Abstract
In recent years Canis familiaris, the domestic dog, has drawn considerable attention as a system in which to investigate the genetics of disease susceptibility, morphology and behavior. Because dogs show remarkable intrabreed homogeneity, coupled with striking interbreed heterogeneity, the dog offers unique opportunities to understand the genetic underpinnings of natural variation in mammals, a portion of which is disease susceptibility. In this review, we highlight the unique features of the dog, such as population diversity and breed structure, that make it particularly amenable to genetic studies. We highlight recent advances in understanding the architecture of the dog genome, which propel the system to the forefront of consideration when selecting a system for disease gene studies. The most notable benefit of using the dog for genetic studies is that dogs get many of the same diseases as humans, with a similar frequency, and the same genetic factors are often involved. We discuss two approaches for localizing disease genes in the dog and provide examples of ongoing studies.
Introduction
The domestic dog, Canis familiaris, reportedly bears over 450 diseases; approximately 360 of which are analogous to human diseases (Patterson, 2000; Parker and Ostrander, 2005; Wayne and Ostrander, 2007). As a spontaneous model for many heritable human diseases, the dog provides an excellent system for the identification and study of disease loci (Parker and Ostrander, 2005; Wayne and Ostrander, 2007; Karlsson and Lindblad-Toh, 2008), particularly cancer loci (Cadieu and Ostrander, 2007). Specific advantages of the dog system include the fact that the dog has a unique population structure, with each breed arising from a limited number of founders (American, 1998). This fact, combined with the frequent use of popular sires, means that each domestic breed is a closed population, with limited locus and disease heterogeneity (Ostrander and Kruglyak, 2000; Ostrander and Wayne, 2005; Parker and Ostrander, 2005; Karlsson and Lindblad-Toh, 2008). As a result, genetic studies in dogs are theoretically simpler and more straightforward than those conducted in complex populations, offering many of the statistical advantages of studies performed in isolated human populations, such as those carried out in Finland or Iceland (Ostrander and Kruglyak, 2000). Additional advantages are offered by the architecture of the dog genome itself (Lindblad-Toh et al., 2005); the dog is known to have long stretches of linkage disequilibrium (LD), reducing the overall number of markers needed to investigate the genome (Sutter et al., 2004; Lindblad-Toh et al., 2005). In addition, analysis of the whole-genome sequence assembly of the dog at both a 2× and 7.8× coverage shows that it is, as expected, more homologous in sequence conservation to humans than mice (Kirkness et al., 2003; Lindblad-Toh et al., 2005). Finally, with humans and dogs sharing a common environment, food, immunologic profile (Storb and Thomas, 1985) and carcinogenic load (Glickman et al., 2004), it is entirely predictable that the domestic dog would emerge as a viable model for cancer genetics.
Breed structure and disease predisposition of dogs
The domestic dog population is divided into over 350 discrete breeds worldwide, with approximately 160 breeds recognized in the USA by the American Kennel Club (AKC) (http://www.akc.org/) (American, 1998). Each breed exhibits a distinct phenotype created by selective breeding practices that took place largely during the Victorian era (Clark and Brace, 1995; Wilcox and Walkowicz, 1995). Today, kennel clubs in both the USA and Europe impose strict restrictions on dog registration; to be an official member of a breed the ancestors of each dog must be registered members as well. Thus, each dog breed represents a closed breeding population of individuals with high levels of phenotypic homogeneity. Not surprisingly, there is reduced genetic diversity within breeds and greater genetic divergence between breeds. Indeed, 27% of the total genetic variance observed in dogs is between breeds, compared with the 5–10% that exists between distinct human populations (Parker et al., 2004).
The strong selection that breeders have imposed in order to produce a homogenous population of individuals with common morphological and behavioral traits has led to an excess of inherited diseases in domestic dogs. Although this is unfortunate for the companion animal community, the fact that many breeds display an excess of disease offers a unique opportunity to identify genes that have been difficult to localize through the study of human families and populations, with cancer providing an excellent example (Cadieu and Ostrander, 2007).
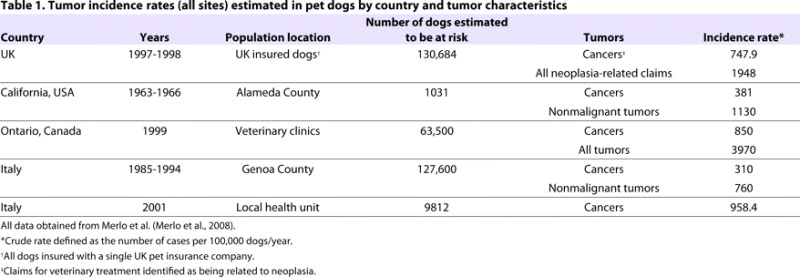
The frequency of occurrence and response to treatment of canine tumors often parallels human neoplasms (Dorn, 1976; Cadieu and Ostrander, 2007; Breen and Modiano, 2008) much better than, for instance, rodent tumors. Similar to in humans, canine tumors usually appear spontaneously, whereas rodent tumors are frequently induced. In terms of the epidemiology of cancer, it is at least as common in dogs as in humans (Table 1). In a necropsy series of 2000 dogs, 23% of all dogs, and 45% of dogs that were over 10 years old, died of cancer (Bronson, 1982; Vail and MacEwen, 2000). The challenge has been to make use of this information to identify genes that are important in both human and companion animal disease; to do this, two general approaches have been used.
Table 1.
Tumor incidence rates (all sites) estimated in pet dogs by country and tumor characteristics
Mapping disease genes in dogs
Finding genes that predispose any disease begins with the identification of a susceptible population. For family-based studies, DNA samples are collected from both affected and unaffected individuals in families segregating an excess of disease. Phenotypes are carefully established and a genome-wide linkage scan is performed using published approaches (Fig. 1) (Acland et al., 1998; Lingaas et al., 1998; Acland et al., 1999). In the past, these scans relied on the use of microsatellite repeat-based markers (Ostrander et al., 2000; Ostrander and Wayne, 2005), and were used to identify the gene for a canine form of a kidney cancer, known as Birt-Hogg-Dubé (BHD) disease, and the genes for several vision disorders (Acland et al., 1998; Acland et al., 1999; Jónasdóttir et al., 2000; Lingaas et al., 2003). Evidence of a locus is indicated by calculation of a Lod (log of the odds) score (Ott, 1976), which indicates the likelihood that a given region of the genome is segregating with the disease state because it is genetically linked, versus the likelihood that the segregation occurs by chance.
Fig. 1.
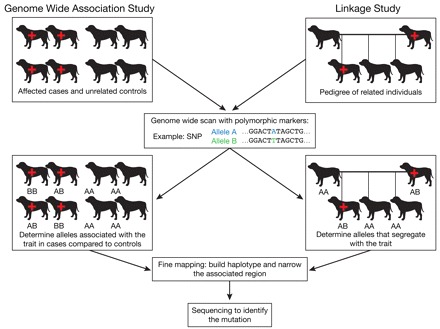
Genome-wide association study (GWAS) versus linkage-based study. A GWAS study compares a dense set of genotypes from animals that have a particular trait (cases) with unrelated controls, in order to ascertain alleles that are associated with the trait. Specific corrections need to be applied to account for factors such as population stratification. By comparison, a linkage study analyzes a genome-wide distribution of markers through multiple generations of a pedigree. Statistical methods are used to find a marker whose alleles segregate with disease status more often then would be expected by chance. Both types of approaches can be successful in identifying a disease locus. Fine mapping with more markers is used to identify a shared pattern of alleles from adjacent markers (haplotype). Sequencing is used to ultimately identify the disease mutation. The red cross symbol indicates an individual that is affected with the disease. The polymorphic markers that are used most frequently for linkage-based studies include SNPs and microsatellites, whereas GWAS studies use SNPs exclusively.
With the completion of a 7.8× draft assembly of the dog genome sequence (Lindblad-Toh et al., 2005), a canine single nucleotide polymorphism (SNP) chip has been developed, producing between 40,000 and 50,000 data points for each individual tested (Karlsson et al., 2007). Not only can the SNP chip be used for linkage-based studies, but this resource has also proven useful for performing genome-wide association studies (GWAS) in which a population of dogs with well-documented disease is compared with a population of healthy controls of the same breed (Fig. 1). This approach is very powerful in dogs, particularly if multiple breeds are used and, for example, has resulted in the mapping of both morphologic (Salmon Hillbertz et al., 2007; Drogemuller et al., 2008; Cadieu et al., 2009; Parker et al., 2009) and disease-associated traits (Wiik, 2008; Awano, 2009). Of note, samples are relatively easy to collect for GWAS, because one can avoid the tedious process of tracking specific family members and any diseased individual who meets a pre-specified set of criteria is usually accepted into the study.
Two key factors have led to the rapid and successful growth of GWAS studies in dogs. The first is that far fewer markers are needed to perform a GWAS study in dogs than, for instance, in humans. This is largely because of the extensive LD that is observed in dogs (Sutter et al., 2004; Lindblad-Toh et al., 2005). Whereas LD in humans rarely extends for more than 10–20 Kb, it is not uncommon for LD in dogs to be in the order of megabases (Sutter et al., 2004; Lindblad-Toh et al., 2005). Thus, whereas human studies require in the order of 500,000 to a million markers, both Sutter et al. and Lind-blad-Toh et al. (Sutter et al., 2004; Lindblad-Toh et al., 2005) predicted, correctly, that 30,000–50,000 biallelic markers would more than suffice for GWAS studies in dogs. This has since been proven by the localization of genes for several canine traits (Salmon Hill-bertz et al., 2007; Drogemuller et al., 2008; Wiik, 2008; Awano, 2009; Cadieu et al., 2009; Parker et al., 2009).
The second advantage to performing genetic mapping studies in the dog is embedded in the breed structure itself. Many dog breeds can be grouped into genetic clusters (Parker et al., 2004; Parker et al., 2007). Genetic loci conferring shared phenotypes within breeds that are members of each cluster can be identified much more readily than when studies are confined to a single breed. In addition, examination of LD in regions of association for multiple, related dog breeds can be used to narrow a crucial region of linkage or association, thus facilitating the task of moving from a linked or associated marker to a gene. This was aptly demonstrated by Parker et al. who used a combination of 20 dog breeds to find an expressed retrogene that is responsible for the chondrodysplasia phenotype in several dog breeds including the Corgi, Basset Hound, Dachshund, etc (Parker et al., 2009). Cadieu et al. also demonstrated the same principle in the search for genes controlling hair type, combining data from dozens of breeds to identify genes controlling fur length, curl and texture (Cadieu et al., 2009).
Cancer
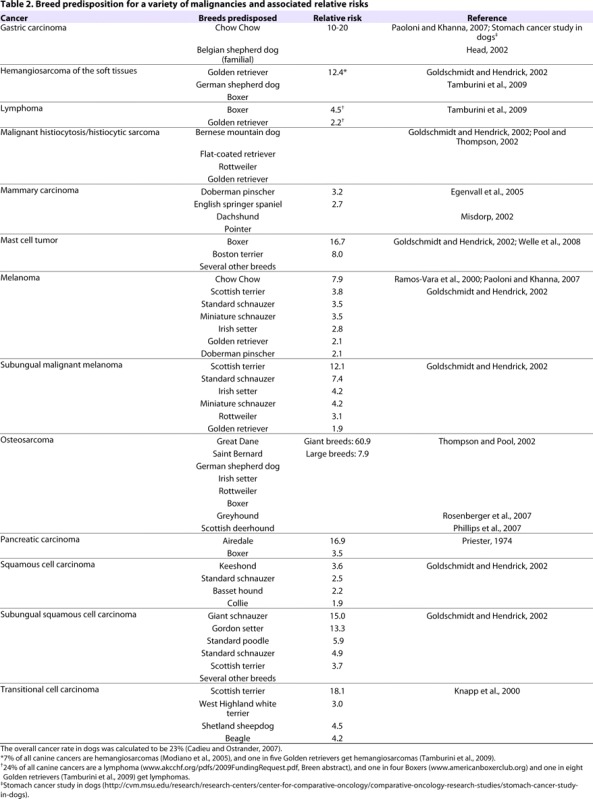
Canine malignancies have been established as strong comparative models for many types of human cancers including transitional cell carcinoma (TCC) of the bladder (Knapp et al., 2000), non-Hodgkin’s lymphoma (Leifer and Matus, 1986; Valli et al., 2006), leukemias, osteosarcoma (Mueller et al., 2007), melanoma (particularly oral melanoma) (MacEwen, 1990; Bergman, 2007) and soft tissue sarcomas (Onions, 1984; Moore and Rosin, 1986; Affolter and Moore, 2000). Mammary (Taylor et al., 1976; Gilbertson et al., 1983) and squamous cell carcinomas (O’Brien, 1992; Henry et al., 2005) have also been reported in the dog. Many of these cancers exhibit an increased prevalence in particular breeds of dogs (Table 2), indicating a genetic predisposition (Cadieu and Ostrander, 2007). Some examples are discussed below.
Table 2.
Breed predisposition for a variety of malignancies and associated relative risks
Renal cystadenocarcinoma and nodular dermatofibrosis in the German shepherd dog
One of the most compelling examples of the use of genome-wide linkage scans to identify canine cancer loci comes from the study of renal cystadenocarcinoma and nodular dermatofibrosis (RCND), a hereditary kidney cancer of the German shepherd dog (GSD) that is characterized by bilateral, multifocal renal tumors, skin nodules and, in females, uterine leiomyomas (Jónasdóttir et al., 2000; Moe et al., 2000). The disease is metastasic in about 50% of cases. In 2000, following a genome-wide linkage scan, the disease locus was mapped to canine chromosome 5 (CFA5) using a large family of several sibships that originated from a single affected popular sire (Fig. 2) (Jónasdóttir et al., 2000). A mutation was found to segregate with affected dogs in exon seven of the folliculin (FLCN) gene (Lingaas et al., 2003). Mutations in folliculin cause a similar disorder in humans, also characterized by renal neoplasias, called BHD syndrome (Schmidt et al., 2001; Nickerson et al., 2002). In dogs, however, tumors develop at a much earlier age and the disease progresses at an accelerated rate, providing an excellent model for the study of this unique cancer. Although this is a relatively rare disease, this study aptly demonstrates the power of popular sires and large mapping families in establishing canine disease models.
Fig. 2.
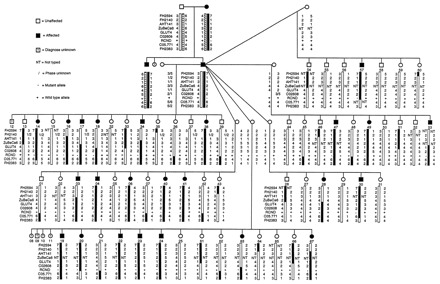
Example of a linkage pedigree. The GSD pedigrees segregating RCND; this is an example of a powerful canine linkage study that was used to identify a cancer locus (Jónasdóttir et al., 2000). Several highly linked microsatellite markers established CFA5 as the location (Jónasdóttir et al., 2000), which was subsequently identified as a mutation in exon 7 of the folliculin gene (Lingaas et al., 2003). Figure reproduced from Jónasdóttir et al. (Jónasdóttir et al., 2000). Copyright (2000) National Academy of Sciences, USA.
Transitional cell carcinoma
Another disorder that lends itself to genetic studies is TCC of the bladder, the most common malignancy of the urinary tract in dogs. Epidemiological studies reveal a number of risk factors, including breed and female gender, as well as environmental factors, such as insecticide exposure (Glickman et al., 1989; Glickman et al., 2004). The tumor is difficult to remove surgically and responds poorly to chemotherapy. The human and canine forms of the disease share many similarities, including similar clinical signs and metastases in 50% of cases, and both express cyclooxygenase-2 in the majority of cases (Glickman et al., 2004).
Only about five major breeds of dog are affected by TCC, with the Scottish terrier having a 20-fold increased risk compared with other dog breeds (Hayes, 1976; Glickman et al., 2004). Other at-risk breeds include the West Highland white terrier, the Shetland sheepdog and the Beagle, each of which are reported to have an increased risk of between threefold and fivefold (Table 2) (Knapp et al., 2000). The West Highland white terrier and Scottish terrier have been shown to share a common phylogenetic lineage (Parker et al., 2007), suggesting that the consideration of these two breeds together is likely to increase the power in a GWAS. With much remaining to be learnt about the genetic factors that predispose to human TCC, studies of the canine disorder are likely to be revealing.
In addition to the value of canine TCC as a genetic model, the disease also serves as a therapeutic model. The cyclooxygenase inhibitor piroxicam has been studied in canine drug trials for the treatment of TCC. One study showed a complete remission in two dogs and a partial remission in four out of 34 dogs (Knapp et al., 1994). Another trial undertaken by Mohammed and colleagues (Mohammed et al., 2002) demonstrated a reduction in tumor volume in 12 out of 18 dogs, suggesting the need for a parallel human trial.
Malignant histiocytosis in the Bernese mountain dog
Although TCC and RCND display the strong comparative power of the canine model, there is also enormous value in the study of canine diseases that do not directly mimic a human disease. For example, malignant histiocytosis (MH), also referred to as disseminated histiocytic sarcoma, is a highly aggressive cancer of the dendritic cells of the soft tissues that occurs in a small number of dog breeds such as the Bernese mountain dog (BMD), with 25% of BMDs succumbing to the disease (Abadie et al., 2009). However, malignant histiocytic neoplasms are extremely rare in the human population (Favara et al., 1997), resulting in little opportunity to study the behavior of aberrant dendritic cells in humans. MH in the BMD thus provides an opportunity to learn about genes that are essential for dendritic cell function, as well as to better understand the utility of dendritic cells in the human immune system.
Appendicular osteosarcoma
For some canine diseases, a human counterpart occurs in only a subset of the population. Osteosarcoma (OSA) occurs most frequently in children and adolescents, with 400 cases per year in the population of the USA that is less than 20 years old; 85% of OSA cases occur in the extremities (Hawkins and Arndt, 2003). However, in dogs, OSA occurs about ten times more frequently (Khanna et al., 2006). Canine OSA cells, as well as human OSA tumors, display chaotic and heterogeneous karyotypes with hypodiploidy, hyperploidy, and structural and numerical chromosomal aberrations. The dog breeds with an increased incidence include the Rottweiler, GSD, Great Dane, Scottish deerhound and Greyhound (Thomas et al., 2009). The latter three are interesting because they are all long-limbed hounds, suggesting that there is a particular physiology in dogs that is associated with disease incidence.
One approach for unraveling the relationship between the disease and its underlying genetics is to look for copy number alterations in tumors (Wang et al., 2002; Rueda and Diaz-Uriarte, 2007). When comparing Rottweiler tumors with Golden retriever tumors, Thomas et al. demonstrated breed-specific differences in copy number aberrations in OSA cells (Thomas et al., 2009). Thomas et al. hypothesized that differences in the genetic backgrounds of each breed could yield hints about the life cycle of the tumor. Indeed, several loci with known tumor suppressor or oncogenic functions, including WT1, TP53 and CDKN2A, displayed either genomic amplifications or deletions that were significantly associated with the specific dog breed.
Advantages of Canis familiaris as a model for genetic susceptibility to disease.
The canine genome exhibits greater homology to the human genome than other mammalian models for disease
The dog population is divided into breeds that were generated by small founder populations and propagated by a closed breeding pool, thereby maintaining a high level of genetic homogeneity within most breeds
The breed structure reduces disease heterogeneity and is an attribute for identifying disease susceptibility loci in a single breed or related breeds
Dogs are susceptible to many of the same diseases as humans, particularly complex diseases like cancer
Cancer occurs spontaneously in dogs, at a rate that is at least equal to human cancer, and often closely mimics characteristics of human tumors
Humans and dogs share many of the same environmental factors that may influence disease onset or progression
Chronic myelogenous leukemia
In some cases, human and canine cancers share the same genetic hallmarks. For instance, Breen and Modiano recently reported that both human and canine chronic myelogenous leukemia (CML) results from the production of a breakpoint cluster region (BCR)-Abl fusion gene product, which is a tyrosine kinase that is involved in cell division and apoptosis (Breen and Modiano, 2008). In humans this so-called ‘Philadelphia chromosome’ involves a translocation event between chromosomes 9 and 22, t(9;22)(q34;q11). HSA 9q34 and 22q11 correspond to CFA 9q25–26.1 and 26q23–24, respectively, in the dog, and the translocation of these loci is denoted as t(9;26)(25–26.1;23–24). Although the numbers are small, studies of tumors from five dogs affected with CML demonstrated that 11–34% of cells carry the same translocation. No published studies exist that evaluate the use of the highly touted drug imatinib, which was developed for human CML and inhibits the breakpoint cluster kinase; however, these data suggest that the dog may be a useful system in which to study appropriate targeted therapies.
Conclusions and long-term issues
In the last 15 years, the domestic dog has emerged as a powerful genetic tool for the study of heritable human diseases. Human disorders associated with immunodeficiency, narcolepsy, metabolic disease, cancer, autoimmune function, vision and epilepsy have all been studied in the dog (Jezyk et al., 1989; Lingaas et al., 1998; Lin et al., 1999; Jónasdóttir et al., 2000; Hungs et al., 2001; Sidjanin et al., 2002; Chase et al., 2005; Clark et al., 2005; Lohi et al., 2005; Zangerl et al., 2006). Advances in several disorders, particularly those associated with vision, sleep and immune function, have informed us about how to better understand the comparable human disease (Acland et al., 1998; Acland et al., 2001; Chabas et al., 2003; Lingaas et al., 2003; Chase et al., 2006). The recent introduction of cancer into the portfolio is exciting and suggests that new and relevant discoveries are around the corner (Khanna et al., 2006).
As more canine cancers are localized at a genomic level, the next steps will be the isolation of specific causative mutations. As we further define the molecular mechanisms responsible for various cancers, the assessment of those that are most similar in humans and canines will aid in targeting drug development efforts. Here, again, the dog will be of use, because it is vital to the development of new treatment modalities (Paoloni and Khanna, 2007). For example, the dog may prove useful in developing new treatments for CML patients who have developed resistance to imatinib, as we now know that the genetic translocations are the same in both humans and canines (Breen and Modiano, 2008). The advantages of the comparative disease approach are at the forefront of the minds of the collective genomics community as we now recognize that a clearer understanding of canine diseases is certain to lead to improved treatment options for both man and his best friend.
Acknowledgments
We thank the dog owners who have contributed DNA samples for our studies for their time and generosity. We gratefully acknowledge the American Kennel Club Canine Health Foundation and the Intramural program of the National Human Genome Research Institute for their support. Deposited in PMC for release after 12 months.
Footnotes
COMPETING INTERESTS
The authors declare no competing financial interests.
REFERENCES
- Abadie J, Hedan B, Cadieu E, De Brito C, Devauchelle P, Bourgain C, Parker HG, Vaysse A, Margaritte-Jeannin P, Galibert F, et al. (2009). Epidemiology, pathology, and genetics of histiocytic sarcoma in the Bernese mountain dog breed. J Hered. 100 Suppl 1, S19–S27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Acland GM, Ray K, Mellersh CS, Gu W, Langston AA, Rine J, Ostrander EA, Aguirre GD. (1998). Linkage analysis and comparative mapping of canine progressive rod-cone degeneration (prcd) establishes potential locus homology with retinitis pigmentosa (RP17) in humans. Proc Natl Acad Sci USA 96, 3048–3053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Acland GM, Ray K, Mellersh CS, Langston AA, Rine J, Ostrander EA, Aguirre GD. (1999). A novel retinal degeneration locus identified by linkage and comparative mapping of canine early retinal degeneration. Genomics 59, 134–142 [DOI] [PubMed] [Google Scholar]
- Acland GM, Aguirre GD, Ray J, Zhang Q, Aleman TS, Cideciyan AV, Pearce-Kelling SE, Anand V, Zeng Y, Maguire AM, et al. (2001). Gene therapy restores vision in a canine model of childhood blindness. Nat Genet. 28, 92–95 [DOI] [PubMed] [Google Scholar]
- Affolter VK, Moore PF. (2000). Canine cutaneous and systemic histiocytosis: reactive histiocytosis of dermal dendritic cells. Am J Dermatopathol. 22, 40–48 [DOI] [PubMed] [Google Scholar]
- American KC. (1998). The Complete Dog Book. New York, NY: Howell Book House [Google Scholar]
- Awano T, Johnson GS, Wade CM, Katz ML, Johnson GC, Taylor JF, Perloski M, Biagi T, Baranowska I, Long S, et al. (2009). Genome-wide association analysis reveals a SOD1 mutation in canine degenerative myelopathy that resembles amyotrophic lateral sclerosis. Proc Natl Acad Sci USA 106, 2794–2799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergman PJ. (2007). Canine oral melanoma. Clin Tech Small Anim Pract. 22, 55–60 [DOI] [PubMed] [Google Scholar]
- Breen M, Modiano JF. (2008). Evolutionarily conserved cytogenetic changes in hematological malignancies of dogs and humans-man and his best friend share more than companionship. Chromosome Res. 16, 145–154 [DOI] [PubMed] [Google Scholar]
- Bronson RT. (1982). Variation in age at death of dogs of different sexes and breeds. Am J Vet Res. 43, 2057–2059 [PubMed] [Google Scholar]
- Cadieu E, Ostrander EA. (2007). Canine genetics offers new mechanisms for the study of human cancer. Cancer Epidemiol Biomarkers Prev. 16, 2181–2183 [DOI] [PubMed] [Google Scholar]
- Cadieu E, Neff M, Quignon P, Walsh K, Chase K, Parker HG, VonHoldt BM, Rhue A, Boyko A, Byers A, et al. (in press). Coat variation in the domestic dog is governed by variants in three genes. Science 326, 150–153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chabas D, Taheri S, Renier C, Mignot E. (2003). The genetics of narcolepsy. Annu Rev Genomics Hum Genet. 4, 459–483 [DOI] [PubMed] [Google Scholar]
- Chase K, Lawler DF, Carrier DR, Lark KG. (2005). Genetic regulation of osteoarthritis: A QTL regulating cranial and caudal acetabular osteophyte formation in the hip joint of the dog (Canis familiaris).. Am J Hum Genet. 135, 334–335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chase K, Sargan D, Miller K, Ostrander EA, Lark KG. (2006). Understanding the genetics of autoimmune disease: two loci that regulate late onset Addison’s disease in Portuguese Water Dogs. Int J Imm. 33, 179–184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark AR, Brace AH. (1995). The International Encyclopedia of Dogs. New York, NY: Howell Book House [Google Scholar]
- Clark LA, Wahl JM, Steiner JM, Zhou W, Ji W, Famula TR, Williams DA, Murphy KE. (2005). Linkage analysis and gene expression profile of pancreatic acinar atrophy in the German Shepherd Dog. Mamm Genome 16, 955–962 [DOI] [PubMed] [Google Scholar]
- Dorn CR. (1976). Epidemiology of canine and feline tumors. Comp Cont Educ Pract Vet. 12, 307–312 [Google Scholar]
- Drogemuller C, Karlsson EK, Hytonen MK, Perloski M, Dolf G, Sainio K, Lohi H, Lindblad-Toh K, Leeb T. (2008). A mutation in hairless dogs implicates FOXI3 in ectodermal development. Science 321, 1462. [DOI] [PubMed] [Google Scholar]
- Egenvall A, Bonnett BN, Ohagen P, Olson P, Hedhammar A, von Euler H. (2005). Incidence of and survival after mammary tumors in a population of over 80,000 insured female dogs in Sweden from 1995 to 2002. Prev Vet Med. 69, 109–127 [DOI] [PubMed] [Google Scholar]
- Favara BE, Feller AC, Pauli M, Jaffe ES, Weiss LM, Arico M, Bucsky P, Egeler RM, Elinder G, Gadner H, et al. (1997). Contemporary classification of histiocytic disorders. The WHO Committee on histiocytic/reticulum cell proliferations. Reclassification working group of the histiocyte society. Med Pediatr Oncol. 29, 157–166 [DOI] [PubMed] [Google Scholar]
- Gilbertson SR, Kurzman ID, Zachrau RE, Hurvitz AI, Black MM. (1983). Canine mammary epithelial neoplasms: biologic implications of morphologic characteristics assessed in 232 dogs. Vet Pathol. 20, 127–142 [DOI] [PubMed] [Google Scholar]
- Glickman LT, Schofer FS, McKee LJ, Reif JS, Goldschmidt MH. (1989). Epidemiologic study of insecticide exposures, obesity, and risk of bladder cancer in household dogs. J Toxicol Environ Health 28, 407–414 [DOI] [PubMed] [Google Scholar]
- Glickman LT, Raghavan M, Knapp DW, Bonney PL, Dawson MH. (2004). Herbicide exposure and the risk of transitional cell carcinoma of the urinary bladder in Scottish Terriers. J Am Vet Med Assoc. 224, 1290–1297 [DOI] [PubMed] [Google Scholar]
- Goldschmidt MH, Hendrick MJ. (2002). Tumors of the skin and soft tissues. In: Tumors in Domestic Animals (ed. Meuten DJ.), pp. 45–118 Ames, Iowa: Blackwell Publishing Professional [Google Scholar]
- Hawkins DS, Arndt CA. (2003). Pattern of disease recurrence and prognostic factors in patients with osteosarcoma treated with contemporary chemotherapy. Cancer 98, 2447–2456 [DOI] [PubMed] [Google Scholar]
- Hayes HJ. (1976). Canine bladder cancer: epidemiologic features. Am J Epidemiol. 104, 673–677 [DOI] [PubMed] [Google Scholar]
- Head KW, Else RW, Dubielzig RR. (2002). Tumors of the Alimentary Tract. In Tumors in Domestic Animals (ed. Meuten DJ.), pp. 401–482 Ames, Iowa: Blackwell Publishing Professional [Google Scholar]
- Henry CJ, Brewer WG, Jr, Whitley EM, Tyler JW, Ogilvie GK, Norris A, Fox LE, Morrison WB, Hammer A, Vail DM, et al. (2005). Canine digital tumors: a veterinary cooperative oncology group retrospective study of 64 dogs. J Vet Intern Med. 19, 720–724 [DOI] [PubMed] [Google Scholar]
- Hungs M, Fan J, Lin L, Lin X, Maki RA, Mignot E. (2001). Identification and functional analysis of mutations in the hypocretin (orexin) genes of narcoleptic canines. Genome Res. 11, 531–539 [DOI] [PubMed] [Google Scholar]
- Jezyk PF, Felsburg PJ, Haskins ME, Patterson DF. (1989). X-linked severe combined immunodeficiency in the dog. Clin Immunol Immunopathol. 52, 173–189 [DOI] [PubMed] [Google Scholar]
- Jónasdóttir TJ, Mellersh CS, Moe L, Heggebø R, Gamlem H, Ostrander EA, Lingaas F. (2000). Genetic mapping of a naturally occurring hereditary renal cancer syndrome in dogs. Proc Natl Acad Sci USA 97, 4132–4137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karlsson EK, Lindblad-Toh K. (2008). Leader of the pack: gene mapping in dogs and other model organisms. Nat Rev Genet. 9, 713–725 [DOI] [PubMed] [Google Scholar]
- Karlsson EK, Baranowska I, Wade CM, Salmon Hillbertz NHC, Zody MC, Anderson N, Biagi TM, Patterson N, Pielberg GR, Kulbokas EJ, et al. (2007). Efficient mapping of mendelian traits in dogs through genome-wide association. Nat Genet. 39, 1321–1328 [DOI] [PubMed] [Google Scholar]
- Khanna C, Lindblad-Toh K, Vail D, London C, Bergman P, Barber L, Breen M, Kitchell B, McNeil E, Modiano JF, et al. (2006). The dog as a cancer model. Nat Biotechnol. 24, 1065–1066 [DOI] [PubMed] [Google Scholar]
- Kirkness EF, Bafna V, Halpern AL, Levy S, Remington K, Rusch DB, Delcher AL, Pop M, Wang W, Fraser CM, et al. (2003). The dog genome: survey sequencing and comparative analysis. Science 301, 1898–1903 [DOI] [PubMed] [Google Scholar]
- Knapp D, Richardson R, Chan T, Bottoms G, Widmer W, DeNicola D, Teclaw R, Bonney P, Kuczek T. (1994). Piroxicam therapy in 34 dogs with transitional cell carcinoma of the urinary bladder. J Vet Intern Med. 8, 273–278 [DOI] [PubMed] [Google Scholar]
- Knapp D, Glickman N, DeNicola D, Bonney P, Lin T, Glickman L. (2000). Naturally-occurring canine transitional cell carcinoma of the urinary bladder: A relevant model of human invasive bladder cancer. Urol Oncol. 5, 47–59 [DOI] [PubMed] [Google Scholar]
- Leifer CE, Matus RE. (1986). Canine lymphoma: clinical considerations. Semin Vet Med Surg (Small Anim) 1, 43–50 [PubMed] [Google Scholar]
- Lin L, Faraco J, Li R, Kadotani H, Rogers W, Lin X, Qiu X, Jong PJd, Nishino S, Mignot E. (1999). The sleep disorder canine narcolepsy is caused by a mutation in the hypocretin (orexin) receptor 2 gene. Cell 98, 365–376 [DOI] [PubMed] [Google Scholar]
- Lindblad-Toh K, Wade CM, Mikkelsen TS, Karlsson EK, Jaffe DB, Kamal M, Clamp M, Chang JL, Kulbokas EJ, 3rd, Zody MC, et al. (2005). Genome sequence, comparative analysis and haplotype structure of the domestic dog. Nature 438, 803–819 [DOI] [PubMed] [Google Scholar]
- Lingaas F, Aarskaug T, Sletten M, Bjerkas I, Grimholt U, Moe L, Juneja RK, Wilton AN, Galibert F, Holmes NG, et al. (1998). Genetic markers linked to neuronal ceroid lipofuscinosis in English setter dogs. Anim Genet. 29, 371–376 [DOI] [PubMed] [Google Scholar]
- Lingaas F, Comstock KE, Kirkness EF, Sorensen A, Aarskaug T, Hitte C, Nickerson ML, Moe L, Schmidt LS, Thomas R, et al. (2003). A mutation in the canine BHD gene is associated with hereditary multifocal renal cystadenocarcinoma and nodular dermatofibrosis in the German Shepherd dog. Hum Mol Genet. 12, 3043–3053 [DOI] [PubMed] [Google Scholar]
- Lohi H, Young EJ, Fitzmaurice SN, Rusbridge C, Chan EM, Vervoort M, Turnbull J, Zhao XC, Ianzano L, Paterson AD, et al. (2005). Expanded repeat in canine epilepsy. Science 307, 81. [DOI] [PubMed] [Google Scholar]
- MacEwen EG. (1990). Spontaneous tumors in dogs and cats: models for the study of cancer biology and treatment. Cancer Metastasis Rev. 9, 125–136 [DOI] [PubMed] [Google Scholar]
- Merlo DF, Rossi L, Pellegrino C, Ceppi M, Cardellino U, Capurro C, Ratto A, Sambucco PL, Sestito V, Tanara G, et al. (2008). Cancer incidence in pet dogs: findings of the Animal Tumor Registry of Genoa, Italy. J Vet Intern Med. 22, 976–984 [DOI] [PubMed] [Google Scholar]
- Misdorp W. (2002). Tumors of the Mammary Gland. In Tumors in Domestic Animals (ed. Meuten DJ.), pp. 575–606 Ames, Iowa: Blackwell Publishing Professional [Google Scholar]
- Modiano JF, Breen M, Burnett RC, Parker HG, Inusah S, Thomas R, Avery PR, Lindblad-Toh K, Ostrander EA, Cutter GC, et al. (2005). Distinct B-cell and T-cell lymphoproliferative disease prevalence among dog breeds indicates heritable risk. Cancer Res. 65, 5654–5661 [DOI] [PubMed] [Google Scholar]
- Moe L, Gamlem H, Jonasdottir TJ, Lingaas F. (2000). Renal microcystic tubular lesions in two 1-year-old-dogs- an early sign on hereditary renal cystadenocarcinomas? J Comp Pathol. 123, 218–221 [DOI] [PubMed] [Google Scholar]
- Mohammed SI, Bennett PF, Craig BA, Glickman NW, Mutsaers AJ, Snyder PW, Widmer WR, DeGortari AE, Bonney PL, Knapp DW. (2002). Effects of the cyclooxygenase inhibitor, piroxicam, on tumor response, apoptosis, and angiogenesis in a canine model of human invasive urinary bladder cancer. Cancer Res. 62, 356–358 [PubMed] [Google Scholar]
- Moore PF, Rosin A. (1986). Malignant histiocytosis of Bernese mountain dogs. Vet Pathol. 23, 1–10 [DOI] [PubMed] [Google Scholar]
- Mueller F, Fuchs B, Kaser-Hotz B. (2007). Comparative biology of human and canine osteosarcoma. Anticancer Res. 27, 155–164 [PubMed] [Google Scholar]
- Nickerson M, Warren M, Toro J, Matrosova V, Glenn G, Turner M, Duray P, Merino M, Choyke P, Pavlovich C, et al. (2002). Mutations in a novel gene lead to kidney tumors, lung wall defects, and benign tumors of the hair follicle in patients with the Birt-Hogg-Dube syndrome. Cancer Cell 2, 157. [DOI] [PubMed] [Google Scholar]
- O’Brien MG, Berg J, Engler SJ. (1992). Treatment by digital amputation of subungual squamous cell carcinoma in dogs: 21 cases (1987–1988). J Am Vet Med Assoc. 201, 759–761 [PubMed] [Google Scholar]
- Onions DE. (1984). A prospective survey of familial canine lymphosarcoma. J Natl Cancer Inst. 72, 909–912 [PubMed] [Google Scholar]
- Ostrander EA, Kruglyak L. (2000). Unleashing the canine genome. Genome Res. 10, 1271–1274 [DOI] [PubMed] [Google Scholar]
- Ostrander EA, Wayne RK. (2005). The canine genome. Genome Res. 15, 1706–1716 [DOI] [PubMed] [Google Scholar]
- Ostrander EA, Galibert F, Patterson DF. (2000). Canine genetics comes of age. Trends Genet. 16, 117–124 [DOI] [PubMed] [Google Scholar]
- Ott J. (1976). A computer program for linkage analysis of general human pedigrees. Am J Hum Genet. 28, 528–529 [PMC free article] [PubMed] [Google Scholar]
- Paoloni MC, Khanna C. (2007). Comparative oncology today. Vet Clin North Am Small Anim Pract. 37, 1023–1032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker H, Kukekova A, Akey D, Goldstein Ol, Kirkness E, Baysac K, Mosher DS, Aguirre G, Acland GM, Ostrander EA. (2007). Breed relationships facilitate fine mapping studies: A 7.8 Kb deletion cosegregates with collie eye anomaly across multiple dog breeds. Genome Res. 17, 1562–1571 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker HG, Ostrander EA. (2005). Canine genomics and genetics: Running with the pack. PLoS Genet. 1, e58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker HG, Kim LV, Sutter NB, Carlson S, Lorentzen TD, Malek TB, Johnson GS, DeFrance HB, Ostrander EA, Kruglyak L. (2004). Genetic structure of the purebred domestic dog. Science 304, 1160–1164 [DOI] [PubMed] [Google Scholar]
- Parker HG, VonHoldt BM, Quignon P, Margulies EH, Shao S, Mosher DS, Spady TC, Elkahloun A, Cargill M, Jones PG, et al. (2009). Expressed fibroblast growth factor 4 (fgf4) retrogene causes breed-defining chondrodysplasia in the domestic dog. Science 325, 995–998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patterson D. (2000). Companion animal medicine in the age of medical genetics. J Vet Internal Med. 14, 1–9 [PubMed] [Google Scholar]
- Phillips JC, Stephenson B, Hauck M, Dillberger J. (2007). Heritability and segregation analysis of osteosarcoma in the Scottish deerhound. Genomics 90, 354–363 [DOI] [PubMed] [Google Scholar]
- Pool RR, Thompson KG. (2002). Tumors of Joints. In Tumors in Domestic Animals (ed. Meuten DJ.), pp. 199–244 Ames, Iowa: Blackwell Publishing Professional [Google Scholar]
- Priester WA. (1974). Data from eleven United States and Canadian colleges of veterinary medicine on pancreatic carcinoma in domestic animals. Cancer Res. 34, 1372–1375 [PubMed] [Google Scholar]
- Ramos-Vara JA, Beissenherz ME, Miller MA, Johnson GC, Pace LW, Fard A, Kottler SJ. (2000). Retrospective study of 338 canine oral melanomas with clinical, histologic, and immunohistochemical review of 129 cases. Vet Pathol. 37, 597–608 [DOI] [PubMed] [Google Scholar]
- Rosenberger JA, Pablo NV, Crawford PC. (2007). Prevalence of and intrinsic risk factors for appendicular osteosarcoma in dogs: 179 cases (1996–2005). J Am Vet Med Assoc. 231, 1076–1080 [DOI] [PubMed] [Google Scholar]
- Rueda OM, Diaz-Uriarte R. (2007). Flexible and accurate detection of genomic copy-number changes from aCGH. PLoS Comput Biol. 3, e122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salmon Hillbertz NHC, Isaksson M, Karlsson EK, Hellmen E, Pielberg GR, Savolainen P, Wade CM, von Euler H, Gustafson U, Hedhammar A, et al. (2007). Duplication of FGF3, FGF4, FGF19 and ORAOV1 causes hair ridge and predisposition to dermoid sinus in Ridgeback dogs. Nat Genet. 39, 1318–1320 [DOI] [PubMed] [Google Scholar]
- Schmidt LS, Warren MB, Nickerson ML, Weirich G, Matrosova V, Toro JR, Turner ML, Duray P, Merino M, Hewitt S, et al. (2001). Birt-Hogg-Dube syndrome, a genodermatosis associated with spontaneous pneumothorax and kidney neoplasia, maps to chromosome 17p11.2. Am J Hum Genet. 69, 876–882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sidjanin DJ, Lowe JK, McElwee JL, Milne BS, Phippen TM, Sargan DR, Aguirre GD, Acland GM, Ostrander EA. (2002). Canine CNGB3 mutations establish cone degeneration as orthologous to the human achromatopsia locus ACHM3. Hum Mol Genet. 11, 1823–1833 [DOI] [PubMed] [Google Scholar]
- Storb R, Thomas ED. (1985). Graft-versus-host disease in dog and man: the Seattle Experience. In Immunological Reviews No. 88 (ed. Müller G.), pp. 215–238 Copenhagen: Munksgaard; [DOI] [PubMed] [Google Scholar]
- Sutter NB, Eberle MA, Parker HG, Pullar BJ, Kirkness EF, Kruglyak L, Ostrander EA. (2004). Extensive and breed-specific linkage disequilibrium in Canis familiaris. Genome Res. 14, 2388–2396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamburini BA, Trapp S, Phang TL, Schappa JT, Hunter LE, Modiano JF. (2009). Gene expression profiles of sporadic canine hemangiosarcoma are uniquely associated with breed. PLoS One 4, e5549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor GN, Shabestari L, Williams J, Mays CW, Angus W, McFarland S. (1976). Mammary neoplasia in a closed beagle colony. Cancer Res. 36, 2740–2743 [PubMed] [Google Scholar]
- Thomas R, Wang HJ, Tsai PC, Langford CF, Fosmire SP, Jubala CM, Getzy DM, Cutter GR, Modiano JF, Breen M. (2009). Influence of genetic background on tumor karyotypes: evidence for breed-associated cytogenetic aberrations in canine appendicular osteosarcoma. Chromosome Res. 17, 365–377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson KG, Pool RR. (2002). Tumors of Bones. In Tumors in Domestic Animals (ed. Meuten DJ.), pp. 245–318 Ames, Iowa: Blackwell Publishing Professional [Google Scholar]
- Vail DM, MacEwen EG. (2000). Spontaneously occurring tumors of companion animals as models for human cancer. Cancer Invest. 18, 781–792 [DOI] [PubMed] [Google Scholar]
- Valli VE, Vernau W, de Lorimier LP, Graham PS, Moore PF. (2006). Canine indolent nodular lymphoma. Vet Pathol. 43, 241–256 [DOI] [PubMed] [Google Scholar]
- Wang TL, Maierhofer C, Speicher MR, Lengauer C, Vogelstein B, Kinzler KW, Velculescu VE. (2002). Digital karyotyping. Proc Natl Acad Sci USA 99, 16156–16161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wayne RK, Ostrander EA. (2007). Lessons learned from the dog genome. Trends Genet. 23, 557–567 [DOI] [PubMed] [Google Scholar]
- Welle MM, Bley CR, Howard J, Rufenacht S. (2008). Canine mast cell tumours: a review of the pathogenesis, clinical features, pathology and treatment. Vet Dermatol. 19, 321–339 [DOI] [PubMed] [Google Scholar]
- Wiik AC, Wade C, Biagi T, Ropstad EO, Bjerkås E, Lindblad-Toh K, Lingaas F. (2008). A deletion in nephronophthisis 4 (NPHP4) is associated with recessive cone-rod dystrophy in standard wire-haired dachshund. Genome Res. 18, 1415–1421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilcox B, Walkowicz C. (1995). Atlas of dog breeds of the world. Neptune City, NJ: T.F.H. Publications [Google Scholar]
- Zangerl B, Goldstein O, Philp AR, Lindauer SJ, Pearce-Kelling SE, Mullins RF, Graphodatsky AS, Ripoll D, Felix JS, Stone EM, et al. (2006). Identical mutation in a novel retinal gene causes progressive rod-cone degeneration in dogs and retinitis pigmentosa in humans. Genomics 88, 551–563 [DOI] [PMC free article] [PubMed] [Google Scholar]


