Abstract
Background
Cocaine dependence is a major public health problem with no available robustly effective pharmacotherapy. This study’s aim was to determine if treatment with sertraline (SERT) or SERT plus gabapentin (GBP) improved treatment retention, depressive symptoms, and/or cocaine use.
Methods
Depressed cocaine-dependent patients (N = 99) were enrolled in a 12-week, double-blind, randomized, placebo (PLA)-controlled, clinical trial and placed in research beds at a residential treatment facility (Recovery Centers of Arkansas). They were randomized by depressive symptom severity and inducted onto 1 of the following while residing at the Recovery Centers of Arkansas: SERT (200 mg/d), SERT (200 mg/d) plus GBP (1200 mg/d), or PLA. Participants transferred to outpatient treatment at the start of their third week, continued receiving study medications or PLA (weeks 3–12), and participated in weekly individual cognitive behavioral therapy. Compliance was facilitated through the use of contingency management procedures. Supervised urine samples were obtained thrice weekly and self-reported mood weekly. At the end of 12 weeks, participants were tapered off the study medication over 5 days and referred to a local treatment program.
Results
Sertraline, but not SERT plus GBP, showed a significantly lower overall percentage of cocaine-positive urine samples compared with that of PLA. A significantly greater percentage of participants experienced relapse in the PLA group (88.9%) compared with that of the SERT group (65.2%). Hamilton depression ratings decreased significantly over time regardless of the treatment group. Retention in treatment did not differ significantly between the treatment groups.
Conclusions
Sertraline plus GBP may not be superior to SERT alone in delaying relapse among abstinent cocaine-dependent individuals undergoing cognitive behavioral therapy.
Keywords: cocaine dependence, randomized clinical trial, placebo control, sertraline, gabapentin, relapse prevention, cognitive behavioral therapy
Cocaine dependence is a major public health problem that is associated with serious medical,1 psychiatric,2 and social3 problems. Despite these compelling factors, no robustly effective pharmacotherapy for cocaine dependence has been developed. Depressive symptoms are common among cocaine-dependent patients4 and have been associated with greater severity of cocaine dependence and impairment5 as well as poor treatment outcome.6 Although most well-controlled trials have had disappointing results with antidepressants in unselected cocaine-dependent patients,7 antidepressants have shown some efficacy in treating depressed subgroups of cocaine-dependent patients8,9 (for exception10).
Dopaminergic11 and serotonergic12 deficits have been shown to occur after long-term cocaine administration, suggesting a neurobiological link to depression, because the dopaminergic system has also been implicated in depression.13 Consequently, administration of antidepressants that inhibit both dopamine and serotonin reuptake would theoretically modulate serotonergic and/or dopaminergic dysfunction associated with cocaine abuse and/or depression. In a recently published study, we were able to demonstrate that the selective serotonin reuptake inhibitor (SSRI) sertraline (SERT), which also blocks reuptake of dopamine,14 significantly delayed time to relapse in recently abstinent cocaine-dependent patients presenting with depressive symptoms.8 These findings suggest that SERT may have utility as a relapse prevention agent for cocaine dependence.
Nevertheless, response to SERT was not complete, with 53.1% of SERT-treated participants versus 70.3% of placebo (PLA)-treated participants having relapsed.8 Thus, the purpose of this study was to determine whether the prior results with SERT alone can be not only replicated, but also enhanced, with the addition of another agent to augment SERT’s effects. More recent evidence suggests that gamma-aminobutyric acid (GABA) function is also an important factor to be considered both in depression15 and cocaine dependence.16 Moreover, ample evidence suggests that cocaine interacts with the GABA system.17 Augmenting the efficacy of antidepressants with GABAergic agents has also been examined for the treatment of depression.18 Thus, we examined the clinical efficacy of the SSRI SERT,19 augmented with a low dose of the GABAergic agent gabapentin (GBP) that promotes release of GABA.20 As in our recently published SERT study,8 a relapse paradigm was used such that participants abstained from cocaine while residing at a residential facility during initial study medication induction and reinstatement of cocaine use was determined during subsequent outpatient treatment.
METHODS
Participants
One hundred fifty-eight male and female cocaine-dependent individuals seeking treatment of cocaine dependence were recruited from the greater Little Rock, Ark area from December of 2005 through April 2009 after giving informed consent to participate in this randomized clinical trial approved by the University of Arkansas for Medical Sciences Human Investigations Review Board. Participants had to meet the Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition (DSM-IV) criteria for cocaine dependence, as determined from the Structured Clinical Interview for DSM-IV, have results for urine toxicologic examination positive for cocaine or benzoylecgonine during the month before study entry, have a history of cocaine use of at least 1 g during the previous 3 months, and have a score greater than 15 on the Hamilton Depression Scale (HAM-D) at the screening visit. Exclusions included medical conditions contraindicating use of SERTor GBP (eg, pregnancy or breast-feeding, significant history of seizures, significant history of head trauma or serious neurological disorders, liver enzyme levels greater than 3 times the reference value, or medications that might have a major interaction with SERT or GBP [eg, use of monoamine oxidase inhibitor within 2 weeks before starting SERT]), current use of psychiatric medications, a current diagnosis of drug (nicotine excluded) or alcohol physiological dependence (ie, reported withdrawal symptoms upon abrupt cessation or, when dependence is suspected, inability to produce negative alcohol breath test results with no symptoms over at least 3 consecutive days), a history of major psychiatric disorder (psychosis, schizophrenia, or bipolar), ill health (eg, major cardiovascular, renal, endocrine, hepatic disorder), current suicidal tendency, and an inability to read and understand the consent form. Women of childbearing age were included provided they had a negative urine pregnancy test result, agreed to use adequate contraception to prevent pregnancy during the study, and agreed to monthly pregnancy tests.
Design and Procedure
Participants were initially evaluated to determine their eligibility to participate in this 12-week randomized, double-blind clinical trial through a 1-week, centralized recruiting/screening procedure. Eligible participants were then randomly assigned to receive SERT, SERT plus GBP, or PLA and admitted to research beds in the Recovery Centers of Arkansas during weeks 1 and 2. Then participants transferred to the Outpatient Treatment Research Unit at the University of Arkansas for Medical Sciences and continued to receive SERT, SERT plus GBP, or PLA during weeks 3 to 12. The data manager performed the randomization using a computerized urn randomization program,21 balancing groups on sex, race, and depressive symptom severity. Only the research pharmacist and the data manager were aware of the medication condition.
During weeks 1 and 2, participants attended the 2-week Recovery Centers of Arkansas Residential Treatment Program. During weeks 3 to 12, subjects participated in weekly 1-hour, manual-driven individual cognitive behavioral therapy.22 The session also provided an opportunity for subjects to review critical issues and problem areas. During weeks 3 to 12, participants attended the outpatient treatment research program at least 3 d/wk to complete study tasks, undergo counseling (weeks 3–12), and receive study medication. Compliance with study requirements during weeks 3 to 12 was facilitated through the use of contingency management procedures, whereby subjects were given monetary compensation for clinic attendance and for returning take-home blister packs. Because dropout during the residential stay was high (32.7%) with the first 67 subjects, monetary compensation was added for participation in the 2-week residential stay, resulting in lower dropout during the residential portion of the study (18.75%) for the remainder of the trial. Supervised urine samples, self-reported adverse effects, and vital signs were obtained thrice weekly; mood and drug use self-reports were obtained once weekly. At the end of 12 weeks, participants were tapered off the study medication and referred to an appropriate treatment program.
Because cocaine abstinence had to be initiated and maintained during weeks 1 and 2, subjects had to submit urine samples negative for cocaine by the beginning of week 2 and were administratively discharged from the study if a urinalysis result became positive for cocaine or other drugs during this time. Subjects were discharged from the trial if they missed attending clinic to receive their weekly medication, missed 3 consecutive supervised gathering of urine samples, or the investigator felt that subjects’ health or well-being was threatened by continuation in the study. Female participants were withdrawn from the study and referred to an appropriate treatment program if a pregnancy test result was positive. Subjects were not discontinued for illicit drug use during the outpatient portion of the trial unless the investigators felt a safety issue for the subject was at hand. Subjects administratively discharged from the study were offered referral to a local treatment program.
Medication
During SERT and GBP induction, subjects received either PLA or an initial dosage of 50 mg SERT hydrochloride once daily via blue opaque capsules starting the day after the admission to the residential unit (day 1 of week 1). This dosage was gradually increased over a 3-week period until subjects received 200 mg once daily.23 Although GBP has not been shown to reduce cocaine-positive urine samples relative to PLA in either non–opioid-dependent24 or methadone-stabilized cocaine-dependent patients,25 the dosage of GBP may have been too high, potentially due to intolerable adverse effects. Thus, we selected the 1200 mg/d dosage because it showed some efficacy relative to PLA during the up-titration of GBP in the trial of Gonzalez et al25 and has been associated with a decrease in subjective effects of self-administered cocaine.26 Participants assigned to receive GBP were given an initial dosage of 200 mg twice daily on days 1 to 5, 400 mg twice daily on days 6 to 10, and then 600 mg twice daily from day 11 on. Subjects were maintained on this dosage for the duration of the trial. If symptoms persisted, subjects’ participation was terminated. All study medications were administered using a double dummy procedure, such that all subjects, regardless of randomization, took the same number of capsules in identical packaging during the 12-week trial. When subjects were transferred to the outpatient program, they were administered capsules once weekly, with take-home dosages given in blister packs to take twice a day for the rest of the week. Subjects were required to return take-home blister packs when they came in to receive their next weekly dosage pack, for which they received monetary compensation. Compliance assessments were made based on client self-report and pill counts. At the end of the 12 weeks, participants were tapered off SERT, SERT and GBP, or PLA over a 5-day period.
Assessments
At intake, participants were interviewed using the Structured Clinical Interview for DSM-IV27 and the Addiction Severity Index, Fifth Edition.28 The clinician-rated version of the HAM-D29 was completed at screening, intake and weekly thereafter. After initial training by a physician investigator, experienced clinical research staff had to show 100% agreement on at least 3 corated interviews to administer the scale and participated in quarterly reviews to prevent drift. Supervised urine samples were obtained thrice weekly and tested for the presence of cocaine metabolite (benzoylecgonine) and other drugs using an Olympus AU640 Emit system (Olympus America Inc, Melville, NY) with a cutoff concentration of 300 ng/mL. Self-report assessments of cocaine (eg, days during which cocaine was used, dimes of cocaine used with a dime roughly equivalent to $10 street worth, or 100 mg of cocaine or crack cocaine) and other drug use were obtained on day 1 of each week using analog scales and 7-day recall method instruments developed in a previous study.30 Participants were monitored at every visit for any adverse symptoms.
Data Analyses
Because participants had to demonstrate 2 weeks of abstinence to continue in the study, and cocaine use, determined by urine toxicology screens, beyond the residential portion of the trial was the primary study outcome, outcome data were analyzed only for those participants who remained abstinent during and completed the 2-week residential stay (Fig. 1). Differences in baseline subject characteristics between treatment groups were determined using t test or its nonparametric analog, Wilcoxon or Kruskal-Wallis test, for continuous variables and Pearson χ2 tests for categorical variables. Hierarchical linear model was used to analyze the percentage of urine samples positive for cocaine over the course of the trial and to examine the changes over time in HAM-D scores. For analyses specifically examining lapse and relapse, our analytic approach was similar to that for a prior study using this paradigm,8 such that lapse is defined as the first cocaine-positive urine sample and relapse as 2 consecutively positive urine samples. In addition, study dropouts after entering the outpatient portion of the trial were considered to have the next 2 urine samples as positive for the purposes of determining lapse and relapse rates at the time they left the study if they had submitted only cocaine-free urine samples up to that point. Otherwise, missing urine samples after dropout and during trial participation were recorded as missing. A Kaplan-Meier survival analysis was performed to test for differences in retention, time to lapse, and time to relapse between treatment groups. Between-group differences in number of days to lapse or relapse were determined by 1-way analysis of variance. Because of the nonnormality of the data, a nonparametric analysis of variance was performed on the ranks of percentage of missing urine samples. After log transformation to achieve normality of the data, repeated measures mixed modeling was used to determine differential changes over time between groups in HAM-D score. The test statistic used was a t statistic to determine the time effect in the model. Depression measures including HAM-D score at week 2, lifetime depression diagnosis, and current depression diagnosis were also included as a covariate or cofactor in the cocaine use analyses previously to determine the differential impact of depressive symptoms on outcomes. For all analyses, P < 0.05 was used to infer statistical significance. The SAS software (SAS System for Windows Version 9.2, SAS Institute Inc, Cary, NC) was used.
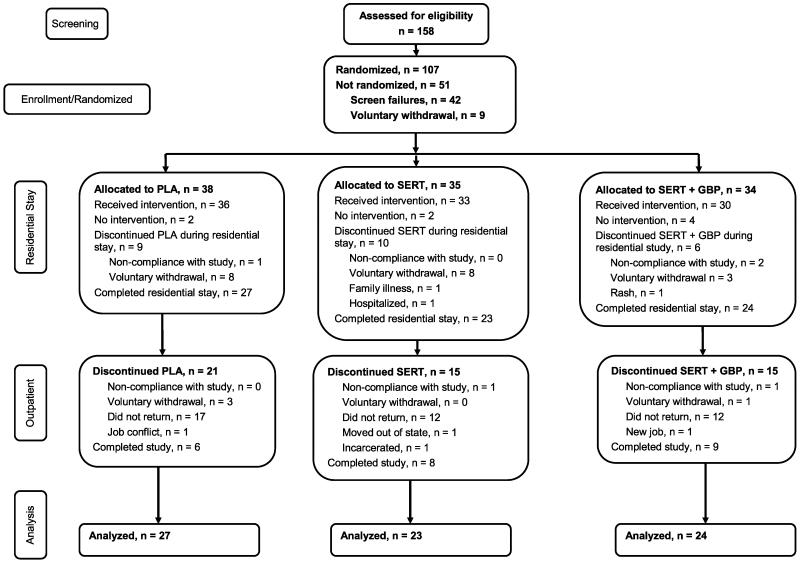
FIGURE 1.
Consort diagram.
RESULTS
Retention, Adverse Events, Missing Data, Compliance
One hundred fifty-eight participants were initially recruited to determine their eligibility to participate in this 12-week randomized, double-blind clinical trial through a 1-week, centralized recruiting/screening procedure (Fig. 1). Of those 116 who were eligible, 107 participants were randomized, and 99 participants entered the residential treatment facility. Of these, 25 (25.2%) dropped out before completing the residential portion of the study, irrespective of treatment group (χ2 = 0.04, df = 1, P = 0.84; Fig. 1). Those who dropped out of the residential portion of the study reported using cocaine a significantly greater number of days in the previous 30 days than those who were retained through the residential stay (median, 27 vs 20 days; rank sum test, P < 0.05), but no other significant baseline differences were observed. During the outpatient portion of the study, participant retention rates did not differ between treatment groups (χ2 = 0.30, df = 2, P = 0.86) with 23 (31.1% of those completing the residential portion of the study) participants completing the entire 12 weeks of the protocol. Fifty-one of those 74 participants who completed the residential stay and were included in the analysis did not complete the outpatient portion of the protocol for the reasons specified in Figure 1. Sertraline participants that were included in the analysis did not differ from those who dropped out during the residential portion of the study in cocaine use frequency in the month before study entry (median, 24 vs 27 days; rank sum test, P = 0.69). Twenty-one study-related adverse events occurred, that is, sexual dysfunction (n = 5), drowsiness (n = 4), nausea/vomiting (n = 3), tremor (n = 2), dizziness (n = 1), anxiety (n = 1), yawning (n = 1), insomnia (n = 1), diarrhea (n = 1), and bruxism (n = 1). One participant in the SERT plus GBP group experienced a potentially life-threatening allergic reaction resulting in emergency department treatment with rapid resolution, immediate withdrawal from the study and subsequent referral to a local drug treatment facility (Fig. 1).
The percentage of missing urine samples differed significantly among the 3 groups during the outpatient portion of the trial (F2 = 3.25, P = 0.044). Using the Tukey-Kramer multiple comparison post hoc procedure for the 3 groups, the SERT plus GBP group (15%) was significantly different from the SERT group (3%; 95% simultaneous confidence limits of the difference between group means were 0.418 and 32.760 but not the PLA group, 8%).
In terms of compliance, the percentage of pills returned (not taken by the participant) did not differ among the PLA (1.19% ± 1.47%), SERT (1.73% ± 2.95%), and SERT plus GBP (0.72% ± 1.07%) groups during the outpatient portion of the trial (χ2 = 0.94, df = 2, P = 0.63).
Baseline Characteristics
The 74 participants who were retained in the study beyond the residential portion of the trial had a mean (SD) age of 39.5 (7.3), 71.6% were African American, 23% were female, 56.8% had a high school education or less, 62.2% were unemployed, 86.5% lived alone, 34.7% were currently alcohol dependent, and 33% did not have a lifetime alcohol use disorder. Groups did not differ in subject characteristics, except for the number of days cocaine was used in the 30 days before study entry and the prevalence of a current diagnosis of major depression. The SERT group used significantly more days with mean (SD) of 22.9 (6.1) than PLA with mean (SD) of 15.4 (9.2) and SERT plus GBP with mean (SD) of 19.1 (10.3) (z = 2.00, P = 0.046); however, this did not seem to be clinically significant as there was no significant difference in the number of dimes per week of cocaine used in the 30 days before study entry (F4 = 0.29, P = 0.89). The SERT plus GBP group had a significantly lower prevalence of current major depression (11.1%) compared with the PLA (50%) and SERT (71.4%) groups (χ2 = 6.21, df = 2, P = 0.045).
Cocaine Use During the Trial
Although the rate at which cocaine-positive urine samples increased over time did not differ between SERT and PLA (P> 0.10), SERT (10.7% ± 23.4%; −2.10 ± 0.9, t = −2.35, P = 0.02), but not SERT plus GBP (21.1% ± 29.8%; −0.72 ± 0.75, t = −0.97, P = 0.33), showed a significantly lower percentage of cocaine-positive urine samples overall compared with the PLA group (29.7% ± 35.6%).
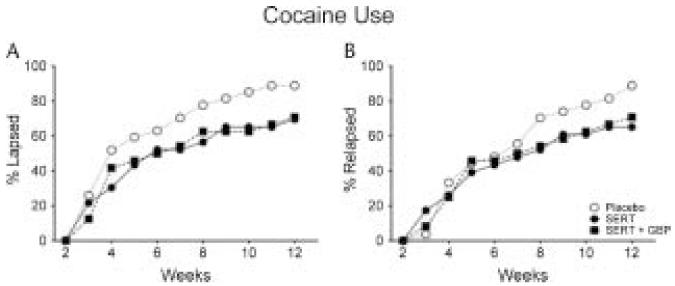
There was a trend toward a significant difference in the percentage of participants who experienced a lapse between PLA and SERT (88.9% vs 69.6%; χ2 = 2.90, df = 1, P = 0.09) and between PLA and SERT plus GBP (88.9% vs 70.8%; χ2 = 2.63, df = 1, P = 0.10) groups (Fig. 2). Furthermore, a significantly greater percentage of participants in the PLA group relapsed (88.9%) relative to the SERT group (65.2%; χ2 = 4.06, df = 1, P = 0.04). The PLA group showed a trend toward a significant difference in the percentage of participants who relapsed relative to the SERT plus GBP group (70.8%; χ2 = 2.63, df = 1, P = 0.10). Among the participants that experienced a lapse or relapse, there was no significant difference in the number of days to lapse (F = 0.04, P = 0.96) and relapse (F = 0.09, P = 0.91) among the SERT (17.62 ± 4.8 and 20.33 ± 5.2), SERT plus GBP (18.06 ± 4.7 and 22.24 ± 4.9), and PLA (16.46 ± 4.0 and 23.12 ± 4.1) groups.
FIGURE 2.
Percentage of participants who lapsed (ie, first urine sample positive for cocaine; panel A) and relapsed (ie, first 2 consecutive urine samples positive for cocaine; panel B) as a function of weeks in the PLA (open circles), SERT (closed circles), and SERT plus GBP (closed squares) during the outpatient portion of the 12-week trial.
Depressive Symptoms
At the time of entry into the residential portion of the study, severity of depressive symptoms did not differ between groups (mean [SD] score, 16.3 [7.2], F2 = 0.33, P = 0.72). Scores on the HAM-D significantly decreased over time during the 12-week study (t381 = −10.09, P < 0.0001) but did not differ between PLA and SERT (t381= 0.08, P = 0.93) or SERT plus GBP (t381 = 0.18, P = 0.85) group (data not shown). The 2 weeks of residential treatment showed a significant decrease in depressive symptom severity scores (t118 = −6.21, P < 0.0001), followed by a more gradual, but still significant, decrease during the outpatient portion (t256 = −3.60, P = 0.0004).
Neither HAM-D score at week 2, a lifetime depression diagnosis, nor a current depression diagnosis interacted with treatment groups to produce differential cocaine use outcomes (P > 0.05; data not shown).
DISCUSSION
Results of this study extend the results of our prior trial with SERT from a more urban to a more rural cocaine-dependent population.8 In the prior trial,8 SERT showed a trend toward a higher proportion of participants who did not relapse (29.7 vs 46.9%), a significantly lower rate of increase over time in cocaine-positive urine samples, and a significantly greater number of days to lapse and relapse relative to PLA. Although the current study does not specifically show a significant delay in time to lapse or relapse when SERT is compared with PLA, there were significantly fewer cocaine-positive urine samples over time in the SERT alone group compared with that of the PLA group. Even more importantly, significantly fewer participants receiving SERT relapsed relative to PLA. Unlike another clinical trial showing that baseline severity of cocaine use impacted outcomes,14 the effect of SERT did not seem to be due to retaining individuals with less severity of cocaine use at baseline, in that the SERT participants that were included in the analysis did not differ from those who dropped out during the residential portion of the study in cocaine use frequency in the month before study entry (median, 24 vs 27 days; rank sum test, P = 0.69). This finding reduces the possibility that SERT’s effects were exclusive to those with lower severity of cocaine dependence. Thus, the results of both studies consistently show that SERT may improve treatment response in this population.
These findings are also consistent with other studies of SSRIs SERT31 and citalopram9 for cocaine dependence. In contrast, prior research with the SSRI fluoxetine has been mixed, with promising results of early trials32,33 not being replicated under rigorous, double-blind, PLA-controlled conditions.10,31 These mixed results could be due to the differing affinities of SSRIs for other receptor sites besides the serotonin transporter34 that may result in differing adverse effects that could undermine treatment outcomes.35
That GBP did not augment the efficacy of SERT to prevent relapse is unexpected. The reasons for this are unclear, but may be because of several factors. First, the dosage of GBP may not have been sufficient for augmenting the effects of SERT. Moreover, given that the rate of cocaine use in the SERT alone group was so low, there was little room for improvement with GBP. In addition, although GABA is considered an important factor in cocaine dependence,36 it may not be as important as serotonin or dopamine in the prevention of relapse. Further work is necessary to shed light whether modulating GABA activity concomitantly with serotonin and dopamine function improves treatment outcomes.
SERT’s effects on drug use did not seem to be related to alleviating depressive symptoms, with symptoms in all 3 groups sharply declining within the first week of the study. This is consistent with results of the fluoxetine clinical trial in depressed cocaine abusers,10 suggesting that psychiatric symptoms at study entry may be more indicative of an acute rather than chronic state. Given the report by Schmitz and associates10 that those with lower depression ratings during a clinical trial of fluoxetine were more likely to have cocaine-free urine samples, SERT may have efficacy for cocaine dependence regardless of depression status.
Limitations of the study include that just over 1 quarter of participants dropped out during the residential stay, so the lack of outcome data with these individuals cannot inform our overall results. Therefore, the fact that only data from those who participated beyond week 2 were analyzed limits the generalizability of these findings to only individuals who are abstinent and receiving SERT for at least 2 weeks. Although retention overall was similar to that observed in another trial with depressed cocaine-dependent participants,37 it was still relatively low (31.1%), another limitation of the current study. Moreover, biomarkers to determine compliance with the study medications were not used; thus, whether differential compliance with taking the study medications occurred across groups cannot be ruled out. In addition, classifying dropouts as treatment failures for the purposes of determining lapse and relapse is very conservative and may discount instances where individuals remained drug free. Nevertheless, given that the length of treatment is negatively associated with treatment outcomes,38 we believe that this strategy was a reasonable approach. Another limitation is that the SERT plus GBP group had a significantly lower prevalence of current, but not lifetime, major depression. It is unclear how this difference in the prevalence of current major depression may have impacted the results of this study, although depression measures did not differentially impact treatment response to the study medications.
Nevertheless, the clinical significance of these results with SERT is clear. Not only did the SERT and PLA groups differ significantly in relapse but also the percentage of SERT-treated participants who did not relapse was more than 3 times greater than PLA-treated participants (34.8% vs 11.1%). In summary, the present study provides additional support for the use of SERT with cognitive behavioral therapy in abstinent cocaine-dependent individuals who initially present with depressive symptoms. These findings add to a growing literature suggesting the potential efficacy of certain SSRIs in treating cocaine dependence.8,9,39 Future trials that directly and systematically evaluate SSRIs in both depressed and nondepressed cocaine-abusing populations and attempt to identify behavioral and biochemical mechanisms mediating treatment response to pharmacological and/or behavioral interventions in cocaine-dependent individuals are warranted to optimize treatment outcomes.
ACKNOWLEDGMENT
The authors thank Summer Alexander for her assistance with the preparation of this study.
This study was funded by grants P50-DA12762 and K05-DA00454 (T.R.K.) and T32 DA022981 (N.S.) from the National Institute on Drug Abuse and GM103425-09 (M.J.M.) from the National Institute of General Medical Services.
Footnotes
AUTHOR DISCLOSURE INFORMATION
The authors declare no conflicts of interest.
REFERENCES
- 1.Maraj S, Figueredo VM, Lynn Morris D. Cocaine and the heart. Clin Cardiol. 2010;33(5):264–269. doi: 10.1002/clc.20746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.US. Department of Health and Human Services . Mental Health: A Report of the Surgeon General-Executive Summary. Substance Abuse and Mental Health Services, NIH, NIMH; Rockville, MD: 1999. [Google Scholar]
- 3.Sorenson JL, Wermuth LA, Gibson DR, et al. Preventing AIDS in Drug Abusers and Their Sexual Partners. Guilford; New York, NY: 1991. [Google Scholar]
- 4.Wild TC, el-Guebaly N, Fischer B, et al. Comorbid depression among untreated illicit opiate users: results from a multisite Canadian study. Can J Psychiatry. 2005;50(9):512–518. doi: 10.1177/070674370505000903. [DOI] [PubMed] [Google Scholar]
- 5.Conner KR, Pinquart M, Holbrook AP. Meta-analysis of depression and substance use and impairment among cocaine users. Drug Alcohol Depend. 2008;98(1-2):13–23. doi: 10.1016/j.drugalcdep.2008.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McKay JR, Pettinati HM, Morrison R, et al. Relation of depression diagnoses to 2-year outcomes in cocaine-dependent patients in a randomized continuing care study. Psychol Addict Behav. 2002;16(3):225–235. [PubMed] [Google Scholar]
- 7.Batki SL, Washburn AM, Delucchi K, et al. A controlled trial of fluoxetine in crack cocaine dependence. Drug Alcohol Depend. 1996;41(2):137–142. doi: 10.1016/0376-8716(96)01233-1. [DOI] [PubMed] [Google Scholar]
- 8.Oliveto A, Poling J, Mancino MJ, et al. Sertraline delays relapse in recently abstinent cocaine-dependent patients with depressive symptoms. Addiction. 2012;107(1):131–141. doi: 10.1111/j.1360-0443.2011.03552.x. PMCID: PMC3237722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Moeller FG, Schmitz JM, Steinberg JL, et al. Citalopram combined with behavioral therapy reduces cocaine use: a double-blind, placebo-controlled trial. Am J Drug Alcohol Abuse. 2007;33(3):367–378. doi: 10.1080/00952990701313686. [DOI] [PubMed] [Google Scholar]
- 10.Schmitz JM, Averill P, Stotts AL, et al. Fluoxetine treatment of cocaine-dependent patients with major depressive disorder. Drug Alcohol Depend. 2001;63(3):207–214. doi: 10.1016/s0376-8716(00)00208-8. [DOI] [PubMed] [Google Scholar]
- 11.Volkow ND, Fowler JS, Wolf AP, et al. Effects of chronic cocaine abuse on postsynaptic dopamine receptors. Am J Psychiatry. 1990;147(6):719–724. doi: 10.1176/ajp.147.6.719. [DOI] [PubMed] [Google Scholar]
- 12.Rossetti ZL, Melis F, Carboni S, et al. Dramatic depletion of mesolimbic extracellular dopamine after withdrawal from morphine, alcohol or cocaine: a common neurochemical substrate for drug dependence. Ann N Y Acad Sci. 1992;654:513–516. doi: 10.1111/j.1749-6632.1992.tb26016.x. [DOI] [PubMed] [Google Scholar]
- 13.Jimerson DC. Role of dopamine mechanisms in the affective disorders. In: Meltzer HY, editor. Psychopharmacology: The Third Generation of Progress. Raven Press; New York, NY: 1987. pp. 505–511. [Google Scholar]
- 14.Simpson DD, Joe GW, Broome KM. A national 5-year follow-up of treatment outcomes for cocaine dependence. Arch Gen Psychiatry. 2002;59(6):538–544. doi: 10.1001/archpsyc.59.6.538. [DOI] [PubMed] [Google Scholar]
- 15.Sanacora G, Mason GF, Krystal JH. Impairment of GABAergic transmission in depression: new insights from neuroimaging studies. Crit Rev Neurobiol. 2000;14(1):23–45. doi: 10.1615/critrevneurobiol.v14.i1.20. [DOI] [PubMed] [Google Scholar]
- 16.Volkow ND, Wang GJ, Fowler JS, et al. Enhanced sensitivity to benzodiazepines in active cocaine-abusing subjects: a PET study. Am J Psychiatry. 1998;155(2):200–206. doi: 10.1176/ajp.155.2.200. [DOI] [PubMed] [Google Scholar]
- 17.McAllister K, Goeders N, Dworkin S. Chronic cocaine modifies brain benzodiazepine receptor densities. NIDA Res Monog. 1988;81:101–108. [PubMed] [Google Scholar]
- 18.Smith WT, Londborg PD, Glaudin V, et al. Is extended clonazepam cotherapy of fluoxetine effective for outpatients with major depression? J Affect Disord. 2002;70(3):251–259. doi: 10.1016/s0165-0327(01)00352-4. [DOI] [PubMed] [Google Scholar]
- 19.Koe BK, Weissman A, Welch WM, et al. Sertraline, 1S,4S-N-methyl-4-(3,4-dichlorophenyl)-1,2,3,4-tetrahydro-1-naphthylamine, a new uptake inhibitor with selectivity for serotonin. J Pharmacol Exp Ther. 1983;226(3):686–700. [PubMed] [Google Scholar]
- 20.Sokolski KN, Green C, Maris DE, et al. Gabapentin as an adjunct to standard mood stabilizers in outpatients with mixed bipolar symptomatology. Ann Clin Psychiatry. 1999;11(4):217–222. doi: 10.1023/a:1022361412956. [DOI] [PubMed] [Google Scholar]
- 21.Wei LJ, Lachin JM. Properties of the urn randomization in clinical trials. Control Clin Trials. 1988;9(4):345–364. doi: 10.1016/0197-2456(88)90048-7. [DOI] [PubMed] [Google Scholar]
- 22.Carroll KM. A Cognitive-Behavioral Approach: Treating Cocaine Addiction. National Institute on Drug Abuse; Rockville, MD: 1998. [Google Scholar]
- 23.Preskorn SH, Lane RM. Sertraline 50 mg daily: the optimal dose in the treatment of depression. Int Clin Psychopharmacol. 1995;10(3):129–141. doi: 10.1097/00004850-199510030-00001. [DOI] [PubMed] [Google Scholar]
- 24.Bisaga A, Aharonovich E, Garawi F, et al. A randomized placebo-controlled trial of gabapentin for cocaine dependence. Drug Alcohol Depend. 2006;81(3):267–274. doi: 10.1016/j.drugalcdep.2005.07.009. [DOI] [PubMed] [Google Scholar]
- 25.Gonzalez G, Desai R, Sofuoglu M, et al. Clinical efficacy of gabapentin versus tiagabine for reducing cocaine use among cocaine-dependent methadone-treated patients. Drug Alcohol Depend. 2007;87(1):1–9. doi: 10.1016/j.drugalcdep.2006.07.003. [DOI] [PubMed] [Google Scholar]
- 26.Hart CL, Haney M, Vosburg SK, et al. Gabapentin does not reduce smoked cocaine self-administration: employment of a novel self-administration procedure. Behav Pharmacol. 2007;18(1):71–75. doi: 10.1097/FBP.0b013e328014139d. [DOI] [PubMed] [Google Scholar]
- 27.First MB, Spitzer RL, Gibbon M, et al. User’s Guide for the Structured Clinical Interview for DSM-IV Personality Disorders (SCID-II) American Psychiatric Press; Washington, DC: 1995. [Google Scholar]
- 28.McLellan AT, Luborsky L, Woody GE, et al. An improved diagnostic evaluation instrument for substance abuse patients. The Addiction Severity Index. J Nerv Ment Dis. 1980;168(1):26–33. doi: 10.1097/00005053-198001000-00006. [DOI] [PubMed] [Google Scholar]
- 29.Hamilton MA. A rating scale for depression. J Neurol Neurosurg Psychiatry. 1960;23:56–82. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kosten T, Oliveto A, Feingold A, et al. Desipramine and contingency management for cocaine and opiate dependence in buprenorphine maintained patients. Drug Alcohol Depend. 2003;70:315–325. doi: 10.1016/s0376-8716(03)00032-2. [DOI] [PubMed] [Google Scholar]
- 31.Winstanley EL, Bigelow GE, Silverman K, et al. A randomized controlled trial of fluoxetine in the treatment of cocaine dependence among methadone-maintained patients. J Subst Abuse Treat. 2011;40(3):255–264. doi: 10.1016/j.jsat.2010.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schmitz JM, Rhoades HM, Elk R, et al. Medication take-home doses and contingency management. Exp Clin Psychopharmacol. 1998;6(2):162–168. doi: 10.1037//1064-1297.6.2.162. [DOI] [PubMed] [Google Scholar]
- 33.Pollack MH, Rosenbaum JF. Fluoxetine treatment of cocaine abuse in heroin addicts. J Clin Psychiatry. 1991;52(1):31–33. [PubMed] [Google Scholar]
- 34.Carrasco JL, Sandner C. Clinical effects of pharmacological variations in selective serotonin reuptake inhibitors: an overview. Int J Clin Pract. 2005;59(12):1428–1434. doi: 10.1111/j.1368-5031.2005.00681.x. [DOI] [PubMed] [Google Scholar]
- 35.Buchman N, Strous RD, Baruch Y. Side effects of long-term treatment with fluoxetine. Clin Neuropharmacol. 2002;25(1):55–57. doi: 10.1097/00002826-200201000-00010. [DOI] [PubMed] [Google Scholar]
- 36.Shorter D, Kosten TR. Novel pharmacotherapeutic treatments for cocaine addiction. BMC Med. 2011;9:119. doi: 10.1186/1741-7015-9-119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.McDowell D, Nunes EV, Seracini AM, et al. Desipramine treatment of cocaine-dependent patients with depression: a placebo-controlled trial. Drug Alcohol Depend. 2005;80(2):209–221. doi: 10.1016/j.drugalcdep.2005.03.026. [DOI] [PubMed] [Google Scholar]
- 38.Higgins ST, Heil SH, Dantona R, et al. Effects of varying the monetary value of voucher-based incentives on abstinence achieved during and following treatment among cocaine-dependent outpatients. Addiction. 2007;102(2):271–281. doi: 10.1111/j.1360-0443.2006.01664.x. [DOI] [PubMed] [Google Scholar]
- 39.Vayalapalli S, Vaughn M, Salles-Shahid K, et al. High-dose citalopram for cocaine dependence in veteran population—a pilot project. Am J Addict. 2011;20(5):485–486. doi: 10.1111/j.1521-0391.2011.00162.x. [DOI] [PubMed] [Google Scholar]