Abstract
Bifidobacteria constitute a specific group of commensal bacteria that inhabit the gastrointestinal tracts of humans and other mammals. Bifidobacterium breve UCC2003 has previously been shown to utilize several plant-derived carbohydrates that include cellodextrins, starch, and galactan. In the present study, we investigated the ability of this strain to utilize the mucin- and human milk oligosaccharide (HMO)-derived carbohydrate sialic acid. Using a combination of transcriptomic and functional genomic approaches, we identified a gene cluster dedicated to the uptake and metabolism of sialic acid. Furthermore, we demonstrate that B. breve UCC2003 can cross feed on sialic acid derived from the metabolism of 3′-sialyllactose, an abundant HMO, by another infant gut bifidobacterial strain, Bifidobacterium bifidum PRL2010.
INTRODUCTION
Bifidobacteria are Gram-positive, anaerobic, typically Y-shaped bacteria that are members of the Bifidobacteriaceae family and the Actinobacteria phylum. Discovered in 1900 (1), bifidobacteria are naturally found in the digestive tracts of mammals and insects but are also isolated from the human oral cavity and sewage (2). The large intestine is the natural habitat for a large and diverse bacterial community, which is known to contribute significantly to the well-being of humans. This positive contribution includes metabolic activities to provide energy and nutrients to the host (3, 4), development of the immune system (5, 6), and protection against pathogenic bacteria (7).
Bifidobacteria are saccharolytic organisms, and their survival and growth in the large intestine require a variety of extracellular and cytoplasmic glycosyl hydrolases, which they employ to metabolize carbohydrates prevalent in this environment. The characteristic central metabolic pathway in bifidobacteria is the fructose-6-phosphate phosphoketolase pathway, also known as the bifid shunt (8). Because of the diversity of carbohydrate sources present in the gastrointestinal tract and the dependence of bifidobacteria on such carbon and energy sources, it is not surprising that an estimated 8% of the typical bifidobacterial genome is devoted to carbohydrate metabolism (2, 9).
Bifidobacterium breve UCC2003 has already been shown to utilize quite a variety of plant-derived poly-, oligo-, and monosaccharides, including cellodextrins, galactan, starch, raffinose, and melezitose (10–15), which reflects the apparent flexibility of the carbohydrate utilization profile of this strain. Interestingly, B. breve strains are numerically prevalent in the gut microbiota of healthy, breast-fed infants (16), although the ability of members of this bifidobacterial species to utilize human milk and host-derived carbohydrates is relatively unexplored (see below).
Sialic acids comprise a large family of nine-carbon monosaccharides called neuraminic acids, the most common of which is N-acetylneuraminic acid (Neu5Ac), for which the name of sialic acid is also used. Approximately 16% of human milk oligosaccharides (HMOs) are sialylated (17), with 3′-sialyllactose and 6′-sialyllactose being abundant sialylated HMO components (18–20). The total sialic acid concentrations are highest in colostrum, reaching levels of 5.04 mmol liter−1, and they subsequently decrease by almost 80% after 3 months (21). Sialic acid also features prominently at the surface-exposed end of human colonic mucin (22), and in healthy adults, approximately 300 μg of sialic acid/mg of colonic mucin is present (23). The concentration of sialic acid in gastric juice has been found to decrease with age, with levels of 100 μg ml−1 in young adults <25 years old compared to less than 20 μg ml−1 in elderly people (24).
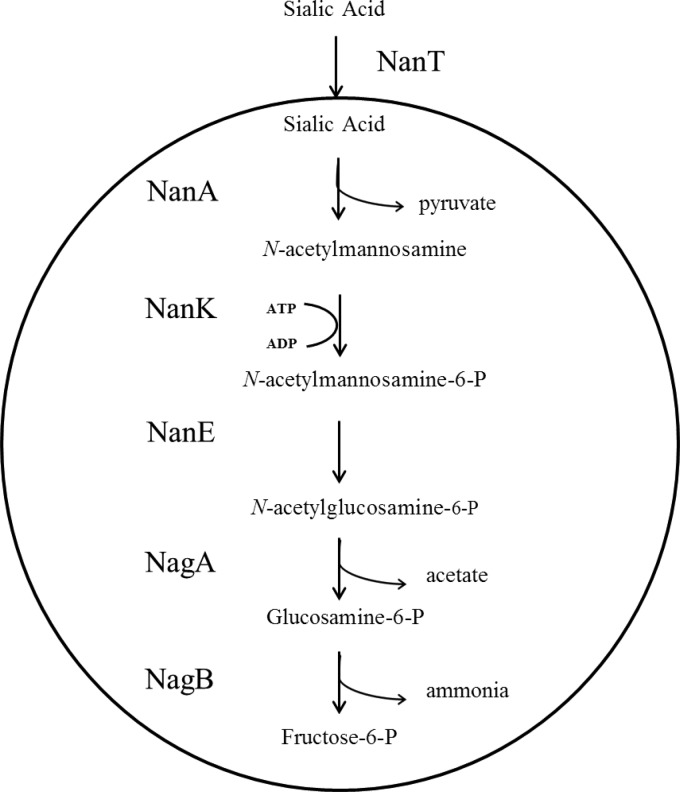
Representative Clostridium perfringens strains were the first bacteria shown to be capable of utilizing sialic acid as the sole carbon source (25, 26). Nevertheless, sialic acid metabolism was first explored in 1983 in Escherichia coli, with the identification of a transporter, designated nanT, and an N-acetylneuraminate lyase, designated nanA (27, 28), followed by the description of the complete, five-step metabolic pathway in 1999 (Fig. 1) (29). Other gut inhabitants, including the pathogen Vibrio cholerae (30) and the commensals Bacteroides fragilis and Lactobacillus sakei (31, 32), have since been shown to utilize sialic acid as a carbon source.
FIG 1.
Metabolism of sialic acid by E. coli as previously described (29). Sialic acid enters the cell through a transporter encoded by the nanT gene. Intracellular sialic acid is cleaved by the nanA-encoded N-acetylneuraminate lyase, producing pyruvate and N-acetylmannosamine. N-Acetylmannosamine is phosphorylated by the nanK-encoded N-acetylmannosamine kinase, forming N-acetylmannosamine-6-phosphate, which is in turn converted to N-acetylglucosamine-6-phosphate by the nanE-encoded N-acetylmannosamine-6-phosphate epimerase. N-Acetylglucosamine-6-phosphate then enters the amino sugar degradation pathway, where this compound is first deacetylated by nagA-encoded N-acetylglucosamine-6-phosphate deacetylase and subsequently converted to fructose-6-phosphate with the concomitant release of ammonia by the nagB-encoded glucosamine-6-phosphate deaminase.
The ability of Bifidobacterium species to metabolize sialic acid has not been widely studied. Genes predicted to be involved in the breakdown of sialic acid, which correspond to those outlined in Fig. 1, were identified in the genome of Bifidobacterium longum subsp. infantis ATCC 15697, and in accordance, this strain was shown to be capable of utilizing sialic acid (33) or the HMO sialylated lacto-N-tetraose as a sole carbon source (34). A recent study of the consumption of HMOs by strains of B. breve revealed that all of those tested can utilize sialylated lacto-N-tetraose (35). Another study examined the utilization of the monosaccharide constituents of HMO by five bifidobacterial strains representing four bifidobacterial species (B. longum, Bifidobacterium bifidum, B. breve, and Bifidobacterium adolescentis) and found that of the strains examined, only B. longum subsp. infantis ATCC 15697 and B. breve ATCC 15700 are capable of utilizing sialic acid (36). This suggests that the ability to utilize sialic acid and/or sialylated HMOs is a species- or strain-specific attribute.
The aim of this study was to investigate how B. breve UCC2003 utilizes sialic acid, a characteristic that is likely to support the ability of particular bifidobacterial species to colonize the infant gut. Furthermore, the manner in which B. breve UCC2003 can cross feed on sialic acid derived from the metabolism of 3′-sialyllactose by B. bifidum PRL2010 was characterized.
MATERIALS AND METHODS
Bacterial strains, plasmids, media, and culture conditions.
The bacterial strains and plasmids used in this study are listed in Table 1. B. breve UCC2003 was routinely cultured in reinforced clostridial medium (Oxoid Ltd., Basingstoke, Hampshire, United Kingdom). B. bifidum PRL2010 was cultured in modified de Man, Rogosa, and Sharpe (mMRS) medium made from first principles (37) but excluding a carbohydrate source and supplemented with 0.05% (wt/vol) l-cysteine HCl and 1% (wt/vol) lactose (unless otherwise specified). Carbohydrate utilization by bifidobacteria was examined in mMRS medium supplemented with 0.05% (wt/vol) l-cysteine HCl and a particular carbohydrate source (0.5%, wt/vol). It was previously shown that without the addition of a carbohydrate, mMRS medium does not support the growth of B. breve (38). The carbohydrates used were lactose (Sigma-Aldrich, Steinheim, Germany), sialic acid, and 3′- and 6′-sialyllactose (Carbosynth, Compton, Berkshire, United Kingdom). A 0.5% (wt/vol) concentration of carbohydrate was considered sufficient to analyze the growth capabilities of a strain on a particular carbon source. Addition of sialic acid at this concentration to mMRS medium resulted in a decrease in the pH to 6.0; hence, the pH was readjusted to 6.8 following the addition of the sugar and the medium was subsequently filter sterilized. Bifidobacterial cultures were incubated under anaerobic conditions in a modular, atmosphere-controlled system (Davidson and Hardy, Belfast, Ireland) at 37°C. E. coli was cultured in Luria-Bertani (LB) broth at 37°C with agitation (39). Lactococcus lactis strains were grown in M17 medium supplemented with 0.5% (wt/vol) glucose at 30°C (40). Where appropriate, the growth medium used contained tetracycline (Tet; 10 μg ml−1), chloramphenicol (Cm; 5 μg ml−1 for L. lactis and E. coli, 2.5 μg ml−1 for B. breve), erythromycin (Em; 100 μg ml−1), or kanamycin (Kan; 50 μg ml−1). Recombinant E. coli cells containing pORI19 were selected on LB agar containing Em and Kan and supplemented with X-Gal (5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside; 40 μg ml−1) and 1 mM IPTG (isopropyl-β-d-thiogalactopyranoside).
TABLE 1.
Bacterial strains and plasmids used in this study
| Strain or plasmid | Relevant feature(s) | Reference or sourcea |
|---|---|---|
| E. coli strains | ||
| XL1-blue | supE44 hsdR17 recA1 gyrA96 thi relA1 lac F′ [proAB+ laclq lacZΔM15 Tn10(Tetr)] | Stratagene |
| XL1-blue/pBC1.2-nanA | XL1-blue containing pBC1.2-nanA | This study |
| EC101 | Cloning host; repA+ kmr | 82 |
| EC101/pNZ-M.BbrII+M.BbrIII | EC101 harboring pNZ8048 derivative containing bbrIIM and bbrIIIM | 45 |
| L. lactis strains | ||
| NZ9000 | MG1363, pepN::nisRK, nisin-inducible overexpression host | 56 |
| NZ9000/pNZ-nanA | NZ9000 containing pNZ-nanA | This study |
| NZ9000/pNZ-nanK | NZ9000 containing pNZ-nanK | This study |
| NZ9000/pNZ-nanE | NZ9000 containing pNZ-nanE | This study |
| NZ9000/pNZ-nagA2 | NZ9000 containing pNZ-nagA2 | This study |
| NZ9000/pNZ-nagB1 | NZ9000 containing pNZ-nagB1 | This study |
| NZ9000/pNZ44-nanA | NZ9000 containing pNZ44-nanA | This study |
| B. breve strains | ||
| UCC2003 | Isolate from nursling stool sample | 47 |
| UCC2003-nanA | pORI19-tet-nanA insertion mutant of UCC2003 | This study |
| UCC2003-nanK | pORI19-tet-nanK insertion mutant of UCC2003 | This study |
| UCC2003-nanB | pORI19-tet-nanB insertion mutant of UCC2003 | This study |
| UCC2003-nanC | pORI19-tet-nanC insertion mutant of UCC2003 | This study |
| UCC2003-nagA2 | pORI19-tet-nagA2 insertion mutant of UCC2003 | This study |
| UCC2003-nanA/pBC1.2-nanA | pORI19-tet-nanA insertion mutant of UCC2003 harboring complementation construct pBC1.2-nanA | This study |
| JCM7017 | Isolate from human feces | JCM |
| JCM7019 | Isolate from adult feces | JCM |
| NCFB2257 | Isolate from infant intestine | NCFB |
| NCFB2258 | Isolate from infant intestine | NCFB |
| NCIMB8815 | Isolate from human feces | NCIMB |
| NIZO658 | Isolate from human feces | NIZO |
| LMG13208 | Isolate from infant intestine | LMG |
| UCC2007 | Isolate from nursling stool sample | UCC |
| UCC2005 | Isolate from infant intestine | UCC |
| NCTC11815 | Isolate from infant intestine | NCTC |
| 461B | Isolate from infant and adult feces | PRL |
| 689B | Isolate from infant feces | 62 |
| 12L | Mother's milk | 62 |
| B. bifidum PRL2010 | Isolate from infant feces | 64 |
| Plasmids | ||
| pAM5 | pBC1-puC19-Tcr | 54 |
| pNZ44 | pNZ8048 containing constitutive p44 promoter from lactococcal chromosome | 53 |
| pNZ44-nanA | pNZ44 harboring nanA downstream of p44 promoter | This study |
| pBC1.2 | pBC1-pSC101-Cmr | 54 |
| pBC1.2-nanA | pBC1-pSC101-Cmr harboring nanA downstream of p44 promoter | This study |
| pORI19 | Emr, RepA−, ori+, cloning vector | 82 |
| pORI19-tet-nanA | Internal 301-bp fragment of nanA and tetW cloned in pORI19 | This study |
| pORI19-tet-nank | Internal 370-bp fragment of nanK and tetW cloned in pORI19 | This study |
| pORI19-tet-nanB | Internal 504-bp fragment of nanB and tetW cloned in pORI19 | This study |
| pORI19-tet-nanC | Internal 355-bp fragment of nanC and tetW cloned in pORI19 | This study |
| pORI19-tet-nagA2 | Internal 402-bp fragment of nagA2 and tetW cloned in pORI19 | This study |
| pNZ8150 | Cmr, nisin-inducible translational fusion vector | 55 |
| pNZ-nanA | Cmr, pNZ8150 derivative containing translational fusion of nanA-containing DNA fragment to nisin-inducible promoter | This study |
| pNZ-nanK | Cmr, pNZ8150 derivative containing translational fusion of nanK-containing DNA fragment to nisin-inducible promoter | This study |
| pNZ-nanE | Cmr, pNZ8150 derivative containing translational fusion of nanE-containing DNA fragment to nisin-inducible promoter | This study |
| pNZ-nagA2 | Cmr, pNZ8150 derivative containing translational fusion of nagA2-containing DNA fragment to nisin-inducible promoter | This study |
| pNZ-nagB1 | Cmr, pNZ8150 derivative containing translational fusion of nagB1-containing DNA fragment to nisin-inducible promoter | This study |
JCM, Japanese Collection of Microorganisms; NCFB, National Collection of Food Bacteria; NCIMB, National Collection of Industrial and Marine Bacteria; NIZO, Netherlands Institute for Dairy Research; LMG, Belgian Coordinated Collection of Microorganisms; UCC, University College Cork; NCTC, National Collection of Type Cultures; PRL, Culture Collection of Probiogenomics, University of Parma.
In order to determine bacterial growth profiles and final optical densities (ODs), 10 ml of freshly prepared mMRS medium including a particular carbohydrate source (see above) was inoculated with 100 μl (1%) of a stationary-phase culture of a particular strain. Uninoculated mMRS medium was used as a negative control. Cultures were incubated anaerobically for 24 to 36 h. Measurements of OD at 600 nm (OD600) were recorded unless otherwise stated.
Nucleotide sequence analysis.
Sequence data were obtained from the Artemis-mediated genome annotations of B. breve UCC2003 (41, 42). Database searches were performed by using the nonredundant sequence database accessible at the National Center for Biotechnology Information website (http://www.ncbi.nlm.nih.gov) with BLAST (43). Sequence analysis was performed by using the Seqbuilder and Seqman programs of the DNASTAR software package (DNASTAR, Madison, WI).
DNA manipulations.
Chromosomal DNA was isolated from B. breve UCC2003 as previously described (44). Plasmid DNA was isolated from E. coli, L. lactis, and B. breve with the Roche High Pure plasmid isolation kit (Roche Diagnostics, Basel, Switzerland). An initial lysis step was performed with 30 mg ml−1 of lysozyme for 30 min at 37°C prior to plasmid isolation from L. lactis or B. breve. The single-stranded oligonucleotide primers used in this study were synthesized by Eurofins (Ebersberg, Germany) (see Table S1 in the supplemental material). Standard PCRs were performed with Taq master mix (Qiagen GmbH, Hilden, Germany). B. breve colony PCRs were carried out as described previously (45). PCR fragments were purified with the Roche High Pure PCR purification kit (Roche Diagnostics). Electroporation of plasmid DNA into E. coli, L. lactis, or B. breve was performed as previously described (39, 46, 47).
Analysis of global gene expression with B. breve DNA microarrays.
Global gene expression was determined during log-phase growth of B. breve UCC2003 in mMRS medium supplemented with 0.5% sialic acid. The transcriptome obtained was compared to that obtained from log-phase B. breve UCC2003 cells grown in mMRS medium supplemented with 0.5% ribose. DNA microarrays containing oligonucleotide primers representing each of the 1,864 open reading frames identified in the genome of B. breve UCC2003 were designed and obtained from Agilent Technologies (Palo Alto, CA). Cell disruption, RNA isolation, RNA quality control, and cDNA synthesis and labeling were performed as described previously (48). Labeled cDNA was hybridized with the Agilent Gene Expression hybridization kit (part no. 5188-5242) as described in the Agilent Two-Color Microarray-Based Gene Expression Analysis v4.0 manual (publication no. G4140-90050). Following hybridization, microarrays were washed in accordance with Agilent's standard procedures and scanned with an Agilent DNA microarray scanner (model G2565A). The scans generated were converted to data files with Agilent's Feature Extraction software (version 9.5). DNA microarray data were processed as previously described (49–51). Differential-expression tests were performed with the Cyber-T implementation of a variant of the t test (52).
Construction of B. breve UCC2003 insertion mutants.
An internal fragment of Bbr_0161 (designated nanK here; 370 bp, representing codons 148 through to 271 of the 336 codons of this gene), Bbr_0164 (designated nanB; 504 bp, representing codons 226 through to 394 of the 521 codons of this gene), Bbr_0165 (designated nanC; 355 bp, representing codons 27 through to 145 of the 319 codons of this gene), Bbr_0168 (designated nanA; 301 bp, representing codons 116 through to 216 of the 321 codons of this gene), and Bbr_1247 (designated nagA2; 402 bp, representing codons 107 through to 241 of the 426 codons of this gene) were amplified by PCR with B. breve UCC2003 chromosomal DNA as the template and primer pairs NanKF and NanKR, NanBF and NanBR, NanCF and NanCR, NanAF and NanAR, and NagA2F and NagA2R, respectively. The insertion mutants were constructed as described previously (45). Site-specific recombination of potential Tetr mutant isolates was confirmed by colony PCR with primers TetWF and TetWR to verify tetW gene integration and primers NanKconfirm, NanBconfirm, NanCconfirm, NanAconfirm, and NagA2confirm (positioned upstream of the selected internal fragments of nanK, nanB, nanC, nanA, and nagA2, respectively) in combination with primer TetWF to confirm integration at the correct chromosomal location.
Complementation of B. breve UCC2003-nanA.
A DNA fragment encompassing nanA was generated by PCR amplification from B. breve UCC2003 chromosomal DNA with Pfu II DNA polymerase (Agilent, Cork, Ireland) and primers NanApNZ44F and NanApNZ44R. The resulting fragment was digested with PstI and XbaI and ligated to similarly digested pNZ44 (53). The ligation mixture was introduced into L. lactis NZ9000 by electrotransformation, and transformants were selected on the basis of Cm resistance. The plasmid contents of a number of Cmr transformants were screened by restriction analysis. The integrity of the cloned insert of one of the recombinant plasmids, designated pNZ44-nanA, was confirmed by sequencing. The nanA coding sequence, together with the constitutive p44 lactococcal promoter, specified by pNZ44, was amplified by PCR from a representative pNZ44-nanA plasmid with Pfu II DNA polymerase and primers P44FORWARD and NanApNZ44R. The resulting DNA fragment was digested with BamHI and XbaI and ligated to similarly digested pBC1.2 (54). The ligation mixture was introduced into E. coli XL1-Blue by electrotransformation, and transformants were selected on the basis of Tet and Cm resistance. Transformants were checked for plasmid content by colony PCR and restriction analysis of plasmid DNA. Positively identified clones were verified by sequencing, and one of the plasmids thus identified was designated pBC1.2-nanA. Plasmid pBC1.2-nanA was introduced into B. breve UCC2003-nanA by electrotransformation, and transformants were selected on the basis of Tet and Cm resistance.
Plasmid constructions.
For the construction of plasmids pNZ-nanK, pNZ-nanE, pNZ-nanA, pNZ-nagB1, and pNZ-nagA2, DNA fragments encompassing the predicted N-acetylmannosamine kinase-encoding gene nanK (Bbr_0161), N-acetylmannosamine-6-phosphate epimerase-encoding gene nanE (Bbr_0162), N-acetylneuraminate lyase-encoding gene nanA (Bbr_0168), glucosamine-6-phosphate deaminase-encoding gene nagB1 (Bbr_0169), and N-acetylglucosamine-6-phosphate deacetylase-encoding gene nagA2 (Bbr_1247) were generated by PCR amplification of chromosomal DNA of B. breve UCC2003 with Pfu II DNA polymerase and primers nanKFOR and nanKREV, nanEFOR and nanEREV, nanAFOR and nanAREV, nagB1FOR and nagB1REV, and nagA2FOR and nagA2REV, respectively. An in-frame His10-encoding sequence was incorporated into forward primers nanKFOR, nanEFOR, and nagA2FOR and reverse primers nanAREV and nagB1REV to facilitate downstream protein purification. The amplicons generated were digested with EcoRV and XbaI and ligated into ScaI- and XbaI-digested, nisin-inducible translational fusion plasmid pNZ8150 (55). The ligation mixtures were introduced into L. lactis NZ9000 by electrotransformation, and transformants were then selected on the basis of Cm resistance. The plasmid contents of a number of Cmr transformants were screened by restriction analysis, and the integrity of positively identified clones was verified by sequencing.
Protein overproduction and purification.
Nisin-inducible gene expression and protein overproduction were performed as described previously (10, 12, 13). In brief, 400 ml of M17 broth supplemented with 0.5% (wt/vol) glucose was inoculated with a 2% inoculum of a particular L. lactis strain, followed by incubation at 30°C until an OD600 of 0.5 was reached, at which point protein expression was induced by the addition of cell-free supernatant of a nisin-producing strain (56), followed by continued incubation for a further 2 h. Cells were harvested by centrifugation, and protein purification was achieved with the PrepEase histidine-tagged protein purification maxikit (USB, Germany) according to the manufacturer's instructions. Elution fractions were analyzed by SDS-polyacrylamide gel electrophoresis. After electrophoresis, the gels were fixed and stained with Coomassie brilliant blue to identify fractions containing the purified protein. The molecular weights of the proteins were estimated by comparison with rainbow-prestained, low-molecular-weight protein markers (New England BioLabs, Herefordshire, United Kingdom). Protein concentrations were determined by the Bradford method (57).
HPTLC.
High-performance thin-layer chromatography (HPTLC) analysis was used for qualitative determination of the activity of each of the purified enzymes. Purified NanAHis, NanKHis, NanEHis, NagA2His, or NagB1His (final concentration, 20 μg ml−1) or a combination thereof was incubated with sialic acid, N-acetylmannosamine, N-acetylmannosamine-6-phosphate, N-acetylglucosamine-6-phosphate, or glucosamine-6-phosphate, respectively. Enzymatic activity assays were performed at 37°C in a total volume of 1 ml with a reaction buffer containing 20 mM Tris-HCl (pH 8.0), 10 mM KCl, and 5 mM MgSO4 · 7H2O along with a 5-mg ml−1 concentration of a particular carbohydrate substrate. This concentration of carbohydrate was considered sufficient for qualitative analysis. In reaction mixtures including NanKHis, 100 μl of 100 mM ATP was added. The reactions were terminated after 24 h by incubation at 65°C for 10 min. HPTLC analysis was performed as previously described (10). An aliquot (1 μl) of the reaction mixture was spotted onto a Silica Gel 60 plate (10 by 10 cm; Merck) with a Nanomat 4 (Camag, Switzerland). The chromatogram was developed with a butanol-isopropanol-water (3:12:4, vol/vol/vol) solvent system in a horizontal developing chamber. Ascending development was repeated twice at room temperature. The plate was allowed to dry in a fume hood and then sprayed evenly with 20% (vol/vol) sulfuric acid in ethanol. The plate was dried and heated to 120°C for 10 min to visualize sugar-representing spots. Reaction products were identified by comparison with relevant carbohydrate standards.
Growth of B. bifidum PRL2010 and B. breve UCC2003 or UCC2003-nanA on 3′-sialyllactose.
An overnight culture of B. bifidum PRL2010 (1%) was used to inoculate mMRS broth supplemented with 0.05% (wt/vol) l-cysteine HCl and 0.5% (wt/vol) 3′-sialyllactose and cultivated for 24 h at 37°C under anaerobic conditions. The cells were removed by centrifugation at 9,000 × g for 5 min. Growth of B. bifidum PRL2010 resulted in a pH decrease to 5.1; therefore, the pH of the cell-free supernatant (CFS) was readjusted to 6.8 and the CFS was filter sterilized. This medium was supplemented with 0.01% (wt/vol) lactose and 0.05% (wt/vol) l-cysteine HCl prior to inoculation with an overnight culture (0.01%) of B. breve UCC2003 or UCC2003-nanA. Growth was monitored for 72 h, with samples taken at 6- or 12-h intervals. All of the samples collected were serially diluted in sterile Ringer solution and plated on reinforced clostridial agar. Viable counts were determined by counting colonies on agar plates with dilutions that yielded between 30 and 300 CFU.
HPAEC-PAD.
Carbohydrate analysis of CFS samples taken at 6- or 12-h intervals during the 72-h growth experiment was performed by high-performance anion-exchange chromatography with pulsed amperometric detection (HPAEC-PAD) with a Dionex ICS-3000 system (Dionex, Sunnyvale, CA). Oligosaccharide fractions (25-μl aliquots) were separated on a CarboPac PA1 analytical-exchange column with dimensions of 250 by 4 mm (Dionex) with a CarboPac PA1 guard column with dimensions of 50 by 4 mm (Dionex) and a pulsed electrochemical detector in the PAD mode. Elution was performed at a constant flow rate of 1.0 ml min−1 at 30°C with the following eluents for the analysis: 200 mM NaOH (A), 100 mM NaOH–550 mM sodium acetate (NaAC) (B), and Milli-Q water (C). The following linear gradient of NaAC with 100 mM NaOH was used: 0 to 50 min, 0 mM; 50 to 51 min, 16 mM; 51 to 56 min, 100 mM; 56.1 to 61 min, 0 mM. Detection was achieved with a Dionex ED40 detector in the PAD mode. The Chromeleon software version 6.70 (Dionex Corporation) was used for the integration and evaluation of the chromatograms obtained. The chromatographic profiles corresponding to 3′-sialyllactose, sialic acid, and lactose were qualitatively examined to evaluate 3′-sialyllactose hydrolysis and subsequent metabolism of lactose and/or sialic acid.
Microarray data accession number.
The microarray data obtained in this study have been deposited in the NCBI Gene Expression Omnibus database under GEO series accession no. GSE56291.
RESULTS
Growth of B. breve strains on sialic acid.
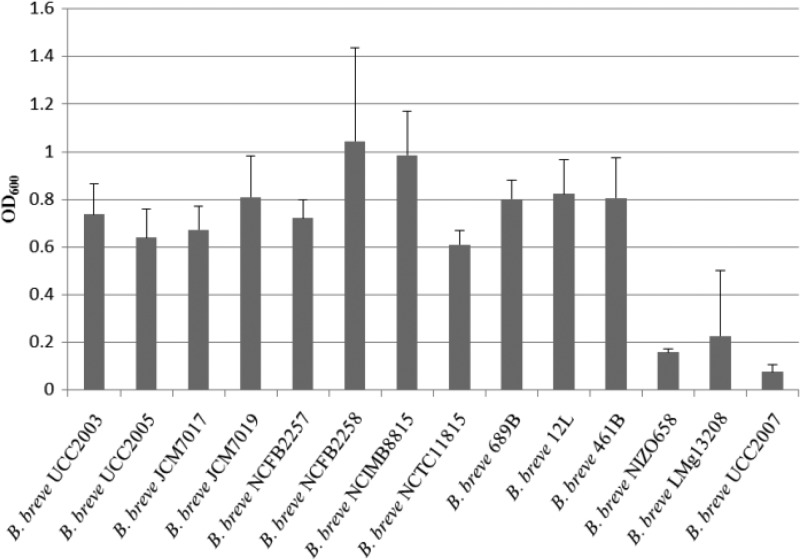
In order to determine if B. breve strains are capable of sialic acid metabolism, the growth of 14 B. breve strains in mMRS medium supplemented with 0.5% (wt/vol) lactose or sialic acid was assessed by measuring the OD600 following 24 h of anaerobic growth at 37°C. All of the B. breve strains were shown to grow well on lactose (OD600 of >1.2) (results not shown). Eleven of the 14 B. breve strains examined, including prototypical strain UCC2003, were capable of moderate growth on sialic acid, reaching OD600s of 0.6 to 0.9, while the three remaining strains were shown to be unable to metabolize sialic acid (Fig. 2).
FIG 2.
Final OD600 values obtained following 24 h of growth of 14 different B. breve strains in mMRS containing 0.5% (wt/vol) sialic acid as the sole carbon source. The results are mean values obtained from two different experiments. Error bars represent standard deviations.
Genome response of B. breve UCC2003 to growth on sialic acid.
In order to investigate which genes are involved in sialic acid metabolism by B. breve UCC2003, global gene expression was determined by microarray analysis during growth of the strain in mMRS medium supplemented with sialic acid and compared with gene expression when it was grown in mMRS medium supplemented with ribose. The pentose sugar ribose was considered an appropriate carbohydrate for comparative transcriptome analysis, as the genes involved in ribose metabolism are known, while it has previously been used in several transcriptome studies of B. breve UCC2003 (10, 11, 13). Analysis of the DNA microarray data revealed that the expression of a gene cluster comprising 12 open reading frames corresponding to locus tags Bbr_0160 to Bbr_0172 was significantly upregulated when B. breve UCC2003 was grown on sialic acid relative to when the strain was grown on ribose (>3.0-fold change, P < 0.001). In addition, two adjacent genes, corresponding to Bbr_1247 (nagA2) and Bbr_1248 (nagB3), were also upregulated (see below; Table 2).
TABLE 2.
Effect of sialic acid on the transcriptome of B. breve UCC2003
| Locus tag (gene) | Predicted function | Level of upregulationa |
|---|---|---|
| Bbr_0160 | Conserved hypothetical protein | 37.32 |
| Bbr_0161 (nanK) | Conserved hypothetical protein in ROK family | 59.01 |
| Bbr_0162 (nanE) | N-Acetylmannosamine-6-phosphate 2-epimerase | 49.91 |
| Bbr_0163 | Hydrolase | 51.55 |
| Bbr_0164 (nanB) | Substrate-binding protein | 367.68 |
| Bbr_0165 (nanC) | ABC transport system permease protein | 451.08 |
| Bbr_0166 (nanD) | ABC transport system ATP-binding protein | 453.7 |
| Bbr_0167 (nanF) | ABC transport system ATP-binding protein | 435.43 |
| Bbr_0168 (nanA) | N-Acetylneuraminate lyase | 435.04 |
| Bbr_0169 (nagB1) | Glucosamine-6-phosphate isomerase | 275.38 |
| Bbr_0171 (nanH) | Sialidase A | 204.69 |
| Bbr_0172 | ATPase | 6.7 |
| Bbr_1247 (nagA2) | N-Acetylglucosamine-6-phosphate deacetylase | 3.07 |
| Bbr_1248 (nagB3) | Glucosamine-6-phosphate isomerase | 4.11 |
The cutoff point is 3-fold with a P value of <0.001.
Genetic organization of the predicted nan-nag gene clusters.
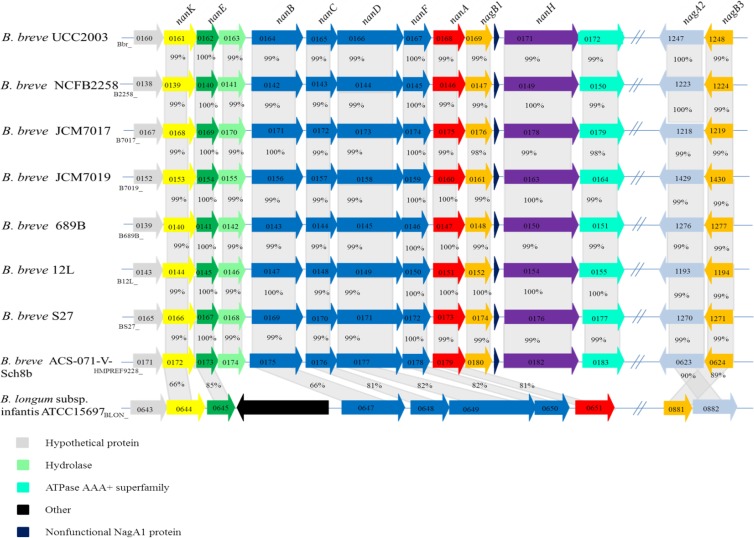
Our presumption, based on the microarray results, was that the products encoded by the Bbr_0160-to-Bbr_0172 gene cluster and those of Bbr_1247 and Bbr_1248, schematically outlined in Fig. 3, are involved in the metabolism of sialic acid in B. breve UCC2003. The first gene of this upregulated cluster, Bbr_0160, encodes a hypothetical protein of unknown function. Bbr_0161, designated nanK here, encodes a putative N-acetylmannosamine kinase that is predicted to be a member of the RhoA-binding kinase (ROK) protein family. The members of this family include transcriptional regulators and sugar kinases (58). Predicted ROK kinases possess a conserved N-terminal ATP-binding motif, DxGxT, that can be identified in the nanK gene of B. breve UCC2003 and is absent from ROK transcriptional regulators (59). The predicted function of NanK is to phosphorylate N-acetylmannosamine, producing N-acetylmannosamine-6-phosphate. Bbr_0162, designated nanE here, is predicted to encode an N-acetylmannosamine-6-phosphate epimerase, the predicted function of which is the conversion of N-acetylmannosamine-6-phosphate to N-acetylglucosamine-6-phosphate. Bbr_0163 encodes a putative hydrolase protein, the function of which, if any, in relation to sialic acid metabolism is unknown. The deduced protein products of Bbr_0164 to Bbr_0167, designated nanB, nanC, nanD, and nanF, respectively, are predicted to represent an ABC transport system including a putative solute-binding protein, a permease, and two ATP-binding proteins. Bbr_0168, designated nanA, encodes a putative N-acetylneuraminate lyase. The predicted function of NanA is the cleavage of sialic acid, producing N-acetylmannosamine and pyruvate. Bbr_0169, designated nagB1, encodes a putative glucosamine-6-phosphate deaminase, the predicted function of which is the conversion of glucosamine-6-phosphate to fructose-6-phosphate, with the concomitant release of ammonia. Bbr_0170 is a pseudogene that is predicted to encode a truncated, nonfunctional NagA protein. Bbr_0171, designated nanH, encodes a predicted sialidase that, because of the lack of a signal sequence at the N terminus, is presumed to be intracellular. Sialidases are responsible for the removal of sialic acid residues from glycans. The nanH gene is followed by Bbr_0172, which encodes a putative ATPase in the AAA+ superfamily of ATPases, a large and diverse family of enzymes that are found in all of the kingdoms of living organisms, where they are essential in various cellular processes such as proteolysis and DNA replication (60). Its function, if any, in sialic acid metabolism by B. breve UCC2003 is obscure. The Bbr_0160-to-Bbr_0172 gene cluster was renamed the nan-nag cluster, as it includes a complete nan system, as defined in 2004 as “one that minimally includes orthologues of genes encoding NanA, NanE, and NanK” (61), as well as a predicted transport system and the nagB1-encoded glucosamine-6-phosphate deaminase. In addition to the nan-nag cluster, the Bbr_1247-Bbr_1248 locus is also predicted to be involved in sialic acid metabolism. Bbr_1247 (designated nagA2) encodes a putative N-acetylglucosamine-6-phosphate deacetylase, the predicted function of which is the removal of acetate from N-acetylglucosamine-6-phosphate, producing glucosamine-6-phosphate. Bbr_1248 (designated nagB3) encodes an additional glucosamine-6-phosphate deaminase (see above). Therefore, within these two loci, whose encompassing genes are transcriptionally induced when B. breve UCC2003 is grown on sialic acid, we can identify elements of a complete sialic acid catabolic pathway corresponding to that previously outlined in E. coli (Fig. 1), with the sole difference being a nanBCDF-encoded ABC transport system instead of the nanT-encoded transporter in E. coli (29). Interestingly, the genome of B. breve UCC2003 contains two adjacent genes, corresponding to locus tags Bbr_0846 and Bbr_0847, designated nagA1 and nagB2 here, which are predicted to encode additional N-acetylglucosamine-6-phosphate deacetylase and glucosamine-6-phosphate deaminase activities, respectively, although these genes were not shown to be significantly upregulated when B. breve UCC2003 was grown on sialic acid. The NagA1 protein is 74% identical to NagA2, while the NagB1 protein is 84% identical to NagB2 and 89% identical to NagB3. On the basis of the comparative genome analysis presented in Fig. 3, the nan-nag cluster is highly conserved among the B. breve strains whose genomes were recently published (62). The same genes can also be identified in B. longum subsp. infantis ATCC 15697, another strain that can utilize sialic acid, although the order of the genes in this particular nan-nag cluster has been slightly rearranged relative to that of B. breve UCC2003 (33) (Fig. 3). There are no other homologs of this cluster within the completed bifidobacterial genomes currently available.
FIG 3.
Comparison of the sialic acid gene cluster of B. breve UCC2003 with corresponding putative sialic acid utilization loci of other bifidobacteria. Each solid arrow represents an open reading frame. The length of each arrow is proportional to the size of the open reading frame, and the gene locus name, which is indicative of its putative function, is shown at the top. Orthologs are the same color. The amino acid sequence identity of each predicted protein to its equivalent protein encoded by B. breve UCC2003, expressed as a percentage, is shown above each arrow.
Disruption of the nanK, nanB, nanC, nanA, and nagA2 genes in B. breve UCC2003.
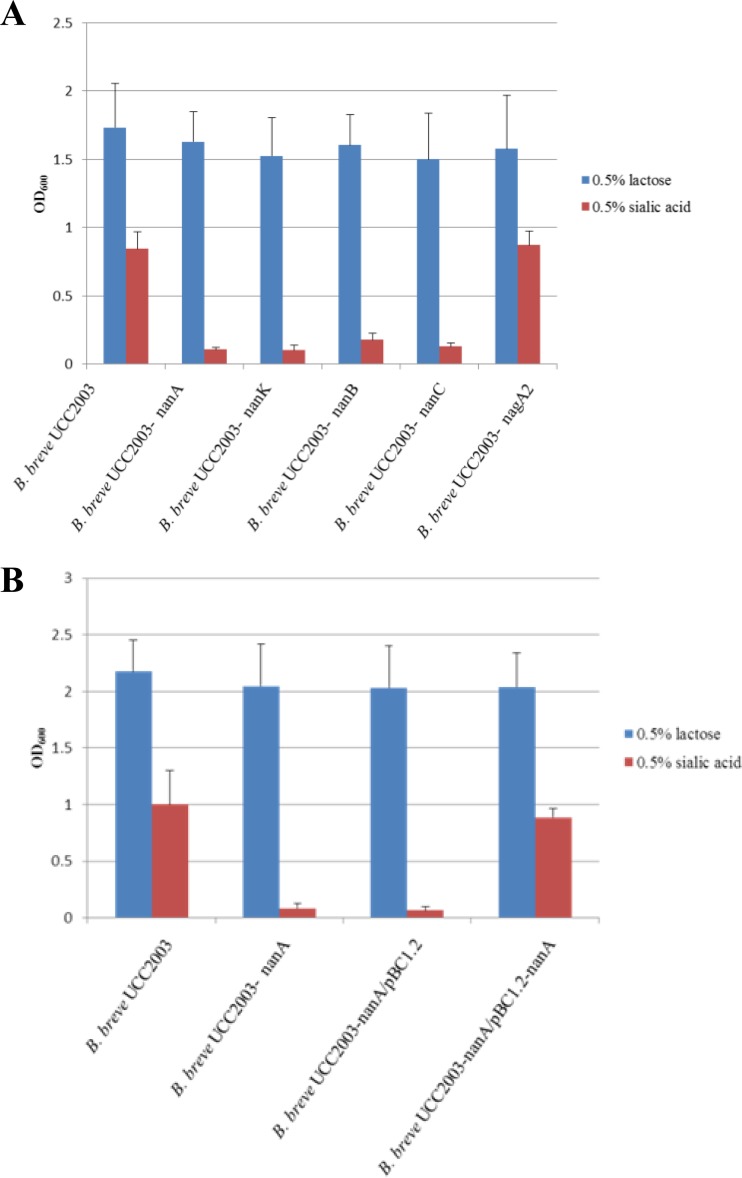
In order to investigate if the disruption of individual genes in the nan cluster would affect the ability of B. breve UCC2003 to utilize sialic acid, nanK, nanB, nanC, nanA, and nagA2 gene insertion mutants were constructed, resulting in B. breve strains UCC2003-nanK, UCC2003-nanB, UCC2003-nanC, UCC2003-nanA, and UCC2003-nagA2, respectively. These strains were compared with B. breve UCC2003 for the ability to grow in mMRS medium supplemented with sialic acid. As expected, and in contrast to the wild type, there was a complete lack of growth of B. breve UCC2003-nanA in medium containing sialic acid (Fig. 4A). In order to demonstrate that the protein product of nanA is uniquely required for sialic acid metabolism in B. breve UCC2003, a complementation experiment was performed. The nanA gene was cloned into pBC1.2 under the control of the constitutive p44 promoter (63) and introduced into B. breve UCC2003-nanA. Expression of NanA in B. breve UCC2003-nanA/pBC1.2-nanA allowed this strain to regain the ability to grow on sialic acid to an OD comparable to that reached by the wild type (Fig. 4B). The B. breve insertion mutants UCC2003-nanK, UCC2003-nanB, and UCC2003-nanC also failed to grow in medium containing sialic acid. However, the B. breve insertion mutant UCC2003-nagA2 reached OD600 levels comparable to those of the wild-type strain during growth on sialic acid. The growth of the insertion mutants was not impaired on lactose, where all of the strains reached final OD600 levels comparable to that reached by the wild-type strain (Fig. 4A).
FIG 4.
(A) Final OD600 values after 24 h of growth of B. breve UCC2003 and the B. breve insertion mutants UCC2003-nanA, UCC2003-nanK, UCC2003-nanB, UCC2003-nanC, and UCC2003-nagA2 in mMRS containing 0.5% (wt/vol) lactose or 0.5% (wt/vol) sialic acid as the sole carbon source. The results are mean values obtained from three separate experiments. Error bars represent standard deviations. (B) Final OD600 values after 24 h of growth of B. breve UCC2003, the insertion mutants B. breve UCC2003-nanA and UCC2003-nanA/pBC1.2, and the complemented insertion mutant B. breve UCC2003-nanA/pBC1.2-nanA in mMRS containing 0.5% (wt/vol) lactose or 0.5% (wt/vol) sialic acid as the sole carbon source. The results are mean values obtained from three separate experiments. Error bars represent standard deviations.
Purification and biochemical characterization of NanA, NanK, NanE, NagA2, and NagB1.
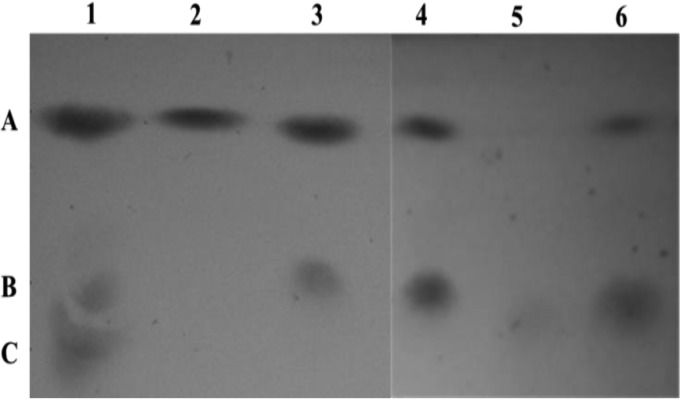
In order to investigate the predicted enzymatic activities of NanA, NanK, NanE, NagA2, and NagB1, the genes encoding the respective enzymes were cloned into L. lactis NZ9000 and subsequently overproduced and purified. The NanAHis, NanKHis, NanEHis, NagA2His, and NagB1His proteins thus produced exhibited the expected molecular masses of 34, 33, 23, 45, and 29 kDa, respectively (results not shown). Biochemical characterization was performed by incubating an individual enzyme or a combination of enzymes with a particular substrate and analyzing the resulting products by HPTLC. The results are consistent with the predicted sialic acid breakdown pathway, as outlined above (Fig. 1) (29). Under the HPTLC conditions used, it was not possible to visualize sialic acid; however, as expected, NanAHis, when incubated with its assumed substrate, was shown to produce a product with mobility properties similar to those of N-acetylmannosamine. The expected by-product of the reaction, pyruvate, was not visible under these conditions. Only in the presence of ATP was NanKHis shown to use N-acetylmannosamine as a substrate and generate a product with (HPTLC) mobility properties consistent with its expected product, N-acetylmannosamine-6-phosphate. Furthermore, when both NanAHis and NanKHis were incubated with sialic acid and ATP, this also allowed the production of a presumed N-acetylmannosamine-6-phosphate. Since it was not possible to visualize N-acetylglucosamine-6-phosphate or glucosamine-6-phosphate under the HPTLC conditions used, NanEHis, NagB1His, and NagA2His were incubated together with N-acetylmannosamine-6-phosphate and, as expected, the combined activities of these three enzymes generated a product exhibiting HPTLC mobility properties consistent with those of fructose-6-phosphate. If any one or two of these enzymes were not included, this product was not generated, thus demonstrating the contribution of each of these three enzymes to this biochemical conversion (results not shown). Finally, when the five enzymes NanAHis, NanKHis, NanEHis, NagA2His, and NagB1His were incubated with only sialic acid as the substrate (plus ATP), a product with HPTLC mobility properties consistent with those of the expected end product fructose-6-phosphate was generated (Fig. 5).
FIG 5.
Hydrolysis of sialic acid to fructose-6-phosphate by the combined activities of purified NanAHis, NanKHis, NanEHis, NagA2His, and NagB1His. Lanes: 1, 5 mg ml−1 (wt/vol) standards (N-acetylmannosamine [A], N-acetylmannosamine-6-phosphate [B], and fructose-6-phosphate [C]) in kinase buffer; 2, NanAHis plus sialic acid; 3, NanKHis plus N-acetylmannosamine; 4, NanAHis and NanKHis plus sialic acid; 5, NanEHis, NagA2His, and NagB1His plus N-acetylmannosamine-6-phosphate; 6, NanAHis, NanKHis, NanEHis, NagA2His, and NagB1His plus sialic acid.
Growth of B. bifidum PRL2010 and B. breve UCC2003 or UCC2003-nanA on 3′-sialyllactose.
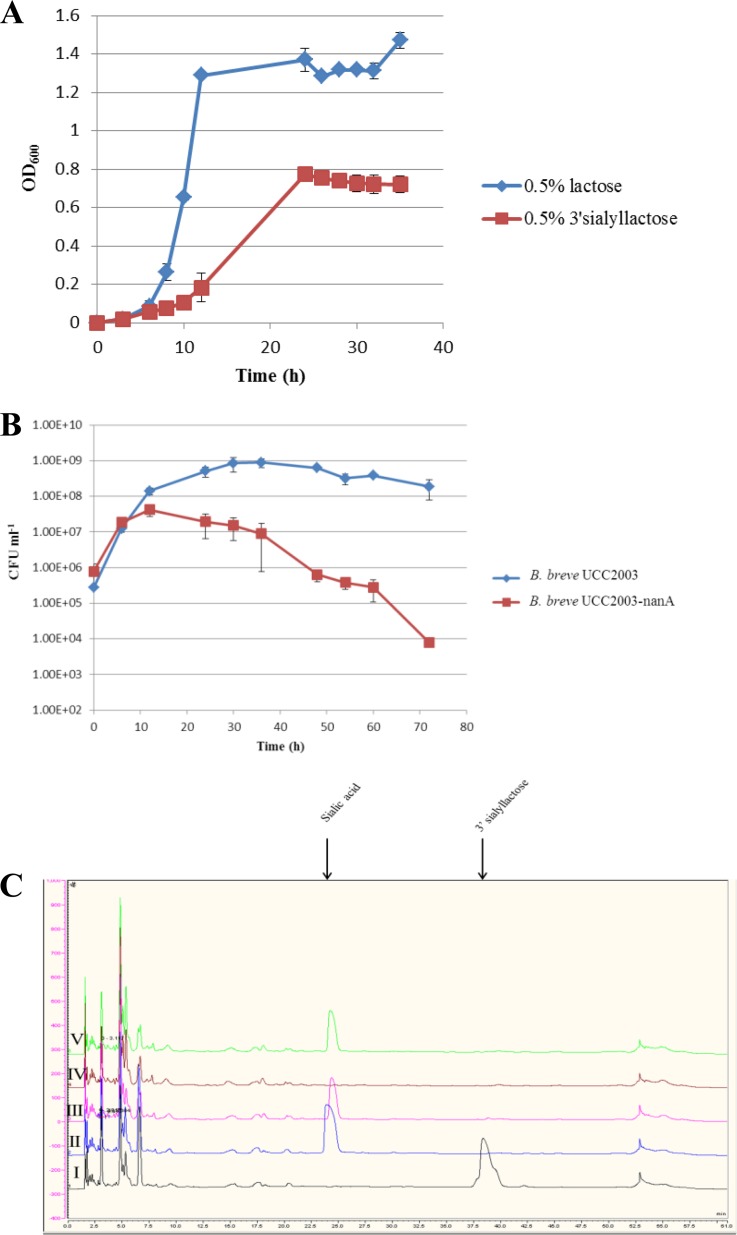
Growth of B. bifidum PRL2010 and B. breve UCC2003 on mMRS medium supplemented with 0.5% (wt/vol) 3′-sialyllactose, a trisaccharide consisting of sialic acid and lactose bound by an α-(2→3) linkage, was first assessed by measuring the OD600 for 36 h under anaerobic conditions. 3′-Sialyllactose was found to support the growth of B. bifidum PRL2010, reaching an OD600 of approximately 0.8 (Fig. 6A). Analysis of the CFS after 24 h of growth by HPAEC-PAD and comparison with nonfermented standards of 3′-sialyllactose, sialic acid, and lactose showed that 3′-sialyllactose was consumed from the medium but that the consumption of this trisaccharide coincided with the accumulation in the medium of a carbohydrate with HPAEC-PAD properties consistent with those of sialic acid. It has previously been shown that B. bifidum PRL2010 encodes two putative extracellular exosialidases on its genome, each presumed to be specific for α-(2→3) or α-(2→6) linkages (64). From this information, we assumed that the extracellular sialidase of B. bifidum PRL2010 cleaves 3′-sialyllactose to produce sialic acid, which it cannot utilize (i.e., B. bifidum PRL2010 does not exhibit growth in a medium containing sialic acid as a sole carbon source [results not shown]), and lactose, which it can utilize, resulting in sialic acid accumulation in the medium. Consistent with this observation, the genome of B. bifidum PRL2010 lacks a sialic acid utilization cluster (64). In contrast, it was found that 3′-sialyllactose and its α-(2→6)-linked counterpart 6′-sialyllactose were unable to support the growth of B. breve UCC2003 (results not shown). In order to establish if B. breve UCC2003 could grow in the 3′-sialyllactose-supplemented mMRS medium in which B. bifidum PRL2010 was previously grown for 24 h (spent medium), a small amount of lactose, 0.01% (wt/vol), was added to the spent medium prior to inoculation to initiate growth. A small inoculum (0.01%) of B. breve UCC2003 was used to allow the strain to undergo multiple growth generations. Growth on lactose was required for the initial increase in cell numbers during the first 12 h; however, HPAEC-PAD analysis confirmed that lactose was fully utilized by B. breve UCC2003 after 12 h of incubation, after which growth and viability were dependent on the ability to utilize the sialic acid in the spent medium. Between 12 and 30 h, B. breve UCC2003 was able to reach a viable count of almost 109 CFU ml−1 from a starting point of 108 CFU ml−1 and was able to maintain these viable cell numbers until 72 h (Fig. 6B). HPAEC analysis of the cell-free supernatant after 30 h of growth shows the absence of the sialic acid-associated peak, implying that the sialic acid released as a result of the exosialidase activity of B. bifidum PRL2010 was entirely utilized to support the growth of B. breve UCC2003 (Fig. 6C). Consistent with this scenario, B. breve UCC2003-nanA (which is incapable of growth in sialic acid [see above]) was shown to be incapable of growth in this spent medium, with viable counts dropping from their initial inoculation number of 105 CFU ml−1 after 72 h (Fig. 6B). As expected, HPAEC analysis demonstrated that sialic acid is not metabolized by B. breve UCC2003-nanA (Fig. 6C).
FIG 6.
(A) Growth profiles of B. bifidum PRL2010 in mMRS containing 0.5% (wt/vol) lactose or 0.5% (wt/vol) 3′-sialyllactose. The results are mean values obtained from two separate experiments. Error bars represent standard deviations. (B) Growth profiles of B. breve UCC2003 and UCC2003-nanA in mMRS containing 0.5% (wt/vol) 3′-sialyllactose previously fermented by B. bifidum PRL2010 for 24 h. The results are mean values obtained from two separate experiments. Error bars represent standard deviations. (C) HPAEC profiles of mMRS plus 0.5% (wt/vol) 3′-sialyllactose (I), mMRS plus 0.5% (wt/vol) sialic acid (II), mMRS plus 0.5% (wt/vol) 3′-sialyllactose after 24 h of growth of B. bifidum PRL2010 (III), the medium from condition III after 30 h of growth of B. breve UCC2003 (IV), and the medium from condition III after 30 h of growth of B. breve UCC2003-nanA (V).
DISCUSSION
B. breve strains represent a dominant commensal group in the breast-fed infant gut microbiota, but it is only recently that the factors that contribute to this dominance have become a subject of scientific scrutiny. It has already been shown that B. breve strains can liberate N-glycans from host glycoproteins and glycoproteins from breast milk (65), and a more recent study has demonstrated that B. breve strains can grow on the HMOs lacto-N-tetraose, lacto-N-neotetraose, and sialylated lacto-N-tetraose (35). In this report, we describe the functional characterization of a locus dedicated to the uptake and utilization of sialic acid. To our knowledge, this is the first study to adopt a functional-genomic approach to gain an understanding of sialic acid metabolism by a Bifidobacterium species.
Of the 14 B. breve strains tested for growth on sialic acid as a sole carbon source, 11 were shown to be capable of growth. DNA microarray analysis of B. breve UCC2003 grown in sialic acid revealed a locus of 12 genes predicted to be involved in sialic acid uptake and catabolism, as well as 2 genes specifying putative N-acetylglucosamine-6-phosphate deacetylase and glucosamine-6-phosphate deaminase, designated nagA2 and nagB3, respectively, located at an unlinked position on the genome. The genome of B. breve UCC2003 also contains additional nagA and nagB genes at the Bbr_0846-Bbr_0847 locus, which were designated nagA1 and nagB2, respectively. Since the enzymatic activities encoded by nagAB genes are also required for the metabolism of amino sugars such as N-acetylglucosamine and N-acetylgalactosamine (66, 67), the presence of multiple nagAB copies on the B. breve UCC2003 genome may reflect the metabolic versatility that allows it to utilize such amino sugars when they are released from host-derived carbohydrates such as mucin and HMO (20, 22). Previously performed comparative genomic hybridization (CGH) analysis revealed that all of the B. breve strains tested harbor the nan-nag genes in their genomes, thus indicating that the ability to utilize sialic acid is well conserved in the B. breve species (42). An apparent inconsistency is the presence of these genes in the genomes of B. breve NIZO 658, LMG13208, and UCC2007, which are unable to grow on sialic acid; however, it must be considered that the nature of CGH analysis means that only the presence or absence of genes is revealed but that of any mutations in a gene or promoter that may result in loss of function is not.
The uptake of sialic acid is likely to be facilitated by an ABC transport system encoded by the nanBCDF genes. Disruption of nanB or nanC in B. breve UCC2003 was shown to result in an impairment of growth on sialic acid, thus proving that this predicted transport system is solely responsible for sialic acid uptake. Previous studies have shown that transport of sialic acid into bacterial cells can be achieved by ABC transport systems, as seen in Haemophilus ducreyi (68), transporters of the major facilitator superfamily of proteins, as in E. coli (28, 69) and Bt. fragilis (31) and/or tripartite ATP-independent periplasmic transporters, as seen first in Haemophilus influenzae (70) and in V. cholerae (71). ABC transport systems have previously been shown to be involved in carbohydrate uptake in B. breve UCC2003 (10, 12, 13, 72).
Similarly, mutations in nanA and nanK were shown to cause impaired growth on sialic acid, implying that the enzymes encoded by these genes are essential for sialic acid metabolism. In contrast, the insertion mutant B. breve UCC2003-nagA2 did not display impaired growth on sialic acid, suggesting either that this gene is not involved in sialic acid metabolism or that another gene is able to compensate for the mutation, a possible candidate for which would be the nagA1 gene mentioned above.
Analysis of the substrate specificities of the enzymes NanA, NanK, NanE, NagA2, and NagB1 indicates that sialic acid metabolism by B. breve UCC2003 occurs by means of a five-step pathway similar to that previously characterized in E. coli (Fig. 1) (29). An analogous pathway has been described in H. influenzae (73), Staphylococcus aureus (74), and L. sakei (32), yet it differs slightly from that found in Bt. fragilis. It was shown that Bt. fragilis does not encode a nanK gene but instead encodes a novel N-acetylmannosamine epimerase, encoded by nanE, which converts N-acetylmannosamine to N-acetylglucosamine, which is then phosphorylated by a rokA-encoded kinase (31). While individual enzymes of this pathway have been purified and characterized previously, such as NanA from C. perfringens (75), H. influenzae (76), and Lactobacillus plantarum (77) and the previously mentioned NanE enzyme from Bt. fragilis (31), to our knowledge, this is the first time that the full catabolic pathway of sialic acid has been reconstituted in vitro.
In order for B. breve UCC2003 to utilize sialic acid, it must first be released from glycoconjugates such as mucin or HMO, in which it is most commonly found. Sialidases, which release sialic acid from glycans, have previously been described for infant-derived bifidobacteria, including two intracellular sialidases from B. longum subsp. infantis ATCC 15697 (34) and an extracellular sialidase from B. bifidum JCM1254 (78). Two predicted extracellular exo-α-sialidases were also identified in the genome of B. bifidum PRL2010 (64). A putative intracellular sialidase is located in the nan-nag locus of B. breve UCC2003, but its substrate is unknown. The inability of B. breve UCC2003 to grow on 3′- or 6′-sialyllactose indicates that this carbohydrate is not the correct substrate for this sialidase, or it may also be that B. breve UCC2003 does not encode an appropriate transport system for its uptake. Its cognate substrate may, in fact, be sialylated lacto-N-tetraose, which has previously been shown to support the growth of various B. breve strains (35).
Interestingly, experiments testing B. breve UCC2003 growth in spent medium in which B. bifidum PRL2010 had previously grown on 3′-sialyllactose (as the sole carbon source) showed that B. breve UCC2003 can cross feed on the sialic acid released by the exosialidase activity of B. bifidum PRL2010. These results suggest that B. bifidum PRL2010 uses its sialidase activity to gain access to the lactose component of this HMO, leaving the sialic acid for B. breve UCC2003 (and others) to forage. These results show that although the sialic acid in the gut is predominantly glycosidically bound (24), a lack of sialidase activity would not necessarily disadvantage the proliferation of a cross-feeding bifidobacterial strain such as B. breve UCC2003 in the infant gut. Similarly, a recent study showed that two pathogens, Salmonella enterica serovar Typhimurium and Clostridium difficile, can scavenge sialic acid released by the sialidase activity of Bacteroides thetaiotaomicron in a gnotobiotic mouse (79). Within the same study, it was shown that antibiotic-treated mice had higher levels of free sialic acid in their ceca than their untreated counterparts. The authors proposed that in the untreated gut, sialic acid is utilized by members of the gut microbiota. Antibiotic treatment causes an acute disturbance of this complex microbiota, yet enough sialidase-producing bacteria remain, resulting in elevated levels of sialic acid, which can be exploited by a sialic acid-utilizing pathogen such as C. difficile (79).
Our results, combined with previous knowledge of the utilization of sialic acid by pathogens, e.g., C. difficile, suggest that the metabolism of sialic acid by certain bifidobacterial strains such as B. breve UCC2003 may provide nutritional immunity and competition for opportunistic pathogens in a healthy gut environment, thus inhibiting or moderating their proliferation. It also suggests a role for sialic acid-utilizing B. breve strains as probiotic prophylaxis, particularly during antibiotic treatment, or in probiotic treatment of C. difficile infection, as recently suggested (80). Recently, B. breve strains were identified as suitable probiotics for the treatment of enteric disorders in infants (81). Our results suggest that a combination of (extracellular) sialidase-encoding B. bifidum strains and sialic acid-utilizing B. breve strains can improve B. breve strain colonization of and persistence in the infant gut, thus potentially conferring the aforementioned benefits on the infant host.
Previous research has shown that B. breve UCC2003, as well as other B. breve strains, is capable of utilizing a number of constituents of an adult, plant-based diet (10–15), yet these data did not explain the prevalence of this species in the infant gut. The present study, which demonstrates how B. breve UCC2003 can utilize sialic acid, advances our understanding of the prevalence of B. breve in the infant gut, where cross feeding may play an important role in the establishment of a microbiota with a high abundance and diversity of bifidobacterial species.
Supplementary Material
ACKNOWLEDGMENTS
The Alimentary Pharmabiotic Centre is a research center funded by Science Foundation Ireland (SFI) through the Irish Government's National Development Plan. We and our work were supported by SFI (grants 07/CE/B1368 and SFI/12/RC/2273) and an HRB postdoctoral fellowship (grant PDTM/20011/9) awarded to M.O.M.
Footnotes
Published ahead of print 9 May 2014
Supplemental material for this article may be found at http://dx.doi.org/10.1128/AEM.01114-14.
REFERENCES
- 1.Tissier H. 1900. Recherches sur la flore intestinale normale pathologique du nourrisson. Ph.D. dissertation University of Paris, Paris, France [Google Scholar]
- 2.Ventura M, Canchaya C, Fitzgerald G, Gupta R, van Sinderen D. 2007. Genomics as a means to understand bacterial phylogeny and ecological adaptation: the case of bifidobacteria. Antonie van Leeuwenhoek 92:265–265. 10.1007/s10482-007-9182-2 [DOI] [PubMed] [Google Scholar]
- 3.Guarner F, Malagelada JR. 2003. Gut flora in health and disease. Lancet 361:512–519. 10.1016/S0140-6736(03)12489-0 [DOI] [PubMed] [Google Scholar]
- 4.LeBlanc JG, Milani C, de Giori GS, Sesma F, van Sinderen D, Ventura M. 2013. Bacteria as vitamin suppliers to their host: a gut microbiota perspective. Curr. Opin. Biotechnol. 24:160–168. 10.1016/j.copbio.2012.08.005 [DOI] [PubMed] [Google Scholar]
- 5.Ventura M, Turroni F, O'Connell Motherway M, MacSharry J, van Sinderen D. 2012. Host-microbe interactions that facilitate gut colonization by commensal bifidobacteria. Trends Microbiol. 20:467–476. 10.1016/j.tim.2012.07.002 [DOI] [PubMed] [Google Scholar]
- 6.Round JL, Mazmanian SK. 2009. The gut microbiota shapes intestinal immune responses during health and disease. Nat. Rev. Immunol. 9:313–323. 10.1038/nri2515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Servin AL. 2004. Antagonistic activities of lactobacilli and bifidobacteria against microbial pathogens. FEMS Microbiol. Rev. 28:405–440. 10.1016/j.femsre.2004.01.003 [DOI] [PubMed] [Google Scholar]
- 8.Scardovi V, Trovatelli ID. 1965. The fructose-6-phosphate shunt as a peculiar pattern of hexose degradation in the genus Bifidobacterium. Ann. Microbiol. 15:19–29 [Google Scholar]
- 9.Schell MA, Karmirantzou M, Snel B, Vilanova D, Berger B, Pessi G, Zwahlen MC, Desiere F, Bork P, Delley M, Pridmore RD, Arigoni F. 2002. The genome sequence of Bifidobacterium longum reflects its adaptation to the human gastrointestinal tract. Proc. Natl. Acad. Sci. U. S. A. 99:14422–14427. 10.1073/pnas.212527599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.O'Connell Motherway M, Fitzgerald GF, van Sinderen D. 2011. Metabolism of a plant derived galactose-containing polysaccharide by Bifidobacterium breve UCC2003. Microb. Biotechnol. 4:403–416. 10.1111/j.1751-7915.2010.00218.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.O'Connell Motherway M, Kinsella M, Fitzgerald GF, van Sinderen D. 2013. Transcriptional and functional characterization of genetic elements involved in galacto-oligosaccharide utilization by Bifidobacterium breve UCC2003. Microb. Biotechnol. 6:67–79. 10.1111/1751-7915.12011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pokusaeva K, O'Connell-Motherway M, Zomer A, MacSharry J, Fitzgerald GF, van Sinderen D. 2011. Cellodextrin utilization by Bifidobacterium breve UCC2003. Appl. Environ. Microbiol. 77:1681–1690. 10.1128/AEM.01786-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.O'Connell KJ, O'Connell Motherway M, O'Callaghan J, Fitzgerald GF, Ross RP, Ventura M, Stanton C, van Sinderen D. 2013. Metabolism of four α-glycosidic linkage-containing oligosaccharides by Bifidobacterium breve UCC2003. Appl. Environ. Microbiol. 79:6280–6292. 10.1128/AEM.01775-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.O'Connell Motherway M, Fitzgerald G, Neirynck S, Ryan S, Steidler L, van Sinderen D. 2008. Characterization of ApuB, an extracellular type II amylopullulanase from Bifidobacterium breve UCC2003. Appl. Environ. Microbiol. 74:6271–6279. 10.1128/AEM.01169-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ryan S, Fitzgerald G, van Sinderen D. 2006. Screening for and identification of starch-, amylopectin-, and pullulan-degrading activities in bifidobacterial strains. Appl. Environ. Microbiol. 72:5289–5296. 10.1128/AEM.00257-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Turroni F, Peano C, Pass DA, Foroni E, Severgnini M, Claesson MJ, Kerr C, Hourihane J, Murray D, Fuligni F, Gueimonde M, Margolles A, De Bellis G, O'Toole PW, van Sinderen D, Marchesi JR, Ventura M. 2012. Diversity of bifidobacteria within the infant gut microbiota. PLoS One 7:e36957. 10.1371/journal.pone.0036957 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ninonuevo M, Park Y, Yin H, Zhang J, Ward R, Clowers B, German B, Freeman S, Killeen K, Grimm R, Lebrilla C. 2006. A strategy for annotating the human milk glycome. J. Agric. Food Chem. 54:7471–7480. 10.1021/jf0615810 [DOI] [PubMed] [Google Scholar]
- 18.Martín-Sosa S, Martín MJ, García-Pardo LA, Hueso P. 2003. Sialyloligosaccharides in human and bovine milk and in infant formulas: variations with the progression of lactation. J. Dairy Sci. 86:52–59. 10.3168/jds.S0022-0302(03)73583-8 [DOI] [PubMed] [Google Scholar]
- 19.Bao Y, Zhu L, Newburg DS. 2007. Simultaneous quantification of sialyloligosaccharides from human milk by capillary electrophoresis. Anal. Biochem. 370:206–214. 10.1016/j.ab.2007.07.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kunz C, Rudloff S, Baier W, Klein N, Strobel S. 2000. Oligosaccharides in human milk: structural, functional, and metabolic aspects. Annu. Rev. Nutr. 20:699–722. 10.1146/annurev.nutr.20.1.699 [DOI] [PubMed] [Google Scholar]
- 21.Wang B, Brand-Miller J, McVeagh P, Petocz P. 2001. Concentration and distribution of sialic acid in human milk and infant formulas. Am. J. Clin. Nutr. 74:510–515 [DOI] [PubMed] [Google Scholar]
- 22.Podolsky DK. 1985. Oligosaccharide structures of human colonic mucin. J. Biol. Chem. 260:8262–8271 [PubMed] [Google Scholar]
- 23.Corfield AP, Myerscough N, Warren BF, Durdey P, Paraskeva C, Schauer R. 1999. Reduction of sialic acid O-acetylation in human colonic mucins in the adenoma-carcinoma sequence. Glycoconj. J. 16:307–317. 10.1023/A:1007026314792 [DOI] [PubMed] [Google Scholar]
- 24.Corfield A, Wagner S, Safe A, Mountford R, Clamp J, Kamerling J, Vliegenthart J, Schauer R. 1993. Sialic acids in human gastric aspirates: detection of 9-O-lactyl- and 9-O-acetyl-N-acetylneuraminic acids and a decrease in total sialic acid concentration with age. Clin. Sci. (Lond.) 84:573–579 [DOI] [PubMed] [Google Scholar]
- 25.Nees S, Schauer R, Mayer F, Ehrlich K. 1976. Purification and characterization of N-acetylneuraminate lyase from Clostridium perfringens. Hoppe-Seyler Z. Physiol. Chem. 357:839–854. 10.1515/bchm2.1976.357.1.839 [DOI] [PubMed] [Google Scholar]
- 26.Nees S, Schauer R. 1974. Induction of neuraminidase from Clostridium perfringens and the co-operation of this enzyme with acylneuraminate pyruvate lyase. Behring Inst. Mitt. 55:68–78 [Google Scholar]
- 27.Vimr ER, Troy FA. 1985. Regulation of sialic acid metabolism in Escherichia coli: role of N-acylneuraminate pyruvate-lyase. J. Bacteriol. 164:854–860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vimr ER, Troy FA. 1985. Identification of an inducible catabolic system for sialic acids (nan) in Escherichia coli. J. Bacteriol. 164:845–853 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Plumbridge J, Vimr E. 1999. Convergent pathways for utilization of the amino sugars N-acetylglucosamine, N-acetylmannosamine, and N-acetylneuraminic acid by Escherichia coli. J. Bacteriol. 181:47–54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Almagro-Moreno S, Boyd EF. 2009. Sialic acid catabolism confers a competitive advantage to pathogenic Vibrio cholerae in the mouse intestine. Infect. Immun. 77:3807–3816. 10.1128/IAI.00279-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Brigham C, Caughlan R, Gallegos R, Dallas MB, Godoy VG, Malamy MH. 2009. Sialic acid (N-acetylneuraminic acid) utilization by Bacteroides fragilis requires a novel N-acetyl mannosamine epimerase. J. Bacteriol. 191:3629–3638. 10.1128/JB.00811-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Anba-Mondoloni J, Chaillou S, Zagorec M, Champomier-Vergès M-C. 2013. Catabolism of N-acetylneuraminic acid, a fitness function of the food-borne lactic acid bacterium Lactobacillus sakei, involves two newly characterized proteins. Appl. Environ. Microbiol. 79:2012–2018. 10.1128/AEM.03301-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sela DA, Chapman J, Adeuya A, Kim JH, Chen F, Whitehead TR, Lapidus A, Rokhsar DS, Lebrilla CB, German JB, Price NP, Richardson PM, Mills DA. 2008. The genome sequence of Bifidobacterium longum subsp. infantis reveals adaptations for milk utilization within the infant microbiome. Proc. Natl. Acad. Sci. U. S. A. 105:18964–18969. 10.1073/pnas.0809584105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sela DA, Li Y, Lerno L, Wu S, Marcobal AM, German JB, Chen X, Lebrilla CB, Mills DA. 2011. An infant-associated bacterial commensal utilizes breast milk sialyloligosaccharides. J. Biol. Chem. 286:11909–11918. 10.1074/jbc.M110.193359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ruiz-Moyano S, Totten SM, Garrido DA, Smilowitz JT, German JB, Lebrilla CB, Mills DA. 2013. Variation in consumption of human milk oligosaccharides by infant gut-associated strains of Bifidobacterium breve. Appl. Environ. Microbiol. 79:6040–6049. 10.1128/AEM.01843-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ward R, Niñonuevo M, Mills D, Lebrilla C, German B. 2007. In vitro fermentability of human milk oligosaccharides by several strains of bifidobacteria. Mol. Nutr. Food Res. 51:1398–1405. 10.1002/mnfr.200700150 [DOI] [PubMed] [Google Scholar]
- 37.de Man JC, Rogosa M, Sharpe ME. 1960. A medium for the cultivation of lactobacilli. J. Appl. Bacteriol. 23:130–135. 10.1111/j.1365-2672.1960.tb00188.x [DOI] [Google Scholar]
- 38.Watson D, O'Connell Motherway M, Schoterman MHC, van Neerven RJJ, Nauta A, van Sinderen D. 2013. Selective carbohydrate utilization by lactobacilli and bifidobacteria. J. Appl. Microbiol. 114:1132–1146. 10.1111/jam.12105 [DOI] [PubMed] [Google Scholar]
- 39.Sambrook J, Fritsch EF, Maniatis T. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY [Google Scholar]
- 40.Terzaghi BE, Sandine WE. 1975. Improved medium for lactic streptococci and their bacteriophages. Appl. Microbiol. 29:807–813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rutherford K, Parkhill J, Crook J, Horsnell T, Rice P, Rajandream M-A, Barrell B. 2000. Artemis: sequence visualization and annotation. Bioinformatics 16:944–945. 10.1093/bioinformatics/16.10.944 [DOI] [PubMed] [Google Scholar]
- 42.O'Connell Motherway M, Zomer A, Leahy SC, Reunanen J, Bottacini F, Claesson MJ, O'Brien F, Flynn K, Casey PG, Moreno Munoz JA, Kearney B, Houston AM, O'Mahony C, Higgins DG, Shanahan F, Palva A, de Vos WM, Fitzgerald GF, Ventura M, O'Toole PW, van Sinderen D. 2011. Functional genome analysis of Bifidobacterium breve UCC2003 reveals type IVb tight adherence (Tad) pili as an essential and conserved host-colonization factor. Proc. Natl. Acad. Sci. U. S. A. 108:11217–11222. 10.1073/pnas.1105380108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ. 1990. Basic local alignment search tool. J. Mol. Biol. 215:403–410. 10.1016/S0022-2836(05)80360-2 [DOI] [PubMed] [Google Scholar]
- 44.O'Riordan K. 1998. Studies on antimicrobial activity and genetic diversity of Bifidobacterium species: molecular characterization of a 5.75 kb plasmid and a chromosomally encoded recA gene homologue from Bifidobacterium breve. Ph.D. dissertation National University of Ireland, Cork, Ireland [Google Scholar]
- 45.O'Connell Motherway M, O'Driscoll J, Fitzgerald GF, van Sinderen D. 2009. Overcoming the restriction barrier to plasmid transformation and targeted mutagenesis in Bifidobacterium breve UCC2003. Microb. Biotechnol. 2:321–332. 10.1111/j.1751-7915.2008.00071.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wells JM, Wilson PW, Le Page RWF. 1993. Improved cloning vectors and transformation procedure for Lactococcus lactis. J. Appl. Bacteriol. 74:629–636. 10.1111/j.1365-2672.1993.tb05195.x [DOI] [PubMed] [Google Scholar]
- 47.Mazé A, O'Connell-Motherway M, Fitzgerald G, Deutscher J, van Sinderen D. 2007. Identification and characterization of a fructose phosphotransferase system in Bifidobacterium breve UCC2003. Appl. Environ. Microbiol. 73:545–553. 10.1128/AEM.01496-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zomer A, Fernandez M, Kearney B, Fitzgerald GF, Ventura M, van Sinderen D. 2009. An interactive regulatory network controls stress response in Bifidobacterium breve UCC2003. J. Bacteriol. 191:7039–7049. 10.1128/JB.00897-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.García de la Nava J, Santaella DF, Alba JC, Carazo JM, Trelles O, Pascual-Montano A. 2003. Engene: the processing and exploratory analysis of gene expression data. Bioinformatics 19:657–658. 10.1093/bioinformatics/btg028 [DOI] [PubMed] [Google Scholar]
- 50.van Hijum SA, Garcia de la Nava J, Trelles O, Kok J, Kuipers OP. 2003. MicroPreP: a cDNA microarray data pre-processing framework. Appl. Bioinformatics 2:241–244 [PubMed] [Google Scholar]
- 51.van Hijum SA, de Jong A, Baerends RJ, Karsens HA, Kramer NE, Larsen R, den Hengst CD, Albers CJ, Kok J, Kuipers OP. 2005. A generally applicable validation scheme for the assessment of factors involved in reproducibility and quality of DNA-microarray data. BMC Genomics 6:77. 10.1186/1471-2164-6-77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Long AD, Mangalam HJ, Chan BY, Tolleri L, Hatfield GW, Baldi P. 2001. Improved statistical inference from DNA microarray data using analysis of variance and a Bayesian statistical framework. Analysis of global gene expression in Escherichia coli K12. J. Biol. Chem. 276:19937–19944. 10.1074/jbc.M010192200 [DOI] [PubMed] [Google Scholar]
- 53.McGrath S, Fitzgerald GF, van Sinderen D. 2001. Improvement and optimization of two engineered phage resistance mechanisms in Lactococcus lactis. Appl. Environ. Microbiol. 67:608–616. 10.1128/AEM.67.2.608-616.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Alvarez-Martín P, O'Connell-Motherway M, van Sinderen D, Mayo B. 2007. Functional analysis of the pBC1 replicon from Bifidobacterium catenulatum L48. Appl. Microbiol. Biotechnol. 76:1395–1402. 10.1007/s00253-007-1115-5 [DOI] [PubMed] [Google Scholar]
- 55.Mierau I, Kleerebezem M. 2005. 10 years of the nisin-controlled gene expression system (NICE) in Lactococcus lactis. Appl. Microbiol. Biotechnol. 68:705–717. 10.1007/s00253-005-0107-6 [DOI] [PubMed] [Google Scholar]
- 56.de Ruyter PG, Kuipers OP, de Vos WM. 1996. Controlled gene expression systems for Lactococcus lactis with the food-grade inducer nisin. Appl. Environ. Microbiol. 62:3662–3667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bradford MM. 1976. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 7:248–254 [DOI] [PubMed] [Google Scholar]
- 58.Titgemeyer F, Reizer J, Reizer A, Saier MH. 1994. Evolutionary relationships between sugar kinases and transcriptional repressors in bacteria. Microbiology 140:2349–2354. 10.1099/13500872-140-9-2349 [DOI] [PubMed] [Google Scholar]
- 59.Conejo M, Thompson S, Miller B. 2010. Evolutionary bases of carbohydrate recognition and substrate discrimination in the ROK protein family. J. Mol. Evol. 70:545–556. 10.1007/s00239-010-9351-1 [DOI] [PubMed] [Google Scholar]
- 60.Ogura T, Wilkinson AJ. 2001. AAA+ superfamily ATPases: common structure—diverse function. Genes Cells 6:575–597. 10.1046/j.1365-2443.2001.00447.x [DOI] [PubMed] [Google Scholar]
- 61.Vimr ER, Kalivoda KA, Deszo EL, Steenbergen SM. 2004. Diversity of microbial sialic acid metabolism. Microbiol. Mol. Biol. Rev. 68:132–153. 10.1128/MMBR.68.1.132-153.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Bottacini F, O'Connell Motherway M, Kuczynski J, O'Connell K, Serafini F, Duranti S, Milani C, Turroni F, Lugli G, Zomer A, Zhurina D, Riedel C, Ventura M, van Sinderen D. 2014. Comparative genomics of the Bifidobacterium breve taxon. BMC Genomics 15:170. 10.1186/1471-2164-15-170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Alvarez-Martin P, O'Connell Motherway M, Turroni F, Foroni E, Ventura M, van Sinderen D. 2012. A two-component regulatory system controls autoregulated serpin expression in Bifidobacterium breve UCC2003. Appl. Environ. Microbiol. 78:7032–7041. 10.1128/AEM.01776-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Turroni F, Bottacini F, Foroni E, Mulder I, Kim J-H, Zomer A, Sanchez B, Bidossi A, Ferrarini A, Giubellini V, Delledonne M, Henrissat B, Coutinho P, Oggioni M, Fitzgerald G, Mills D, Margolles A, Kelly D, van Sinderen D, Ventura M. 2010. Genome analysis of Bifidobacterium bifidum PRL2010 reveals metabolic pathways for host-derived glycan foraging. Proc. Natl. Acad. Sci. U. S. A. 107:19514–19519. 10.1073/pnas.1011100107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Garrido D, Nwosu C, Ruiz-Moyano S, Aldredge D, German JB, Lebrilla CB, Mills DA. 2012. Endo-β-N-acetylglucosaminidases from infant gut-associated bifidobacteria release complex N-glycans from human milk glycoproteins. Mol. Cell. Proteomics 11:775–785. 10.1074/mcp.M112.018119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.White R. 1968. Control of amino sugar metabolism in Escherichia coli and isolation of mutants unable to degrade amino sugars. Biochem. J. 106:847–858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Reizer J, Ramseier TM, Reizer A, Charbit A, Saier MH. 1996. Novel phosphotransferase genes revealed by bacterial genome sequencing: a gene cluster encoding a putative N-acetylgalactosamine metabolic pathway in Escherichia coli. Microbiology 142:231–250. 10.1099/13500872-142-2-231 [DOI] [PubMed] [Google Scholar]
- 68.Post DM, Mungur R, Gibson BW, Munson RS., Jr 2005. Identification of a novel sialic acid transporter in Haemophilus ducreyi. Infect. Immun. 73:6727–6735. 10.1128/IAI.73.10.6727-6735.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Martinez J, Steenbergen S, Vimr E. 1995. Derived structure of the putative sialic acid transporter from Escherichia coli predicts a novel sugar permease domain. J. Bacteriol. 177:6005–6010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Allen S, Zaleski A, Johnston JW, Gibson BW, Apicella MA. 2005. Novel sialic acid transporter of Haemophilus influenzae. Infect. Immun. 73:5291–5300. 10.1128/IAI.73.9.5291-5300.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Chowdhury N, Norris J, McAlister E, Lau SYK, Thomas GH, Boyd EF. 2012. The VC1777–VC1779 proteins are members of a sialic acid-specific subfamily of TRAP transporters (SiaPQM) and constitute the sole route of sialic acid uptake in the human pathogen Vibrio cholerae. Microbiology 158:2158–2167. 10.1099/mic.0.059659-0 [DOI] [PubMed] [Google Scholar]
- 72.Pokusaeva K, Neves AR, Zomer A, O'Connell-Motherway M, MacSharry J, Curley P, Fitzgerald GF, van Sinderen D. 2010. Ribose utilization by the human commensal Bifidobacterium breve UCC2003. Microb. Biotechnol. 3:311–323. 10.1111/j.1751-7915.2009.00152.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Vimr E, Lichtensteiger C, Steenbergen S. 2000. Sialic acid metabolism's dual function in Haemophilus influenzae. Mol. Microbiol. 36:1113–1123. 10.1046/j.1365-2958.2000.01925.x [DOI] [PubMed] [Google Scholar]
- 74.Olson ME, King JM, Yahr TL, Horswill AR. 2013. Sialic acid catabolism in Staphylococcus aureus. J. Bacteriol. 195:1779–1788. 10.1128/JB.02294-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Krüger D, Schauer R, Traving C. 2001. Characterization and mutagenesis of the recombinant N-acetylneuraminate lyase from Clostridium perfringens. Eur. J. Biochem. 268:3831–3839. 10.1046/j.1432-1327.2001.02297.x [DOI] [PubMed] [Google Scholar]
- 76.Lilley GG, Barbosa JA, Pearce LA. 1998. Expression in Escherichia coli of the putative N-acetylneuraminate lyase gene (nanA) from Haemophilus influenzae: overproduction, purification, and crystallization. Protein Expr. Purif. 12:295–304. 10.1006/prep.1997.0841 [DOI] [PubMed] [Google Scholar]
- 77.Sánchez-Carrón G, García-García MI, López-Rodríguez AB, Jiménez-García S, Sola-Carvajal A, García-Carmona F, Sánchez-Ferrer A. 2011. Molecular characterization of a novel N-acetylneuraminate lyase from Lactobacillus plantarum WCFS1. Appl. Environ. Microbiol. 77:2471–2478. 10.1128/AEM.02927-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kiyohara M, Tanigawa K, Chaiwangsri T, Katayama T, Ashida H, Yamamoto K. 2011. An exo-α-sialidase from bifidobacteria involved in the degradation of sialyloligosaccharides in human milk and intestinal glycoconjugates. Glycobiology 21:437–447. 10.1093/glycob/cwq175 [DOI] [PubMed] [Google Scholar]
- 79.Ng KM, Ferreyra JA, Higginbottom SK, Lynch JB, Kashyap PC, Gopinath S, Naidu N, Choudhury B, Weimer BC, Monack DM, Sonnenburg JL. 2013. Microbiota-liberated host sugars facilitate post-antibiotic expansion of enteric pathogens. Nature 502:96–99. 10.1038/nature12503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Ley R. 2014. Harnessing microbiota to kill a pathogen: the sweet tooth of Clostridium difficile. Nat. Med. 20:248–249. 10.1038/nm.3494 [DOI] [PubMed] [Google Scholar]
- 81.Aloisio I, Santini C, Biavati B, Dinelli G, Cencič A, Chingwaru W, Mogna L, Gioia D. 2012. Characterization of Bifidobacterium spp. strains for the treatment of enteric disorders in newborns. Appl. Microbiol. Biotechnol. 96:1561–1576. 10.1007/s00253-012-4138-5 [DOI] [PubMed] [Google Scholar]
- 82.Law J, Buist G, Haandrikman A, Kok J, Venema G, Leenhouts K. 1995. A system to generate chromosomal mutations in Lactococcus lactis which allows fast analysis of targeted genes. J. Bacteriol. 177:7011–7018 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.