Abstract
In heart cells, Ca2+ released from the internal storage unit, the sarcoplasmic reticulum (SR) through ryanodine receptor (RyR2) channels is the predominant determinant of cardiac contractility. Evidence obtained in recent years suggests that SR Ca2+ release is tightly regulated not only by cytosolic Ca2+ but also by intra-store Ca2+ concentration. Specifically, Ca2+-induced Ca2+ release (CICR) that relies on auto-catalytic action of Ca2+ at the cytosolic side of RyR2s is precisely balanced and counteracted by RyR2 deactivation dependent on a reciprocal decrease of Ca2+ at the luminal side of RyR2s. Dysregulation of this inherently unstable Ca2+ signaling is considered to be an underlying cause of triggered arrhythmias, and is associated with genetic and acquired forms of sudden cardiac death. In this article, we present an overview of recent advances in our understanding of the regulatory role luminal Ca2+ plays in Ca2+ handling, with a particular emphasis on the role of Ca2+ release refractoriness in aberrant Ca2+ release. This article is part of a Special Issue entitled “Calcium Signaling in Heart”.
Keywords: Luminal calcium, Sarcoplasmic reticulum, Ryanodine receptor, Calsequestrin, Calcium-induced calcium-release
1. Introduction
During each heartbeat, coordinated contraction and subsequent relaxation of the billions of cardiomyocytes in the mammalian heart is attained through a synchronized effort of two high fidelity signaling systems: The first is mediated by the action potential (AP) and the other by intracellular Ca2+ transient. In the former case, opening of voltage-dependent Na+ channels generates a depolarizing inward Na+ current that initiates the AP [1]. In the latter, Ca2+ influx through voltage-sensitive L-type Ca2+ channels in response to electrical depolarization activates Ca2+-sensitive ryanodine receptors channels (RyR2) on the surface of the sarcoplasmic reticulum (SR), leading to a self-regenerating process known as Ca2+-induced Ca2+ release (CICR), which initiates contraction [2]. Although the intracellular Ca2+ storage site, the SR, takes up only 3.5% of the total myocyte volume, it contains sufficient Ca2+ not only for the generation of systolic contraction but also for a sizable physiological SR Ca2+ reserve [3]. This in part is due to the presence of the SR Ca2+-adenosine triphosphatase (SERCA) pump on the SR membrane that raises the free intra-SR (luminal) Ca2+ concentration ([Ca2+]) close to 1 mM [3], as well as to the low affinity, high capacity luminal Ca2+ buffering protein calsequestrin (CASQ2) that virtually doubles the SR Ca2+ storage capacity [4].
It is important to note that both membrane depolarization and CICR, because of their positive feedback mechanisms, are intrinsically prone to instabilities and spontaneous activation. For this reason, these high fidelity, self-regenerating signaling processes require effective means for signal termination and containment. For instance, the termination of the AP and the resultant restoration of the resting membrane potential is achieved by the inactivation of Na+ and Ca2+ channels as well as by the repolarizing currents carried by multitude of K+ channels that include the delayed rectifier current with its multiple components [5]. Resting membrane potential between APs, on the other hand, is stabilized by the inward rectifier K+ current. Analogously, termination of SR Ca2+ release and restoration of the diastolic Ca2+ level within the cytosol of the cardiomyocyte is predominantly attained through inactivation/deactivation of RyR2s as well as by SERCA-dependent resequestration of Ca2+ into the SR, and to a lesser extent by extrusion of Ca2+ by the sarcolemmal Na+-Ca2+ exchanger (NCX) [6]. These processes responsible for signal termination ensure that the membrane potential and CICR remain functionally silent or refractory during the diastolic period preventing thereby inappropriately timed APs or contractions.
The concepts of refractoriness and repolarization reserve have been key to understanding the pathophysiology and treatment of rhythm disorders associated with abnormal membrane excitability [7,8]. Despite, growing evidence indicating that regulation of RyR2 by luminal Ca2+ is critical for controlling physiologic SR Ca2+ release (for recent reviews see references [4,9–11]) key questions as to the molecular components involved in luminal Ca2+ regulation of SR Ca2+ release and its refractoriness in the development of cardiac disease remains to be resolved. In this review, we will highlight recent advances that have furthered our understanding of the mechanisms underlying the control of SR Ca2+ release, termination and refractoriness by the interaction of luminal Ca2+ with RyR2 complex. We will also discuss some of the important unresolved issues regarding these processes in normal physiologic and cardiac disease conditions.
2. Luminal Ca2+ governs its own release
In cardiomyocytes during contractions, where most of the Ca2+ required for contractile activation is supplied by the SR, luminal [Ca2+] itself is an important determinant of contractility. However, as suggested by the initial studies, intra-SR Ca2+ is not merely acting as a passive reservoir of available Ca2+, but plays anactive role in controlling the Ca2+ release process [4,12–15]. More precisely, high luminal [Ca2+] (load) facilitates Ca2+ release from the SR, while a sharp decrease of release is observed at reduced SR [Ca2+] load [16,17]. Two principal mechanisms have been proposed by which this regulation could be accomplished. The first is the modulation of RyR2 activity via RyR2 luminal Ca2+ sensing sites. The other, termed feed-through, posits that leak of Ca2+ from the SR (via RyR2) then alters cytosolic [Ca2+] in the vicinity of the “leaky” RyR2 and thereby affects the open probability of that very same and neighboring RyR2s via cytosolic Ca2+ sensing sites [4]. Delineating the contributions of these mechanisms in isolated myocytes is complicated by the difficulty of controlling [Ca2+] on both sides of the RyR2 channel and by the possibility that these mechanisms may operate in tandem.
Compelling evidence for the regulation of RyR2 gating by luminal Ca2+ was obtained in planar lipid bilayers, which allowed not only a direct control over the luminal environment surrounding RyR2s but also prevented the possibility of the feed-through effects [18,19]. It was possible in these studies to isolate the true luminal Ca2+ effects and minimize the possibility of feed-through mechanism(s) by either setting a cytosolic-to-luminal electrochemical Ca2+ gradient [18] or by verifying the luminal localization of the gating effect by trypsin digestion of the luminal aspects of RyR2 [20]. These studies demonstrated that RyR2 open probability changes as a function of luminal Ca2+ with a Kd value of about 1 mM, which corresponds to the SR [Ca2+] in a cardiomyocyte at rest [18,21]. Taken together, these studies suggest that in addition to CICR, luminal effects contribute to the regulatory role of SR Ca2+ load on Ca2+ transient/release by potentiating this effect at elevated SR Ca2+ loads. Importantly, these studies also raised the possibility that reduced luminal [Ca2+] could serve as a negative regulator of RyR2 by inhibiting Ca2+ release at decreased SR Ca2+ content [18].
3. Luminal Ca2+ alters the sensitivity of cytosolic Ca2+ activation site
A number of studies using isolated cardiomyocytes and reconstituted RyR2s demonstrated that luminal Ca2+ alters the sensitivity of RyR2 to cytosolic Ca2+. Single channel studies demonstrated that rather than uniformly scaling RyR2 activity at different cytosolic [Ca2+], increases in luminal Ca2+ shift the cytosolic Ca2+ sensitivity to lower [Ca2+] [18,22,23]. As already mentioned, such investigation of the interplay between the cystosolic and luminal Ca2+ on RyR2 function is challenging in the setting of isolated myocytes. To better understand the mechanisms of SR Ca2+ release regulation by Ca2+ on both sides of the SR membrane, Stevens et al. [24] investigated the effects of a wide range (1–100 µM) of cytosolic [Ca2+] on SR Ca2+ release in permeabilized cardiomyocytes by monitoring luminal [Ca2+]with Fluo-5N, a low-affinity Ca2+ indicator. At any given cytosolic [Ca2+], including levels as high as 50 µM, luminal Ca2+ evidenced periodic oscillations. Since feed-through effects of luminal Ca2+ on SR Ca2+ release should beminimal at such high cytosolic [Ca2+], these intra-SR Ca2+ oscillations were attributed to RyR2 alternating between low and high cytosolic Ca2+-sensitivity states as determined by the filling status of the SR (low and high Ca2+ load, respectively). A similar conclusion regarding dynamic allosteric regulation of RyR2 functional activity by cytosolic and luminal Ca2+ was also reached by MacQuaide et al. [25] in their analysis of the effects of tetracaine on spontaneous Ca2+ release in cardiomyocytes. Of note, a recent study by Tencerová et al. [26] demonstrated that luminal Ca2+ influences RyR2 gating by allosterically modulating affinity of the adenosine-5′-triphosphate (ATP) binding site and thereby RyR2 activation by ATP and low levels of cytosolic [Ca2+]s (<500 nM). Taken together, these results are consistent with the view that RyR2 is an allosteric protein whose activity is regulated not only by luminal Ca2+ but also by numerous ligands, both endogenous (Mg2+, ATP, calmodulin) and exogenous (caffeine, ryanodine, tetracaine) that modulate each others' effects on RyR2 activity [27].
Conversely, Jiang et al. [28] using recombinant channels expressed in HEK cells reported that luminal Ca2+ acts without influencing cytosolic Ca2+ sensitivity of RyR2. Furthermore, these investigators reported that the application of caffeine, considered a sensitizer of cytosolic activation sites [27], actuated RyR2 Ca2+ release by sensitizing the luminal sites without affecting cytosolic sensitivity. However, these results were challenged by the work of Porta et al. [29] which reaffirmed previous observations that caffeine indeed acts by sensitizing the RyR2 cytosolic activation sites [27]. Thus, most available evidence suggests that luminal Ca2+ allosterically influences the sensitivity of RyR2 to cytosolic Ca2+. As discussed below, the mode of action by which RyR2 is regulated by luminal Ca2+ is relevant to understanding the mechanistic control of SR Ca2+ release by intra-store Ca2+ in normal physiology and disease.
4. Luminal Ca2+ and SR Ca2+ release termination
Although Ca2+ release from the SR should be a self-limiting process because of the restricted size of the Ca2+ store, only a fraction of available Ca2+ is released during a regular Ca2+ transient, thus leaving a substantial Ca2+ reserve in the SR [30,31]. Growing evidence, both theoretical and experimental, points to the importance of luminal Ca2+ for release termination. Computer simulations by Sobie et al. [15] demonstrated the feasibility of local depletion of SR Ca2+ as a signal for the closure of RyR2. At the same time, Terentyev et al. [12,13] used low affinity Ca2+ buffers directed to the SR to demonstrate that changes in luminal [Ca2+] play a critical role in Ca2+ release termination and refractoriness, whereby a decline in the SR Ca2+ store resulted in functional deactivation of RyR2s. Subsequent introduction of luminal Ca2+ measurement techniques (with SR-entrapped low-affinity Ca2+ indicators) made possible direct detection of Ca2+ depletion during both unitary Ca2+ release events (Ca2+ sparks) as well as during cell-wide Ca2+ transients [32,33]. Through paired measurements of cytosolic and luminal [Ca2+], it has been demonstrated that Ca2+ spark termination occurs at a certain steady-state threshold of intra-SR [Ca2+] [34–36]. These findings thus offered further support for the role of luminal [Ca2+] in release termination. However, it is important to note that this seemingly premature termination of SR Ca2+ release in cardiac myocytes that does not completely deplete the SR of Ca2+ can also have other explanations. For example, activation of only a fraction of release sites, a scenario where all the activated sites become fully depleted while idle sites maintain their corresponding SR Ca2+ content, could also account for the partial release of the total SR [Ca2+]. Consistent with this notion, Hake et al. [37] using mathematical modeling to compare the true extent of local junctional SR depletion as reported by Ca2+ blinks concluded that blinks may underestimate the extent of local SR depletion. Critical to the role of SR depletion in termination of local Ca2+ release is the concept of Ca2+ mobility within the SR. Indeed, high Ca2+ mobility would tend to prevent severe depletion by hastening Ca2+ redistribution from adjacent SR elements. On the other hand, low Ca2+ mobility would facilitate SR emptying by restricting the aforementioned redistribution of Ca2+ within the SR network. However, studies of the intra-SR diffusion yielded conflicting results with nearly an order of magnitude difference in the apparent diffusion coefficients (8 µm2/s [38] vs. 60 µm2/s [39,40]). Resolving the precise role of local SR Ca2+ depletion in termination of Ca2+ release will require future investigation into Ca2+ diffusion through the SR network as well as further advances in the techniques for measurement of luminal Ca2+ in cardiac myocytes.
5. Refractoriness of SR Ca2+ release
Following the discharge of Ca2+ from the SR, the release mechanism enters a refractory period when it is unresponsive to stimulation by cytosolic Ca2+ [41,42]. There is a growing consensus that this refractoriness of Ca2+ signaling involves a change of [Ca2+] on the luminal side of the SR [4,12,14,24,42,43]. For example, experimental interventions that either accelerate or slow SR refilling corresponding accelerate or slow Ca2+ release restitution, respectively, independent of changes in cytosolic Ca2+ [12,13,43]. Importantly, the functional recovery of release lags significantly behind refilling of the SR Ca2+ store, an observation made on the level of focal as well as global SR Ca2+ release using both direct and indirect methods for assessing intra-SR Ca2+ recovery [14,43–46].
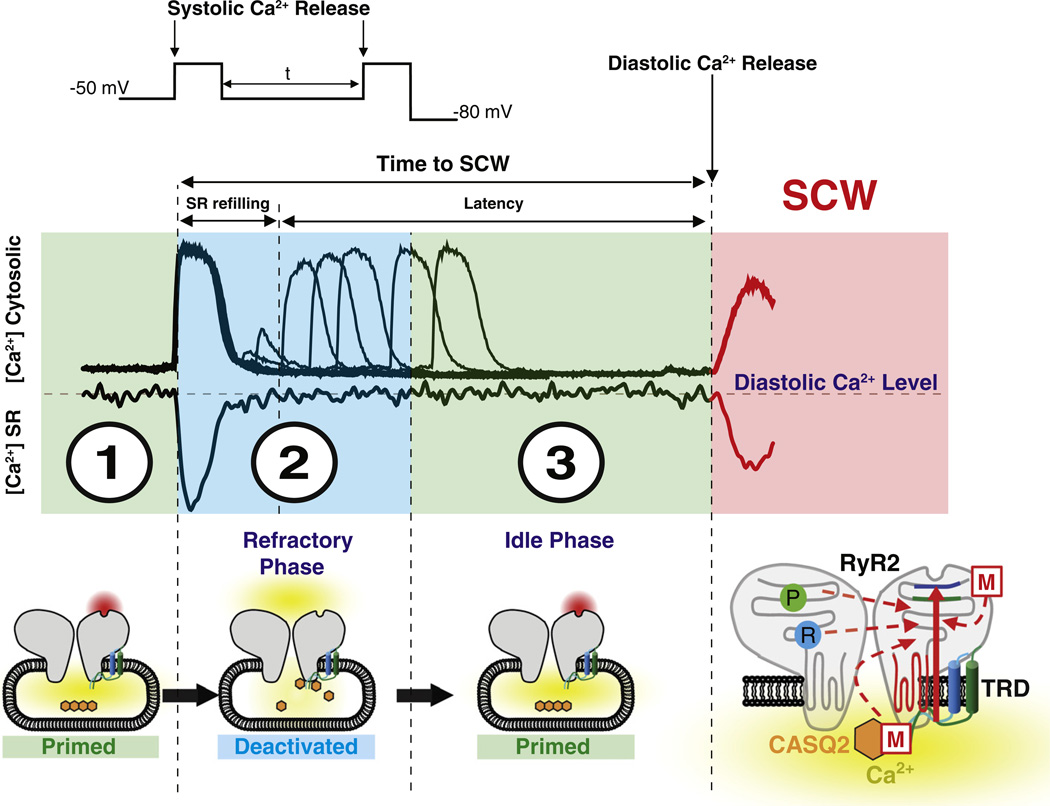
The specific components of Ca2+ signaling restitution following SR Ca2+ release were recently defined by Belevych et al. [45] by concomitant assessment of L-type Ca2+ current along with cytosolic and luminal Ca2+ recorded from myocytes isolated from normal and post-infarction canine hearts. As illustrated in Fig. 1, SR Ca2+ release is accompanied by a transient decrease in luminal Ca2+ content. The total time from this initial release to a spontaneous Ca2+ release event is composed of a period of refilling of the SR Ca2+ stores and a subsequent latency period during which the intra-SR Ca2+ levels remain constant. Two-pulse experiments demonstrated that the latency period is comprised of a refractory phase and an “idle” phase. As suggested by theoretical analysis performed by Maltsev et al. [47], the idle period can be attributed to a delay required for synchronization of stochastic release sites that have recovered from refractoriness through a process resembling a phase transition from unitary events to global Ca2+ release. Of note, the refractoriness and not the idle period are significantly shortened in post-infarction myocytes thereby accounting for increased vulnerability of these myocytes to diastolic spontaneous Ca2+ waves. The distinction between a refilling time and a latency period has also been described on the level of Ca2+ spark. Using both isolated cardiomyocytes as well as computational simulations, Ramay et al. [48] separated the recovery of Ca2+ spark amplitude from recovery of spark triggering probability for recurring events triggered by ryanodine. These investigators concluded that the former depended only on local SR refilling whereas the latter depended on both refilling and on recovery from RyR2 refractoriness. Accordingly, mathematical theory developed by Rovetti et al. [49] proposed that luminal Ca2+-dependent refractoriness of release units is one of the important variables required for stability of CICR that governs the appearance of arrhythmogenic phenomena such Ca2+ waves and alternans. Thus, in contrast to Ca2+ release termination for which the role of functional effects of luminal Ca2+ on RyR2 gating is yet to be distinguished from SR Ca2+ depletion, release refractoriness does have a distinct component due to the effects upon RyR2 gating (i.e. deactivation).
Fig. 1.
Prior to membrane depolarization, the SR Ca2+ interacts with CASQ2 functionally detaching it from the RyR2 complex rendering RyR2 primed for activation (primed cytosolic RyR2 activation site highlighted in red) by cytosolic Ca2+ (1). During RyR2 activation and the resulting systolic Ca2+ release, SR Ca2+ content is partially reduced thereby facilitating CASQ2 interaction with the RyR2 complex [via triadin (TRD) and/or junctin (JNT) (2). This interaction results in an allosteric inhibition of cytosolic activation site of the RyR2. The time between systolic SR Ca2+ release and spontaneous diastolic Ca2+ release is composed by refilling of SR Ca2+ store and latency during which the Ca2+ within the SR remains constant (2 and 3). The refractory phase reflects the time required for recovery of RyR2 from luminal Ca2+ deactivation and can be determined by a 2-pulse protocol (insets 1 and 2). At the completion of the refractory phase, refilling of the SR Ca2+ stores leads to dissociation of CASQ2 rendering RyR2 again functionally primed (primed cytosolic RyR2 activation site highlighted in red, 3) underlying thereby an idle phase during which stochastic activation of the recovered SR Ca2+ release sites triggers spontaneous diastolic Ca2+ waves (SCW). Phosphorylation (P) and redox modification (R) of RyR2 along with mutations in CASQ2 or the SR Ca2+ release complex (M) promote shortened recovery of RyR2 from luminal Ca2+ deactivation reducing thereby the refractory period and the time to spontaneous diastolic Ca2+ waves.
6. The intermediary luminal Ca2+ sensor
Consistent with the presence of a RyR2 Ca2+-mediated modulation site on the luminal side of the SR, it is possible that luminal Ca2+ can directly regulate SR Ca2+ release by binding directly to the luminal side of RyR2 or via intermediate luminal auxiliary proteins such as triadin (TRD), junctin (JNT) and CASQ2. The notion of direct luminal regulation was further advanced in experiments using recombinant RyR2 variants (expressed in HEK cells devoid of RyR2 luminal auxiliary proteins) with altered responsiveness to luminal Ca2+ [28,50,51]. Based on these experiments it was proposed that direct activation of RyR2 by luminal Ca2+ independent of accessory proteins and effects of cytosolic Ca2+ causes the release of Ca2+ from the SR [28,50]. However, a body of evidence has accumulated, suggesting that intermediate regulatory proteins play a role in modulation of SR Ca2+ release by luminal Ca2+ [13,22,52–57]. Moreover as noted above, most available evidence indicates that luminal Ca2+ acts by allosterically affecting RyR2's responsiveness to cytosolic Ca2+, and hence CICR, rather than by directly activating the RyR2 [24–27].
Studies from several laboratories have demonstrated that CASQ2 is capable of conferring luminal Ca2+ sensitivity to RyR2s reconstituted in planar lipid bilayers [22,52–54]. The effects of CASQ2 are dependent on the presence of luminal TRD and JNT and are influenced by cytosolic factors such as ATP. In RyR2s activated by cytosolic Ca2+ and ATP association with CASQ2 has been reported to decrease RyR2 activity at low luminal Ca2+ (<1 mM) and facilitate the channel response to high luminal Ca2+ [52,54]. The effects of CASQ2 were mediated by TRD (and/or JNT) as they relied on the presence of luminal TRD and JNT [52]. In the absence of activating ATP, CASQ2 conferred luminal Ca2+ dependency by increasing RyR2 activity at high (>1 mM) but not low luminal Ca2+ (at which open probability was close to zero) [22]. In both settings (i.e. in the presence or absence of ATP) the ability of CASQ2 and luminal Ca2+ to modulate RyR2 function was compromised by arrhythmogenic mutations linked to Catecholaminergic Polymorphic Ventricular Tachycardia (CPVT) [22,35]. Future RyR2 reconstitution studies will have to address the precise contributions of direct Ca2+ binding and intermediate luminal Ca2+ sensor(s) on the RyR2 as well as the role of intracellular affecters (including ATP, Mg2+, calmodulin) in modulation of RyR2 complex gating by luminal Ca2+.
The role of CASQ2 in Ca2+ handling has been further demonstrated in studies utilizing myocytes deficient in CASQ2 [13,55,56] and those expressing inhibitory peptides targeting CASQ2-TRD interaction [57]. These studies revealed that the absence of CASQ2 or specifically targeting CASQ2 interaction with the RyR2 complex leads to dysregulated SR Ca2+ release. Such alterations in the function of the RyR2 complex manifest an increased frequency of diastolic Ca2+ sparks and Ca2+ waves. Taken together these results provided further evidence that CASQ2 can act as a luminal Ca2+ sensor that cross-talks (possibly through TRD) with RyR2, thereby regulating SR Ca2+ release and its termination and/or refractoriness by stabilizing the deactivation of RyR2.
Based on the combination of studies conducted using single RyR2 and myocyte reviewed above it was proposed that RyR2 modulation by CASQ2 and luminal Ca2+ involves the following steps [24]: At low luminal [Ca2+], CASQ2 interacts with the RyR2 complex rendering the cytosolic activation site on RyR2 allosterically inhibited (Fig. 1). Refilling the SR Ca2+ stores by SERCA during the recovery (restitution) phase once again increases luminal Ca2+, causing CASQ2 to dissociate from the RyR2s rendering these SR Ca2+ release channels once more sensitized for yet another activation by cytosolic Ca2+. There is also evidence that has implicated other proteins such as TRD [56,58], JNT [59] and histidine-rich Ca2+ binding protein [60] in regulation of RyR2. The potential role of these proteins in mediating the effects of luminal Ca2+ on RyR2 function, as well as that of Ca2+-dependent CASQ2 polymerization-depolymerization as part of the luminal signaling cascade [35] requires further investigation.
7. The luminal Ca2+ threshold level for spontaneous SR Ca2+ release revisited
Over forty years ago Fabiato & Fabiato [2] reported that in addition to CICR, mechanically skinned cardiac cells can exhibit another form of SR Ca2+ release that occurs secondary to increased SR Ca2+ load, independent of a cytosolic Ca2+ trigger. These unprovoked, aberrant SR Ca2+ releases, also visualized as propagating Ca2+ waves, have been examined in many subsequent studies. A common observation that emerged from these investigations was that whenever luminal Ca2+ reached a “threshold” level, this resulted in spontaneous Ca2+ release [61,62] Moreover, this apparent intra-SR threshold has been reported to be reduced in cardiac disease as a consequence of either genetic mutations or acquired modifications in the RyR2 channel complex thus accounting for the increased arrhythmogenic propensity in these cell types [28,61,63,64]. These results have sometimes been taken as an indication that spontaneous Ca2+ release is directly activated by luminal Ca2+ independent of canonical CICR, a process referred to as store-overload-induced Ca2+ release (SOICR) [28,50,51]. This view however may not be sufficiently justified.
As recent studies have demonstrated with the aid of direct luminal Ca2+ monitoring, spontaneous Ca2+ release does not occur once the intra-SR Ca2+ returns to its baseline level, but a distinct time delay is present before another spontaneous Ca2+ release event may occur (Fig. 1) [45,48,65]. Importantly, this latency to spontaneous Ca2+ release is shortened in disease despite lowered diastolic intra-SR [Ca2+] [45,65]. Thus direct monitoring of [Ca2+] SR failed to confirm existence of a luminal [Ca2+] threshold as a sufficient factor for activation of diastolic Ca2+ release. The concept of threshold SR Ca2+ content while being useful for characterization of SR Ca2+ release at steady-state, it may be less applicable to beat-to-beat Ca2+ cycling dynamics as occurs in the beating heart and in arrhythmias. The aforementioned behavior further challenges the notion of direct activation of Ca2+ release by luminal Ca2+, and as alluded to earlier instead points to recovery from refractoriness as the underlying mechanism responsible for the timing of spontaneous Ca2+ release. A correlation between incidents of diastolic release and SR refilling rate consistent with a role for store-dependent refractoriness has been also demonstrated in intact beating hearts [46].
Considering the intrinsic instability of CICR, which requires mechanisms able to exquisitely control it, it is difficult to envision a physiologically useful role for direct luminal Ca2+ activation of RyR2. Rather than operating as a stimulator of Ca2+ release, luminal modulation appears to constrain RyR2 activity when intra-SR Ca2+ level is low such as following SR Ca2+ release. As growing evidence suggest [12–14,43,45], following Ca2+ release the decrease of luminal Ca2+ provides a signal for deactivation of RyR2s thereby contributing to diastolic refractoriness of Ca2+ release. Thus CICR that relies on auto-catalytic action of Ca2+ at the cytosolic side of the SR is precisely balanced and counteracted by a deactivation mechanism dependent on a reciprocal decrease of Ca2+ inside the SR. Importantly, the impairment of this regulatory mechanism accounts for the spontaneous Ca2+ release observed in various pathologies as discussed below.
8. Genetic and acquired arrhythmias associated with altered luminal Ca2+ control of SR Ca2+ release
The importance of RyR2 modulation by luminal Ca2+ in healthy heart is highlighted by recent findings indicating that genetic defects in RyR2 and its auxiliary luminal proteins including CASQ2 and TRD are associated with inherited arrhythmias such CPVT [66–68]. CPVT has been attributed to “leaky” RyR2s resulting in spontaneous Ca2+ release during diastole [51,69,68].Whereas the link between spontaneous Ca2+ release and the consequent membrane depolarization (delayed after depolarization, DAD) is well established and involves NCX stimulated by elevated cytosolic Ca2+ [62,70–72], the mechanism responsible for spontaneous Ca2+ release in CPVT has been an area of intense investigation. Based on studies using recombinant RyR2, Chen and colleagues suggested that spontaneous release associated with CPVT is due to direct activation of SR Ca2+ release by luminal Ca2+ [28,51]. However, CASQ2 and other auxiliary luminal proteins were absent from the experimental preparations used by these investigators making their interpretation of the role of luminal Ca2+ in a physiological milieu difficult. Furthermore, as already stated the concept of direct activation of RyR2 by luminal Ca2+ is not supported in cardiomyocytes by direct experimental measurements of spontaneous Ca2+ release either on the level of cytosolic or luminal Ca2+ dynamics [45,65]. Conversely, evidence provided by other laboratories suggests that rather than acting by direct luminal Ca2+ activation, CPVT-associated genetic mutations of CASQ2 destabilize the deactivation of RyR2 [44,68,73,74]. This is accomplished by an alteration of luminal Ca2+ control of RyR2 and thereby shortening Ca2+ release refractoriness resulting in premature spontaneous Ca2+ release (Fig. 1).
Growing evidence suggests that similar alterations in RyR2 function as those ascribed to CPVT-associated mutations accompany some of the much more common acquired forms of cardiac diseases including both ischemic and non-ischemic cardiomyopathy. In the case of these acquired diseases, post-translational modification of RyR2s by either phosphorylation (via Protein Kinase A and/or Calmodulin-dependent Protein Kinase II) and/or by reactive oxygen and nitrogen species have been implicated as molecular mechanisms of altered Ca2+ release function [45,64,65,73,75]. Moreover, research into alterations of RyR2 complex in these acquired forms of cardiac disease provided new mechanistic insight into the role of altered Ca2+ signaling and its refractoriness in arrhythmogenesis. Specifically, RyR2 phosphorylation and oxidation evidenced in post-infarction cardiomyocytes resulted in shortened Ca2+ signaling refractoriness that contributed to the increased rate of spontaneous Ca2+ waves observed in these cells [45]. Thus it appears that genetic arrhythmogenic mutations within the components of the RyR2 complex along with posttranslational modifications of the Ca2+ release channel associated with acquired cardiac disease act through a common mechanism, which affects the RyR2 luminal to cytosolic Ca2+ regulatory axis (Fig. 1).
Taken together these studies highlight the importance of RyR2 function and its regulation by its auxiliary luminal proteins and luminal Ca2+ in the maintenance of dynamic stability of SR Ca2+ release. The loss of such regulatory mechanism results in shortened Ca2+ release refractoriness that in turn underlies arrhythmogenic spontaneous Ca2+ release and DADs. Refractoriness has been extensively investigated in relation to electrical repolarization and its reserve [8,76]. By analogy it appears Ca2+ release refractoriness and CICR stability reserve hold similar promise for understanding and treatment of arrhythmias. Future studies will need to elucidate the role of Ca2+ release refractoriness as well as other mechanism(s) responsible for synchronization of Ca2+ waves and the ensuing membrane potential depolarization between individual cardiac cells.
Acknowledgments
Funding
This work was supported by National Institutes of Health grants HL074045 and HL063043 (to S.G.), HL089836 (to C.A.C.).
Glossary
- AP
Action potential
- ATP
Adenosine-5′-triphosphate
- [Ca2+]
Ca2+ concentration
- CICR
Ca2+-induced Ca2+ release
- CASQ2
Calsequestrin
- JNT
Junctin
- NCX
Na+–Ca2+ exchanger
- RyR2
Ryanodine receptors channels
- SR
Sarcoplasmic reticulum
- SERCA
SR Ca2+-adenosine triphosphatase
- SOICR
Store-overload-induced Ca2+ release
- TRD
Triadin
Footnotes
Disclosures
None declared.
References
- 1.Kleber AG, Rudy Y. Basic mechanisms of cardiac impulse propagation and associated arrhythmias. Physiol Rev. 2004;84:431–488. doi: 10.1152/physrev.00025.2003. [DOI] [PubMed] [Google Scholar]
- 2.Fabiato A, Fabiato F. Contractions induced by a calcium-triggered release of calcium from the sarcoplasmic reticulum of single skinned cardiac cells. J Physiol. 1975;249:469–495. doi: 10.1113/jphysiol.1975.sp011026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bers D. Excitation–contraction coupling and cardiac contractile force. 2nd ed. Dordrecht: Springer; 2001. [Google Scholar]
- 4.Györke S, Terentyev D. Modulation of ryanodine receptor by luminal calcium and accessory proteins in health and cardiac disease. Cardiovasc Res. 2008;77:245–255. doi: 10.1093/cvr/cvm038. [DOI] [PubMed] [Google Scholar]
- 5.Rudy Y. Modelling the molecular basis of cardiac repolarization. Europace. 2007;9(Suppl. 6):vi17–vi19. doi: 10.1093/europace/eum202. [DOI] [PubMed] [Google Scholar]
- 6.Bers DM. Cardiac excitation–contraction coupling. Nature. 2002;415:198–205. doi: 10.1038/415198a. [DOI] [PubMed] [Google Scholar]
- 7.Michael G, Xiao L, Qi X-Y, Dobrev D, Nattel S. Remodelling of cardiac repolarization: how homeostatic responses can lead to arrhythmogenesis. Cardiovasc Res. 2009;81:491–499. doi: 10.1093/cvr/cvn266. [DOI] [PubMed] [Google Scholar]
- 8.Varró A, Baczkó I. Cardiac ventricular repolarization reserve: a principle for understanding drug-related proarrhythmic risk. Br J Pharmacol. 2011;164:14–36. doi: 10.1111/j.1476-5381.2011.01367.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stern MD, Cheng H. Putting out the fire: what terminates calcium-induced calcium release in cardiac muscle? Cell Calcium. 2004;35:591–601. doi: 10.1016/j.ceca.2004.01.013. [DOI] [PubMed] [Google Scholar]
- 10.Sobie EA, Lederer WJ. Dynamic local changes in sarcoplasmic reticulum calcium: physiological and pathophysiological roles. J Mol Cell Cardiol. 2012;52:304–311. doi: 10.1016/j.yjmcc.2011.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Györke S, Hagen BM, Terentyev D, Lederer WJ. Chain-reaction Ca2+ signaling in the heart. J Clin Invest. 2007;117:1758–1762. doi: 10.1172/JCI32496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Terentyev D, Viatchenko-Karpinski S, Valdivia HH, Escobar AL, Györke S. Luminal Ca2+controls termination and refractory behavior of Ca2+-induced Ca2+ release in cardiac myocytes. Circ Res. 2002;91:414–420. doi: 10.1161/01.res.0000032490.04207.bd. [DOI] [PubMed] [Google Scholar]
- 13.Terentyev D, Viatchenko-Karpinski S, Györke I, Volpe P, Williams SC, Györke S. Calsequestrin determines the functional size and stability of cardiac intracellular calcium stores: mechanism for hereditary arrhythmia. Proc Natl Acad Sci U S A. 2003;100:11759–11764. doi: 10.1073/pnas.1932318100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sobie EA, Song L-S, Lederer WJ. Local recovery of Ca2+ release in rat ventricular myocytes. J Physiol. 2005;565:441–447. doi: 10.1113/jphysiol.2005.086496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sobie EA, Dilly KW, dos Santos Cruz J, Lederer WJ, Jafri MS. Termination of cardiac Ca2+ sparks: an investigative mathematical model of calcium-induced calcium release. Biophys J. 2002;83:59–78. doi: 10.1016/s0006-3495(02)75149-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bassani JW, Yuan W, Bers DM. Fractional SR Ca release is regulated by trigger Ca and SR Ca content in cardiac myocytes. Am J Physiol. 1995;268:C1313–C1319. doi: 10.1152/ajpcell.1995.268.5.C1313. [DOI] [PubMed] [Google Scholar]
- 17.Shannon TR, Ginsburg KS, Bers DM. Potentiation of fractional sarcoplasmic reticulum calcium release by total and free intra-sarcoplasmic reticulum calcium concentration. Biophys J. 2000;78:334–343. doi: 10.1016/S0006-3495(00)76596-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Györke I, Györke S. Regulation of the cardiac ryanodine receptor channel by luminal Ca2+ involves luminal Ca2+ sensing sites. Biophys J. 1998;75:2801–2810. doi: 10.1016/S0006-3495(98)77723-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ching LL, Williams AJ, Sitsapesan R. Evidence for Ca2+ activation and inactivation sites on the luminal side of the cardiac ryanodine receptor complex. Circ Res. 2000;87:201–206. doi: 10.1161/01.res.87.3.201. [DOI] [PubMed] [Google Scholar]
- 20.Hill AP, Sitsapesan R. DIDS modifies the conductance, gating, and inactivation mechanisms of the cardiac ryanodine receptor. Biophys J. 2002;82:3037–3047. doi: 10.1016/S0006-3495(02)75644-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lukyanenko V, Györke I, Györke S. Regulation of calcium release by calcium inside the sarcoplasmic reticulum in ventricular myocytes. Pflugers Arch. 1996;432:1047–1054. doi: 10.1007/s004240050233. [DOI] [PubMed] [Google Scholar]
- 22.Qin J, Valle G, Nani A, Nori A, Rizzi N, Priori SG, et al. Luminal Ca2+ regulation of single cardiac ryanodine receptors: insights provided by calsequestrin and its mutants. J Gen Physiol. 2008;131:325–334. doi: 10.1085/jgp.200709907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Laver DR. Ca2+ stores regulate ryanodine receptor Ca2+ release channels via luminal and cytosolic Ca2+ sites. Biophys J. 2007;92:3541–3555. doi: 10.1529/biophysj.106.099028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stevens SCW, Terentyev D, Kalyanasundaram A, Periasamy M, Györke S. Intra-sarcoplasmic reticulum Ca2+ oscillations are driven by dynamic regulation of ryanodine receptor function by luminal Ca2+ in cardiomyocytes. J Physiol. 2009;587:4863–4872. doi: 10.1113/jphysiol.2009.175547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.MacQuaide N, Dempster J, Smith GL. Assessment of sarcoplasmic reticulum Ca2+ depletion during spontaneous Ca2+ waves in isolated permeabilized rabbit ventricular cardiomyocytes. Biophys J. 2009;96:2744–2754. doi: 10.1016/j.bpj.2008.12.3944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tencerová B, Zahradníková A, Gaburjáková J, Gaburjáková M. Luminal Ca2+ controls activation of the cardiac ryanodine receptor by ATP. J Gen Physiol. 2012;140:93–108. doi: 10.1085/jgp.201110708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Meissner G. Regulation of mammalian ryanodine receptors. Front Biosci. 2002;7:d2072–d2080. doi: 10.2741/A899. [DOI] [PubMed] [Google Scholar]
- 28.iang D, Xiao B, Yang D, Wang R, Choi P, Zhang L, et al. RyR2 mutations linked to ventricular tachycardia and sudden death reduce the threshold for store-overload-induced Ca2+ release (SOICR) Proc Natl Acad Sci U S A. 2004;101:13062–13067. doi: 10.1073/pnas.0402388101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Porta M, Zima AV, Nani A, Diaz-Sylvester PL, Copello JA, Ramos-Franco J, et al. Single ryanodine receptor channel basis of caffeine's action on Ca2+ sparks. Biophys J. 2011;100:931–938. doi: 10.1016/j.bpj.2011.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bassani JW, Bassani RA, Bers DM. Twitch-dependent SR Ca accumulation and release in rabbit ventricular myocytes. Am J Physiol. 1993;265:C533–C540. doi: 10.1152/ajpcell.1993.265.2.C533. [DOI] [PubMed] [Google Scholar]
- 31.Delbridge LM, Satoh H, Yuan W, Bassani JW, Qi M, Ginsburg KS, et al. Cardiacmyocyte volume, Ca2+ fluxes, and sarcoplasmic reticulum loading in pressure-overload hypertrophy. Am J Physiol. 1997;272:H2425–H2435. doi: 10.1152/ajpheart.1997.272.5.H2425. [DOI] [PubMed] [Google Scholar]
- 32.Brochet DXP, Yang D, Di Maio A, Lederer WJ, Franzini-Armstrong C, Cheng H. Ca2+ blinks: rapid nanoscopic store calcium signaling. Proc Natl Acad Sci U S A. 2005;102:3099–3104. doi: 10.1073/pnas.0500059102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shannon TR, Guo T, Bers DM. Ca2+ scraps: local depletions of free [Ca2+] in cardiac sarcoplasmic reticulum during contractions leave substantial Ca2+ reserve. Circ Res. 2003;93:40–45. doi: 10.1161/01.RES.0000079967.11815.19. [DOI] [PubMed] [Google Scholar]
- 34.Zima AV, Picht E, Bers DM, Blatter LA. Termination of cardiac Ca2+ sparks: role of intra-SR [Ca2+], release flux, and intra-SR Ca2+ diffusion. Circ Res. 2008;103:e105–e115. doi: 10.1161/CIRCRESAHA.107.183236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Terentyev D, Kubalova Z, Valle G, Nori A, Vedamoorthyrao S, Terentyeva R, et al. Modulation of SR Ca release by luminal Ca and calsequestrin in cardiac myocytes: effects of CASQ2 mutations linked to sudden cardiac death. Biophys J. 2008;95:2037–2048. doi: 10.1529/biophysj.107.128249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Domeier TL, Blatter LA, Zima AV. Alteration of sarcoplasmic reticulum Ca2+ release termination by ryanodine receptor sensitization and in heart failure. J Physiol. 2009;587:5197–5209. doi: 10.1113/jphysiol.2009.177576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hake J, Edwards AG, Yu Z, Kekenes-Huskey PM, Michailova AP, McCammon JA, et al. Modeling cardiac calcium sparks in a three-dimensional reconstruction of a calcium release unit. J Physiol. 2012;590:4403–4422. doi: 10.1113/jphysiol.2012.227926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Swietach P, Spitzer KW, Vaughan-Jones RD. Ca2+ mobility in the sarcoplasmic reticulum of ventricular myocytes is low. Biophys J. 2008;95:1412–1427. doi: 10.1529/biophysj.108.130385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wu X, Bers DM. Sarcoplasmic reticulum and nuclear envelope are one highly interconnected Ca2+ store throughout cardiac myocyte. Circ Res. 2006;99:283–291. doi: 10.1161/01.RES.0000233386.02708.72. [DOI] [PubMed] [Google Scholar]
- 40.Picht E, Zima AV, Shannon TR, Duncan AM, Blatter LA, Bers DM. Dynamic calcium movement inside cardiac sarcoplasmic reticulum during release. Circ Res. 2011;108:847–856. doi: 10.1161/CIRCRESAHA.111.240234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cheng H, Lederer MR, Lederer WJ, Cannell MB. Calcium sparks and [Ca2+]i waves in cardiac myocytes. Am J Physiol. 1996;270:C148–C159. doi: 10.1152/ajpcell.1996.270.1.C148. [DOI] [PubMed] [Google Scholar]
- 42.DelPrincipe F, Egger M, Niggli E. Calcium signalling in cardiac muscle: refractoriness revealed by coherent activation. Nat Cell Biol. 1999;1:323–329. doi: 10.1038/14013. [DOI] [PubMed] [Google Scholar]
- 43.Szentesi P, Pignier C, Egger M, Kranias EG, Niggli E. Sarcoplasmic Reticulum Ca2+ Refilling Controls Recovery From Ca2+–Induced Ca2+ Release Refractoriness in Heart Muscle. Circ Res. 2004;95:807–813. doi: 10.1161/01.RES.0000146029.80463.7d. [DOI] [PubMed] [Google Scholar]
- 44.Kornyeyev D, Petrosky AD, Zepeda B, Ferreiro M, Knollmann B, Escobar AL. Calsequestrin 2 deletion shortens the refractoriness of Ca2+ release and reduces rate-dependent Ca2+-alternans in intact mouse hearts. J Mol Cell Cardiol. 2012;52:21–31. doi: 10.1016/j.yjmcc.2011.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Belevych AE, Terentyev D, Terentyeva R, Ho H-T, Gyorke I, Bonilla IM, et al. Shortened Ca2+ signaling refractoriness underlies cellular arrhythmogenesis in a postinfarction model of sudden cardiac death. Circ Res. 2012;110:569–577. doi: 10.1161/CIRCRESAHA.111.260455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wasserstrom JA, Shiferaw Y, Chen W, Ramakrishna S, Patel H, Kelly JE, et al. Variability in timing of spontaneous calcium release in the intact rat heart is determined by the time course of sarcoplasmic reticulum calcium load. Circ Res. 2010;107:1117–1126. doi: 10.1161/CIRCRESAHA.110.229294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Maltsev AV, Maltsev VA, Mikheev M, Maltseva LA, Sirenko SG, Lakatta EG, et al. Synchronization of stochastic Ca2+ release units creates a rhythmic Ca2+ clock in cardiac pacemaker cells. Biophys J. 2011;100:271–283. doi: 10.1016/j.bpj.2010.11.081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ramay HR, Liu OZ, Sobie EA. Recovery of cardiac calcium release is controlled by sarcoplasmic reticulum refilling and ryanodine receptor sensitivity. Cardiovasc Res. 2011;91:598–605. doi: 10.1093/cvr/cvr143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rovetti R, Cui X, Garfinkel A, Weiss JN, Qu Z. Spark-induced sparks as a mechanism of intracellular calcium alternans in cardiac myocytes. Circ Res. 2010;106:1582–1591. doi: 10.1161/CIRCRESAHA.109.213975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Jiang D, Chen W, Wang R, Zhang L, Chen SRW. Loss of luminal Ca2+ activation in the cardiac ryanodine receptor is associated with ventricular fibrillation and sudden death. Proc Natl Acad Sci U S A. 2007;104:18309–18314. doi: 10.1073/pnas.0706573104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Priori SG, Chen SRW. Inherited dysfunction of sarcoplasmic reticulum Ca2+ handling and arrhythmogenesis. Circ Res. 2011;108:871–883. doi: 10.1161/CIRCRESAHA.110.226845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Györke I, Hester N, Jones LR, Györke S. The role of calsequestrin, triadin, and junctin in conferring cardiac ryanodine receptor responsiveness to luminal calcium. Biophys J. 2004;86:2121–2128. doi: 10.1016/S0006-3495(04)74271-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Qin J, Valle G, Nani A, Chen H, Ramos-Franco J, Nori A, et al. Ryanodine receptor luminal Ca2+ regulation: swapping calsequestrin and channel isoforms. Biophys J. 2009;97:1961–1970. doi: 10.1016/j.bpj.2009.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Beard NA, Casarotto MG, Wei L, Varsányi M, Laver DR, Dulhunty AF. Regulation of ryanodine receptors by calsequestrin: effect of high luminal Ca2+ and phosphorylation. Biophys J. 2005;88:3444–3454. doi: 10.1529/biophysj.104.051441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Knollmann BC, Chopra N, Hlaing T, Akin B, Yang T, Ettensohn K, et al. Casq2 deletion causes sarcoplasmic reticulum volume increase, premature Ca2+ release, and catecholaminergic polymorphic ventricular tachycardia. J Clin Invest. 2006;116:2510–2520. doi: 10.1172/JCI29128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Chopra N, Yang T, Asghari P, Moore ED, Huke S, Akin B, et al. Ablation of triadin causes loss of cardiac Ca2+ release units, impaired excitation-contraction coupling, and cardiac arrhythmias. Proc Natl Acad Sci U S A. 2009;106:7636–7641. doi: 10.1073/pnas.0902919106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Terentyev D, Cala SE, Houle TD, Viatchenko-Karpinski S, Gyorke I, Terentyeva R, et al. Triadin overexpression stimulates excitation–contraction coupling and increases predisposition to cellular arrhythmia in cardiac myocytes. Circ Res. 2005;96:651–658. doi: 10.1161/01.RES.0000160609.98948.25. [DOI] [PubMed] [Google Scholar]
- 58.Terentyev D, Viatchenko-Karpinski S, Vedamoorthyrao S, Oduru S, Györke I, Williams SC, et al. Protein protein interactions between triadin and calsequestrin are involved in modulation of sarcoplasmic reticulum calcium release in cardiac myocytes. J Physiol. 2007;583:71–80. doi: 10.1113/jphysiol.2007.136879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Altschafl BA, Arvanitis DA, Fuentes O, Yuan Q, Kranias EG, Valdivia HH. Dual role of junctin in the regulation of ryanodine receptors and calcium release in cardiac ventricular myocytes. J Physiol. 2011;589:6063–6080. doi: 10.1113/jphysiol.2011.215988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Pritchard TJ, Kranias EG. Junctin and the histidine-rich Ca2+ binding protein: potential roles in heart failure and arrhythmogenesis. J Physiol. 2009;587:3125–3133. doi: 10.1113/jphysiol.2009.172171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kubalova Z, Györke I, Terentyeva R, Viatchenko-Karpinski S, Terentyev D, Williams SC, et al. Modulation of cytosolic and intra-sarcoplasmic reticulum calcium waves by calsequestrin in rat cardiac myocytes. J Physiol. 2004;561:515–524. doi: 10.1113/jphysiol.2004.073940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Venetucci LA, Trafford AW, O'Neill SC, Eisner DA. The sarcoplasmic reticulum and arrhythmogenic calcium release. Cardiovasc Res. 2008;77:285–292. doi: 10.1093/cvr/cvm009. [DOI] [PubMed] [Google Scholar]
- 63.Kubalova Z, Terentyev D, Viatchenko-Karpinski S, Nishijima Y, Györke I, Terentyeva R, et al. Abnormal intrastore calcium signaling in chronic heart failure. Proc Natl Acad Sci U S A. 2005;102:14104–14109. doi: 10.1073/pnas.0504298102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Curran J, Brown KH, Santiago DJ, Pogwizd S, Bers DM, Shannon TR. Spontaneous Ca waves in ventricular myocytes from failing hearts depend on Ca2+-calmodulin-dependent protein kinase II. J Mol Cell Cardiol. 2010;49:25–32. doi: 10.1016/j.yjmcc.2010.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Belevych AE, Terentyev D, Terentyeva R, Nishijima Y, Sridhar A, Hamlin RL, et al. The relationship between arrhythmogenesis and impaired contractility in heart failure: role of altered ryanodine receptor function. Cardiovasc Res. 2011;90:493–502. doi: 10.1093/cvr/cvr025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Cerrone M, Colombi B, Santoro M, di Barletta MR, Scelsi M, Villani L, et al. Bidirectional ventricular tachycardia and fibrillation elicited in a knock-in mouse model carrier of a mutation in the cardiac ryanodine receptor. Circ Res. 2005;96:e77–e82. doi: 10.1161/01.RES.0000169067.51055.72. [DOI] [PubMed] [Google Scholar]
- 67.Roux-Buisson N, Cacheux M, Fourest-Lieuvin A, Fauconnier J, Brocard J, Denjoy I, et al. Absence of triadin, a protein of the calcium release complex, is responsible for cardiac arrhythmia with sudden death in human. Hum Mol Genet. 2012;21:2759–2767. doi: 10.1093/hmg/dds104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Györke S. Molecular basis of catecholaminergic polymorphic ventricular tachycardia. Heart Rhythm. 2009;6:123–129. doi: 10.1016/j.hrthm.2008.09.013. [DOI] [PubMed] [Google Scholar]
- 69.Knollmann BC. New roles of calsequestrin and triadin in cardiac muscle. J Physiol. 2009;587:3081–3087. doi: 10.1113/jphysiol.2009.172098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Laurita KR, Rosenbaum DS. Mechanisms and potential therapeutic targets for ventricular arrhythmias associated with impaired cardiac calcium cycling. J Mol Cell Cardiol. 2008;44:31–43. doi: 10.1016/j.yjmcc.2007.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Radwański PB, Veeraraghavan R, Poelzing S. Cytosolic calcium accumulation and delayed repolarization associated with ventricular arrhythmias in a guinea pig model of Andersen–Tawil syndrome. Heart Rhythm. 2010;7:1428–1435. doi: 10.1016/j.hrthm.2010.03.044. [DOI] [PubMed] [Google Scholar]
- 72.Radwański PB, Poelzing S. NCX is an important determinant for premature ventricular activity in a drug-induced model of Andersen–Tawil syndrome. Cardiovasc Res. 2011;92:57–66. doi: 10.1093/cvr/cvr180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Terentyev D, Györke I, Belevych AE, Terentyeva R, Sridhar A, Nishijima Y, et al. Redox modification of ryanodine receptors contributes to sarcoplasmic reticulum Ca2+ leak in chronic heart failure. Circ Res. 2008;103:1466–1472. doi: 10.1161/CIRCRESAHA.108.184457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Liu OZ, Lederer WJ, Sobie EA. Does the Goldilocks principle apply to calcium release restitution in heart cells? J Mol Cell Cardiol. 2012;52:3–6. doi: 10.1016/j.yjmcc.2011.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Bovo E, Lipsius SL, Zima AV. Reactive oxygen species contribute to the development of arrhythmogenic Ca2+ waves during β-adrenergic receptor stimulation in rabbit cardiomyocytes. J Physiol. 2012;590:3291–3304. doi: 10.1113/jphysiol.2012.230748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Qu Z, Weiss JN. Dynamics and cardiac arrhythmias. J Cardiovasc Electrophysiol. 2006;17:1042–1049. doi: 10.1111/j.1540-8167.2006.00567.x. [DOI] [PubMed] [Google Scholar]