Abstract
Spider silk is a biomaterial with impressive mechanical properties, resulting in various potential applications. Recent research has focused on producing synthetic spider silk fibers with the same mechanical properties as the native fibers. For this study, three proteins based on the Argiope aurantia Major ampullate Spidroin 2 consensus repeat sequence were expressed, purified and spun into fibers. A number of post-spin draw conditions were tested to determine the effect of each condition on the mechanical properties of the fiber. In all cases, post-spin stretching improved the mechanical properties of the fibers. Aqueous isopropanol was the most effective solution for increasing extensibility, while other solutions worked best for each fiber type for increasing tensile strength. The strain values of the stretched fibers correlated with the length of the proline-rich protein sequence. Structural analysis, including X-ray diffraction and Raman spectroscopy, showed surprisingly little change in the initial as-spun fibers compared with the post-spin stretched fibers.
Keywords: Synthetic spider silk fibers, Mechanical properties, Post-spinning, Dragline, Argiope aurantia
1. Introduction
Spider silk is a biomaterial with unique mechanical properties. Spiders are dependent on their silk for survival, and as a result, spider silks have evolved over the past 400 million years into a biomaterial with remarkable mechanical properties (Gosline et al., 1986). Orb-web weaving spiders can make six different types of silk and a glue (Vollrath, 1992). Each type of silk is produced in a separate gland in the spider’s abdomen and is uniquely suited for its purpose (Vollrath, 1992; Gosline et al., 1999). For instance, major ampullate silk (also known as dragline silk), which provides a lifeline for the spider, is the strongest of the silks. In contrast, flagelliform silk, which makes up the capture spiral of the spider’s web, is very elastic in order to absorb the energy of a flying insect without breaking.
Due to the differing mechanical properties of spider silk, there are various potential applications for it ranging from military to medical (Altman et al., 2003; Kluge et al., 2008; Lewis, 2006). However, mass production of natural silks is not possible because spiders are territorial and difficult to raise in captivity. Thus, current research is focused on creating synthetic spider silk fibers with mechanical properties comparable to or exceeding those of native spider silk (Scheibel, 2004; Vendrely and Scheibel, 2007).
Research on spider silk over the past few decades has started to reveal the molecular elements that produce its mechanical properties (Gatesy et al., 2001). Spider silk proteins are made up of hundreds of tandem repeats of specific, highly conserved amino acids motifs (Hu et al., 2006; Hayashi and Lewis, 1998; Garb et al., 2006). Different amino acid motifs contribute specific mechanical properties to the fiber (Teulé et al., 2007; Hayashi et al., 1999). Major ampullate silk, the strongest type of silk, is made up of two proteins: Major ampullate Spidroin 1 (MaSp1) (Xu and Lewis, 1990) and Major ampullate Spidroin 2 (MaSp2) (Hinman et al., 1992). These two proteins each lend strength and elasticity to the fiber through unique tandemly repeated amino acid motifs, which are highly conserved throughout the orb-web weaving spider species. MaSp1 contains the GGX (X=L, Y, Q, and A) motif and (A)n, and MaSp2 contains the GPGXX (X=G, Q, and Y) motif and (A)n. Structural studies on silk have revealed the secondary structures created by these motifs. The (GA)n and (A)n motifs have been shown to create anti-parallel beta sheets which align parallel to the fiber’s axis (Simmons et al., 1996; Hijirida et al., 1996; Jelinski et al., 1999; Parkhe et al., 1997; Rathore and Sogah, 2001) and create tensile strength and toughness in the fiber. The GGX motif forms a GlyII helix (Holland et al., 2008; van Beek et al., 2002), and the GPGXX motif forms a spring-like type II β-turn that creates elasticity in the fiber (Hayashi and Lewis, 1998; Jenkins et al., 2010).
To date, many studies focusing on the silk of Nephila clavipes have established a basic understanding of the relationship between structure and function in the different types of silk. Currently, researchers are looking at the silk of other spider species to determine why there are differences in mechanical properties between species (Ayoub et al., 2007; Blackledge and Hayashi, 2006; Gorb et al., 2006). While the silks of these species have many similarities, there are some important differences. The dragline silk of N. clavipes is composed of 81% MaSp1 and 19% MaSp2, and Argiope aurantia is 41% MaSp1 and 59% MaSp2 (Brooks et al., 2005). A. aurantia dragline silk was shown to have a greater tensile strength and Young’s modulus than the dragline silk of N. clavipes; however, there was no significant difference in the extensibilities (Brooks et al., 2005). This result is unexpected considering that A. aurantia has roughly three times the amount of MaSp2 in its dragline silk as N. clavipes does, and MaSp2 exhibits the proline containing amino acid motif GPGXX, which forms beta-turn secondary structure that confers elasticity on the fiber (Jenkins et al., 2010; Hayashi and Lewis, 1998; Urry et al., 1995). N. clavipes MaSp2 silk contains many repetitions of the GPGXX motif, while A. aurantia contains the GGX motif in addition to a variation of the GPGXX motif (Table 1).
Table 1.
MaSp2 repeat units for Argiope aurantia and Nephila clavipes are shown (Brooks et al., 2005).
| Species | MaSp2 repeat unit |
|---|---|
| Argiope aurantia | [GGY][GPGAGQQ][GPGSQ][GPGSGGQQ][GPGQQ]GPYGPSAAAAAAAA |
| Nephila clavipes | [GPGQQGPGGY][GPGQQGPGGY][GPGQQ]GPSGPGSAAAAAAAA |
While the repeat unit of N. clavipes is very consistently GPGQQGPGGY (shown in brackets in Table 1), the repeat unit of A. aurantia has two or three extra amino acids inserted making its repeat five, seven or eight amino acids (also shown in brackets in Table 1). The poly-alanine motif present in both A. aurantia and N. clavipes forms β-sheets that contribute strength to the fiber (Simmons et al., 1996; Rathore and Sogah, 2001).
In order to further elucidate the impact of the MaSp2 primary structure of A. aurantia on the mechanical properties of its dragline fibers, three proteins were created based on the MaSp2 silk sequence of A. aurantia (Brooks et al., 2008). These proteins were designed to have an increasing elasticity to strength motif ratio. The protein designated 1E has one elasticity motif to one strength motif, while 2E has two elasticity motifs to one strength motif and 3E has a three to one ratio. Because 3E has the highest elasticity to strength motif ratio, it was hypothesized that it would form the most elastic and the least strong of the three types of fibers. It was conversely hypothesized that 1E would yield the strongest, least elastic fibers given that it had the most poly-alanine. In addition to testing the effects of varying the primary structure, a wide variety of post-spin stretch conditions were also tested.
2. Materials and methods
2.1. Gene cloning
The two original synthetic genes were synthesized by Midland Reagents (Midland, TX) and used to make three monomer units with an increasing number of elasticity motifs before interruption by a linker (GPYGPS) and a polyalanine (A8) (Brooks et al., 2008). Monomer units (Table 2) were generated using a compatible but non-regenerable cloning strategy (Lewis et al., 1996). This strategy was then used to duplicate the monomer units to create three synthetic silk genes (1Ex16, 2Ex12, and 3Ex8) of about 2 kb each. These genes were originally expressed from pET30a (Novagen; San Diego, CA); however this resulted in low levels of expression. For this study, these three constructs were removed from pET30a using NcoI and BlpI and ligated into pET19k (a modified pET19b vector (Novagen; San Diego, CA) in which the ampicillin resistance gene was replaced with the kanamycin resistance gene from the pET26b vector (Novagen; San Diego, CA)) (Teulé et al., 2009). In this cloning strategy, the N-terminal 10 histidine tag in pET19k was removed and the C-terminal six histidine tag from pET30a was moved into pET19k for purification purposes. Restriction enzymes used in gene cloning were purchased from New England Biolabs (Ipswich, MA).
Table 2.
Synthetic silk protein sequences with gene and protein sizes.
| Protein name | Monomer unit | Repeat times | DNA size (bp) | Protein size (kDa) |
|---|---|---|---|---|
| 1E | (GGYGPGAGQQGPGSQGPGSGGQQGPGGQ)1GPYGPSA8 | 16 | 2223 | 80 |
| 2E | (GGYGPGAGQQGPGSQGPGSGGQQGPGGQ)2GPYGPSA8 | 12 | 2601 | 91 |
| 3E | (GGYGPGAGQQGPGSQGPGSGGQQGPGGQ)3GPYGPSA8 | 8 | 2433 | 86.5 |
2.2. Protein expression
The three final constructs were expressed from pET19k in BL21(DE3) Escherichia coli (Novagen). Positive colonies were tested with small-scale (5 ml) protein expression in LB media with 50 μg/ml kanamycin to determine which colonies produced the highest levels of silk protein. Colonies were grown in LB media plus kanamycin in a shaking incubator at 37 °C to an OD600 of 0.8 at which time silk protein expression was induced with isopropylthiogalactoside (IPTG; Biosynth AG, Switzerland) as per the manufacturer’s instructions, and bacteria were harvested in a manner similar to that described below for large scale expression.
Colonies with the highest synthetic silk protein yield were selected and grown on a larger scale in a 19.5 L BioFlo® 415 Sterilizable-In-Place Fermentor (New Brunswick Scientific; Edison, NJ). Single colonies were used to grow a 1 L starter culture in LB media with 50 mg kanamycin in a shaking incubator at 37 °C. This culture was allowed to grow for 4–6 h before it was centrifuged at 3500 rpm for 20 min (Allegra™ 6KR, Beckman Coulter; Brea, CA). Cells were re-suspended in 100 ml of LB media and used to inoculate the larger fermentor culture which was grown overnight using manufacturer recommended media plus 50 mg/L kanamycin to an OD600 of 15–20 at which time synthetic silk protein expression was induced by the addition of IPTG according to the manufacturer’s instructions. Cells were grown for an additional 4 h before they were harvested by centrifugation at 3500 rpm for 20 min (Allegra™ 6KR, Beckman Coulter; Brea, CA). Cell pellets were re-suspended in lysis buffer (10 mM MgCl2, 10 mM NaCl, and 50 mM Tris–HCl; pH 8.0) with 0.25 mg/ml lysozyme at a weight to volume ratio of 1:3 (cell pellet:buffer). Extracts were frozen at −80 °C for at least 24 h.
2.3. Protein purification
Cell extracts were thawed and sonicated (Misonix Sonicator 3000, Misonix Inc.; Farmingdale, NY) at 75 W for 6–8 min to lyse cells completely. Extracts were heat treated at 80 °C for 20 min to precipitate the heat unstable E. coli proteins leaving the silk protein, which was known to be heat stable, in solution. Extracts were centrifuged at 9500 rpm for 30 min at room temperature (Beckman J2-21M, Beckman Coulter; Brea, CA). The supernatant was diluted 1:1 with 2× binding buffer (10 mM imidazole, 1 M NaCl, and 40 mM Tris; pH 8.0). The extract was loaded onto an affinity column, and the silk protein was purified with immobilized metal (Nickel) affinity chromatography (IMAC) using an AKTA Explorer (GE Healthcare; Piscataway, NJ). The affinity column was washed with binding buffer (5 mM imidazole, 0.5 M NaCl, and 20 mM Tris; pH 8.0) and then washed with 40 mM imidazole, 0.5 M NaCl, and 20 mM Tris; pH 8.0 for 1E and 3E or 20 mM imidazole, 0.5 M NaCl, and 20 mM Tris; pH 8.0 for 2E. All silk proteins were eluted from the column with 250 mM imidazole, 0.5 M NaCl, and 20 mM Tris; pH 7.9. Eluted fractions containing silk protein were pooled and dialyzed against dH2O using dialysis tubing with a 12–14 kDa molecular weight cut off (Spectrum Laboratories, Inc.; Rancho Dominguez, CA) in 4 L of deionized water for at least 2 days with four or more water changes. The dialysates were lyophilized to dry protein powders.
2.4. Electrophoretic analysis
Electrophoresis of DNA during cloning was performed using 1.0% agarose gels at 100 V in Tris acetate EDTA (TAE) buffer (40 mM Tris acetate and 1 mM EDTA). Gels were stained with ethidium bromide (0.5 μg/ml) for 10–15 min and de-stained for 10 min in dH2O. Gels were visualized using UV light.
Protein gel electrophoresis was performed using pre-cast 4–20% SDS-PAGE gels (Pierce Biotechnology; Rockford, IL) run at 100 V with Tris–HEPES-SDS running buffer. Proteins were visualized in the gel using Bio-Safe Coomassie (Bio-Rad; Hercules, CA). Western blots were done by transferring proteins overnight at 35 mA to a PVDF membrane according to the manufacturer’s instructions (Immobilon-P, EMD Millipore; Billerica, MA). Synthetic silk proteins were detected with a His-tag monoclonal antibody (Novagen; San Diego, CA).
2.5. Fiber spinning
Spin dopes were made by dissolving lyophilized protein powder in 1,1,1,3,3,3-hexafluoro-2-propanol (HFIP; TCI America, Portland, OR) in glass vials at a weight protein to volume HFIP ratio from 45% to 60%. The protein was allowed to dissolve for at least 48 h with occasional rolling of the tube. Then the cap on the vial was loosened to allow evaporation of the HFIP in order to increase the viscosity of the dope. The dope was loaded into a 1 ml Hamilton Gastight® syringe (Hamilton company; Reno, NV) fitted with a 10 cm long piece of PEEK tubing (inner diameter: 0.25 mm; Upchurch Scientific; Oak Harbor, WA). Fibers were extruded at a syringe plunger speed of 0.5 mm/min using a fiber spinning machine made by DACA Instruments (Santa Barbara, CA) into a 100% isopropanol coagulation bath. The end of the fiber was picked up in the coagulation bath using forceps and the fibers were spooled on a winder.
2.6. Post-spin treatment
A wide variety of post-spin treatments were tested on 1E, 2E, and 3E as-spun fibers. Small pieces (~2 cm) of the as-spun fiber were placed in each of the various solutions and stretched from both ends using tweezers. Stretched fibers were taken out of the solution and placed on a piece of paper to dry overnight. Fibers that had a tendency to contract after stretching were constrained until they dried enough to hold their shape. Solutions tested for post-spin draw included dH2O, isopropanol (IPA), ethanol (EtOH), methanol (MeOH), 1.2 M ammonium sulfate, and different mixtures of these. Different draw ratios were also tested from 2 × to 7 ×. When optimal solutions and draw ratios were determined, the addition of heat to the post-spin treatment was tested. Solutions were heated to 60 °C in a water bath and the dish used for post-spin stretching was placed in a water bath during stretching to ensure the solution stayed at 60 °C.
2.7. Mechanical testing
Fibers were cut and mounted on C-shaped mechanical testing cards with a 19 mm square hole in the middle as shown in Fig. 1. The white line in Fig. 1 shows where the fiber would be mounted for testing. Microscope pictures were taken of each fiber using a 40 × objective lens (Nikon Eclipse E200 microscope, Nikon Instruments Inc.; Melville, NY). Fiber diameters were measured using ImageJ 1.42q (National Institute of Health; Bethesda, Maryland) at nine different places along the length of the fiber and averaged to calculate the diameter of the fiber. Mechanical testing was done at room temperature and ~15% humidity on an MTS Synergie 100 (MTS Corporation; Eden Prairie, MN) using a 10 g load cell (Transducer Techniques; Temecula, CA) with a pulling rate of 10 mm/min and 30 Hz frequency data collection. Data was plotted and stress strain curves created in Microsoft Excel 2010, and the Energy to Break was calculated using the trapezoid rule to determine the area under the curve.
Fig. 1.

Picture of the MTS Synergie 100 mechanical testing system. The white line shows where the fiber would be mounted on the card for testing.
All mechanical tests for each treatment group were averaged, a standard deviation derived from the data (n≥14 for each group) and Minitab 16 was used to analyze the data.
3. Results and discussion
3.1. Electrophoresis
Cloning was checked by restriction digest and agarose gel electrophoresis (Fig. 2A) and confirmed by DNA sequencing (Macromolecular Core Equipment Facility, University of Wyoming). The size of the proteins produced during fermentation was checked by SDS-PAGE electrophoresis followed by Coomassie blue staining (Fig. 2B), and the proteins were detected on Western blots using a monoclonal anti-histidine tag antibody (data not shown).
Fig. 2.

Agarose and SDS-PAGE gels for 1E, 2E, and 3E. (A) Agarose gel showing the NcoI and BlpI restriction enzyme digests of the synthetic silk genes in pET19k. Lower bands are the synthetic silk gene inserts and upper bands are the pET19k vector (5.8 kb). (B) SDS-PAGE (polyacrylamide gel electrophoresis) of proteins purified by IMAC and stained with Biosafe Coomassie.
3.2. Post-spin stretching
A variety of post-spin draw conditions were tested for 1E, 2E and 3E as-spun fibers. All as-spun fibers were soluble in 100% water, and different concentrations of water and alcohol were optimal in the solutions used for the post-spin stretching of different fibers. The highest concentration of water was used that would not dissolve the fiber. Water plays a critical role in the post-spin stretching process. It acts as a plasticizer, allowing the glycine-rich regions to mobilize which can lead to more optimal molecular conformation and chain orientation (Seidel et al., 1998, 2000). At this time, it is unclear why different alcohols improved different mechanical properties; however, it is clear that water is a necessary component of the post-spin stretching solution as stretching in 100% alcohol was unsuccessful for all types of alcohols and fibers tested here.
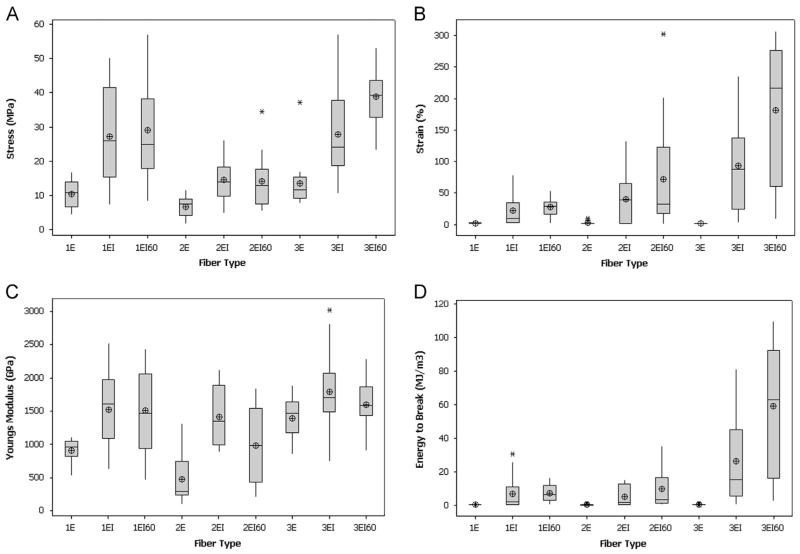
Aqueous isopropanol (85% IPA with 15% water for 1E and 2E; 75% IPA with 25% water for 3E) was effective for post-spin stretching of all three fiber types, while aqueous methanol (85% MeOH with 15% water) was effective only for 1E fibers and aqueous ethanol (85% EtOH with 15% water) only for 3E fibers. For solutions that were effective (meaning fibers could be stretched without dissolving), post-spin stretching was done both at room temperature and at 60 °C to determine the effect of heat on the stretching process and the subsequent mechanical properties of the fibers. Mechanical data for both as-spun and post-spin stretched fibers is shown in the boxplots in Fig. 3 as well as in Tables 1–3 in Supplementary Materials.
Fig. 3.
Average mechanical properties for as-spun, IPA post-spin stretched and IPA at 60 °C post-spin stretched fibers. Boxplots show the range of values of all fibers within a treatment group for stress (A), strain (B), Young’s modulus (C) and energy to break (D). The boxes show the middle 50% of the data also called the interquartile range. The whiskers extending from the top and bottom of each box show the rest of the data. Any data point farther than 1.5 times away from the interquartile range was considered an outlier and denoted with an asterisk. The circle with cross hairs indicates the mean and the horizontal line across each box represents the median. Each treatment group had n≥14.
3.3. As-spun fibers
The average diameters and morphology for the 1E, 2E and 3E as-spun fibers varied. The 1E fibers were white and opaque, while the 3E fibers were more translucent and shiny. The 2E fibers were in between the 1E and 3E fibers in translucence. The two different groups of 1E as-spun fibers, one group (n=18) spun from a 56% wt/vol spin dope and the other (n=16) from a 48% wt/vol spin dope, had a large difference in their average diameters at 106.0±4.8 μm and 54.2±2.7 μm, respectively (Supplementary Materials, Table 1). The average diameter for the 2E as-spun fibers (n=16) was 106.4±10.8 μm, and for the 3E as-spun fibers (n=16), it was 62.1±3.3 (Supplementary Materials, Tables 2 and 3).
In comparing the tensile strengths of the as-spun fibers, the group averages were similar for 1E spun from the 48% dope (14.4±4.4 MPa), 1E spun from the 56% dope (10.4±4.0 MPa), 2E (6.6±2.8 MPa) and 3E (13.6±6.7 MPa). Comparing the best individual fibers within each group, a 3E fiber was the strongest (37.2 MPa) (Fig. 3A; Supplementary Materials, Tables 1–3). This result was surprising considering 3E has the lowest percentage of poly-alanine, and poly-alanine forms β-sheets, which create strength in the fiber (An et al., 2011; Teulé et al., 2012). Next highest was a 1E fiber spun from the 48% wt/vol spin dope (23.0 MPa) followed by a 1E fiber spun from the more viscous 56% wt/vol spin dope (16.8 MPa) (Fig. 3A; Supplementary Materials, Table 1). The strongest 2E as-spun fiber had the lowest tensile strength (11.7 MPa) (Fig. 3A; Supplementary Materials, Table 2). These results are unexpected as it would seem that 2E should fall between 1E and 3E for both strength and extensibility. It may be that there is an optimal combination of these amino acid motifs for the correct protein structure or assembly of the fiber. Overall, 2E fibers were the weakest even after post-spin stretching so perhaps that protein’s primary structure did not allow proper alignment of the polyalanine motifs into β-sheets.
The Young’s modulus or stiffness of the 1E, 2E and 3E as-spun fibers was relatively similar except for a 3E fiber that was an outlier with the highest modulus (9.17 GPa), while the group average was much lower (1.88±1.91 GPa) (Fig. 3C; Supplementary Materials, Tables 1–3). A 1E fiber spun from the 48% wt/vol spin dope had the next highest Young’s modulus (2.52 GPa, group average 1.69±0.44 GPa). The next highest modulus was for a 2E as-spun fiber (1.32 GPa, group average 0.47±0.36 GPa) followed by a 1E fiber spun from the 56% wt/vol spin dope (1.11 GPa, group average 0.91±0.17 GPa).
Like the tensile strength values, the average extensibilities of the 1E, 2E and 3E as-spun fibers were not much different: 1E spun from the 48% wt/vol spin dope (0.8±0.3%), 1E spun from the 56% wt/vol spin dope (1.5±0.8%), 2E (1.9±2.3%), and 3E (1.2±0.5%) (Fig. 3B; Supplementary Materials, Tables 1–3). The resulting energy to break or toughness values followed the same pattern with the 1E fibers spun from the 48% wt/vol spin dope having a slightly lower average (0.06±0.03 MJ/m3) than the other as-spun fibers: 1E spun from the 56% spin dope (0.11±0.10 MJ/m3), 2E (0.10±0.19 MJ/m3), and 3E (0.10±0.13 MJ/m3) (Fig. 3D; Supplementary Materials, Tables 1–3). Looking at the best individual fibers, it was surprising that a 2E fiber was the most extensible (8.9%) and the toughest (0.67 MJ/m3) of all the as-spun fibers. The next best extensibility (3.3%) and toughness (0.38 MJ/m3) were for a 1E fiber spun from the 56% wt/vol spin dope (Supplementary Materials, Table 1). A 3E fiber followed (2.4%), while a different 3E fiber was actually the second toughest (0.60 MJ/m3). Last was a 1E fiber spun from the 48% wt/vol spin dope (1.3% and toughness of 0.11 MJ/m3) (Supplementary Materials, Table 1).
The hypothesis was that 1E would be the strongest and the least extensible given that it has the lowest elasticity to strength motif ratio, and conversely, that 3E would be the most extensible and the weakest. In comparing the two sets of 1E fibers, the less viscous spin dope resulted in stronger, less extensible fibers roughly half the diameter of the fibers spun from the more viscous dope. The fibers spun from the less viscous dope were more brittle, so this may explain why they would not extend as far before breaking. The fact that the 1E fibers spun from the 48% wt/vol dope were the least extensible confirm that part of the hypothesis; however, 3E fibers had the greatest tensile strengths which disproves the other part of the hypothesis. 3E was hypothesized to be the most extensible and the least strong, and neither of these proved true for the as-spun fibers.
Overall, the extensibilities and therefore the energy to break values for the as-spun fibers are quite low when compared with those from the post-spin stretched fibers. The as-spun fibers were very difficult to handle as they were very brittle and easily broken during processing; however, once the fibers had been post-spin stretched, they were easier to handle as they were more flexible and robust in spite of the fact that the diameters decreased after post-spin stretch. The likely explanation for these results is that the proteins were not in the required conformations in the as-spun fibers for optimal extensibility. Post-spin stretching in an aqueous solution created mobility within the glycine-rich regions of the fiber allowing for increased secondary structure and alignment.
3.4. Isopropanol post-spin stretch
The average diameters for the isopropanol (IPA) post-spin stretched fibers were about half the diameters for the as-spun fibers, suggesting changes in molecular conformation. The 1E fibers (n=19) had an average diameter of 43.4±7.5 μm (Supplementary Materials, Table 1). 2E IPA post-spin stretched fibers (n=15) had an average diameter of 43.4±12.2 μm (Supplementary Materials, Table 2). The 3E fibers (n=17) had an average diameter of 32.4±8.4 μm (Supplementary Materials, Table 3).
With aqueous isopropanol (IPA) as a post-spin stretching solution, different draw ratios and concentrations of isopropanol to water were optimal for each fiber type: 85% IPA with a 4–6 × stretch was optimal for 1E, 85% IPA with a 2× stretch for 2E and 75% IPA with a 3 × stretch was best for 3E. The IPA post-spin stretch increased stress and strain values for all three fiber types. The average strength values of the as-spun fibers increased by over two fold following the IPA post-spin stretch: 2.6 fold for 1E, 2.2 fold for 2E and 2.3 fold for 3E (Fig. 3A; Supplementary Materials, Tables 1–3). In looking at the individual fibers, a 3E fiber was again the strongest (57.1 MPa, group average of 27.9±11.9 MPa) (Fig. 3A; Supplementary Materials, Table 3). Next was a 1E fiber spun from the 56% wt/vol spin dope (50.3 MPa, group average of 27.1±12.5 MPa), and 2E was the weakest (26.1 MPa, group average of 14.6±6.3 MPa) (Fig. 3A; Supplementary Materials, Tables 1 and 2). Although the group averages for 1E and 3E were very close, it was surprising that a 3E fiber was the strongest because it had the lowest percentage of poly-alanine. The explanation likely involves both the protein sequence and the intermolecular interactions within the fiber. Perhaps the primary structure of the 3E protein allowed it to achieve a higher level of the needed molecular interactions to create strength within the fiber.
Young’s moduli followed the same pattern as the strength values for the IPA post-spin stretched fibers with a 3E fiber having the highest modulus (3.02 GPa, group average of 1.79±0.59 GPa). A 1E fiber spun from the 56% wt/vol spin dope was next (2.52 GPa, group average of 1.69±0.44 GPa) followed by a 2E fiber (2.13 GPa, group average was 1.42±0.41 GPa) (Fig. 3C, Supplementary Materials, Tables 1–3).
IPA post-spin stretching produced a significant increase in the strain and energy to break values for all three fiber types (Supplementary Materials, Tables 1–3). The extent of the increase in extensibility correlated to the ratio of elasticity to strength amino acid motifs with 3E having the greatest increase (77.5 fold), 2E next (21.1 fold) and 1E the least (14.7 fold). A 3E fiber was the most extensible (235.2%, group average of 93.0±67.1%), a 2E was next (133.0%, group average of 40.2±40.4%) and 1E was least extensible (78.5%, group average of 22.0±26.0%) (Fig. 3B; Supplementary Materials, Tables 1–3). These data fit the original hypothesis that the length of the β-turn region would control the fiber extensibility. 3E was expected to be the most extensible based on the fact that it had the highest ratio of elastic to strength amino acid motifs, and conversely, 1E was hypothesized to be the strongest and least extensible. The same 3E fiber that was the most extensible was also the toughest of the IPA post-spin stretched fibers (81.14 MJ/m3, group average of 26.21±25.75 MJ/m3) (Fig. 3D; Supplementary Materials, Table 3). A 1E fiber was next toughest (30.34 MJ/m3, group average of 6.67±9.09 MJ/m3) and a 2E fiber followed (15.18 MJ/m3, group average was 4.89±5.58 MJ/m3) (Fig. 3D; Supplementary Materials, Tables 1 and 2).
3.5. Isopropanol post-spin stretch at 60 °C
Heating the isopropanol/water to 60 °C for the post-spin stretch made improvements in certain mechanical properties for some fibers. The diameters of the fibers stretched in IPA at 60 °C were comparable to those of the fibers stretched in IPA at room temperature (Supplementary Materials, Tables 1–3). When stretched in a heated bath, fibers were more malleable and as a result, they stretched further. Sometimes, fibers became so soft that they would pull apart during stretching, and reducing the percentage of water in the post-spin stretch solution solved this problem. For 3E, 85% IPA worked better at 60 °C than 75% IPA. Heating the IPA post-spin stretch solution slightly improved the maximum stress values for 1E (57.1 MPa compared to 50.3 MPa in IPA/water at room temperature) and 2E (34.4 MPa compared to 26.1 MPa in IPA/water at room temperature), but not for 3E (53.2 MPa compared to 57.1 MPa in IPA/water at room temperature); however, in looking at the average tensile strengths, they increased the most for the 3E fibers (Fig. 3A; Supplementary Materials, Tables 1–3).
The decrease in maximum tensile strength for 3E could be attributed to a number of factors: the addition of heat, the change in IPA concentration from 75% to 85% or the increase in the draw ratio from 3 × to 4 ×. 2E was the weakest as it was for as-spun and the 85% IPA post-spin draw. It may also be that the increased temperature allowed the proteins in the 1E and 2E fibers to be more flexible and achieve a more organized state whereas 3E had sufficient flexibility to achieve that structure during post-spin stretching at room temperature. The diameters of the 3E as-spun fibers were a little over half that of the 1E and 2E as-spun fibers, so perhaps the IPA/water was able to penetrate deeper into the 3E fibers at room temperature allowing for greater mobility and resulting in better molecular orientation. Heating the aqueous IPA solution may have allowed greater penetration into the 1E and 2E fibers resulting in stronger fibers.
Like tensile strength, heating the IPA post-spin stretch solution had mixed results on the other mechanical properties. The maximum Young’s moduli decreased slightly for all fiber types in the heated versus room temperature IPA post-spin stretch (Supplementary Materials, Tables 1–3). For strain, heating the IPA/water post-spin stretch solution substantially improved the maximum values for 2E (302.7% compared to 133.0% in IPA/water at room temperature) and 3E (306.7% compared to 235.2% in IPA/water at room temperature) but not for 1E (53.2% compared to 78.5% in IPA/water at room temperature). Energy to break values followed the same trend with increases in toughness for 2E (35.41 MJ/m3 compared to 15.18 MJ/m3 at room temperature) and 3E (109.73 MJ/m3 compared to 81.14 MJ/m3 at room temperature) but not for 1E (16.54 MJ/m3 compared to 30.34 MJ/m3 for IPA/water at room temperature) (Supplementary Materials, Tables 1–3).
3.6. Methanol post-spin stretch
Certain post-spin stretching solutions were only effective for one fiber type. For instance, 85% methanol was effective for 1E fibers that were spun from a 48% wt/vol spin dope but not the 1E fibers spun from a 56% wt/vol spin dope or 2E or 3E fibers. A 4X stretch in 85% methanol resulted in the 1E fiber with the highest tensile strength (80.7 MPa) (Fig. 4A) and Young’s modulus (4.26 GPa) of all the 1E fibers; however, the maximum extensibility produced by the methanol post-spin stretch (10.6%) was over seven times less than that produced by the IPA post-spin stretch (78.5%) (Supplementary Materials, Table 1).
Fig. 4.
Stress–strain curves showing the best individual 1E, 2E and 3E fibers for stress (A) and strain (B). Note different scales in (A) and (B).
3.7. Ethanol post-spin stretch
Ethanol post-spin stretching was effective only for 3E fibers. Fibers were stretched three times their original length in 75% ethanol. Ethanol post-spin stretching created 3E fibers with greater tensile strengths than the as-spun fibers, and heating the ethanol to 60 °C for post-spin stretching created the 3E fiber with the highest tensile strength (96.3 MPa) (Fig. 4A). However, maximum extensibility produced by the ethanol post-spin stretch at 60 °C (91.4%) was over three times less than the highest strain value created by the IPA post-spin stretch at 60 °C (306.7%) (Supplementary Materials, Table 3).
3.8. Structural analysis
Biophysical analysis of as-spun fibers and fibers stretched in isopropanol using both Raman (data not shown) and X-ray diffraction (1E is shown in Supplementary Materials, Figs. 1–5 and data not shown for 2E and 3E) showed the presence of substantial β-sheet formation in both conditions for all three proteins. There was no detectable increase in β-sheet secondary structure following post-spin stretching when compared to the as-spun fibers. There was a detectable increase in orientation of the β-sheets parallel to the fiber axis but it was not substantial.
3.9. As-spun fibers versus post-spin stretched fibers
While structural analysis showed no increase in the amount of β-sheet secondary structure following post-spin stretching, it is likely that the stretching process created extensive changes in the molecular conformation of the as-spun fibers. Evidence supporting this includes the drastic reduction in fiber diameters following stretching as well as the clear improvements in mechanical properties. The use of post-spin stretch led to improved mechanical properties for fibers of all three protein repeat sequences, 1E, 2E and 3E. However, different post-spin stretch conditions lead to greater increases in different mechanical properties for each fiber type. For instance, an aqueous isopropanol post-spin stretch was best for increasing the extensibilities of all three fiber types. Aqueous isopropanol has been an effective post-spin stretch solution for other synthetic silk fibers as well (An et al., 2011; Teulé et al., 2012; Adrianos et al., 2013). In contrast, for 1E, a post-spin stretch in aqueous methanol produced the strongest fiber, and for 3E, the strongest fiber was produced using an aqueous ethanol post-spin stretch. Although these post-spin stretch treatments created improvements in tensile strength, they led to very little improvement in extensibility.
In comparing the mechanical properties of the 1E, 2E and 3E as-spun fibers with those that were post-spin stretched, the post-spin stretched fibers had better mechanical properties regardless of the solution used for stretching. This may be due to the fact that post-spin stretching more closely emulates the spinning apparatus of the spider. The spider pulls its silk from the spinnerets, while the method used for this study involved extruding the silk through a needle. Also, the spider spins aqueously, meaning that water likely plays an important role in the spinning process, and perhaps that may explain water’s critical role in the post-spin stretching process. So even though the necessary amino acid motifs are present within the fiber, the method of spinning and the combination of amino acid motifs may not be optimal to create fibers with the best mechanical properties possible. Post-spin stretching leads to better organization of the secondary structural motifs within the fiber and thus better mechanical properties (An et al., 2011; Teulé et al., 2012).
4. Conclusions
Post-spin stretching clearly improved the mechanical properties for 1E, 2E and 3E fibers, and different post-spin stretch conditions enhanced specific mechanical properties. The hypothesis that the poly-alanine content would control the tensile strength was not true for these proteins. However, the hypothesis that the longer the β-turn regions the greater the extensibility was shown to be true, but only after post-spin stretching in a water-containing bath. Water is thought to induce mobility in the GPGXX regions (An et al., 2011; Teulé et al., 2012). Thus, it appears that for these proteins, the molecular interactions needed to achieve maximal mechanical properties depend strongly on the non-β-sheet regions. Water and stretching are required to achieve the best mechanical properties. Post-spin stretching in a water containing bath may mimic the spider’s spinning process with greater fidelity than the spinning process alone.
In the future, it may be possible to create designer fibers with the mechanical properties needed for specific applications. Engineering these mechanical properties starts with the primary structure of the protein and extends to the post-spin stretching of the fibers in appropriate solutions with the optimal draw ratios. These parameters are specific to each protein and fiber type and thus must be determined for each new situation. The one common theme that is evident from this and other studies is that isopropanol with some percentage of water is an effective post-spin stretch solution for a variety of fiber types (An et al., 2011; Teulé et al., 2012; Adrianos et al., 2013). Isopropanol was the post-spin stretch solution that created the best extensibility for all three fiber types in this study.
Supplementary Material
Acknowledgments
The authors would like to acknowledge support for the work done in the Lewis laboratory from the National Institute of Health (NIH) Award no. EB000490, the Department of Energy (DOE) Award no. DE-SC0004791, and the Department of Defense Air Force Office of Scientific Research (AFOSR) Award no. FA9550-09-1-0717. Jeffery Yarger would like to acknowledge support from the Department of Defense AFOSR under Award no. FA9550-10-1-0275 and the National Science Foundation (NSF), Division of Materials Research under Award no. DMR-0805197. Use of the Advanced Photon Source was supported by the U.S. Department of Energy, Basic Energy Sciences, Office of Science, under Contract no. DE-AC02-06CH11357. Use of the BioCARS Sector 14 was also supported by grants from the National Center for Research Resources (5P41RR007707) and the National Institute of General Medical Sciences (8P41GM103543) from the National Institutes of Health. The authors would also like to thank Dr. Michael Hinman for help with mechanical testing data analysis and valuable advice on this manuscript and Dr. Kenneth Gerow for providing insight into the statistical analysis of the data. Any opinions, findings, or conclusions presented in this publication are those of the author(s) and do not necessarily reflect the views of the NIH, DOE, NSF or AFOSR.
Appendix A. Supplementary material
Supplementary data associated with this article can be found in the online version at http://dx.doi.org/10.1016/j.jmbbm.2013.09.002.
References
- Adrianos SL, Teulé F, Hinman MB, Jones JA, Weber W, Yarger JL, Lewis RV. Nephila clavipes flagelliform silk-like GGX motifs contribute to extensibility and spacer motifs contribute to strength in synthetic spider silk fibers. Biomacromolecules. 2013;14:1751–1760. doi: 10.1021/bm400125w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altman GH, Diaz F, Jakuba C, Calabro T, Horan RL, Chen J, et al. Silk-based biomaterials. Biomaterials. 2003;24:401–416. doi: 10.1016/s0142-9612(02)00353-8. [DOI] [PubMed] [Google Scholar]
- An B, Hinman MB, Holland GP, Yarger JL, Lewis RV. Inducing beta-sheets formation in synthetic spider silk fibers by aqueous post-spin stretching. Biomacromolecules. 2011;12:2375–2381. doi: 10.1021/bm200463e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayoub NA, Garb JE, Tinghitella RM, Collin MA, Hayashi CY. Blueprint for a high-performance biomaterial: full-length spider dragline silk genes. PloS ONE. 2007;2:e514. doi: 10.1371/journal.pone.0000514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackledge TA, Hayashi CY. Silken toolkits: biomechanics of silk fibers spun by the orb web spider Argiope argentata (Fabricius 1775) Journal of Experimental Biology. 2006;209:2452–2461. doi: 10.1242/jeb.02275. [DOI] [PubMed] [Google Scholar]
- Brooks AE, Steinkraus HB, Nelson SR, Lewis RV. An investigation of the divergence of major ampullate silk fibers from Nephila clavipes and Argiope aurantia. Biomacromolecules. 2005;6:3095–3099. doi: 10.1021/bm050421e. [DOI] [PubMed] [Google Scholar]
- Brooks AE, Stricker SM, Joshi SB, Kamerzell TJ, Middaugh CR, Lewis RV. Properties of synthetic spider silk fibers based on Argiope aurantia MaSp2. Biomacromolecules. 2008;9:1506–1510. doi: 10.1021/bm701124p. [DOI] [PubMed] [Google Scholar]
- Garb JE, Dimauro T, Vo V, Hayashi CY. Silk genes support the single origin of orb webs. Science. 2006;312:1762. doi: 10.1126/science.1127946. [DOI] [PubMed] [Google Scholar]
- Gatesy J, Hayashi C, Motriuk D, Woods J, Lewis R. Extreme diversity, conservation, and convergence of spider silk fibroin sequences. Science. 2001;291:2603–2605. doi: 10.1126/science.1057561. [DOI] [PubMed] [Google Scholar]
- Gorb SN, Niederegger S, Hayashi CY, Summers AP, Votsch W, Walther P. Biomaterials: silk-like secretion from tarantula feet. Nature. 2006;443:407. doi: 10.1038/443407a. [DOI] [PubMed] [Google Scholar]
- Gosline JM, DeMont ME, Denny MW. The structure and properties of spider silk. Endeavour. 1986;10:37–43. [Google Scholar]
- Gosline JM, Guerette PA, Ortlepp CS, Savage KN. The mechanical design of spider silks: from fibroin sequence to mechanical function. Journal of Experimental Biology. 1999;202:3295–3303. doi: 10.1242/jeb.202.23.3295. [DOI] [PubMed] [Google Scholar]
- Hayashi CY, Lewis RV. Evidence from flagelliform silk cDNA for the structural basis of elasticity and modular nature of spider silks. Journal of Experimental Biology. 1998;275:773–784. doi: 10.1006/jmbi.1997.1478. [DOI] [PubMed] [Google Scholar]
- Hayashi CY, Shipley NH, Lewis RV. Hypotheses that correlate the sequence, structure, and mechanical properties of spider silk proteins. International Journal of Biological Macromolecules. 1999;24:271–275. doi: 10.1016/s0141-8130(98)00089-0. [DOI] [PubMed] [Google Scholar]
- Hijirida D, Do K, Michal CA, Wong S, Zax DB, Jelinski L. 13C NMR of Nephila clavipes major ampullate silk gland. Biophysical Journal. 1996;71:3442–3447. doi: 10.1016/S0006-3495(96)79539-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinman M, Dong Z, Xu M, Lewis RV. Spider silk: a mystery starting to unravel. Results and Problems in Cell Differentiation. 1992;19:227–254. doi: 10.1007/978-3-540-47207-0_8. [DOI] [PubMed] [Google Scholar]
- Holland GP, Creager MS, Jenkins JE, Lewis RV, Yarger JL. Determining secondary structure in spider dragline silk by carbon–carbon correlation solid-state NMR spectroscopy. Journal of the American Chemical Society. 2008;130:9871–9877. doi: 10.1021/ja8021208. [DOI] [PubMed] [Google Scholar]
- Hu X, Vasanthavada K, Kohler K, McNary S, Moore AM, Vierra CA. Molecular mechanisms of spider silk. Cellular and Molecular Life Sciences. 2006;63:1986–1999. doi: 10.1007/s00018-006-6090-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jelinski LW, Blye A, Liivak O, Michal C, LaVerde G, Seidel A, et al. Orientation, structure, wet-spinning, and molecular basis for supercontraction of spider dragline silk. International Journal of Biological Macromolecules. 1999;24:197–201. doi: 10.1016/s0141-8130(98)00085-3. [DOI] [PubMed] [Google Scholar]
- Jenkins JE, Creager MS, Butler EB, Lewis RV, Yarger JL, Holland GP. Solid-state NMR evidence for elastin-like beta-turn structure in spider dragline silk. Chemical Communications (Cambridge) 2010;46:6714–6716. doi: 10.1039/c0cc00829j. [DOI] [PubMed] [Google Scholar]
- Kluge JA, Rabotyagova O, Leisk GG, Kaplan DL. Spider silks and their applications. Trends in Biotechnology. 2008;26:244–251. doi: 10.1016/j.tibtech.2008.02.006. [DOI] [PubMed] [Google Scholar]
- Lewis RV. Spider silk: ancient ideas for new biomaterials. Chemical Reviews. 2006;106:3762–3774. doi: 10.1021/cr010194g. [DOI] [PubMed] [Google Scholar]
- Lewis RV, Hinman M, Kothakota S, Fournier MJ. Expression and purification of a spider silk protein: a new strategy for producing repetitive proteins. Protein Expression and Purification. 1996;7:400–406. doi: 10.1006/prep.1996.0060. [DOI] [PubMed] [Google Scholar]
- Parkhe AD, Seeley SK, Gardner K, Thompson L, Lewis RV. Structural studies of spider silk proteins in the fiber. Journal of Molecular Recognition. 1997;10:1–6. doi: 10.1002/(SICI)1099-1352(199701/02)10:1<1::AID-JMR338>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- Rathore O, Sogah DY. Self-assembly of beta-sheets into nanostructures by poly(alanine) segments incorporated in multiblock copolymers inspired by spider silk. Journal of the American Chemical Society. 2001;123:5231–5239. doi: 10.1021/ja004030d. [DOI] [PubMed] [Google Scholar]
- Scheibel T. Spider silks: recombinant synthesis, assembly, spinning, and engineering of synthetic proteins. Microbial Cell Factories. 2004;3:14. doi: 10.1186/1475-2859-3-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seidel A, Liivak O, Jelinski LW. Artificial spinning of spider silk. Macromolecules. 1998;31:6733–6736. [Google Scholar]
- Seidel A, Liivak O, Calve S, Adaska J, Ji G, Yang Z, et al. Regenerated spider silk: processing, Properties, and Structure. Macromolecules. 2000;33:775–780. [Google Scholar]
- Simmons AH, Michal CA, Jelinski LW. Molecular orientation and two-component nature of the crystalline fraction of spider dragline silk. Science. 1996;271:84–87. doi: 10.1126/science.271.5245.84. [DOI] [PubMed] [Google Scholar]
- Teulé F, Furin WA, Cooper AR, Duncan JR, Lewis RV. Modifications of spider silk sequences in an attempt to control the mechanical properties of the synthetic fibers. Journal of Materials Science. 2007;42:8974–8985. [Google Scholar]
- Teulé F, Cooper AR, Furin WA, Bittencourt D, Rech EL, Brooks A, et al. A protocol for the production of recombinant spider silk-like proteins for artificial fiber spinning. Nature Protocols. 2009;4:341–355. doi: 10.1038/nprot.2008.250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teulé F, Addison B, Cooper AR, Ayon J, Henning RW, Benmore CJ, et al. Combining flagelliform and dragline spider silk motifs to produce tunable synthetic biopolymer fibers. Biopolymers. 2012;97:418–431. doi: 10.1002/bip.21724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urry DW, Luan CH, Peng SQ. Molecular biophysics of elastin structure, function and pathology. Proceedings of the Ciba Foundation Symposium. 1995;192:4922. doi: 10.1002/9780470514771.ch2. (discussion-30) [DOI] [PubMed] [Google Scholar]
- van Beek JD, Hess S, Vollrath F, Meier BH. The molecular structure of spider dragline silk: folding and orientation of the protein backbone. Proceedings of the National Academy of Sciences of the United States of America. 2002;99:10266–10271. doi: 10.1073/pnas.152162299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vendrely C, Scheibel T. Biotechnological production of spider-silk proteins enables new applications. Macromolecular Bioscience. 2007;7:401–409. doi: 10.1002/mabi.200600255. [DOI] [PubMed] [Google Scholar]
- Vollrath F. Spider webs and silks. Scientific American. 1992;266:70–76. [Google Scholar]
- Xu M, Lewis RV. Structure of a protein superfiber: spider dragline silk. Proceedings of the National Academy of Sciences of the United States of America. 1990;87:7120–7124. doi: 10.1073/pnas.87.18.7120. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.