Abstract
We previously reported that 2,3,7,8-Tetrachlorodibenzo-p-dioxin (TCDD) increased the levels of several cytochrome P450 metabolites of the omega-6 polyunsaturated fatty acids (PUFAs), arachidonic acid (ARA) and linoleic acid in the serum, liver, lung and spleen of C57BL/6 mice in an aryl hydrocarbon receptor (AHR)-dependent fashion. These increases correlated with increased levels of CYP1A1, CYP1A2 and/or CYP1B1. In the current study, we measured 77 oxylipins, including 59 that we had not measured previously, and demonstrate that TCDD also markedly increases the levels of many epoxide and diol metabolites of the omega-3 PUFAs, α-linolenic acid, eicosapentaenoic acid (EPA) and docasahexaenoic acid (DHA) in these mice. Since these epoxide metabolites have been reported to have opposite effects on angiogenesis, tumor growth and tumor metastasis compared with the equivalent metabolites of omega-6 PUFA, these observations have important implications with regard to the potential involvement of the cytochrome P450 metabolites of PUFAs in mediating the biological effects of TCDD and other agonists of AHR.
Keywords: TCDD, dioxin, oxylipins, eicosanoids, omega-3, AHR
INTRODUCTION
2,3,7,8-Tetrachlorodibenzo-p-dioxin (TCDD) is an environmental pollutant which exerts a vast number of toxic effects in mammals. All the toxic effects of TCDD are mediated by the aryl hydrocarbon receptor (AHR). However, beyond AHR binding, the mechanism(s) of TCDD toxicity are largely unresolved. A great number of other environmental pollutants also bind and activate AHR, including polyhalogenated dibenzofurans, certain polyhalogenated biphenyls (PHB), and polycyclic aromatic hydrocarbons (PAH) (Denison et al. 2011; Gasiewicz and Henry 2011; Puga 2011). After binding TCDD or other agonists, the AHR activates the transcription of a number of genes. The most massively upregulated of these are CYP1A1, CYP1A2 and CYP1B1. These enzymes are well-known for their ability to metabolize polycyclic aromatic hydrocarbons to genotoxic derivatives. Human CYP1A1, CYP1A2 and CYP1B1 can also metabolize arachidonic acid (ARA) and other polyunsaturated fatty acids (PUFAs) in vitro.
ARA and other PUFAs are mainly metabolized via three pathways: the “cyclooxygenase”, “lipoxygenase” and “cytochrome P450 “epoxidation/hydroxylation” pathways. The immediate products from the metabolism of ARA by the P450’s include four cis-epoxyeicosatrienoic acids (EETs) and certain hydroxyeicosatrienoic acids (HETEs). The epoxide metabolites can be further metabolized, particularly by soluble epoxide hydrolase, which converts them to the dihydroxyeicosatrienoic acids (DHETs). Other PUFAs, including linoleic acid (C18:2 ω6), α-linolenic acid (C18:3, ω3), eicosapentaenoic acid (EPA, C20:5 ω3), and docosahexaenoic acid (DHA, C22:6 ω3) are metabolized in a similar fashion (Fer et al. 2008; Konkel and Schunck 2011). We recently analyzed the levels of up to twenty-five eicosanoids in five organs/tissues of male and female wild-type and Ahr null mice treated or untreated with TCDD (Bui et al. 2012). Our major observations can be summarized as follows:
TCDD increased the levels of many metabolites of the cytochrome P450 epoxidation/hydroxylation pathway in the serum, liver, lung and spleen, but not the heart in both sexes.
TCDD treatment increased the levels of several eicosanoids that are generally categorized as lipoxygenase products (but which can also be generated by particular cytochrome P450s) in the serum, liver and spleen but not the lungs or heart.
TCDD did not increase eicosanoid levels in Ahr−/− knockout mice, demonstrating that AHR mediates most if not all the effects of TCDD on the eicosanoids
The levels of the Cyp1a1 and Cyp1b1 mRNAs were increased by TCDD treatment in the wild-type mice in the liver, lung and heart (although the mRNA levels were 10-fold or more lower in the heart). The Cyp1a2 mRNA increased only in the liver. No induction of any of these enzymes occurred in the Ahr−/− mouse. These cytochromes P450 are likely to be responsible for much of the increases in these metabolites in the various organs/tissues after TCDD treatment.
In the current study, we measured the levels of 77 oxylipins in the lungs, livers and hearts of male wild-type C57BL/6 mice treated intraperitoneally with 50 μg/kg TCDD, including 59 oxylipins that we had not previously measured.
MATERIALS AND METHODS
Administration of TCDD to mice
The husbandry and treatment of mice has been described previously (Bui et al. 2012). Briefly, 50 μg/kg of TCDD in corn oil (obtained from Ralph’s supermarket, Los Angeles, used at 100 μl/mouse) was administered by intraperitoneal injection of C57BL/6 male mice two to three months old. Corn oil was used as vehicle control. The data were obtained from 3 to 6 mice for each organ and treatment.
Chemical and reagents
The abbreviations used for the compounds are those listed in the “Lipid Metabolites and Pathways Strategy” website (http://www.lipidmaps.org/data/structure/LMSDFuzzySearch.php?&Name=1920EPDPE&Mode=ProcessTextOntologySearch&s).
Extraction of fatty acids from different tissues
Each tissue was cut into pieces and then frozen immediately in liquid nitrogen. After addition of 10 uL of anti-oxidant cocktail solution (0.2 mg/mL of butylated hydroxytoluene (BHT) and EDTA) and addition of 10 uL of 100 nM isotope internal standard solution (including d4 6-ketoPGF1α, d4 PGE2, d4 TXB2, d4 LTB4, d11 14,15-DiHETrE, d4 9-HODE, d8 5HETE and d11 11(12)-EpETrE), 100 mg of each tissue sample was homogenized with 400 uL of ice-cold methanol and 0.1% of acetic acid and 0.1% of BHT at 30 Hz for 10 min and then stored at −80°C overnight. The next day, the homogenates were centrifuged at 10,000 rpm for 10 min. The supernatants were collected and the remaining pellets were washed with 100 μL of ice-cold methanol with 0.1 % of acetic acid and 0.1% of BHT and centrifuged. The supernatants of each sample were combined and diluted with 2 mL of H2O and load onto solid phase extraction (SPE) cartridges. Then, the SPE protocol was used to extract the fatty acids as described previously (Yang et al. 2009).
LC/MS/MS analysis
The LC/MS/MS analyses were carried out on an Agilent 1200 SL UHPLC system (Santa Clara, CA) coupled with AB Sciex 4000 QTRAP system (Foster City, CA) under negative MRM mode. The detailed method were described previously (Zivkovic et al. 2012).
Statistical Analysis
Data were analyzed using the ABI software and Microsoft Excel, and significance was evaluated using Student’s t-test.
RESULTS AND DISCUSSION
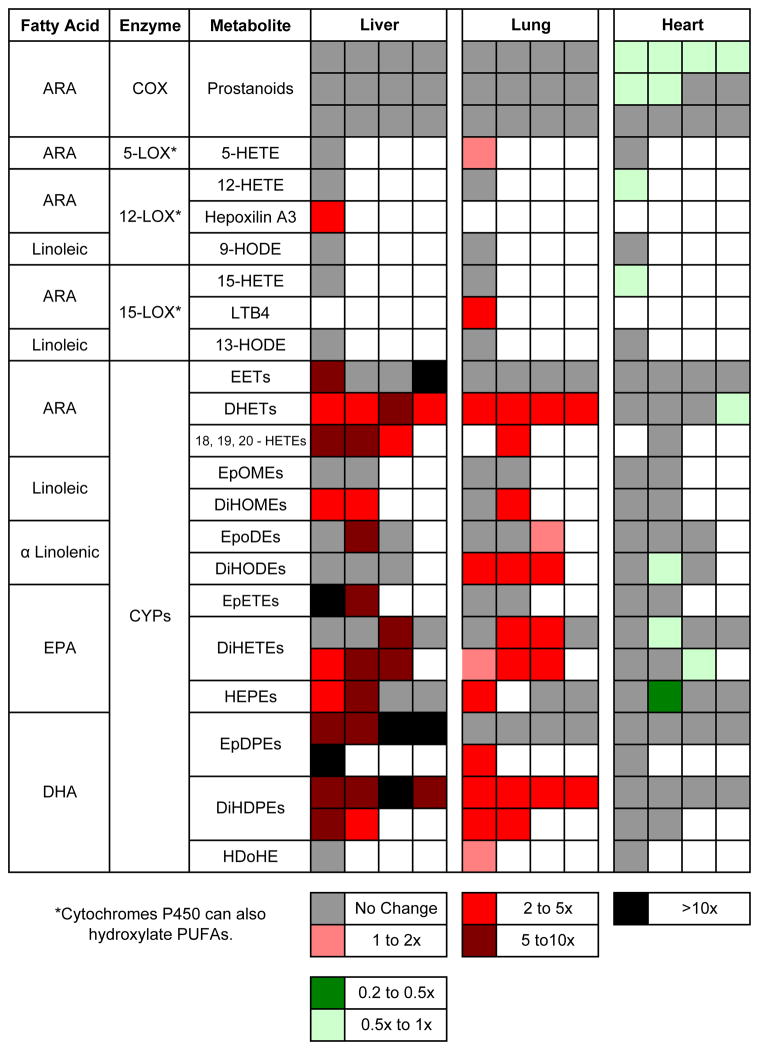
We measured the levels of 77 oxylipins in the lungs, livers and hearts of male wild-type C57BL/6 mice treated with 50 μg/kg TCDD or treated with vehicle (corn oil) for three days (Supplementary Table 1). We had previously reported on the levels of 25 oxylipins in organs obtained from these same mice in analyses performed at UCLA. Some of the 18 latter oxylipins were among the 77 compounds analyzed at the University of California, Davis, while 59 were newly analyzed. Although the absolute values for the amounts of an individual oxylipin measured at UCLA and UC Davis differed in several cases, the effects of TCDD on the levels of the individual oxylipins were in general agreement. Figure summarizes the data for certain of the oxylipins that we measured for the mice analyzed in this paper (see Supplementary Table 1). For 5-HETE, 12-HETE, hepoxilin A3, 9,10-diHOME and 12,13-diHOME the Table also incorporates the data on these mice from our previous paper (Bui et al., 2012). The data for 18-HETE, 19-HETE and 20-HETE are taken from our previous paper. The following are important observations from these data.
TCDD treatment increased the levels of a number of oxylipins in the liver and lung, but with the exception of one oxylipin, did not increase any of these compounds in the heart. In fact, the levels of several oxylipins were modestly reduced in the heart by TCDD.
TCDD did not increase the levels of any of the twelve prostanoids measured in the liver or lung (or heart). This suggests that the increases in the levels of the other metabolites in the liver and lung, discussed below, are not ascribable to TCDD induction of phospholipase A2. It is also consistent with our observation that under our experimental conditions, TCDD did not increase the levels of cyclooxygenase 2 in any organ or tissue examined (Bui et al. 2012).
In the liver, TCDD increased the levels of several epoxides of ARA (EETs), α-linolenic acid (EpODEs), EPA (EpETEs) and DHA (EpDPEs), in several cases more than ten-fold. TCDD increased the levels in the liver of an even greater number of the corresponding diols (HETEs, DiHOMEs, DiHETEs, DiHDPEs and DiHDPEs, respectively), which are generated from the epoxides by soluble epoxide hydrolase.
In the lung, TCDD increased the level of the most abundant epoxide of DHA (19,20-EpDPE), but otherwise did not significantly increase the levels of the other epoxides. The absence of the other EpDPE metabolites may also be due to the fact that they are better substrates of soluble epoxide hydrolase than 19,20-EpDPE (Morisseau and Hammock 2013). However TCDD did increase the levels of nearly all the diols of ARA, α-linolenic acid, EPA and DHA. This indicates that TCDD does generate epoxides in the lung but these are rapidly metabolized to the corresponding diols by soluble epoxide hydrolase.
TCDD increased the levels of some of the hydroxyl metabolites of ARA, EPA and DHA (HETEs, HEPEs and HDoHEs, respectively) in both the liver and lung.
It should be noted that the changes in levels in particular regions or cell types of the liver and lung may be much greater than appears from the levels measured in the whole organ. This is likely to be particularly true in the case of the lung, which contains dozens of different cell types.
The current studies extend our previous studies by demonstrating that the levels of many metabolites of the ω-3 PUFA are also increased by TCDD in the liver and lungs of mice, as we previously observed for several of the equivalent ω-6 metabolites. Epidemiological and preclinical evidence supports that a diet rich in omega-3 dietary fatty acids is correlated with reduced risks of several diseases, including heart diseases and cancer (Rose and Connolly 1999; Wolk et al. 2006; Berquin et al. 2007; Hall et al. 2008; Brasky et al. 2010). Of interest, the epoxides of omega-3 PUFA have the opposite effects from the epoxides of ARA on tumor growth, angiogenesis and metastasis (Zhang et al. 2013). The increased levels of the epoxides and hydroxyl derivatives of the PUFAs elicited by TCDD in the liver and lung are likely to have biological consequences. This will be the subject of future investigations.
Supplementary Material
Figure 1. “Heat Map” summarizing data from this paper and Bui et al., 2012 on the changes in oxylipin levels elicited by TCDD in mice.
Each square represents a different metabolite.
Acknowledgments
Supported by NIEHs grants R01ES015384, R01 ES002710 and P42 ES004699, and NIH/NIDDK grant U24 DK097154. BDH is a George and Judy Marcus Fellow of the American Asthma Society. We thank Alvin Teng for help in preparing the manuscript.
References
- Denison MS, Soshilov AA, He G, DeGroot DE, Zhao B. Exactly the same but different: promiscuity and diversity in the molecular mechanisms of action of the aryl hydrocarbon (dioxin) receptor. Toxicol Sci. 2011;124(1):1–22. doi: 10.1093/toxsci/kfr218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gasiewicz TA, Henry EC. History of Research on the AHR. Hoboken, N.J: Wiley; 2011. [Google Scholar]
- Puga A. Perspectives on the potential involvement of the AH receptor-dioxin axis in cardiovascular disease. Toxicol Sci. 2011;120(2):256–261. doi: 10.1093/toxsci/kfq393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fer M, Dreano Y, Lucas D, Corcos L, Salaun JP, Berthou F, Amet Y. Metabolism of eicosapentaenoic and docosahexaenoic acids by recombinant human cytochromes P450. Arch Biochem Biophys. 2008;471(2):116–125. doi: 10.1016/j.abb.2008.01.002. [DOI] [PubMed] [Google Scholar]
- Konkel A, Schunck WH. Role of cytochrome P450 enzymes in the bioactivation of polyunsaturated fatty acids. Biochim Biophys Acta. 2011;1814(1):210–222. doi: 10.1016/j.bbapap.2010.09.009. [DOI] [PubMed] [Google Scholar]
- Bui P, Solaimani P, Wu X, Hankinson O. 2,3,7,8-Tetrachlorodibenzo-p-dioxin treatment alters eicosanoid levels in several organs of the mouse in an aryl hydrocarbon receptor-dependent fashion. Toxicol Appl Pharmacol. 2012;259(2):143–151. doi: 10.1016/j.taap.2011.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J, Schmelzer K, Georgi K, Hammock BD. Quantitative profiling method for oxylipin metabolome by liquid chromatography electrospray ionization tandem mass spectrometry. Anal Chem. 2009;81(19):8085–8093. doi: 10.1021/ac901282n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zivkovic AM, Yang J, Georgi K, Hegedus C, Nording ML, O’Sullivan A, German JB, Hogg RJ, Weiss RH, Bay C, Hammock BD. Serum oxylipin profiles in IgA nephropathy patients reflect kidney functional alterations. Metabolomics. 2012;8(6):1102–1113. doi: 10.1007/s11306-012-0417-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morisseau C, Hammock BD. Impact of soluble epoxide hydrolase and epoxyeicosanoids on human health. Annu Rev Pharmacol Toxicol. 2013;53:37–58. doi: 10.1146/annurev-pharmtox-011112-140244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose DP, Connolly JM. Omega-3 fatty acids as cancer chemopreventive agents. Pharmacol Ther. 1999;83(3):217–244. doi: 10.1016/s0163-7258(99)00026-1. [DOI] [PubMed] [Google Scholar]
- Wolk A, Larsson SC, Johansson JE, Ekman P. Long-term fatty fish consumption and renal cell carcinoma incidence in women. JAMA. 2006;296(11):1371–1376. doi: 10.1001/jama.296.11.1371. [DOI] [PubMed] [Google Scholar]
- Berquin IM, Min Y, Wu R, Wu J, Perry D, Cline JM, Thomas MJ, Thornburg T, Kulik G, Smith A, Edwards IJ, D’Agostino R, Zhang H, Wu H, Kang JX, Chen YQ. Modulation of prostate cancer genetic risk by omega-3 and omega-6 fatty acids. J Clin Invest. 2007;117(7):1866–1875. doi: 10.1172/JCI31494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall MN, Chavarro JE, Lee IM, Willett WC, Ma J. A 22-year prospective study of fish, n-3 fatty acid intake, and colorectal cancer risk in men. Cancer Epidemiol Biomarkers Prev. 2008;17(5):1136–1143. doi: 10.1158/1055-9965.EPI-07-2803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brasky TM, Lampe JW, Potter JD, Patterson RE, White E. Specialty supplements and breast cancer risk in the VITamins And Lifestyle (VITAL) Cohort. Cancer Epidemiol Biomarkers Prev. 2010;19(7):1696–1708. doi: 10.1158/1055-9965.EPI-10-0318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang G, Panigrahy D, Mahakian LM, Yang J, Liu JY, Stephen Lee KS, Wettersten HI, Ulu A, Hu X, Tam S, Hwang SH, Ingham ES, Kieran MW, Weiss RH, Ferrara KW, Hammock BD. Epoxy metabolites of docosahexaenoic acid (DHA) inhibit angiogenesis, tumor growth, and metastasis. Proc Natl Acad Sci U S A. 2013;110(16):6530–6535. doi: 10.1073/pnas.1304321110. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.