Introduction
An organotypic culture system (OCS) allows for the in vitro growth of complex biological tissues in a way that replicates part of their normal physiology and function. Because epidermis and other skin components, such as hair follicles, can be readily maintained in vitro, OCSs have gained broad popularity in dermatological research. Compared with traditional “on-a-plastic” cultures, where individual dissociated cells quickly lose all but a few of their original in vivo properties, cells in OCSs can engage in elaborate behaviors, such as the growth of new hairs, which requires complex coordination of cell division, differentiation, and migration. OCSs enable human skin to be studied with approaches, such as genetic manipulations, that are otherwise unsafe and unethical in human subjects. As such, skin OCSs are powerful as an experimental platform in preclinical dermatological research, helping to validate mechanisms of diseases and test the therapeutic potential of candidate drugs. This article provides an overview of organotypic skin culture techniques with special emphasis on stratified epidermis and hair follicle in vitro systems.
Organotypic cultures in epidermal research
The key advantage of OCSs over the traditional “on-a-plastic” systems is in their ability to reproduce the three-dimensional stratified space within which skin cells normally live and function in vivo. In its very basic form, the skin consists of a collagen-rich stroma dominated by fibroblasts and topped with stratified epidermis. Epidermal disorders are among the most prevalent, often life-threatening, and mechanistically complex pathologies of the human skin. Understandably, there is a great deal of interest in an in vitro culture system that can support the formation of nearly normal stratified epidermis (Stark et al., 1999), and mimic epidermal pathologies, such as psoriasis (Figure 1) (Barker et al., 2004) or epidermal cancer metastasis (Ridky et al., 2010).
Figure 1. Reconstituted epidermal organotypic culture systems (OCSs).
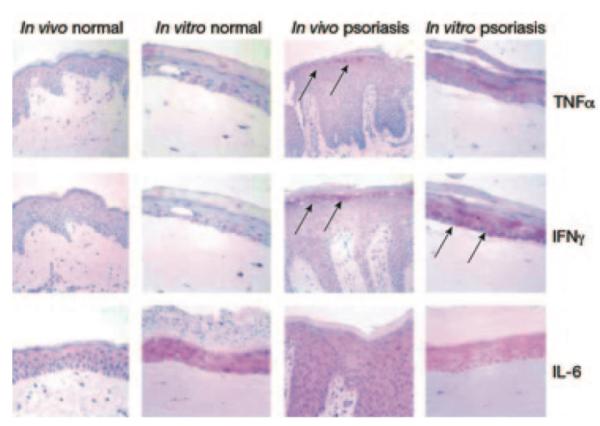
The OCSs, reconstituted with keratinocytes and fibroblasts derived from a human donor with psoriasis (far right), displayed high expression of tumor necrosis factor α (TNF-α) and IFN-γ cytokines in epidermis (pink areas, arrows), similar to psoriasis skin (arrows). IL-6 remains high in both normal and psoriasis OCSs. Reprinted with permission from Barker et al. (2004).
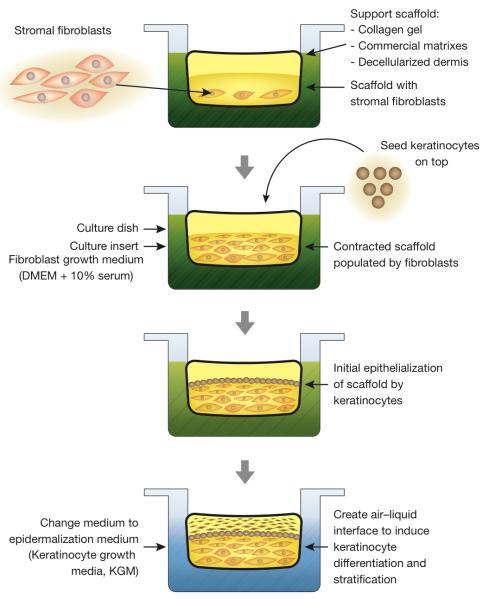
Traditionally, epidermal OCSs are started by adding fibroblast support cells to vacant scaffolds, ranging from a simple collagen gel made fresh in the laboratory to an array of commercially available premade matrices, to the actual skin dermis previously stripped of its original cells. Fibroblasts are allowed time to populate the scaffold, at which point epidermal keratinocytes are seeded on top of it. Stratified differentiation of keratinocytes is then induced by elevating the scaffold above the liquid-air interface (Figure 2). The relative simplicity and ease with which epidermal OCSs can be established under standard laboratory settings, makes them an obvious system of choice in research projects tasked with studying signaling mechanisms and screening therapeutic candidates for human epidermal disorders.
Figure 2.
Flowchart of organotypic epidermal culture system.
Once a basic culture technique is in place, the behavior of human epidermis can be studied and compared under a variety of disease-like scenarios. For example, Ridky et al. (2010) modeled the process of oncogene-induced epidermal neoplasia by replacing normal epidermal cells in an OCS with cells virally transduced to overexpress mutant cell cycle proteins. This way, transduced epidermal cells bypassed normal cell cycle checkpoint mechanisms, mimicking genetic alterations commonly observed during spontaneous malignant transformation of human epidermis in vivo. This in vitro epidermal neoplasia model system reproduced basement membrane invasion, the key step during in vivo epidermal cancer metastasis. Ridky et al. (2010) also showed that epidermal neoplasia OCS is suitable for systematically screening cancer inhibitors on the basis of their ability to block basement membrane invasion. Under similar experimental conditions, anti-cancer potential of other therapeutic agents, such as soluble peptides, neutralizing antibodies, or small hairpin RNAs, can be evaluated and compared.
Other cells, in addition to keratinocytes and fibroblasts, can be incorporated into and studied within the context of skin OCSs. For example, by adding normal or malignant melanocytes, one can study the mechanisms of epidermal pigmentation or melanoma progression (Eves et al., 2000). Also, immune cells, such as macrophages, can be added into OCS scaffolds to study more complex in vivo-like epidermal-dermal-immune signaling interactions in in vitro settings (Bechetoille et al., 2011).
Organotypic culture in hair follicle research
An alternative to engineering organotypic cultures through their de novo reassembly from dissociated cells and extracellular matrix is to culture freshly isolated intact tissues in a way that preserves part of their function and physiological responses. Owing to their inherent complexity, most adult tissues cannot be easily maintained in vitro without undergoing rapid deterioration. Few exceptions exist, and the hair follicle is one of such tissues that can continue to grow new hair, seemingly uninterrupted, for up to 2 weeks after its dissection and placement in a dish. Since they were originally described by Philpott et al. (1990), human hair follicle OCSs have become a favorite experimental systems in preclinical studies on anti-alopecia compound screening. The popularity of human hair follicle OCSs is partly attributed to the fact that mouse hairs grow differently from human hairs, remaining in active growth for less than 2 weeks compared to 3-5 years in human scalp. Further, mice do not develop alopecia in response to androgenic hormones, thus failing to replicate the key signaling step in the pathogenesis of human androgenetic alopecia.
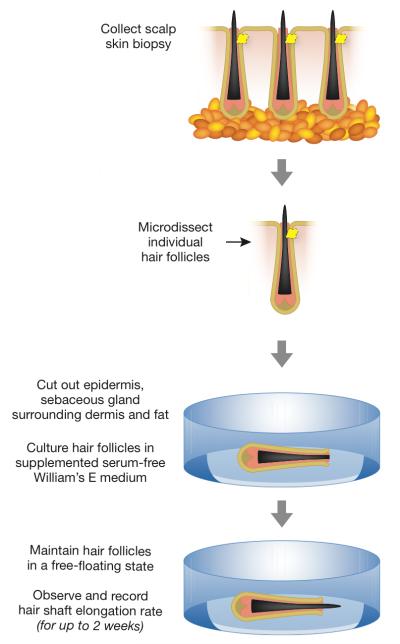
Human hair follicle OCSs are easy to set up and detailed experimental protocols have been made available (Figure 3) (Tobin, 2011). In short, hair follicles are microdissected from freshly isolated scalp skin biopsies and selected for culture on the basis of their morphology. Only intact, undamaged follicles that display mature growth phase morphology (aka anagen) are typically selected. Anagen hair follicles are then cultured in a free-floating state, typically, in serum-free William’s E medium supplemented with glutamine, hydrocortisone and insulin. If undamaged during the initial OCS setup, follicles will continue growing new hair in vitro, and the hair elongation rate can be easily measured and compared between different experimental conditions by means of time-lapse photography and simple morphometry. Care should be taken to examine cultures regularly for signs of premature follicle involution (aka catagen), which is when normal growth activities cease, yet the hair shaft appears to be elongating as it is physically extruded out from the follicle. A set of morphological criteria has been recently described, providing a simple guide for distinguishing anagen from catagen hair follicles in vitro (Kloepper et al., 2010). Also, care should be taken to maintain cultured follicles in a free-floating state. If attached to the dish surface, epithelial and dermal cells can start growing and spreading out from the follicle, altering its morphology and disrupting its hair-growing activity (Tobin, 2011).
Figure 3.
Flowchart of in vitro hair follicle culture.
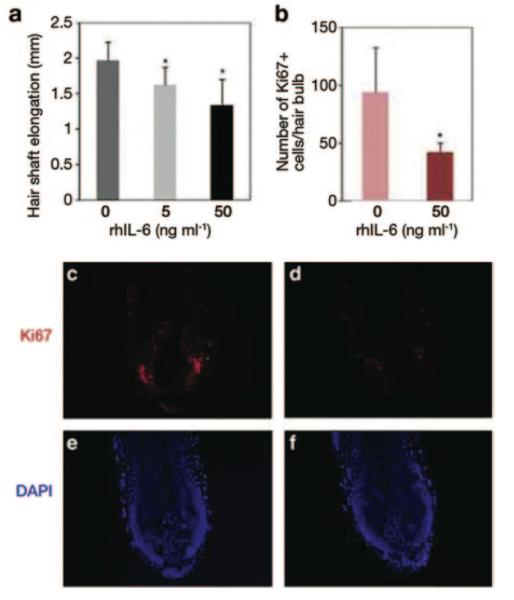
Once these conditions are optimized, hair follicle OCSs can become an important tool to uncover the mechanism of human hair growth pathologies. Progressive miniaturization of human scalp hairs upon androgenetic alopecia is believed to occur as the result of changes in the growth factor composition secreted by dermal papilla cells of the hair follicle in response to their exposure to high levels of testosterone or its metabolite, dihydrotestosterone. For example, Kwack et al. (2012) have shown that interleukin-6, prominently produced by dermal papilla cells of the balding scalp, exerts a strong inhibitory effect on hair shaft elongation by human hair follicles in vitro (Figure 4). A decrease in hair shaft elongation rate and induction of premature catagen involution are the two main readouts indicative of the inhibitory properties of a given molecule. Opposite in vitro results can indicate potential hair growth-promoting effects.
Figure 4. The effect of IL-6 in humans hair follicle culture.
(a) Addition of recombinant human IL-6 into human hair follicle cultures significantly inhibits the hair shaft elongation rate as compared with control. (b—f) The number of proliferating cells in the matrix of cultured human hair follicles, as measured by immunostaining for Ki-67 marker, significantly decreased in the presence of IL-6 (b). Also compare Ki-67 expression in (d) (IL-6 treated) versus (c) (control). Reprinted with permission from Kwack et al. (2012).
Limitations of organotypic skin culture
Although OCSs are powerful research tools for studying human skin, it is important to be aware of their limitations. OCSs engineered from scratch, such as epidermal OCSs, recapitulate only part of normal skin organization and function. Their microanatomy is simpler than that of native skin. They consist of a much simpler cell type repertoire and lack signaling feedback normally coming to skin from a variety of systemic sources. For this reason, skin OCSs cannot replicate complex inflammatory reactions, making them unsuitable for studying wound healing. Ideally, data from OCS experiments should be validated in vivo. Thus, human-on-mouse skin xenografts are commonly used for this purpose. Additionally, the composition of the culture media can affect the behavior of cultured cells. For this reason, serum-free culture medium should be chosen over serum-supplemented ones when possible. Biologically active compounds found in serum, such as fetal bovine serum, cannot be fully accounted for and can potentially interfere with the experiments when drug-treated cultures are being compared with controls.
Organotypic hair follicle cultures also have their limitations. Whereas in vivo hair follicles cycle through consecutive phases of growth (anagen), involution (catagen) and rest (telogen), in vitro they can only maintain a limited-in-duration state of growth or undergo involution. The key hair cycle event of telogen-to-anagen transition, when dormant hair follicle stem cells undergo activation, has not being reliably recapitulated in vitro. It is also important to consider that depending on the proposed mechanism of action, the effect of anti-alopecia compounds might not necessarily register in hair cultures because in vitro conditions have been developed for physiologically normal anagen hair follicles, which already grow at nearly the maximum possible rate. In the future, it will be important to adapt culture conditions for alopecia-affected hair follicles derived from genetically susceptible patients.
Emerging organotypic culture techniques
In an effort to overcome some of the inherent OCS limitations, such as inefficient tissue perfusion, the so-called “on-a-chip” approaches are starting to emerge. Through integration of microfluidic technologies, “on-a-chip” approaches deliver a constant steady-state flow of culture medium to tissues. Additionally, bioengineering techniques that can reconstitute endothelial micro-vascular tissue networks in vitro are now being developed. When these networks are allowed to anastomose to the preengineered microfluidic channels, this approach can support in vivo-like perfusion and physical scaling of OCS size, which currently is largely limited by the inefficiency of passive diffusion (Sakaguchi et al., 2013).
What organotypic culture does
Allows for the study of complex in vivo-like behavior of skin cells under in vitro settings.
Allows for the study of human skin with research techniques otherwise unsafe and unethical in human subjects.
Can be used to simulate human skin diseases and study their mechanisms.
Can be used for screening the therapeutic potential of new drug compounds on human tissues.
Limitations
It recapitulates only part of normal skin organization and function.
Complex, systemic responses, such as wound healing, cannot be reliably studied.
The size of cultured tissues is constrained by inefficient diffusion of nutrients from culture media.
Studying disease states in vitro requires tissues from disease-affected donors.
Supplementary Material
Acknowledgements
M.V.P. is supported by the Edward Mallinckrodt Jr. Foundation Research Grant and the Dermatology Foundation Research Grant. C.F.G.J. is supported by the NIH MBRS-IMSD training grant (GM055246).
References
- 1.Barker CL, McHale MT, Gillies AK, Waller J, Pearce DM, Osborne J, Hutchinson PE, Smith GM, Pringle JH. The development and characterization of an in vitro model of psoriasis. J Invest Dermatol. 2004;123:892–901. doi: 10.1111/j.0022-202X.2004.23435.x. [DOI] [PubMed] [Google Scholar]
- 2.Bechetoille N, Vachon H, Gaydon A, Boher A, Fontaine T, Schaeffer E, Decossas M, André-Frei V, Mueller CG. A new organotypic model containing dermal-type macrophages. Exp Dermatol. 2011;20:1035–1037. doi: 10.1111/j.1600-0625.2011.01383.x. [DOI] [PubMed] [Google Scholar]
- 3.Eves P, Layton C, Hedley S, Dawson RA, Wagner M, Morandini R, Ghanem G, Mac Neil S. Characterization of an in vitro model of human melanoma invasion based on reconstructed human skin. Br J Dermatol. 2000;142:210–222. doi: 10.1046/j.1365-2133.2000.03287.x. [DOI] [PubMed] [Google Scholar]
- 4.Kloepper JE, Sugawara K, Al-Nuaimi Y, Gaspar E, van Beek N, Paus R. Methods in hair research: how to objectively distinguish between anagen and catagen in human hair follicle organ culture. Exp Dermatol. 2010;19:305–312. doi: 10.1111/j.1600-0625.2009.00939.x. [DOI] [PubMed] [Google Scholar]
- 5.Kwack MH, Ahn JS, Kim MK, Kim JC, Sung YK. Dihydrotestosterone-inducible IL-6 inhibits elongation of human hair shafts by suppressing matrix cell proliferation and promotes regression of hair follicles in mice. J Invest Dermatol. 2012;132:43–49. doi: 10.1038/jid.2011.274. [DOI] [PubMed] [Google Scholar]
- 6.Philpott MP, Green MR, Kealey T. Human hair growth in vitro. J Cell Sci. 1990;97:463–471. doi: 10.1242/jcs.97.3.463. [DOI] [PubMed] [Google Scholar]
- 7.Ridky TW, Chow JM, Wong DJ, Khavari PA. Invasive three-dimensional organotypic neoplasia from multiple normal human epithelia. Nat Med. 2010;16:1450–1455. doi: 10.1038/nm.2265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sakaguchi K, Shimizu T, Horaguchi S, Sekine H, Yamato M, Umezu M, Okano T. In vitro engineering of vascularized tissue surrogates. Sci Rep. 2013;3:1316. doi: 10.1038/srep01316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stark HJ, Baur M, Breitkreutz D, Mirancea N, Fusenig NE. Organotypic keratinocyte cocultures in defined medium with regular epidermal morphogenesis and differentiation. J Invest Dermatol. 1999;112:681–691. doi: 10.1046/j.1523-1747.1999.00573.x. [DOI] [PubMed] [Google Scholar]
- 10.Tobin DJ. Ex vivo organ culture of human hair follicles: a model epithelial-neuroectodermal-mesenchymal interaction system. Methods Mol Biol. 2011;695:213–227. doi: 10.1007/978-1-60761-984-0_14. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.