Abstract
Rationale
Diastolic spontaneous Ca2+ waves (DCWs) are recognized as important contributors to triggered arrhythmias. DCWs are thought to arise when [Ca2+] in sarcoplasmic reticulum ([Ca2+]SR) reaches a certain threshold level, which might be reduced in cardiac disease as a consequence of sensitization of ryanodine receptors (RyR2s) to luminal Ca2+.
Objective
We investigated the mechanisms of DCW generation in myocytes from normal and diseased hearts, using a canine model of post–myocardial infarction ventricular fibrillation (VF).
Methods and Results
The frequency of DCWs, recorded during periodic pacing in the presence of a β-adrenergic receptor agonist isoproterenol, was significantly higher in VF myocytes than in normal controls. Rather than occurring immediately on reaching a final [Ca2+]SR, DCWs arose with a distinct time delay after attaining steady [Ca2+]SR in both experimental groups. Although the rate of [Ca2+]SR recovery after the SR Ca2+ release was similar between the groups, in VF myocytes the latency to DCWs was shorter, and the [Ca2+]SR at DCW initiation was lower. The restitution of depolarization-induced Ca2+ transients, assessed by a 2-pulse protocol, was significantly faster in VF myocytes than in controls. The VF-related alterations in myocyte Ca2+ cycling were mimicked by the RyR2 agonist, caffeine. The reducing agent, mercaptopropionylglycine, or the CaMKII inhibitor, KN93, decreased DCW frequency and normalized restitution of Ca2+ release in VF myocytes.
Conclusions
The attainment of a certain threshold [Ca2+]SR is not sufficient for the generation of DCWs. Postrelease Ca2+ signaling refractoriness critically influences the occurrence of DCWs. Shortened Ca2+ signaling refractoriness due to RyR2 phosphorylation and oxidation is responsible for the increased rate of DCWs observed in VF myocytes and could provide a substrate for synchronization of arrhythmogenic events at the tissue level in hearts prone to VF.
Keywords: excitation-contraction coupling, ryanodine receptor, Ca2+ waves, arrhythmia, refractoriness
Sudden cardiac death resulting from ventricular tachyarrhythmias (ventricular tachycardia or ventricular fibrillation [VF]) remains the leading cause of mortality accounting for between 250 000 and 300 000 deaths in the United States each year.1 It has been established that a large proportion of sudden cardiac death occurs after myocardial infarction (MI).2 Abnormal Ca2+ handling has been implicated in a broad range of cardiac arrhythmias, including post-MI malignant ventricular arrhythmias.3–5 However, the specific mechanisms contributing to these arrhythmias have not been fully elucidated.
In the beating heart, most of the Ca2+ required for cardiac contractile activation is released from the sarcoplasmic reticulum (SR) via ryanodine receptor (RyR2) channels in response to Ca2+ entry during the systolic action potential (AP). After activation, SR Ca2+ release is terminated, due at least in part to an inhibitory effect of reduced SR [Ca2+] on the RyR2 channels, that is, store-dependent deactivation.6 This mechanism also maintains refractoriness of Ca2+ signaling during the diastolic period,7–9 allowing the Ca2+ released into the cytosol to be effectively resequestered by the SR to be liberated again during the next cardiac cycle.
In disease settings, SR Ca2+ release can occur spontaneously rather than being triggered by the systolic action potential. Spontaneous Ca2+ releases, which occur in the form of diastolic self-propagating Ca2+ waves (DCWs), contribute to cardiac arrhythmogenesis by eliciting phase 4 oscillations of membrane potential known as delayed after-depolarizations (DADs) and extrasystolic action potentials leading to triggered activity. Changes in the SR Ca2+ content and/or sensitivity of RyR2s to luminal Ca2+ have been recognized as important factors in the generation of DCWs.10 It has been suggested that spontaneous Ca2+ release arises whenever [Ca2+]SR reaches a certain threshold level10–12 and that this threshold is reduced in cardiac disease as a consequence of either genetic mutations in the RyR2 channel complex [ie, catecholaminergic polymorphic ventricular tachycardia (CPVT) caused by mutations in RyR2 or calsequestrin (CSQ2)] or acquired modifications in RyR2, leading to enhanced activation of RyR2s by luminal Ca2+.6,13 However, nearly all data supporting this view have been obtained either by steady-state recordings from RyR2 channels or in resting (ie, nonstimulated) cells, and therefore provide little information about spontaneous Ca2+ release during physiologically relevant Ca2+ cycling.
The goal of the present study was to investigate the molecular mechanisms of cellular arrhythmogenesis during physiologically relevant pacing using a well-established canine model of post-MI VF and sudden cardiac death.14 To this end, we performed simultaneous measurements of cytosolic and luminal Ca2+ changes during pacing-induced Ca2+ cycling in myocytes isolated from normal and diseased hearts. Additionally, we investigated the role of modifications of RyR2s by phosphorylation and oxidation in arrhythmogenesis in myocytes from heart in which VF was inducible. We found that spontaneous Ca2+ release was indeed influenced by levels of intra-SR Ca2+. However, rather than occurring once a final [Ca2+]SR level had been reached, DCWs arose with a distinct time delay (latency) that was significantly shortened in cells from diseased hearts when compared with myocytes from control hearts. The shortened latency in VF myocytes is attributable to the modification of RyR2s by CaMKII-phosphorylation and oxidation.
Methods
Model Description
The principles governing the care and use of animals, as expressed in the Guide for the Care and Use of Laboratory Animals published by the US National Institute of Health (NIH publication No. 85–323, revised 1996) and as adopted by the American Physiological Society, were followed at all times during this study. In addition, the Ohio State University Institutional Animal Care and Use Committee approved all animal procedures used in this study.
The surgical procedures, and the arrhythmia risk stratification test (exercise plus ischemia) used to classify the dogs as to susceptibility to VF have been previously described.14,15 Briefly, heartworm-free mixed breed dogs (male/female, 2–3 years of age) were anesthetized and a myocardial infarction was induced via 2-stage occlusion of the left anterior descending coronary artery. The ligation of this vessel produced an anterolateral infarction (≈17% of left ventricular mass),14 from the region between the papillary muscle and the apex. At the time of the surgery, a pulsed Doppler flow transducer and a vascular occluder were placed around the left circumflex coronary artery. After a 3- to 4-week recovery period, susceptibility to VF was assessed using a standardized exercise plus ischemia test.14,15 This exercise plus ischemia test has been shown to induce VF reproducibly (up to at least 5 months) in those animals exhibiting an initial positive test result (≈60% of the total post-MI animals).14 The animals with a positive test were promptly resuscitated and at least 1 week later, the animals were euthanized and the heart removed for preparation of ventricular myocytes (see below). Studies were only performed on the susceptible (ie, VF+, n=18) dogs. Myocytes were also obtained from control (ie, noninfarcted) dogs (n=14).
Ca2+ Imaging and Electrophysiology
Myocytes were isolated from the left lateral ventricular midmyocardium as previously described.16 Whole-cell patch clamp recordings of AP were performed with an Axopatch 200B amplifier (Molecular Devices, Sunnyvale, CA) using the external solution (mmol/L): 140 NaCl, 5.4 KCl, 2.0 CaCl2, 0.5 MgCl2, 10 HEPES, and 5.6 glucose (pH 7.4). Patch pipettes were filled with the following solution (mmol/L): 90 K-aspartate, 50 KCl, 5 MgATP, 5 NaCl, 1 MgCl2, 0.1Tris GTP, 10 HEPES; pH 7.2. Potassium salts of the following Ca2+indicators were added to a pipette solution to monitor cytosolic Ca2+: Fluo-3 (0.06 mmol/L) or Rhod-2 (0.1 mmol/L) or Fluo-4FF (0.2 mmol/L) (Life Technologies, Grand Island, NY) Intracellular Ca2+ imaging was performed using an Olympus Fluoview 1000 and a Nikon A1R confocal microscopes. Free intra-SR Ca2+ levels were measured by loading myocytes with 10 μmol/L Fluo-5N AM (Life Technologies, Grand Island, NY) for 3 to 3.5 hours at 37°C. The SR Ca2+ levels and intra-SR Ca2+ dynamics can be significantly affected by alterations in the SR volume and/or changes in the level of CSQ2, a major intra-SR Ca2+ buffer. We assessed potential changes in the SR spatial organization by comparing fluorescence recovery after photobleaching (FRAP) of SR-entrapped fluo-5N in control and VF myocytes.17 As shown in Online Figure I, fluo-5N FRAP rate was not different in control and VF myocytes suggesting that no significant alterations in the SR spatial organization occurred in VF. We also measured the levels of CSQ2 in control and VF. As shown in Online Figure II, the level of CSQ2 was not significantly altered in VF. To monitor cytosolic Ca2+ in field-stimulation experiments, myocytes were loaded with 9 μmol/L Rhod-2 AM for 25 minutes at room temperature; 30–60 minutes was allowed for deesterification.
Reagents
All reagents were from Sigma-Aldrich, Inc (St. Louis, MO), unless otherwise indicated.
Analysis
Results are presented as mean±SEM. Statistical significance was evaluated either by the appropriate Student t test or by 1-way ANOVA with Tukey post hoc test where appropriate. A probability value of <0.05 was considered significant.
Results
Frequency of DCWs and DADs Are Increased in VF Myocytes
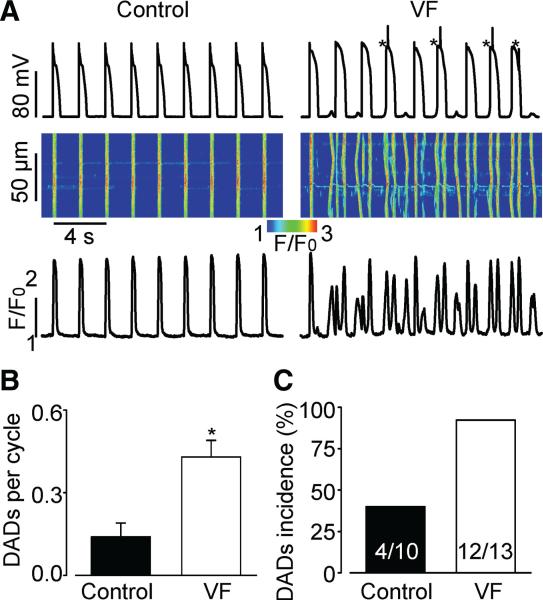
We examined the propensity toward proarrhythmic Ca2+ waves and DADs in a model of postinfarction sudden cardiac death (VF). As we previously reported, VF hearts in this model are characterized by abnormally high RyR2 activity but normal SERCA and NCX function.18 First, we performed recordings of cytosolic Ca2+ and membrane potential in paced (at 0.5 Hz) VF and control myocytes in the presence of the β-adrenergic receptor agonist isoproterenol (100 nmol/L). As shown in Figure 1A, paced VF myocytes exhibited frequent DCWs and corresponding DADs. On average, the frequency of occurrence of DADs in VF myocytes was 3-times higher than in control myocytes (Figure 1B). Additionally, DADs occurred in nearly all VF myocytes examined but only in 40% of control cells (Figure 1C).
Figure 1. Frequency of spontaneous diastolic Ca2+ waves (DCWs) and delayed afterdepolarizations (DADs) are increased in VF myocytes.
A, Representative recordings of membrane potential with corresponding line-scan images and temporal profiles of Rhod-2 fluorescence recorded in control and VF myocytes stimulated at 0.5 Hz in the presence of 100 nmol/L isoproterenol, a β-adrenergic receptor agonist. Asterisks mark action potentials triggered by DADs. B, Average frequency of DADs recorded in VF myocytes (0.43±0.06 per cycle, n=13) was significantly higher when compared with controls (0.14±0.05 per cycle, n=10, P<0.05). C, Number of cells displaying DCWs and DADs is higher in VF than in control.
Attainment of a Certain [Ca2+SR Is Insufficient for Diastolic Release
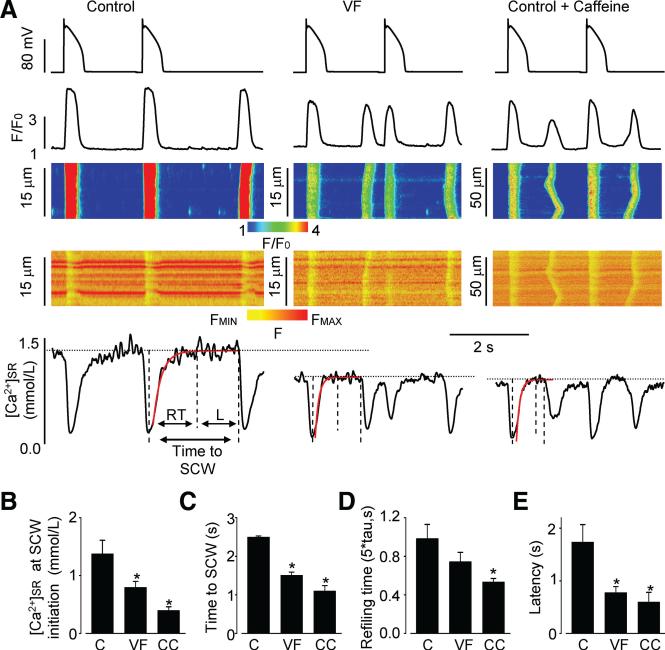
It is generally assumed that Ca2+ waves arise when [Ca2+] inside the SR attains a certain critical level or “threshold.”10–12 To test whether DCWs observed in our myocyte experiments were associated with luminal Ca2+ reaching a critical level, we recorded intra-SR Ca2+ levels during DCW generation using the low-affinity Ca2+ indicator fluo-5N entrapped in the SR in VF versus control myocytes. We standardized the conditions for examining DCW generation by using a Ca2+ loading protocol composed of a train of standard AP-clamp pulses. Using this protocol, DCWs occurred consistently after stimulation in both VF and control myocytes, although in VF myocytes DCWs arose with a significantly shorter time delay after termination of stimulation (Figure 2A and 2C). As indicated by the intra-SR Fluo-5N fluorescence signal, myocytes indeed exhibited a certain critical [Ca2+]SR level at which DCWs arose and this [Ca2+]SR was significantly lower in VF myocytes than in control cells (Figure 2A and 2B). Notably, in neither control nor VF myocytes did DCWs occur as soon as [Ca2+]SR recovered to its final baseline level. Instead there was a distinct delay or latency between [Ca2+]SR reaching the baseline and the onset of DCW. After a Ca2+ wave, baseline [Ca2+]SR declined as expected, 19 because some of the Ca2+ that forms the wave is removed from the cell by NCX, thus depleting the SR.
Figure 2. Susceptibility of VF myocytes to DCWs is associated with decreased [Ca2+]SR and shortened time interval between SR Ca2+ depletion and DCW initiation.
A, Action potential clamp with corresponding line-scan images and temporal profiles of Rhod-2 and Fluo-5N fluorescence recorded in myocytes from control, VF, and control treated with caffeine (0.4 mmol/L) groups. Cells were stimulated at 0.5 Hz, using a control AP waveform (upper panels). Time interval between SR Ca2+ depletion and DCW initiation can be divided into 2 periods marked by dashed vertical lines: refilling time (RT) and latency period (L, [Ca2+]SR remains constant). Post-depletion SR Ca2+ recovery was fit to exponential functions (red traces) with time constants of 256, 84, and 85 ms for control, VF, and control plus caffeine myocytes, respectively. RT was calculated as five times the fitted exponential time constant to correspond to >99% recovery. Dotted horizontal lines indicate the steady-state SR Ca2+ level at which DCWs occur in each experimental group. Bar graphs demonstrate average data for SR Ca2+ levels at initiation of DCWs (B), time after systolic SR Ca2+ depletion and DCW initiation (C), SR refilling time (D), and latency period (E) that were recorded in control (C, n=6–7), VF (n=10–11), and control plus caffeine (CC, n=7–8) groups. *P<0.05 versus control.
The shortened time to DCW could be due to either a faster refilling of the SR Ca2+ store and/or to shortening of the latency period at an already steady-state [Ca2+]SR in VF myocytes compared with controls. As shown in Figure 2, the average SR Ca2+ refilling time was not significantly different between control and VF myocytes (Figure 2D). In contrast, the latency period (at steady [Ca2+]SR) was significantly shorter in VF myocytes with respect to control (Figure 2E).
The functional state of the Ca2+ release channels and the probability of spontaneous Ca2+ release are known to depend on the SR Ca2+ content.6 To assess the role of different baseline SR Ca2+ levels in determining the probability and timing of DCWs in VF vs. control myocytes, we varied [Ca2+]SR in control myocytes by varying the Ca2+ loading protocol. As shown in Online Figure III, lowering SR Ca2+ in control myocytes towards the levels observed in VF cells led to an increase in latency and to a decrease in the incidence of DCWs. Therefore, the fact that the time delay to DCWs is shorter in VF myocytes despite the reduced diastolic SR Ca2+ level suggests impairment of the ability of depleted diastolic [Ca2+]SR to deactivate RyR2s in VF myocytes.
Collectively, these results show for the first time that spontaneous Ca2+ waves do not arise as a result of luminal Ca2+ activating RyR2 at a certain critical [Ca2+]SR threshold as is commonly assumed. Rather these data suggest that DCWs are associated with the failure of RyR2s to remain closed or inactivated/refractory during diastole.
Caffeine Mimics the Impact of VF on Myocyte Ca2+ Handling
To assess further the potential role of altered RyR2 functional activity in the generation of DCWs, we examined the effects of the RyR agonist caffeine20 on DCW occurrence and on changes in both cytosolic and SR luminal Ca2+ in control myocytes. Experiments were performed in myocytes exposed to 0.4–1 mmol/L caffeine and 100 nmol/L isoproterenol using the same AP-clamp simulation protocols as in the experiments in control and VF myocytes (Figure 2). Under these conditions, caffeine significantly reduced the [Ca2+]SR at which DCWs occurred and shortened both the overall time delay and latency (at a constant [Ca2+]SR) to DCWs in control myocytes (Figure 2B and 2C) to values similar to the alterations observed in VF myocytes. Of note, in addition to shortening latency, caffeine also shortened the SR Ca2+ refilling rate by a small but significant degree (Figure 2D).
SR Ca2+ Release Refractoriness Is Shortened in VF Myocytes
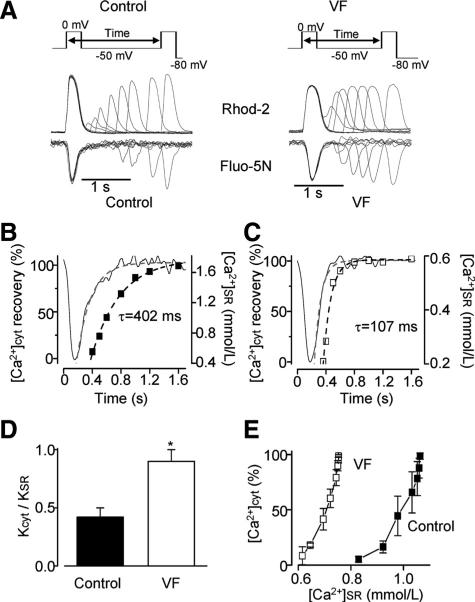
To test directly the hypothesis that the shortened latency to DCW in VF myocytes involves impaired diastolic refractoriness of RyR2s, we used a 2-pulse protocol as depicted in Figure 3 (upper panels). In these experiments, by varying the interpulse interval, we determined the rate of restitution of the cytosolic Ca2+ transient amplitude, while simultaneously measuring [Ca2+]SR recovery in VF versus control myocytes. Of note, recovery of the peak L-type Ca2+ current recorded using the 2-pulse protocol was not different between control and VF myocytes (Online Figure IV). Consistent with the diminished refractoriness hypothesis, the rate of restitution of cytosolic Ca2+ transients was significantly faster in VF myocytes than in control (Figure 3A through 3D). In addition, comparison of Ca2+ transient restitution rates with [Ca2+]SR recovery rates within the same myocyte groups revealed that while in control myocytes, Ca2+ transient recovery lagged substantially behind SR Ca2+ refilling, the rates of these 2 processes were similar in VF myocytes (Figure 3A–D). To gain further insight into the role of [Ca2+]SR in the different Ca2+ release restitution properties in VF and control cells we plotted the amplitude of Ca2+ transient as a function of [Ca2+]SR during recovery in the 2 myocyte groups (Figure 3E). The release-[Ca2+]SR dependency in VF myocyte is markedly shifted to lower [Ca2+]SR values with respect to control. This shows that while even relatively small Ca2+ store reductions are able to effectively restrain SR Ca2+ release in control myocytes, this control mechanism is impaired in VF myocytes, in which nearly normal SR Ca2+ release is preserved at much lower intra-SR Ca2+ levels. Collectively, these data suggest that the SR Ca2+ release refractoriness is significantly shortened in VF myocytes compared with controls. This decrease of diastolic refractoriness in VF myocytes is attributable to reduced ability of RyR2 to become deactivated on the decline of [Ca2+]SR that follows SR Ca2+ release.
Figure 3. Ca2+ signaling refractoriness is shortened in VF myocytes.
A, Upper panels show the voltage protocol used to measure recovery of the amplitude of cytosolic Ca2+ transient ([Ca2+]CYT) from SR Ca2+-dependent deactivation. Lower panels show representative recordings of Rhod-2 and Fluo-5N fluorescence during 2-pulse experiments. Time-course of [Ca2+]CYT (squares) and [Ca2+]SR (black line) recovery is shown for control (B) and VF (C) myocytes. Dashed lines represent exponential fits to the data. D, Rate of recovery of the amplitude of [Ca2+]CYT (KCYT) was normalized to the rate of recovery of [Ca2+]SR (KSR) for control (n=3) and VF (n=5) myocytes. E, [Ca2+]CYT was plotted as a function of [Ca2+]SR for control (n=3) and VF (n=5) myocytes during 2-pulse experiments described in A through C. *P<0.05 versus control.
Oxidation and Phosphorylation of RyR2s Contribute to Shortened Ca2+ Release Refractoriness
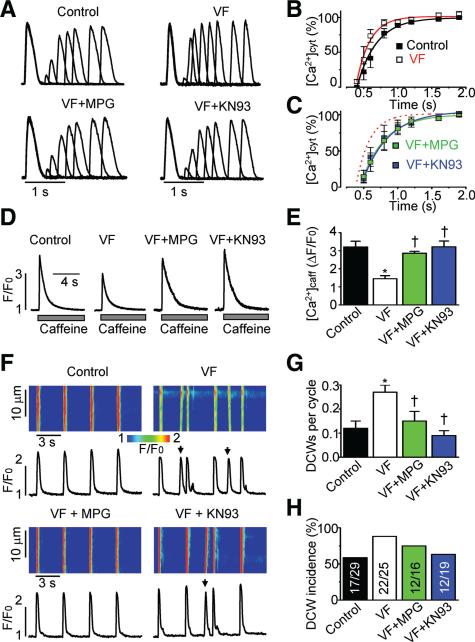
We previously demonstrated that cardiomyocytes in the VF model used here are characterized by increased reactive oxygen species, increased levels of RyR2 thiol oxidation and augmented redox-sensitive RyR-mediated SR Ca2+ leak.18 Therefore, we tested the effect of the reducing agent N-2-mercaptopropionyl glycine (MPG) on proarrhythmic alterations of Ca2+ handling. Figure 4 shows that treating VF myocytes with 0.75 mmol/L MPG significantly reduced the propensity for DCW generation and restored both the restitution of Ca2+ release and the SR Ca2+ content to control levels. These improvements of parameters of myocyte Ca2+ handling were associated with a significant increase in free thiol content and an improvement of functional activity of single RyR2 channels from VF hearts treated with MPG (Online Figure V).
Figure 4. Treatment with the reducing agent MPG or with the CaMKII inhibitor KN93 normalized recovery of cytosolic Ca2+ transient, restored the SR Ca2+ content toward control values, and significantly reduced the frequency of DCWs recorded in VF myocytes.
A, Representative recordings of cytosolic Ca2+ transients imaged using Fluo-4FF during 2-pulse experiments (shown in Figure 3) in voltage-clamped control and VF myocytes and VF myocytes treated with either 0.75 mmol/L MPG or 1 μmol/L KN93. B and C, Average amplitude of the cytosolic Ca2+ transients presented as a function of interpulse time. The recovery of the amplitude was fitted to a mono exponential function with time constants of 287±16 ms in control (n=7), 197±28 ms in VF untreated (n=3–4), 342±33 ms in VF myocytes treated with MPG (n=4–5), and 380±40 ms in VF treated with KN93 (n=4–5). D, Representative recordings of cytosolic Ca2+ transients induced by 10 mmol/L caffeine imaged with Fluo-4FF in voltage-clamped control and VF myocytes. E, average amplitude of caffeine-induced Ca2+ transients in control (n=8) and VF (n=8) myocytes, and VF myocytes treated with either 0.75 mmol/L MPG (n=6) or 1 μmol/L KN93 (n=4). F, Representative line-scan images and temporal profiles of Rhod-2 fluorescence recorded in control and VF myocytes, and in VF myocytes treated with either 0.75 mmol/L MPG, a reducing agent, or 1 μmol/L KN-93, a CaMKII inhibitor. Cells were field-stimulated at 0.3 Hz. G, Frequency of DCWs (marked with arrows in F) was calculated for control (n=29) and VF myocytes (n=25) and VF myocytes treated with either MPG (n=19) or KN-93 (n=16). H, Proportion of cells displaying DCWs in each experimental group. All data presented in this figure were obtained in the presence of 100 nmol/L isoproterenol, a β-adrenergic receptor agonist. *P<0.05 versus control; †P<0.05 versus VF untreated.
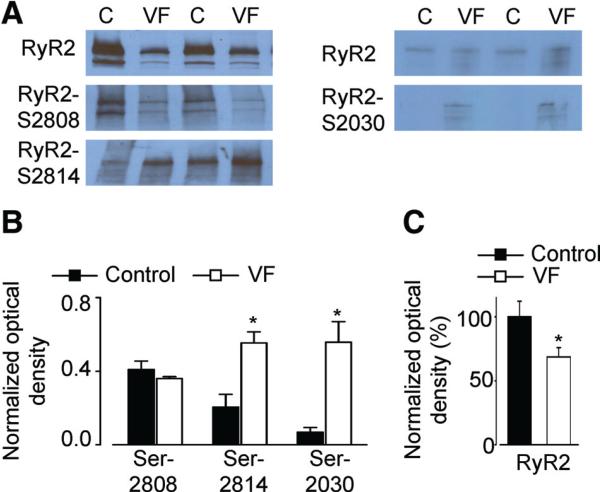
Increased phosphorylation of RyR2s by PKA or CaMKII has been implicated in increased susceptibility to cellular arrhythmias.6,21 Therefore, we also tested whether the phosphorylation of RyR2s was altered in VF myocytes. As illustrated in Figure 5, RyR2s from VF myocytes showed increased phosphorylation at Ser-2014, a CaMKII-dependent phosphorylation site, when compared with controls. Of the PKA-dependent phosphorylation sites Ser-2030 and Ser-2808,22,23 only the former showed a significant increase in VF hearts compared with control (Figure 5). The RyR2 protein appeared to be decreased by ≈30% in VF samples. In VF myocytes, CaMKII inhibition with 1 μmol/L KN-93 restored toward control values the kinetics of restitution of the cytosolic Ca2+ transient and the SR Ca2+ content, and significantly reduced the frequency of DCWs (Figure 4). Taken together, these data strongly support the critical role of excessive oxidation and CaMKII phosphorylation of RyR2s in altered refractoriness of SR Ca2+ release observed in VF myocytes.
Figure 5. Phosphorylation levels of RyR2 are altered in VF.
A, Representative Western blots showing phosphorylation of RyR2s at Ser-2030 and Ser-2808 (PKA-dependent) and Ser-2814 (CaMKII-dependent) phosphorylation sites in control and in VF myocytes measured with phospho-specific antibodies. B, Data pooled for Ser 2030 (n=5), Ser-2808 (n=7), and Ser-2814 (n=4) experiments, respectively. C, Average data show signifi-cant decrease in the amount of RyR2 in VF (n=8) compared with control (n=8). *P<0.05 versus control.
Discussion
DCWs and DADs are recognized as important contributors to the pathogenesis of triggered arrhythmias, including CPVT and post-MI VF.3,24 Although alterations in RyR2 regulation induced by both cytosolic and SR luminal Ca2+ have been implicated in arrhythmogenesis, the specific mechanisms responsible for Ca2+ wave generation and factors accounting for increased propensity toward DCWs in cardiac disease remain to be fully elucidated. In the present study, we used a canine model of post-MI tachyarrhythmia to show that susceptibility to DCWs is determined by alterations in the time- and Ca2+ store-dependent properties of RyR2s that contribute to the diastolic refractoriness of release required for normal myocyte Ca2+ cycling. The shortened RyR2 refractoriness is attributable to modifications of the channel protein by CaMKII phosphorylation and thiol oxidation. These findings provide new mechanistic insights into the control of SR Ca2+ release in the normal heart and illustrate how alterations in these control mechanisms lead to the arrhythmogenesis associated with acquired defects in the RyR2 channel.
Role of Shortened Ca2+ Release Refractoriness in Arrhythmogenesis
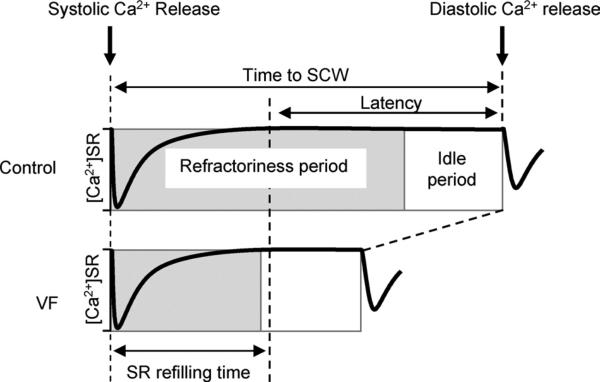
The current study examined the factors that account for the loss of dynamic stability of SR Ca2+ release in paced ventricular myocytes from hearts susceptible to malignant tachyarrhythmias. Our findings are schematically illustrated in Figure 6, showing the main components of the time period preceding DCWs after systolic Ca2+ release for control and VF myocytes. Based on the results of experiments with monitoring of [Ca2+]SR (Figure 2), the total time to DCW is comprised of a period of refilling of the SR Ca2+ store and a subsequent delay or latency period during which [Ca2+]SR remains constant. Whereas the SR Ca2+ refilling time was not significantly different between VF and control myocytes, the latency to DCWs at a constant [Ca2+]SR was significantly shortened in VF myocytes. The latency in the development of DCWs after reaching a steady-state [Ca2+]SR could result from RyR2s being refractory (RyR2s are completely or partially unresponsive) and/or may involve an idle period (during which RyR2 functional activity is completely recovered and Ca2+ waves can be initiated at any time by stochastic Ca2+ sparks). The duration of Ca2+-induced Ca2+ release (CICR) restitution measured in 2-pulse experiments (Figure 3) suggests that most of the latency to DCW in control myocytes can be attributed to RyR2 refractoriness. In VF myocytes, CICR restitution closely follows the SR Ca2+ refilling time. The relatively short idle periods after the functional recovery of RyR2s and before the onset of DCWs are similar between VF and control myocytes (Figure 6). The idle period is likely to reflect a delay associated with “spontaneous” activation of stochastic release sites recovered from refractoriness, ultimately leading to the generation of diastolic Ca2+ release.25
Figure 6. Schematic representation of the time delay between systolic SR Ca2+ depletion and DCW in control and VF myocytes.
The time delay between systolic SR Ca2+ depletion and DCW is comprised of a period of refilling of the SR Ca2+ store and a latency period during which [Ca2+]SR remains constant. The SR Ca2+ refilling time was not significantly different between control and VF myocytes, whereas latency was significantly shorter in VF myocytes (Figure 2). The refractory period reflects the time required for recovery of RyR2s from the SR Ca2+-dependent deactivation. In control myocytes, the refractory period determined in 2-pulse experiments was 2 times longer than the SR Ca2+ refilling time (Figure 3). In VF myocytes the refractory period was approximately equal to the SR Ca2+ refilling time (Figure 3). In both control and VF groups, the refractory period is followed by an idle period, during which stochastic activation of the recovered SR Ca2+ release sites triggers a DCW.
Although DCWs are considered to be the basis of triggered arrhythmias,3,24,26 the mechanism responsible for synchronization of Ca2+ waves and the ensuing membrane potential oscillations, between individual cardiac cells as required for the generation of ectopic action potentials remain to be elucidated. Our study demonstrated that DCWs in VF myocytes occur with a high degree of probability, reaching on average 0.5 per cycle (Figure 1), with the timing after systolic depletion tightly clustered around a median value of 1.5 seconds (standard deviation 0.3 seconds; Figure 2). Given their high probability and uniform timing, DCW rather than being spontaneous or random events seem to occur in a manner predetermined by refilling of the SR Ca2+ store and accelerated restitution of SR Ca2+ release. Thus, reduced refractoriness of Ca2+ signaling combined with uniform refilling of the SR after systolic SR Ca2+ release could provide a mechanism for temporal alignment of DCWs in multiple myocytes. We suggest that this synchronicity of abnormal Ca2+ release in multiple myocytes may be necessary for triggered activity and resulting tachyarrhythmias in VF hearts. This interpretation is consistent with recent studies by Wasserstrom et al,27 that showed that refilling of the SR Ca2+ store contributes to synchronization of Ca2+ waves and DADs in cardiac muscle during triggered activity induced by Ca2+ overload.
Mechanism of Ca2+ Release Refractoriness and Its Impairment in VF Myocytes
In addition to demonstrating the importance of refractoriness in preventing arrhythmogenic DCWs, our study provides insights into the mechanism of diminished refractoriness in VF myocytes that underlies the increased arrhythmogenic potential of myocytes from VF hearts. Previous studies from our and other laboratories have suggested that SR Ca2+ release is followed by a period of decreased store responsiveness (ie, refractoriness) due to lowered [Ca2+]SR that in turn, reduces RyR2 sensitivity to cytosolic Ca2+ (ie, store-mediated deactivation).6,8,28 In the present study, we showed that the luminal Ca2+-dependence of CICR during restitution is markedly shifted toward lower luminal Ca2+ concentrations, suggesting that the ability of reduced luminal Ca2+ to inhibit SR Ca2+ release is impaired in VF myocytes (Figure 3E). Furthermore, sensitization of RyR2s to cytosolic Ca2+, caused by low doses of caffeine,29,30 significantly reduced the latency to DCWs in normal cells in a manner similar to that observed in VF myocytes (Figure 2). These results support the notion that Ca2+ release refractoriness involves SR Ca2+ store-dependent changes in RyR2 responsiveness to cytosolic Ca2+ and, moreover, that this mechanism is altered in VF.
Impaired Ca2+ Release Refractoriness Versus Direct Activation by Luminal Ca2+ as Mechanisms for DCWs
It has been previously proposed that diastolic Ca2+ release is a result of luminal Ca2+ activating RyR2 directly at luminally accessible sites, a mechanism referred to as store-overload-induced SR Ca2+ release.11 One important premise of this mechanism is that diastolic Ca2+ release arises as soon as the SR Ca2+ content reaches a critical level, that is, “threshold.”11 In contrast to this expectation, our present study with direct monitoring of [Ca2+]SR in paced myocytes from VF and control hearts showed that DCWs do not arise immediately on [Ca2+]SR reaching a final “threshold” level but instead occur with a substantial time delay after the attainment of a baseline [Ca2+]SR (Figure 2). This delay or latency was significantly shorter in VF myocytes. In addition, increasing the sensitivity of RyR2s to cytosolic Ca2+ by low doses of caffeine,29,30 significantly shortened the latency to DCWs in control cells. These results demonstrate unequivocally that increased susceptibility to self-regenerating CICR, rather than direct activation of RyR2s by luminal Ca2+, underlies the occurrence of DCWs and the increased arrhythmogenic propensity. These results are consistent with previous reports that luminal Ca2+ rather than activating RyR2 directly, acts by allosterically influencing RyR2 sensitivity to cytosolic Ca2+.31,32
An important implication of these results is that given the slow restitution and long delay to DCWs, a substantial fraction of RyR2s would be expected to reside in a Ca2+-desensitized state (high level of steady-state refractoriness) thus preventing spontaneous Ca2+ waves in normal myocytes except at very slow stimulation rates. This possibility is supported by the results of theoretical studies that showed that simulation of the dynamic properties cardiac EC coupling required that most RyR2s reside in a refractory state during diastole.33 In MI and other disease settings, however, the ability of RyR2s to deactivate is diminished (a low level of steady-state refractoriness), thereby decreasing the latency to DCWs and leading to DCWs even at relatively fast pacing rates.
Roles of Phosphorylation and Redox Modification of RyR2s
Previous reports attributed proarrhythmic alterations in RyR2 function to either hyperphosphorylation by CAMKII or redox modifications of the channel protein in various disease settings.21,34,35 Our present study demonstrates that both of these mechanisms are involved in post-MI VF through affecting the refractory state of RyR2s. The vulnerability of RyR2 to oxidation is not surprising given the fact that it contains about 90 cysteines.36 Interestingly, targeting these mechanisms individually, by either an antioxidant treatment or CaMKII inhibition, led to nearly complete normalization of Ca2+ handling parameters including RyR2 refractoriness. Moreover the effect of combined antioxidant and CaMKII inhibition treatments in VF myocytes was not significantly different from that observed with either treatment alone (data not shown). Thus there was no additive effect of these interventions. One possible explanation for this result is that both types of modifications are required to induce an energetically unfavorable RyR2 conformation, such as “domain unzipping,”37 associated with the increased RyR2 functional activity in VF. Altered RyR2 interdomain interactions caused by phosphorylation and redox modification of the channel protein have been previously reported to contribute to the hyperactive RyR2 phenotype associated with cardiac disease.38
Relationship to Arrhythmogenesis in CPVT and Heart Failure
The mechanism of arrhythmogenesis in VF myocytes appears to be analogous to that described for CPVT linked to mutations in RyR2 and CASQ2.13 Indeed, in both disease settings, arrhythmogenesis is associated with altered luminal Ca2+ regulation of RyR2 and shortened refractoriness and is facilitated by β-adrenergic receptor stimulation.6,13,39 The proarrhythmic effects of isoproterenol in both CPVT and VF myocytes could be attributed to a combination of (1) phosphorylation of RyR2s by CaMKII that would further contribute to altered RyR2 function, rendering them more sensitized and less refractory; and (2), an enhanced SERCA-mediated SR Ca2+ uptake that leads also to facilitated restitution from store-dependent deactivation. Thus, genetic and acquired defects in the RyR2 complex appear to be involved in a range of arrhythmia disorders, which are associated with dysregulated SR Ca2+ release. The basic pathophysiology of these arrhythmias hinges on the loss of Ca2+ signaling stability, due to the failure of RyR2 channels to deactivate and maintain appropriate refractoriness.
Our present results are consistent with accelerated Ca2+ release refractoriness observed in a canine model of chronic heart failure,40 although Ca2+ transient restitution was slowed in a rat model of heart failure.41 One possible explanation for these different results is a better preserved SR Ca2+ content and more extensive RyR2 phosphorylation in the canine as opposed to the rat studies performed with and without isoproterenol, respectively.
Conclusions
In summary, our study provides a unifying mechanistic framework for understanding Ca2+ handling in both normal physiology and disease settings. In normal myocytes, store-mediated deactivation of release is required for the stabilization of CICR, a process that is intrinsically prone to self-regeneration. Impairment of this stabilizing mechanism results in decreased Ca2+ signaling refractoriness, and increased propensity of myocytes to arrhythmogenic diastolic Ca2+ release and DADs in myocytes from VF hearts. RyR2 luminal Ca2+ regulation appears to be a site of integration for various physiological and pathophysiological influences on RyR2s, including cytosolic Ca2+, phosphorylation, redox modification, and genetic mutations, and may present a common target for treatment of different forms of cardiac diseases associated with altered RyR2 function.
Supplementary Material
Novelty and Significance.
What Is Known?
In multiple pathologies associated with both genetic and acquired defects in the cardiac ryanodine receptor (RyR2) channel, arrhythmias result from aberrant Ca2+ release from the sarcoplasmic reticulum (SR) in the form of spontaneous diastolic Ca2+ waves.
Normal control of SR Ca2+ release involves Ca2+-dependent activation of RyR2s followed by their store-dependent deactivation rendering the RyR2s refractory during diastole.
Spontaneous diastolic Ca2+ waves are thought to arise when [Ca2+] in the SR ([Ca2+]SR) exceeds a critical threshold level thereby directly activating RyR2s; however, direct experimental conformation of this proposed mechanism is lacking.
What New Information Does This Article Contribute?
Intra-SR [Ca2+] during arrhythmogenic Ca2+ waves are recorded in cardiac myocytes isolated from post–myocardial infarction (MI) canine hearts prone to ventricular fibrillation (VF).
Ca2+ waves do not arise immediately on [Ca2+]SR reaching its final Ca2+ level, but rather occur with a distinct time delay that is markedly shorter in myocytes from post-MI hearts.
Increased predisposition toward Ca2+ waves in myocytes from post-MI hearts is due to diminished/shortened refractoriness of RyR2, caused by reduced ability of RyR2s to become deactivated by a decline in luminal Ca2+ after systolic SR Ca2+ release.
Impaired refractory behavior of RyR2s is attributable to posttranslational modification of the RyR2 protein by both Ca2+/calmodulin-dependent protein kinase (CaMKII)-dependent phosphorylation as well as oxidation.
Intracellular Ca2+ waves are known to play a key role in the pathophysiology of triggered ventricular arrhythmias. Although the SR Ca2+ load plays an important role in the genesis of Ca2+ waves, the specific mechanisms accounting for increased propensity toward Ca2+ waves in cardiac disease remain to be fully elucidated. In the present study, we monitored intra-SR Ca2+ levels during proarrhythmic Ca2+ waves in myocytes isolated from canine hearts with healed myocardial infarction prone to malignant arrhythmias. Our study demonstrated that the susceptibility for Ca2+ waves is determined by impairment of store-dependent deactivation of RyR2s, the mechanism that normally prevents SR Ca2+ release from occurring during the diastolic period. The shortened RyR2 refractoriness in post-MI myocytes is attributable to modifications of the channel protein by both CaMKII–dependent phosphorylation and thiol oxidation. Our findings provide a unifying conceptual framework for understanding Ca2+ handling in normal and diseased heart settings. Specifically, in normal myocytes, store-mediated deactivation of RyR2 stabilizes Ca2+-induced Ca2+ release that is intrinsically prone to self-regeneration. Impairment of this stabilizing mechanism in myocytes from diseased hearts results in decreased Ca2+ signaling refractoriness and increased propensity of myocytes for arrhythmogenic diastolic SR Ca2+ release.
Acknowledgements
We thank Jeanne Green for excellent technical assistance.
Sources of Funding
This work was supported by National Institutes of Health grants HL074045 and HL063043 (to S.G.), HL089836 (to C.A.C.), and HL068609 and HL086700 (to G.E.B.).
Non-standard Abbreviations and Acronyms
- AP
action potential
- CICR
Ca2+-induced Ca2+ release
- CPVT
catecholaminergic polymorphic ventricular tachycardia
- DAD
delayed afterdepolarization
- DCW
diastolic spontaneous Ca2+ wave
- MI
myocardial infarction
- ROS
reactive oxygen species
- RyR
ryanodine receptor
- SERCA
sarcoplasmic reticulum Ca2+ ATPase
- SR
sarcoplasmic reticulum
- VF
ventricular fibrillation
Footnotes
Disclosures
None.
References
- 1.Lloyd-Jones D, Adams RJ, Brown TM, Carnethon M, Dai S, De Simone G, Ferguson TB, Ford E, Furie K, Gillespie C, Go A, Greenlund K, Haase N, Hailpern S, Ho PM, Howard V, Kissela B, Kittner S, Lackland D, Lisabeth L, Marelli A, McDermott MM, Meigs J, Mozaffarian D, Mussolino M, Nichol G, Roger VL, Rosamond W, Sacco R, Sorlie P, Roger VL, Thom T, Wasserthiel-Smoller S, Wong ND, Wylie-Rosett J. Heart disease and stroke statistics: 2010 update: a report from the American Heart Association. Circulation. 2010;121:e46–e215. doi: 10.1161/CIRCULATIONAHA.109.192667. [DOI] [PubMed] [Google Scholar]
- 2.Bunch TJ, Hohnloser SH, Gersh BJ. Mechanisms of sudden cardiac death in myocardial infarction survivors: insights from the randomized trials of implantable cardioverter-defibrillators. Circulation. 2007;115:2451–2457. doi: 10.1161/CIRCULATIONAHA.106.683235. [DOI] [PubMed] [Google Scholar]
- 3.Ter Keurs HE, Boyden PA. Calcium and arrhythmogenesis. Physiol Rev. 2007;87:457–506. doi: 10.1152/physrev.00011.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Laurita KR, Rosenbaum DS. Mechanisms and potential therapeutic targets for ventricular arrhythmias associated with impaired cardiac calcium cycling. J Mol Cell Cardiol. 2008;44:31–43. doi: 10.1016/j.yjmcc.2007.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Weiss JN, Nivala M, Garfinkel A, Qu Z. Alternans and arrhythmias: from cell to heart. Circ Res. 2011;108:98–112. doi: 10.1161/CIRCRESAHA.110.223586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gyorke S, Terentyev D. Modulation of ryanodine receptor by luminal calcium and accessory proteins in health and cardiac disease. Cardiovasc Res. 2008;77:245–255. doi: 10.1093/cvr/cvm038. [DOI] [PubMed] [Google Scholar]
- 7.Terentyev D, Viatchenko-Karpinski S, Valdivia HH, Escobar AL, Gyorke S. Luminal Ca2+ controls termination and refractory behavior of Ca2+induced Ca2+ release in cardiac myocytes. Circ Res. 2002;91:414–420. doi: 10.1161/01.res.0000032490.04207.bd. [DOI] [PubMed] [Google Scholar]
- 8.Szentesi P, Pignier C, Egger M, Kranias EG, Niggli E. Sarcoplasmic reticulum Ca2+ refilling controls recovery from Ca2+-induced Ca2+ release refractoriness in heart muscle. Circ Res. 2004;95:807–813. doi: 10.1161/01.RES.0000146029.80463.7d. [DOI] [PubMed] [Google Scholar]
- 9.Sobie EA, Song LS, Lederer WJ. Local recovery of Ca2+ release in rat ventricular myocytes. J Physiol. 2005;565:441–447. doi: 10.1113/jphysiol.2005.086496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Venetucci LA, Trafford AW, O'Neill SC, Eisner DA. The sarcoplasmic reticulum and arrhythmogenic calcium release. Cardiovasc Res. 2008;77:285–292. doi: 10.1093/cvr/cvm009. [DOI] [PubMed] [Google Scholar]
- 11.Jiang D, Chen W, Wang R, Zhang L, Chen SR. Loss of luminal Ca2+ activation in the cardiac ryanodine receptor is associated with ventricular fibrillation and sudden death. Proc Natl Acad Sci U S A. 2007;104:18309–18314. doi: 10.1073/pnas.0706573104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stern MD, Capogrossi MC, Lakatta EG. Spontaneous calcium release from the sarcoplasmic reticulum in myocardial cells: mechanisms and consequences. Cell Calcium. 1988;9:247–256. doi: 10.1016/0143-4160(88)90005-x. [DOI] [PubMed] [Google Scholar]
- 13.Kashimura T, Briston SJ, Trafford AW, Napolitano C, Priori SG, Eisner DA, Venetucci LA. In the RyR2(R4496C) mouse model of CPVT, beta-adrenergic stimulation induces Ca waves by increasing SR Ca content and not by decreasing the threshold for Ca waves. Circ Res. 2010;107:1483–1489. doi: 10.1161/CIRCRESAHA.110.227744. [DOI] [PubMed] [Google Scholar]
- 14.Billman GE. A comprehensive review and analysis of 25 years of data from an in vivo canine model of sudden cardiac death: implications for future anti-arrhythmic drug development. Pharmacol Ther. 2006;111:808–835. doi: 10.1016/j.pharmthera.2006.01.002. [DOI] [PubMed] [Google Scholar]
- 15.Schwartz PJ, Billman GE, Stone HL. Autonomic mechanisms in ventricular fibrillation induced by myocardial ischemia during exercise in dogs with healed myocardial infarction: an experimental preparation for sudden cardiac death. Circulation. 1984;69:790–800. doi: 10.1161/01.cir.69.4.790. [DOI] [PubMed] [Google Scholar]
- 16.Sridhar A, Nishijima Y, Terentyev D, Terentyeva R, Uelmen R, Kukielka M, Bonilla IM, Robertson GA, Gyorke S, Billman GE, Carnes CA. Repolarization abnormalities and afterdepolarizations in a canine model of sudden cardiac death. Am J Physiol Regul Integr Comp Physiol. 2008;295:R1463–R1472. doi: 10.1152/ajpregu.90583.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wu X, Bers DM. Sarcoplasmic reticulum and nuclear envelope are one highly interconnected Ca2+ store throughout cardiac myocyte. Circ Res. 2006;99:283–291. doi: 10.1161/01.RES.0000233386.02708.72. [DOI] [PubMed] [Google Scholar]
- 18.Belevych AE, Terentyev D, Viatchenko-Karpinski S, Terentyeva R, Sridhar A, Nishijima Y, Wilson LD, Cardounel AJ, Laurita KR, Carnes CA, Billman GE, Gyorke S. Redox modification of ryanodine receptors underlies calcium alternans in a canine model of sudden cardiac death. Cardiovasc Res. 2009;84:387–395. doi: 10.1093/cvr/cvp246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Diaz ME, Trafford AW, O'Neill SC, Eisner DA. A measurable reduction of sr Ca content follows spontaneous Ca release in rat ventricular myocytes. Pflugers Arch. 1997;434:852–854. doi: 10.1007/s004240050475. [DOI] [PubMed] [Google Scholar]
- 20.Venetucci LA, Trafford AW, Eisner DA. Increasing ryanodine receptor open probability alone does not produce arrhythmogenic calcium waves: threshold sarcoplasmic reticulum calcium content is required. Circ Res. 2007;100:105–111. doi: 10.1161/01.RES.0000252828.17939.00. [DOI] [PubMed] [Google Scholar]
- 21.Xie LH, Chen F, Karagueuzian HS, Weiss JN. Oxidative stress-induced afterdepolarizations and calmodulin kinase II signaling. Circ Res. 2009;104:79–86. doi: 10.1161/CIRCRESAHA.108.183475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Benkusky NA, Weber CS, Scherman JA, Farrell EF, Hacker TA, John MC, Powers PA, Valdivia HH. Intact beta-adrenergic response and unmodified progression toward heart failure in mice with genetic ablation of a major protein kinase A phosphorylation site in the cardiac ryanodine receptor. Circ Res. 2007;101:819–829. doi: 10.1161/CIRCRESAHA.107.153007. [DOI] [PubMed] [Google Scholar]
- 23.Xiao B, Zhong G, Obayashi M, Yang D, Chen K, Walsh MP, Shimoni Y, Cheng H, Ter Keurs H, Chen SR. Ser-2030, but not Ser-2808, is the major phosphorylation site in cardiac ryanodine receptors responding to protein kinase A activation upon beta-adrenergic stimulation in normal and failing hearts. Biochem J. 2006;396:7–16. doi: 10.1042/BJ20060116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pogwizd SM, Bers DM. Cellular basis of triggered arrhythmias in heart failure. Trends Cardiovasc Med. 2004;14:61–66. doi: 10.1016/j.tcm.2003.12.002. [DOI] [PubMed] [Google Scholar]
- 25.Maltsev AV, Maltsev VA, Mikheev M, Maltseva LA, Sirenko SG, Lakatta EG, Stern MD. Synchronization of stochastic Ca2+ release units creates a rhythmic Ca2+ clock in cardiac pacemaker cells. Biophys J. 2011;100:271–283. doi: 10.1016/j.bpj.2010.11.081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fujiwara K, Tanaka H, Mani H, Nakagami T, Takamatsu T. Burst emergence of intracellular Ca2+ waves evokes arrhythmogenic oscillatory depolarization via the Na+-Ca2+ exchanger: simultaneous confocal recording of membrane potential and intracellular Ca2+ in the heart. Circ Res. 2008;103:509–518. doi: 10.1161/CIRCRESAHA.108.176677. [DOI] [PubMed] [Google Scholar]
- 27.Wasserstrom JA, Shiferaw Y, Chen W, Ramakrishna S, Patel H, Kelly JE, O'Toole MJ, Pappas A, Chirayil N, Bassi N, Akintilo L, Wu M, Arora R, Aistrup GL. Variability in timing of spontaneous calcium release in the intact rat heart is determined by the time course of sarcoplasmic reticulum calcium load. Circ Res. 2010;107:1117–1126. doi: 10.1161/CIRCRESAHA.110.229294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cheng H, Lederer WJ. Calcium sparks. Physiol Rev. 2008;88:1491–1545. doi: 10.1152/physrev.00030.2007. [DOI] [PubMed] [Google Scholar]
- 29.O'Neill SC, Eisner DA. A mechanism for the effects of caffeine on Ca2+ release during diastole and systole in isolated rat ventricular myocytes. J Physiol. 1990;430:519–536. doi: 10.1113/jphysiol.1990.sp018305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Porta M, Zima AV, Nani A, Diaz-Sylvester PL, Copello JA, Ramos-Franco J, Blatter LA, Fill M. Single ryanodine receptor channel basis of caffeine's action on Ca2+ sparks. Biophys J. 2011;100:931–938. doi: 10.1016/j.bpj.2011.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stevens SC, Terentyev D, Kalyanasundaram A, Periasamy M, Gyorke S. Intra-sarcoplasmic reticulum Ca2+ oscillations are driven by dynamic regulation of ryanodine receptor function by luminal Ca2+ in cardiomyocytes. J Physiol. 2009;587:4863–4872. doi: 10.1113/jphysiol.2009.175547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.MacQuaide N, Dempster J, Smith GL. Assessment of sarcoplasmic reticulum Ca2+ depletion during spontaneous Ca2+ waves in isolated permeabilized rabbit ventricular cardiomyocytes. Biophys J. 2009;96:2744–2754. doi: 10.1016/j.bpj.2008.12.3944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Huertas MA, Smith GD, Gyorke S. Ca2+ alternans in a cardiac myocyte model that uses moment equations to represent heterogeneous junctional SR Ca2+. Biophys J. 2010;99:377–387. doi: 10.1016/j.bpj.2010.04.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zima AV, Blatter LA. Redox regulation of cardiac calcium channels and transporters. Cardiovasc Res. 2006;71:310–321. doi: 10.1016/j.cardiores.2006.02.019. [DOI] [PubMed] [Google Scholar]
- 35.Currie S. Cardiac ryanodine receptor phosphorylation by CaM Kinase II: keeping the balance right. Front Biosci. 2009;14:5134–5156. doi: 10.2741/3591. [DOI] [PubMed] [Google Scholar]
- 36.Xu L, Eu JP, Meissner G, Stamler JS. Activation of the cardiac calcium release channel (ryanodine receptor) by poly-S-nitrosylation. Science. 1998;279:234–237. doi: 10.1126/science.279.5348.234. [DOI] [PubMed] [Google Scholar]
- 37.Ikemoto N, Yamamoto T. Regulation of calcium release by interdomain interaction within ryanodine receptors. Front Biosci. 2002;7:d671–d683. doi: 10.2741/A803. [DOI] [PubMed] [Google Scholar]
- 38.Mochizuki M, Yano M, Oda T, Tateishi H, Kobayashi S, Yamamoto T, Ikeda Y, Ohkusa T, Ikemoto N, Matsuzaki M. Scavenging free radicals by low-dose carvedilol prevents redox-dependent Ca2+ leak via stabilization of ryanodine receptor in heart failure. J Am Coll Cardiol. 2007;49:1722–1732. doi: 10.1016/j.jacc.2007.01.064. [DOI] [PubMed] [Google Scholar]
- 39.Kornyeyev D, Petrosky AD, Zepeda B, Ferreiro M, Knollmann B, Escobar AL. Calsequestrin 2 deletion shortens the refractoriness of Ca2+ release and reduces rate-dependent Ca2+-alternans in intact mouse hearts. J Mol Cell Cardiol. 2012;52:21–31. doi: 10.1016/j.yjmcc.2011.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Belevych AE, Terentyev D, Terentyeva R, Nishijima Y, Sridhar A, Hamlin RL, Carnes CA, Györke S. The relationship between arrhythmo-genesis and impaired contractility in heart failure: role of altered ryanodine receptor function. Cardiovasc Res. 2011;90:493–502. doi: 10.1093/cvr/cvr025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wasserstrom JA, Sharma R, Kapur S, Kelly JE, Kadish AH, Balke CW, Aistrup GL. Multiple defects in intracellular calcium cycling in whole failing rat heart. Circ Heart Fail. 2009;2:223–232. doi: 10.1161/CIRCHEARTFAILURE.108.811539. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.