Abstract
Use of granulocyte colony stimulating factor (G-CSF)–mobilized peripheral blood hematopoietic progenitor cells (HPC) has largely replaced bone marrow (BM) as a source of stem cells for both autologous and allogeneic cell transplantation. With G-CSF alone, up to 35% of patients are unable to mobilize sufficient numbers of CD34 cells/kg to ensure successful and consistent multi-lineage engraftment and sustained hematopoietic recovery. To this end, research is ongoing to identify new agents or combinations which will lead to the most effective and efficient stem cell mobilization strategies, especially in those patients who are at risk for mobilization failure. We describe both established agents and novel strategies at various stages of development. The latter include but are not limited to drugs that target the SDF-1/CXCR4 axis, S1P agonists, VCAM/VLA-4 inhibitors, parathyroid hormone, proteosome inhibitors, Groβ, and agents that stabilize HIF. While none of the novel agents have yet gained an established role in HPC mobilization in clinical practice, many early studies exploring these new pathways show promising results and warrant further investigation.
Keywords: Stem cell mobilization, G-CSF, Plerixafor, SDF-1, Parathyroid hormone
Introduction
Hematopoietic cell transplantation is an important and often life-saving treatment for many hematological malignancies and select solid tumors as means of reconstitution of blood cells following high dose chemotherapy.1-5 Use of granulocyte colony stimulating factor (G-CSF)-mobilized peripheral blood stem cells (PBSC) has largely replaced bone marrow (BM) as a source of stem cells for both autologous and allogeneic cell transplantation. In spite of the increased number of CD34+ stem cells obtained after G-CSF mobilization compared to BM harvests, one still needs to obtain a minimum number of CD34/kg (~ ≥2 × 106) to ensure successful and consistent multi-lineage engraftment and sustained hematopoietic recovery.
The dose of CD34+ cells infused is an important predictor of both neutrophil and platelet engraftment. For autologous stem cell transplantation (ASCT), an optimal CD34+ cell dose which leads to rapid and sustained recovery is thought to be >5 × 106/kg.6,7 On the other hand, 2 × 106 CD34+ cells/kg is accepted as the minimum threshold below which consistent and rapid multilineage engraftment, especially of platelets, may not take place.
For allogeneic stem cell transplants (AlloSCT), a CD34+ cell dose ≥4.2 – 4.5×106/kg is associated with improved overall survival in the matched unrelated donor setting, without increased risk of acute or chronic graft vs. host disease (GVHD), while higher doses (>8-14 × 106 cells/kg) have been associated with increased risk of GVHD.8,9 Likewise, higher CD34+ cell doses (>9.1 × 106) have also been associated with increased risk of chronic GVHD after reduced intensity AlloSCT from HLA-matched siblings. 10
The success of stem cell mobilization is also dependent on both the total dose and the type of chemotherapy as well as radiation administered prior to autologous stem cell collection (Table 1). Studies have identified multiple chemotherapeutic agents which increase the risk of poor stem cell mobilization, including melphalan,11 Lenalidomide,12,13 Fludarabine,14,15 Chlorambucil, 16 Carmustine,17 Hyper-CVAD 18 and DHAP.19
Table 1.
Clinical Factors that Hinder Stem Cell Mobilization
| Risk Factor | Postulated Mechanism | Reference |
|---|---|---|
| Increasing age | Age-related reduced HSC reserve | [20, 25] |
| Low BM cellularity | Reflects low HSC reserve | [20] |
| Low baseline platelet count | Reflects low HSC reserve | [20-23] |
| Prior chemotherapy | Direct toxicity to HSCs and BM niche | [12-19] |
| Prior radiation therapy | Direct toxicity to HSCs and BM niche | [11] |
| Diabetes mellitus/Impaired glucose tolerance | Possible direct alteration of the hematopoietic niche via sympathetic denervation; Baseline low peripheral CD34+ cell count. | [26-28] |
Abbreviations: HSC, hematopoietic stem cell; BM, bone marrow.
A limited BM reserve as indicated by a low platelet count prior to mobilization,20-23 low bone marrow cellularity,20 baseline low peripheral blood CD34+ numbers,24 and age 25 are other risk factors for poor PBSC mobilization. Diabetes mellitus and impaired glucose tolerance have recently been identified as independent risk factors for poor mobilization.26-28 The mechanisms underlying these observations remain enigmatic but are thought to be related to a baseline low peripheral CD34+ cell count in these patients 27 and direct alteration of the hematopoietic niche via a sympathetic denervation syndrome associated with diabetes mellitus.28
Bone Marrow Niche
The BM niche is a highly organized microenvironment which anchors HSCs and regulates their self-renewal, proliferation and trafficking (Figure 1). Structurally, the niche is formed by supporting cells that engage in direct cell-cell interaction with stem cells and provide chemical signals that support their survival. 29,30
Figure 1. Bone Marrow Niche.
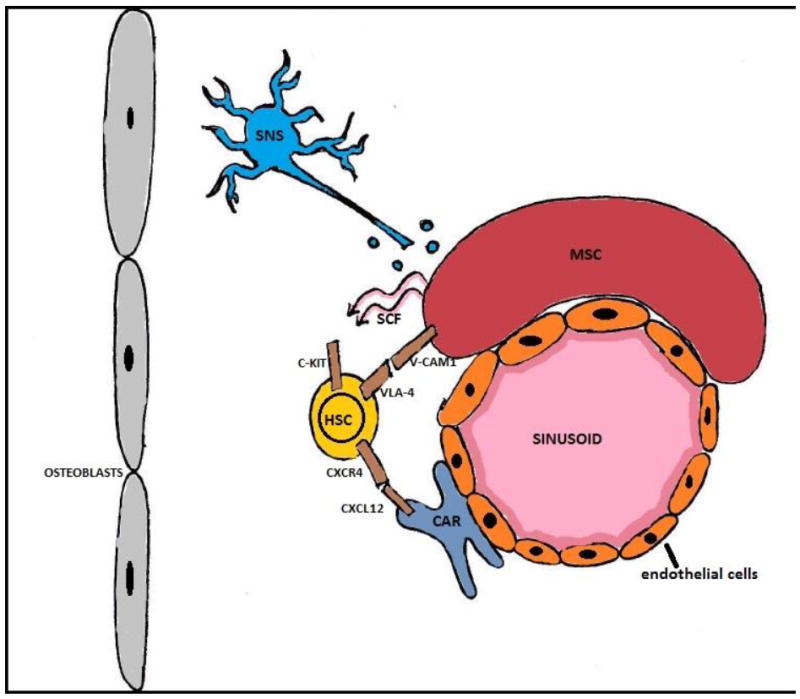
Abbreviations: CAR: CXCL12 abundant reticular cells; HSC: hematopoietic stem cell; MSC: menenchymal stem cell; SCF: stem cell factor; SNS: sympathetic nervous system; V-CAM1: vascular cellular adhesion molecule 1; VLA-4: very late antigen-4.
The niche has been subdivided into vascular and endosteal compartments. Within the vascular niche, sinusoidal endothelial cells and nestin+ mesenchymal stem cells (MSC) express adhesion molecules such as vascular cellular adhesion molecule 1 (VCAM-1), which binds to HSC receptor α4β1, and stem cell factor (SCF), which binds c-kit on HSC surface. Depletion of nestin+ MSCs increases mobilization of bone marrow HSC toward extramedullary sites and decreases homing of the PBSC to the BM.31 Nestin+ MSCs within the vascular niche associate closely with the adrenergic fibers of the sympathetic nervous system (SNS). Release of SNS neurotransmitters induces metalloproteinase MT1-MMP expression and MMP-2 activity,32 which then mediate the cleavage of important tethers (CXCR4, VLA4, VCAM-1, SCF) holding the HSC in the BM niche, thus promoting HSC egress from the bone marrow.33-35
In addition to these interactions, the binding of stromal derived factor-1 (SDF-1, also known as CXCL-12) to its receptor (CXCR4) on HSC plays a key role in the HSC retention within the bone marrow.36 SDF-1 is produced predominantly by reticular cells termed CXCL-12 abundant reticular cells (CAR) 37 but also by nestin+ MSCs and osteoblasts.38
The endosteal niche is located closer to the trabecular or cortical bone and is composed of osteoblasts and osteoclasts. Osteoblasts produce several factors, including angiopoetin 1 (Ang-1), which promotes tight adhesion of HSCs to the niche,39 and thrombopoietin (TPO), which has been shown to mediate HSC quiescence.40 Recent studies have also described the importance of niche macrophages in the expression of various HSC retention factors, including CXCL-12.41-43
HSC maintenance, proliferation, differentiation, and egress is a balanced process achieved through tight homeostatic neural and hormonal regulation. The release of progenitor cells from the bone marrow to the peripheral blood occurs constitutively at a very low level 44 but is amplified at times of stress.45
Standard Approach to Hematopoietic Stem Cell Mobilization (Table 2)
Table 2.
Approved Agents for Hematopoietic Progenitor Cell Mobilization
| Drugs | Mechanism of Action | Name | Side Effects | Clinical Trial Status |
|---|---|---|---|---|
| G-CSF | Down regulation of CXCL12 in BM osteoblasts; release of proteases | Filgrastim | Bone pain, fever, elevation in alk phos, nausea | Approved for HPC mobilization |
| GM-CSF | Possible alterations in adhesion molecules on HPC surface | Sargramostim | Fever, hypertension, headache, rash, malaise, diarrhea, nausea | Approved for HPC mobilization |
| Plerixafor | Reversible inhibition of CXCR4 | Injection site reactions, diarrhea, nausea, fatigue, headache, muscle/joint pain | Approved for HPC mobilization in combination with G-CSF in NHL and myeloma | |
| SCF | Down-regulation of c-kit on HPC surface | Ancestim | Injection site reactions, diffuse erythema, bone pain, flu-like symptoms, rare mast-cell mediated systemic toxicity | Approved for use in Canada and New Zealand but not currently available in the United States |
Abbreviations: G-CSF, granulocyte colony stimulating factor; BM, bone marrow; Alk phos, alkaline phosphatase; GM-CSF, Granulocyte- Macrophage Colony Stimulating Factor; HPC, hematopietic progenitor cell; NHL, non-Hodgkin Lymphoma; SCF, Stem Cell Factor.
Granulocyte Colony Stimulating Factor
G-CSF remains the most commonly-used agent for HSC mobilization in the clinic. Several mechanisms have been proposed to explain how this cytokine affects functional changes within the bone marrow, resulting in stem cell mobilization. These include the promotion of granulocyte expansion and both protease-dependent and independent attenuation of adhesion molecules and disruption of the SDF-1/CXCR4 axis.33,34,46-49 Levesque et al described release of proteolytic enzymes, neutrophil elastase (NE) and cathepsin G (CG), in mice following administration of G-CSF. These enzymes have been shown to cleave various molecules responsible for HSC retention in the bone marrow, including VCAM-1, 34,47 SDF-1 and CXCR4 35,46 and c-Kit.50 G-CSF has also been shown to reduce SDF-1 mRNA expression and inhibit osteoblast activity, leading to a decrease in the SDF-1 levels. 51
Several studies in the mid 1990’s established the role of G-CSF in HSC mobilization for autologous stem cell rescue. These studies found that when compared with transplantation of BM HSCs, transplantation with G-CSF-mobilized PB HSCs resulted in greater stem cell yields, reduced time to platelet and neutrophil recovery, reduced requirements of post-transplant platelet transfusions,52,53 and slight reduction in the length of hospitalization.53 Similar to mobilization of autologous HSC, mobilization of allogeneic HSC with G-CSF resulted in faster neutrophil and platelet engraftment 54-57 which translated to modest reduction in the overall costs in some reports.55
The most commonly used dose of G-CSF is 10 μg/kg/day given by subcutaneous injection, with leukapheresis starting on day 5. Both G-CSF and stem cell collection are continued daily until an adequate HSC count is collected. In patients with plasma cell myeloma and non-Hodgkin lymphoma (NHL) who tend to be a heavily pretreated group, mobilization with G-CSF alone has been reported to result in a mobilization failure rate (defined as <2×106 cells/kg after 3-5 days of apheresis) of up to 23%. In order to improve the stem cell yield and reduce the number of apheresis sessions required, both the novel agents and chemotherapy have been combined with G-CSF in heavily pre-treated patients. Some of this data will be discussed below.
Granulocyte- Macrophage Colony Stimulating Factor (GM-CSF)
GM-CSF (sargramostim, Leukine, Bayer Healthcare Pharmaceuticals, Seattle, WA, USA) plays a very limited role in HSC mobilization today. GM-CSF mobilized significantly fewer CD34+ cells than G-CSF in healthy subjects.58 Moreover, in patients with NHL, mobilization with GM-CSF resulted in a lower CD34+ cell yield than mobilization with G-CSF.59 GM-CSF mobilization yields smaller numbers of CD34+ cells but a higher percentage of CD14+ monocytes, dendritic cells and CD4+ CD25+ regulatory T cells,60 which modulate the immune system and have the potential to lower the rates of acute GVHD. However, only limited evidence is available suggesting the correlation between GM-CSF and lower rates of acute GVHD in patients.61
Plerixafor
SDF-1/CXCR4 interaction plays a key role in HSC quiescence and retention within the bone marrow.62,63 Plerixafor (Mozobil, AMD3100) is a bicyclam molecule which reversibly inhibits SDF-1 binding to CXCR4, promoting HSC mobilization. Plerixafor is approved to be used in combination with G-CSF for stem cell mobilization in patients with myeloma and lymphoma.
In a phase I study, Devine et al showed that a single subcutaneous injection of Plerixafor resulted in a 6-fold increase in the circulating CD34+ cells in patients with NHL and myeloma.64 The first phase II study of Plerixafor compared safety and efficacy of mobilization with Plerixafor and G-CSF to G-CSF alone.65 G-CSF was administered at 10 μg/kg for 5 days followed by apheresis on day 5. Plerixafor was administered subcutaneously at 160-240 μg/kg on days 4 or 5, 6 hours prior to apheresis. The study demonstrated the superiority of the combination regimen, which mobilized up to 50% more CD34+ cells and reduced the number of apheresis procedures required to reach the minimum and optimal doses of CD34+ cells compared to G-CSF alone.
Several additional phase II studies established the efficacy of plerixafor in heavily pretreated patients with NHL, Hodgkin lymphoma (HL) and myeloma.66-69 In one such study,66 22 transplant-eligible patients with HL underwent mobilization with a combination of G-CSF 10 μg/kg daily and plerixafor 240 μg/kg subcutaneously, 10-11 hours prior to apheresis. Fifteen patients (68%) collected ≥5×106 CD34+ cells/kg while 21 patients (95%) achieved the minimum threshold of ≥2×106 CD34+ cells in a median of 2 apheresis sessions. These results were superior to those of 98 consecutive historical controls with HL at the investigating institution, 22% of whom had failed to achieve a minimum collection of 2×106 CD34+ cells in fewer than 5 apheresis procedures and only 15% of whom had collected >5×106 CD34+ cells/kg. In another study, a combination of G-CSF and Plerixafor led to successful HSC mobilization in 18 of 20 transplant-eligible patients who had failed prior mobilization attempts.67 Seventeen of these patients required only one apheresis procedure to collect the adequate numbers of CD34+ cells.
Two phase III, multicenter, randomized, double-blinded, placebo-controlled studies established the superiority of plerixafor plus G-CSF over G-CSF alone in patients with NHL and myeloma.70,71 In the NHL trial,70 the primary endpoint of ≥5 × 106 CD34+ cells was reached in 59% of patients in the combination arm compared with 20% in the G-CSF plus placebo arm. Likewise, 130/150 (87%) patients in the combination arm achieved the secondary end-point of ≥2 × 106 CD34+ cell compared with only 70/148 (47%) in the G-CSF plus placebo arm (p < 0.001). Median time to engraftment was similar in both groups and the combination was well tolerated. In the myeloma trial,71 71.6% (106/148) of patients in the plerixafor plus G-CSF group met the primary endpoint of collecting ≥6 × 106 CD34+ cells/kg in ≤2 aphereses. Primary endpoint was met in only 34.4% (53/154) of patients in the G-CSF-alone group.
Allogeneic Stem Cell Transplantation
Plerixafor was also tested in healthy sibling donors in the setting of allogeneic stem cell transplantation.72 A single injection of Plerixafor 240 μg/kg subcutaneously followed by leukapheresis 4 hours later. yielded a minimum goal of 2 × 106 CD34+ cells/kg of recipient body weight in 66% (17/25) after a single 20 liter apheresis. The goal number of CD34+ cells was collected in 91% (22/24) of donors after 2 leukapheresis sessions. Engraftment was multilineage and stable without any episodes of primary or late graft failure. Actuarial rates of acute and chronic GVHD were also acceptable and slightly lower but not statistically lower than matched sibs receiving G-CSF-mobilized allografts. Several recent trials have been initiated at Washington University and through the Center for International Blood and Marrow Transplant Research (CIBMTR) to assess the toxicities and efficacy of intravenous Plerixafor for mobilization of normal donors for allogeneic stem cell transplantation. The results of the trial at Washington University were recently presented and suggest similar kinetics and magnitude of stem cell mobilization by intravenous vs. subcutaneous Plerixafor.73
Timing of Apheresis with Plerixafor
Initial pharmacokinetic studies in healthy volunteers demonstrated peak PB CD34+ counts 9 hours following subcutaneous Plerixafor 240 μg/kg.74,75 Peak effect of Plerixafor occurred at 10 hours when administered in combination with G-CSF in healthy volunteers.76 Further studies in patients with lymphoma and myeloma revealed similar results when plerixafor was combined with G-CSF.77 A recent study examined the optimal timing of leukapheresis in patients who had failed at least two prior HSC mobilizations without Plerixafor. These patients were treated with daily G-CSF 10 μg/kg/day for 5 days, followed by a single subcutaneous injection of plerixafor 240 μg/kg on day 5. Serial PB CD34+ cell counts were obtained every 3 hours, and apheresis was initiated when the CD34+ cell count reached 10 × 106 CD34+ cells/kg. The goal number of PB CD34+ cells was reached as early as at 3 hours following Plerixafor dose and began to decline at 8-12 hours, suggesting the need for earlier monitoring of PB CD34+ cells in poor mobilizers.78
Cost
High cost associated with plerixafor has restricted its universal use. Several institutions have developed risk-adapted algorithms for optimal utilization of the drug, in which plerixafor is administered only to those patients who are at high risk for mobilization failure.79-84 The latter has been defined by a predetermined PB CD34+ count threshold, below which continuation of G-CSF-only mobilization is unlikely to yield adequate CD34+ cell numbers. In general, PB CD34+ count of <10-15 × 106/L of blood following at least 4-5 days of mobilization with G-CSF alone has been used.
Maziarz and colleagues performed a post hoc retrospective analysis of the landmark phase III study which established superiority of mobilization with Plerixafor and G-CSF over G-CSF alone in patients with NHL.70 They examined mobilization outcomes in the two treatment arms in patients stratified by PB CD34+ cell count (<5, 5-9, 10-14, 15-19 or ≥20 CD34+ cells/μL) as measured following 4 days of mobilization with G-CSF alone. The probability of transplantation without a rescue mobilization in plerixafor-treated patients was the greatest in groups with PB CD34+ cell counts of <5, 5-9, and 10-14.84
Such just-in-time approaches have been shown to increase minimum and optimal CD34+ yield, decrease mobilization failures and the number of apheresis days and limit costs associated with repeat mobilizations. In addition, such strategies enhance engraftment and decrease transfusions in poor mobilizers.
Stem Cell Factor
Stem cell factor is a hematopoietic growth factor also known as c-kit ligand (KITL), which is produced by endothelial cells and perivascular stromal cells in the bone marrow niche. Differential splicing of SCF leads to the soluble and membrane-bound forms of the protein, the latter of which is important in HSC maintenance.85 Membrane-bound SCF exerts its action by binding to c-kit, a tyrosine kinase receptor expressed on normal hematopoietic cells.86 This interaction activates multiple downstream signals, including VLA-4-mediated BM HSC adhesion in humans.87 C-Kit receptor may be proteolytically cleaved from the surface of hematopoietic cells and circulate as s-Kit in normal human plasma. Binding of circulating s-Kit to SCF on HSC surface blocks c-Kit/SCF interaction,88 which may be exploited in stem cell mobilization.
A recombinant human SCF (Ancestim: Stemgen, Amgen INC, Thousand Oaks, CA, USA) used in combination with G-CSF has been shown to increase stem cell yield in poor mobilizers.89-97 More recently, a retrospective analysis from France examined a cohort of 550 patients who had failed prior mobilization with G-CSF or a combination of G-CSF and chemotherapy. The group consisted of myeloma, non-Hodgkin and Hodgkin lymphoma, refractory chronic lymphocytic leukemia and select solid tumor patients. Ancestim administered in combination with G-CSF with or without chemotherapy led to reaching a target threshold of >2 × 106 CD34+ cells/kg in 31% of patients.98 Injection site reactions due to Ancestim have been observed in vast majority of patients across studies. More severe reactions resulting from widespread mast cell degranulation are rare but have limited the use of this agent. Acestim is approved for use in Canada and New Zealand but is not currently available in the United States.
Chemotherapy
Chemotherapy is commonly used for stem cell mobilization in autologous stem cell transplantation. Specific agents chosen are generally disease-specific, used to both reduce the tumor burden and enhance mobilization.
Cyclophosphamide (CY) is the most commonly used chemotherapeutic agent and has been tested at various doses. In myeloma patients treated with conventional chemotherapy, mobilization with high dose CY (7 g/m2) led to greater toxicity without clear evidence of superiority when compared with intermediate (3-4 g/m2) and low doses (1-2 g/m2). 99,100 Comparison of low and intermediate doses has yielded conflicting results. In one study, these two regimens produced a similar PB HSC yield when combined with G-CSF, but the intermediate dose was associated with higher toxicity.101 However, in patients in whom tandem transplantation was planned, intermediate dose CY mobilized more effectively and in fewer apheresis sessions than the low dose.102 One retrospective analysis comparing the two regimens in patients treated with novel induction therapies (immunomodulatory agents and proteasome inhibitors) found more mobilization failures with the low-dose regimen.103
In lymphoma, combination chemotherapy is commonly used for stem cell mobilization in autologous stem cell transplantation setting. Regimens include DHAP,104 ESHAP,105-107 combination of CY, G-CSF, and etoposide 108 and a combination of ifosphamide, epirubicin and etoposide,109 among others.
Novel or Experimental Agents (Table 3)
Table 3.
Novel Agents Currently Being Tested for Hematopoietic Progenitor Cell Mobilization
| Drugs/Pathways | Mechanism of Action | Specific Agents | Clinical Trial Status in HPC Mobilization |
|---|---|---|---|
| CXCL12/CXCR4 modulators | CXCR4 antagonists | POL6326 | Phase I, II in progress |
| BKT-140 | Phase I/IIA completed | ||
| TG-0054 | Phase II in progress | ||
| Neutralization of CXCL12 | NOX-A12 | Phase II in progress | |
| S1P Agonists | Alteration of S1P gradient between PB and BM, which may counteract HSC retention in the BM | SEW2871 | Animal Studies |
| VCAM/VLA-4 Inhibitors | Inhibition of VLA-4 mediated HSC adhesion to VCAM-1 within the bone marrow stroma | BIO 5192 | Animal studies |
| Parathyroid Hormone | Stimulation of niche osteoblasts which in turn release endogenous G-CSF | Phase I completed | |
| Proteosome Inhibitors | Possible alteration of the VLA-4/VCAM-1 pathway | Bortezomib | Phase III in progress |
| Groβ | Release of proteases that alter HSC adhesion to the BM niche | SB-251353 | Animal Studies |
| Stabilization of HIF | Expression of VEGF A in the BM sinusoids, leading to vasodilatation | FG-4497 | Animal Studies |
Abbreviations: S1P, Sphingosine-1-phosphate; PB, peripheral blood; VCAM/VLA-4, vascular cell adhesion molecule-1/Very Late Antigen 4; MS, multiple sclerosis; HIF, hypoxia inducible factor.
Alternative drugs that target the SDF-1/CXCR4 axis
As reviewed by Rettig et al, alternative drugs that modulate the SDF-1/CXCR4 axis have shown promising results in early human studies.110 POL6326 (Polyphor, Allschwil, Switzerland) is a synthetic cyclic peptide which reversibly inhibits CXCR4. In a phase I study in healthy volunteers, this agent was well-tolerated and effective. Early results of an ongoing phase II study in newly diagnosed myeloma patients indicate sufficient stem cell mobilization in 66% of patients in 1 or 2 apheresis sessions. The drug was well tolerated at all doses tested, up to 1200 μg/kg delivered over 2 hours, with adverse events limited to minor infusion site reactions.111 This drug is also being tested to mobilize normal donors for allogeneic stem cell transplant in the United States.
BKT 140 (4F-benzoyl-TN14003; Biokine Therapeutics, Rehovit, Israel) (T-140) is a highly selective CXCR4 antagonist, originally designed to inhibit binding of the human immunodeficiency virus (HIV) to CXCR4. In mice, this agent induced up to a 10-fold increase in PB progenitor cells, which peaked at 1-2 hours following the administration of the dose. BKT 140 synergized with G-CSF, leading to a 78-fold increase in PB progenitor cells over controls, higher than seen with a combination of Plerixafor and G-CSF,112 In a phase I/IIA dose-escalation trial in myeloma patients, BTK 140 was well-tolerated and resulted in a dose-dependent increase in the mean absolute PB CD34+ cells. The highest dose tested (900 μg/kg) resulted in a mean PB CD34+ count of 20.6 × 106/kg, reducing the number of apheresis sessions required from 2.25 with lower doses to 1.113
In a phase I study, TG-0054 (Taigen Biotechnology, Taipei, Taiwan) resulted in a 3-14 fold increase in the PB CD34+ cell counts starting at 2 hours and peaking 4-6 hours following administration of the agent, followed by a gradual decline to baseline at 24 hours. Maximum tolerated dose was not reached (MTD) although a plateau in CD34+ mobilization was observed with the higher doses.114 A Phase II study evaluating safety, pharmacokinetics, and hematopoietic stem cell mobilization in patients with myeloma, NHL and HL was completed in October 2011.
NOX-A12 (NOXXON Pharma, Berlin, Germany) binds to SDF-1, inhibiting its binding to CXCR4. In a mouse model, this agent induced reversible mobilization of HSCs and synergized with G-CSF to yield long-term repopulating HSC that successfully engrafted primary and secondary lethally-irradiated recipients.115 A single center dose escalation study of NOX-A12 in 48 healthy subjects was completed in May 2010. A phase I study of NOX-A12 alone and in combination of G-CSF was also completed in January 2011. NOX-A12 is currently being studied for its role in sensitizing malignant cells to chemotherapy in chronic lymphocytic leukemia (CLL).116-118 It’s effect in inhibiting myeloma cell dissemination to distant BM niches in early plasma cell dyscrasias is also being explored.119
Sphingosine-1-phosphate (S1P) agonists
Sphingosine-1-phosphate is a bioactive phospholipid stored and released into peripheral blood mainly by erythrocytes,120,121 S1P acts as a ligand to five G-protein-coupled receptors (S1PR1 –S1PR5)122 and plays a key role in immune surveillance and differentiation. While plasma S1P levels remain high, most tissue S1P is quickly degraded and dephosphorylated by tissue-resident enzymes,123 resulting in a gradient which is important in lymphocyte egress from lymphoid organs.124 HSCs also express S1P receptors, signaling through which has been shown to enhance their mobilization from non-lymphoid peripheral tissues to draining lymphatics.125 Steady level of S1P in the plasma creates a gradient which continuously attracts BM HSCs and is counteracted by HSC interactions within the BM niche. It has been proposed that mechanisms that either weaken the effect of the niche on HSC retention or those that increase HSC attraction to the plasma will lead to HSC mobilization into the peripheral blood.126
Increasing evidence shows the importance of the complement cascade in HSC mobilization. Specifically, Lee et al described C5-mediated increase in bone marrow proteolysis, leading to HSC egress into the peripheral blood.127 In addition, it has been proposed that membrane-attack complex (MAC) generated during the terminal steps of the complement cascade leads to erythrocyte lysis and subsequent increase in plasma S1P levels, which enhances HSC egress from the bone marrow.126
Recently, Mierzejewska and colleagues found that phenylhydrazine-induced hemolysis in mice together with AMD3100 was able to mobilize twice as many HSC as AMD3100 alone. They attributed this difference in part to an elevated plasma S1P level resulting from hemolysis, which acts as a critical chemoattractant to the bone marrow HSCs. In addition, they report direct correlation between mobilization of HSCs and the level of complement activation (measured by MAC level), which is thought to counteract CXCR4-related bone marrow HSC retention.128
Juarez and colleagues demonstrated that while an elevation in the plasma S1P level was not required for mobilization, administration of a SIP1 agonist SEW2871 one hour before AMD3100 resulted in dose-dependent mobilization of HSCs, which was further enhanced by co-administration of G-CSF. Mobilization did not increase significantly with SEW2871 alone or in combination with G-CSF, in the absence of AMD3100.129
VCAM/VLA4 inhibitors
Integrins are a structurally and functionally diverse family of transmembrane glycoproteins that mediate cell-cell and cell-matrix interactions in a wide range of biological contexts. Eighteen different α and eight different β subunits exist in vertebrates, giving rise to 24 different non-covalently-bound αβ heterodimers 130,131 which are able to bind a wide variety of ligands.132 One such heterodimer expressed in hematopoietic stem cells, α4β1, termed very late antigen 4(VLA-4), mediates HSC adhesion to vascular cell adhesion molecule-1 (VCAM-1) within the bone marrow stroma.133 In preclinical studies, administration of anti-VLA-4 antibodies resulted in mobilization of HSC progenitors into the bloodstream.134,135
Natalizumab, a recombinant humanized monoclonal antibody against α4 subunit of VLA-4, approved for treatment of multiple sclerosis (MS) and Crohn’s disease, has been found to increase peripheral blood CD34+ cells in patients with relapsing-remitting MS.136-138 Zohen et al showed a gradual increase in the circulating CD34+ cells in MS patients, with a maximal concentration of 10.4 CD34+ cells/μL 72 hours following administration of Natalizumab.137 Jing et al demonstrated a 7-fold increase in PB CD34+ cells and a 7-fold, dose-dependent increase in BM CD34+ cells in patients with MS treated with Natalizumab, with a maximum absolute count reached on day 4 following treatment.136 Moreover, concurrent VLA-4 and CXCR4 blockade has been shown to have a greater than an additive effect in stem cell mobilization in primates, when compared with either agent alone.139 Unfortunately, Natalizumab-induced elevation in PB CD34+ cells persists at least 1 month following administration of the drug, which limits its use in healthy donors.136-138
BIO5192, small molecule inhibitor of VLA-4, resulted in a rapid 30-fold increase in PB HSC in mice, which peaked within 30-60 minutes of the BIO5192 dose. Additive effect on PB HSC mobilization was noted when BIO5192 was combined with plerixafor or plerixafor plus G-CSF.140 This molecule has not been studied in humans but warrants further investigation.
As reviewed by Rettig et al, several other small molecule inhibitors of VLA-4 are being studied in clinical trials for their efficacy in diseases such as MS, asthma, and inflammatory bowel disease.110 While no data has been published on the effect of these drugs on stem cell mobilization, further studies may reveal benefit.
Parathyroid hormone (PTH)
Over the past several decades, studies have shown the important regulatory effects of PTH on bone. Brunner et al demonstrated a positive correlation between PTH levels in patients with pituitary adenomas and a number of circulating HSCs, which decreased to a normal level following resection of the adenoma.141 In subsequent studies, Brunner et al compared the effects of PTH and G-CSF on HSC mobilization in mice. Stimulation with PTH showed a 1.5-9.8 fold increase in PB HSC, compatible with that produced by G-CSF. However, unlike G-CSF, PTH resulted in a constant level of CD34+ stem cells.142 In a Phase I study, patients who had failed one or two mobilization attempts for autologous stem cell transplantation were treated with escalating doses of PTH over 14 days, followed by filgrastim 10μg/kg on days 10-14. PTH was well-tolerated and resulted in adequate mobilization in 47% of patients who had failed 1 prior mobilization and 40% of patients who had failed 2 prior mobilization attempts.143 Further studies are necessary to establish the role of PTH in stem cell mobilization.
Proteosome inhibitors
Proteosome inhibitors have emerged as leading agents in the treatment of plasma cell myeloma. One of these agents, Bortezomib, has also been noted to have efficacy in stem cell mobilization. In one study, bortezomib resulted in a 6.8-fold increase in the peripheral blood CFU-Cs in mice, which was significantly higher than 0.8-fold increase seen with placebo. However, no statistically significant difference was seen in the number of mobilized HSPC with bortezomib vs. placebo when the same experiment was carried out in VLA-4 knockout mice. This led the authors to conclude that bortezomib mobilization probably involves the VLA-4/VCAM-1 axis. The study also showed that combining bortezomib with G-CSF or AMD3100 in mice resulted in the mobilization of significantly higher number of CFU-Cs than produced by G-CSF or AMD3100 alone.144
A recent phase II study evaluated the role of bortezomib induction and stem cell mobilization in 38 myeloma patients who had an incomplete response to or relapse following previous immunomodulatory drug-based induction. The study unexpectedly found enhanced CD34+ stem cell yield with the addition of bortezomib to cyclophosphamide and filgrastim. Twenty-three of the 27 (85%) patients treated with a bortezomib-based induction regimen were able to collect a median of 23.2 × 106 cells/kg (range 6.8 × 106 to 294 × 106) within a median of 1 collection day (range 1-5).145
Groβ
Groβ is a member of CXC chemokine family which stimulates chemotaxis and activation of neutrophils by binding to the CXCR2 receptor.146 In preclinical studies, SB-251353, recombinant N-terminal 4–amino acid truncated form of the human chemokine Groβ, mobilized HSC within 15 minutes following administration. Moreover, one day of G-CSF treatment followed by a single dose of SB-251353 resulted in PB HSC numbers equal to that produced by 4 days of G-CSF treatment. When compared with transplantation with HSCs mobilized by G-CSF, SB-251353-mobilized HSCs resulted in faster neutrophil and platelet recovery in mice.147 Fukuda et al showed that PB HSCs mobilized by SB-251353 alone or in combination with G-CSF contained significantly more primitive hematopoietic cells with enhanced engraftment and repopulation activity. These cells adhered better to VCAM-1+ endothelial cells and homed more efficiently to the marrow in vivo.148 Further research is necessary to determine the efficacy and potential toxicities of this agent in humans.
Stabilization of Hypoxia-inducible Factor (HIF)
In addition to stromal cells and their chemical signals which support HSCs within the bone marrow niche, hypoxia is emerging as an important factor for BM HSC quiescence and self-renewal. HSCs with long-term reconstitution ability have been reported to reside in the poorly-perfused, most hypoxic areas of the bone marrow,149,150 where their quiescence is supported by the expression of hypoxia-inducible factor. HIF is a heterodimeric transcription factor composed of an O2-labile α subunit and stable β subunit.151 In HSCs, HIF-1α subunit is expressed in hypoxic conditions and associates with the β subunit, forming a heterodimer, which then translocates to the nucleus and activates transcription of many important genes. When the O2 concentration rises above 2%, the HIF-1α subunit is quickly degraded by ubiquitination, preventing the formation of an active transcription factor.152,153
HIF has been noted to play a key role in neutrophil function 154and induction of expression of SDF-1 155 and VEGF-A.156-158 HSC mobilization with G-CSF and cyclophosphamide in mice has been noted to promote expansion of hypoxia within the BM microenvironment, which leads to stabilization of HIF-1α. HIF-1α in turn induces expression of vascular endothelial growth factor (VEGF) A in the BM sinusoids, leading to vasodilation and enhancement in HSC mobilization.156 A recent study found that stabilization of HIF-1α with FG-4497, when combined with G-CSF and Plerixafor, led to a 6-fold increase in mobilization of HSCs in mice when compared with a combination of G-CSF and Plerixafor alone. FG-4497 inhibits propyl hydroxylases (PHD) which usually hydroxylate HIF-1α in normoxic conditions, leading to its ubiquitination and eventually degradation.159 Therefore, PHD inhibitors may represent a novel therapeutic strategy to increase HSC yield in poor mobilizers.
Conclusion
Use of G-CSF-mobilized PBSC has largely replaced BM as a source of stem cells for both autologous and allogeneic stem cell transplantation. Research is ongoing to identify new agents or combinations which will lead to the most effective and efficient stem cell mobilization strategies, especially in those patients who are at risk for mobilization failure. G-CSF remains the most commonly-used agent for HSC mobilization in the clinic, with Plerixafor added to G-CSF in patients who are at high risk for poor mobilization. This review describes several novel pathways and therapeutic agents in HSC mobilization which are currently being explored in animal and early human studies. These include but are not limited to alternative drugs that target the SDF-1/CXCR4 axis, S1P agonists, VCAM/VLA-4 inhibitors, parathyroid hormone, proteosome inhibitors, Groβ, and agents that stabilize HIF. While none of the novel agents described above have yet gained an established role in stem cell mobilization in clinical practice, many early studies exploring these new pathways show promising results and warrant further investigation.
Footnotes
Conflict of Interest: None.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Attal M, Harousseau JL, Stoppa AM, Sotto JJ, Fuzibet JG, Rossi JF, et al. A prospective, randomized trial of autologous bone marrow transplantation and chemotherapy in multiple myeloma. Intergroupe Francais du Myelome. N Engl J Med. 1996;335:91–7. doi: 10.1056/NEJM199607113350204. [DOI] [PubMed] [Google Scholar]
- 2.Child JA, Morgan GJ, Davies FE, Owen RG, Bell SE, Hawkins K, et al. High-dose chemotherapy with hematopoietic stem-cell rescue for multiple myeloma. N Engl J Med. 2003;348:1875–83. doi: 10.1056/NEJMoa022340. [DOI] [PubMed] [Google Scholar]
- 3.Schmitz N, Pfistner B, Sextro M, Sieber M, Carella AM, Haenel M, et al. Aggressive conventional chemotherapy compared with high-dose chemotherapy with autologous haemopoietic stem-cell transplantation for relapsed chemosensitive Hodgkin’s disease: a randomised trial. Lancet. 2002;359:2065–71. doi: 10.1016/S0140-6736(02)08938-9. [DOI] [PubMed] [Google Scholar]
- 4.Philip T, Guglielmi C, Hagenbeek A, Somers R, Van der Lelie H, Bron D, et al. Autologous bone marrow transplantation as compared with salvage chemotherapy in relapses of chemotherapy-sensitive non-Hodgkin’s lymphoma. N Engl J Med. 1995;333:1540–5. doi: 10.1056/NEJM199512073332305. [DOI] [PubMed] [Google Scholar]
- 5.Rohatiner AZ, Nadler L, Davies AJ, Apostolidis J, Neuberg D, Matthews J, et al. Myeloablative therapy with autologous bone marrow transplantation for follicular lymphoma at the time of second or subsequent remission: long-term follow-up. J Clin Oncol. 2007;25:2554–9. doi: 10.1200/JCO.2006.09.8327. [DOI] [PubMed] [Google Scholar]
- 6.Weaver CH, Hazelton B, Birch R, Palmer P, Allen C, Schwartzberg L, et al. An analysis of engraftment kinetics as a function of the CD34 content of peripheral blood progenitor cell collections in 692 patients after the administration of myeloablative chemotherapy. Blood. 1995;86:3961–9. [PubMed] [Google Scholar]
- 7.Reiffers J, Faberes C, Boiron JM, Marit G, Foures C, Ferrer AM, et al. Peripheral blood progenitor cell transplantation in 118 patients with hematological malignancies: analysis of factors affecting the rate of engraftment. J Hematother. 1994;3:185–91. doi: 10.1089/scd.1.1994.3.185. [DOI] [PubMed] [Google Scholar]
- 8.Pulsipher MA, Chitphakdithai P, Logan BR, Leitman SF, Anderlini P, Klein JP, et al. Donor, recipient, and transplant characteristics as risk factors after unrelated donor PBSC transplantation: beneficial effects of higher CD34+ cell dose. Blood. 2009;114:2606–16. doi: 10.1182/blood-2009-03-208355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Baron F, Maris MB, Storer BE, Sandmaier BM, Panse JP, Chauncey TR, et al. High doses of transplanted CD34+ cells are associated with rapid T-cell engraftment and lessened risk of graft rejection, but not more graft-versus-host disease after nonmyeloablative conditioning and unrelated hematopoietic cell transplantation. Leukemia. 2005;19:822–8. doi: 10.1038/sj.leu.2403718. [DOI] [PubMed] [Google Scholar]
- 10.Gorin NC, Labopin M, Boiron JM, Theorin N, Littlewood T, Slavin S, et al. Results of genoidentical hemopoietic stem cell transplantation with reduced intensity conditioning for acute myelocytic leukemia: higher doses of stem cells infused benefit patients receiving transplants in second remission or beyond--the Acute Leukemia Working Party of the European Cooperative Group for Blood and Marrow Transplantation. J Clin Oncol. 2006;24:3959–66. doi: 10.1200/JCO.2006.05.5855. [DOI] [PubMed] [Google Scholar]
- 11.Boccadoro M, Palumbo A, Bringhen S, Merletti F, Ciccone G, Richiardi L, et al. Oral melphalan at diagnosis hampers adequate collection of peripheral blood progenitor cells in multiple myeloma. Haematologica. 2002;87:846–50. [PubMed] [Google Scholar]
- 12.Kumar S, Dispenzieri A, Lacy MQ, Hayman SR, Buadi FK, Gastineau DA, et al. Impact of lenalidomide therapy on stem cell mobilization and engraftment post-peripheral blood stem cell transplantation in patients with newly diagnosed myeloma. Leukemia. 2007;21:2035–42. doi: 10.1038/sj.leu.2404801. [DOI] [PubMed] [Google Scholar]
- 13.Popat U, Saliba R, Thandi R, Hosing C, Qazilbash M, Anderlini P, et al. Impairment of filgrastim-induced stem cell mobilization after prior lenalidomide in patients with multiple myeloma. Biol Blood Marrow Transplant. 2009;15:718–23. doi: 10.1016/j.bbmt.2009.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tournilhac O, Cazin B, Lepretre S, Divine M, Maloum K, Delmer A, et al. Impact of frontline fludarabine and cyclophosphamide combined treatment on peripheral blood stem cell mobilization in B-cell chronic lymphocytic leukemia. Blood. 2004;103:363–5. doi: 10.1182/blood-2003-05-1449. [DOI] [PubMed] [Google Scholar]
- 15.Laszlo D, Galieni P, Raspadori D, Tozzi M, Lauria F, Martinelli G. Fludarabine combination regimen severely affected peripheral blood stem cell mobilization. Acta Haematol. 2004;111:228–9. doi: 10.1159/000077572. [DOI] [PubMed] [Google Scholar]
- 16.Nickenig C, Dreyling M, Hoster E, Ludwig WD, Dorken B, Freund M, et al. Initial chemotherapy with mitoxantrone, chlorambucil, prednisone impairs the collection of stem cells in patients with indolent lymphomas--results of a randomized comparison by the German Low-Grade Lymphoma Study Group. Ann Oncol. 2007;18:136–42. doi: 10.1093/annonc/mdl348. [DOI] [PubMed] [Google Scholar]
- 17.Clark RE, Brammer CG. Previous treatment predicts the efficiency of blood progenitor cell mobilisation: validation of a chemotherapy scoring system. Bone Marrow Transplant. 1998;22:859–63. doi: 10.1038/sj.bmt.1701461. [DOI] [PubMed] [Google Scholar]
- 18.Keane C, Gibbs S, Seymour JF, Mills AK, Grimmett K, Van Kuilenberg R, et al. The Hyper-CVAD chemotherapy regimen has an adverse long-term impact on the ability to mobilize peripheral blood stem cells, which can be readily circumvented by using the early cycles for mobilization. Hematol Oncol. 2006;24:159–63. doi: 10.1002/hon.784. [DOI] [PubMed] [Google Scholar]
- 19.Olivieri A, Brunori M, Capelli D, Montanari M, Massidda D, Gini G, et al. Salvage therapy with an outpatient DHAP schedule followed by PBSC transplantation in 79 lymphoma patients: an intention to mobilize and transplant analysis. Eur J Haematol. 2004;72:10–7. doi: 10.1046/j.0902-4441.2004.00171.x. [DOI] [PubMed] [Google Scholar]
- 20.Hosing C, Saliba RM, Ahlawat S, Korbling M, Kebriaei P, Alousi A, et al. Poor hematopoietic stem cell mobilizers: a single institution study of incidence and risk factors in patients with recurrent or relapsed lymphoma. Am J Hematol. 2009;84:335–7. doi: 10.1002/ajh.21400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kuittinen T, Nousiainen T, Halonen P, Mahlamaki E, Jantunen E. Prediction of mobilisation failure in patients with non-Hodgkin’s lymphoma. Bone Marrow Transplant. 2004;33:907–12. doi: 10.1038/sj.bmt.1704466. [DOI] [PubMed] [Google Scholar]
- 22.Putkonen M, Rauhala A, Pelliniemi TT, Remes K. Sepsis, low platelet nadir at mobilization and previous IFN use predict stem cell mobilization failure in patients with multiple myeloma. Cytotherapy. 2007;9:548–54. doi: 10.1080/14653240701508429. [DOI] [PubMed] [Google Scholar]
- 23.Ozkurt ZN, Yegin ZA, Suyani E, Aki SZ, Acar K, Yagci M, et al. Factors affecting stem cell mobilization for autologous hematopoietic stem cell transplantation. J Clin Apher. 2010;25:280–6. doi: 10.1002/jca.20246. [DOI] [PubMed] [Google Scholar]
- 24.Corso A, Varettoni M, Mangiacavalli S, Zappasodi P, Klersy C, Rusconi C, et al. Bone marrow CD34+ cell count is predictive for adequate peripheral progenitor cell collection. Leukemia research. 2005;29:159–63. doi: 10.1016/j.leukres.2004.05.018. [DOI] [PubMed] [Google Scholar]
- 25.Shimizu N, Asai T, Hashimoto S, Narita M, Kobayashi M, Ito M, et al. Mobilization factors of peripheral blood stem cells in healthy donors. Ther Apher. 2002;6:413–8. doi: 10.1046/j.1526-0968.2002.00463.x. [DOI] [PubMed] [Google Scholar]
- 26.Fadini GP, Pucci L, Vanacore R, Baesso I, Penno G, Balbarini A, et al. Glucose tolerance is negatively associated with circulating progenitor cell levels. Diabetologia. 2007;50:2156–63. doi: 10.1007/s00125-007-0732-y. [DOI] [PubMed] [Google Scholar]
- 27.Fadini GP, Boscaro E, de Kreutzenberg S, Agostini C, Seeger F, Dimmeler S, et al. Time course and mechanisms of circulating progenitor cell reduction in the natural history of type 2 diabetes. Diabetes Care. 2010;33:1097–102. doi: 10.2337/dc09-1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ferraro F, Lymperi S, Mendez-Ferrer S, Saez B, Spencer JA, Yeap BY, et al. Diabetes impairs hematopoietic stem cell mobilization by altering niche function. Sci Transl Med. 2011;3:104ra1. doi: 10.1126/scitranslmed.3002191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li L, Xie T. Stem cell niche: structure and function. Annu Rev Cell Dev Biol. 2005;21:605–31. doi: 10.1146/annurev.cellbio.21.012704.131525. [DOI] [PubMed] [Google Scholar]
- 30.Lin H. The stem-cell niche theory: lessons from flies. Nat Rev Genet. 2002;3:931–40. doi: 10.1038/nrg952. [DOI] [PubMed] [Google Scholar]
- 31.Mendez-Ferrer S, Michurina TV, Ferraro F, Mazloom AR, Macarthur BD, Lira SA, et al. Mesenchymal and haematopoietic stem cells form a unique bone marrow niche. Nature. 2010;466:829–34. doi: 10.1038/nature09262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Spiegel A, Shivtiel S, Kalinkovich A, Ludin A, Netzer N, Goichberg P, et al. Catecholaminergic neurotransmitters regulate migration and repopulation of immature human CD34+ cells through Wnt signaling. Nat Immunol. 2007;8:1123–31. doi: 10.1038/ni1509. [DOI] [PubMed] [Google Scholar]
- 33.Heissig B, Hattori K, Dias S, Friedrich M, Ferris B, Hackett NR, et al. Recruitment of stem and progenitor cells from the bone marrow niche requires MMP-9 mediated release of kit-ligand. Cell. 2002;109:625–37. doi: 10.1016/s0092-8674(02)00754-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Levesque JP, Takamatsu Y, Nilsson SK, Haylock DN, Simmons PJ. Vascular cell adhesion molecule-1 (CD106) is cleaved by neutrophil proteases in the bone marrow following hematopoietic progenitor cell mobilization by granulocyte colony-stimulating factor. Blood. 2001;98:1289–97. doi: 10.1182/blood.v98.5.1289. [DOI] [PubMed] [Google Scholar]
- 35.Levesque JP, Hendy J, Takamatsu Y, Simmons PJ, Bendall LJ. Disruption of the CXCR4/CXCL12 chemotactic interaction during hematopoietic stem cell mobilization induced by GCSF or cyclophosphamide. The Journal of clinical investigation. 2003;111:187–96. doi: 10.1172/JCI15994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Broxmeyer HE. Chemokines in hematopoiesis. Curr Opin Hematol. 2008;15:49–58. doi: 10.1097/MOH.0b013e3282f29012. [DOI] [PubMed] [Google Scholar]
- 37.Sugiyama T, Kohara H, Noda M, Nagasawa T. Maintenance of the hematopoietic stem cell pool by CXCL12-CXCR4 chemokine signaling in bone marrow stromal cell niches. Immunity. 2006;25:977–88. doi: 10.1016/j.immuni.2006.10.016. [DOI] [PubMed] [Google Scholar]
- 38.Ehninger A, Trumpp A. The bone marrow stem cell niche grows up: mesenchymal stem cells and macrophages move in. The Journal of experimental medicine. 2011;208:421–8. doi: 10.1084/jem.20110132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Arai F, Hirao A, Ohmura M, Sato H, Matsuoka S, Takubo K, et al. Tie2/angiopoietin-1 signaling regulates hematopoietic stem cell quiescence in the bone marrow niche. Cell. 2004;118:149–61. doi: 10.1016/j.cell.2004.07.004. [DOI] [PubMed] [Google Scholar]
- 40.Qian H, Buza-Vidas N, Hyland CD, Jensen CT, Antonchuk J, Mansson R, et al. Critical role of thrombopoietin in maintaining adult quiescent hematopoietic stem cells. Cell Stem Cell. 2007;1:671–84. doi: 10.1016/j.stem.2007.10.008. [DOI] [PubMed] [Google Scholar]
- 41.Chow A, Lucas D, Hidalgo A, Mendez-Ferrer S, Hashimoto D, Scheiermann C, et al. Bone marrow CD169+ macrophages promote the retention of hematopoietic stem and progenitor cells in the mesenchymal stem cell niche. The Journal of experimental medicine. 2011;208:261–71. doi: 10.1084/jem.20101688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Christopher MJ, Rao M, Liu F, Woloszynek JR, Link DC. Expression of the G-CSF receptor in monocytic cells is sufficient to mediate hematopoietic progenitor mobilization by G-CSF in mice. The Journal of experimental medicine. 2011;208:251–60. doi: 10.1084/jem.20101700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Winkler IG, Sims NA, Pettit AR, Barbier V, Nowlan B, Helwani F, et al. Bone marrow macrophages maintain hematopoietic stem cell (HSC) niches and their depletion mobilizes HSCs. Blood. 2010;116:4815–28. doi: 10.1182/blood-2009-11-253534. [DOI] [PubMed] [Google Scholar]
- 44.Wright DE, Wagers AJ, Gulati AP, Johnson FL, Weissman IL. Physiological migration of hematopoietic stem and progenitor cells. Science. 2001;294:1933–6. doi: 10.1126/science.1064081. [DOI] [PubMed] [Google Scholar]
- 45.Kollet O, Dar A, Lapidot T. The multiple roles of osteoclasts in host defense: bone remodeling and hematopoietic stem cell mobilization. Annu Rev Immunol. 2007;25:51–69. doi: 10.1146/annurev.immunol.25.022106.141631. [DOI] [PubMed] [Google Scholar]
- 46.Petit I, Szyper-Kravitz M, Nagler A, Lahav M, Peled A, Habler L, et al. G-CSF induces stem cell mobilization by decreasing bone marrow SDF-1 and up-regulating CXCR4. Nature immunology. 2002;3:687–94. doi: 10.1038/ni813. [DOI] [PubMed] [Google Scholar]
- 47.Levesque JP, Hendy J, Takamatsu Y, Williams B, Winkler IG, Simmons PJ. Mobilization by either cyclophosphamide or granulocyte colony-stimulating factor transforms the bone marrow into a highly proteolytic environment. Exp Hematol. 2002;30:440–9. doi: 10.1016/s0301-472x(02)00788-9. [DOI] [PubMed] [Google Scholar]
- 48.Pelus LM, Bian H, King AG, Fukuda S. Neutrophil-derived MMP-9 mediates synergistic mobilization of hematopoietic stem and progenitor cells by the combination of G-CSF and the chemokines GRObeta/CXCL2 and GRObetaT/CXCL2delta4. Blood. 2004;103:110–9. doi: 10.1182/blood-2003-04-1115. [DOI] [PubMed] [Google Scholar]
- 49.Levesque JP, Liu F, Simmons PJ, Betsuyaku T, Senior RM, Pham C, et al. Characterization of hematopoietic progenitor mobilization in protease-deficient mice. Blood. 2004;104:65–72. doi: 10.1182/blood-2003-05-1589. [DOI] [PubMed] [Google Scholar]
- 50.Levesque JP, Hendy J, Winkler IG, Takamatsu Y, Simmons PJ. Granulocyte colony-stimulating factor induces the release in the bone marrow of proteases that cleave c-KIT receptor (CD117) from the surface of hematopoietic progenitor cells. Exp Hematol. 2003;31:109–17. doi: 10.1016/s0301-472x(02)01028-7. [DOI] [PubMed] [Google Scholar]
- 51.Semerad CL, Christopher MJ, Liu F, Short B, Simmons PJ, Winkler I, et al. G-CSF potently inhibits osteoblast activity and CXCL12 mRNA expression in the bone marrow. Blood. 2005;106:3020–7. doi: 10.1182/blood-2004-01-0272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Nademanee A, Sniecinski I, Schmidt GM, Dagis AC, O’Donnell MR, Snyder DS, et al. High-dose therapy followed by autologous peripheral-blood stem-cell transplantation for patients with Hodgkin’s disease and non-Hodgkin’s lymphoma using unprimed and granulocyte colony-stimulating factor-mobilized peripheral-blood stem cells. J Clin Oncol. 1994;12:2176–86. doi: 10.1200/JCO.1994.12.10.2176. [DOI] [PubMed] [Google Scholar]
- 53.Schmitz N, Linch DC, Dreger P, Goldstone AH, Boogaerts MA, Ferrant A, et al. Randomised trial of filgrastim-mobilised peripheral blood progenitor cell transplantation versus autologous bone-marrow transplantation in lymphoma patients. Lancet. 1996;347:353–7. doi: 10.1016/s0140-6736(96)90536-x. [DOI] [PubMed] [Google Scholar]
- 54.Bensinger WI, Martin PJ, Storer B, Clift R, Forman SJ, Negrin R, et al. Transplantation of bone marrow as compared with peripheral-blood cells from HLA-identical relatives in patients with hematologic cancers. N Engl J Med. 2001;344:175–81. doi: 10.1056/NEJM200101183440303. [DOI] [PubMed] [Google Scholar]
- 55.Blaise D, Kuentz M, Fortanier C, Bourhis JH, Milpied N, Sutton L, et al. Randomized trial of bone marrow versus lenograstim-primed blood cell allogeneic transplantation in patients with early-stage leukemia: a report from the Societe Francaise de Greffe de Moelle. J Clin Oncol. 2000;18:537–46. doi: 10.1200/JCO.2000.18.3.537. [DOI] [PubMed] [Google Scholar]
- 56.Couban S, Simpson DR, Barnett MJ, Bredeson C, Hubesch L, Howson-Jan K, et al. A randomized multicenter comparison of bone marrow and peripheral blood in recipients of matched sibling allogeneic transplants for myeloid malignancies. Blood. 2002;100:1525–31. doi: 10.1182/blood-2002-01-0048. [DOI] [PubMed] [Google Scholar]
- 57.Schmitz N, Beksac M, Hasenclever D, Bacigalupo A, Ruutu T, Nagler A, et al. Transplantation of mobilized peripheral blood cells to HLA-identical siblings with standard-risk leukemia. Blood. 2002;100:761–7. doi: 10.1182/blood-2001-12-0304. [DOI] [PubMed] [Google Scholar]
- 58.Lane TA, Law P, Maruyama M, Young D, Burgess J, Mullen M, et al. Harvesting and enrichment of hematopoietic progenitor cells mobilized into the peripheral blood of normal donors by granulocyte-macrophage colony-stimulating factor (GM-CSF) or G-CSF: potential role in allogeneic marrow transplantation. Blood. 1995;85:275–82. [PubMed] [Google Scholar]
- 59.Gazitt Y, Shaughnessy P, Liu Q. Differential mobilization of CD34+ cells and lymphoma cells in non-Hodgkin’s lymphoma patients mobilized with different growth factors. Journal of hematotherapy & stem cell research. 2001;10:167–76. doi: 10.1089/152581601750098453. [DOI] [PubMed] [Google Scholar]
- 60.Gazitt Y, Shaughnessy P, Devore P. Mobilization of dendritic cells and NK cells in non-Hodgkin’s lymphoma patients mobilized with different growth factors. Journal of hematotherapy & stem cell research. 2001;10:177–86. doi: 10.1089/152581601750098471. [DOI] [PubMed] [Google Scholar]
- 61.Devine SM, Brown RA, Mathews V, Trinkaus K, Khoury H, Adkins D, et al. Reduced risk of acute GVHD following mobilization of HLA-identical sibling donors with GM-CSF alone. Bone Marrow Transplant. 2005;36:531–8. doi: 10.1038/sj.bmt.1705091. [DOI] [PubMed] [Google Scholar]
- 62.Nie Y, Han YC, Zou YR. CXCR4 is required for the quiescence of primitive hematopoietic cells. The Journal of experimental medicine. 2008;205:777–83. doi: 10.1084/jem.20072513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Tzeng YS, Li H, Kang YL, Chen WC, Cheng WC, Lai DM. Loss of Cxcl12/Sdf-1 in adult mice decreases the quiescent state of hematopoietic stem/progenitor cells and alters the pattern of hematopoietic regeneration after myelosuppression. Blood. 2011;117:429–39. doi: 10.1182/blood-2010-01-266833. [DOI] [PubMed] [Google Scholar]
- 64.Devine SM, Flomenberg N, Vesole DH, Liesveld J, Weisdorf D, Badel K, et al. Rapid mobilization of CD34+ cells following administration of the CXCR4 antagonist AMD3100 to patients with multiple myeloma and non-Hodgkin’s lymphoma. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2004;22:1095–102. doi: 10.1200/JCO.2004.07.131. [DOI] [PubMed] [Google Scholar]
- 65.Flomenberg N, Devine SM, Dipersio JF, Liesveld JL, McCarty JM, Rowley SD, et al. The use of AMD3100 plus G-CSF for autologous hematopoietic progenitor cell mobilization is superior to G-CSF alone. Blood. 2005;106:1867–74. doi: 10.1182/blood-2005-02-0468. [DOI] [PubMed] [Google Scholar]
- 66.Cashen A, Lopez S, Gao F, Calandra G, MacFarland R, Badel K, et al. A phase II study of plerixafor (AMD3100) plus G-CSF for autologous hematopoietic progenitor cell mobilization in patients with Hodgkin lymphoma. Biology of blood and marrow transplantation : journal of the American Society for Blood and Marrow Transplantation. 2008;14:1253–61. doi: 10.1016/j.bbmt.2008.08.011. [DOI] [PubMed] [Google Scholar]
- 67.Fowler CJ, Dunn A, Hayes-Lattin B, Hansen K, Hansen L, Lanier K, et al. Rescue from failed growth factor and/or chemotherapy HSC mobilization with G-CSF and plerixafor (AMD3100): an institutional experience. Bone marrow transplantation. 2009;43:909–17. doi: 10.1038/bmt.2008.409. [DOI] [PubMed] [Google Scholar]
- 68.Stiff P, Micallef I, McCarthy P, Magalhaes-Silverman M, Weisdorf D, Territo M, et al. Treatment with plerixafor in non-Hodgkin’s lymphoma and multiple myeloma patients to increase the number of peripheral blood stem cells when given a mobilizing regimen of G-CSF: implications for the heavily pretreated patient. Biology of blood and marrow transplantation : journal of the American Society for Blood and Marrow Transplantation. 2009;15:249–56. doi: 10.1016/j.bbmt.2008.11.028. [DOI] [PubMed] [Google Scholar]
- 69.Calandra G, McCarty J, McGuirk J, Tricot G, Crocker SA, Badel K, et al. AMD3100 plus G-CSF can successfully mobilize CD34+ cells from non-Hodgkin’s lymphoma, Hodgkin’s disease and multiple myeloma patients previously failing mobilization with chemotherapy and/or cytokine treatment: compassionate use data. Bone marrow transplantation. 2008;41:331–8. doi: 10.1038/sj.bmt.1705908. [DOI] [PubMed] [Google Scholar]
- 70.DiPersio JF, Micallef IN, Stiff PJ, Bolwell BJ, Maziarz RT, Jacobsen E, et al. Phase III prospective randomized double-blind placebo-controlled trial of plerixafor plus granulocyte colony-stimulating factor compared with placebo plus granulocyte colony-stimulating factor for autologous stem-cell mobilization and transplantation for patients with non-Hodgkin’s lymphoma. J Clin Oncol. 2009;27:4767–73. doi: 10.1200/JCO.2008.20.7209. [DOI] [PubMed] [Google Scholar]
- 71.DiPersio JF, Stadtmauer EA, Nademanee A, Micallef IN, Stiff PJ, Kaufman JL, et al. Plerixafor and G-CSF versus placebo and G-CSF to mobilize hematopoietic stem cells for autologous stem cell transplantation in patients with multiple myeloma. Blood. 2009;113:5720–6. doi: 10.1182/blood-2008-08-174946. [DOI] [PubMed] [Google Scholar]
- 72.Devine SM, Vij R, Rettig M, Todt L, McGlauchlen K, Fisher N, et al. Rapid mobilization of functional donor hematopoietic cells without G-CSF using AMD3100, an antagonist of the CXCR4/SDF-1 interaction. Blood. 2008;112:990–8. doi: 10.1182/blood-2007-12-130179. [DOI] [PubMed] [Google Scholar]
- 73.Schroeder MA, Lopez S, Rettig MP, Trinkaus K, Westervelt P, DiPersio JF. High Dose Sargramostim (GM-CSF) Combined with IV Plerixafor for the Mobilization of Peripheral Blood Stem Cells (PBSC) From Normal HLA-Matched Allogeneic Sibling Donors Results in Hypercoagulability. ASH Annual Meeting Abstracts; 2012. p. 4095. [Google Scholar]
- 74.Liles WC, Broxmeyer HE, Rodger E, Wood B, Hubel K, Cooper S, et al. Mobilization of hematopoietic progenitor cells in healthy volunteers by AMD3100, a CXCR4 antagonist. Blood. 2003;102:2728–30. doi: 10.1182/blood-2003-02-0663. [DOI] [PubMed] [Google Scholar]
- 75.Hubel K, Liles WC, Broxmeyer HE, Rodger E, Wood B, Cooper S, et al. Leukocytosis and Mobilization of CD34+ Hematopoietic Progenitor Cells by AMD3100, a CXCR4 Antagonist. Support Cancer Ther. 2004;1:165–72. doi: 10.3816/SCT.2004.n.008. [DOI] [PubMed] [Google Scholar]
- 76.Liles WC, Rodger E, Broxmeyer HE, Dehner C, Badel K, Calandra G, et al. Augmented mobilization and collection of CD34+ hematopoietic cells from normal human volunteers stimulated with granulocyte-colony-stimulating factor by single-dose administration of AMD3100, a CXCR4 antagonist. Transfusion. 2005;45:295–300. doi: 10.1111/j.1537-2995.2005.04222.x. [DOI] [PubMed] [Google Scholar]
- 77.Stewart DA, Smith C, MacFarland R, Calandra G. Pharmacokinetics and pharmacodynamics of plerixafor in patients with non-Hodgkin lymphoma and multiple myeloma. Biology of blood and marrow transplantation : journal of the American Society for Blood and Marrow Transplantation. 2009;15:39–46. doi: 10.1016/j.bbmt.2008.10.018. [DOI] [PubMed] [Google Scholar]
- 78.Lefrere F, Mauge L, Rea D, Ribeil JA, Dal Cortivo L, Brignier AC, et al. A specific time course for mobilization of peripheral blood CD34+ cells after plerixafor injection in very poor mobilizer patients: impact on the timing of the apheresis procedure. Transfusion. 2013;53:564–9. doi: 10.1111/j.1537-2995.2012.03744.x. [DOI] [PubMed] [Google Scholar]
- 79.Vishnu P, Roy V, Paulsen A, Zubair AC. Efficacy and cost-benefit analysis of risk-adaptive use of plerixafor for autologous hematopoietic progenitor cell mobilization. Transfusion. 2012;52:55–62. doi: 10.1111/j.1537-2995.2011.03206.x. [DOI] [PubMed] [Google Scholar]
- 80.Li J, Hamilton E, Vaughn L, Graiser M, Renfroe H, Lechowicz MJ, et al. Effectiveness and cost analysis of “just-in-time” salvage plerixafor administration in autologous transplant patients with poor stem cell mobilization kinetics. Transfusion. 2011;51:2175–82. doi: 10.1111/j.1537-2995.2011.03136.x. [DOI] [PubMed] [Google Scholar]
- 81.Costa LJ, Alexander ET, Hogan KR, Schaub C, Fouts TV, Stuart RK. Development and validation of a decision-making algorithm to guide the use of plerixafor for autologous hematopoietic stem cell mobilization. Bone marrow transplantation. 2011;46:64–9. doi: 10.1038/bmt.2010.78. [DOI] [PubMed] [Google Scholar]
- 82.Smith VR, Popat U, Ciurea S, Nieto Y, Anderlini P, Rondon G, et al. Just-in-time rescue plerixafor in combination with chemotherapy and granulocyte-colony stimulating factor for peripheral blood progenitor cell mobilization. Am J Hematol. 2013;88:754–7. doi: 10.1002/ajh.23499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Farina L, Spina F, Guidetti A, Longoni P, Ravagnani F, Dodero A, et al. Peripheral blood CD34+ cell monitoring after cyclophosphamide and granulocyte-colony-stimulating factor: an algorithm for the pre-emptive use of plerixafor. Leuk Lymphoma. 2013 doi: 10.3109/10428194.2013.802783. [DOI] [PubMed] [Google Scholar]
- 84.Maziarz RT, Nademanee AP, Micallef IN, Stiff PJ, Calandra G, Angell J, et al. Plerixafor plus granulocyte colony-stimulating factor improves the mobilization of hematopoietic stem cells in patients with non-Hodgkin lymphoma and low circulating peripheral blood CD34+ cells. Biol Blood Marrow Transplant. 2013;19:670–5. doi: 10.1016/j.bbmt.2013.01.005. [DOI] [PubMed] [Google Scholar]
- 85.Ding L, Saunders TL, Enikolopov G, Morrison SJ. Endothelial and perivascular cells maintain haematopoietic stem cells. Nature. 2012;481:457–62. doi: 10.1038/nature10783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Broudy VC. Stem cell factor and hematopoiesis. Blood. 1997;90:1345–64. [PubMed] [Google Scholar]
- 87.Levesque JP, Leavesley DI, Niutta S, Vadas M, Simmons PJ. Cytokines increase human hemopoietic cell adhesiveness by activation of very late antigen (VLA)-4 and VLA-5 integrins. The Journal of experimental medicine. 1995;181:1805–15. doi: 10.1084/jem.181.5.1805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Dahlen DD, Lin NL, Liu YC, Broudy VC. Soluble Kit receptor blocks stem cell factor bioactivity in vitro. Leukemia research. 2001;25:413–21. doi: 10.1016/s0145-2126(00)00122-3. [DOI] [PubMed] [Google Scholar]
- 89.Herbert KE, Morgan S, Prince HM, Westerman DA, Wolf MM, Carney DA, et al. Stem cell factor and high-dose twice daily filgrastim is an effective strategy for peripheral blood stem cell mobilization in patients with indolent lymphoproliferative disorders previously treated with fludarabine: results of a Phase II study with an historical comparator. Leukemia. 2009;23:305–12. doi: 10.1038/leu.2008.302. [DOI] [PubMed] [Google Scholar]
- 90.To LB, Bashford J, Durrant S, MacMillan J, Schwarer AP, Prince HM, et al. Successful mobilization of peripheral blood stem cells after addition of ancestim (stem cell factor) in patients who had failed a prior mobilization with filgrastim (granulocyte colony-stimulating factor) alone or with chemotherapy plus filgrastim. Bone Marrow Transplant. 2003;31:371–8. doi: 10.1038/sj.bmt.1703860. [DOI] [PubMed] [Google Scholar]
- 91.Prosper F, Sola C, Hornedo J, Arbona C, Menendez P, Orfao A, et al. Mobilization of peripheral blood progenitor cells with a combination of cyclophosphamide, r-metHuSCF and filgrastim in patients with breast cancer previously treated with chemotherapy. Leukemia. 2003;17:437–41. doi: 10.1038/sj.leu.2402750. [DOI] [PubMed] [Google Scholar]
- 92.Stiff P, Gingrich R, Luger S, Wyres MR, Brown RA, LeMaistre CF, et al. A randomized phase 2 study of PBPC mobilization by stem cell factor and filgrastim in heavily pretreated patients with Hodgkin’s disease or non-Hodgkin’s lymphoma. Bone Marrow Transplant. 2000;26:471–81. doi: 10.1038/sj.bmt.1702531. [DOI] [PubMed] [Google Scholar]
- 93.Moskowitz CH, Stiff P, Gordon MS, McNiece I, Ho AD, Costa JJ, et al. Recombinant methionyl human stem cell factor and filgrastim for peripheral blood progenitor cell mobilization and transplantation in non-Hodgkin’s lymphoma patients--results of a phase I/II trial. Blood. 1997;89:3136–47. [PubMed] [Google Scholar]
- 94.Basser RL, To LB, Begley CG, Maher D, Juttner C, Cebon J, et al. Rapid hematopoietic recovery after multicycle high-dose chemotherapy: enhancement of filgrastim-induced progenitor-cell mobilization by recombinant human stem-cell factor. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 1998;16:1899–908. doi: 10.1200/JCO.1998.16.5.1899. [DOI] [PubMed] [Google Scholar]
- 95.Facon T, Harousseau JL, Maloisel F, Attal M, Odriozola J, Alegre A, et al. Stem cell factor in combination with filgrastim after chemotherapy improves peripheral blood progenitor cell yield and reduces apheresis requirements in multiple myeloma patients: a randomized, controlled trial. Blood. 1999;94:1218–25. [PubMed] [Google Scholar]
- 96.Shpall EJ, Wheeler CA, Turner SA, Yanovich S, Brown RA, Pecora AL, et al. A randomized phase 3 study of peripheral blood progenitor cell mobilization with stem cell factor and filgrastim in high-risk breast cancer patients. Blood. 1999;93:2491–501. [PubMed] [Google Scholar]
- 97.Glaspy JA, Shpall EJ, LeMaistre CF, Briddell RA, Menchaca DM, Turner SA, et al. Peripheral blood progenitor cell mobilization using stem cell factor in combination with filgrastim in breast cancer patients. Blood. 1997;90:2939–51. [PubMed] [Google Scholar]
- 98.Lapierre V, Rossi JF, Heshmati F, Azar N, Vekhof A, Makowski C, et al. Ancestim (r-metHuSCF) plus filgrastim and/or chemotherapy for mobilization of blood progenitors in 513 poorly mobilizing cancer patients: the French compassionate experience. Bone Marrow Transplant. 2011;46:936–42. doi: 10.1038/bmt.2010.231. [DOI] [PubMed] [Google Scholar]
- 99.Fitoussi O, Perreau V, Boiron JM, Bouzigon E, Cony-Makhoul P, Pigneux A, et al. A comparison of toxicity following two different doses of cyclophosphamide for mobilization of peripheral blood progenitor cells in 116 multiple myeloma patients. Bone Marrow Transplant. 2001;27:837–42. doi: 10.1038/sj.bmt.1702879. [DOI] [PubMed] [Google Scholar]
- 100.Goldschmidt H, Hegenbart U, Wallmeier M, Hohaus S, Haas R. Factors influencing collection of peripheral blood progenitor cells following high-dose cyclophosphamide and granulocyte colony-stimulating factor in patients with multiple myeloma. Br J Haematol. 1997;98:736–44. doi: 10.1046/j.1365-2141.1997.2783095.x. [DOI] [PubMed] [Google Scholar]
- 101.Jantunen E, Putkonen M, Nousiainen T, Pelliniemi TT, Mahlamaki E, Remes K. Low-dose or intermediate-dose cyclophosphamide plus granulocyte colony-stimulating factor for progenitor cell mobilisation in patients with multiple myeloma. Bone Marrow Transplant. 2003;31:347–51. doi: 10.1038/sj.bmt.1703840. [DOI] [PubMed] [Google Scholar]
- 102.Hiwase DK, Bollard G, Hiwase S, Bailey M, Muirhead J, Schwarer AP. Intermediate-dose CY and G-CSF more efficiently mobilize adequate numbers of PBSC for tandem autologous PBSC transplantation compared with low-dose CY in patients with multiple myeloma. Cytotherapy. 2007;9:539–47. doi: 10.1080/14653240701452800. [DOI] [PubMed] [Google Scholar]
- 103.Hamadani M, Kochuparambil ST, Osman S, Cumpston A, Leadmon S, Bunner P, et al. Intermediate-dose versus low-dose cyclophosphamide and granulocyte colony-stimulating factor for peripheral blood stem cell mobilization in patients with multiple myeloma treated with novel induction therapies. Biol Blood Marrow Transplant. 2012;18:1128–35. doi: 10.1016/j.bbmt.2012.01.005. [DOI] [PubMed] [Google Scholar]
- 104.Pavone V, Gaudio F, Guarini A, Perrone T, Zonno A, Curci P, et al. Mobilization of peripheral blood stem cells with high-dose cyclophosphamide or the DHAP regimen plus G-CSF in non-Hodgkin’s lymphoma. Bone Marrow Transplant. 2002;29:285–90. doi: 10.1038/sj.bmt.1703364. [DOI] [PubMed] [Google Scholar]
- 105.Lee JL, Kim S, Kim SW, Kim EK, Kim SB, Kang YK, et al. ESHAP plus G-CSF as an effective peripheral blood progenitor cell mobilization regimen in pretreated non-Hodgkin’s lymphoma: comparison with high-dose cyclophosphamide plus G-CSF. Bone Marrow Transplant. 2005;35:449–54. doi: 10.1038/sj.bmt.1704798. [DOI] [PubMed] [Google Scholar]
- 106.Akhtar S, Tbakhi A, Humaidan H, El Weshi A, Rahal M, Maghfoor I. ESHAP + fixed dose G-CSF as autologous peripheral blood stem cell mobilization regimen in patients with relapsed or refractory diffuse large cell and Hodgkin’s lymphoma: a single institution result of 127 patients. Bone Marrow Transplant. 2006;37:277–82. doi: 10.1038/sj.bmt.1705239. [DOI] [PubMed] [Google Scholar]
- 107.Watts MJ, Ings SJ, Leverett D, MacMillan A, Devereux S, Goldstone AH, et al. ESHAP and G-CSF is a superior blood stem cell mobilizing regimen compared to cyclophosphamide 1.5 g m(-2) and G-CSF for pre-treated lymphoma patients: a matched pairs analysis of 78 patients. Br J Cancer. 2000;82:278–82. doi: 10.1054/bjoc.1999.0915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Mollee P, Pereira D, Nagy T, Song K, Saragosa R, Keating A, et al. Cyclophosphamide, etoposide and G-CSF to mobilize peripheral blood stem cells for autologous stem cell transplantation in patients with lymphoma. Bone Marrow Transplant. 2002;30:273–8. doi: 10.1038/sj.bmt.1703653. [DOI] [PubMed] [Google Scholar]
- 109.Pocali B, De Simone M, Annunziata M, Palmieri S, D’Amico MR, Copia C, et al. Ifosfamide, epirubicin and etoposide (IEV) regimen as salvage and mobilization therapy for refractory or early relapsing patients with aggressive non-Hodgkin’s lymphoma. Leuk Lymphoma. 2004;45:1605–9. doi: 10.1080/10428190410001683651. [DOI] [PubMed] [Google Scholar]
- 110.Rettig MP, Ansstas G, DiPersio JF. Mobilization of hematopoietic stem and progenitor cells using inhibitors of CXCR4 and VLA-4. Leukemia. 2012;26:34–53. doi: 10.1038/leu.2011.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Schmitt S, Weinhold N, Dembowsky K, Neben K, Witzens-Harig M, Braun M, et al. First Results of a Phase-II Study with the New CXCR4 Antagonist POL6326 to Mobilize Hematopoietic Stem Cells (HSC) In Multiple Myeloma (MM). ASH Annual Meeting Abstracts; 2010. p. 824. [Google Scholar]
- 112.Abraham M, Biyder K, Begin M, Wald H, Weiss ID, Galun E, et al. Enhanced unique pattern of hematopoietic cell mobilization induced by the CXCR4 antagonist 4F-benzoyl-TN14003. Stem Cells. 2007;25:2158–66. doi: 10.1634/stemcells.2007-0161. [DOI] [PubMed] [Google Scholar]
- 113.Nagler A, Shimoni A, Avivi I, Rowe JM, Beider K, Hardan I, et al. BKT140 Is a Novel CXCR4 Antagonist with Stem Cell Mobilization and Antimyeloma Effects: An Open-Label First Human Trial In Patients with Multiple Myeloma Undergoing Stem Cell Mobilization for Autologous Transplantation. ASH Annual Meeting Abstracts; 2010. p. 2260. [Google Scholar]
- 114.Chung DT, Chang L-W, Huang Y-H, Tsai C-Y, Hsu C-H, King C-HR, et al. TG-0054, a Novel and Potent Stem Cell Mobilizer, Displays Excellent PK/PD and Safety Profile in Phase I Trial. ASH Annual Meeting Abstracts; 2009. p. 866. [Google Scholar]
- 115.Scheller M, Schwoebel F, Vossmeyer D, Leutz A. Rapid and Efficient Mobilization of Murine Hematopoietic Stem and Progenitor Cells with Nox-A12, a New Spiegelmers(R)-Based CXCR4/SDF-1(CXCL12) Antagonist. ASH Annual Meeting Abstracts; 2011. p. 2995. [Google Scholar]
- 116.Gobbi M, Caligaris-Cappio F, Montillo M, Vauleon S, Zollner S, Dummler T, et al. Phase IIa Study of the Anti-CXCL12/SDF-1 Spiegelmer(R) Nox-A12 Alone and Combined with Bendamustine/Rituximab in Patients with Relapsed Chronic Lymphocytic Leukemia (CLL). ASH Annual Meeting Abstracts; 2012. p. 4593. [Google Scholar]
- 117.Zboralski D, Hoellenriegel J, Maasch C, Kruschinski A, Burger JA. Mode of Action of the SDF-1/CXCL12 Inhibiting Spiegelmer(R) Nox-A12 and Its Impact On Chronic Lymphocytic Leukemia (CLL) Cell Motility and Chemosensitization. ASH Annual Meeting Abstracts; 2012. p. 318. [Google Scholar]
- 118.Hoellenriegel J, Zboralski D, Estrov Z, Wierda WG, Keating M, Kruschinski A, et al. The Spiegelmer Nox-A12, a Novel SDF-1 (CXCL12) Inhibitor, and Its Effects on Chronic Lymphocytic Leukemia (CLL) Cell Migration. ASH Annual Meeting Abstracts; 2011. p. 3878. [Google Scholar]
- 119.Roccaro AM, Sacco A, Ungari M, Maiso P, Manier S, Quang P, et al. In Vivo Targeting of Stromal-Derived Factor-1 As a Strategy to Prevent Myeloma Cell Dissemination to Distant Bone Marrow Niches. ASH Annual Meeting Abstracts; 2012. p. 440. [Google Scholar]
- 120.Hanel P, Andreani P, Graler MH. Erythrocytes store and release sphingosine 1-phosphate in blood. FASEB J. 2007;21:1202–9. doi: 10.1096/fj.06-7433com. [DOI] [PubMed] [Google Scholar]
- 121.Ohkawa R, Nakamura K, Okubo S, Hosogaya S, Ozaki Y, Tozuka M, et al. Plasma sphingosine-1-phosphate measurement in healthy subjects: close correlation with red blood cell parameters. Ann Clin Biochem. 2008;45:356–63. doi: 10.1258/acb.2007.007189. [DOI] [PubMed] [Google Scholar]
- 122.Sanchez T, Hla T. Structural and functional characteristics of S1P receptors. J Cell Biochem. 2004;92:913–22. doi: 10.1002/jcb.20127. [DOI] [PubMed] [Google Scholar]
- 123.Peest U, Sensken SC, Andreani P, Hanel P, Van Veldhoven PP, Graler MH. S1P-lyase independent clearance of extracellular sphingosine 1-phosphate after dephosphorylation and cellular uptake. J Cell Biochem. 2008;104:756–72. doi: 10.1002/jcb.21665. [DOI] [PubMed] [Google Scholar]
- 124.Schwab SR, Pereira JP, Matloubian M, Xu Y, Huang Y, Cyster JG. Lymphocyte sequestration through S1P lyase inhibition and disruption of S1P gradients. Science. 2005;309:1735–9. doi: 10.1126/science.1113640. [DOI] [PubMed] [Google Scholar]
- 125.Massberg S, Schaerli P, Knezevic-Maramica I, Kollnberger M, Tubo N, Moseman EA, et al. Immunosurveillance by hematopoietic progenitor cells trafficking through blood, lymph, and peripheral tissues. Cell. 2007;131:994–1008. doi: 10.1016/j.cell.2007.09.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Ratajczak MZ, Lee H, Wysoczynski M, Wan W, Marlicz W, Laughlin MJ, et al. Novel insight into stem cell mobilization-plasma sphingosine-1-phosphate is a major chemoattractant that directs the egress of hematopoietic stem progenitor cells from the bone marrow and its level in peripheral blood increases during mobilization due to activation of complement cascade/membrane attack complex. Leukemia. 2010;24:976–85. doi: 10.1038/leu.2010.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Lee HM, Wu W, Wysoczynski M, Liu R, Zuba-Surma EK, Kucia M, et al. Impaired mobilization of hematopoietic stem/progenitor cells in C5-deficient mice supports the pivotal involvement of innate immunity in this process and reveals novel promobilization effects of granulocytes. Leukemia. 2009;23:2052–62. doi: 10.1038/leu.2009.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Mierzejewska K, Kucia M, Ratajczak J, Ratajczak MZ. Novel Evidence That Hematopoietic Stem/Progenitor Cells (HSPCs) Are Mobilized During Hemolysis in an Erythrocyte Lysis-Derived, Sphingosine-1-Phosphate (S1P)-Dependent manner--the Crucial Involvement of Complement Cascade (CC) Activation and Attenuation of CXCR4 Retention Signaling. ASH Annual Meeting Abstracts; 2012. p. 3189. [Google Scholar]
- 129.Juarez JG, Harun N, Thien M, Welschinger R, Baraz R, Pena AD, et al. Sphingosine-1-phosphate facilitates trafficking of hematopoietic stem cells and their mobilization by CXCR4 antagonists in mice. Blood. 2012;119:707–16. doi: 10.1182/blood-2011-04-348904. [DOI] [PubMed] [Google Scholar]
- 130.Hynes RO. Integrins: versatility, modulation, and signaling in cell adhesion. Cell. 1992;69:11–25. doi: 10.1016/0092-8674(92)90115-s. [DOI] [PubMed] [Google Scholar]
- 131.Springer TA. Traffic signals for lymphocyte recirculation and leukocyte emigration: the multistep paradigm. Cell. 1994;76:301–14. doi: 10.1016/0092-8674(94)90337-9. [DOI] [PubMed] [Google Scholar]
- 132.Humphries JD, Byron A, Humphries MJ. Integrin ligands at a glance. J Cell Sci. 2006;119:3901–3. doi: 10.1242/jcs.03098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Elices MJ, Osborn L, Takada Y, Crouse C, Luhowskyj S, Hemler ME, et al. VCAM-1 on activated endothelium interacts with the leukocyte integrin VLA-4 at a site distinct from the VLA-4/fibronectin binding site. Cell. 1990;60:577–84. doi: 10.1016/0092-8674(90)90661-w. [DOI] [PubMed] [Google Scholar]
- 134.Papayannopoulou T, Nakamoto B. Peripheralization of hemopoietic progenitors in primates treated with anti-VLA4 integrin. Proc Natl Acad Sci U S A. 1993;90:9374–8. doi: 10.1073/pnas.90.20.9374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Vermeulen M, Le Pesteur F, Gagnerault MC, Mary JY, Sainteny F, Lepault F. Role of adhesion molecules in the homing and mobilization of murine hematopoietic stem and progenitor cells. Blood. 1998;92:894–900. [PubMed] [Google Scholar]
- 136.Jing D, Oelschlaegel U, Ordemann R, Holig K, Ehninger G, Reichmann H, et al. CD49d blockade by natalizumab in patients with multiple sclerosis affects steady-state hematopoiesis and mobilizes progenitors with a distinct phenotype and function. Bone marrow transplantation. 2010;45:1489–96. doi: 10.1038/bmt.2009.381. [DOI] [PubMed] [Google Scholar]
- 137.Zohren F, Toutzaris D, Klarner V, Hartung HP, Kieseier B, Haas R. The monoclonal anti-VLA-4 antibody natalizumab mobilizes CD34+ hematopoietic progenitor cells in humans. Blood. 2008;111:3893–5. doi: 10.1182/blood-2007-10-120329. [DOI] [PubMed] [Google Scholar]
- 138.Bonig H, Wundes A, Chang KH, Lucas S, Papayannopoulou T. Increased numbers of circulating hematopoietic stem/progenitor cells are chronically maintained in patients treated with the CD49d blocking antibody natalizumab. Blood. 2008;111:3439–41. doi: 10.1182/blood-2007-09-112052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Bonig H, Watts KL, Chang KH, Kiem HP, Papayannopoulou T. Concurrent blockade of alpha4-integrin and CXCR4 in hematopoietic stem/progenitor cell mobilization. Stem Cells. 2009;27:836–7. doi: 10.1002/stem.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Ramirez P, Rettig MP, Uy GL, Deych E, Holt MS, Ritchey JK, et al. BIO5192, a small molecule inhibitor of VLA-4, mobilizes hematopoietic stem and progenitor cells. Blood. 2009;114:1340–3. doi: 10.1182/blood-2008-10-184721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Brunner S, Theiss HD, Murr A, Negele T, Franz WM. Primary hyperparathyroidism is associated with increased circulating bone marrow-derived progenitor cells. Am J Physiol Endocrinol Metab. 2007;293:E1670–5. doi: 10.1152/ajpendo.00287.2007. [DOI] [PubMed] [Google Scholar]
- 142.Brunner S, Zaruba MM, Huber B, David R, Vallaster M, Assmann G, et al. Parathyroid hormone effectively induces mobilization of progenitor cells without depletion of bone marrow. Exp Hematol. 2008;36:1157–66. doi: 10.1016/j.exphem.2008.03.014. [DOI] [PubMed] [Google Scholar]
- 143.Ballen KK, Shpall EJ, Avigan D, Yeap BY, Fisher DC, McDermott K, et al. Phase I trial of parathyroid hormone to facilitate stem cell mobilization. Biology of blood and marrow transplantation : journal of the American Society for Blood and Marrow Transplantation. 2007;13:838–43. doi: 10.1016/j.bbmt.2007.03.007. [DOI] [PubMed] [Google Scholar]
- 144.Ghobadi A, Holt M, Ritchey J, DiPersio JF. The Effect of Bortezomib (B) Alone or in Combination with Other Agents for Stem Cell Mobilization in Mice. ASH Annual Meeting Abstracts; 2012. p. 583. [Google Scholar]
- 145.Niesvizky R, Mark TM, Ward M, Jayabalan DS, Pearse RN, Manco M, et al. Overcoming the response plateau in multiple myeloma: a novel bortezomib-based strategy for secondary induction and high-yield CD34+ stem cell mobilization. Clin Cancer Res. 2013;19:1534–46. doi: 10.1158/1078-0432.CCR-12-1429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Haskill S, Peace A, Morris J, Sporn SA, Anisowicz A, Lee SW, et al. Identification of three related human GRO genes encoding cytokine functions. Proc Natl Acad Sci U S A. 1990;87:7732–6. doi: 10.1073/pnas.87.19.7732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.King AG, Horowitz D, Dillon SB, Levin R, Farese AM, MacVittie TJ, et al. Rapid mobilization of murine hematopoietic stem cells with enhanced engraftment properties and evaluation of hematopoietic progenitor cell mobilization in rhesus monkeys by a single injection of SB-251353, a specific truncated form of the human CXC chemokine GRObeta. Blood. 2001;97:1534–42. doi: 10.1182/blood.v97.6.1534. [DOI] [PubMed] [Google Scholar]
- 148.Fukuda S, Bian H, King AG, Pelus LM. The chemokine GRObeta mobilizes early hematopoietic stem cells characterized by enhanced homing and engraftment. Blood. 2007;110:860–9. doi: 10.1182/blood-2006-06-031401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Winkler IG, Barbier V, Wadley R, Zannettino AC, Williams S, Levesque JP. Positioning of bone marrow hematopoietic and stromal cells relative to blood flow in vivo: serially reconstituting hematopoietic stem cells reside in distinct nonperfused niches. Blood. 2010;116:375–85. doi: 10.1182/blood-2009-07-233437. [DOI] [PubMed] [Google Scholar]
- 150.Parmar K, Mauch P, Vergilio JA, Sackstein R, Down JD. Distribution of hematopoietic stem cells in the bone marrow according to regional hypoxia. Proc Natl Acad Sci U S A. 2007;104:5431–6. doi: 10.1073/pnas.0701152104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Wang GL, Semenza GL. Purification and characterization of hypoxia-inducible factor 1. J Biol Chem. 1995;270:1230–7. doi: 10.1074/jbc.270.3.1230. [DOI] [PubMed] [Google Scholar]
- 152.Bruick RK, McKnight SL. A conserved family of prolyl-4-hydroxylases that modify HIF. Science. 2001;294:1337–40. doi: 10.1126/science.1066373. [DOI] [PubMed] [Google Scholar]
- 153.Jaakkola P, Mole DR, Tian YM, Wilson MI, Gielbert J, Gaskell SJ, et al. Targeting of HIF-alpha to the von Hippel-Lindau ubiquitylation complex by O2-regulated prolyl hydroxylation. Science. 2001;292:468–72. doi: 10.1126/science.1059796. [DOI] [PubMed] [Google Scholar]
- 154.Cramer T, Yamanishi Y, Clausen BE, Forster I, Pawlinski R, Mackman N, et al. HIF-1alpha is essential for myeloid cell-mediated inflammation. Cell. 2003;112:645–57. doi: 10.1016/s0092-8674(03)00154-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Ceradini DJ, Kulkarni AR, Callaghan MJ, Tepper OM, Bastidas N, Kleinman ME, et al. Progenitor cell trafficking is regulated by hypoxic gradients through HIF-1 induction of SDF-1. Nat Med. 2004;10:858–64. doi: 10.1038/nm1075. [DOI] [PubMed] [Google Scholar]
- 156.Levesque JP, Winkler IG, Hendy J, Williams B, Helwani F, Barbier V, et al. Hematopoietic progenitor cell mobilization results in hypoxia with increased hypoxia-inducible transcription factor-1 alpha and vascular endothelial growth factor A in bone marrow. Stem Cells. 2007;25:1954–65. doi: 10.1634/stemcells.2006-0688. [DOI] [PubMed] [Google Scholar]
- 157.Forsythe JA, Jiang BH, Iyer NV, Agani F, Leung SW, Koos RD, et al. Activation of vascular endothelial growth factor gene transcription by hypoxia-inducible factor 1. Mol Cell Biol. 1996;16:4604–13. doi: 10.1128/mcb.16.9.4604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Semenza GL. Regulation of mammalian O2 homeostasis by hypoxia-inducible factor 1. Annual review of cell and developmental biology. 1999;15:551–78. doi: 10.1146/annurev.cellbio.15.1.551. [DOI] [PubMed] [Google Scholar]
- 159.Forristal CE, Nowlan B, Barbier V, Winkler IG, Walkinshaw G, Levesque J-P. FG-4497, a Pharmacological Stabilizer of HIF-1{alpha} Protein, Synergistically Enhances Hematopoietic Stem Cells (HSC) Mobilization in Response to G-CSF and Plerixafor. ASH Annual Meeting Abstracts; 2012. p. 216. [Google Scholar]


