Abstract
GIL01, Bam35, GIL16, AP50, and Wip1 are tectiviruses preying on the Bacillus cereus group. Despite the significant contributions of phages in different biological processes, little is known about the dealings taking place between tectiviruses and their Gram-positive bacterial hosts. Therefore, this work focuses on characterizing the interactions between tectiviruses and the B. cereus group by assessing their occurrence and genetic diversity and evaluating their host range. To study the occurrence of tectiviruses in the B. cereus group, 2,000 isolates were evaluated using primers designed to be specific to two variable regions detected in previously described elements. PCR and propagation tests revealed that tectivirus-like elements occurred in less than 3% of the isolates. Regardless of this limited distribution, several novel tectiviruses were found, and partial DNA sequencing indicated that a greater diversity exists within the family Tectiviridae. Analyses of the selected variable regions, along with their host range, showed that tectiviruses in the B. cereus group can be clustered mainly into two different groups: the ones infecting B. anthracis and those isolated from other B. cereus group members. In order to address the host range of some novel tectiviruses, 120 strains were tested for sensitivity. The results showed that all the tested tectiviruses produced lysis in at least one B. cereus sensu lato strain. Moreover, no simple relationship between the infection patterns of the tectiviruses and their diversity was found.
INTRODUCTION
The Bacillus cereus group comprises seven closely related species according to the current taxonomy: B. cereus sensu stricto (referred to herein as B. cereus), B. anthracis, B. thuringiensis, B. mycoides, B. pseudomycoides, B. weihenstephanensis, and B. cytotoxicus (1, 2). Although these species are genetically very close, they also display highly specialized lifestyles with distinct ecological niches and virulence spectra, which are often directly associated with plasmids, particularly large ones (1). For example, in B. anthracis, the causative agent of the lethal disease anthrax, the toxin components and capsule genes responsible for full virulence are located on the 182-kb pXO1 and 95-kb pXO2 plasmids, respectively (3). The entomopathogenic properties and main characteristics that distinguish B. thuringiensis, a bacterium used worldwide as a bioinsecticide (4), are due to large plasmids carrying cry genes (5). Moreover, B. cereus is known mainly to be an opportunistic pathogen of mammals, causing food-associated intoxications manifested by either diarrheal or emetic syndromes. The latter syndrome is caused by a cyclic dodecadepsipeptide called cereulide, whose genetic determinants are plasmid borne (6, 7). The remaining members of the B. cereus group are differentiated on the basis of gross morphological characteristics (i.e., rhizoidal growth for B. mycoides and B. pseudomycoides) and physiological ones (i.e., psychrotolerance for B. weihenstephanensis, thermotolerance for B. cytotoxicus), which seem to be encoded by chromosomal genes. Although little is known about the plasmid-borne features in these four members of the B. cereus group, their potential contribution to different ecotypes and pathotypes cannot be disregarded.
The close relationship among the different members of the B. cereus group has been established through studies based on phylogenetic analysis of single or multiple gene markers and, recently, from data provided by multiple whole-genome sequencing projects (8). In addition, extensive genomic studies conducted on strains of B. cereus, B. thuringiensis, and B. anthracis have suggested that it may be more appropriate to consider them varieties of a single generic species from which various ecotypes and pathotypes have evolved (9–12). To further complicate this issue, seven major phylogenetic subdivisions can be distinguished among the seven members of the B. cereus group, but strains of B. cereus and B. thuringiensis are scattered in the majority of these phylogenetic clusters (13–15). However, B. anthracis and cereulide-producing B. cereus isolates seem to appear as clonal populations restricted to particular clades (14, 16, 17).
Members of the B. cereus group are known to be associated with species-specific bacteriophages (phages) either as prophages integrated into the chromosome or as independently replicating elements (18; A. Gillis and J. Mahillon, unpublished data). To date, phages residing as linear plasmids and undergoing a lysogenic stage in the B. cereus group have been identified as belonging to the family Tectiviridae (Lat. tectus, covered). This family includes tail-less phages that have a lipid membrane/vesicle beneath the icosahedral protein shell and that are formed of approximately equal amounts of virus-encoded proteins and lipids derived from the host cell plasma membrane. Upon infection, the lipid membrane becomes a tail-like structure used in genome delivery. The approximately 15-kb linear double-stranded DNA (dsDNA) genome has long inverted terminal repeat sequences (∼100 bp) and is coiled within the lipid membrane (20).
The Tectiviridae can be subdivided into two groups according to the host that they infect. The PRD1-like phages infect Gram-negative enterobacteria such as Escherichia coli or Salmonella enterica, while GIL01, Bam35, GIL16, AP50, and Wip1 are representatives of temperate tectiviruses preying on the B. cereus group (18, 21–25; A. Gillis and J. Mahillon, unpublished data). The second group of phages also exhibits strong similarity to the B. cereus linear plasmid pBClin15 (23, 26). Tectiviruses infecting Gram-negative bacteria are known to lyse the host cell only at completion of their infectious cycle, not establishing a prophage state within the bacterial host. Both tectivirus groups have a similar genome size and organization, yet they have no detectable sequence similarity at the nucleotide level (20, 21).
All six PRD1-like tectiviruses infecting Gram-negative bacteria display a very high level of sequence identity (91.9 to 99.8%) (21). Although the six fully sequenced tectivirus-like elements from the B. cereus group are also conserved, their pairwise nucleotide sequence identity appears to be more diverse, ranging from 23.5 to 100% (23, 25). Moreover, genome alignments of this group of tectiviruses indicated the existence of two highly variable regions (HVRs). In AP50, the first and second HVRs are located between coding DNA sequences (CDSs) 8 to 10 and 28 to 30, respectively (24). In GIL01, the first HVR is adjacent to an inducible promoter region that controls the expression of structural and lytic genes (27). Recently, five tectiviruses (i.e., Sole, Sand, Sato, Emet, and Lima) have been uncovered in the B. cereus group using primers that target the first HVR, including the promoter region (28). This region was shown to harbor small and unique genes with no orthologs (ORFans) in otherwise well-conserved phages, suggesting that the acquisition of those ORFans may provide a source of genetic diversity within this group of phages (28).
Since the occurrence of tectiviruses in the B. cereus group remains poorly characterized, the main goals of this study were (i) to assess other variable, ORFans-free regions that are suitable for large screenings, (ii) to evaluate the prevalence and distribution of tectiviruses in a worldwide collection of strains belonging mainly to the B. cereus group, and (iii) to analyze the genetic diversity of several novel putative tectiviruses. The results greatly extend the existing view of the occurrence and genetic diversity of tectiviruses in the B. cereus group, as well as their host range, indicating that a greater genetic diversity than was previously thought exists within the Tectiviridae.
MATERIALS AND METHODS
Bacterial collection and culture conditions. (i) Bacterial collection.
A collection comprising 2,000 isolates was compiled for this study. This bacterial set includes all seven recognized members of the B. cereus group and other Gram-positive bacterial species (including, among others, Bacillus subtilis, Bacillus licheniformis, Paenibacillus polymyxa, Listeria spp., and Staphylococcus spp.). The B. cereus group species partition was settled on the basis of phenotypic and pathogenic traits, using the current taxonomy, as follows (Table 1): B. cereus, B. anthracis, B. thuringiensis, B. weihenstephanensis, and B. cytotoxicus were limited to well-characterized strains mostly received from collections worldwide; B. mycoides-B. pseudomycoides was used for those isolates already identified to be members of these species or presenting typical rhizoid growth; and B. cereus emetic pathotype and B. weihenstephanensis emetic pathotype were restricted to isolates typified to be cereulide producers and/or harboring the genetic determinants for cereulide production. For isolates that did not fulfill these categories and had partial taxonomical information, B. cereus sensu lato was used to avoid possible misidentification and, consequently, misinterpretation. The bacterial set contains isolates from environmental and clinical samples, as well as isolates from diverse foodstuffs and food-borne outbreaks. Relevant features of a subset of the isolates used in this work are shown in Table 2. Details on the origins of the remaining isolates can be obtained from the authors upon request.
TABLE 1.
Origin and environmental distribution of strains screened for the presence of tectivirus-like elements
| Bacterial species | No. of isolates from the following source: |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Water | Soil | Plants | Insects | Food toxi-infa | Foodstuffs | Clinical | Others | Unknown | Total | |
| B. cereus sensu lato | 29 | 457 | 0 | 65 | 0 | 465 | 4 | 75 | 49 | 1,144 |
| B. cereus | 1 | 158 | 0 | 5 | 19 | 84 | 21 | 6 | 31 | 325 |
| B. cereus emetic pathotype | 0 | 0 | 0 | 0 | 14 | 28 | 4 | 29 | 0 | 75 |
| B. thuringiensis | 0 | 32 | 13 | 16 | 0 | 0 | 1 | 16 | 115 | 193 |
| B. anthracis | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 14 | 13 | 27 |
| B. mycoides-B. pseudomycoides | 35 | 95 | 2 | 7 | 0 | 3 | 0 | 8 | 19 | 169 |
| B. weihenstephanensis | 0 | 1 | 0 | 0 | 0 | 4 | 0 | 0 | 2 | 7 |
| B. weihenstephanensis emetic pathotype | 0 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 |
| B. cytotoxicus | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 1 | 0 | 2 |
| Other Gram-positive bacteria | 0 | 0 | 0 | 9 | 0 | 1 | 0 | 4 | 42 | 56 |
| Total | 65 | 745 | 15 | 102 | 34 | 585 | 30 | 153 | 271 | 2,000 |
toxi-inf, toxic infection (strains related to food-borne outbreaks).
TABLE 2.
Bacillus cereus group hosts for tectiviruses identified in this study
| Straina | Source of isolation, strain serovar | Country of origin | Prophage | DNA amplification |
Strain source or referencee | |
|---|---|---|---|---|---|---|
| DNA polB (RR region) | mur1 (PL region) | |||||
| B. cereus sensu lato | ||||||
| 9616 | Honey from rape flowers | Belgium | Honey16 | + | + | MIAE |
| 10279 | Honey from Rubus flowers | Belgium | Honey79 | + | + | MIAE |
| 10292 | Honey from linden flowers | Belgium | Honey92 | + | + | MIAE |
| AFR036 | Starchy food | Ivory Coast | Africa36 | − | + | MIAE |
| AFR119 | Starchy food | Ivory Coast | Africa19 | − | + | MIAE |
| Bc1561 | Cooked chicken, food poisoning | Brazil | Chick61 | + | + | MIAE |
| Bc1570 | Food for intubation feeding | Brazil | Tube70 | + | + | MIAE |
| Bc1573 | Food for intubation feeding | Brazil | Tube73 | + | + | MIAE |
| Bc1575 | Food for intubation feeding | Brazil | Tube75 | + | + | MIAE |
| Bc1576 | Uncooked chicken, food poisoning | Brazil | Chick76 | − | + | MIAE |
| COR1 | Coriander spice | NAf | Coriander | + | + | MIAE |
| Cum1 | Cumin spice | NA | Cumin | + | + | MIAE |
| EO9 3328 | NA | NA | Nath28 | + | + | MIAE |
| Miel2 | Honey | Belgium | Honey02 | + | + | MIAE |
| Miel3 | Honey | Belgium | Honey03 | + | + | MIAE |
| Miel9 | Honey | Belgium | Honey09 | + | + | MIAE |
| R30 | Cooked rice | Belgium | Rice30 | + | + | MIAE |
| TP08 Gr6 | Environment | Belgium | Stud86 | + | + | MIAE |
| VD166 | Soil | United Arab Emirates | Soleb | − | + | 60 |
| VD184 | Soil | United Arab Emirates | Sandb | + | + | 60 |
| B. cereus | ||||||
| ATCC 21281 (AH229)g | Soil | NA | Soil29 | + | + | ATCC |
| B23c,h | Food-poisoning case | Canada | Can23 | + | + | S. Kasatiya |
| Bc021 (ISP 2954) | Lamb meat | Belgium | Lamb | + | + | SIPH |
| Bc090 (Bc1568) | Vanilla custard | Belgium | Vanilla | − | + | MIAE |
| MHI 1687 | Rice | Germany | Rice87 | + | + | DVS |
| MHI 2385 | NA | Germany | Ger85 | + | + | DVS |
| B. cereus emetic pathotype | ||||||
| 5958d | Boiled pasta, food poisoning | Belgium | Simila | + | + | 48 |
| 5964a | Pasta salad, food poisoning | Belgium | Fusilli | + | + | 48 |
| 5964b | Pasta salad, food poisoning | Belgium | Farfalle | + | + | 48 |
| 5975c | Vomit, food poisoning | Belgium | Emetb | + | + | 48 |
| AND1284 (10329) | Pasta | Belgium | Satob | − | + | 6 |
| B. thuringiensis | ||||||
| HD1 | Insect larva, reference B. thuringiensis serovar kurstaki | France | Edouard | + | + | BGSC |
| HD133 (4J3) | Insect larva, B. thuringiensis serovar aizawai/pacificus | United Kingdom | Aiza33 | + | + | BGSC |
| HD231 (4D14)c | Wild type isolate, B. thuringiensis serovar kurstaki | NA | Kurs31 | + | + | BGSC |
| ATCC 35646 | Sewage, reference strain, B. thuringiensis serovar israelensis | Israel | pBth35646d | + | + | A. Sorokin |
| IBL 4222 | Cat, B. thuringiensis serovar israelensis | NA | Cat22 | + | + | USDA |
| T14 034c | NA, B. thuringiensis serovar israelensis | Brazil | Bra34 | + | + | IEBC |
| T14 477c | NA, B. thuringiensis serovar israelensis | NA | Isra77 | + | + | IEBC |
| BMG 1.7 | Soil | Tunisia | Tun17 | + | + | 61 |
| DBT 012c | Cauliflower phylloplane | Denmark | Caul12 | + | + | B. Hansen |
| DBT 027 | Cauliflower phylloplane | Denmark | Caul27 | + | + | B. Hansen |
| DBT 066 | Cauliflower phylloplane | Denmark | Caul66 | + | + | B. Hansen |
| DBT 088 | Cauliflower cabbage larva | Denmark | Cabb88 | + | + | B. Hansen |
| DBT 111 | Cauliflower phylloplane | Denmark | Caul11 | + | + | B. Hansen |
| DBT 120 | Cauliflower phylloplane | Denmark | Caul20 | − | + | B. Hansen |
| DBT 123 | Cauliflower phylloplane | Denmark | Caul23 | + | + | B. Hansen |
| DBT 685 | White cabbage phylloplane | Denmark | Cabb85 | + | + | B. Hansen |
| DBT 764 | White cabbage phylloplane | Denmark | Cabb64 | + | + | B. Hansen |
| DBT 776 | White cabbage phylloplane | Denmark | Cabb76 | + | + | B. Hansen |
| Bt5c | NA, B. thuringiensis serovar alesti | Sweden | Ales05 | + | + | A. Lövgren |
| B. mycoides-B. pseudomycoides | ||||||
| VDm028 | Soil | Greenland | Lutum | + | + | 60 |
| VDm034 | Soil | Greenland | Limab | + | − | 60 |
Alternative strain names are given in parentheses.
Phages have been partially characterized by Jalasvuori et al. (28).
Strains were reported to be positive for GIL01-related elements by dot blotting or PCR (22).
Phage pBth35646 has been already designated by Sozhamannan et al. (24).
MIAE, Laboratory of Food and Environmental Microbiology, Université Catholique de Louvain, Louvain-la-Neuve, Belgium; ATCC, American Type Culture Collection, Manassas, VA; S. Kasatiya, Ottawa Public Health Laboratory (OPHL), Ottawa, Ontario, Canada; SIPH, Scientific Institute of Public Health, Brussels, Belgium; DVS, Department of Veterinary Sciences, Faculty of Veterinary Medicine, Ludwig Maximilians University, Munich, Germany; BGSC, Bacillus Genetic Stock Center, Columbus, OH; A. Sorokin, Institut Micalis, INRA, Jouy-en-Josas, France; USDA, U.S. Department of Agriculture, Beltsville, MD; IEBC, International Entomopathogenic Bacillus Center, Pasteur Institute, Paris, France; B. Hansen, National Environmental Research Institute (NERI), Roskilde, Denmark; A. Lövgren, University of Stockholm, Stockholm, Sweden.
NA, no information available.
This strain has been classified as B. cereus, even though it presents activity against mosquito larvae and hybridizes with the cry1a crystal toxin gene from B. thuringiensis (62).
This strain has been classified as B. cereus, but it produces typical B. thuringiensis crystal protein inclusions (data not shown).
(ii) Susceptible hosts.
Strains GBJ002, HER1410, and HER1047 were used as susceptible hosts for tectiviral infections (22, 23, 28, 29). Strain GBJ002 is derived from B. thuringiensis serovar israelensis 4Q7 that is cured of its plasmids and that has chromosomal resistance to nalidixic acid (Nal) (30). HER1410 was identified as B. thuringiensis serovar thuringiensis, whereas HER1047 has been classified as B. cereus.
(iii) Media and culture conditions.
Bacteria were grown overnight at 30°C on lysogenic broth (LB; also referred to elsewhere as Luria-Bertani) medium (5 g/liter NaCl, 5 g/liter yeast extract, 10 g/liter tryptone) solidified with 1.4% (wt/vol) agar, unless otherwise indicated. B. anthracis strains were grown overnight at 37°C on Columbia agar plates supplemented with 5% sheep blood (CSB) (Bio-Rad).
Whole-genome alignments and PCR primer design.
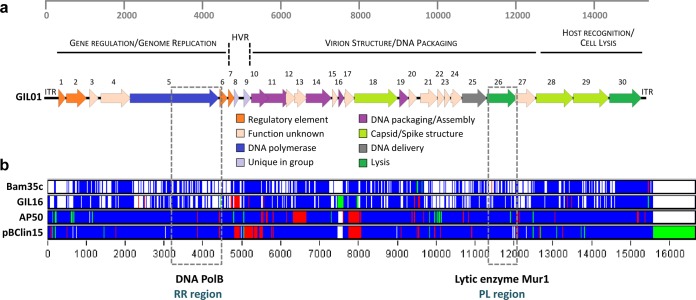
Whole-genome sequence alignments of the fully sequenced tectiviral elements (GIL01, Bam35, and GIL16 from B. thuringiensis, AP50 infecting B. anthracis in a specific manner, and plasmid pBClin15 from B. cereus) were performed using the ClustalW program included in DAMBE software (31). Alignments were manually analyzed at the single nucleotide level in order to identify variable regions. The ClustalW alignment file generated from the Multifasta alignment was summarized for visualization using Base-By-Base software (Fig. 1) by use of a model described elsewhere (24, 32, 33).
FIG 1.
Graphical representation of selected variable regions. (a) Genetic map of phage GIL01. Three gene modules based on functional grouping and similarities to other Tectiviridae in the B. cereus group are displayed. Predicted genes are represented as block arrows, and the color key at the bottom indicates postulated functions. CDS numbers are indicated. The ruler at the top represents base pairs in the GIL01 genome. ITR, inverted terminal repeat; HVR, highly variable region. (b) Whole-genome nucleotide alignments of Gram-positive bacterial tectivirus elements. For visualization, the ClustalW alignment file generated from the Multifasta alignment was summarized using Base-By-Base software (32, 33) after a model given previously (24). The GIL01 genome was used as the base sequence, and changes in other tectiviral genomes are color coded as follows: white, nucleotide identity; blue, single nucleotide polymorphism; green, insertions; and red, deletions. The ruler at the bottom represents base pairs in the whole-genome nucleotide sequence alignments. Selected variable regions (see the text), the RR and the PL regions, which mainly target the genes encoding DNA polymerase B (DNA PolB) and N-acetyl-muramidase (Mur1), respectively, are highlighted by gray dashed rectangles.
Two sets of degenerated primers (Tect1-Tect2 and Tect3-Tect4; Table 3) were designed on the basis of two selected variable regions with pseudoconserved ends that allowed primer anchoring. The selected regions included genes that are involved in genome replication and that encode the virion structure (Fig. 1). Degenerated primers were designed so that all possible nucleotide sequences present in tectiviral reference genomes were included. Primers Tect1-Tect2 partially target the DNA polB gene and CDS 6, whereas primers Tect3-Tect4 target the mur1 gene and CDS 27.
TABLE 3.
Characteristics of primer pairs used in this study
| Primer paira | Sequence (5′ to 3′)b | Positionsc | Major gene targeted | Expected amplicon size (bp) | Annealing temp (°C) |
|---|---|---|---|---|---|
| Tect1 (f) | GAAGGTGTWTGGACRTTHCC | 3162–4442 | DNA polB | 1,280 | 56 |
| Tect2 (r) | ACYHGCCATDTCYCGCAC | ||||
| Tect3 (f) | CCGGYGGWWTRTCMGGDCTTG | 11096–11838 | mur1 | 742 | 59 |
| Tect4 (r) | AAGTCCATHGCCATGCCTTGG |
f and r, forward and reverse primers, respectively.
Key to symbols: W, A + T; R, A + G; H, A + T + C; Y, C + T; D, G + A + T; M, A + C.
Positions correspond to the primer annealing sites in the GIL01 genome.
Total DNA extractions.
Total DNA from all bacteria, except B. anthracis, was prepared as described earlier by Hansen and Hendriksen (34). Briefly, one bacterial colony was resuspended in 200 μl of Tris-EDTA (pH 8.0) buffer. Bacteria were lysed by incubation at 100°C for 10 min and cooled for 5 min on ice, and cell debris was removed by centrifugation at 15,000 × g for 10 min. The DNA-containing supernatant was transferred to a new Microfuge tube and stored at 4°C for immediate utilization.
B. anthracis DNA was extracted by using a DNeasy blood and tissue kit (Qiagen) and adding to the manufacturer's recommendations a prior lysis step, as follows: one single bacterial colony growing on a CSB-agar plate was recovered and resuspended in 180 μl lysis buffer (20 mM Tris, pH 8.0, 2 mM EDTA, pH 8.0, 1.2% Triton X-100). Lysozyme (4 mg; Sigma) and RNase A (4 μl of 100 mg/ml; Sigma) were added. The suspension was vortex mixed and incubated for 30 min at 37°C. Proteinase K (25 μl; Sigma) was added, and the instructions indicated in the kit were followed.
PCR screening, cloning, and sequencing.
DNA extractions were screened for the presence of tectivirus-related elements by PCR using the Tect1-Tect2 and Tect3-Tect4 primer pairs (Table 3). DNA extractions from strains harboring GIL01, GIL16, and pBClin15 were used as positive controls. PCR amplicons were either purified directly using a GenElute PCR cleanup kit (Sigma) or gel excised and further purified by use of a QIAquick gel extraction kit (Qiagen). Purified amplicons were inserted by TA ligation into the pCR4-TOPO cloning vector (Invitrogen) and electroporated into E. coli TOP10 electrocompetent cells, following the manufacturer's instructions. Plasmid DNA was extracted from selected positive transformants by using a GenElute plasmid miniprep kit (Sigma) and sequenced directly using both strands. Sequencing reactions were performed at the Macrogen sequencing facility (The Netherlands).
Sequence comparison analyses.
Multiple-nucleotide-sequence alignments of tectiviral elements were obtained by use of the MUSCLE program (35), which is built into the MEGA (v5.2) program (36). Consensus sequences for each tectiviral isolate were translated in frame into amino acids for the Mur1 and DNA polymerase B (DNA PolB) proteins. Pairwise p-distance matrices were calculated, and phylogenetic analyses were conducted with MEGA. A graphical representation of the percentage of pairwise nucleotide sequence identity was made using the SDT (v1.0) program (37). The general time-reversible plus gamma model of nucleotide substitution was chosen using MODELTEST (38), implemented online as FindModel at http://www.hiv.lanl.gov/content/sequence/findmodel/findmodel.html. The maximum likelihood (ML) method was employed to build phylogenetic trees using a value of 1,000 bootstrap resamplings. The criterion used for subdividing sequences into separate subclades was bootstrap support of greater than 60%. For optimal phylogenetic reconstruction, alignments for ML trees were previously cured by use of the Gblocks (39) online version hosted at the Phylogeny.fr website (40), using the following parameters: minimum sequences for flank position, 85%; maximum number of contiguous nonconserved positions, 8; minimum block length, 10; and no gaps in final blocks. It must be noted that all sequence alignments performed with sequences obtained using primers Tect1-Tect2 were previously cured by Gblocks in order to exclude alignment segments that had too many variable positions or gaps. Deduced DNA PolB and Mur1 amino acid sequences from noncured alignments were manually examined to identify conserved residues.
A recombination analysis was performed on both cured and noncured alignments of the nucleotide and deduced amino acid sequences for DNA PolB and Mur1, using the Genetic Algorithm for Recombination Detection (GARD) method (41) with default settings (available at http://www.datamonkey.org/).
Phage propagation.
Phage stocks were obtained by treating 20 ml of a mid-log-phase bacterial culture with 100 ng/ml of mitomycin C (AppliChem) for 1 h, and the culture was then centrifuged at 4,500 rpm for 10 min. The pellet was washed twice with 5 ml of 0.01 M MgSO4. The bacterial cells were resuspended in 20 ml fresh LB medium, and phage induction was allowed to progress for 2 h at 30°C and 120 rpm. After centrifugation, the supernatants were filtered through 0.22-μm-pore-size filter membranes (Millipore) and the titer was determined by the double-layer agar method or the filtered supernatants were analyzed by spot assays. For lysogeny confirmation, susceptible hosts GBJ002, HER1410, and HER1047 were grown to mid-exponential phase and 200 μl was infected with equal volumes of 10-fold serial dilutions of filtered phage stocks. Bacterium-phage mixes were incubated for 30 min and plated with molten LB-top agar (0.6% [wt/vol]) onto LB-agar plates. The plates were incubated overnight at 30°C and visually screened for PFU. Single turbid plaques were randomly selected (n = 3), picked with sterile toothpicks, and subjected to three rounds of single-plaque purification on their respective new host. Final lysogens growing on LB-agar were screened by PCR with primers Tect1-Tect2 and Tect3-Tect4.
Phage host range assessments and analyses.
The ability of 23 tectiviruses to infect other hosts was evaluated by spotting assays. Briefly, sterile-filtered phage stocks were adjusted to a concentration of approximately 1 × 105 PFU. Phages Sato and Lima were used without concentration adjustment due to the lack of a known host. Bacterial lawns were prepared as follows: 100 μl mid-exponential-phase bacterial cultures grown in LB liquid medium were added to 4 ml molten LB-top agar, the culture and medium were mixed gently, and the mixture was placed onto LB-agar plates. The contents of the plates were allowed to solidify and dry for 20 min at room temperature. A drop (spot) of 10 μl phage suspension was added on top of the bacterial lawn, allowed to dry for 20 min, and incubated overnight at 30°C for all tested hosts, except B. anthracis (37°C). Positive bacterial lysis was indicated by a clearing of the lawn encompassing the original spot. One hundred fifteen isolates belonging to the B. cereus group and five Gram-positive bacteria which did not harbor tectivirus-related elements were evaluated (see Table S1 in the supplemental material). Three independent spotting assays were performed, and a positive result for lysis was considered if lysis developed in a minimum of two assays.
In order to determine if bacterial host sensitivities can be used to distinguish among the tested tectiviral isolates, a principal coordinates analysis (PCoA) was undertaken using the software package InfoStat (version 2011; InfoStat Group, College of Agricultural Sciences, National University of Cordoba, Argentina). Scale values for PCoA were based on the detection of bacterial lysis, as follows: absence of lysis, 0; lysis by turbid plaques, 1; and lysis by clear plaques, 2.
Nucleotide sequence accession numbers.
The nucleotide sequences determined in this study were submitted to the GenBank database under accession numbers KF905330 to KF905357 for DNA polB (28 sequences) and KF905358 to KF905408 for mur1 (51 sequences) (Table 4). The GenBank accession numbers for reference genomes are AJ536073 for GIL01, AY257527 for Bam35c, AY701338 for GIL16c, EU408779 for AP50, KF188458 for Wip1, and AE016878 for pBClin15.
TABLE 4.
GenBank accession numbers for RR and PL regions, which partially code for the DNA PolB and Mur1 proteins, respectively
| Gene and prophage | GenBank accession no. |
|---|---|
| DNA polB | |
| Honey16 | KF905330 |
| Honey79 | KF905331 |
| Chick61 | KF905332 |
| Tube70 | KF905333 |
| Tube73 | KF905334 |
| Tube75 | KF905335 |
| Coriander | KF905336 |
| Cumin | KF905337 |
| Honey02 | KF905338 |
| Honey03 | KF905339 |
| Honey09 | KF905340 |
| Rice30 | KF905341 |
| Sand | KF905342 |
| Lamb | KF905343 |
| Rice87 | KF905344 |
| Simila | KF905345 |
| Fusilli | KF905346 |
| Farfalle | KF905347 |
| Emet | KF905348 |
| Edouard | KF905349 |
| pBth35646 | KF905350 |
| Cat22 | KF905351 |
| Bra34 | KF905352 |
| Tun17 | KF905353 |
| Caul12 | KF905354 |
| Ales05 | KF905355 |
| Lutum | KF905356 |
| Lima | KF905357 |
| mur1 | |
| Honey16 | KF905358 |
| Honey79 | KF905359 |
| Honey92 | KF905360 |
| Africa36 | KF905361 |
| Africa19 | KF905362 |
| Chick61 | KF905363 |
| Tube70 | KF905364 |
| Tube73 | KF905365 |
| Tube75 | KF905366 |
| Chick76 | KF905367 |
| Coriander | KF905368 |
| Cumin | KF905369 |
| Nath28 | KF905370 |
| Honey02 | KF905371 |
| Honey03 | KF905372 |
| Honey09 | KF905373 |
| Rice30 | KF905374 |
| Stud86 | KF905375 |
| Sole | KF905376 |
| Sand | KF905377 |
| Soil29 | KF905378 |
| Can23 | KF905379 |
| Lamb | KF905380 |
| Vanilla | KF905381 |
| Rice87 | KF905382 |
| Ger85 | KF905383 |
| Simila | KF905384 |
| Fusilli | KF905385 |
| Farfalle | KF905386 |
| Emet | KF905387 |
| Sato | KF905388 |
| Edouard | KF905389 |
| Aiza33 | KF905390 |
| Kurs31 | KF905391 |
| pBth35646 | KF905392 |
| Cat22 | KF905393 |
| Bra34 | KF905394 |
| Isra77 | KF905395 |
| Tun17 | KF905396 |
| Caul12 | KF905397 |
| Caul27 | KF905398 |
| Caul66 | KF905399 |
| Cabb88 | KF905400 |
| Caul11 | KF905401 |
| Caul20 | KF905402 |
| Caul23 | KF905403 |
| Cabb85 | KF905404 |
| Cabb64 | KF905405 |
| Cabb76 | KF905406 |
| Ales05 | KF905407 |
| Lutum | KF905408 |
RESULTS
Uncovering tectiviral variable regions using whole-genome alignments.
In order to identify new tectiviral variable regions, other than the HVRs previously found (24, 28), whole-genome nucleotide sequence alignments of the fully sequenced tectivirus-like elements (phages GIL01, Bam35, GIL16, and AP50 and plasmid pBClin15) were performed and compared (Fig. 1). Phage Wip1 was not included in the alignments because its genome was not available at the time (25).
On the basis of these whole-genome alignments, two variable regions were retained for the following reasons: (i) localization in different modules of the tectivirus genome (Fig. 1a), (ii) the target genes code for orthologous proteins in the five analyzed tectivirus-like elements, avoiding the existence of ORFans, and (iii) the presence of pseudoconserved upstream and downstream regions that allowed the design of degenerated primers. Tectiviruses possess a modular genome that can be divided into a replication-regulation (RR) region that encodes proteins involved in phage genome replication and regulation, ensuring the replication of the phage as a plasmid throughout the lysogenic cycle, and a packaging-lysis (PL) region that can be divided into a module encoding virion structural and DNA packaging proteins and another module encoding genes responsible for host recognition and lysis. Therefore, the first variable region identified in this work, located within the module that contains replication and regulatory genes (coordinates 3162 to 4442 in GIL01), is referred to herein as the “RR region.” This region codes for the C-terminal end of the DNA polymerase B (DNA PolB) protein (CDS 5) and for the N-terminal part of the LexA-like protein (CDS 6). The second variable region is located within the module containing the host recognition and cell lysis genes (coordinates 11096 to 11838 in GIL01) and is herein referred to as the “PL region.” The second region primarily targets the N-acetyl-muramidase-coding gene (mur1; CDS 26 in GIL01) (42) but also partially targets CDS 27, for which the predicted function is related to the pentameric spike base involved in host recognition (24).
Tectivirus-like elements are rare across the B. cereus group.
A total of 2,000 isolates from throughout the world comprising all seven recognized members of the B. cereus group and other Gram-positive bacterial species was compiled for this study (Table 1). Bacterial isolates were isolated from diverse sources, with a high prevalence being from foodstuffs, or were obtained from different laboratories and international collections representing a large geographical area (countries from Europe, Asia, the Americas, and Africa). Using two sets of primers, it was possible to identify 52 isolates of the B. cereus group harboring tectivirus-like elements (Table 2). It is important to note that the strains harboring phages Sole, Sand, Sato, Emet, and Lima (28) were included in the screening (Table 2) to validate the selection of the two variable regions and, as a consequence, to have their nucleotide sequences in these regions. The positive bacterial isolates belonged to the following categories: B. cereus sensu lato, B. cereus, B. cereus emetic pathotype, B. thuringiensis, and B. mycoides-B. pseudomycoides. No tectivirus-like elements were detected either in B. anthracis, B. weihenstephanensis, and B. cytotoxicus or in other Gram-positive bacteria. However, the likelihood that tectiviruses would be identified in B. anthracis, B. weihenstephanensis, and B. cytotoxicus and in other Gram-positive bacteria was low because few isolates were investigated.
Although a large collection of bacterial isolates was examined, the distribution of tectivirus-like elements in the environment tends to be very limited within the B. cereus group, showing an occurrence of only 2.7%. Beside the 10 members already known, including Sole, Sand, Sato, Emet, and Lima, 47 novel putative tectiviruses were discovered in the present work.
The tectivirus-like elements detected are able to carry out an infective process.
In order to resolve the infectious nature of the tectivirus-like elements detected using the two selected variable regions, previously known susceptible hosts (B. thuringiensis strains GBJ002 and HER1410 and B. cereus strain HER1047) (22, 23, 28, 29) were infected with phage stocks. Lysis tests demonstrated the production of turbid plaques by all phages tested in at least one strain, indicating that these are temperate phages (prophages) capable of lysogeny (Table 5). Moreover, for each phage, turbid plaques were randomly selected (n = 3) and purified by subsequent rounds of infection in their respective new host, and the final infected lysogens were screened by PCR to confirm positive tectiviral signals (data not shown). Hence, tectivirus-like elements were noted as prophages in Table 2 and named mainly on the basis of their source of isolation. It should be noted that despite several attempts, it was not possible to infect strains GBJ002, HER1410, and HER1047 with either phage Sato from B. cereus sensu lato or phage Lima from B. mycoides-B. pseudomycoides (Table 5). They were, however, kept in the data set for further analysis.
TABLE 5.
Host range determined by tectivirus spottinga
Phages GIL01 and GIL16 and 21 tectiviruses found in this study were tested for plaque formation in strains devoid of tectivirus-like elements. Gray cells, detection of lysis by turbid plaques; black cells, detection of lysis by clear plaques; white cells, the absence of lysis. Strains above the dashed line are known to be hosts for tectiviruses. Only the results for sensitive strains are shown. For the complete data set, see Table S1 in the supplemental material.
The first column specifies the species of the B. cereus group tested (Bc, B. cereus; Bc-sl, B. cereus sensu lato; BcE, B. cereus emetic pathotype; BcE-C, B. cereus emetic pathotype plasmid pCER270 cured; Bt, B. thuringiensis; Bt-C, B. thuringiensis plasmid cured; Bm, B. mycoides; Bpm, B. pseudomycoides; Bw, B. weihenstephanensis).
The PL region showed a low divergence between tectiviral isolates.
In a first endeavor to study the diversity of tectiviruses preying on the B. cereus group, the PL region, which partially targets the mur1 gene and CDS 27, was chosen mainly because it is involved in host lysis and was amplified for all the phages, except Lima (Table 2). Therefore, 51 isolates were sequenced for this region. Sequences were obtained from the genomes of GIL01, Bam35c, GIL16c, AP50, Wip1, and pBClin15 retrieved from the GenBank database. Preliminary sequence analyses indicated that some phages displayed identical nucleotide sequences. Since their source of isolation might be related (Table 2) and in order to avoid biases due to clonality, isolates Honey03, Caul11, Caul23, Caul27, Caul66, and Cabb76 were not considered in further analyses.
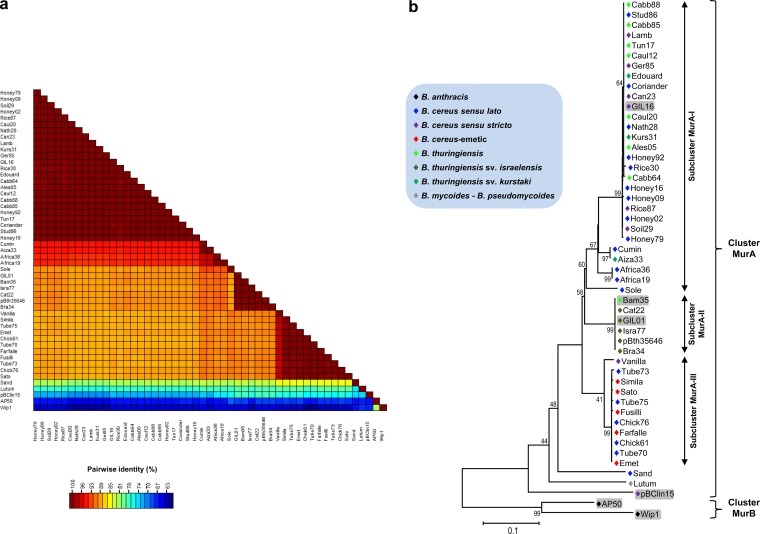
Analysis of the sequence of the PL region indicated that the different tectiviruses share between 62.2 and 99.8% nucleotide sequence identity, with several isolates being closely related to each other (Fig. 2a; see Table S2 in the supplemental material). Maximum likelihood (ML) assessments among the isolates revealed that two main clusters were formed, with most phages falling into cluster MurA (Fig. 2b). Cluster MurB contained only phages AP50 and Wip1. The nucleotide and amino acid sequences of representative members of the two clusters indicated that they share only 66.5 and 67.1% identities, respectively (see Table S2 in the supplemental material).
FIG 2.
Evolutionary relationships displayed by the PL region. (a) Graphical representation of percent pairwise nucleotide sequence identity of the PL region. The color scale bar indicates the percentage of nucleotide sequence identity among the 51 tectiviral isolates. (b) Maximum likelihood tree showing the genetic relationships among the new tectiviral isolates. The general time-reversible plus gamma model of nucleotide substitution was used to build the phylogenetic tree based on the PL region. Bootstrap values (1,000 iterations) above 60% are indicated for each node. Fifty-one nucleotide sequences and 581 cured positions were used for the analysis. The blue box indicates the color key for Bacillus species harboring the respective phages.
Sequences within cluster MurA could be subdivided into three subclusters, despite their close relationship (Fig. 2b). Subcluster MurA-I contained the majority of the isolates, and isolates in this subcluster were related to GIL16. This subcluster consisted of a mix of phages isolated from B. cereus sensu lato, B. cereus, and B. thuringiensis strains. Subcluster MurA-II encompassed a homogeneous group of GIL01-like phages, all of which were isolated from B. thuringiensis serovar israelensis strains, with a single exception, Bam35, which is reported to have been isolated from B. thuringiensis serovar alesti (43). Subcluster MurA-III comprised isolates related to Sato and Emet, two phages from B. cereus cereulide-producing (emetic) strains. Phages Simila, Fusilli, and Farfalle, all of which were derived from other emetic strains, also grouped in the latter subcluster. Their pairwise nucleotide sequence calculations revealed an identity greater than 99% (Fig. 2a). They also shared more than 99% DNA sequence identity with other phages isolated from clinical food-related material (i.e., Chick61 and Chick76 harbored by bacterial isolates involved in food intoxication cases and Tube70, Tube73, and Tube75 found in B. cereus isolates from food for intubation feeding). Subcluster MurA-III also contained phage Vanilla, which shared approximately 97% DNA sequence identity with the other isolates present in this group (Fig. 2a). In addition, cluster MurA harbored the more diverse tectivirus-related element pBClin15 and phages Sand and Lutum, with Lutum being isolated from B. mycoides-B. pseudomycoides. These three isolates shared less than 72 and 80% nucleotide and amino acid sequence identity, respectively, with the other members of cluster MurA (Fig. 2a; see Table S2 in the supplemental material).
The Mur1 nucleotide and deduced amino acid sequences were analyzed for potential recombination events by use of the GARD algorithm (41). No evidence of recombination between tectiviruses was found in this region, most likely due to its low divergence.
The RR region reveals higher tectiviral diversity.
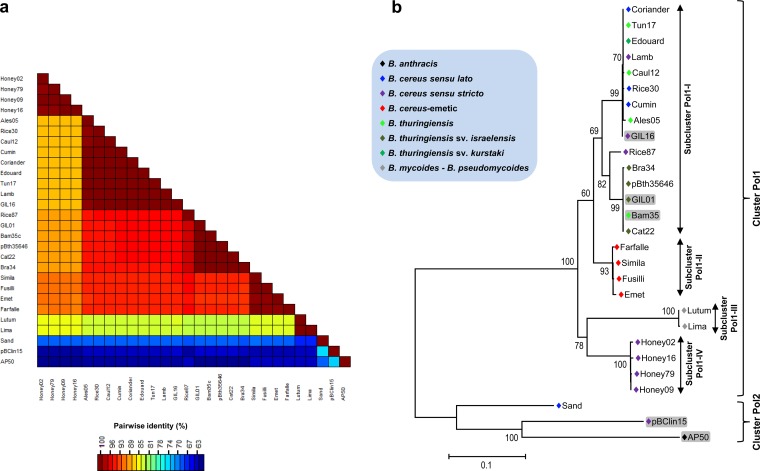
On the basis of the different clusters and groups formed with the PL region, the RR region of 28 representative tectiviruses was chosen to be analyzed in detail in order to better define their relationships. Sequences were obtained from the GIL01, Bam35c, GIL16c, AP50, and pBClin15 genomes retrieved from the GenBank database, while this region of 23 other isolates was sequenced.
Since preliminary analyses showed that the RR region has a greater diversity than the PL region, all the sequence alignments involving this region were cured with the aim of eliminating poorly aligned positions and divergent regions that may not be homologous or may have been saturated by multiple substitutions (39), which would affect the quality of the analysis outputs. The nucleotide sequence identities for this region ranged from 59% to 99.9% (Fig. 3a; see Table S3 in the supplemental material). ML evaluations also separated the selected tectiviruses into two major clusters (Fig. 3b) that shared 59 and 41% nucleotide and amino acid sequence identities, respectively (see Table S3 in the supplemental material). Similar to the PL region, most of the studied tectivirus elements fell into cluster Pol1. In contrast, the RR region grouped phage Sand and the tectivirus element pBClin15 together with AP50 into cluster Pol2.
FIG 3.
Evolutionary relationships displayed by the RR region. (a) Graphical representation of percent pairwise nucleotide sequence identity of the RR region. The color scale bar indicates the percent nucleotide sequence identity among the 28 tectiviral isolates. (b) Maximum likelihood tree showing the genetic relationships among representatives of new tectiviral isolates. The general time-reversible plus gamma model of nucleotide substitution was used to build the phylogenetic tree based on the RR region. Bootstrap values (1,000 iterations) above 60% are indicated for each node. Twenty-eight nucleotide sequences and 1,087 cured positions were used for the analysis. The blue box indicates the color key for Bacillus species harboring the respective phages.
As shown in Fig. 3b, sequences within cluster Pol1 could be subdivided into four subclusters. Subcluster Pol1-I encompassed isolates closely related to GIL01 and GIL16 phages, while subcluster Pol1-II included tectiviruses isolated from B. cereus emetic strains, for which pairwise nucleotide sequence identity calculations showed more than 98% identity (Fig. 3a). Moreover, isolates Chick61, Tube70, Tube73, and Tube75, which clustered with the B. cereus emetic phages using the PL region, were also compared using the RR region, confirming that they are all likely to group, independently of the region evaluated (data not shown). Subcluster Pol1-III contained the two phages isolated from B. mycoides-B. pseudomycoides strains: Lutum and Lima. Interestingly, Lima shared 99.9 and 100% nucleotide and amino acid sequence identities with Lutum, respectively (see Table S3 in the supplemental material), even though its PL region was not amplified by the set of primers used, possibly indicating a lower degree of conservation in the mur1 gene or that these two phages could have undergone genetic exchanges during coinfections of the same host. Finally, subcluster Pol1-IV contained phages isolated from B. cereus recovered from honey samples coming from different origins (Fig. 3b; Table 2). Besides, the relationships among the 28 selected phages were evaluated using pairwise calculations of only the deduced C-terminal region of DNA PolB. Even though the percentage of changes between subclusters Pol1-I and Pol1-II at the amino acid level was less than that at the nucleotide level (see Fig. S1 and Table S3 in the supplemental material), all phages found in B. cereus honey-related isolates shared among them more than 99% sequence identity and were separated from their other siblings by amino acid sequence changes of at least 6%.
One recombination breakpoint was detected between the selected phages with the GARD algorithm, using both cured and noncured alignments of the nucleotide sequence or the deduced C-terminal DNA PolB sequence. In particular, in noncured amino acid alignments, this breakpoint was located at position 198 (see Fig. S2a in the supplemental material), corresponding to residue E549 of the GIL01 complete DNA PolB protein. The recombination breakpoint divided the analyzed C-terminal DNA PolB region into two segments (segment 1, residues 1 to 198; segment 2, residues 199 to 388), which in turn reflected two different clustering subtrees (see Fig. S2b in the supplemental material).
Conservation of essential residues in Mur1 and DNA PolB proteins.
It was then investigated if the observed nucleotide substitutions could drive changes in some important residues that are essential for the enzymatic activity of Mur1 and DNA PolB proteins or, on the contrary, if these essential residues are conserved among the tectiviral proteins regardless of their DNA divergence.
The mur1 gene encodes a 250-amino-acid polypeptide with a calculated molecular mass of 26.5 kDa (42). In general, the N-terminal catalytic domain of this enzyme contains two amino acids residues, E and D, which were shown to be necessary for the enzymatic activity of this enzyme family (44). Therefore, in order to study the conservation of these two residues, the deduced amino acid sequences of the Mur1 proteins from 51 independent tectiviral isolates were aligned and compared. Residues E and D were fully conserved in the catalytic domain, even though some amino acid substitutions were present in this domain (see Fig. S3 in the supplemental material). Among the 210 deduced Mur1 amino acid residues, 81 were strictly conserved in the 51 tectiviral isolates.
In GIL01, CDS 5 encodes a 735-amino-acid DNA PolB protein, whereas in the tectiviral model phage PRD1, the gene I product is a 553-amino-acid DNA PolB protein. It has been demonstrated that at least three PRD1 DNA PolB regions are conserved among other polymerases belonging to the RNA-primed DNA polymerase subfamily (45, 46). In particular, PRD1 has the sequence motif YCDTDSI in region I and the sequence motif KX6YGKF in region II (45–47). On the basis of what is known for the well-studied PRD1 phage, the deduced C-terminal halves of the DNA PolB proteins of the 28 previously selected tectiviruses (residues 352 to 735 in GIL01) were aligned and compared. Notably, the YCDTDS residues, in the active site of the protein (region I), were conserved in all 28 studied phages (see Fig. S4 in the supplemental material). However, residue I was detected only in isolates grouped under cluster Pol2 by ML analyses (i.e., Sand, pBClin15, and AP50), whereas in cluster Pol1 this residue was replaced by C, with two single exceptions, phages Lutum and Lima, isolated from B. mycoides-B. pseudomycoides strains (in which I was replaced by T). Moreover, region II sequence motif KX6YGKF was also detected in all the studied isolates. The conserved sequence motif KLMQNALYGKF (residues 403 to 413 in GIL01 DNA PolB) was displayed in region II in all isolates except the three isolates belonging to cluster Pol2. Specifically, residue M was replaced by residue V in Sand and residue I in pBClin15 and AP50, whereas residue A was replaced by residue S in Sand and AP50. Overall, 128 strictly conserved residues were found in the 388-amino-acid sequence alignments of the C-terminal half of the DNA PolB protein.
Tectivirus host range analysis.
In order to assess the host range of 21 new tectiviruses found in this work, along with that of phages GIL01 and GIL16, 115 strains belonging to the B. cereus group and five other Gram-positive bacteria which did not harbor tectivirus-related elements were tested for effective infection and lysis using the drop plaque assay system. The spotting host range showed that all tested phages were able to produce lysis in more than one strain. Table 5 presents the results for 46 strains, among 120 tested, that were sensitive to the phages tested (for the complete data set, see Table S1 in the supplemental material). Two different host lysis responses could be observed, and the production of either turbid or clear plaques largely depended on the bacterial host examined. None of the B. anthracis strains or the five other Gram-positive bacteria were infected by the tectiviruses tested (see Table S1 in the supplemental material).
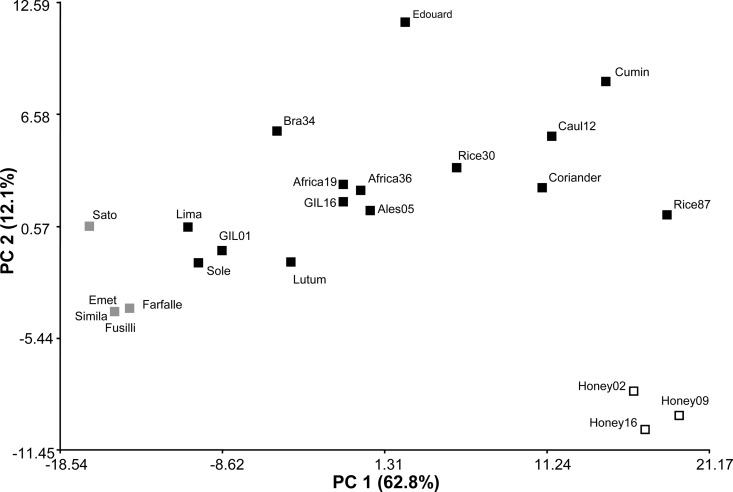
A principal coordinates analysis (PCoA) was then performed in order to determine if the tectivirus infection patterns could be used to distinguish among them. Using the scale values described in Materials and Methods, the multidimensional scaling PCoA revealed that most of the isolates appear dispersed in the plot (Fig. 4). More than 74% of the variability could be explained by principal coordinates 1 and 2 (62.8 and 12.1%, respectively). Outstandingly, two small groups were formed, and these were represented by phages isolated from B. cereus emetic strains and phages found in B. cereus honey-related isolates (Fig. 4). In addition, it can be noted that B. cereus emetic phages Farfalle, Simila, Fusilli, and Emet have a similar host range that can partially be explained not only by the high degree of DNA identity found in the PL and RR regions but also by the clonal evolutionary lineage described for their original B. cereus emetic host strains (16, 48). Along the same lines, subcluster Pol1-IV, represented in PCoA by phages Honey02, Honey09, and Honey16, was also supported by their host range. Nevertheless, further studies are required to better define the interactions taking place between these honey-related phages and their respective hosts.
FIG 4.
Scatter plot of principal coordinates analysis (PCoA) (axis PC 1 versus axis PC 2) on the basis of the tectivirus spotting host range. Twenty-three tectiviruses and 120 strains were used for host range determination. Squares, tectiviral isolates; gray squares, tectiviruses isolated from B. cereus emetic pathotype strains; white squares, tectiviruses harbored in a B. cereus strain recovered from honey samples; black squares, tectiviruses from different origins that did not cluster. PC, principal coordinates.
DISCUSSION
In this study, the occurrence, distribution, and diversity of tectiviruses infecting the B. cereus group have been assessed. In previous work, it was found that, using some specific HVRs, it was possible to discover novel tectiviruses (28). Therefore, the present study focused on selecting variable regions that were suitable for an extensive screening of a broad collection of strains. Two variable regions were selected on the basis of whole-genome nucleotide sequence alignments of the fully sequenced tectivirus-like elements. The first variable region, the RR region, targeted the DNA polB gene in the replication-regulation module, whereas the second region, the PL region, targeted the N-acetyl-muramidase-coding gene (mur1). PCR screenings using the two selected variable regions of a worldwide collection of strains revealed that tectivirus-like elements are rare across the B. cereus group, showing an occurrence of less than 3%. Despite this low rate of occurrence, 47 novel putative tectiviruses, besides the 10 already known, were uncovered in the present work.
Among the bacterial isolates harboring tectivirus-like elements, it was not possible to pinpoint a clear association between the incidence of this type of mobile genetic element (MGE) and the environmental host source, since they were isolated from niches as diverse as mammals, soil samples, insect larvae, foodstuffs, and food-related outbreaks. This low rate of occurrence among tectiviruses specifically preying hitherto on the B. cereus group was not unforeseen. Most phages reported to infect B. cereus group members belong to the order Caudovirales, where the families Myoviridae and Siphoviridae have numerous representatives (18, 49; Gillis and Mahillon, unpublished). Outside this virus order, dsDNA phages are rare, and among them, the tectiviruses are, so far, the only ones infecting members of the B. cereus group (Gillis and Mahillon, unpublished).
In order to study the tectiviral diversity in the B. cereus group, the PL region of 51 tectiviruses was sequenced. Despite the fact that this region a priori showed a genetic divergence between tectiviral isolates that was not so high, its pseudoconserved ends make it ideal for primer anchoring and, therefore, for screening a large collection of bacteria without compromising the occurrence of these types of phages. Besides, it is discriminatory enough to separate closely related tectiviruses, such as GIL01 and GIL16, into different clusters. Moreover, to evaluate if the first HVR (CDSs 8 to 10 in AP50) described previously by Sozhamannan et al. (24) was amplified from the novel tectiviruses found in the present work, the primer set described by Jalasvuori et al. (28) was used. However, for many of the 52 tectiviruses, no amplification products could be detected (data not shown). Consequently, the choice of the PL region for large screenings was valid and is to be recommended.
Two main clusters were formed using the PL region, with the majority of phage isolates falling into cluster MurA (Fig. 2b). Inside cluster MurA, three other subclusters were formed, where subcluster MurA-I contained the majority of isolates, which were related to GIL16. Prior to this study, it was assumed that GIL01-like phages were more abundant than GIL16-like tectiviruses in the B. cereus group, since two of the fully sequenced tectiviruses (i.e., Bam35 and GIL01) were almost identical.
Interestingly, phages in cluster MurB (i.e., AP50 and Wip1) are the only tectiviruses reported to infect B. anthracis (24, 25, 50–52), whereas cluster MurA encompasses phages isolated from other members of the B. cereus group. Phages AP50 and Wip1 were isolated from soil and earthworm (Eisenia fetida) intestine samples, respectively, using B. anthracis Sterne as the host strain throughout the initial isolation and enrichment processes (51, 53). Albeit 27 B. anthracis strains fitting into different canonical single nucleotide polymorphism lineages were used in the present work (data not shown; S. Derzelle and P. Wattiau, personal communication), none were positive for tectiviruses residing as prophages. Additionally, an in silico survey using B. anthracis genomes available in the GenBank database was performed, but no sequence with a tectiviral signature was found (data not shown). Consequently, natural bacterial hosts harboring AP50- and Wip1-related phages are still not known.
Furthermore, the RR region of 28 tectiviruses was also sequenced, and it was found that this region displays a greater genetic diversity than the PL region. Similar to the PL region, the RR region is divided into two clusters, with the majority of isolates falling into cluster Pol1. Remarkably, cluster Pol2 grouped together phages AP50 and Sand with plasmid pBClin15. One recombination breakpoint was detected among the tectivirus-like elements using the RR region. Recombination is an important process in the evolution of viruses that shapes the genome architecture and the genetic structure of populations at numerous levels (54) and accounts for most of the genetic diversity detected among viruses. Therefore, it would be interesting to investigate the contribution of this particular recombination event to the rearrangement of the DNA polB gene and decipher to what extent it accounts for the tectiviral diversity observed.
It is worth noting that phages Africa36, Africa19, Chick76, Sole, Vanilla, Sato, and Caul20 were not identified by the primers for the RR region (Table 2), albeit these phages have very closely related PL regions. Other sets of primers that have been used to detect tectiviruses (22, 28) were also employed in this study to compare and confirm the bona fide tectiviral sequences for these phages (data not shown). Recently, it was shown that the DNA polB gene from phage Wip1 is present on the complementary strand, and its sequence identity with respect to the sequences of other DNA polB genes found in previously described tectiviruses is very low (25). Thus, different sets of primers were designed on the basis of the sequence of the Wip1 DNA polB gene, but no amplification products could be detected (data not shown). These results suggest that a greater diversity than was previously thought might exist among tectiviruses preying on the B. cereus group. Furthermore, for the PRD1-like phages of Gram-negative bacteria, it was determined that a high degree of genome conservation occurs in the DNA polB gene, except close to the gene end (21), analogous to the tectivirus divergence revealed in this work using the RR region.
Since the PL and RR regions target genes encoding two proteins with important enzymatic activities, the conservation of essential residues in Mur1 and PolB was also analyzed. In general, the N-terminal domain of the N-acetyl-muramidase contains the catalytic activity that acts on the sugar moiety of the bacterial wall, while the C-terminal cell binding domain binds to a specific substrate (usually carbohydrate) found in the cell wall of the host bacterium (55). The N-terminal catalytic domain contains the residues E and D that are necessary for its enzymatic activity (44). Residues E and D were found to be fully conserved in the catalytic domain of Mur1 in the 51 analyzed tectiviruses. Interestingly, the C-terminal binding domain of Mur1 was less conserved (see Fig. S3 in the supplemental material), most probably because it recognizes the cell wall of different members of the B. cereus group. It is worth noting that in cluster MurB members (i.e., AP50 and Wip1) the terminal part of the amino acid alignments are considered gaps, mainly because the total length of the Mur1 protein is shorter than that of the Mur1 protein of members of cluster MurA (218 amino acids in AP50 and Wip1 versus 250 amino acids in GIL01), changing the C-terminal domain, but not the catalytic domain, regardless of the size of the protein.
Although small, it has been shown that PRD1 DNA PolB is a multifunctional enzyme with at least three enzymatic activities: (i) terminal protein-dGMP covalent complex formation, (ii) DNA chain elongation activity, and (iii) 3′ → 5′ exonuclease activity (45). It has also been demonstrated that at least three PRD1 DNA PolB regions are conserved among other polymerases belonging to the same family (45, 46). In particular, PRD1 has the sequence motif YCDTDSI in region I, forming part of the active site where Mg2+-binding D residues are in equivalent structural positions (45, 47). The YCDTDS residues were found to be conserved in the studied tectiviruses. Interestingly, residue I was present only in AP50, Sand, and pBClin15, tectivirus-like elements belonging to the cluster Pol2. Besides, PRD1 region II is involved in polymerization as well as protein-primed initiation activities and has the sequence motif KX6YGKF (46). This sequence motif was also found to be conserved among the tectiviruses analyzed in this work, even though some amino acid substitutions were present.
The ability of 23 tectiviruses to infect 120 strains belonging mainly to the B. cereus group was evaluated by spotting assays. This method allows description of a spotting bactericidal host range (56) and relies on the formation of visualized plaques and/or lysis zones encompassing the phage suspension spot, allowing analysis of numerous samples (57, 58). Among the tested strains, 46 were infected by at least one tectivirus (Table 5). Overall, the tested tectiviruses displayed some degree of specificity for particular hosts. It was not possible to pinpoint a clear association between the phage infection pattern and the Bacillus species, indicating that these phages are probably able to infect only specific bacterial strains from closely related hosts, unlike the PRD1-like phages that have a broad host range (59). Interestingly however, none of the evaluated B. anthracis strains were infected by the tectiviruses tested (see Table S1 in the supplemental material), which suggests, in this specific case, a relationship between the clustering formed using genomic data and their host range. In other words, it seems that cluster Mur1 members can infect all members of the B. cereus group, except B. anthracis, whereas phages AP50 and Wip1 have been reported to be highly specific to B. anthracis (24, 25, 50–52).
As a final observation, the family Tectiviridae comprises a relatively rare, yet interesting group of phages whose distinctive morphological feature is that they are tail-less phages having a lipid membrane that forms a vesicle beneath the icosahedral protein capsid (20). This family has not yet been assigned to an order. However, under its current taxonomic description, several phages that do not share any DNA sequence similarity have been grouped, and this grouping is an issue that could be debatable. The current list of approved species in the genus Tectivirus, based on the International Committee on Taxonomy of Viruses (ICTV) (http://www.ictvonline.org), includes PRD1 infecting enterobacteria, Bam35 and AP50 infecting members of the B. cereus group, and even Thermus phage P37-14 (20). The ICTV species demarcation criteria for this family are based on sequence comparisons between PRD1 and Bam35, and since there is almost no detectable similarity, their taxonomy relies on a similar genome organization and a similar location of key genes, alongside their morphology (20, 21). In the near future, it would be pertinent to evaluate the possibility of dividing this taxon into at least two genera (e.g., Enterotectivirus and Bacillitectivirus), which will facilitate their appropriate characterization.
Concluding remarks.
The outstanding problem with the taxonomy of the B. cereus group raises questions not only about precise species identification but also in the description of their MGEs. Moreover, the particular contribution of phages to the evolution and appearance of the diverse ecotypes and pathotypes among B. cereus group members has been disregarded, with a few exceptions. Overall, the present work (i) expands the view of the occurrence of tectiviral prophages in members of this bacterial group, providing evidence for (ii) genetic diversity greater than that previously observed for these MGEs and (iii) addressing the question whether their host range can contribute to their differentiation. In summary, the genomic data and biological behavior show that, so far, tectiviruses preying on the B. cereus group are divided into two mayor clades: tectiviruses infecting B. anthracis strains and tectiviruses isolated from other B. cereus group members. In addition, the genomic data exposed that diversity and apparent relationships between tectiviruses differ with the analyzed region and if genes are subjected to different evolutionary processes, like recombination. As a consequence, the mur1 gene is proposed to be used in large screenings for the occurrence of tectiviruses, whereas the DNA polB gene can be used to analyze the bona fide diversity. From an evolutionary perspective, the results indicate that the DNA polB gene has undergone genetic recombination, although the actual contribution of this event remains to be further explored.
Supplementary Material
ACKNOWLEDGMENTS
We are deeply indebted to Pierre Wattiau (Veterinary and Agrochemical Research Centre, Brussels, Belgium) for providing the B. anthracis strain collection used in this study and allowing access to biosafety level 3 laboratory facilities. We gratefully acknowledge Mieke Van Hessche for her excellent technical assistance with B. anthracis-related experiments. We are also thankful to Gustavo Romay for his critical reading of the manuscript and his helpful comments.
This work was supported by the Foundation for Training in Industrial and Agricultural Research (FRIA; a grant to A.G.), the National Fund for Scientific Research (FNRS), and the Université Catholique de Louvain (grants to J.M.).
Footnotes
Published ahead of print 2 May 2014
Supplemental material for this article may be found at http://dx.doi.org/10.1128/AEM.00912-14.
REFERENCES
- 1.Jensen GB, Hansen BM, Eilenberg J, Mahillon J. 2003. The hidden lifestyles of Bacillus cereus and relatives. Environ. Microbiol. 5:631–640. 10.1046/j.1462-2920.2003.00461.x [DOI] [PubMed] [Google Scholar]
- 2.Guinebretière MH, Auger S, Galleron N, Contzen M, De Sarrau B, De Buyser ML, Lamberet G, Fagerlund A, Granum PE, Lereclus D, De Vos P, Nguyen-The C, Sorokin A. 2013. Bacillus cytotoxicus sp. nov. is a novel thermotolerant species of the Bacillus cereus group occasionally associated with food poisoning. Int. J. Syst. Evol. Microbiol. 63:31–40. 10.1099/ijs.0.030627-0 [DOI] [PubMed] [Google Scholar]
- 3.Okinaka R, Cloud K, Hampton O, Hoffmaster A, Hill K, Keim P, Koehler T, Lamke G, Kumano S, Manter D, Martinez Y, Ricke D, Svensson R, Jackson P. 1999. Sequence, assembly and analysis of pX01 and pX02. J. Appl. Microbiol. 87:261–262. 10.1046/j.1365-2672.1999.00883.x [DOI] [PubMed] [Google Scholar]
- 4.Bravo A, Likitvivatanavong S, Gill SS, Soberon M. 2011. Bacillus thuringiensis: a story of a successful bioinsecticide. Insect Biochem. Mol. Biol. 41:423–431. 10.1016/j.ibmb.2011.02.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schnepf E, Crickmore N, Van Rie J, Lereclus D, Baum J, Feitelson J, Zeigler DR, Dean DH. 1998. Bacillus thuringiensis and its pesticidal crystal proteins. Microbiol. Mol. Biol. Rev. 62:775–806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hoton FM, Andrup L, Swiecicka I, Mahillon J. 2005. The cereulide genetic determinants of emetic Bacillus cereus are plasmid-borne. Microbiology 151:2121–2124. 10.1099/mic.0.28069-0 [DOI] [PubMed] [Google Scholar]
- 7.Ehling-Schulz M, Fricker M, Grallert H, Rieck P, Wagner M, Scherer S. 2006. Cereulide synthetase gene cluster from emetic Bacillus cereus: structure and location on a mega virulence plasmid related to Bacillus anthracis toxin plasmid pXO1. BMC Microbiol. 6:20. 10.1186/1471-2180-6-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Van der Auwera GA, Feldgarden M, Kolter R, Mahillon J. 2013. Whole-genome sequences of 94 environmental isolates of Bacillus cereus sensu lato. Genome Announc. 1(5):e00380-13. 10.1128/genomeA.00380-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Helgason E, Caugant DA, Lecadet MM, Chen Y, Mahillon J, Lovgren A, Hegna I, Kvaloy K, Kolsto AB. 1998. Genetic diversity of Bacillus cereus/B. thuringiensis isolates from natural sources. Curr. Microbiol. 37:80–87. 10.1007/s002849900343 [DOI] [PubMed] [Google Scholar]
- 10.Helgason E, Østad OA, Caugant DA, Johansen HA, Fouet A, Mock M, Hegna I, Kolstø A-B. 2000. Bacillus anthracis, Bacillus cereus, and Bacillus thuringiensis—one species on the basis of genetic evidence. Appl. Environ. Microbiol. 66:2627–2630. 10.1128/AEM.66.6.2627-2630.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Helgason E, Tourasse NJ, Meisal R, Caugant DA, Kolstø A-B. 2004. Multilocus sequence typing scheme for bacteria of the Bacillus cereus group. Appl. Environ. Microbiol. 70:191–201. 10.1128/AEM.70.1.191-201.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ticknor LO, Kolstø A-B, Hill KK, Keim P, Laker MT, Tonks M, Jackson PJ. 2001. Fluorescent amplified fragment length polymorphism analysis of Norwegian Bacillus cereus and Bacillus thuringiensis soil isolates. Appl. Environ. Microbiol. 67:4863–4873. 10.1128/AEM.67.10.4863-4873.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Guinebretière MH, Velge P, Couvert O, Carlin F, Debuyser ML, Nguyen-The C. 2010. Ability of Bacillus cereus group strains to cause food poisoning varies according to phylogenetic affiliation (groups I to VII) rather than species affiliation. J. Clin. Microbiol. 48:3388–3391. 10.1128/JCM.00921-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tourasse NJ, Helgason E, Klevan A, Sylvestre P, Moya M, Haustant M, Okstad OA, Fouet A, Mock M, Kolsto AB. 2011. Extended and global phylogenetic view of the Bacillus cereus group population by combination of MLST, AFLP, and MLEE genotyping data. Food Microbiol. 28:236–244. 10.1016/j.fm.2010.06.014 [DOI] [PubMed] [Google Scholar]
- 15.Maughan H, Van der Auwera G. 2011. Bacillus taxonomy in the genomic era finds phenotypes to be essential though often misleading. Infect. Genet. Evol. 11:789–797. 10.1016/j.meegid.2011.02.001 [DOI] [PubMed] [Google Scholar]
- 16.Ehling-Schulz M, Svensson B, Guinebretiere MH, Lindback T, Andersson M, Schulz A, Fricker M, Christiansson A, Granum PE, Martlbauer E, Nguyen-The C, Salkinoja-Salonen M, Scherer S. 2005. Emetic toxin formation of Bacillus cereus is restricted to a single evolutionary lineage of closely related strains. Microbiology 151:183–197. 10.1099/mic.0.27607-0 [DOI] [PubMed] [Google Scholar]
- 17.Didelot X, Barker M, Falush D, Priest FG. 2009. Evolution of pathogenicity in the Bacillus cereus group. Syst. Appl. Microbiol. 32:81–90. 10.1016/j.syapm.2009.01.001 [DOI] [PubMed] [Google Scholar]
- 18.Lee J-H, Shin H, Ryu S. 2014. Characterization and comparative genomic analysis of bacteriophages infecting members of the Bacillus cereus group. Arch. Virol. 159:871–874. 10.1007/s00705-013-1920-3 [DOI] [PubMed] [Google Scholar]
- 19. Reference deleted.
- 20.Oksanen HM, Bamford DH. 2012. Family Tectiviridae, p 317–321 In King AMQ, Adams MJ, Carstens EB, Lefkowitz EJ. (ed), Virus taxonomy: classification and nomenclature of viruses. Ninth report of the International Committee on Taxonomy of Viruses. Academic Press, San Diego, CA [Google Scholar]
- 21.Saren A-M, Ravantti JJ, Benson SD, Burnett RM, Paulin L, Bamford DH, Bamford JKH. 2005. A snapshot of viral evolution from genome analysis of the Tectiviridae family. J. Mol. Biol. 350:427–440. 10.1016/j.jmb.2005.04.059 [DOI] [PubMed] [Google Scholar]
- 22.Verheust C, Jensen G, Mahillon J. 2003. pGIL01, a linear tectiviral plasmid prophage originating from Bacillus thuringiensis serovar israelensis. Microbiology 149:2083–2092. 10.1099/mic.0.26307-0 [DOI] [PubMed] [Google Scholar]
- 23.Verheust C, Fornelos N, Mahillon J. 2005. GIL16, a new Gram-positive tectiviral phage related to the Bacillus thuringiensis GIL01 and the Bacillus cereus pBClin15 elements. J. Bacteriol. 187:1966–1973. 10.1128/JB.187.6.1966-1973.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sozhamannan S, McKinstry M, Lentz SM, Jalasvuori M, McAfee F, Smith A, Dabbs J, Ackermann H-W, Bamford JKH, Mateczun A, Read TD. 2008. Molecular characterization of a variant of Bacillus anthracis-specific phage AP50 with improved bacteriolytic activity. Appl. Environ. Microbiol. 74:6792–6796. 10.1128/AEM.01124-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kan S, Fornelos N, Schuch R, Fischetti VA. 2013. Identification of a ligand on the Wip1 bacteriophage highly specific for a receptor on Bacillus anthracis. J. Bacteriol. 195:4355–4364. 10.1128/JB.00655-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Strömsten NJ, Benson SD, Burnett RM, Bamford DH, Bamford JK. 2003. The Bacillus thuringiensis linear double-stranded DNA phage Bam35, which is highly similar to the Bacillus cereus linear plasmid pBClin15, has a prophage state. J. Bacteriol. 185:6985–6989. 10.1128/JB.185.23.6985-6989.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fornelos N, Bamford JKH, Mahillon J. 2011. Phage-borne factors and host LexA regulate the lytic switch in phage GIL01. J. Bacteriol. 193:6008–6019. 10.1128/JB.05618-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jalasvuori M, Palmu S, Gillis A, Kokko H, Mahillon J, Bamford JKH, Fornelos N. 2013. Identification of five novel tectiviruses in Bacillus strains: analysis of a highly variable region generating genetic diversity. Res. Microbiol. 164:118–126. 10.1016/j.resmic.2012.10.011 [DOI] [PubMed] [Google Scholar]
- 29.Ravantti JJ, Gaidelyte A, Bamford DH, Bamford JKH. 2003. Comparative analysis of bacterial viruses Bam35, infecting a gram-positive host, and PRD1, infecting gram-negative hosts, demonstrates a viral lineage. Virology 313:401–414. 10.1016/S0042-6822(03)00295-2 [DOI] [PubMed] [Google Scholar]
- 30.Jensen G, Andrup L, Wilcks A, Smidt L, Poulsen O. 1996. The aggregation-mediated conjugation system of Bacillus thuringiensis subsp. israelensis: host range and kinetics of transfer. Curr. Microbiol. 33:228–236. 10.1007/s002849900105 [DOI] [PubMed] [Google Scholar]
- 31.Xia X. 2013. DAMBE5: a comprehensive software package for data analysis in molecular biology and evolution. Mol. Biol. Evol. 30:1720–1728. 10.1093/molbev/mst064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Brodie R, Smith A, Roper R, Tcherepanov V, Upton C. 2004. Base-By-Base: single nucleotide-level analysis of whole viral genome alignments. BMC Bioinformatics 5:96. 10.1186/1471-2105-5-96 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hillary W, Lin S-H, Upton C. 2011. Base-By-Base version 2: single nucleotide-level analysis of whole viral genome alignments. Microb. Inform. Exp. 1:2. 10.1186/2042-5783-1-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hansen BM, Hendriksen NB. 2001. Detection of enterotoxic Bacillus cereus and Bacillus thuringiensis strains by PCR analysis. Appl. Environ. Microbiol. 67:185–189. 10.1128/AEM.67.1.185-189.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Edgar RC. 2004. MUSCLE: multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res. 32:1792–1797. 10.1093/nar/gkh340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tamura K, Peterson D, Peterson N, Stecher G, Nei M, Kumar S. 2011. MEGA5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol. Biol. Evol. 28:2731–2739. 10.1093/molbev/msr121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Muhire B, Martin D, Brown J, Navas-Castillo J, Moriones E, Zerbini FM, Rivera-Bustamante R, Malathi VG, Briddon R, Varsani A. 2013. A genome-wide pairwise-identity-based proposal for the classification of viruses in the genus Mastrevirus (family Geminiviridae). Arch. Virol. 158:1411–1424. 10.1007/s00705-012-1601-7 [DOI] [PubMed] [Google Scholar]
- 38.Posada D, Crandall KA. 1998. MODELTEST: testing the model of DNA substitution. Bioinformatics 14:817–818. 10.1093/bioinformatics/14.9.817 [DOI] [PubMed] [Google Scholar]
- 39.Castresana J. 2000. Selection of conserved blocks from multiple alignments for their use in phylogenetic analysis. Mol. Biol. Evol. 17:540–552. 10.1093/oxfordjournals.molbev.a026334 [DOI] [PubMed] [Google Scholar]
- 40.Dereeper A, Guignon V, Blanc G, Audic S, Buffet S, Chevenet F, Dufayard J-F, Guindon S, Lefort V, Lescot M, Claverie J-M, Gascuel O. 2008. Phylogeny.fr: robust phylogenetic analysis for the non-specialist. Nucleic Acids Res. 36:W465–W469. 10.1093/nar/gkn180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kosakovsky Pond SL, Posada D, Gravenor MB, Woelk CH, Frost SDW. 2006. Automated phylogenetic detection of recombination using a genetic algorithm. Mol. Biol. Evol. 23:1891–1901. 10.1093/molbev/msl051 [DOI] [PubMed] [Google Scholar]
- 42.Verheust C, Fornelos N, Mahillon J. 2004. The Bacillus thuringiensis phage GIL01 encodes two enzymes with peptidoglycan hydrolase activity. FEMS Microbiol. Lett. 237:289–295. 10.1111/j.1574-6968.2004.tb09709.x [DOI] [PubMed] [Google Scholar]
- 43.Ackermann HW, Roy R, Martin M, Murthy MR, Smirnoff WA. 1978. Partial characterization of a cubic Bacillus phage. Can. J. Microbiol. 24:986–993. 10.1139/m78-162 [DOI] [PubMed] [Google Scholar]
- 44.Nambu T, Minamino T, Macnab RM, Kutsukake K. 1999. Peptidoglycan-hydrolyzing activity of the FlgJ protein, essential for flagellar rod formation in Salmonella typhimurium. J. Bacteriol. 181:1555–1561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jung GH, Leavitt MC, Hsieh JC, Ito J. 1987. Bacteriophage PRD1 DNA polymerase: evolution of DNA polymerases. Proc. Natl. Acad. Sci. U. S. A. 84:8287–8291. 10.1073/pnas.84.23.8287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhu W, Leavitt MC, Jung G, Ito J. 1994. Mutagenesis of a highly conserved lysine 340 of the PRD1 DNA polymerase. Biochim. Biophys. Acta 1219:260–266. 10.1016/0167-4781(94)90047-7 [DOI] [PubMed] [Google Scholar]
- 47.Albà MM. 2001. Replicative DNA polymerases. Genome Biol. 2:REVIEWS3002. 10.1186/gb-2001-2-1-reviews3002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Dierick K, Van Coillie E, Swiecicka I, Meyfroidt G, Devlieger H, Meulemans A, Hoedemaekers G, Fourie L, Heyndrickx M, Mahillon J. 2005. Fatal family outbreak of Bacillus cereus-associated food poisoning. J. Clin. Microbiol. 43:4277–4279. 10.1128/JCM.43.8.4277-4279.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.King AMQ, Adams MJ, Carstens EB, Lefkowitz EJ. (ed). 2012. Virus taxonomy: classification and nomenclature of viruses. Ninth report of the International Committee on Taxonomy of Viruses. Academic Press, San Diego, CA [Google Scholar]
- 50.Nagy E, Pragai B, Ivanovics G. 1976. Characteristics of phage AP50, an RNA phage containing phospholipids. J. Gen. Virol. 32:129–132. 10.1099/0022-1317-32-1-129 [DOI] [PubMed] [Google Scholar]
- 51.Schuch R, Fischetti VA. 2009. The secret life of the anthrax agent Bacillus anthracis: bacteriophage-mediated ecological adaptations. PLoS One 4:e6532. 10.1371/journal.pone.0006532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Schuch R, Pelzek AJ, Kan S, Fischetti VA. 2010. Prevalence of Bacillus anthracis-like organisms and bacteriophages in the intestinal tract of the earthworm Eisenia fetida. Appl. Environ. Microbiol. 76:2286–2294. 10.1128/AEM.02518-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Nagy E, Ivanovics G. 1972. Defective lysogeny in Bacillus anthracis Sterne strains. Acta Microbiol. Acad. Sci. Hung. 19:69–76 [PubMed] [Google Scholar]
- 54.Posada D, Crandall KA. 2001. Evaluation of methods for detecting recombination from DNA sequences: computer simulations. Proc. Natl. Acad. Sci. U. S. A. 98:13757–13762. 10.1073/pnas.241370698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Fischetti VA. 2011. Exploiting what phage have evolved to control gram-positive pathogens. Bacteriophage 1:188–194. 10.4161/bact.1.4.17747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hyman P, Abedon ST. 2010. Bacteriophage host range and bacterial resistance. Adv. Appl. Microbiol. 70:217–248. 10.1016/S0065-2164(10)70007-1 [DOI] [PubMed] [Google Scholar]
- 57.Mazzocco A, Waddell TE, Lingohr E, Johnson R. 2009. Enumeration of bacteriophages using the small drop plaque assay system. Methods Mol. Biol. 501:81–85. 10.1007/978-1-60327-164-6_9 [DOI] [PubMed] [Google Scholar]
- 58.Kutter E. 2009. Phage host range and efficiency of plating. Methods Mol. Biol. 501:141–149. 10.1007/978-1-60327-164-6_14 [DOI] [PubMed] [Google Scholar]
- 59.Bradley DE, Rutherford EL. 1975. Basic characterization of a lipid-containing bacteriophage specific for plasmids of the P, N, and W compatibility groups. Can. J. Microbiol. 21:152–163. 10.1139/m75-023 [DOI] [PubMed] [Google Scholar]
- 60.Hoton FM, Fornelos N, N′Guessan E, Hu X, Swiecicka I, Dierick K, Jääskeläinen E, Salkinoja-Salonen M, Mahillon J. 2009. Family portrait of Bacillus cereus and Bacillus weihenstephanensis cereulide-producing strains. Environ. Microbiol. Rep. 1:177–183. 10.1111/j.1758-2229.2009.00028.x [DOI] [PubMed] [Google Scholar]
- 61.Cherif A, Ouzari H, Daffonchio D, Cherif H, Ben Slama K, Hassen A, Jaoua S, Boudabous A. 2001. Thuricin 7: a novel bacteriocin produced by Bacillus thuringiensis BMG1.7, a new strain isolated from soil. Lett. Appl. Microbiol. 32:243–247. 10.1046/j.1472-765X.2001.00898.x [DOI] [PubMed] [Google Scholar]
- 62.Carlson CR, Caugant DA, Kolstø A-B. 1994. Genotypic Diversity among Bacillus cereus and Bacillus thuringiensis strains. Appl. Environ. Microbiol. 60:1719–1725 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.