin
| C. difficile strain | Antibiotic | Concn(s) tested (× MIC) | RIa |
|
|---|---|---|---|---|
| Sample A | Sample B | |||
| BI | Surotomycin | 8 | 4.4E−09 | <5.4E−09 |
| 16, 32 | <4.4E−09 | <5.4E−09 | ||
| Vancomycin | 8, 16, 32 | <4.4E−09 | <5.4E−09 | |
| Rifampin | 8 | <4.4E−09 | 3.2E−08 | |
| 16 | 1.3E−08 | 5.4E−09 | ||
| BK | Surotomycin | 8 | <4.2E−08 | <6.0E−08 |
| 16 | <4.2E−08 | 6.0E−08 | ||
| 32 | <4.2E−08 | <6.0E−08 | ||
| Vancomycin | 8, 16, 32 | <4.2E−08 | <6.0E−08 | |
| Rifampin | 8 | <4.2E−08 | 1.2E−07 | |
| 16 | 4.2E−08 | <6.0E−08 | ||
| K | Surotomycin | 8, 16, 32 | <5.8E−09 | <4.9E−09 |
| Vancomycin | 8, 16, 32 | <5.8E−09 | <4.9E−09 | |
| Rifampin | 8 | <5.8E−09 | 9.9E−09 | |
| 16 | 1.7E−08 | 9.9E−09 | ||
| QC | Surotomycin | 8, 16, 32 | <6.5E−09 | <5.5E−09 |
| Vancomycin | 8, 16, 32 | <6.5E−09 | <5.5E−09 | |
| Rifampin | 8 | 1.3E−08 | 1.1E−08 | |
| 16 | 2.6E−08 | 5.5E−09 | ||
RI, resistance incidence. The average numbers of CFU/ml plated for the C. difficile strains were as follows: 1.9E+08 to 2.3E+08 for strain BI, 1.7E+07 to 2.4E+07 for strain BK, 1.7E+08 to 2.0E+08 for strain K, and 1.6E+08 to 1.8E+08 for strain QC.
Table 3 demonstrates observed RI values of surotomycin, vancomycin, and rifampin against E. faecalis and E. faecium strains. The RI for surotomycin was below the limit of detection for three of the four strains tested at all three concentrations. Three resistant mutants were isolated using the VREfs strain (RI of 3.2−9 to <9.09−10) with MICs 4-fold higher than that of the naive control (final MIC of 1 μg/ml). The RIs for VSEfs and VSEfm were below the limit of detection for vancomycin at 8, 16, and 32× the MIC; the RI for vancomycin was not tested for the VRE. Rifampin had RI frequencies of 10−6 to 10−8 against VSEfs and VREfs where all rifampin isolates had MICs 32- to >64-fold higher than those of the naive controls. No cross-resistance to vancomycin, rifampin, ampicillin, metronidazole, or moxifloxacin was observed in the C. difficile, E. faecalis, or E. faecium RI isolates.
TABLE 3.
Resistance incidence of vancomycin-susceptible and -resistant E. faecalis and E. faecium strains against surotomycin, vancomycin, and rifampin
| Enterococcal strain | Antibiotic | Concn(s) tested (× MIC) | RIa |
|
|---|---|---|---|---|
| Sample A | Sample B | |||
| VSEfs | Surotomycin | 8, 16, 32 | <1.33E−09 | <2.34E−09 |
| Vancomycin | 8, 16, 32 | <1.33E−09 | <2.34E−09 | |
| Rifampin | 8, 16 | >1.3E−06 | >2.3E−07 | |
| VSEfm | Surotomycin | 8, 16, 32 | <2.9E−09 | <1.97E−09 |
| Vancomycin | 8, 16, 32 | <2.9E−09 | <1.97E−09 | |
| VREfs | Surotomycin | 8 | 3.2E−09 | <9.09E−10 |
| 16 | <1.6E−09 | 9.1E−10 | ||
| 32 | <1.6E−09 | <9.09E−10 | ||
| Rifampin | 8 | >8.0E−07 | >1.6E−07 | |
| 16 | 1.4E−08 | 7.8E−08 | ||
| VREfm | Surotomycin | 8, 16, 32 | <3.39E−09 | <4.04E−09 |
The average numbers of CFU/ml plated for vancomycin-susceptible and -resistant E. faecalis and E. faecium strains were as follows: 4.3E+08 to 7.5E+08 for VSEfs, 3.5E+08 to 5.1E+08 for VSEfm, 6.3E+08 to 1.1E+09 for VREfs, and 2.4E+08 to 3.0E+08 for VREfm.
Multistep resistance.
Multistep resistance under selective pressure was evaluated under anaerobic conditions by serial passage over 15 days. The highest concentration of drug permitting growth was determined daily for each sample. Figure 1 displays serial passage results of C. difficile BI, BK, K, and QC strains against surotomycin, vancomycin, and rifampin. C. difficile BI, K, and QC grew in a maximum of 4 μg/ml surotomycin during passage, while the BK strain grew in 16 to 32 μg/ml surotomycin by day 15 (Fig. 1A). After three daily drug-free transfers, broth MIC values were 2 to 4 μg/ml (4- to 8-fold higher than those of naive controls) for C. difficile K, 4 to 8 μg/ml (8- to 16-fold higher than those of naive controls) for C. difficile BI and QC, and 8 to >16 μg/ml (16- to 32-fold higher than those of naive controls) for C. difficile BK. C. difficile BI, BK, K, and QC grew in a maximum of 8 μg/ml vancomycin (Fig. 1B); broth MIC values were 2 to 8 μg/ml (4- to 16-fold higher than those of naive controls). Rapid generation of rifampin resistance was observed, with the exception of C. difficile K (sample 1) and QC (sample 1) (Fig. 1C).
FIG 1.
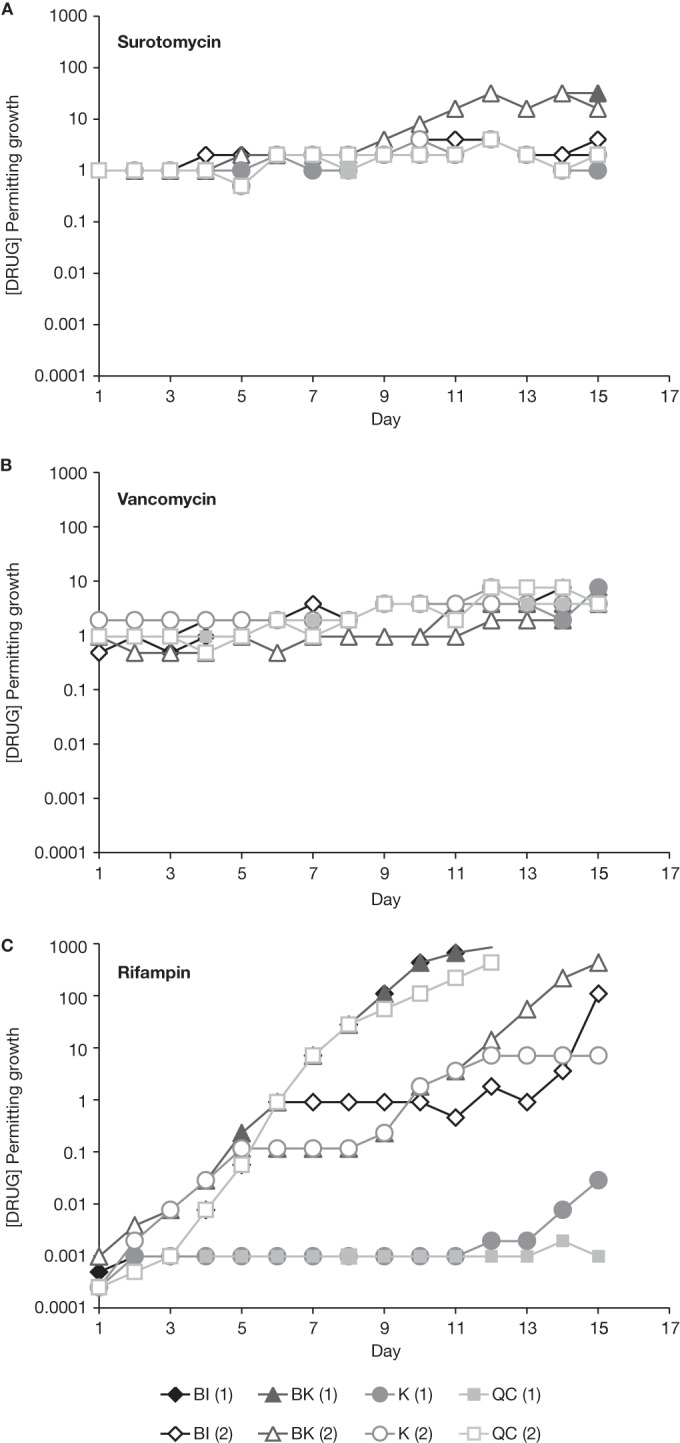
Serial passage of C. difficile BI, BK, K, and QC strains against surotomycin, vancomycin, and rifampin. The concentration of drug permitting growth is shown on the y axes, and time (in days) is shown on the x axes.
Serial passage results of vancomycin-susceptible and -resistant E. faecalis and E. faecium against surotomycin, vancomycin, and rifampin are shown in Fig. 2. VSEfs, VSEfm, VREfs, and VREfm grew in a maximum of 8 μg/ml surotomycin (Fig. 2A). Broth MIC values were 2 μg/ml (4-fold higher than those of naive controls) for VSEfs, 4 to 8 μg/ml (8- to 16-fold higher than those of naive controls) for VSEfm, 4 μg/ml (16-fold higher than those of naive controls) for VREfs, and 4 to 16 μg/ml (4- to 16-fold higher than those of naive controls) for VREfm. VSEfs and VSEfm grew in a maximum of 4 μg/ml vancomycin (Fig. 2B); broth MIC values were 1 μg/ml (1- to 2-fold compared to those of naive controls). Consistent with C. difficile, rapid generation of rifampin resistance was observed in VSEfs and VSEfm (Fig. 2C); MIC values confirmed resistance (>64 μg/ml [>2- to >128-fold higher than those of naive controls]).
FIG 2.
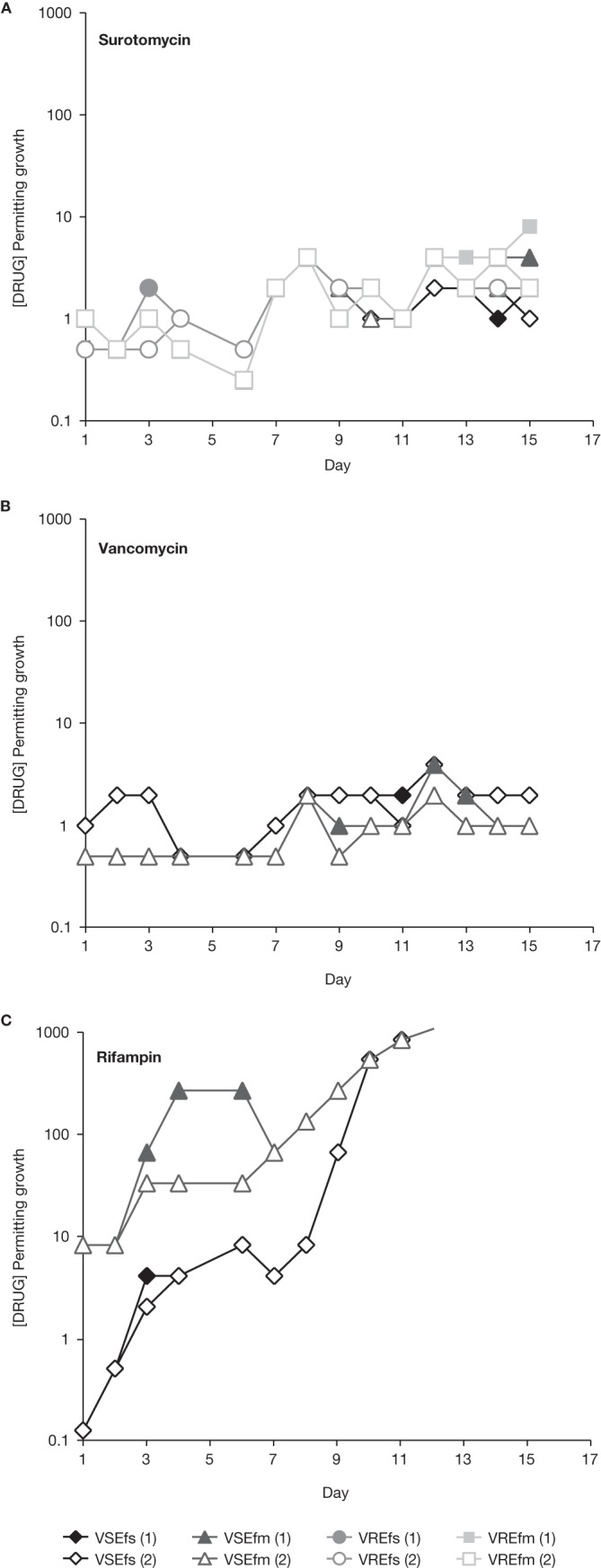
Serial passage of vancomycin-susceptible and -resistant E. faecalis and E. faecium against surotomycin, vancomycin, and rifampin.
The isolates selected in the surotomycin serial passage were not cross-resistant to vancomycin, rifampin, ampicillin, metronidazole, or moxifloxacin. Following serial passage in surotomycin, VREfm became susceptible to vancomycin (MIC shifted from >64 μg/ml at day 1 to 0.5 μg/ml at day 15).
In vitro bactericidal activity.
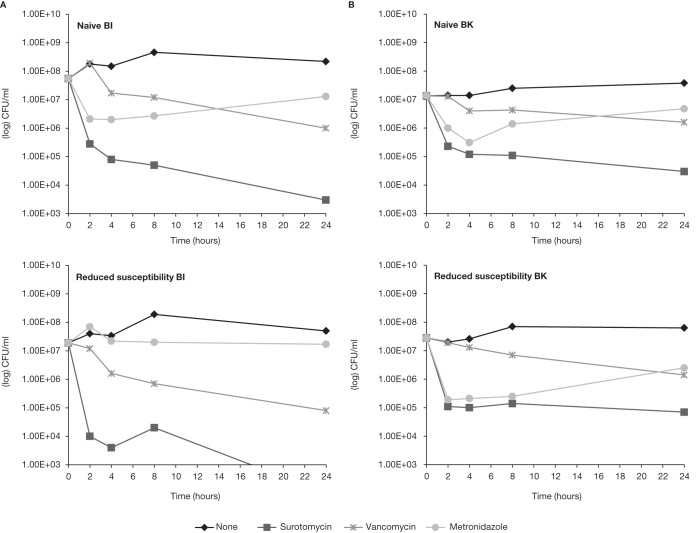
The in vitro bactericidal activity of surotomycin, vancomycin, and metronidazole against naive and reduced-susceptibility isolates, selected by serial passage over 15 days in the presence of increasing concentrations of surotomycin, was evaluated. Surotomycin at 20× the MIC demonstrated equally rapid bactericidal activity (≥3-log-unit reduction in CFU/ml in ≤8 h) against naive and reduced-susceptibility isolates of C. difficile (BI, K, and QC), except for C. difficile BK, which demonstrated 2.6-log-unit reductions for both naive and reduced-susceptibility isolates (Fig. 3; time-kill analysis of C. difficile BI and BK presented). At 20× the MIC, surotomycin demonstrated a ≥4-log-unit reduction in CFU/ml in ≤4 h in both naive and reduced-susceptibility isolates of enterococci, including VRE isolates (Fig. 4; time-kill analysis of VSEfs presented). In contrast, neither vancomycin nor metronidazole at 20× the MIC displayed bactericidal activity (defined by achieving a 3-log-unit reduction in 24 h) against naive or strains with reduced susceptibility to surotomycin.
FIG 3.
Time-kill analysis of surotomycin, vancomycin, and metronidazole at 20× the MIC against naive and reduced-susceptibility isolates of C. difficile BI (A) and BK (B). The surotomycin MICs for naive and reduced-susceptibility isolates of C. difficile BI were 0.5 and 8 μg/ml, respectively, and the surotomycin MICs for naive and reduced-susceptibility isolates of C. difficile BK were 0.5 and 32 μg/ml, respectively.
FIG 4.
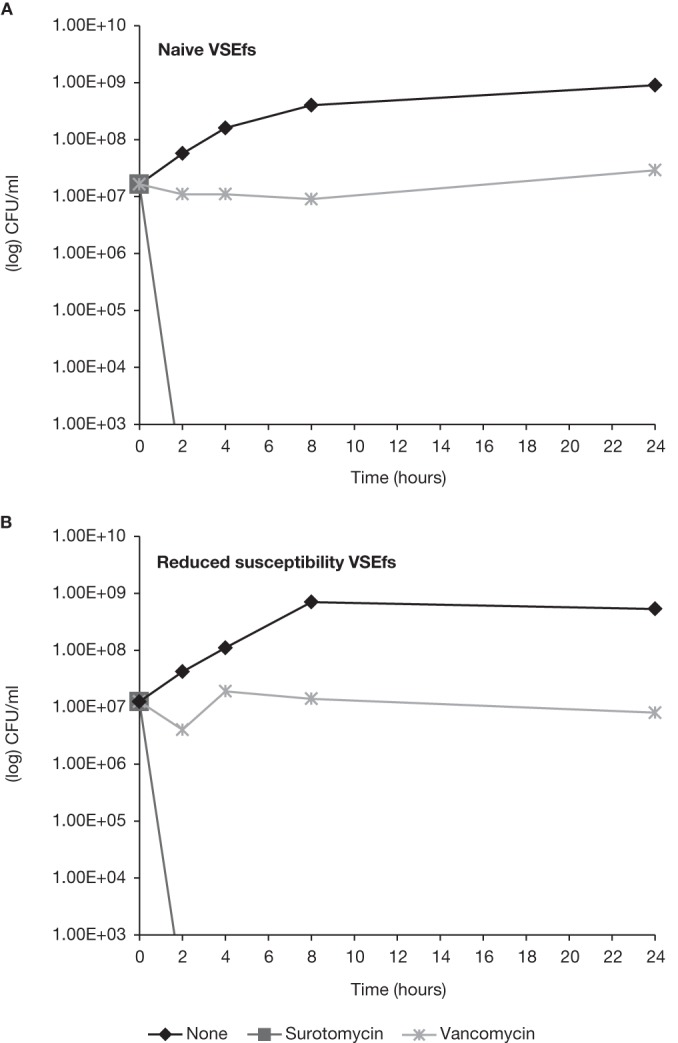
Time-kill analysis of surotomycin and vancomycin at 20× the MIC against naive and reduced-susceptibility isolates of VSEfs. The surotomycin MICs for naive and reduced-susceptibility isolates of VSEfs were 0.5 and 2 μg/ml, respectively.
DISCUSSION
Despite current treatments, morbidity and mortality rates in patients with C. difficile-associated diarrhea (CDAD) remain high. The first-line treatment options for CDAD, vancomycin and metronidazole, are equally effective among patients with mild disease with clinical response rates of ∼90%, while vancomycin appears superior to metronidazole (97% versus 76% [P = 0.02]) for the treatment of patients with severe CDAD (6). However, unacceptable recurrence rates and emergence of C. difficile isolates with elevated MICs to vancomycin and metronidazole, suggesting potential reduced susceptibility (21, 22), highlight the need for new agents.
Surotomycin is a novel, cyclic lipopeptide with Gram-positive activity against C. difficile and limited activity against Gram-negative pathogens (11, 12). In a recent phase 2 clinical trial comparing surotomycin (125 mg or 250 mg b.i.d.) with oral vancomycin, clinical cure rates at end of treatment were similar across treatment groups: 92.4% and 86.6% for the 125-mg b.i.d. and 250-mg b.i.d. surotomycin groups, respectively, and 89.4% for the oral vancomycin group. However, lower rates of recurrence were observed with 125 mg of surotomycin b.i.d. (27.9%) and 250 mg of surotomycin b.i.d. (17.2%) compared with oral vancomycin (35.6%). Furthermore, the rates of sustained cure, defined as cure at the end of the 10-day treatment period with no recurrence during 4-week follow-up, were improved with surotomycin at 125 mg b.i.d. and 250 mg b.i.d. (66.7% and 70.1%, respectively) compared with oral vancomycin (56.1%) (13, 23). Development of resistance to surotomycin in C. difficile is likely to be a rare event, as previously demonstrated in an earlier in vitro serial passage study (10).
In this in vitro study, emergence of spontaneous resistance and potential multistep resistance in C. difficile, E. faecalis, and E. faecium was evaluated. Enterococci are common gastrointestinal commensal organisms in humans, known for their high level of intrinsic antibiotic resistance and propensity to acquire and disseminate resistance mechanisms (24), therefore providing a rationale for evaluation of VSE and VRE. The rate of spontaneous resistance to surotomycin at 8, 16, and 32× the MIC was either low or below the limit of detection in C. difficile and enterococci, including VRE. Under selective pressure with surotomycin, C. difficile BI, K, and QC remained relatively unchanged over 15 days of serial passage, while C. difficile BK remained unchanged until approximately day 9. Strain BK cells grew in 16 to 32 μg/ml at the end of day 15 (MIC values of 8 to >16 μg/ml [8- to 16-fold increase than those of the naive controls]). VSEfs, VSEfm, VREfs, and VREfm remained relatively unchanged over 15 days of serial passage, growing in a maximum of 8 μg/ml surotomycin (MIC values 2 to 16 μg/ml [2- to 16-fold increase than those of the naive controls]).
During a recent phase 2 clinical trial of patients with CDAD, stool samples were collected from 26 patients treated with either surotomycin (125 mg b.i.d. or 250 mg b.i.d.) or oral vancomycin. Surotomycin concentrations in fecal samples were >1,000 μg/g (data on file at Cubist Pharmaceuticals, Lexington, MA, USA), which is significantly higher than the highest MIC recorded in any of the follow-up isolates during this study. Therefore, the emergence of C. difficile resistance to surotomycin during treatment of CDAD is likely to be infrequent.
Surotomycin maintained potent MIC activity and bactericidal activity against both naive and reduced-susceptibility isolates of C. difficile, E. faecalis, and E. faecium, selected by 15-day serial passage. Furthermore, of the isolates tested, no cross-resistance was observed to various classes of antibiotics. These findings are consistent with clinical observations in phase 1 and phase 2 trials (data on file at Cubist Pharmaceuticals, Lexington, MA, USA), in which surotomycin did not select for C. difficile resistance to other cyclic lipopeptides or other antibiotic drug classes. Taken together, these in vitro data suggest that the emergence of resistance to surotomycin against C. difficile, E. faecalis, and E. faecium (VSE and VRE) is likely to be rare, supporting continued clinical development of this agent for the treatment of CDAD.
ACKNOWLEDGMENTS
We acknowledge StemScientific for providing writing and editorial support, funded by Cubist Pharmaceuticals.
Footnotes
Published ahead of print 5 May 2014
REFERENCES
- 1.Kelly CP, Pothoulakis C, LaMont JT. 1994. Clostridium difficile colitis. N. Engl. J. Med. 330:257–262. 10.1056/NEJM199401273300406 [DOI] [PubMed] [Google Scholar]
- 2.Bartlett JG. 2002. Clinical practice. Antibiotic-associated diarrhea. N. Engl. J. Med. 346:334–339. 10.1056/NEJMcp011603 [DOI] [PubMed] [Google Scholar]
- 3.Bouza E. 2012. Consequences of Clostridium difficile infection: understanding the healthcare burden. Clin. Microbiol. Infect. 18(Suppl 6):5–12. 10.1111/1469-0691.12064 [DOI] [PubMed] [Google Scholar]
- 4.Wiegand PN, Nathwani D, Wilcox MH, Stephens J, Shelbaya A, Haider S. 2012. Clinical and economic burden of Clostridium difficile infection in Europe: a systematic review of healthcare-facility-acquired infection. J. Hosp. Infect. 81:1–14. 10.1016/j.jhin.2012.02.004 [DOI] [PubMed] [Google Scholar]
- 5.McDonald LC, Killgore GE, Thompson A, Owens RC, Jr, Kazakova SV, Sambol SP, Johnson S, Gerding DN. 2005. An epidemic, toxin gene-variant strain of Clostridium difficile. N. Engl. J. Med. 353:2433–2441. 10.1056/NEJMoa051590 [DOI] [PubMed] [Google Scholar]
- 6.Zar FA, Bakkanagari SR, Moorthi KM, Davis MB. 2007. A comparison of vancomycin and metronidazole for the treatment of Clostridium difficile-associated diarrhea, stratified by disease severity. Clin. Infect. Dis. 45:302–307. 10.1086/519265 [DOI] [PubMed] [Google Scholar]
- 7.Musher DM, Aslam S, Logan N, Nallacheru S, Bhaila I, Borchert F, Hamill RJ. 2005. Relatively poor outcome after treatment of Clostridium difficile colitis with metronidazole. Clin. Infect. Dis. 40:1586–1590. 10.1086/430311 [DOI] [PubMed] [Google Scholar]
- 8.Pépin J, Valiquette L, Gagnon S, Routhier S, Brazeau I. 2007. Outcomes of Clostridium difficile-associated disease treated with metronidazole or vancomycin before and after the emergence of NAP1/027. Am. J. Gastroenterol. 102:2781–2788. 10.1111/j.1572-0241.2007.01539.x [DOI] [PubMed] [Google Scholar]
- 9.Louie TJ, Miller MA, Mullane KM, Weiss K, Lentnek A, Golan Y, Gorbach S, Sears P, Shue YK, OPT-80-003 Clinical Study Group 2011. Fidaxomicin versus vancomycin for Clostridium difficile infection. N. Engl. J. Med. 364:422–431. 10.1056/NEJMoa0910812 [DOI] [PubMed] [Google Scholar]
- 10.Mascio CTM, Mortin LI, Howland KT, Van Praagh ADG, Zhang S, Arya A, Chuong CL, Kang C, Li T, Silverman JA. 2012. In vitro and in vivo characterization of CB-183,315, a novel lipopeptide antibiotic for the treatment of Clostridium difficile. Antimicrob. Agents Chemother. 56:5023–5030. 10.1128/AAC.00057-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Citron DM, Tyrrell KL, Merriam CV, Goldstein EJC. 2012. In vitro activities of CB-183,315, vancomycin, and metronidazole against 556 strains of Clostridium difficile, 445 other intestinal anaerobes, and 56 Enterobacteriaceae species. Antimicrob. Agents Chemother. 56:1613–1615. 10.1128/AAC.05655-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Snydman DR, Jacobus NV, McDermott LA. 2012. Activity of a novel cyclic lipopeptide, CB-183,315, against resistant Clostridium difficile and other gram-positive aerobic and anaerobic intestinal pathogens. Antimicrob. Agents Chemother. 56:3448–3452. 10.1128/AAC.06257-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Patino H, Stevens C, Louie T, Bernado P, Friedland I. 2011. Efficacy and safety of the lipopeptide CB-183,315 for the treatment of Clostridium difficile infection, abstr K-205a. Abstr. 51st Intersci. Conf. Antimicrob. Agents Chemother. American Society for Microbiology, Washington, DC [Google Scholar]
- 14.Mascio C, Chesnel L, Thorne G, Silverman J. 2012. CB-183,315 demonstrates low in vitro frequency of spontaneous or multistep resistance in Clostridium difficile, Enterococcus faecalis and E. faecium, abstr. P41, p117. Abstr. 4th Int, Clostridium difficile Symp [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mascio C, Chesnel L, Thorne G, Silverman J. 2013. Surotomycin (CB-183,315) demonstrates rapid bactericidal activity against strains of Clostridium difficile, Enterococcus faecalis and E. faecium subjected to 15 days of serial passage under anaerobic conditions, abstr. F-627. Abstr. 53rd Intersci. Conf. Antimicrob. Agents Chemother. American Society for Microbiology, Washington, DC [Google Scholar]
- 16.Clinical and Laboratory Standards Institute. 2007. Methods for antimicrobial susceptibility testing of anaerobic; approved standard M11-A7. Clinical and Laboratory Standards Institute, Wayne, PA [Google Scholar]
- 17.Citron DM, Goldstein EJ. 2011. Reproducibility of broth microdilution and comparison to agar dilution for testing CB-183,315 against clinical isolates of Clostridium difficile. Diagn. Microbiol. Infect. Dis. 70:554–556. 10.1016/j.diagmicrobio.2011.04.012 [DOI] [PubMed] [Google Scholar]
- 18.Silverman JA, Oliver N, Andrew T, Li T. 2001. Resistance studies with daptomycin. Antimicrob. Agents Chemother. 45:1799–1802. 10.1128/AAC.45.6.1799-1802.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gonzales M, Pepin J, Frost EH, Carrier JC, Sirard S, Fortier LC, Valiquette L. 2010. Faecal pharmacokinetics of orally administered vancomycin in patients with suspected Clostridium difficile infection. BMC Infect. Dis. 10:363–369. 10.1186/1471-2334-10-363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bolton RP, Culshaw MA. 1986. Faecal metronidazole concentrations during oral and intravenous therapy for antibiotic associated colitis due to Clostridium difficile. Gut 27:1169–1172. 10.1136/gut.27.10.1169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Peláez T, Alcalá L, Alonso R, Rodríguez-Créixems M, García-Lechuz JM, Bouza E. 2002. Reassessment of Clostridium difficile susceptibility to metronidazole and vancomycin. Antimicrob. Agents Chemother. 46:1647–1650. 10.1128/AAC.46.6.1647-1650.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Baines SD, O'Connor R, Freeman J, Fawley WN, Harmanus C, Mastrantonio P, Kuijper EJ, Wilcox MH. 2008. Emergence of reduced susceptibility to metronidazole in Clostridium difficile. J. Antimicrob. Chemother. 62:1046–1052. 10.1093/jac/dkn313 [DOI] [PubMed] [Google Scholar]
- 23.Chesnel L, Sambol S, Gerding D, Patino H, Thorne G, Silverman J. 2012. Treatment of CDAD with oral CB-183 315: time to recurrence, relapse and reinfection rates compared with vancomycin. Clin. Microbiol. Infect. 18(Suppl 3):380. 10.1111/j.1469-0691.2012.03802.x [DOI] [Google Scholar]
- 24.Gilmore MS, Lebreton F, van Schaik W. 2013. Genomic transition of enterococci from gut commensals to leading causes of multidrug-resistant hospital infection in the antibiotic era. Curr. Opin. Microbiol. 16:10–16. 10.1016/j.mib.2013.01.006 [DOI] [PMC free article] [PubMed] [Google Scholar]