Abstract
Lactococcus lactis subsp. cremoris strains are used globally for the production of fermented dairy products, particularly hard cheeses. Believed to be of plant origin, L. lactis strains that are used as starter cultures have undergone extensive adaptation to the dairy environment, partially through the acquisition of extrachromosomal DNA in the form of plasmids that specify technologically important phenotypic traits. Here, we present a detailed analysis of the eight plasmids of L. lactis UC509.9, an Irish dairy starter strain. Key industrial phenotypes were mapped, and genes that are typically associated with lactococcal plasmids were identified. Four distinct, plasmid-borne bacteriophage resistance systems were identified, including two abortive infection systems, AbiB and AbiD1, thereby supporting the observed phage resistance of L. lactis UC509.9. AbiB escape mutants were generated for phage sk1, which were found to carry mutations in orf6, which encodes the major capsid protein of this phage.
INTRODUCTION
Lactococcus lactis strains have been exploited for millennia for the fermentation of dairy products. In modern fermentations, the species is used mostly in defined starter mixes to produce fermented dairy products with consistent organoleptic properties (1).
It is widely believed that the original niche of L. lactis strains is plant based (2). Adaptation to the nutrient-rich dairy environment is reflected in the genomes of industrially exploited strains. Reduced genome sizes, in addition to a higher number of pseudogenes and transposase-encoding genes, highlight the extent of genome decay among industrial lactococcal genomes compared to their plant-associated brethren (2–4). The process of adaptation to the dairy environment via genome decay was recently experimentally demonstrated by monitoring sequence changes in the chromosome of a lactococcal plant isolate used in a dairy fermentation (2). Reductive evolution appears to be especially pronounced for L. lactis subsp. cremoris strains, which tend to have smaller genomes than L. lactis subsp. lactis strains (5) and which are almost exclusively found in dairy fermentation environments, with rare reports of their isolation from plant material (5–7).
Another feature of industrial lactococcal isolates is their extensive plasmid complement (8). Important dairy-associated phenotypes, such as lactose utilization and casein hydrolysis, have long been known to be carried on plasmids (9). A study of 150 L. lactis dairy strains from New Zealand showed that they all possessed a substantial number of plasmids, typically between 6 and 14 (5). The recent availability of the entire extrachromosomal sequence data for several strains has revealed multiple large plasmids (up to 80 kb) that carry genes for a diverse range of functions, including plasmid conjugation and mobilization, exopolysaccharide (EPS) production, bacteriophage resistance, heavy metal resistance, and citrate utilization (10–13).
Bacteriophages that infect L. lactis have been extensively studied due to their associated negative impact on dairy fermentations (14). Owing to their application potential, lactococcal phage resistance systems are among the most intensely characterized antiphage systems (15). Restriction/modification (R/M) and abortive infection (Abi) systems appear to be common bacteriophage resistance mechanisms within L. lactis and are frequently carried on plasmids (16). In contrast, CRISPR-cas systems appear to be very rare in L. lactis, with the only known representative being carried on a plasmid (17).
While individual modes of action remain unknown for many Abi systems, their defining feature is preventing phage proliferation by interfering with some critical aspect of the phage lytic cycle (15, 16). Their mode of action not only impacts phage proliferation, but also leads to the death of the host. This limits the number of progeny produced, thereby protecting the wider bacterial population. There are currently over 20 described lactococcal Abi systems, which affect different stages of phage multiplication, such as major capsid protein production (in the case of AbiC) (18), DNA replication (for AbiA) (19), and transcription (in the case of AbiG) (20). Since these Abi proteins display low (if any at all) levels of similarity to other proteins, their mode of action is usually difficult to predict. Genome sequencing of bacteriophage Abi escape mutants has led to the identification of the molecular triggers, modes of action, and possible interaction sites for several Abi proteins, such as AbiD1 (21), AbiQ (22), AbiT (23), and AbiV (24).
L. lactis subsp. cremoris UC509 is an Irish industrial starter strain isolated in the 1980s from a mixed starter culture (25). It is the lysogenic host to the well-studied lactococcal P335 group phage Tuc2009 (26–28). Recently, the genome and entire plasmid complement of its Tuc2009-cured derivative, L. lactis UC509.9, were sequenced (4). Here, we provide a detailed analysis of the eight plasmids of L. lactis UC509.9 and its plasmid-carried bacteriophage resistance systems.
MATERIALS AND METHODS
Strains used in this study and growth conditions.
Bacterial strains used in this study are listed in Table 1. L. lactis strains were grown in M17 broth or agar (Oxoid, United Kingdom), supplemented with 5 g/liter glucose and incubated overnight at 30°C. Where necessary, tetracycline (Sigma, United Kingdom) was added to growth media at a concentration of 5 μg/ml. For induction of genes that were placed under the transcriptional control of a nisin-inducible promoter (see below), growth medium was supplemented with a 1:2,000 dilution of the cell-free supernatant of the nisin-producing strain L. lactis NZ9700 (29).
TABLE 1.
Strains, plasmids, and bacteriophages used in this study
| Strain, plasmid, or phage | Relevant feature(s) | Reference(s) or source |
|---|---|---|
| Lactococcus lactis subsp. cremoris strains | ||
| UC509.9 | Harbors plasmids pCIS1 to -8, host to bacteriophages Tuc2009 and c2 | 4, 25 |
| UC509.9S1 | L. lactis UC509.9 derivative cured of pCIS3 and pCIS4 | This work |
| UC509.9S2 | L. lactis UC509.9 derivative cured of pCIS1, pCIS3, and pCIS4 | This work |
| NZ9000 | L. lactis MG1363 derivative containing nisRK; host to sk1, jj50, 712, c2 | 29 |
| 158 | Alternative host to bacteriophage Tuc2009 | 64 |
| NZ7000 | Nisin-producing L. lactis strain | 29 |
| Escherichia coli One Shot TOP10 | F− mcrA Δ(mrr-hsdRMS-mcrBC) φ80lacZ ΔM15 ΔlacX74 recA1 araΔ139 Δ(ara-leu)7697 galU galK rpsL (Strr) endA1 nupG | Invitrogen |
| Plasmids | ||
| pPTPL | E. coli-L. lactis promoter-probe vector, Tetr | 34, 35 |
| pPTPLabiD1UC509.9 | pPTPL containing abiD from pCIS8 | This work |
| pPTPi | E. coli-L. lactis shuttle vector, PnisA, Tetr | 35 |
| pPTPiabiBUC509.9 | pPTPi containing abiB from pCIS8 | This work |
| Bacteriophages | ||
| Tuc2009 | P335 species, propagated on 158 | 26 |
| c2 | c2 species, propagated on NZ9000 | 65 |
| sk1 | 936 species, propagated on NZ9000 | 61 |
| jj50 | 936 species, propagated on NZ9000 | 66 |
| 712 | 936 species, propagated on NZ9000 | 66 |
| SK1833-3 | sk1 derivative, AbiB escape mutant | This work |
| SK1833-4 | sk1 derivative, AbiB escape mutant | This work |
| SK1833-5 | sk1 derivative, AbiB escape mutant | This work |
| SK1833-6 | sk1 derivative, AbiB escape mutant | This work |
| SK1833-7 | sk1 derivative, AbiB escape mutant | This work |
| SK1833-8 | sk1 derivative, AbiB escape mutant | This work |
Bacteriophage assays.
Bacteriophages used in this study are listed in Table 1. Bacteriophages were propagated on their respective host strains as previously described, and lysates were maintained at 4°C (30). Spot assays and plaque assays were performed using the overlay method (31). Center-of-infection assays and one-step growth curves were performed in triplicate as previously described (22).
Cloning.
Construction of all plasmids was performed in Escherichia coli One Shot TOP 10 (Invitrogen). All primers were ordered from Eurofins MWG (Ebersberg, Germany). The predicted abi genes, abiBUC509.9 (uc509_p8051) and abiD1UC509.9 (uc509_p80062), identified from the L. lactis UC509.9 plasmid complement, were amplified using KOD DNA polymerase (Invitrogen). Primers for amplification of abiBUC509.9 and abiD1UC509.9 (Table 1) contained BamHI (forward primer) and SphI (reverse primer) restriction sites to allow insertion into the low-copy-number, nisin-inducible vector pPTPi (to generate plasmid pPTPiabiBUC509.9) or plasmid pPTPL (to generate plasmid pPTPLabiDUC509.9), respectively (Table 1) (32, 33). The generated recombinant plasmids were then introduced into L. lactis NZ9000 by electrotransformation.
Plasmid DNA isolation.
Plasmid DNA was isolated using the GeneJET plasmid prep kit (Thermo Scientific, Ireland) with the following modifications for isolation from L. lactis. Cells from a 10-ml overnight culture were harvested (10 min, 3,600 relative centrifugal force [RCF]), resuspended in 250 μl of protoplast buffer (20 mM Tris-HCl [pH 7.5], 5 mM EDTA, 0.75 M sucrose, 10 mg/ml lysozyme [Sigma, United Kingdom], 50 U/ml mutanolysin [Sigma, United Kingdom]), and incubated for 30 min at 37°C. Treated cells were harvested at 3,600 RCF for 10 min, the supernatant was discarded, and the procedure was continued as recommended by the GeneJET kit manufacturer. DNA was visualized via UV transillumination of 0.6% agarose gels stained with ethidium bromide.
Plasmid curing.
Protoplast-induced plasmid curing (34) was carried out as follows: 20-ml cell cultures (optical density at 600 nm, ∼0.35) were harvested by centrifugation (3,600 RCF, 10 min) and washed once in sterile, deionized water. The pellet was resuspended in 5 ml of SGM17 (GM17 plus 0.3 M sucrose), lysozyme was added to a final concentration of 10 mg/ml, and the mixture was incubated for 30 min at 37°C. The cells were harvested by centrifugation at 3,600 RCF for 10 min and washed twice in SGM17. The final pellet was resuspended in 1 ml SGM17, and serial dilutions were plated on SGM17 agar plates. Plates were incubated for 48 h at 30°C. Individual colonies were then picked and screened for plasmid loss by plasmid DNA isolation and subsequent agarose gel electrophoresis as described above. Confirmation of plasmid curing was performed by PCR analysis, using plasmid-specific primers (primer combinations pcis1F [GATATTCCATTTATTCGTTCTG] and pcis1R [AATTTCCTTGTCCACCTTG] to detect plasmid pCIS1; pcis3F [CAAGCCCTAGACCAATTCAG] and pcis3R [GACTCCCAGGTTGTCCA] to detect plasmid pCIS3; pcis4F [CAGAAACTTGGCTTGGATAG] and pcis4R [ATGGCCCGTACTGGATCG] to detect pCIS4. Plasmid-curing PCR confirmation conditions were 95°C for 10 min followed by 30 cycles of 95°C for 15 s, 51°C for 30 s, and 72°C for 1 min, followed by a final extension step at 72°C for 7 min.
Isolation and genome sequencing strategy for AbiB escape mutants.
To isolate AbiBUC509.9 escape mutants of phage sk1, a 1 × 108 PFU per ml sk1 lysate (propagated on L. lactis NZ9000) was used to challenge nisin-induced L. lactis NZ9000 harboring pPTPiabiBUC509.9 by plaque assay. Plaques with a normal appearance (i.e., 2 mm in size and clear, as opposed to pin-point and hazy) were isolated from the resulting overnight lawns, and phages from these plaques (representing presumed sk1-derived AbiBUC509.9 escape mutants) were propagated on cultures of nisin-induced L. lactis NZ9000 pPTPiabiBUC509.9 until complete lysis had occurred. This procedure was repeated three times for each of the six independently isolated mutants (Table 1).
Genome sequencing of one of the AbiBUC509.9 escape mutants of sk1, named SK1833, was performed by using a Sanger sequencing approach (performed by Eurofins MWG, Ebersberg, Germany), employing the bacteriophage sk1 genome sequence (accession number NC_001835.1) for primer design and overlapping, PCR-generated sections of the SK1833 genome as templates. All PCR products were generated using high-fidelity KOD DNA polymerase (Invitrogen), and each amplified region was sequenced at least twice from two independent PCRs. Reads were assembled and mutations identified by using the SeqMan package (version 5.0; DNAStar Lasergene). Mutations were confirmed by sequencing the same region of the predecessor sk1 phage.
Bioinformatic analysis.
The nucleotide sequences of the plasmids of L. lactis UC509.9 were accessed through GenBank (accession numbers CP003165 [pCIS1], CP003164 [pCIS2], CP003163 [pCIS3], CP003162 [pCIS4], CP003161 [pCIS5], CP003160 [pCIS6], CP003159 [pCIS7], and CP003158 [pCIS8]) (4). Putative open reading frames (ORFs) were identified by using Prodigal version 2.0 ORF prediction (35) and BLASTP (36), and the resulting identified ORFs were inspected by using Artemis (37) with manual checking and editing. Domain searches and functional searches of each ORF were performed using the Pfam (38), KEGG (39), and COG databases (40).
Nucleotide sequence accession number.
The genome sequence of SK1833 was submitted to GenBank and assigned accession number KF676640.
RESULTS
General plasmid features.
L. lactis UC509.9 was previously shown to contain eight plasmids, named pCIS1 to pCIS8, ranging in size from 4.3 kb to over 80.5 kb and representing the largest lactococcal plasmid complement sequenced to date, with pCIS8 representing the largest lactococcal plasmid sequenced to date (4). The 6.1-kb plasmid of L. lactis UC509.9, named pCIS3, had previously been sequenced and characterized, revealing that it carries a magnesium transporter and a type I restriction-modification HsdS specificity subunit (41). Total G+C content ranged between 30.1% and 38.5%, which is similar to the GC content of the L. lactis UC509.9 genome (35.8%) and other lactococcal plasmids (8). The total plasmid content represents 202,302 bp of extrachromosomal DNA harboring 193 ORFs, 127 of which can be assigned a putative function, while 25 appear to harbor mutations that render such ORFs pseudogenes. In terms of the total genome (chromosome and plasmids), the plasmid complement represents 9% of total genomic DNA (and 8.5% of the total number of protein-encoding genes).
The majority of these ORFs display high homology with genes located on other lactococcal plasmids or chromosomes. The identified ORFs on plasmids pCIS2 and pCIS4 encode, aside from replication functions (see below), hypothetical proteins with no assignable phenotypic traits. Functions frequently identified on other lactococcal plasmids, such as those encoding EPS production, cold shock proteins, and heavy metal transport (8), are absent from the L. lactis UC509.9 plasmid complement. No single plasmid appears to specify a complete set of proteins required for conjugation or mobilization, suggesting that none of the L. lactis UC509.9 plasmids is (self-)transmissible.
All L. lactis UC509.9 plasmids are predicted to replicate via a theta-type replication mechanism, based on sequence similarity with known theta-type replicons (10, 42). Ten highly homologous theta-type replication protein-encoding genes with associated origins of replication were detected on the eight plasmids, with three such replication functions carried by the biggest plasmid, pCIS8, suggesting that pCIS8 is the result of cointegration of three smaller plasmids. Eight of the replicons belong to the Rep_3 superfamily (PF01051) and RepB_C (PF06430) pfam families and possess characteristic 2.5 22-bp sequence repeats located upstream of the corresponding start codon of the replication protein-encoding gene, whereas the remaining two replicons (represented on pCIS4 and pCIS7) belong exclusively to the Rep_3 superfamily, where the replication protein-encoding gene is preceded by 5 22-bp sequence repeats.
Important technological phenotypes.
Many traits are desirable for L. lactis starter strains to proliferate in the dairy environment, several of which have been described previously (8). Table 2 describes identified L. lactis UC509.9 plasmid-carried properties with industrial significance.
TABLE 2.
Important dairy phenotypes encoded by the plasmid complement of L. lactis UC509.9a
| Plasmid | Gene(s) | Product(s) | Function |
|---|---|---|---|
| pCIS1 | hsdS | Type I restriction enzyme specificity subunit | Bacteriophage resistance |
| pCIS3 | hsdS | Type I restriction enzyme specificity subunit | Bacteriophage resistance |
| corA | CorA family magnesium and cobalt transporter | Increased Mg2+ uptake | |
| pCIS5 | uc509_p5006 | 2-Dehydropantoate 2-reductase | |
| pCIS6 | uc509_p6010 | Peptidase E | Degradation of casein products |
| uc509_p6011 | Pyrrolidone-carboxylate peptidase | Degradation of casein products | |
| corA | CorA family magnesium and cobalt transporter | Increased Mg2+ uptake | |
| dld | d-Lactate dehydrogenase | ATP conversion | |
| uc509_p6021 | Pyridine nucleotide-disulfide oxidoreductase | ||
| prtP/M | Cell envelope proteinase and maturase | Degradation of casein | |
| uc509_p6021b | Glycosylhydrolase | Oligosaccharide degradation | |
| uc509_p6030 | Major facilitator family transporter | ATP generation | |
| pCIS7 | umuC(1) | Low-fidelity polymerase | Stress response |
| uc509_p7009/10 | para-Aminobenzoate synthase component I/II | Folate precursor biosynthesis | |
| umuC(2) | Low-fidelity polymerase | Stress response | |
| uc509_p7029-33 | Lactococcin A biosynthesis and transport | Bacteriocin production | |
| pCIS8 | mntH | Mn2+/Fe2+ transporter | Increased Mn2+ uptake |
| uspA | Universal stress protein | Stress response | |
| lacABCDFEGX | Lactose PEP-PTS utilization operon | Lactose fermentation | |
| pepF | Oligopeptidase F | Degradation of casein products | |
| oppOACBFD | Oligopeptide uptake | Degradation of casein products | |
| aldC | α-Acetolactate decarboxylase | α-Acetolactate degradation | |
| abiB | Abortive infection protein B | Bacteriophage resistance | |
| uc509_p8059a | CHW repeat/cell adhesion domain peptidase | Degradation of casein products | |
| abiD1 | Abortive infection protein D1 | Bacteriophage resistance | |
| uc509_p8069 | Peptidase E | Degradation of casein products | |
| uc509_p8086 | BRCT domain protein | Stress response |
Plasmids pCIS2 and pCIS4 carry genes mostly harboring hypothetical proteins or pseudogenes and therefore were not included in the table.
Not previously associated with L. lactis plasmids.
Two essential phenotypes are required for rapid growth in milk by L. lactis strains: lactose metabolism and casein metabolism (43). The full set of genes that are predicted to specify the lactose-specific phosphoenolpyruvate-phosphotransferase system (PEP-PTS), i.e., lacABCDFEGX, and the putative divergently transcribed repressor-carrying gene, lacR, are located on the largest plasmid, pCIS8. The ability to degrade and metabolize casein is dependent on a cell wall-associated protease and an oligopeptide transport system (44). Similar to other lactococcal plasmid complements (10), this ability is carried across two plasmids, pCIS6 and pCIS8, which harbor genes for the oligopeptide permease (Opp) (45) and the proteinase/maturase system (PrtP/M) (46), respectively.
Flavor development and cheese ripening are dependent on various intracellular enzymes, such as peptidases and amino acid decarboxylases, acting upon casein degradation products once they have been transported into the cell by the oligopeptide system (47). Multiple putative peptidases are carried on the L. lactis UC509.9 plasmid complement. In addition to the chromosomally located pepF, a second copy of this gene, which encodes oligoendopeptidase F, is located on pCIS8 (uc509_p8028). Genes for proteins with peptidase family S51 and dipeptidase E domains are located on pCIS8 (uc509_p8069) and pCIS6 (uc509_p6010), respectively. Also carried on pCIS6 is a predicted pyroglutamyl-peptidase (uc509_p6011) that is responsible for the removal of pyroglutamate from the N termini of peptides.
Bacteriocins are small, ribosomally synthesized antimicrobial peptides produced by lactic acid bacteria (48). Interestingly, the sequence of pCIS7 reveals the presence of two clusters that specify two putative bacteriocins and their associated export and immunity functions; however, only one cluster (uc509_p7029 to uc509_p7033) appears to be intact, since the second cluster (uc509_p7038 to uc509_p7042) contains a bacteriocin-processing protein gene, lcnC (uc509_p7042), in which an internal stop codon is present, thus representing a pseudogene. The intact cluster contains ORFs with homology to the lactococcin A (LcnA)-specifying gene cluster present in L. lactis WM4 (49). This is comprised of four genes, which govern bacteriocin synthesis (lcnA), processing and secretion (lcnCD), and immunity (lciA). Consistent with the above findings, L. lactis UC509.9 was shown to produce at least one protease-sensitive antibacterial activity, which was effective against the bacteriocin-sensitive indicator strain, L. lactis HP (data not shown). In addition, pCIS8 also harbors a gene for a putative EntA_Immun (PF08951) family protein that is thought to confer broad range class II bacteriocin immunity (50).
Characterization of bacteriophage resistance in L. lactis UC509.9.
L. lactis UC509.9 is a highly bacteriophage-resistant strain that is sensitive to infection by bacteriophages Tuc2009 and c2 while being insensitive to all tested 936 group phages currently in our collection (51). The observed bacteriophage resistance may be partly due to plasmid-harbored bacteriophage restriction systems. Based on similarity searches, we identified four bacteriophage resistance systems on three L. lactis UC509.9 plasmids (see below). These resistance systems are present in addition to two predicted chromosomally encoded phage resistance systems, representing a predicted, yet undefined type I R/M system and the type II ScrFI (100% amino acid identity [52]) R/M system. Several experiments were performed in order to define the contribution of the plasmid-carried systems to the overall phage resistance of L. lactis UC509.9.
Stacking of type I R/M systems.
Two genes encoding predicted HsdS specificity subunits of type I restriction-modification systems are located on pCIS1 and pCIS3, the latter of which has previously been studied in detail (41). Type I restriction systems consist of a HsdR restriction subunit, HsdM methylation subunit and a HsdS specificity subunit, all three of which are chromosomally-encoded in L. lactis UC509.9 as three adjacent genes (uc509_0476 to uc509_0478). The holoenzyme of such a Type I R/M system consists of a heterooligomer of HsdM and HsdR subunits, and may utilize different HsdS subunits to broaden its specificity and phage-resistance efficiency (41, 53, 54).
To determine the effect of (loss of) multiple type I specificity subunits on bacteriophage efficiency of plaquing (EOP), plasmid curing by protoplast formation was undertaken to generate strains lacking one or both plasmid-carrried hsdS genes (Fig. 1). Confirmation of plasmid loss was performed by plasmid-specific PCR (data not shown). These derivatives were then challenged with bacteriophage Tuc2009 or c2, which had been propagated on L. lactis 158 and L. lactis NZ9000, respectively, to remove DNA methylation, which would render the bacteriophages insensitive to L. lactis UC509.9 R/M systems. The obtained results (Table 3) showed that the loss of pCIS3 (L. lactis UC509.9S1) leads to a 1,000-fold increase in the EOP for Tuc2009 and a modest 10-fold increase in the EOP for c2, compared to the EOP on L. lactis UC509.9 harboring the complete plasmid complement. The combined effect of the loss of both plasmids/hsdS genes (L. lactis UC509.9S2) further increased the c2 E.O.P. by 100-fold (i.e., the EOP was 1,000-fold higher than that obtained for L. lactis UC509.9), whereas the combined effect on Tuc2009 was minimal (Table 3). Additionally, pCIS4 was lost from both L. lactis UC509.9S1 and UC509.9S2 during plasmid curing (Fig. 1). The loss of pCIS4 does not appear to have any effect on Tuc2009 or C2 infection (data not shown), which is consistent with the observation that pCIS4 does not carry any obvious bacteriophage resistance mechanisms. Confirmation of failure to cure the larger plasmids, pCIS7 and pCIS8, which are not visible by means of standard agarose gel electrophoresis (Fig. 1), was determined with pCIS7- and pCIS8-specific PCR primers (data not shown).
FIG 1.
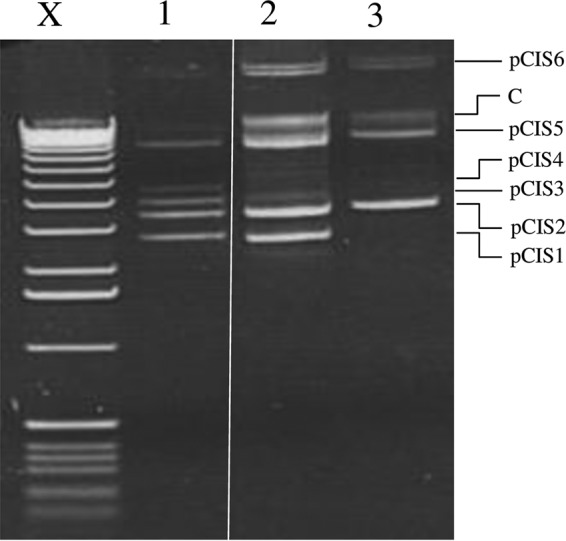
Plasmid profiles of L. lactis UC509.9 and its plasmid-cured derivatives. X, molecular weight marker X (Roche, Germany); lane 1, UC509.9; lane 2, UC509.9S1 (cured of pCIS3 and pCIS4); lane 3, UC509.9S2 (cured of pCIS1, pCIS3, and pCIS4). C, chromosomal DNA.
TABLE 3.
EOP values for L. lactis 158-propagated Tuc2009 and L. lactis NZ9000-propagated c2 on L. lactis UC509.9 (two plasmid-carried copies of hsdS) and plasmid-cured derivatives UC509.9S1 (one plasmid-carried copy of hsdS) and UC509.9S2 (no plasmid-carried hsdS)a
| Strain | EOP |
|
|---|---|---|
| Tuc2009 | c2 | |
| NZ9000 | NA | 1 ± 0.2 |
| 158 | 1 ± 0.1 | NA |
| UC509.9 | (4.1 ± 0.1) × 10−4 | (1.7 ± 0.6) × 10−5 |
| UC509.9S1 | 0.1 ± 0.6 | (1.3 ± 0.6) × 10−4 |
| UC509.9S2 | 0.9 ± 0.2 | (6.8 ± 0.2) × 10−3 |
Results are means ± standard errors and are representative of three independent repeat experiments. NA, not applicable.
L. lactis UC509.9 abortive infection systems.
The largest L. lactis UC509.9 plasmid, pCIS8, harbors clear homologs of abiD1 (21) and abiB (55) (whose protein products exhibit 58 and 94% amino acid identity, respectively); for this reason, they are designated abiD1UC509.9 and abiBUC509.9, respectively. As L. lactis UC509.9 is not susceptible to 936 group bacteriophages of our collection, which is possibly (and partially) due to the presence of these Abi systems, we attempted to cure pCIS8 from L. lactis UC509.9. However, this could not be achieved. Therefore, we employed an alternative strategy whereby the individual abi genes were cloned and expressed in the 936 group-sensitive host L. lactis NZ9000 to determine their effectiveness.
The abiD1UC509.9 gene and its associated upstream region, previously shown to be important for the AbiD abortive infection phenotype (56), were cloned into the low-copy-number vector pPTPL to generate the plasmid pPTPLabiD1uc509.9. L. lactis NZ9000 harboring pPTPLabiD1uc509.9 was challenged with 936 group bacteriophages and was found to provide very moderate, but noticeable and reproducible, resistance against sk1 and 712 (Table 4).
TABLE 4.
EOP values for various 936 bacteriophages on L. lactis NZ9000 harboring pPTPL or pPTPLabiD1UC509.9a
| Phage | pPTPL |
pPTPLabiD1UC509.9 |
||
|---|---|---|---|---|
| EOP on NZ9000 | Plaque size (mm) | EOP on NZ9000 | Plaque size (mm) | |
| sk1 | 1 ± 0.1 | 1.5 ± 0.6 | 0.65 ± 0.3 | ≤0.2 |
| 712 | 1 ± 0.1 | 1.5 ± 0.5 | 0.74 ± 0.1 | ≤0.2 |
| c2 | 1 ± 0.1 | 3 ± 0.6 | 0.65 ± 0.1 | 2 ± 0.5 |
Results are means ± standard errors and are representative of three independent repeat experiments.
Transcription of abiB is dependent on a constitutive promoter (57), while overexpression of AbiB from a high-copy-number plasmid has previously been described as toxic for L. lactis (16). To avoid similar problems with uncontrolled expression of AbiBUC509.9, the corresponding gene was cloned into the low-copy-number, nisin-inducible vector pPTPi. Nisin-induced transcription of abiBUC509.9 from pPTPi did not appear to cause any bacteriostatic effect (data not shown). L. lactis NZ9000 expressing AbiBUC509.9 was challenged with 936 group phages (Table 1). The AbiBUC509.9 system was shown to be highly effective at interfering with sk1 and jj50 proliferation (Table 5), reducing the EOP by approximately 105-fold. Curiously, 936 group bacteriophage 712 appears unaffected by AbiBUC509.9.
TABLE 5.
EOP values and associated mutations of sk1 and sk1-derived AbiBUC509.9 escape mutants on L. lactis NZ9000 cells with or without nisin-induced expression of AbiBUC509.9
| Phagea | EOP on NZ9000 pPTPiabiBUC509.9 |
Gene | Mutation |
|||
|---|---|---|---|---|---|---|
| Uninduced | Induced | Reference positionb | Nucleotide change(s) | Substitution(s) | ||
| sk1 | 1 ± 0.2 | (1.8 ± 0.2) × 10−5 | NA | NA | NA | NA |
| SK1833 | 1 ± 0.2 | 0.8 ± 0.2 | orf6 | 4670 | C → T | L110F |
| orf6 | 4729 | T → C | Synonymous | |||
| SK1834 | 1 ± 0.2 | 0.9 ± 0.1 | orf6 | 4587 | C → T | S82L |
| orf6 | 4703 | G → A | T121A | |||
| SK1835 | 1 ± 0.11 | 0.91 ± 0.1 | orf6 | 4604 | G → A | E88K |
| SK1836 | 1 ± 0.1 | 1 ± 0.2 | orf6 | 4604 | G → A | E88K |
| orf6 | 4712 | C → A | L124I | |||
| SK1837 | 1 ± 0.2 | 0.96 ± 0.1 | orf6 | 4604 | G → A | E88K |
| orf6 | 4734 | C → T | S131L | |||
| SK1838 | 1 ± 0.3 | 0.6 ± 0.3 | orf6 | 4587 | C → T | S82L |
| 712 | 1 ± 0.1 | 1 ± 0.1 | NA | NA | NA | NA |
| c2 | 1 ± 0.1 | 0.1 ± 0.2 | NA | NA | NA | NA |
Bold type denotes a phage whose whole genome was determined. The EOP results are means ± standard errors and are representative of three independent repeat experiments. NA, not applicable.
Nucleotide position, based on the sk1 reference genome (accession number NC_001835.1).
Characterization of an AbiBUC509.9 escape mutant.
Six sk1 escape mutants resistant to AbiBUC509.9 were randomly isolated and propagated in a host that expresses AbiBUC509.9. All six mutants exhibited near-complete insensitivity to AbiBUC509.9 (Table 5).
One escape mutant, SK1833, was further examined to assess its ability to circumvent the action of AbiBUC509.9. One-step growth curves were performed, and the number of effective centers of infection of SK1833 was calculated on L. lactis NZ9000 cells with and without the nisin-induced expression of AbiBUC509.9. The burst size of sk1 on L. lactis NZ9000 harboring pPTPiAbiBUC509 without induction and thus not expressing AbiBUC509.9 was 153 ± 16 virions (mean ± standard error), with an associated latent period of 40 ± 1 min. Similar results were obtained for escape mutant SK1833, with a burst size of 192 ± 17 and a latent period of 41 ± 3 min. Due to aborted infection, the burst size and latent period of sk1 on L. lactis NZ9000 expressing AbiBUC509.9 could not be measured. SK1833, on the other hand, was capable of replication on L. lactis NZ9000 expressing AbiBUC509.9, though with a somewhat reduced burst size of 95 ± 18 and a latent period of 42 ± 1 min. The number of effective centers of infection was also determined for SK1833: in the presence of AbiBUC509.9, the majority of infected cells released virions (80% ± 28%), whereas this number was determined to be 100% (± 10%) in the absence of AbiBUC509.9. These results suggest that mutations obtained by SK1833 as a result of a selective pressure to bypass the antiphage activities of AbiBUC509.9-expressing cells have a relatively small impact on bacteriophage fitness.
To determine the underlying mutations incurred by the AbiBUC509.9 escape mutant, the complete genome of SK1833 was sequenced and compared to that of sk1, from which it was derived. Sequencing revealed two single-nucleotide changes in orf6, which encodes the major capsid protein (23) (Table 5). One of the mutations was synonymous, and therefore the amino acid sequence was unaffected. Sanger sequencing of orf6 of sk1 confirmed that these mutations were not present in the ancestor phage. Sequencing of orf6 from the five other AbiBUC509.9 escape mutants all revealed either single or double mutations located at the 5′ end of orf6 (Table 5).
DISCUSSION
L. lactis UC509.9, a prophage-cured derivative of an Irish dairy starter strain, possesses the smallest lactococcal chromosome currently known; however, the strain harbors an extensive plasmid complement, which includes pCIS8, the largest lactococcal plasmid sequenced to date. The number of plasmids is higher than the six suspected plasmids originally identified by plasmid profiling (41). This difference may be due to poor separation of similarly sized/large plasmids and/or to degradation of the large plasmids pCIS7 and pCIS8 during plasmid isolation. Prophage curing of UC509 was facilitated by mitomycin C exposure, which has long been known to induce plasmid loss (58). Upon generation of UC509.9, no phenotypic changes were observed between UC509 and UC509.9 (except for those related to the loss of Tuc2009), although this does not preclude the possibility that mutations among the resident plasmids may have occurred relative to UC509. Attempts to cure all plasmids from L. lactis UC509.9 by using protoplastation and other methods were unsuccessful, indicating that some of the UC509.9 plasmids carry genes for as-yet-uncharacterized stability functions.
L. lactis UC509.9 is a highly phage-resistant strain. To date, only two phages are known to successfully infect L. lactis UC509.9, the P335 group phage Tuc2009 and phage c2, the namesake of its group (59). No 936 group phage from our collection (51), which are ubiquitous in dairy manufacturing environments and cause the majority of dairy fermentation failures (14), have been found to infect L. lactis UC509.9. This latter phage insensitivity could be, in part, due to the multitude of phage resistance mechanisms both encoded by the L. lactis UC509.9 chromosome (see below) and carried on its plasmids. L. lactis UC509.9 is predicted to produce three type I HsdS restriction enzyme subunits, of which one is chromosomally encoded, while the remaining two are plasmid-borne (specified on plasmids pCIS1 and pCIS3). The HsdS subunit is known to determine the specificity of the type I restriction subunit HsdR, which together with the methylase (HsdM) subunit creates a holoenzyme restriction enzyme. The ability of lactococcal strains such as UC509.9 to “stack” hsdS subunits has been shown previously (41, 53, 54) and was corroborated here by plasmid curing, whereby multiple type I specificities increased the restriction capability, and thus phage resistance, of the strain (Table 3). The differences in sensitivity to the presence of HsdS subunits are likely due to a combination of factors. First, c2 and Tuc2009 were propagated on two different L. lactis strains which may have different indigenous R/M systems, and this may cause different protective methylation of these two phages. Second, there are likely differences in the frequencies of the unknown restriction sites of type I systems between the genomes of Tuc2009 and c2.
L. lactis UC509.9 harbors two plasmid-carried, functional Abi systems, AbiB and AbiD1. It has previously been reported that AbiD1 is effective against c2 and 936 group phages (21, 56), whereas AbiB has been reported to be effective against 936 group phages only (16). Our results demonstrated that AbiBUC509.9 andAbiD1UC509.9 are both functional and effective against 936 group phages. The EOPs of 936 group phages on AbiBUC509.9 were severely reduced, whereas the decrease in the EOPs of 936 group phages on cells harboring AbiD1UC509.9 was modest, with plaques appearing pin-point and hazy, suggesting that the burst size of the bacteriophage was affected, which is in agreement with previous results (56). The AbiD1 system is believed to be activated by the gene product of orf1, which is located in the middle region of 936 phage bIL66 (21). There are no homologs of bIL66 orf1 in c2 or the P335 group phage Tuc2009, which may explain why these phages can still infect UC509.9, although it has been shown for AbiQ that unrelated gene products from different lactococcal phage groups may play a recognition or activation role for a given Abi system (22).
Many Abi systems do not exhibit similarity to functionally characterized proteins (other than to other Abi systems) or domains, making postulations regarding their mode of action difficult (16). We generated and characterized bacteriophage escape mutants of sk1 that were insensitive to AbiBUC509.9. Interestingly, previous attempts to obtain escape mutants for AbiB were not successful (16). Similar to the unrelated AbiT system, we found that AbiBUC509.9 escape mutants harbor single- or double-nucleotide changes in orf6, the major capsid protein (23). Unlike AbiT-mediated escape mutants, where all identified mutations affected the C-terminal part of the translated product of the affected gene, AbiBUC509.9-mediated escape mutants carry genetic alterations located within a 100-bp region of the 5′ portion of orf6.
It has previously been shown that infection of AbiB-expressing cells results in the degradation of all phage transcripts during expression of the structural region of 936 group phages, leading to the suggestion that AbiB either possesses a latent RNase activity or activates a cellular RNase (55). Additionally, blocking of phage protein synthesis during infection of cells harboring abiB, by using chloramphenicol, demonstrated that a phage protein is required during the early stages of infection to confer the AbiB phenotype (16, 60). Considering these previously reported findings, and the fact that multiple degradation products were detected by Parreira and colleagues (55), in combination with our results, which revealed single- or double-nucleotide mutations in orf6, clearly suggests that insensitivity to AbiB is not due to mutations in RNase restriction sites. Therefore, it is possible that synthesis and/or activation of AbiBUC509.9 is facilitated by (a portion of) the major capsid protein. Previous analysis of phage transcripts during infection of AbiB cells detected extensive degradation of phage transcripts 15 min postinfection. orf6 resides in the late sk1 transcript region, which is predicted to be expressed 15 min postinfection on the basis of Northern analysis, therefore correlating well with previous data (61).
Abi systems that require activation by either enhancement of translational stability or unmasking of a latent activity have previously been described. As mentioned above, expression of AbiD1 is positively regulated by the presence of the bIL66 orf1 gene product, which stabilizes abiD1-encompassing mRNA, a phenomenon that does not occur in escape mutants of bIL66 (21). Activation of latent Abi mechanisms in the presence of a phage gene product has also been demonstrated in E. coli. Induction of latent anticodon RNase activity of the Abi tRNA ribotoxin PrrC is regulated by a small phage T4-carried gene product, termed Stp (62). PrrC-mediated escape mutants of phage T4 were shown to carry mutations in stp. Further experiments aimed at elucidating the AbiBUC509.9 mechanism of activation and subsequent action are ongoing.
As mentioned above, the parent strain of L. lactis UC509.9, L. lactis UC509, harbors the prophage Tuc2009 (27). Tuc2009 carries genes for a well-characterized superinfection exclusion protein (Sie2009), which confers resistance to certain phages belonging to the 936 group (63). In addition to these, the chromosomally encoded type II restriction enzyme ScrF1 (52) endows L. lactis UC509 with a sixth phage resistance mechanism. Based on the combined results of our EOP experiments (Tables 3, 4, and 5), we predict that these resistance mechanisms make the strain virtually impenetrable to 936 group bacteriophages, although additional, chromosomally or plasmid-carried phage resistance systems can of course not be ruled out.
ACKNOWLEDGMENT
This work was funded through a Science Foundation Ireland (SFI) Principal Investigator award (08/IN.1/B1909) to D.V.S.
Footnotes
Published ahead of print 9 May 2014
REFERENCES
- 1.de Vos WM. 2011. Systems solutions by lactic acid bacteria: from paradigms to practice. Microb. Cell Fact. 10(Suppl 1):S2. 10.1186/1475-2859-10-S1-S2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bachmann H, Starrenburg MJ, Molenaar D, Kleerebezem M, van Hylckama Vlieg JE. 2012. Microbial domestication signatures of Lactococcus lactis can be reproduced by experimental evolution. Genome Res. 22:115–124. 10.1101/gr.121285.111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Makarova K, Slesarev A, Wolf Y, Sorokin A, Mirkin B, Koonin E, Pavlov A, Pavlova N, Karamychev V, Polouchine N, Shakhova V, Grigoriev I, Lou Y, Rohksar D, Lucas S, Huang K, Goodstein DM, Hawkins T, Plengvidhya V, Welker D, Hughes J, Goh Y, Benson A, Baldwin K, Lee JH, Diaz-Muniz I, Dosti B, Smeianov V, Wechter W, Barabote R, Lorca G, Altermann E, Barrangou R, Ganesan B, Xie Y, Rawsthorne H, Tamir D, Parker C, Breidt F, Broadbent J, Hutkins R, O'Sullivan D, Steele J, Unlu G, Saier M, Klaenhammer T, Richardson P, Kozyavkin S, Weimer B, Mills D. 2006. Comparative genomics of the lactic acid bacteria. Proc. Natl. Acad. Sci. U. S. A. 103:15611–15616. 10.1073/pnas.0607117103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ainsworth S, Zomer A, de Jager V, Bottacini F, van Hijum SA, Mahony J, van Sinderen D. 2013. Complete genome of Lactococcus lactis subsp. cremoris UC509.9, host for a model lactococcal P335 bacteriophage. Genome Announc. 1(1):e00119-12. 10.1128/genomeA.00036-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kelly WJ, Ward LJ, Leahy SC. 2010. Chromosomal diversity in Lactococcus lactis and the origin of dairy starter cultures. Genome Biol. Evol. 2:729–744. 10.1093/gbe/evq056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Klijn N, Weerkamp AH, de Vos WM. 1995. Detection and characterization of lactose-utilizing Lactococcus spp. in natural ecosystems. Appl. Environ. Microbiol. 61:788–792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Salama MS, Musafija-Jeknic T, Sandine WE, Giovannoni SJ. 1995. An ecological study of lactic acid bacteria: isolation of new strains of Lactococcus including Lactococcus lactis subspecies cremoris. J. Dairy Sci. 78:1004–1017. 10.3168/jds.S0022-0302(95)76716-9 [DOI] [Google Scholar]
- 8.Mills S, McAuliffe OE, Coffey A, Fitzgerald GF, Ross RP. 2006. Plasmids of lactococci: genetic accessories or genetic necessities? FEMS Microbiol. Rev. 30:243–273. 10.1111/j.1574-6976.2005.00011.x [DOI] [PubMed] [Google Scholar]
- 9.Efstathiou JD, McKay LL. 1977. Inorganic salts resistance associated with a lactose-fermenting plasmid in Streptococcus lactis. J. Bacteriol. 130:257–265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Siezen RJ, Renckens B, van Swam I, Peters S, van Kranenburg R, Kleerebezem M, de Vos WM. 2005. Complete sequences of four plasmids of Lactococcus lactis subsp. cremoris SK11 reveal extensive adaptation to the dairy environment. Appl. Environ. Microbiol. 71:8371–8382. 10.1128/AEM.71.12.8371-8382.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fallico V, Ross RP, Fitzgerald GF, McAuliffe O. 2012. Novel conjugative plasmids from the natural isolate Lactococcus lactis subspecies cremoris DPC3758: a repository of genes for the potential improvement of dairy starters. J. Dairy. Sci. 95:3593–3608. 10.3168/jds.2011-5255 [DOI] [PubMed] [Google Scholar]
- 12.Bolotin A, Quinquis B, Ehrlich SD, Sorokin A. 2012. Complete genome sequence of Lactococcus lactis subsp. cremoris A76. J. Bacteriol. 194:1241–1242. 10.1128/JB.06629-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gorecki RK, Koryszewska-Baginska A, Golebiewski M, Zylinska J, Grynberg M, Bardowski JK. 2011. Adaptative potential of the Lactococcus lactis IL594 strain encoded in its 7 plasmids. PLoS One 6(7):e22238. 10.1371/journal.pone.0022238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mahony J, Murphy J, van Sinderen D. 2012. Lactococcal 936-type phages and dairy fermentation problems: from detection to evolution and prevention. Front. Microbiol. 3:335. 10.3389/fmicb.2012.00335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Labrie SJ, Samson JE, Moineau S. 2010. Bacteriophage resistance mechanisms. Nat. Rev. Microbiol. 8:317–327. 10.1038/nrmicro2315 [DOI] [PubMed] [Google Scholar]
- 16.Chopin MC, Chopin A, Bidnenko E. 2005. Phage abortive infection in lactococci: variations on a theme. Curr. Opin. Microbiol. 8:473–479. 10.1016/j.mib.2005.06.006 [DOI] [PubMed] [Google Scholar]
- 17.Millen AM, Horvath P, Boyaval P, Romero DA. 2012. Mobile CRISPR/Cas-mediated bacteriophage resistance in Lactococcus lactis. PLoS One 7:e51663. 10.1371/journal.pone.0051663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Klaenhammer TR, Sanozky RB. 1985. Conjugal transfer from Streptococcus lactis ME2 of plasmids encoding phage resistance, nisin resistance and lactose-fermenting ability: evidence for a high-frequency conjugative plasmid responsible for abortive infection of virulent bacteriophage. J. Gen. Microbiol. 131:1531–1541 [DOI] [PubMed] [Google Scholar]
- 19.Hill C, Miller LA, Klaenhammer TR. 1990. Nucleotide sequence and distribution of the pTR2030 resistance determinant (hsp) which aborts bacteriophage infection in lactococci. Appl. Environ. Microbiol. 56:2255–2258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.O'Connor L, Tangney M, Fitzgerald GF. 1999. Expression, regulation, and mode of action of the AbiG abortive infection system of Lactococcus lactis subsp. cremoris UC653. Appl. Environ. Microbiol. 65:330–335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bidnenko E, Chopin A, Ehrlich SD, Chopin MC. 2009. Activation of mRNA translation by phage protein and low temperature: the case of Lactococcus lactis abortive infection system AbiD1. BMC Mol. Biol. 10:4. 10.1186/1471-2199-10-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Samson JE, Belanger M, Moineau S. 2013. Effect of the abortive infection mechanism and type III toxin/antitoxin system AbiQ on the lytic cycle of Lactococcus lactis phages. J. Bacteriol. 195:3947. 10.1128/JB.00296-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Labrie SJ, Tremblay DM, Moisan M, Villion M, Magadan AH, Campanacci V, Cambillau C, Moineau S. 2012. Involvement of the major capsid protein and two early-expressed phage genes in the activity of the lactococcal abortive infection mechanism AbiT. Appl. Environ. Microbiol. 78:6890–6899. 10.1128/AEM.01755-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Haaber J, Samson JE, Labrie SJ, Campanacci V, Cambillau C, Moineau S, Hammer K. 2010. Lactococcal abortive infection protein AbiV interacts directly with the phage protein SaV and prevents translation of phage proteins. Appl. Environ. Microbiol. 76:7085–7092. 10.1128/AEM.00093-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Costello V. 1988. Characterization of bacteriophage-host interactions in Streptococcus cremoris UC503 and related lactic streptococci. Ph.D. thesis National University of Ireland, University College Cork, Cork, Ireland [Google Scholar]
- 26.Seegers JFML, Mc Grath S, O'Connell-Motherway M, Arendt EK, van de Guchte M, Creaven M, Fitzgerald GF, van Sinderen D. 2004. Molecular and transcriptional analysis of the temperate lactococcal bacteriophage Tuc2009. Virology 329:40–52. 10.1016/j.virol.2004.07.003 [DOI] [PubMed] [Google Scholar]
- 27.Collins B, Bebeacua C, Mahony J, Blangy S, Douillard F, Veesler D, Cambillau C, van Sinderen D. 2013. Structure and functional analysis of the host recognition device of lactococcal phage Tuc2009. J. Virol. 87:8429–8440. 10.1128/JVI.00907-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stockdale SR, Mahony J, Courtin P, Chapot-Chartier MP, van Pijkeren JP, Britton RA, Neve H, Heller KJ, Aideh B, Vogensen FK, van Sinderen D. 2013. The lactococcal phages Tuc2009 and TP901-1 incorporate two alternate forms of their tail fibre into their virions for infection specialization. J. Biol. Chem. 288:5581–5590. 10.1074/jbc.M112.444901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kuipers OP, de Ruyter PGGA, Kleerebezem M, de Vos WM. 1998. Quorum sensing-controlled gene expression in lactic acid bacteria. J. Biotechnol. 64:15–21. 10.1016/S0168-1656(98)00100-X [DOI] [Google Scholar]
- 30.Mahony J, McGrath S, Fitzgerald GF, van Sinderen D. 2008. Identification and characterization of lactococcal-prophage-carried superinfection exclusion genes. Appl. Environ. Microbiol. 74:6206–6215. 10.1128/AEM.01053-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lillehaug D. 1997. An improved plaque assay for poor plaque-producing temperate lactococcal bacteriophages. J. Appl. Microbiol. 83:85–90. 10.1046/j.1365-2672.1997.00193.x [DOI] [PubMed] [Google Scholar]
- 32.Burgess C, O'Connell-Motherway M, Sybesma W, Hugenholtz J, van Sinderen D. 2004. Riboflavin production in Lactococcus lactis: potential for in situ production of vitamin-enriched foods. Appl. Environ. Microbiol. 70:5769–5777. 10.1128/AEM.70.10.5769-5777.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.O'Driscoll J, Glynn F, Cahalane O, O'Connell-Motherway M, Fitzgerald GF, Van Sinderen D. 2004. Lactococcal plasmid pNP40 encodes a novel, temperature-sensitive restriction-modification system. Appl. Environ. Microbiol. 70:5546–5556. 10.1128/AEM.70.9.5546-5556.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gasson MJ. 1983. Plasmid complements of Streptococcus lactis NCDO 712 and other lactic streptococci after protoplast-induced curing. J. Bacteriol. 154:1–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hyatt D, Chen GL, Locascio PF, Land ML, Larimer FW, Hauser LJ. 2010. Prodigal: prokaryotic gene recognition and translation initiation site identification. BMC Bioinformatics 8:119. 10.1186/1471-2105-11-119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Altschul SF, Madden TL, Schaffer AA, Zhang JH, Zhang Z, Miller W, Lipman DJ. 1997. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 25:3389–3402. 10.1093/nar/25.17.3389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rutherford K, Parkhill J, Crook J, Horsnell T, Rice P, Rajandream MA, Barrell B. 2000. Artemis: sequence visualization and annotation. Bioinformatics 16:944–945. 10.1093/bioinformatics/16.10.944 [DOI] [PubMed] [Google Scholar]
- 38.Finn RD, Mistry J, Tate J, Coggill P, Heger A, Pollington JE, Gavin OL, Gunasekaran P, Ceric G, Forslund K, Holm L, Sonnhammer EL, Eddy SR, Bateman A. 2010. The Pfam protein families database. Nucleic Acids Res. 38:D211–D222. 10.1093/nar/gkp985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kanehisa M, Goto S. 2000. KEGG: Kyoto encyclopedia of genes and genomes. Nucleic Acids Res. 28:27–30. 10.1093/nar/28.1.27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tatusov RL, Fedorova ND, Jackson JD, Jacobs AR, Kiryutin B, Koonin EV, Krylov DM, Mazumder R, Mekhedov SL, Nikolskaya AN, Rao BS, Smirnov S, Sverdlov AV, Vasudevan S, Wolf YI, Yin JJ, Natale DA. 2003. The COG database: an updated version includes eukaryotes. BMC Bioinformatics 4:41. 10.1186/1471-2105-4-41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Seegers JF, van Sinderen D, Fitzgerald GF. 2000. Molecular characterization of the lactococcal plasmid pCIS3: natural stacking of specificity subunits of a type I restriction/modification system in a single lactococcal strain. Microbiology 146:435–443 http://mic.sgmjournals.org/content/146/2/435.long [DOI] [PubMed] [Google Scholar]
- 42.Kiewiet R, Bron S, de Jonge K, Venema G, Seegers JF. 1993. Theta replication of the lactococcal plasmid pWVO2. Mol. Microbiol. 10:319–327. 10.1111/j.1365-2958.1993.tb01958.x [DOI] [PubMed] [Google Scholar]
- 43.McKay LL, Baldwin KA. 1974. Simultaneous loss of proteinase- and lactose-utilizing enzyme activities in Streptococcus lactis and reversal of loss by transduction. Appl. Microbiol. 28:342–346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yu W, Gillies K, Kondo JK, Broadbent JR, McKay LL. 1996. Loss of plasmid-mediated oligopeptide transport system in lactococci: another reason for slow milk coagulation. Plasmid 35:145–155. 10.1006/plas.1996.0017 [DOI] [PubMed] [Google Scholar]
- 45.Yu W, Gillies K, Kondo JK, Broadbent JR, McKay LL. 1995. Plasmid-mediated oligopeptide transport system in lactococci. Dev. Biol. Stand. 85:509–521 [PubMed] [Google Scholar]
- 46.Savijoki K, Ingmer H, Varmanen P. 2006. Proteolytic systems of lactic acid bacteria. Appl. Microbiol. Biot. 71:394–406. 10.1007/s00253-006-0427-1 [DOI] [PubMed] [Google Scholar]
- 47.Steele J, Broadbent J, Kok J. 2013. Perspectives on the contribution of lactic acid bacteria to cheese flavor development. Curr. Opin. Biotechnol. 24:135–141. 10.1016/j.copbio.2012.12.001 [DOI] [PubMed] [Google Scholar]
- 48.Cotter PD, Hill C, Ross RP. 2005. Bacteriocins: developing innate immunity for food. Nat. Rev. Microbiol. 3:777–788. 10.1038/nrmicro1273 [DOI] [PubMed] [Google Scholar]
- 49.Stoddard GW, Petzel JP, Vanbelkum MJ, Kok J, Mckay LL. 1992. Molecular analyses of the lactococcin A gene-cluster from Lactococcus lactis subsp. lactis biovar. diacetylactis Wm4. Appl. Environ. Microbiol. 58:1952–1961 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Johnsen L, Dalhus B, Leiros I, Nissen-Meyer J. 2005. 1.6-Å crystal structure of EntA-im. A bacterial immunity protein conferring immunity to the antimicrobial activity of the pediocin-like bacteriocin enterocin A. J. Biol. Chem. 280:19045–19050. 10.1074/jbc.M501386200 [DOI] [PubMed] [Google Scholar]
- 51.Mahony J, Kot W, Murphy J, Ainsworth S, Neve H, Hansen LH, Heller KJ, Sørensen SJ, Hammer K, Cambillau C, Vogensen FK, van Sinderen D. 2013. Investigation of the relationship between lactococcal host cell wall polysaccharide genotype and 936 phage receptor binding protein phylogeny. Appl. Environ. Microbiol. 79:4385–4392. 10.1128/AEM.00653-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Davis R, van der Lelie D, Mercenier A, Daly C, Fitzgerald GF. 1993. ScrFI restriction-modification system of Lactococcus lactis subsp. cremoris UC503: cloning and characterization of two ScrFI methylase genes. Appl. Environ. Microbiol. 59:777–785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.O'Sullivan D, Twomey DP, Coffey A, Hill C, Fitzgerald GF, Ross RP. 2000. Novel type I restriction specificities through domain shuffling of HsdS subunits in Lactococcus lactis. Mol. Microbiol. 36:866–875. 10.1046/j.1365-2958.2000.01901.x [DOI] [PubMed] [Google Scholar]
- 54.Schouler C, Gautier M, Ehrlich SD, Chopin MC. 1998. Combinational variation of restriction modification specificities in Lactococcus lactis. Mol. Microbiol. 28:169–178 [DOI] [PubMed] [Google Scholar]
- 55.Parreira R, Ehrlich SD, Chopin MC. 1996. Dramatic decay of phage transcripts in lactococcal cells carrying the abortive infection determinant AbiB. Mol. Microbiol. 19:221–230. 10.1046/j.1365-2958.1996.371896.x [DOI] [PubMed] [Google Scholar]
- 56.Anba J, Bidnenko E, Hillier A, Ehrlich D, Chopin MC. 1995. Characterization of the lactococcal abiD1 gene coding for phage abortive infection. J. Bacteriol. 177:3818–3823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Cluzel PJ, Chopin A, Ehrlich SD, Chopin MC. 1991. Phage abortive infection mechanism from Lactococcus lactis subsp. lactis, expression of which is mediated by an Iso-ISS1 element. Appl. Environ. Microbiol. 57:3547–3551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Dunn N, Gunsalus I. 1973. Transmissible plasmid coding early enzymes of naphthalene oxidation in Pseudomonas putida. J. Bacteriol. 114:974–979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Deveau H, Labrie SJ, Chopin MC, Moineau S. 2006. Biodiversity and classification of lactococcal phages. Appl. Environ. Microbiol. 72:4338–4346. 10.1128/AEM.02517-05 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Parreira R. 1996. Characterization of the abiB-encoded phage abortive infection mechanism of Lactococcus lactis subsp. lactis. Ph.D. dissertation. Université de Paris XI, Paris, France [Google Scholar]
- 61.Chandry PS, Moore SC, Boyce JD, Davidson BE, Hillier AJ. 1997. Analysis of the DNA sequence, gene expression, origin of replication and modular structure of the Lactococcus lactis lytic bacteriophage sk1. Mol. Microbiol. 26:49–64. 10.1046/j.1365-2958.1997.5491926.x [DOI] [PubMed] [Google Scholar]
- 62.Snyder L. 1995. Phage-exclusion enzymes: a bonanza of biochemical and cell biology reagents? Mol. Microbiol. 15:415–420. 10.1111/j.1365-2958.1995.tb02255.x [DOI] [PubMed] [Google Scholar]
- 63.McGrath S, Seegers JF, Fitzgerald GF, van Sinderen D. 1999. Molecular characterization of a phage-encoded resistance system in Lactococcus lactis. Appl. Environ. Microbiol. 65:1891–1899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Jarvis AW. 1984. Differentiation of lactic streptococcal phages into phage species by DNA-DNA homology. Appl. Environ. Microbiol. 47:343–349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lubbers MW, Waterfield NR, Beresford TP, Le Page RW, Jarvis AW. 1995. Sequencing and analysis of the prolate-headed lactococcal bacteriophage c2 genome and identification of the structural genes. Appl. Environ. Microbiol. 61:4348–4356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Mahony J, Deveau H, Mc Grath S, Ventura M, Canchaya C, Moineau S, Fitzgerald GF, van Sinderen D. 2006. Sequence and comparative genomic analysis of lactococcal bacteriophages jj50, 712 and P008: evolutionary insights into the 936 phage species. FEMS Microbiol. Lett. 261:253–261. 10.1111/j.1574-6968.2006.00372.x [DOI] [PubMed] [Google Scholar]


