Abstract
In this study, we designed and evaluated a microalgal pretreatment method using cellulolytic bacteria that naturally degrades microalgae in their native habitat. Bacterial strains were isolated from each of two mollusk species in a medium containing 1% carboxymethyl cellulose agar. We selected nine bacterial strains that had endoglucanase activity: five strains from Mytilus chilensis, a Chilean mussel, and four strains from Mesodesma donacium, a clam found in the Southern Pacific. These strains were identified phylogenetically as belonging to the genera Aeromonas, Pseudomonas, Chryseobacterium, and Raoultella. The cellulase-producing capacities of these strains were characterized, and the degradation of cell walls in Botryococcus braunii and Nannochloropsis gaditana was tested with “whole-cell” cellulolytic experiments. Aeromonas bivalvium MA2, Raoultella ornithinolytica MA5, and Aeromonas salmonicida MC25 degraded B. braunii, and R. ornithinolytica MC3 and MA5 degraded N. gaditana. In addition, N. gaditana was pretreated with R. ornithinolytica strains MC3 and MA5 and was then subjected to an anaerobic digestion process, which increased the yield of methane by 140.32% and 158.68%, respectively, over that from nonpretreated microalgae. Therefore, a “whole-cell” cellulolytic pretreatment can increase the performance and efficiency of biogas production.
INTRODUCTION
Microalgae have been used throughout history in industrial applications owing to the variety of products of interest that can be generated from this resource (1). For biofuel production, microalgae have distinct advantages, including growth to a high density, generation of a denser biomass per hectare than even the best oilseed crops (2), and the fact that they do not compete with food crops for soil (1, 2). In recent years, microalgal biomass has become an option for alternative biofuel production (3). However, to produce biofuels that compete directly with traditional energy sources, such as biodiesel or biogas, the cost of microalgal biomass production must be reduced (4).
The biogas produced from microalgal biomass is generated by a process of anaerobic digestion. The biochemical composition of microalgae, which includes the trace elements iron, cobalt, and zinc (5), meets the general nutrient requirements of anaerobic microbiota; thus, incubation of microalgal biomass with anaerobic microbes can stimulate methanogenesis (6). The amount of biogas produced depends on the microalgal species used, because the relative proportions of proteins, carbohydrates, and lipids contained in microalgal cells influence the action of methanogenic bacteria (7). Another factor that may affect the methanogenic potential of microalgae is the protease resistance of their cell walls, which limits the effectiveness of the microorganisms present in the anaerobic digesters in metabolizing the intracellular components of the microalgae (6, 8).
The cell walls of many species of microalgae are multilayered and contain a relatively large proportion of cellulose. Cellulose is a linear polymer of β-1,4-d-anhydroglucopyranose units, which makes it very stable and resistant to degradation (9). To allow methanogenic bacteria to gain access to the intracellular contents of microalgae, biomass is first subjected to pretreatment of the cell wall; this pretreatment increases both overall biodegradability and methane production (6). The most commonly used pretreatments include high-pressure homogenization, sulfuric acid, microwave-induced bead beating, and autoclaving (10, 11). However, most of these methods require an abundance of energy and thus are expensive (12), increasing the cost of biofuel production. Pretreatment with commercial cellulases to degrade cellulose during biogas production is rarely employed and has not been tested on an industrial scale because the enzymes are cost-prohibitive and cannot be reused (12–14).
The purpose of this study was to identify bacteria capable of specifically degrading the microalgal cell wall, which is composed mainly of cellulose. In previous studies, researchers have isolated cellulolytic bacteria and fungi from terrestrial environments, such as compost, ruminant feces, and vegetable waste (15). Moreover, they also found cellulolytic bacteria in marine environments, such as Teredinibacter turnerae, isolated from mollusks called “shipworms,” which is capable of degrading cellulose from wood (16). However, these particular bacteria have not yet been used to pretreat microalgal biomass, because the microalgal cell wall is not composed exclusively of cellulose. We focused on the isolation, identification, and characterization of marine bacteria with cellulolytic capacity isolated from the guts of filtering bivalve mollusks for use in “whole-cell” enzymatic pretreatment of the cell wall in microalgal biomass for biogas production. Bacterial species from invertebrates and bivalve mollusks have not been studied for their abilities to degrade microalgal cell walls. Most marine bivalves and invertebrates depend on microalgae during their life cycles (17). Like fish and crustacean larvae, bivalves feed directly on microalgae (18). We thus hypothesized that the digestive systems of filtering bivalve mollusks contain symbiotic microorganisms that have cellulolytic activity and other enzymatic degradative activities; these activities could thus promote the hydrolysis of the microalgal cell wall and therefore increase the nutrient bioavailability of microalgal biomass.
MATERIALS AND METHODS
Collection and preparation of shellfish samples.
Samples were obtained from the guts of the following filter-feeding bivalve mollusks: Mytilus chilensis, Protothaca thaca, and Mesodesma donacium. All were collected from the fish market in Antofagasta, Chile. For this purpose, 1 g of the gut from each species was weighed, diluted in 9 ml of marine saline solution, and homogenized using a Stomacher 80 lab blender for 2 min. The resulting homogenate was used as the initial sample for plate streaking in a selective carboxymethyl cellulose (CMC) agar medium.
Bacterial growth medium and conditions.
Homogenized mollusk gut solutions were streaked onto plates with a CMC agar selective medium modified from the work of Samira et al. (19), consisting of minimal medium supplemented with 10 g liter−1 CMC, 1 g liter−1 KH2PO4, 0.5 g liter−1 MgSO4·7H2O, 20 g liter−1 NaCl, 0.01 g liter−1 FeSO4·7H2O, 0.01 g liter−1 MnSO4·H2O, 0.3 g liter−1 NH4NO3, and 15 g liter−1 agar. The plates were incubated at 30°C for 7 days. Colonies that grew in this selective medium were transferred to Luria-Bertani agar medium supplemented with 2% NaCl and were incubated at 30°C for 3 days. The colonies that grew after the third isolation were inoculated into 20 ml of Luria-Bertani medium supplemented with 2% NaCl and were incubated at 30°C for 3 days with constant stirring at 125 rpm. These bacterial cultures were used as inocula in subsequent experiments.
Screening of cellulase-producing bacteria.
Each bacterial culture was inoculated onto CMC agar and was incubated at 30°C for 7 days. Gram staining was performed to visualize cellulolytic activity (20). The CMC agar plates were flooded with Gram's iodine at room temperature for 3 min; the excess was removed, and the diameter of the halo degradation around each colony was measured. The strains that formed hydrolysis zones were selected for use in subsequent assays. The positive control for cellulase activity was 0.5 U ml−1 cellulase from Aspergillus niger (Sigma).
Growth and biochemical profile.
Each bacterial strain was grown in 100 ml Luria-Bertani medium supplemented with 2% NaCl at 30°C, and absorbance at 600 nm was measured at intervals of 24 h for 5 days. Also, direct microscopic cell counting was performed using a Neubauer chamber. Biochemical characterization was performed using the API 20E assay (Biomerieux Inc.).
DNA extraction and molecular identification using 16S rRNA genes.
Genomic DNA was extracted and purified using the Power Soil DNA purification kit (Mo Bio). The 16S rRNA gene was amplified from genomic DNA using primers F27 (5′-AGAGTTTGATCMTGGCTCAG-3′) and R1492 (5′-GGTTACCTTGTTACGACTT-3′). The PCR conditions were as follows: initial denaturation at 95°C for 5 min, followed by 35 cycles of denaturation at 95°C for 30 s, annealing at 55°C for 30 s, and extension at 72°C for 1 min, with a final extension at 72°C for 5 min. The PCR products were purified with the QIAquick gel extraction kit (Qiagen). Then the products were sequenced by Macrogen Inc. (South Korea). Sequences were analyzed with the Bioedit Sequence Alignment Editor, assembled by using ChromasPro 1.5 software, and subsequently analyzed using BLASTN software against the nonredundant database available in GenBank (http://www.ncbi.nlm.nih.gov/GenBank/index.htm) with a cutoff of 1 × 10−5.
Filter paper degradation assay.
The filter paper assay was performed as described by Lu et al. (21). Each selected strain was incubated with Whatman no. 1 filter paper (1- by 6-cm strips; mass, 0.5 g) in 40 ml of a modified version of the minimal medium described by Samira et al. (19) [5 g liter−1 KH2PO4, 0.2 g liter−1 MgSO4·7H2O, 20 g liter−1 NaCl, 2.5 g liter−1 yeast extract, and 0.6 g liter−1 (NH4)2SO4] at 30°C for 7 days with constant stirring at 125 rpm. The culture medium was then removed, and the filter paper was sonicated for 3 min at 60 Hz and was washed with distilled water to remove bacteria that adhered to the paper. This procedure was repeated five times. Then each paper was dried at 45°C for 30 min, until a constant weight was reached. The percentage of degradation of the filter paper was calculated by comparing the initial weight to the final weight.
Disruption of microalgal cells with a “whole-cell” bacterial pretreatment.
We tested the microalgal biomass of Botryococcus braunii UTEX572 cultured under outdoor batch conditions in UMA2 medium in 0.4-m3 photobioreactors as described by Bazaes et al. (22). Nannochloropsis gaditana CCMP527 was subjected to continuous culture at a concentration of approximately 2 g liter−1 in F/2 medium (23) in a 2-liter photobioreactor at 20°C with continuous light. (N. gaditana culture was performed at the University of La Frontera, Temuco, Chile.)
Each bacterial strain was cultured individually in minimal medium supplemented with yeast extract [2.5 g liter−1 yeast extract, 5 g liter−1 K2HPO4, 20 g liter−1 NaCl, 0.2 g liter−1 MgSO4·7H2O, and 0.6 g liter−1 (NH4)2SO4] for 48 h at 30°C and 125 rpm. After incubation, the biomass of each bacterium in 1 g liter−1 was diluted in 5 ml medium and was mixed with 5 ml of the B. braunii or N. gaditana culture in the stationary phase. The negative control corresponded to minimal medium with microalgal biomass but without bacteria.
The microalga-bacterium mixtures were incubated at 30°C for 72 h (for B. braunii) or 96 h (for N. gaditana). Every 24 h, a 1-ml sample of each mixture was taken. The success of the treatment of B. braunii was assessed by the addition of calcofluor white stain (Sigma), which specifically stains the cellulose in the cell wall (24) that has been degraded. These samples were incubated for 16 h in the dark and were analyzed using epifluorescence and bright-field microscopy (with an Olympus BX52 microscope). The extent of degradation was calculated as the ratio of intact cells to total cells.
The average N. gaditana cell size is smaller than the average B. braunii cell size, and therefore, calcofluor white staining of the cell wall does not allow one to detect the rupture of N. gaditana cells. Instead, the intact cells that maintained their chlorophyll autofluorescence were counted in a Neubauer chamber. The initial cell count corresponded to day 1, and the final count corresponded to day 3 (72 h). The strains that most effectively disrupted the cell wall were selected for use in enzymatic pretreatment prior to anaerobic digestion of microalgal biomass.
“Whole-cell” enzymatic pretreatment of N. gaditana.
For enzymatic pretreatment, cultures of each selected cellulolytic bacterial strain and a culture of microalgal biomass of stationary-stage N. gaditana were obtained. The two cultures were mixed in a proportion of 1:1 (vol/vol), and the mixture was incubated for 48 h at 30°C with constant stirring at 125 rpm. Then the microalgal biomass was subjected to anaerobic digestion for biogas production.
Anaerobic digestion assay.
The anaerobic digestion assay utilized microalgal biomass (20 g liter−1) and a culture of each cellulolytic bacterial strain (1 g liter−1). The N. gaditana biomass was rinsed to remove the salts present in the culture, because salts can inhibit the growth of methanogenic bacteria (25, 26). To concentrate both cultures, we used an ultrafiltration system with a 0.03-μm tubular X-flow type filter (Norit, The Netherlands). The test for biochemical methane potential was performed using a microbial inoculum of anaerobic mud obtained from an anaerobic digester for wastewater treatment at the CCU brewery plant, Temuco, Chile.
Anaerobic digestion vials (50 ml) were used. In each vial, the substrate was added at a concentration of 2 g liter−1 in anaerobic mud in a 1:1 (wt/wt) proportion with the pretreated microalgal biomass, with 0.5 ml yeast extract, 0.5 ml of NaHCO3 (50 g liter−1), 50 μl of nutrients (65 mg liter−1 NH4Cl, 18.5 mg liter−1 KH2PO4, 4 mg liter−1 CaCl·2H2O, 5.7 mg liter−1 MgSO4·7H2O, 20 g liter−1 yeast extract, and 50 g liter−1 NaHCO3), and distilled water for a final volume of 50 ml. A flow of N2-CO2 (80:20 [vol/vol]) was applied to each vial to expel O2. Each bottle was sealed and was incubated for 30 days at 35°C. The following controls were also included: (i) each selected bacterial strain added separately, (ii) microalgae without pretreatment, and (iii) a negative control with water added rather than a substrate.
During anaerobic digestion, the changes in headspace gas pressure in each vial were measured with a pressure sensor (Cole Parmer), and the composition of headspace gases was determined using a gas chromatograph with a thermal conductivity detector (Clarus 580; PerkinElmer). The composition was evaluated for 25 days of incubation. These values were used to determine the amount of methane produced from each substrate.
Data processing.
To measure methane production from the substrate for each “whole-cell” enzymatic pretreatment, it was necessary to consider the presence of two different biomasses, namely, the bacterial and microalgal biomasses, in a 1:2 (wt/wt [expressed in grams of volatile suspended solids {VSS}]) proportion. In addition, for each vial that contained nonpretreated microalgal biomass, the substrate had twice the weight of VSS (in grams) as vials with bacterium-pretreated microalgae. VSS were measured according to method 2540 of Standard Methods for the Examination of Water and Wastewater (27) using a muffle furnace (Thermolyne type 1300 furnace). To determine the amount of methane (expressed in milliliters per gram of VSS) produced from pretreated microalgae, the following equation was used:
Statistical analysis.
All experiments were performed in triplicate. Mean methane production levels in different samples were compared by one-way analysis of variance followed by Tukey tests for statistical significance using Statgraphics Plus software, version 5.1 (Centurion). In all cases, differences with a P value of <0.05 were considered significant.
Nucleotide sequence accession numbers.
The 16S rRNA gene sequences that we obtained for the strains isolated from M. chilensis and M. donacium were uploaded to GenBank with the following accession numbers: KF436942 (strain MA2), KF436943 (strain MA3), KF436944 (strain MA5), KF436945 (strain MA11), KF436946 (strain MC3), KF436947 (strain MC18), KF436948 (strain MC21), KF436949 (strain MC23), and KF436950 (strain MC25).
RESULTS
Selection and isolation of cellulolytic bacteria.
Cellulolytic bacteria were isolated using CMC agar selective medium in which cellulose was the sole carbon source. From the homogenized solutions extracted from the guts of M. chilensis, M. donacium, and P. thaca mollusks, 54 bacterial strains were obtained: 29 strains from M. chilensis, 12 from M. donacium, and 13 from P. thaca. Nine strains showed endoglucanase activity after staining with Gram's iodine (Fig. 1): from M. chilensis, strains MC3, MC18, MC21, MC23, and MC25, and from M. donacium, strains MA2, MA3, MA5, and MA11 (Fig. 1). None of the strains isolated from P. thaca formed degradation halos on CMC agar plates after the staining assay. Of the strains isolated from M. chilensis, those that produced the longest clear-zone diameters were MC3 (1.8 cm) and MC25 (1.5 cm); the strains from M. donacium that produced the longest clear-zone diameters were MA2 (1.1 cm) and MA11 (0.9 cm) (Table 1).
FIG 1.

Effect of Gram's iodine stain on the cellulolytic zone in plates with CMC agar. (A through E) Degradation halos of strains isolated from Mytilus chilensis. (A) MC3; (B) MC18; (C) MC21; (D) MC23; (E) MC25. (F through I) Degradation halos of strains isolated from Mesodesma donacium. (F) MA2; (G) MA3; (H) MA5; (I) MA11. (J) Aspergillus niger cellulase (Sigma) served as the positive control for cellulase activity.
TABLE 1.
Diameters of CMC degradation halos and phylogenetic identification of cellulolytic strains isolated from Mytilus chilensis and Mesodesma donacium
| Bacterial strain | Host species | Species with sequence homologya | E value | Similarity (%) | Identity (%) | Homolog GenBank accession no. | Diam of halo (cm) |
|---|---|---|---|---|---|---|---|
| MC3 | Mytilus chilensis | Raoultella ornithinolytica | 0.0 | 100 | 99 | NR_102983.1 | 1.8 |
| MC18 | Mytilus chilensis | Chryseobacterium sp. | 0.0 | 100 | 99 | JQ660045.1 | 1.1 |
| MC21 | Mytilus chilensis | Chryseobacterium sp. | 0.0 | 98 | 96 | JQ660045.1 | 1.0 |
| MC23 | Mytilus chilensis | Aeromonas bivalvium | 0.0 | 100 | 99 | DQ504430.1 | 1.1 |
| MC25 | Mytilus chilensis | Aeromonas salmonicida | 0.0 | 99 | 98 | AB472980.1 | 1.5 |
| MA2 | Mesodesma donacium | Aeromonas bivalvium | 0.0 | 100 | 99 | DQ504430.1 | 1.1 |
| MA3 | Mesodesma donacium | Pseudomonas pseudoalcaligenes | 0.0 | 99 | 99 | HE575911.1 | 0.6 |
| MA5 | Mesodesma donacium | Raoultella ornithinolytica | 0.0 | 100 | 99 | CP004142.1 | 0.8 |
| MA11 | Mesodesma donacium | Klebsiella sp. | 0.0 | 100 | 99 | GU290323.1 | 0.9 |
Sequence homology was determined with BLASTN.
Phylogenetic identification of cellulolytic bacteria.
Phylogenetic analysis (Table 1) based on 16S rRNA gene sequences revealed that strains MC3 and MA5 belong to the species Raoultella ornithinolytica, with 99% identity in each case. R. ornithinolytica is a Gram-negative bacterium generally found in aquatic environments and associated with diseases such as enteric fever in humans (28).
Strains MC23, MC25, and MA2 showed a large degree of 16S rRNA gene sequence similarity with Aeromonas species, with 98 to 99% identity. These species are associated with human diseases such as gastroenteritis and respiratory infections, are commonly found in aquatic environments (mainly in the guts of mollusks and fish), and have the ability to produce virulence factors (29). To date, there is little information on Aeromonas bivalvium, because it has only recently been described (30).
Strains MC18 and MC21 showed 99% and 96% 16S rRNA gene sequence identity, respectively, with Chryseobacterium spp., which, like the species mentioned above, have been described in aquatic environments. However, only certain Chryseobacterium species are pathogenic to humans, causing disease mainly in neonates and immunocompromised individuals (31). Strain MA3 showed 99% identity with Pseudomonas pseudoalcaligenes, a species associated with the cyanide bioremediation process (32) (Table 1). The species corresponding to MC18, MC21, and MA3 have not been described as having cellulolytic activity.
Biochemical profile.
Strain MC3 (R. ornithinolytica) and strains MC18 and MC21 (Chryseobacterium sp.) grew more slowly at 20°C than at 30°C. Additionally, the stationary phase was reached by all selected strains at 48 h with incubation at 30°C and constant stirring at 125 rpm (data not shown). A biochemical profile was determined for each selected species (Table 2). However, only the A. bivalvium strains MA2 and MC23, Aeromonas salmonicida MC25, R. ornithinolytica MA5, and Klebsiella sp. strain MA11 were able to ferment or oxidize glucose, mannitol, sucrose, and arabinose. R. ornithinolytica MA5 and Klebsiella sp. MA11 were positive for all fermentation/oxidation tests and showed the abilities to produce lysine decarboxylase, urease, and NO2 and to metabolize citrate.
TABLE 2.
Biochemical profiles of strains isolated using API 20E
| Test | Reaction and/or enzyme | Aeromonas bivalvium (MC23) | Aeromonas salmonicida (MC25) | Aeromonas bivalvium (MA2) | Raoultella ornithinolytica (MA5) | Klebsiella sp. (MA11) |
|---|---|---|---|---|---|---|
| ONPG | β-Galactosidase (ortho-nitrophenyl-β-d-galactopyranosidase) | + | + | + | + | − |
| ADH | Arginine dihydrolase | + | + | + | − | − |
| LDC | Lysine decarboxylase | − | − | − | + | + |
| ODC | Ornithine decarboxylase | − | − | − | − | − |
| CIT | Citrate utilization | − | − | − | + | + |
| H2S | H2S production | − | − | − | − | − |
| URE | Urease | − | − | − | + | + |
| TDA | Tryptophan deaminase | + | + | + | + | + |
| IND | Indole production | + | + | + | + | + |
| VP | Acetoin production (Voges-Proskauer test) | − | + | + | + | + |
| GEL | Gelatinase | + | + | + | − | − |
| GLU | Fermentation/oxidation (glucose) | + | + | + | + | + |
| MAN | Fermentation/oxidation (mannitol) | + | + | + | + | + |
| INO | Fermentation/oxidation (inositol) | − | − | − | + | + |
| SOR | Fermentation/oxidation (sorbitol) | − | − | − | + | + |
| RHA | Fermentation/oxidation (rhamnose) | − | − | − | + | + |
| SAC | Fermentation/oxidation (saccharose) | + | + | + | + | + |
| MEL | Fermentation/oxidation (melibiose) | − | − | − | + | + |
| AMY | Fermentation/oxidation (amygdalin) | − | − | + | + | + |
| ARA | Fermentation/oxidation (arabinose) | + | + | + | + | + |
| Nitrate reduction GLU tube | NO2 production | − | − | + | + | + |
Filter paper degradation assay.
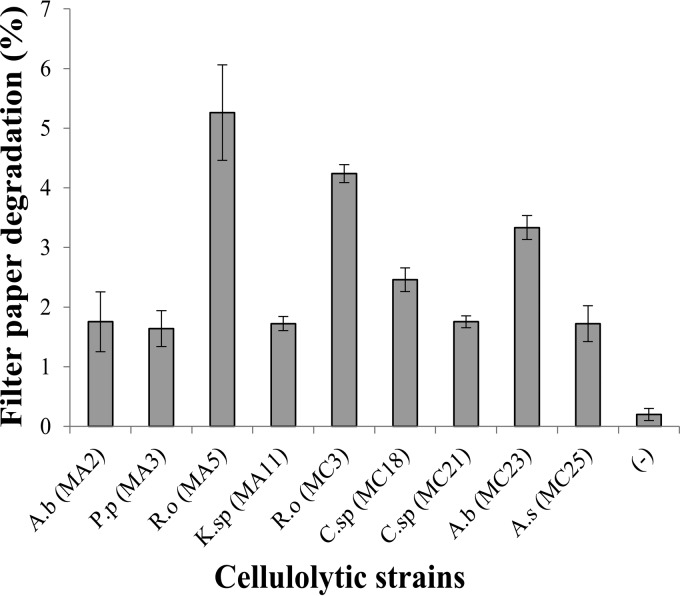
All strains degraded filter paper to some degree, but the most efficient strains were R. ornithinolytica MA5 (5.26% degradation) and MC3 (4.23%) and A. bivalvium MC23 (3.51%) (Fig. 2).
FIG 2.
Filter paper degradation by cellulolytic strains. A.b, Aeromonas bivalvium; P.p, Pseudomonas pseudoalcaligenes; R.o, Raoultella ornithinolytica; K.sp, Klebsiella sp.; C.sp, Chryseobacterium sp.; A.s, Aeromonas salmonicida. Each bar represents the average of results for three replicates per sample. Error bars, standard deviations. All of the results for strains in this assay were significantly different (P, <0.05) from that with the negative control (−, no bacteria).
Degradation of B. braunii.
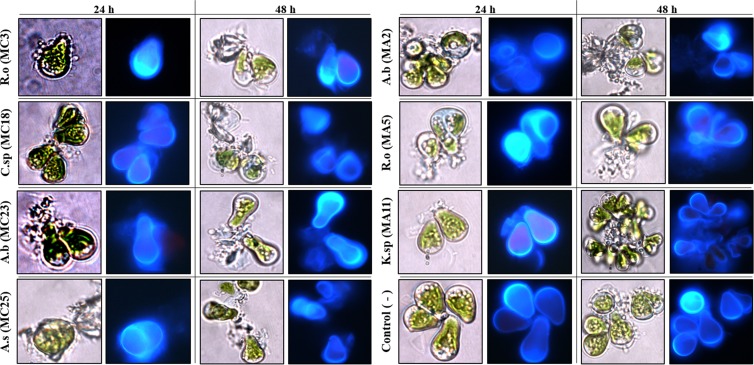
The effect of the “whole-cell” pretreatment was determined by using calcofluor white to dye the microalgal cell wall (Fig. 3). Observation using bright-field and epifluorescence microscopy at 24 and 48 h of pretreatment revealed that A. salmonicida MC25, A. bivalvium MA2, and R. ornithinolytica MA5 altered the morphology of the microalgal cell wall. Furthermore, only dyed cell wall fragments were observed. This indicates that these three strains effectively promoted the degradation of the cell wall of B. braunii.
FIG 3.
Degradation of B. braunii cell walls after pretreatment with cellulose-degrading strains. Bright-field and epifluorescence microscopic visualization was performed at 24 and 48 h of pretreatment. Samples were stained with calcofluor white. R.o, Raoultella ornithinolytica; A.b, Aeromonas bivalvium; C.sp, Chryseobacterium sp.; K.sp, Klebsiella sp.; A.s, Aeromonas salmonicida.
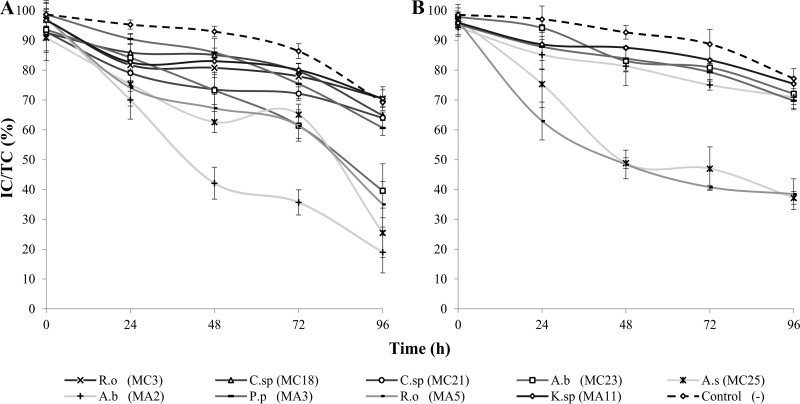
The ratio of intact cells to total cells decreased in all cases between 24 and 48 h of pretreatment, in contrast to the ratio for the control (Fig. 4). A. salmonicida MC25, A. bivalvium MA2, and R. ornithinolytica MA5 showed the greatest microalgal degradation. Survival decreased in the negative control during the test owing to the absence of aeration and the low light level.
FIG 4.
Survival of B. braunii after degradation by cellulolytic bacteria, determined by counting the intact cells (IC) and total cells (TC) over 96 h. (A) Assay at 30°C; (B) assay at 20°C. A.b, Aeromonas bivalvium; P.p, Pseudomonas pseudoalcaligenes; R.o, Raoultella ornithinolytica; K.sp, Klebsiella sp.; C.sp, Chryseobacterium sp.; A.s, Aeromonas salmonicida. Error bars show the standard deviations for three replicates per sample.
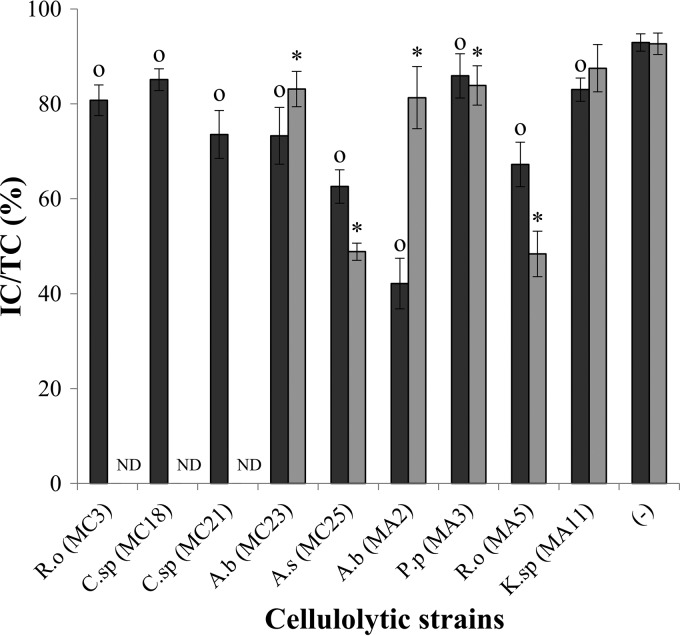
Moreover, important differences in degradation were observed at different temperatures for some strains (Fig. 5). A. salmonicida MC25 and R. ornithinolytica MA5 were more effective at degrading B. braunii at 20°C than at 30°C. However, A. bivalvium MA2, which showed the greatest difference between the temperatures of all strains tested, was more effective at 30°C. Importantly, R. ornithinolytica strain MC3 and Chryseobacterium sp. strains MC18 and MC21 did not grow optimally at 20°C, and therefore, they were not considered in this comparison.
FIG 5.
Survival of B. braunii after degradation by cellulolytic bacteria, expressed as the ratio of intact cells (IC) to total cells (TC), throughout a 48-h incubation at 20°C (gray bars) or 30°C (black bars). A.b, Aeromonas bivalvium; P.p, Pseudomonas pseudoalcaligenes; R.o, Raoultella ornithinolytica; K.sp, Klebsiella sp.; C.sp, Chryseobacterium sp.; A.s, Aeromonas salmonicida. Each bar represents the average of results for three replicates per sample. Error bars, standard deviations. Significant differences (P, < 0.05) between the negative control (−) and the treatment at 20°C (*) or 30°C (O) are indicated. ND, not determined.
Degradation of N. gaditana.
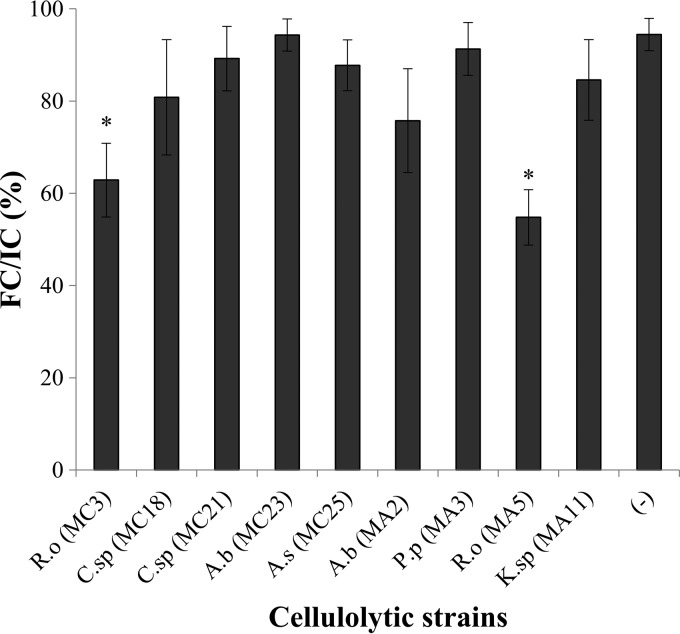
The ratio of final cells to initial cells indicated that the microalgae had a lower cellular survival rate in the presence of most bacterial strains tested than in the presence of the control, with the exception of A. bivalvium MC23 and P. pseudoalcaligenes MA3 (Fig. 6). The strains that produced the greatest N. gaditana degradation were R. ornithinolytica MC3 and MA5, with final cell survival rates of 62.8% and 54.7%, respectively.
FIG 6.
Degradation of N. gaditana by a “whole-cell” enzymatic pretreatment. IC, initial cells at 0 h; FC, final cells after 72 h of pretreatment. A.b, Aeromonas bivalvium; P.p, Pseudomonas pseudoalcaligenes; R.o, Raoultella ornithinolytica; K.sp, Klebsiella sp.; C.sp, Chryseobacterium sp.; A.s, Aeromonas salmonicida. Each bar represents the average of results for three replicates per sample. Error bars, standard deviations. Asterisks indicate significant differences (P, <0.05) between the negative control (−) and the treatment at 30°C.
Effect of microalgal pretreatment on biogas production.
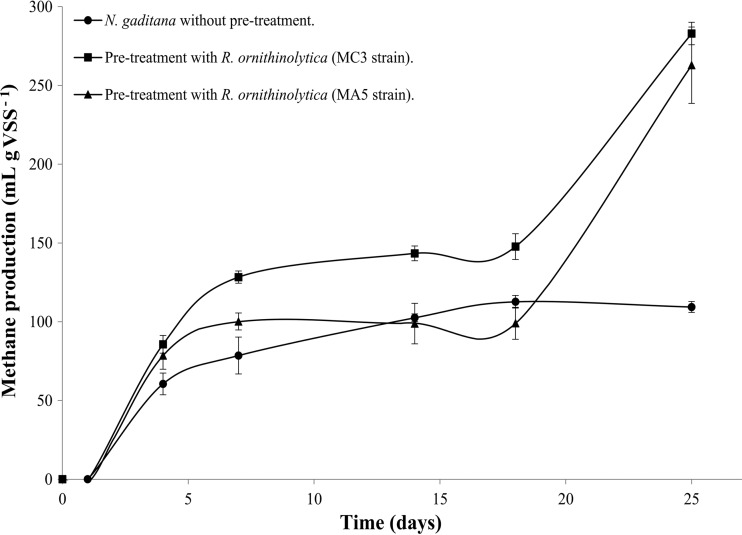
The R. ornithinolytica strains MC3 and MA5 were used in the pretreatment assay because they degraded both species of microalgae well but degraded N. gaditana most effectively. Figure 7 shows levels of methane production from microalgal biomass without pretreatment and from N. gaditana biomass exposed to “whole-cell” pretreatment with R. ornithinolytica strain MC3 or MA5. After 25 days of incubation, the rate of methane production from the nonpretreated microalgae was 109.37 ml g VSS−1, whereas production from biomass pretreated with R. ornithinolytica strain MC3 or MA5 was 262.84 ml g VSS−1 or 282.92 ml g VSS−1, respectively.
FIG 7.
Comparison of methane production from nonpretreated microalgal biomass with methane production from microalgal biomass enzymatically pretreated with cellulolytic “whole-cell” bacteria. Error bars show the standard deviations for three replicates per sample.
DISCUSSION
In this study, despite promising results for CMC agar degradation assays, filter paper degradation, and the degradation of the two microalgal species tested, there appeared to be species-specific differences underlying the results for both the filter paper assay and the N. gaditana degradation assays. In the CMC assay, A. salmonicida strain MC25 presented a large degradation halo of 1.5 cm (Fig. 1) that coincided with the degradation of B. braunii (survival, 48.85% at 20°C) (Fig. 4 and 5). In filter paper assays (Fig. 2) and the N. gaditana degradation assay (Fig. 6), however, strain MC25 did not have the same degradation efficiency. In the same way, A. bivalvium strain MA2 did not degrade CMC agar as efficiently as some other strains (Fig. 1), but it degraded B. braunii with great efficiency (survival, 42.13% at 30°C) (Fig. 4 and 5). The differences observed may be attributed to interspecies differences and are probably due to other enzymatic activities not evaluated in this study.
Moreover, if we compare the effects of the different bacterial strains on microalgal degradation, A. salmonicida strain MC25 and A. bivalvium strain MA2 degraded B. braunii most efficiently (Fig. 4 and 5); however, they did not degrade N. gaditana efficiently (Fig. 6). The R. ornithinolytica strains MC3 and MA5 were the most efficient at degrading N. gaditana (Fig. 6), but MC3 was one of the strains that degraded B. braunii least effectively (Fig. 4 and 5). The differences observed in microalgal degradation could be due to differences in environmental salinity, which, in turn, could affect the metabolism of cellulolytic bacteria and influence the level of microalgal degradation.
The R. ornithinolytica strains MC3 and MA5 produced substantial cell wall degradation in most assays. Moreover, MA5 was positive for most of the specific enzymatic activities in our biochemical characterization, such as expression of β-galactosidase and urease, glucose fermentation/oxidation, and metabolism of mannitol, inositol, sucrose, and arabinose (Table 2). Moreover, strain MA5 was also positive for lysine decarboxylase and tryptophan deaminase, suggesting the presence of peptidase activity. According to the phylogenetic analysis, strains MC3 and MA5 were identified as R. ornithinolytica based on the facts that they produced an arginine dihydrolase (33), were negative for ornithine decarboxylase (34), were positive for lysine decarboxylase and urease (33), and produced citrate. This is consistent with our biochemical characterization results. Other species were identified as belonging to the genus Aeromonas (Table 1), such as A. bivalvium. This species has been isolated previously from bivalve mollusks and has been found to be part of the cellulolytic microbiota predominant in herbivorous fish (35). The remaining species identified have been found in marine environments and also as opportunistic pathogens in humans (29, 31). However, there is no documentation showing that any of these species is capable of degrading cellulose. Moreover, with the exception of P. pseudoalcaligenes, biotechnological applications do not exist for these bacterial species.
In contrast to our study, most research on the isolation of cellulolytic activity in bacteria has been performed in terrestrial environments. However, some studies of marine organisms have described the bacterium Teredinibacter turnerae, isolated from mollusks called “shipworms” that degrade cellulose from the wooden boats to which they adhere (16). However, these bacteria have not been tested for their ability to degrade the microalgal cell wall or applied as a pretreatment.
Other methods of cellular disruption have been used as pretreatments for biogas production. Generally, pretreatments are utilized to increase methane production (14). Schimpf and Valbuena, as well as Ziemiński et al. (36, 37), tested the enzymatic pretreatment of lignocellulosic biomass, which generated an ∼20% increase in methane production over that with nonpretreated biomass. Grala et al. carried out studies with macroalgal biomass (Pilayella, Ectocarpus, and Enteromorpha) using a commercial multienzyme complex as a pretreatment; the amount of methane generated from pretreated biomass was 63.63% higher than that from nonpretreated biomass (38). Our proposed “whole-cell” enzymatic pretreatment for anaerobic digestion is an alternative to previous methods. Moreover, this kind of enzymatic pretreatment is more cost-effective and simpler because it uses whole bacteria, thereby eliminating the need to purify cellulases.
Using microalgae as a substrate in methane production may alter and inhibit the anaerobic digestion process because of (i) ammonium toxicity caused by the high protein content of microalgae, which affects acetoclastic methanogenic bacteria (6, 39), and (ii) the high sodium concentration (140 mM) present in the culture medium, which can inhibit anaerobic bacterial activity (25). Therefore, methane production might be affected if the biomass is from marine microalgae such as N. gaditana, which requires a culture medium with a high NaCl concentration (from 0.5 to 1 M) (26). One means of eliminating salts and avoiding toxicity is to use ultrafiltration membranes, which we indeed utilized in our study.
Pretreatment of microalgal biomass with the R. ornithinolytica cellulolytic strain MA5 or MC3 increased methane production by 140.32% and 158.68%, respectively, over that with nonpretreated biomass. Methane production using pretreatments was developed in two phases: the first phase was from day 1 to day 7, corresponding to the conversion of easily biodegradable substrates, and the second was from day 18 through day 25, corresponding to the slower bioconversion of the portion of the substrates that is more resistant to biodegradation (40).
Based on our results, we conclude that using bacteria with cellulolytic activity as a “whole-cell” enzymatic pretreatment of microalgal biomass from N. gaditana increases methane production to 262.84 ml CH4 g VSS−1 and 282.92 ml CH4 g VSS−1, levels higher than that for nonpretreated biomass (109.37 ml CH4 g VSS−1). These results differ from the methane production results described by Sanchez and Travieso (41), who obtained 315 to 350 ml methane g VSS−1 from Chlorella vulgaris microalgal biomass without pretreatment in a batch reactor. However, methane production is directly related to microalgal cell composition (proteins, carbohydrates, and lipids), which depends both on the species and on the environment (6). Finally, it can be inferred that, owing to the environment from which the bacteria were obtained and the assay temperature (30°C), energy costs were lower with our “whole-cell” enzymatic pretreatment than with the commercial enzymes that are available (commercial enzymes have maximal activity over a temperature range of 50 to 55°C) (42, 43).
ACKNOWLEDGMENTS
This study was funded by Fondecyt 1120488, CICITEM R10C1004, the University of Antofagasta, and the University of La Frontera.
Footnotes
Published ahead of print 2 May 2014
REFERENCES
- 1.Parmar A, Singh NK, Pandey A, Gnansounou E, Madamwar D. 2011. Cyanobacteria and microalgae: a positive prospect for biofuels. Bioresour. Technol. 102:10163–10172. 10.1016/j.biortech.2011.08.030 [DOI] [PubMed] [Google Scholar]
- 2.Singh J, Gu S. 2010. Commercialization potential of microalgae for biofuels production. Renew. Sust. Energ. Rev. 14:2596–2610. 10.1016/j.rser.2010.06.014 [DOI] [Google Scholar]
- 3.Li Y, Horsman M, Wu N, Lan CQ, Dubois-Calero N. 2008. Biofuels from microalgae. Biotechnol. Prog. 24:815–820. 10.1021/bp070371k [DOI] [PubMed] [Google Scholar]
- 4.Chisti Y. 2007. Biodiesel from microalgae. Biotechnol. Adv. 25:294–306. 10.1016/j.biotechadv.2007.02.001 [DOI] [PubMed] [Google Scholar]
- 5.Grobbelaar JU. 2004. Algal nutrition: mineral nutrition, p 97–115 In Richmond A. (ed), Handbook of microalgal culture: biotechnology and applied phycology. Wiley-Blackwell, Oxford, United Kingdom [Google Scholar]
- 6.Sialve B, Bernet N, Bernard O. 2009. Anaerobic digestion of microalgae as a necessary step to make microalgal biodiesel sustainable. Biotechnol. Adv. 27:409–416. 10.1016/j.biotechadv.2009.03.001 [DOI] [PubMed] [Google Scholar]
- 7.Illman A, Scragg A, Shales S. 2000. Increase in Chlorella strains calorific values when grown in low nitrogen medium. Enzyme Microb. Technol. 27:631–635. 10.1016/S0141-0229(00)00266-0 [DOI] [PubMed] [Google Scholar]
- 8.Angelidaki I, Sanders W. 2004. Assessment of the anaerobic biodegradability of macropollutants. Rev. Environ. Sci. Biotechnol. 3:117–129. 10.1007/s11157-004-2502-3 [DOI] [Google Scholar]
- 9.Percival Zhang Y-H, Himmel ME, Mielenz JR. 2006. Outlook for cellulase improvement: screening and selection strategies. Biotechnol. Adv. 24:452–481. 10.1016/j.biotechadv.2006.03.003 [DOI] [PubMed] [Google Scholar]
- 10.Bougrier C, Albasi C, Delgenès JP, Carrère H. 2006. Effect of ultrasonic, thermal and ozone pre-treatments on waste activated sludge solubilisation and anaerobic biodegradability. Chem. Eng. Process. 45:711–718. 10.1016/j.cep.2006.02.005 [DOI] [Google Scholar]
- 11.Halim R, Harun R, Danquah MK, Webley PA. 2012. Microalgal cell disruption for biofuel development. Appl. Energy 91:116–121. 10.1016/j.apenergy.2011.08.048 [DOI] [Google Scholar]
- 12.Sander K, Murthy G. 2009. Enzymatic degradation of microalgal cell walls. An ASABE meeting presentation. Paper no. 1035636. American Society of Agricultural and Biological Engineers, St Joseph, MI. 10.13031/2013.27044 [DOI] [Google Scholar]
- 13.Chisti Y, Moo-Young M. 1986. Disruption of microbial cells for intracellular products. Enzyme Microb. Technol. 8:194–204. 10.1016/0141-0229(86)90087-6 [DOI] [Google Scholar]
- 14.Gerken HG, Donohoe B, Knoshaug EP. 2013. Enzymatic cell wall degradation of Chlorella vulgaris and other microalgae for biofuels production. Planta 237:239–253. 10.1007/s00425-012-1765-0 [DOI] [PubMed] [Google Scholar]
- 15.Doi RH. 2008. Cellulases of mesophilic microorganisms: cellulosome and noncellulosome producers. Ann. N. Y. Acad. Sci. 1125:267-279. 10.1196/annals.1419.002 [DOI] [PubMed] [Google Scholar]
- 16.Distel DL, Morrill W, MacLaren-Toussaint N, Franks D, Waterbury J. 2002. Teredinibacter turnerae gen. nov., sp. nov., a dinitrogen-fixing, cellulolytic, endosymbiotic gamma-proteobacterium isolated from the gills of wood-boring molluscs (Bivalvia: Teredinidae). Int. J. Syst. Evol. Microbiol. 52:2261–2269. 10.1099/ijs.0.02184-0 [DOI] [PubMed] [Google Scholar]
- 17.Guedes AC, Malcata FX. 2012. Nutritional value and uses of microalgae in aquaculture. In Muchlisin Z. (ed), Aquaculture. InTech, Rijeka, Croatia. 10.5772/30576 [DOI] [Google Scholar]
- 18.Robert R, Trintignac P. 1997. Microalgues et nutrition larvaire en écloserie de mollusques. Haliotis 26:1–13 [Google Scholar]
- 19.Samira M, Mohammad R, Gholamreza G. 2011. Carboxymethyl-cellulase and filter-paperase activity of new strains isolated from Persian Gulf. Microbiol. J. 1:8–16. 10.3923/mj.2011.8.16 [DOI] [Google Scholar]
- 20.Kasana RC, Salwan R, Dhar H, Dutt S, Gulati A. 2008. A rapid and easy method for the detection of microbial cellulases on agar plates using Gram‘s iodine. Curr. Microbiol. 57:503–507. 10.1007/s00284-008-9276-8 [DOI] [PubMed] [Google Scholar]
- 21.Lu WJ, Wang HT, Yang SJ, Wang ZC, Nie YF. 2005. Isolation and characterization of mesophilic cellulose-degrading bacteria from flower stalks-vegetable waste co-composting system. J. Gen. Appl. Microbiol. 51:353–360. 10.2323/jgam.51.353 [DOI] [PubMed] [Google Scholar]
- 22.Bazaes J, Sepulveda C, Acién G, Morales J, Gonzales L, Rivas M, Riquelme C. 2012. Outdoor pilot-scale production of Botryococcus braunii in panel reactors. J. Appl. Phycol. 24:1353–1360. 10.1007/s10811-012-9787-3 [DOI] [Google Scholar]
- 23.Guillard RR, Ryther JH. 1962. Studies of marine planktonic diatoms. I. Cyclotella nana Hustedt, and Detonula confervacea (Cleve) Gran. Can. J. Microbiol. 8:229–239 [DOI] [PubMed] [Google Scholar]
- 24.Pouneva I. 1997. Evaluation of algal culture viability and physiological state by fluorescent microscopic methods. Bulg. J. Plant Physiol. 23:67–76 [Google Scholar]
- 25.Rinzema A, van Lier J, Lettinga G. 1988. Sodium inhibition of acetoclastic methanogens in granular sludge from a UASB reactor. Enzyme Microb. Technol. 10:24–32. 10.1016/0141-0229(88)90094-4 [DOI] [Google Scholar]
- 26.Chen PH. 1987. Factors influencing methane fermentation of micro-algae. Ph.D. thesis University of California, Berkeley, CA [Google Scholar]
- 27.Clesceri LS, Greenberg AE, Eaton AD. (ed). 1998. Standard methods for the examination of water and wastewater, 20th ed. American Public Health Association, Washington, DC [Google Scholar]
- 28.Morais VP, Daporta MT, Bao AF, Campello MG, Andrés GQ. 2009. Enteric fever-like syndrome caused by Raoultella ornithinolytica (Klebsiella ornithinolytica). J. Clin. Microbiol. 47:868–869. 10.1128/JCM.01709-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Parker JL, Shaw JG. 2011. Aeromonas spp. clinical microbiology and disease. J. Infect. 62:109–118. 10.1016/j.jinf.2010.12.003 [DOI] [PubMed] [Google Scholar]
- 30.Miñana-Galbis D, Farfán M, Fusté MC, Lorén JG. 2007. Aeromonas bivalvium sp. nov., isolated from bivalve molluscs. Int. J. Syst. Evol. Microbiol. 57:582–587. 10.1099/ijs.0.64497-0 [DOI] [PubMed] [Google Scholar]
- 31.Bernardet JF, Vancanneyt M, Matte-Tailliez O, Grisez L, Tailliez P, Bizet C, Nowakowski M, Kerouault B, Swings J. 2005. Polyphasic study of Chryseobacterium strains isolated from diseased aquatic animals. Syst. Appl. Microbiol. 28:640–660. 10.1016/j.syapm.2005.03.016 [DOI] [PubMed] [Google Scholar]
- 32.Huertas MJ, Sáez LP, Roldán MD, Luque-Almagro VM, Martínez-Luque M, Blasco R, Castillo F, Moreno-Vivián C, García-García I. 2010. Alkaline cyanide degradation by Pseudomonas pseudoalcaligenes CECT5344 in a batch reactor. Influence of pH. J. Hazard. Mater. 179:72–78. 10.1016/j.jhazmat.2010.02.059 [DOI] [PubMed] [Google Scholar]
- 33.García-Lozano T, Plá FJP, Oroval EA. 2013. Raoultella ornithinolytica en infecciones de las vías urinarias. Estudio clínico y microbiológico de una serie de 4 pacientes con neoplasias. Med. Clin. (Barcelona, Spain) 141:138–139. 10.1016/j.medcli.2012.11.021 [DOI] [PubMed] [Google Scholar]
- 34.Park JS, Hong KH, Lee HJ, Choi SH, Song SH, Song K, Kim HB, Park KU, Song J, Kim E. 2011. Evaluation of three phenotypic identification systems for clinical isolates of Raoultella ornithinolytica. J. Med. Microbiol. 60:492–499. 10.1099/jmm.0.020768-0 [DOI] [PubMed] [Google Scholar]
- 35.Jiang Y, Xie C, Yang G, Gong X, Chen X, Xu L, Bao B. 2011. Cellulase-producing bacteria of Aeromonas are dominant and indigenous in the gut of Ctenopharyngodon idellus (Valenciennes). Aquacult. Res. 42:499–505. 10.1111/j.1365-2109.2010.02645.x [DOI] [Google Scholar]
- 36.Schimpf U, Valbuena R. 2009. Increase in efficiency of biomethanation by enzyme application, p 44 In Köhler S. (ed), Wie Viel Biogas Steckt in Pflanzen? Bornimer Agrartechnische Berichte, Potsdam-Bornim, Germany [Google Scholar]
- 37.Ziemiński K, Romanowska I, Kowalska M. 2012. Enzymatic pretreatment of lignocellulosic wastes to improve biogas production. Waste Manag. 32:1131–1137. 10.1016/j.wasman.2012.01.016 [DOI] [PubMed] [Google Scholar]
- 38.Grala A, Zielinski M, Debowski M, Dudek M. 2012. Effects of hydrothermal depolymerization and enzymatic hydrolysis of algae biomass on yield of methane fermentation process. Pol. J. Environ. Stud. 21:363–368 [Google Scholar]
- 39.Angelidaki I, Ahring BK. 1993. Thermophilic anaerobic digestion of livestock waste: the effect of ammonia. Appl. Microbiol. Biotechnol. 38:560–564 [DOI] [PubMed] [Google Scholar]
- 40.Rao MS, Singh SP, Singh AK, Sodha MS. 2000. Bioenergy conversion studies of the organic fraction of MSW: assessment of ultimate bioenergy production potential of municipal garbage. Appl. Energy 66:75–87. 10.1016/S0306-2619(99)00056-2 [DOI] [Google Scholar]
- 41.Sanchez EP, Travieso L. 1993. Anaerobic digestion of Chlorella vulgaris for energy production. Resour. Conserv. Recycl. 9:127–132. 10.1016/0921-3449(93)90037-G [DOI] [Google Scholar]
- 42.Martínez-Anaya C, Balcázar-López E, Dantán-González E, Folch-Mallol JL. 2008. Fungal cellulases. Biological aspects and applications in the energy industry. Rev. Latinoam. Microbiol. 50:119–131 [Google Scholar]
- 43.Lynd LR, Weimer PJ, van Zyl WH, Pretorius IS. 2002. Microbial cellulose utilization: fundamentals and biotechnology. Microbiol. Mol. Biol. Rev. 66:506–577. 10.1128/MMBR.66.3.506-577.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]