Abstract
Docosahexaenoic acid (22:6n-3) is the major brain n-3 polyunsaturated fatty acid and it is possible that docosahexaenoic acid is anti-inflammatory in the brain as it is known to be in other tissues. Using a combination of models including the fat-1 transgenic mouse, chronic dietary n-3 PUFA modulation in transgenic and wildtype mice, and acute direct brain infusion, we demonstrated that unesterified docosahexaenoic acid attenuates neuroinflammation initiated by intracerebroventricular lipopolysaccharide. Hippocampal neuroinflammation was assessed by gene expression and immunohistochemistry. Further, docosahexaenoic acid protected against lipopolysaccharide-induced neuronal loss. Acute intracerebroventricular infusion of unesterified docosahexaenoic acid or its 12/15-lipoxygenase product and precursor to protectins and resolvins, 17S-hydroperoxy-docosahexaenoic acid, mimics anti-neuroinflammatory aspects of chronically increased unesterified docosahexaenoic acid. LCMS/MS revealed that neuroprotectin D1 and several other docosahexaenoic acid-derived specialized pro-resolving mediators are present in the hippocampus. Acute icv infusion of 17S-hydroperoxydocosahexaenoic acid increases hippocampal neuroprotectin D1 levels concomitant to attenuating neuroinflammation. These results show that unesterified docosahexaenoic acid is protective in a lipopolysaccharide-initiated mouse model of acute neuroinflammation, at least in part, via its conversion to specialized pro-resolving mediators; these docosahexaenoic acid stores may provide novel targets for the prevention and treatment(s) of neurological disorders with a neuroinflammatory component.
Keywords: unesterified docosahexaenoic acid, neuroinflammation
INTRODUCTION
Docosahexaenoic acid (DHA; 22:6n-3) is the most abundant n-3 polyunsaturated fatty acid (n-3 PUFA) in the brain and is considered essential for optimal retinal and neural development, likely due to its unique molecular structure that facilitates neural signaling (Crawford et al. 2013, Hoffman et al. 2009). The importance of DHA in the maintenance of neurological health and disease prevention remains of considerable interest (Cole et al. 2010). Epidemiological studies suggest there is an inverse association between fish or n-3 PUFA intake and risk of neurological disorders and several, though not all, randomized controlled trials show benefits of n-3 PUFA supplementation (Calon 2011, Appleton et al. 2010, Balanza-Martinez et al. 2011).
There are several proposed mechanisms for the protective role of n-3 PUFA in neurological disorders. For instance, DHA upregulates anti-apoptotic factors Akt, Bcl-2 and Bfl-1 in vitro (Akbar et al. 2005, Lukiw et al. 2005). DHA increases cortical brain derived neurotrophic factor (Rao et al. 2007b), promotes synaptic plasticity (Lafourcade et al. 2011), and via its ethanolamide metabolites promotes neurite growth and synaptogenesis (Kim et al. 2011b). Beyond the aforementioned pathways, it is possible that DHA acts via an anti-neuroinflammatory mechanism, since DHA is known to be anti-inflammatory in non-neural tissues, and because most, if not all, neurological disorders have a neuroinflammatory component (Aid & Bosetti 2011).
In the past decade, specialized pro-resolving mediators (SPM) have been identified as a potent group of autocoids produced endogenously from the enzymatic oxygenation of DHA and other PUFA. Thus, SPM have provided a major mechanism for the well-documented beneficial actions of DHA (Calder 2009, Serhan et al. 2008). While most of arachidonic acid (ARA; 20:4n-6)-derived lipid mediators, including prostaglandins and leukotrienes, are generally pro-inflammatory, SPM derived from n-3 PUFA, such as eicosapentaenoic acid (EPA; 20:5n-3) and DHA, are anti-inflammatory and pro-resolving (Serhan et al. 2008, Serhan et al. 2002). Pro-resolution is an active process that reduces inflammation and returns a tissue to homeostasis, and is driven by its own mediators and signaling pathways. For a mediator to be pro-resolving it counter regulates inflammation and stimulates the clearance of inflammatory and cellular debris (Serhan & Petasis 2011). DHA is converted by resolving inflammatory exudates into several families of SPM including resolvins, protectins, and maresins, which play similar but distinct roles in inflammation and resolution (Serhan et al. 2008). To date, these mediators have been investigated in a number of tissues and disease models. Neither DHA nor DHA-derived SPM have been tested in an in vivo model of neuroinflammation, although total brain DHA is known to reduce brain inflammation resulting from insults such as ischemia-reperfusion (Lalancette-Hebert et al. 2011), and systemic inflammation (Mingam et al. 2008).
D-series resolvins are produced following the action of two lipoxygenases (LO) on unesterified DHA. 15-lipoxygenase-1 (15-LO) first catalyzes the oxygenation of DHA at carbon 17 with the S chirality to produce 17S-hydroperoxy-DHA (17S-HpDHA; 17S-hydroperoxy-docosa-4Z,7Z,10Z,13Z,15E,19Z-hexaenoic acid). Resolvin D1 and D2 are formed, via the action of a second 5-lipoxygenase (5-LO), from a 7(8)S-epoxide intermediate (Yang et al. 2011). In animal models, RvD1 inhibits neutrophil infiltration in peritonitis (Sun et al. 2007, Kasuga et al. 2008), protects from ischemia-reperfusion induced kidney damage (Duffield et al. 2006), reduces oxidative-stress induced inflammation (Spite et al. 2009b), and reduces post-operative and inflammation-induced pain (Xu et al. 2010, Huang et al. 2011). RvD2 inhibits neutrophil infiltration in murine sepsis and peritonitis (Spite et al. 2009a).
17S-HpDHA is also a precursor to neuroprotectin D1 (NPD1; 10R,17S-dihydroxydocosa-4Z,7Z,11E,13E,15Z,19Z-hexaenoic acid). 17S-HpDHA undergoes enzymatic epoxidation followed by hydrolysis to produce NPD1. NPD1 is also coined protectin D1 (PD1) when referring to its production outside the nervous system. PD1/NPD1 has protective effects in the retina (Mukherjee et al. 2004, Sheets et al. 2010), experimental brain ischemia-reperfusion (Marcheselli et al. 2003), and a triple transgenic mouse model of Alzheimer's disease (Zhao et al. 2011). Compared to age-matched neurologically normal human brain, patients with moderate Alzheimer’s disease have two-fold less unesterified DHA in their hippocampi, as well as two-fold lower 15-LO expression and twenty-fold less PD1/NPD1 (Lukiw et al. 2005).
In the second lipoxygenase-initated route using molecular oxygen, maresin 1 (7R,14S-dihydroxydocosa-4Z,8E,10E,12Z,16Z,19-hexaenoic acid; MaR1) is formed via human 12-LO, an enzyme present in macrophages and platelets (Serhan et al. 2009). MaR1 displays the potent anti-inflammatory and proresolving properties of SPM, stimulates tissue regeneration and controls pain (Serhan et al. 2012), yet MaR1 has not yet been reported in the brain.
DHA and its SPM are anti-inflammatory in non-neural tissues, but only indirect evidence exists for their anti-inflammatory effects in the brain (Orr et al. 2012). We therefore tested the effect of increased DHA and 17S-HpDHA levels in a mouse model of local, lipopolysaccharide (LPS)-induced neuroinflammation. Herein we provide the first report of murine hippocampal unesterified DHA levels, captured using high-energy microwave irradiation, in relation to neuroinflammation. Our analyses are focused in the hippocampus due to its sensitivity in our model of neuroinflammation, its importance in neurodegenerative diseases like Alzheimer's disease, and since PD1/NPD1 is present in the mouse and human hippocampi (Lukiw et al. 2005, Marcheselli et al. 2003). In chronic studies we used transgenic and dietary models to increase brain unesterified DHA and found neuroinflammation was significantly attenuated. Using a direct infusion model, here we find that acute increases in unesterified DHA and 17SHpDHA are sufficient to attenuate neuroinflammation. SPM derived from DHA were identified in the hippocampus, with higher concentrations of PD1/NPD1 during attenuated neuroinflammation.
METHODS
All procedures were carried out in accordance to the policies set out by the Canadian Council on Animal Care and were approved by the Animal Ethics Committee at the University of Toronto, Toronto, Canada (Canadian Council on Animal Care. 1993). Mice were housed 3-4 per cage in a facility in which temperature (21°C), humidity and light cycle (12 h light/dark) were controlled, and had ad libitum access to food and water.
Diets
Mice were fed one of three diets: 10% safflower oil diet (SO; D04092701; Research Diets Inc.), or 2% menhaden oil, 8% safflower oil diet (FO; D04092702; Research Diets Inc.), or standard chow (Teklad 2018; Harlan). Both the SO and FO diets are modified AIN-93-G. The SO diet contains 0.03 and 0.09% of EPA and DHA, respectively, while the FO diet contains 1.97 and 1.61% of EPA and DHA, respectively (Supplementary Table 1). The standard chow contained 5.99% α-linolenate (18:3n-3), 0.06% EPA, and 0.06% DHA (Supplementary Table 1). Fatty acid composition was measured by GC-FID.
Animals
Fat-1 mice and wildtype littermates
Experimental mice were obtained by mating male fat-1 mice (C57BL/6 x C3H background) (Kang et al. 2004) with female C57BL/6 wildtype mice from Charles River Laboratories Canada. Genotyping was performed as previously reported (Orr et al. 2010). Male F1 progeny were used in these studies. Due to several generations of backcrossing with C57BL/6, our F1 progeny are 76.2 ± 2.5% C57BL/6 as determined using the Mouse MD Linkage Panel (n=10; Illumina, Inc.). There is no difference between fat-1 mice (76.4 ± 3.3% C57BL/6; n=5) and wildtype littermates (76.1 ± 1.7% C57BL/6; n=5). Dams were fed the SO diet to reduce transfer of n-3 PUFA to offspring via the placenta or milk. Fat-1 and wildtype littermate F1 progeny were weaned at 21 days of age onto either the SO or FO diet, randomly, depending on the study.
C57BL/6 mice
C57BL/6 mice that underwent dietary manipulation were obtained with their dams at 10 days of age from Charles River Laboratories. Dams were immediately placed on the SO diet to reduce n-3 PUFA intake and subsequent transfer to offspring via milk. Mice were weaned at 21 days of age onto either the same SO diet or to the FO diet. C57BL/6 mice that were acutely infused with fatty acids post-LPS were obtained at eleven weeks of age from Charles River Laboratories and fed standard chow.
Total phospholipid and unesterified fatty acid analysis
A separate group of mice from each study was euthanized at 12 weeks of age for hippocampal fatty acid analysis. To maintain integrity of the unesterified fatty acid pool, mice were euthanized by head-focused, high-energy microwave irradiation (6 kW, 0.88–0.99 s, Muromachi brain fixation system, Stoelting Co.). Total lipids were extracted from hippocampi according to the method of Folch (Folch et al. 1957). Total phospholipids and unesterified fatty acids were measured according to previously published methods (Chen et al. 2011).
Intracerebroventricular administration of LPS
At 12 weeks of age, mice were anesthetized with isoflurane gas (3% induction, 1-2% maintenance) and their head secured into a stereotaxic frame (Stoelting). Before the incision was made, 50 μl of 0.1% Sensorcaine was injected subcutaneously at the incision site. The skull was exposed and a small hole was drilled (-1.0 mm medial/lateral and -0.5 mm anterior/posterior from bregma). A 1 μl injection of vehicle (saline) or LPS (5 μg in 1 μl; E. coli serotype 055:B5; Sigma-Aldrich, St. Louis, MO) was infused at a constant rate over a 5 minute period into the right lateral ventricle (-2.3 dorsal/ventral) using a 33-gauge beveled injection needle (World Precision Instruments). The needle was slowly drawn out of the brain to avoid infusate backflow. Twenty-four hours after the injection, mice were euthanized by CO2, brains were excised, and hippocampi were isolated then stored at -80°C until analysis.
Implantation of osmotic pump
One approach required the insertion of an icv catheter attached to an osmotic pump (model 1003D; Alzet, DURECT Corporation) that delivered treatment over 24h immediately post-LPS. Treatments included artificial cerebrospinal fluid (aCSF, vehicle; 150 mM Na, 3 mM K, 1.4 mM Ca, 0.8 mM Mg, 1 mM P, 155 mM Cl), 40 μg of DHA (#90310, Cayman Chemical), 1 μg of 17S-HpDHA (#13185; Cayman Chemical), 40 μg ARA (U-71-A, Nu-Chek Prep Inc.), or 40 μg DPAn-6 (U-102-A, Nu-Chek Prep Inc.). Mice were first treated with acute saline or LPS as described above. Immediately following saline or LPS administration, while still under anesthetic, an icv catheter was placed at the same coordinate and attached to an osmotic pump containing one of the treatments. Osmotic pumps were implanted subcutaneously on the dorsal side, posterior to the head.
Radioactive tracer
Two mice were implanted with an osmotic pump containing 10 μCi of 14C-DHA ([1-14C]-DHA, specific activity: 52 mCi mmol-1) in order to determine where unesterified fatty acids diffuse within the brain. Twenty four hours following catheter implantation, mice were euthanized and the brain dissected into hippocampus, cortex, and the remainder of the brain. Total lipids were extracted and separated by thin layer chromatography to isolate total phospholipid and unesterified fatty acid fractions. Radioactivity was quantified by a Packard TRI-CARB2900TR liquid scintillation analyzer (Packard) with a detector efficiency of 48.8%. Radioactivity was expressed in units of decays per minute (dpm); then converted to nCi (g wet weight tissue)-1.
Gene expression analysis
Total RNA was isolated from hippocampi using Trizol (Invitrogen) according to the manufacturer's protocol. RNA purity and quantity were determined using the 260 nm to 280 nm UV absorbance ratio and 260 nm absorbance, respectively, measured with a Nanodrop 1000 (NanoDrop Technologies Inc.). One microgram of RNA was reverse transcribed using a High Capacity cDNA Reverse Transcription Kit (Applied Biosystems).
Quantitative real-time PCR was performed using the TaqMan Low Density Array (TLDA; Applied Biosystems) on the 7900HT Real-Time PCR System (Applied Biosystems). Briefly, 50 ng of cDNA was diluted with RNAse-free water to a volume of 50 μL and combined with an equal volume of TaqMan Universal PCR Master Mix (Applied Biosystems), then loaded onto a pre-configured 384-well TLDA plate according to the manufacturer's protocol. TaqMan Gene Expression Assays were used to assess ARA cascade enzymes including Group IVA calcium-dependent cytosolic phospholipase A2 (cPLA2; assay ID Mm00447040_m1), cyclooxygenase-2 (COX-2; Mm00478374_m1), and microsomal prostaglandin E synthase (mPGES Mm00452105_m1); cytokines interleukin-1β (IL-1β; Mm00434228_m1), interleukin-6 (IL-6; Mm00446190_m1), and tumor necrosis factor-α (TNF-α; Mm00443258_m1); chemokines chemokine (c-c motif) ligand 2 and 3 (CCL2 and CCL3, respectively; Mm00441242_m1 and Mm00441258_m1, respectively); activated microglial cell markers clusters of differentiation 11b and 45 (CD11b and CD45, respectively; Mm00434455_m1 and Mm00448463_m1, respectively); astrocyte marker glial fibrillary acidic protein (GFAP; Mm00546086_m1 ); and oxidative stress markers inducible nitric oxide synthase (iNOS; Mm00440485_m1) and cytochrome b-245, beta polypeptide (CYBB; Mm00432775_m1). Endogenous controls measured included 18S RNA and phosphoglycerate kinase 1 (PGK1; Mm00435617_m1). All genes were normalized to PGK1. Similar results were found when normalizing to 18S.
Immunohistochemistry
For immunohistochemistry, mice were euthanized by transcardial perfusion with heparin-containing phosphate buffered saline followed by a 4% paraformaldehyde solution. Brains were post-fixed overnight in the same paraformaldehyde solution and then placed in a 30% sucrose solution, frozen in 2-propyl butane (-60 °C) and stored at -80 °C. Fixed frozen brains were sectioned (30 μm) using a cryostat Leica CM1950 (Leica Microsystems).
Fluoro-jade B (FJB) (Millipore) was used to identify degenerating neurons following the protocol provided by the company. FJB positive cells were counted in the hippocampus within the dentate gyrus and quantified as previously described (Choi & Bosetti 2009).
Anti-GFAP (GA5) mouse primary antibody (1:400, Cell Signaling, Beverly, MA) and anti-ionized calcium binding adaptor molecule 1 (Iba1; 2 μg ml-1; Wako Chemicals) were used as markers of astrocytes and macrophages/microglia respectively using the Vectastain ABC kit following the protocol provided by the same company (Vector Laboratories). The number of positive GFAP and Iba1 cells was counted within 0.3 mm2 of dentate gyrus hippocampus area in 3 consecutive sections from each brain; two blinded investigators repeated the counting.
LC-MS/MS
Mouse hippocampi were homogenized by adding 0.5 mL cold methanol. Deuterated internal standards (d4-PGE2 (m/z 355>193), d8-5S-HETE (m/z 327>116), and d4-LTB4 (m/z 339>197)) were added to each homogenate. After protein precipitation for 12 hr, samples were extracted by SPE columns and methyl formate fractions were taken for LC-MS/MS (for detailed methods see (Yang et al. 2011)).
LC-MS/MS was performed with an Agilent 1100 HPLC (Agilent Technologies) equipped with an Agilent Eclipse Plus C-18 column (4.6 mm×50 mm×1.8 μm) paired with an ABI Sciex Instruments 5500 QTRAP linear ion trap triple quadrupole mass spectrometer (Applied Biosystems). Instrument control and data acquisition were performed using AnalystTM 1.5 software (Applied Biosystems). The mobile phase consisted of methanol/water/acetic acid (55/45/0.01; v/v/v) and was ramped to 88/12/0.01 (v/v/v) after 10 min, 100/0/0.01 (v/v/v) after 18 min, and 55:45:0.01 (v/v/v) after 1 minute to wash and equilibrate the column. Mass spectrometry analyses were carried out in negative ion mode using multiple reaction monitoring (MRM) of established specific transitions for 17-HDHA (m/z 343>245), PD1/NPD1 (m/z 359>153), MaR1 (m/z 359>250), RvD1 (m/z 375>215), RvD2 (m/z 375>233), RvD5 (m/z 359>199), 14-HDHA (m/z 343>205), PGE2 (m/z 351>189), PGD2 (m/z 351>189), LXB4 (m/z 351>221), and 15-HETE (m/z 319>179). The criteria used for identification of mediators of interest were matching specific retention time and 6 diagnostic ions to available synthetic standards (Yang et al. 2011, Hong et al. 2007). Quantification was performed using standard calibration curves for each compound, which are linear (r2=0.98-0.99), and determined using mixtures containing 12.5, 25, 50, and 100 pg of each compound. Recovery was calculated using deuterated internal standards.
Statistics
Results are expressed as means ± SEM. Fatty acid concentrations were compared by Student’s t-test. Gene expression and immunohistochemistry means in the first three studies were compared by twoway ANOVA. Significant interactions were further analyzed by one-way ANOVA with Bonferroni's multiple comparisons post-hoc test. In the osmotic pump study, gene expression means were compared by one-way ANOVA with a Bonferroni's multiple comparison post-hoc. PD1/NPD1 was analyzed by Student's t-test to compare treatment (DHA or 17S-HpDHA) to control (aCSF). Statistical significance was set as p<0.05 for all analyses.
RESULTS
Fat-1 mice have higher hippocampal DHA levels
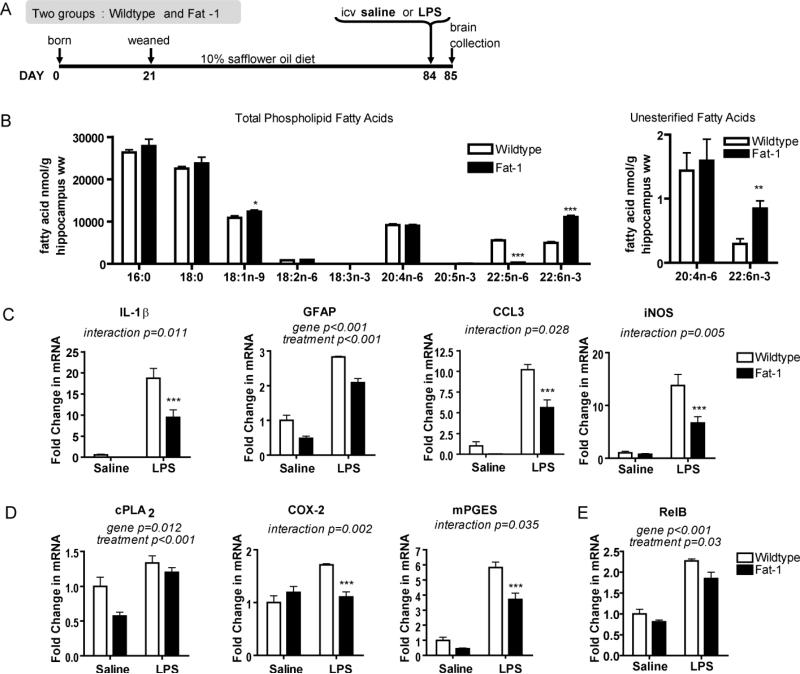
In our first experimental approach we used the fat-1 transgenic mouse, which carries an n-3 fatty acid desaturase from C. elegans that enables the endogenous conversion of n-6 to n-3 fatty acids (Kang et al. 2004). Use of a transgene eliminates dietary confounders that are introduced when modulating n-3 PUFA through different diets. We characterized the brain fatty acid profile of fat-1 mice and their wildtype littermates. All mice were fed a 10% safflower oil (SO) diet from weaning, as were their dams throughout breeding and lactation (Fig. 1A). Under ischemic conditions, calcium-dependent phospholipases A2 cleave fatty acids from membrane phospholipids, dramatically increasing unesterified fatty acid concentrations. Therefore to preserve the integrity of the unesterified fatty acid pool, mice were euthanized by head-focused, high-energy microwave irradiation. Fat-1 mice have twice the amount of DHA in their total phospholipids with a much lower amount of docosapentaenoic acid n-6 (DPAn-6; 22:5n-6) compared to wildtype littermates (p<0.001; Fig. 1B). Wildtype littermates are n-3 PUFA inadequate, indicated by their high level of phospholipid DPAn-6, which is preferentially esterified into membranes in the absence of DHA. There were no other phospholipid fatty acid differences, except for the slightly higher oleic acid (18:1n-9) concentrations in fat-1 mice compared to wildtype littermates (p=0.05).
FIGURE 1.
Fatty acid and neuroinflammatory profile of the fat-1 mouse. (A) Study design. F0 mice (C57BL/6 females and fat-1 males) and F1 progeny (male fat-1 mice and their wildtype littermates) consumed 10% safflower oil (SO) diet. At 12 weeks of age mice received an intracerebroventricular (icv) injection of lipopolysaccharide (LPS) or vehicle (saline). Twenty-four hours following surgery, brains were collected and hippocampi isolated for analysis. (B) Hippocampal wet weight (ww) fatty acid concentrations (nmol g-1) of wildtype and fat-1 mice consuming the SO diet. 18:3n-3 was not detected in either group, 20:5n-3 was not detected in wildtype mice. n=5 independent samples per group. Significantly different means represented by *p<0.05, **p<0.01 and ***p<0.001 (Student's t-test). (C) Hippocampal gene expression of neuroinflammatory markers interleukin-1β (IL-1β), glial fibrillary acidic protein (GFAP), chemokine (c-c motif) ligand 3 (CCL3), and inducible nitric oxide synthase (iNOS) are significantly attenuated in LPS-treated fat-1 mice compared to LPS-treated wildtype littermates. (D) Arachidonic acid cascade markers, calcium-dependent cytosolic phospholipase A2 (cPLA2), cyclooxygenase-2 (COX-2), and microsomal prostaglandin E synthase (mPGES) are significantly down-regulated in fat-1 mice compared to wildtype littermates. (E) Hippocampal gene expression of NF-κB subunit RelB. Gene expression in C, D, and E compared by 2-way ANOVA followed by Bonferroni's post-hoc if a significant interaction was found. n=5-7 independent samples per group. Differences between wildtype and fat-1 within treatment (icv saline or LPS): ***p<0.001. All data are mean ± SEM.
The unesterified fatty acid pool is gaining recognition as the active pool from which fatty acids are metabolized to bioactive mediators, and in the brain it is 5,000-10,000 times smaller than the total phospholipid pool. We found that fat-1 mice have significantly higher levels of unesterified DHA compared to wildtype littermates, but that there is no difference in unesterified ARA (Fig. 1B).
Fat-1 mice display attenuated neuroinflammatory responses
To test if increased DHA is protective against neuroinflammation, fat-1 mice and their wildtype littermates were challenged with an acute 5 μg icv injection of LPS. LPS was injected directly into the left lateral cerebral ventricle of mice because systemic injections cause robust systemic inflammation, changes in body temperature, and increased mortality (Rudaya et al. 2005). We did not observe any significant difference in body temperatures of LPS-treated (36.7 ± 0.4 °C) versus vehicle-treated mice (36.8 ± 0.7 °C), confirming that our LPS dose directly activated brain innate immunity while minimizing other side effects like altered temperature or peripheral immune signals to the brain that might be attenuated by increases in peripheral DHA (Yong & Rivest 2009, Calder 2009). Saline-infused mice were used to control for neuroinflammation resulting from surgery. The study design is described in Fig. 1A, with age at surgery, inflammatory agent, dose, and time points based on published literature (Choi et al. 2008).
We found either i) a significant interactive effect of genotype (fat-1 or wildtype littermates) and treatment (saline or LPS), or ii) significant main effects of genotype and treatment on the gene expression of cytokines, chemokines, and other neuroinflammatory markers, all indicating an attenuated neuroinflammatory response in fat-1 mice. The attenuated increase in pro-inflammatory cytokine IL-1β, astrocyte marker GFAP, chemokine CCL3, and oxidative stress marker iNOS in LPS-treated fat-1 mice compared to LPS-treated wildtype littermates is shown in Fig. 1C. Similar results were seen with pro-inflammatory cytokines IL-6 and TNF-α, chemokine CCL2, activated microglia markers CD11b and CD45, and microbicidal oxidase system component CYBB (data not shown). The decreased expression of these pro-inflammatory markers is partly explained by a decreased expression of RelB, a subunit of NF-kB, a pro-inflammatory transcription factor (Fig 1E).
The ARA cascade is activated during neuroinflammation and mRNA and protein levels of ARA cascade enzymes are upregulated in response to DHA deprivation (Choi et al. 2008, Rao et al. 2007a). cPLA2 preferentially cleaves ARA out of the phospholipid membrane into its unesterified form where it is available for metabolism. Fat-1 mice have significantly lower cPLA2 gene expression in the hippocampus compared to wildtype littermates (Fig. 1D). COX-2 and mPGES, in sequence, metabolize ARA to prostaglandin E2 (PGE2). LPS-treated fat-1 mice have significantly lower gene expression of both COX-2 and mPGES compared to LPS-treated wildtype littermates (Fig. 1D).
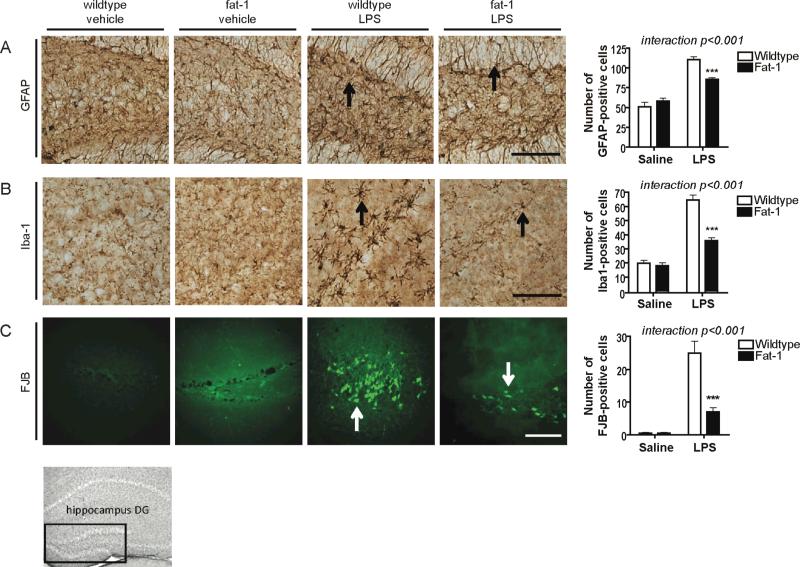
In addition to gene expression measures, we measured glial activation and neuronal degeneration by immunohistochemistry (Fig. 2). GFAP and Iba1 were measured in the dentate gyrus. GFAP protein reflected gene expression results in that LPS-treated fat-1 mice had a significantly attenuated increase compared to LPS-treated wildtype littermates (Fig. 2A). Iba1 is expressed on the surface of microglia and its expression increases with microglial activation. Iba1 protein expression showed lowered microglial activation in fat-1 mice following LPS compared to wildtype littermates (Fig. 2B), confirming CD11b and CD45 gene expression measures. Similar results were found with Iba1 and GFAP in the CA1 region of the hippocampus (data not shown). FJB staining indicates significantly less neuronal degeneration in the dentate gyrus of fat-1 mice following LPS compared to wildtype littermates (Fig. 3C). Fat-1 mice have increased neurogenesis compared to their wildtype littermates (He et al. 2009) therefore the decreased neuronal degeneration cannot be attributed to a decreased number of initial neurons. As expected, there was no marked neuronal degeneration in saline treated wildtype and fat-1 mice. Interestingly, saline-treated fat-1 mice had significantly lower gene expression of CYBB, GFAP, CD11b, CD45, mPGES, and cPLA2 compared to saline-treated wildtype littermates (Student's t-test, p<0.05), indicating that DHA affects inflammation pathways separate from its established roles in neurotrophic and neural apoptosis pathways (Akbar et al. 2005, Lukiw et al. 2005, Rao et al. 2007b, Kim et al. 2011b, Lafourcade et al. 2011).
FIGURE 2.
Effect of fat-1 genotype on neuroinflammatory and neurodegenerative markers in the dentate gyrus (DG) by immunohistochemistry (IHC). Representative photomicrographs and quantitative analysis of (A) glial fibrillary acidic protein (GFAP), (B) ionized calcium binding adaptor molecule 1 (Iba1), and (C) fluoro-jade B (FJB) in wildtype and fat-1 mice 24 h after icv injection of vehicle (saline) or LPS. Analyzed by 2-way ANOVA followed by 1-way ANOVA with Bonferroni's post-hoc if a significant interaction was found. Differences between wildtype and fat-1 within treatment (icv saline or LPS): ***p<0.001. n=8 independent samples per group. Scale bars = 100 μm.
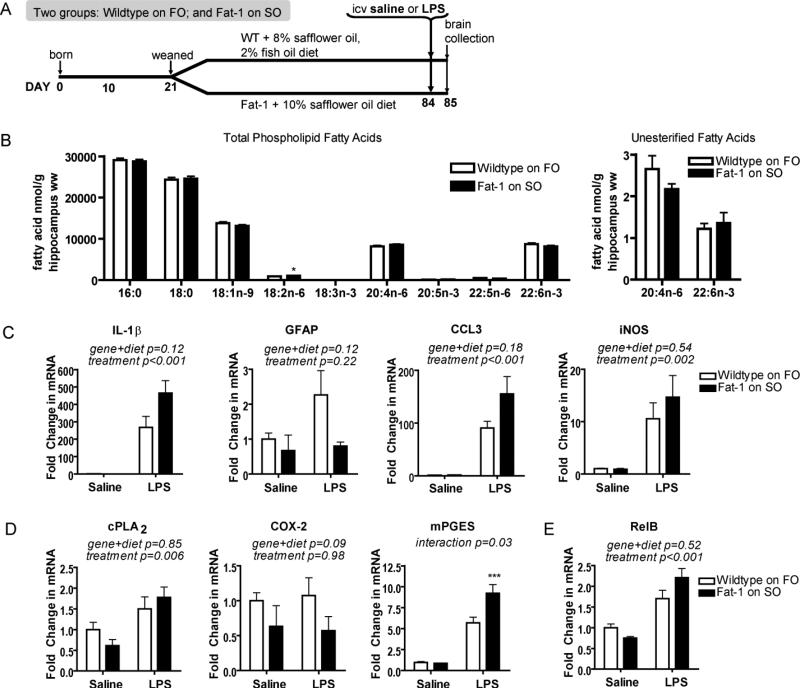
FIGURE 3.
Fatty acid and neuroinflammatory profile of wildtype littermates fed FO compared to fat-1 mice. (A) Study design is the same as in Fig. 1A except at weaning wildtype mice are placed on a 2% fish oil, 8% safflower oil (FO) diet. Fat-1 mice remained on a 10% safflower oil diet (SO). (B) Hippocampal wet weight (ww) fatty acid concentrations (nmol g-1) of FO-fed wildtype mice and SO-fed fat-1 mice. 18:3n-3 was not detected in either group. n= 9 independent samples per group. Significantly different means represented by *p<0.05 (Student's t-test). (C) Hippocampal neuroinflammatory responses of SO-fed fat-1 mice and FO-fed wildtype littermates 24 h following icv administration of lipopolysaccharide (LPS) or vehicle (saline). Gene expression of neuroinflammatory markers interleukin-1β (IL-1β), glial fibrillary acidic protein (GFAP), chemokine (c-c motif) ligand 3 (CCL3), and inducible nitric oxide synthase (iNOS) is not significantly different between LPS-treated SO-fed fat-1 mice and LPS-treated FO-fed wildtype littermates. (D) Arachidonic acid cascade markers, calcium-dependent cytosolic phospholipase A2 (cPLA2), cyclooxygenase-2 (COX-2), and microsomal prostaglandin E synthase (mPGES) are similar in SO-fed fat-1 mice and FO-fed wildtype littermates. (E) Hippocampal gene expression of NF-kB subunit RelB. Gene expression in C, D, and E compared by 2-way ANOVA followed by Bonferroni's post-hoc if a significant interaction was found. Differences between fat-1 and wildtype littermates within treatment (icv saline or LPS): ***p<0.001. n=4-6 independent samples per group. All data are mean ± SEM.
FO diet restores hippocampal DHA of wildtype littermates
The fat-1 mouse is a useful model of increased body n-3 PUFA levels, but as a transgenic model we must be cautious not to over-interpret its applicability to physiologically relevant effects. Further, there may be off-targets of the transgene that can account for the reduced neuroinflammation. In our second experimental approach we address these issues by using a second, dietary model to increase brain n-3 PUFA. Earlier findings from our lab show that whole brain total DHA concentrations of fat-1 mice can be mimicked by feeding a 2% fish oil, 8% safflower oil (FO) diet to their n-3 PUFA inadequate wildtype littermates (Orr et al. 2010). For this experiment, wildtype littermates were weaned onto the FO diet, while fat-1 mice were maintained on the SO diet (see study design Fig. 3A). As expected, there were no significant differences in phospholipid fatty acid profile, or unesterified ARA or DHA between fat-1 mice on the SO and wildtype littermates on the FO diet, except a statistically higher level of phospholipid linoleic acid (18:2n-6) in fat-1 mice on the SO diet compared to wildtype littermates on the FO diet (Fig. 3B). Having raised hippocampal DHA levels through a second approach, we could go on to test if abrogating the difference in hippocampal DHA levels also abrogated the difference in the neuroinflammatory response to LPS.
FO diet restores protection in wildtype littermates
To determine if mimicking the fatty acid profile of fat-1 mice is sufficient to attenuate the neuroinflammatory response in wildtype littermates fed the FO diet, we challenged mice with icv LPS. We found no significant differences in the expression of the IL-1β, GFAP, CCL3, or iNOS genes between LPS-treated wildtype littermates on the FO diet and LPS-treated fat-1 mice on the SO diet (Fig. 3C). There was also no difference in RelB gene expression (Fig. 3E). The only significant finding was an effect of LPS. There were similar findings in IL-6, TNF-β, CD11b, CD45, CCL2, and CYBB where the only significant effect was of LPS treatment (data not shown). cPLA2 also showed a similar effect (Fig. 3D). COX-2 did not respond to LPS. mPGES, on the other hand, was significantly attenuated in wildtype littermates fed the FO diet compared to fat-1 mice fed SO diet. Interestingly, compared to icv saline treated wildtype littermates on SO diet, icv saline treated wildtype littermates on FO diet experience less neuroinflammation, exemplified by IL-1β levels of saline- and LPS-treated mice (Fig. 1C and Fig. 3C).
FO diet increases phospholipid DHA in n-3 PUFA adequate mice
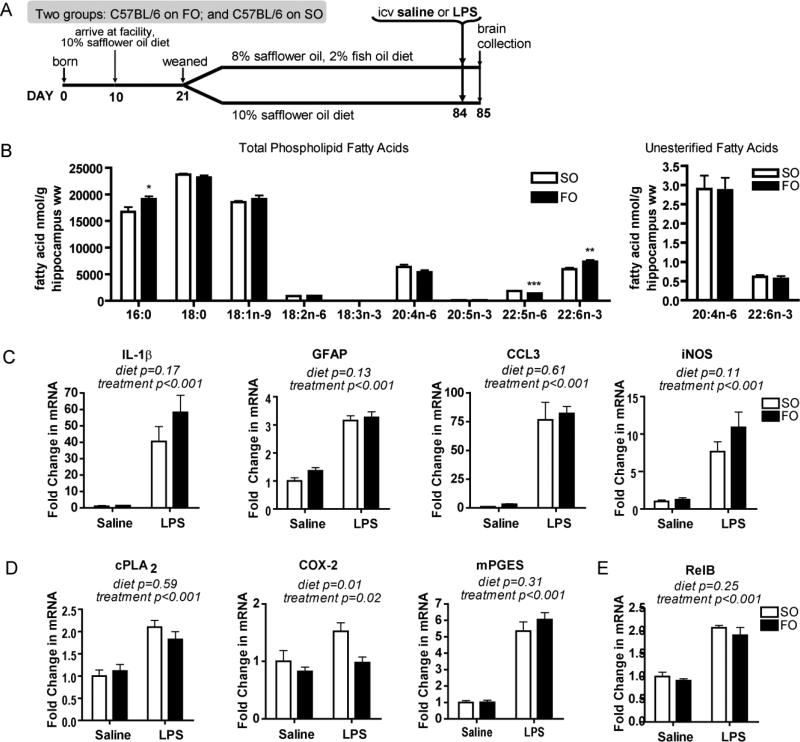
Having shown that the fat-1 mouse has an attenuated neuroinflammatory response, which is mimicked by feeding fish oil to its n-3 PUFA inadequate wildtype littermates, we set out to determine if a dietary intervention in n-3 PUFA adequate C57BL/6 mice could attenuate neuroinflammation. Brain DHA has a long half-life that is increased in times of n-3 PUFA deprivation (DeMar et al. 2004). To address this, C57BL/6 mice and their dams were placed on the SO diet at 10 days of age upon arrival to our facility (Fig. 4A). At weaning, mice were randomized to either the SO or FO diet. A subset of mice was euthanized at 12 weeks of age to determine their hippocampal fatty acid profile. FO-fed C57BL/6 mice had significantly higher phospholipid DHA levels of 7,315 ± 244 ng/g hippocampus compared to 5,951 ± 339 ng/g hippocampus in SO-fed C57BL/6 mice (p<0.01), and an expected equally lowered level of phospholipid DPAn-6 (p<0.001). There was no significant difference in either unesterified ARA or DHA levels (Fig. 4B). The ability to modulate unesterified DHA when feeding the FO diet to wildtype littermates of fat-1 mice (Fig. 3), when these same diets did not affect C57BL/6 mice, is likely due to the state of n-3 PUFA inadequacy in wildtype littermates of fat-1 mice prior to n-3 PUFA feeding. Of note, basal unesterified fatty acid amounts are difficult to compare between studies due to their low concentrations, and experiment-to-experiment variability.
FIGURE 4.
Fatty acid and neuroinflammatory profile of C57BL/6 mice on SO and FO diets. (A) Study design. C57BL/6 mice consumed 10% safflower oil diet (SO) from 10 days of age. At weaning mice were randomized to either the SO diet or a 2% fish oil, 8% safflower oil (FO) diet. Remainder of design is similar to Fig. 1A. (B) Hippocampal (wet weight, ww) fatty acid concentrations (nmol g-1) of SO- or FO-fed C57BL/6 mice. 18:3n-3 was not detected in either group. n= 8 independent samples per group. Significantly different means represented by *p<0.05, **p<0.01, ***p<0.001 (Student's t-test). (C) Hippocampal neuroinflammatory responses of SO- or FO-fed C57BL/6 mice 24 h following icv administration of lipopolysaccharide (LPS) or vehicle (saline). Gene expression of neuroinflammatory markers, interleukin-1β (IL-1β), glial fibrillary acidic protein (GFAP), chemokine (c-c motif) ligand 3 (CCL3), and inducible nitric oxide synthase (iNOS) is not significantly different between LPS-treated SO-fed mice and LPS-treated FO-fed mice. (D) Arachidonic acid cascade markers, calcium-dependent cytosolic phospholipase A2 (cPLA2), cyclooxygenase-2 (COX-2), and microsomal prostaglandin E synthase (mPGES) are similar in SO-fed and FO-fed C57BL/6. (E) Hippocampal gene expression of NF-kB subunit RelB. Gene expression in C, D, and E compared by 2-way ANOVA followed by Bonferroni’s post-hoc if a significant interaction was found. n=6-8 independent samples per group. All data are mean ± SEM.
Neuroinflammation is not altered by increased phospholipid DHA
To determine whether a 23% increase in total phospholipid DHA, but not unesterified DHA, results in an attenuated neuroinflammatory response, we challenged 12 week old FO- and SO-fed C57BL/6 mice with an icv injection of LPS (Fig. 4A). There was no difference in the neuroinflammatory response of FO-fed mice compared to SO-fed mice as measured by a complement of gene expression markers including IL-1β, GFAP, CCL3, iNOS, and RelB (Fig. 4). There were also no differences in CCL2, CYBB, IL-6, CD11b, CD45, or TNF-β (data not shown). Also, there were no differences in ARA cascade enzymes, with the exception of COX-2 (Fig. 4D), which is known to decrease with increased n-3 PUFA brain levels (Rao et al. 2007a). These results indicate that modulating the unesterified DHA pool is necessary to affect neuroinflammation.
icv DHA or 17S-HpDHA attenuates neuroinflammation
In the earlier studies, chronically increased unesterified DHA resulted in attenuated neuroinflammatory responses. Chronic increases in body n-3 PUFA levels can have effects on many pathways, including on glucose tolerance which can in turn affect astrocyte function (Smith et al. 2010). We sought to determine if an acute increase in hippocampal unesterified DHA during neuroinflammation would attenuate the response, in order to minimize the impact of n-3 PUFA modulation on other, largely non-inflammatory pathways. N-3 PUFA adequate C57BL/6 mice on standard chow were challenged with LPS then immediately implanted with an icv catheter delivering 40 μg of DHA, 1 μg of its metabolite 17SHpDHA, or vehicle (aCSF) over 24 hours (Fig. 5A). Doses are in the range of those reported to effect ischemia-reperfusion (Marcheselli et al. 2003). 17S-HpDHA was chosen since it is the precursor to both protectins and D-series resolvins. To ensure that icv-delivered DHA reaches the hippocampus, we implanted two mice with catheters delivering 10 μCi of 14C-DHA. Radioactivity was detectible in hippocampal unesterified fatty acids, with low but detectible levels also found in total phospholipids (Supplementary Figure 1), indicating we could test the hypothesis that increased hippocampal unesterified DHA levels would attenuate the neuroinflammatory response. No previous study has measured the distribution of icv-infused unesterified DHA in mice, however, other fatty acids like ARA and erucic acid are more highly esterified upon entry into the brain (Golovko & Murphy 2006).
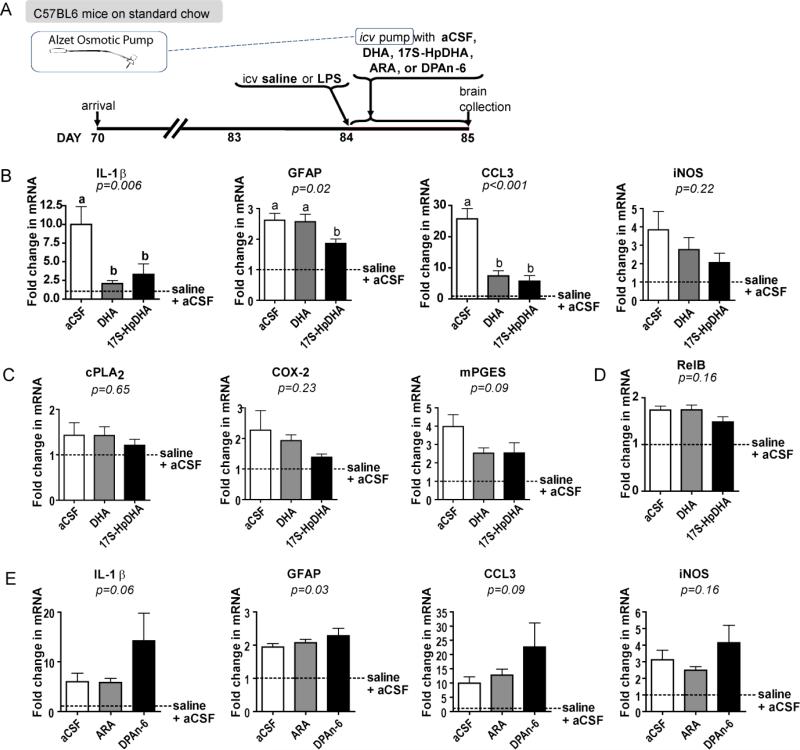
FIGURE 5.
Neuroinflammatory profile of mice infused with DHA or 17-HpDHA. (A) At 12 weeks of age mice received an intracerebroventricular (icv) injection of lipopolysaccharide (LPS) followed immediately by the implantation of an icv catheter at the same coordinate. Catheters were attached to pumps containing 40 μg DHA, 1 μg 17S-HpDHA, 40 μg ARA, 40 μg DPAn-6, or vehicle (aCSF). (B) Hippocampal neuroinflammatory responses of mice treated with aCSF [white bars], 40 μg of DHA [gray bars], or 1 μg 17S-HpDHA [black bars] for 24 h post-LPS. Gene expression of neuroinflammatory markers shown are interleukin-1β (IL-1β), glial fibrillary acidic protein (GFAP), chemokine (c-c motif) ligand 3 (CCL3), and inducible nitric oxide synthase (iNOS). There is a significantly attenuated neuroinflammatory response in mice treated with DHA or 17S-HpDHA post-LPS compared to vehicle with no effect on the ARA cascade markers (C) cytosolic phospholipase A2 (cPLA2), cyclooxygenase-2 (COX-2), and microsomal prostaglandin E synthase (mPGES). (D) Hippocampal gene expression of NF-kB subunit RelB. (E) In a separate study, mice were implanted with a pump containing aCSF [white bars], ARA [gray bars], or DPAn-6 [black bars]. Gene expression of neuroinflammatory markers shown are IL-1β, GFAP, CCL3, and iNOS. ARA and DPAn-6 have no effect on neuroinflammatory markers. One-way ANOVA p values are italicized in each graph; letters signify a significant (p<0.05) difference between groups by Bonferroni's multiple comparisons post-hoc test. Fold change is relative to mice that underwent a sham surgery (icv injection of saline then implantation of an aCSF-containing pump [dotted line]. n=4-5 independent samples per group. All data are mean ± SEM.
Expression of neuroinflammatory markers including IL-1β and CCL3 were significantly attenuated in the hippocampi of DHA- and 17S-HpDHA-infused mice following LPS compared to control (Fig. 5B). Similar results were seen for CYBB, CD45, and TNF-α (data not shown). 17S-HpDHA alone attenuated GFAP (Fig. 5B) and CD11b (data not shown). CCL2, iNOS, IL-6, and RelB were not significantly altered by DHA or 17S-HpDHA treatment post-LPS, indicating chronic increases in unesterified DHA may have a more broad effect on inflammatory pathways compared to acute increases in unesterified DHA and 17S-HpDHA. Interestingly, acute infusion of DHA or 17S-HpDHA did not uniformly modulate ARA cascade enzyme gene expression as was seen in our chronic transgenic and dietary models (Fig. 5C); suggesting that down-regulating the ARA cascade is not necessary for protection.
DHA and 17S-HpDHA selectively attenuate neuroinflammation
To determine if the attenuation of neuroinflammation observed in mice infused with DHA and 17S-HpDHA is selective, we infused mice with 40 μg of ARA, 40 μg of DPAn-6, or vehicle in the same study design. There was no significant difference in the gene expression of neuroinflammatory markers (Fig. 5D). GFAP was one exception with significant differences between treatments (p=0.03).
SPM in the hippocampus
Novel families of bioactive lipid mediators were recently identified and characterized (Serhan et al. 2008, Serhan et al. 2012, Hong et al. 2003). These DHA-derived autacoids, termed resolvins, protectins, and maresins, are thought to underlie most of the protective and beneficial actions of DHA in settings where inflammation is involved (Serhan et al. 2008, Serhan et al. 2012). In this study, we analyzed these lipid mediators using LC-MS/MS in samples of the mouse hippocampus (Fig. 6). Mice were euthanized with head-focused, high-energy microwave irradiation. In initial experiments we established that several lipid mediators increase dramatically in the hippocampus during ischemia at death and are not degraded by microwave irradiation, consistent with previous reports (Murphy 2010, Golovko & Murphy 2008).
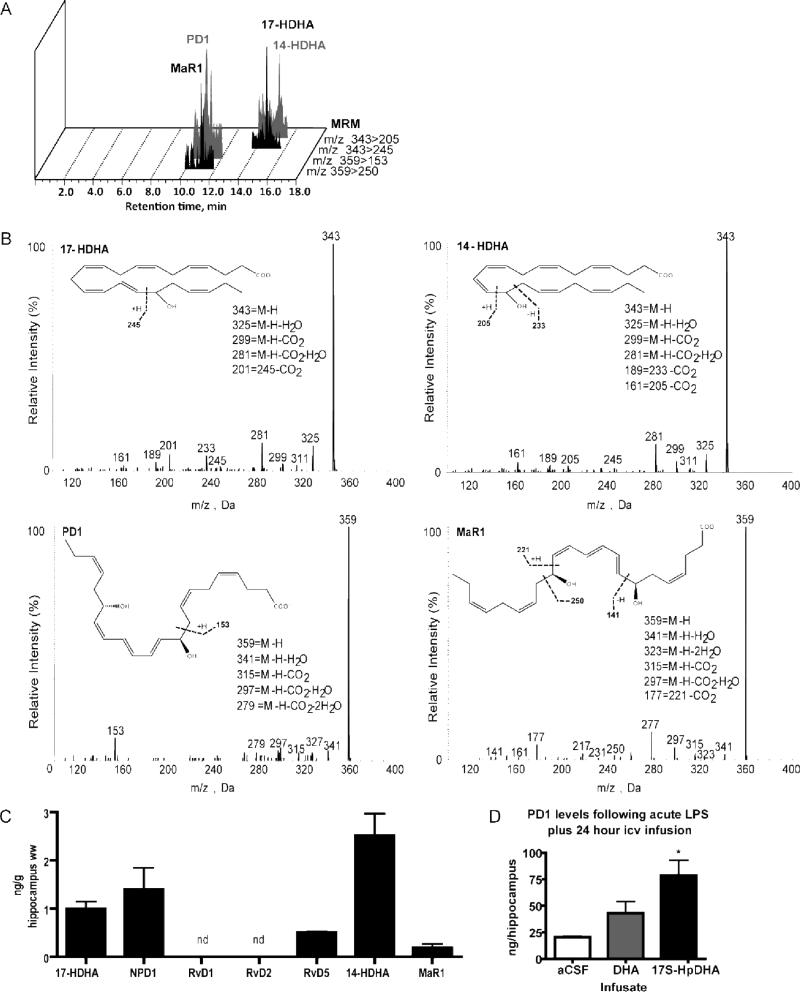
FIGURE 6.
Specialized proresolving mediators (SPM) derived from DHA in the mouse hippocampus: LC-MS/MS lipid mediator identification. (A) MRM chromatograms (343>245, 343>205, 359>153 and 359>250). (B) Representative tandem mass spectra of 17-hydroxy-DHA (17-HDHA), 14-hydroxy-DHA (14-HDHA), neuroprotectin D1 (PD1/NPD1), and maresin1 (MaR1) in samples of mouse hippocampus. (C) DHA-derived SPM in the mouse hippocampus; n=3. (D) Mice were acutely injected icv with 5 μg LPS then implanted with an icv pump containing artificial cerebrospinal fluid (aCSF), 40 μg DHA, or 1 μg 17S-hydroperoxy-DHA (17S-HpDHA) (see treatment design Fig. 5A). PD1/NPD1 measured by LCMS/MS after 24 h. PD1/NPD1 levels were significantly increased with 17S-HpDHA infusion compared to aCSF (p<0.01). Unesterified DHA treatment did not increase PD1/NPD1 levels (p=0.07). n=2 independent samples per group. All results are expressed as the mean ± SEM.
PD1/NPD1 was identified in the mouse hippocampus (Fig. 6C). 17-hydroxy-DHA (17-HDHA), a biosynthesis marker of 17S-HpDHA production, was also identified. Interestingly, for the first time, 14-hydroxy-DHA (14-HDHA), the marker for 14S-HpDHA production the precursor of MaR1, and MaR1 were found in the mouse hippocampus. RvD5 was identified, but neither RvD1 nor RvD2, adding these present data to initial experiments showing RvD1 and RvD2 are not present at basal conditions in the hippocampus nor in response to ischemia at death (Fig. 6C). The absence of RvD1 and RvD2 suggests that they are not responsible for the attenuated neuroinflammation in our experiments and possibly that the leukocytes that produce them did not infiltrate the tissue.
As expected, prostaglandin D2 (PGD2) and PGE2 were present in the hippocampus at low levels (Supplementary Figure 2B). Lipoxin B4 (LXB4) was also identified in the hippocampus along with 15-hydroxyeicosatetraenoic acid (15-HETE), a marker of the LX pathway (supplementary Fig. 2C). EPA-derived product resolvin E1 as well as 5- and 18-hydroxy-EPA were detected in the hippocampus. This was unexpected since it is well established that EPA itself occurs at low to nearly nondetectible levels in neural tissue (Chen et al. 2011). E-series resolvins and mono-hydroxy-EPAs, which possess anti-inflammatory properties in non-neural tissues, may therefore play an important role in brain inflammation and should be explored in future research.
PD1/NPD1 increases with 17S-HpDHA administration
We established that neuroinflammation could be attenuated by icv infusion of unesterified DHA and 17S-HpDHA for 24 hours post-LPS (Fig. 5). We treated mice according to the described study design (Fig. 5A) to determine whether PD1/NPD1 is increased in the hippocampus of mice infused with unesterified DHA or 17S-HpDHA following LPS. There was an increase in hippocampal PD1/NPD1 levels in mice treated with 17S-HpDHA compared to control (p=0.01) (Fig. 6D). PD1/NPD1 in unesterified DHA-treated mice was not increased (p=0.07). There was no difference in brain PD1 levels between unesterified DHA and 17S-HpDHA-infused mice.
DISCUSSION
DHA protects against neuronal damage in several models of brain injury and disease, however, this study was the first to show that brain DHA, specifically unesterified DHA, directly attenuates neuroinflammation. Through a series of chronic and acute studies we have individually increased phospholipid and unesterified DHA levels and identified unesterified DHA as necessary to attenuate neuroinflammation. Increases in phospholipid DHA are not sufficient to attenuate neuroinflammation, although this pool is an important source of unesterified DHA through deacylation. It is important to note that DHA release from the phospholipid membrane is enzymatically-mediated via PLA2, possibly explaining the discrepancy between these pools. Unesterified DHA is a precursor to several families of SPM, which are each anti-inflammatory, pro-resolving molecules. We identified SPM 14-HDHA, MaR1, and RvD5 in the brain for the first time, but neither RvD1 nor RvD2, demonstrating that DHA metabolism in the brain is unique from other tissues and requires independent study. The production of DHA-derived SPM, exemplified by PD1/NPD1, appears to be augmented by directly infusing the brain with their precursor 17S-HpDHA. 17S-HpDHA-infused mice had the most attenuated neuroinflammation, suggesting that DHA is anti-neuroinflammatory, at least in part, via its local conversion to SPM.
This study is unique because we injected LPS directly into the brain lateral ventricle, therefore initiating a primarily local brain inflammatory response. Furthermore, LPS-induced increases in COX-2, mPGES and IL-1β mRNA were of a similar magnitude to those reported in post-mortem brain tissues from patients with bipolar disorder or Alzheimer's disease (Lukiw et al. 2005, Kim et al. 2011a). Earlier studies have found that DHA and DHA-derived lipid mediators decrease inflammatory markers in the central nervous system following systemic LPS administration (Mingam et al. 2008), brain ischemiareperfusion (Lalancette-Hebert et al. 2011, Marcheselli et al. 2003) and spinal cord injury (Huang et al. 2007), however, we cannot conclude from these earlier studies that DHA was acting directly in neuroinflammatory pathways, since they also show a consistent attenuation of primary injury (Orr et al. 2012). Further, the relative attenuation of neuroinflammatory markers in mice with higher levels of unesterified DHA (Fig. 1 and 5) are of a similar magnitude as the attenuation seen in these other models (Orr et al. 2012). Downregulation of RelB expression suggests that the anti-inflammatory effect of unesterified DHA is partly mediated via a downregulation of NF-kB signaling. The present results provide evidence that unesterified DHA directly attenuates neuroinflammation. Increasing phospholipid DHA by 23% (Fig. 4B) is not sufficient to attenuate neuroinflammation, raising the importance of DHA pool differences in studies measuring the effect of brain DHA concentrations on a biological outcome. 17SHpDHA also directly attenuates neuroinflammation, suggesting that unesterified DHA is anti-neuroinflammatory at least in part by acting as a precursor to bioactive SPM. The brain SPM levels of mice following chronic modulations such as in our genetic and dietary models would help determine to what degree these bioactive mediators were responsible for the anti-neuroinflammatory effects observed, and should be addressed in future studies. Interestingly, unesterified DHA and 17S-HpDHA have protective effects in n-3 PUFA adequate mice, arguing against the hypothesis that the use of n-3 PUFA is limited to conditions of inadequacy.
Only recently has there been an interest in enzymatically oxygenated n-3 PUFA-derived SPM. To date, complete lipid mediator profiles have been established in select tissues and disease models. PD1/NPD1 and 17-HDHA have been observed in the hippocampus following ischemia-reperfusion (Marcheselli et al. 2003), PD1/NPD1 in the hippocampus of a triple transgenic mouse model of Alzheimer's disease (Zhao et al. 2011), and PD1/NPD1 in the hippocampus of patients with Alzheimer's disease and neurologically normal controls (Lukiw et al. 2005). Notably, there is a lack of RvD1 and RvD2 in the hippocampus, whether under basal conditions or in response to ischemia, therefore it is unlikely that they mediated the protection from neuroinflammation in our LPS model. Because 17SHpDHA treatment mimicked the gene expression effects of increasing unesterified DHA and also appeared to enhance the production of PD1/NPD1, it suggests that the 17S-HpDHA conversion to PD1/NPD1 pathway is responsible for a portion of the protective impact of unesterified DHA in our LPS models.
One confounder when chronically increasing brain DHA levels is the closely related decrease in DPAn-6 levels, an association that was confirmed in our studies. Little is known about the biological effect of DPAn-6 (Akbar et al. 2005). In addition, chronically reduced brain DHA levels increase ARA metabolism and may exacerbate neuroinflammation indirectly via this mechanism (Rao et al. 2007a). Several ARA cascade enzymes and pro-inflammatory ARA mediators are upregulated in response to icv LPS, including increased activity and mRNA of cPLA2, ARA turnover, and PGE2 levels (Choi et al. 2008, Rosenberger et al. 2004), while inhibiting ARA metabolism reduces neuroinflammatory markers (Choi et al. 2008, Basselin et al. 2011, Golovko et al. 2009). The results of our acute icv infusion of 17SHpDHA allow us to attribute direct anti-neuroinflammatory effects to DHA-derived biosynthesis of local autacoids. Not all inflammatory markers were altered by unesterified DHA or 17S-HpDHA infusion, including genes of the ARA cascade (Fig. 5), suggesting that some of the anti-neuroinflammatory effects of DHA observed in our chronic study using fat-1 mice (Fig. 1) are due to chronic modulation of inflammatory pathways including the ARA cascade and its prostaglandin products. Finally, ARA and DPAn-6 were injected acutely. There was no effect of ARA or DPAn-6. Thus, unesterified DHA and 17SHpDHA are attenuating neuroinflammation, although chronic modulation of ARA pathways and a potentially detrimental effect of DPAn-6 are potential contributing mechanisms and cannot be discounted.
The present results show that chronically low DHA predisposes the brain to more robust and damaging icv LPS-induced inflammation. This may be one mechanism by which n-3 PUFA supplementation is beneficial in the prevention and treatment of chronic neurological disorders with a neuroinflammatory component. Direct infusion of unesterified DHA or 17S-HpDHA also attenuates neuroinflammation, elucidating novel pathways in the brain through which DHA is exerting its anti-inflammatory effects.
Supplementary Material
ACKNOWLEDGEMENTS
The authors thank Thad Vickery and Jonathan FitzGerald (CNS lab BWH), as well as Liz Cumyn (HTM lab) for their excellent technical assistance. The authors also thank Dr. Fei Gao for providing helpful discussions.
FUNDING
This work was supported by the Canadian Institutes of Health Research [RPB]; the International Life Sciences Institute [RPB]; the James H Cummings Foundation [RPB]; and the Scottish Rite Charitable Foundation [RPB]. This work was also supported by the National Institutes of Health [grant number 1P01GM095467 to CNS]. SKO received financial support from the Natural Sciences and Engineering Research Council of Canada.
REFERENCES
- Aid S, Bosetti F. Targeting cyclooxygenases-1 and -2 in neuroinflammation: Therapeutic implications. Biochimie. 2011;93:46–51. doi: 10.1016/j.biochi.2010.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akbar M, Calderon F, Wen Z, Kim HY. Docosahexaenoic acid: a positive modulator of Akt signaling in neuronal survival. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:10858–10863. doi: 10.1073/pnas.0502903102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Appleton KM, Rogers PJ, Ness AR. Updated systematic review and meta-analysis of the effects of n-3 long-chain polyunsaturated fatty acids on depressed mood. The American journal of clinical nutrition. 2010;91:757–770. doi: 10.3945/ajcn.2009.28313. [DOI] [PubMed] [Google Scholar]
- Balanza-Martinez V, Fries GR, Colpo GD, Silveira PP, Portella AK, Tabares-Seisdedos R, Kapczinski F. Therapeutic use of omega-3 fatty acids in bipolar disorder. Expert Rev Neurother. 2011;11:1029–1047. doi: 10.1586/ern.11.42. [DOI] [PubMed] [Google Scholar]
- Basselin M, Ramadan E, Chen M, Rapoport SI. Anti-inflammatory effects of chronic aspirin on brain arachidonic acid metabolites. Neurochemical research. 2011;36:139–145. doi: 10.1007/s11064-010-0282-4. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Calder PC. Polyunsaturated fatty acids and inflammatory processes: new twists in an old tale. Biochimie. 2009 doi: 10.1016/j.biochi.2009.01.008. [DOI] [PubMed] [Google Scholar]
- Calon F. Omega-3 polyunsaturated fatty acids in Alzheimer's disease: key questions and partial answers. Curr Alzheimer Res. 2011;8:470–478. doi: 10.2174/156720511796391881. [DOI] [PubMed] [Google Scholar]
- Canadian Council on Animal Care . Guide to the care and use of experimental animals. Canadian Council on Animal Care; Ottawa: 1993. [Google Scholar]
- Chen CT, Liu Z, Bazinet RP. Rapid de-esterification and loss of eicosapentaenoic acid from rat brain phospholipids: an intracerebroventricular study. J Neurochem. 2011;116:363–373. doi: 10.1111/j.1471-4159.2010.07116.x. [DOI] [PubMed] [Google Scholar]
- Choi SH, Bosetti F. Cyclooxygenase-1 null mice show reduced neuroinflammation in response to beta-amyloid. Aging (Albany NY) 2009;1:234–244. doi: 10.18632/aging.100021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi SH, Langenbach R, Bosetti F. Genetic deletion or pharmacological inhibition of cyclooxygenase-1 attenuate lipopolysaccharide-induced inflammatory response and brain injury. Faseb J. 2008;22:1491–1501. doi: 10.1096/fj.07-9411com. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole GM, Ma QL, Frautschy SA. Dietary fatty acids and the aging brain. Nutr Rev. 2010;68(Suppl 2):S102–111. doi: 10.1111/j.1753-4887.2010.00345.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawford MA, Leigh Broadhurst C, Guest M, Nagar A, Wang Y, Ghebremeskel K, Schmidt WF. A quantum theory for the irreplaceable role of docosahexaenoic acid in neural cell signalling throughout evolution. Prostaglandins, leukotrienes, and essential fatty acids. 2013;88:5–13. doi: 10.1016/j.plefa.2012.08.005. [DOI] [PubMed] [Google Scholar]
- DeMar JC, Jr., Ma K, Bell JM, Rapoport SI. Half-lives of docosahexaenoic acid in rat brain phospholipids are prolonged by 15 weeks of nutritional deprivation of n-3 polyunsaturated fatty acids. J Neurochem. 2004;91:1125–1137. doi: 10.1111/j.1471-4159.2004.02789.x. [DOI] [PubMed] [Google Scholar]
- Duffield JS, Hong S, Vaidya VS, Lu Y, Fredman G, Serhan CN, Bonventre JV. Resolvin D series and protectin D1 mitigate acute kidney injury. J Immunol. 2006;177:5902–5911. doi: 10.4049/jimmunol.177.9.5902. [DOI] [PubMed] [Google Scholar]
- Folch J, Lees M, Sloane Stanley GH. A simple method for the isolation and purification of total lipides from animal tissues. The Journal of biological chemistry. 1957;226:497–509. [PubMed] [Google Scholar]
- Golovko MY, Barcelo-Coblijn G, Castagnet PI, Austin S, Combs CK, Murphy EJ. The role of alpha-synuclein in brain lipid metabolism: a downstream impact on brain inflammatory response. Mol Cell Biochem. 2009;326:55–66. doi: 10.1007/s11010-008-0008-y. [DOI] [PubMed] [Google Scholar]
- Golovko MY, Murphy EJ. Uptake and metabolism of plasma-derived erucic acid by rat brain. J Lipid Res. 2006;47:1289–1297. doi: 10.1194/jlr.M600029-JLR200. [DOI] [PubMed] [Google Scholar]
- Golovko MY, Murphy EJ. An improved LC-MS/MS procedure for brain prostanoid analysis using brain fixation with head-focused microwave irradiation and liquid-liquid extraction. J Lipid Res. 2008;49:893–902. doi: 10.1194/jlr.D700030-JLR200. [DOI] [PubMed] [Google Scholar]
- He C, Qu X, Cui L, Wang J, Kang JX. Improved spatial learning performance of fat-1 mice is associated with enhanced neurogenesis and neuritogenesis by docosahexaenoic acid. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:11370–11375. doi: 10.1073/pnas.0904835106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman DR, Boettcher JA, Diersen-Schade DA. Toward optimizing vision and cognition in term infants by dietary docosahexaenoic and arachidonic acid supplementation: a review of randomized controlled trials. Prostaglandins, leukotrienes, and essential fatty acids. 2009;81:151–158. doi: 10.1016/j.plefa.2009.05.003. [DOI] [PubMed] [Google Scholar]
- Hong S, Gronert K, Devchand PR, Moussignac RL, Serhan CN. Novel docosatrienes and 17S-resolvins generated from docosahexaenoic acid in murine brain, human blood, and glial cells. Autacoids in anti-inflammation. The Journal of biological chemistry. 2003;278:14677–14687. doi: 10.1074/jbc.M300218200. [DOI] [PubMed] [Google Scholar]
- Hong S, Lu Y, Yang R, Gotlinger KH, Petasis NA, Serhan CN. Resolvin D1, protectin D1, and related docosahexaenoic acid-derived products: Analysis via electrospray/low energy tandem mass spectrometry based on spectra and fragmentation mechanisms. Journal of the American Society for Mass Spectrometry. 2007;18:128–144. doi: 10.1016/j.jasms.2006.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang L, Wang CF, Serhan CN, Strichartz G. Enduring prevention and transient reduction of postoperative pain by intrathecal resolvin D1. Pain. 2011;152:557–565. doi: 10.1016/j.pain.2010.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang WL, King VR, Curran OE, Dyall SC, Ward RE, Lal N, Priestley JV, Michael-Titus AT. A combination of intravenous and dietary docosahexaenoic acid significantly improves outcome after spinal cord injury. Brain. 2007;130:3004–3019. doi: 10.1093/brain/awm223. [DOI] [PubMed] [Google Scholar]
- Kang JX, Wang J, Wu L, Kang ZB. Transgenic mice: fat-1 mice convert n-6 to n-3 fatty acids. Nature. 2004;427:504. doi: 10.1038/427504a. [DOI] [PubMed] [Google Scholar]
- Kasuga K, Yang R, Porter TF, Agrawal N, Petasis NA, Irimia D, Toner M, Serhan CN. Rapid appearance of resolvin precursors in inflammatory exudates: novel mechanisms in resolution. J Immunol. 2008;181:8677–8687. doi: 10.4049/jimmunol.181.12.8677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim HW, Rapoport SI, Rao JS. Altered arachidonic acid cascade enzymes in postmortem brain from bipolar disorder patients. Molecular psychiatry. 2011a;16:419–428. doi: 10.1038/mp.2009.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim HY, Moon HS, Cao D, Lee J, Kevala K, Jun SB, Lovinger DM, Akbar M, Huang BX. N-Docosahexaenoylethanolamide promotes development of hippocampal neurons. Biochem J. 2011b;435:327–336. doi: 10.1042/BJ20102118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lafourcade M, Larrieu T, Mato S, et al. Nutritional omega-3 deficiency abolishes endocannabinoid-mediated neuronal functions. Nat Neurosci. 2011;14:345–350. doi: 10.1038/nn.2736. [DOI] [PubMed] [Google Scholar]
- Lalancette-Hebert M, Julien C, Cordeau P, Bohacek I, Weng YC, Calon F, Kriz J. Accumulation of dietary docosahexaenoic acid in the brain attenuates acute immune response and development of postischemic neuronal damage. Stroke; a journal of cerebral circulation. 2011;42:2903–2909. doi: 10.1161/STROKEAHA.111.620856. [DOI] [PubMed] [Google Scholar]
- Lukiw WJ, Cui JG, Marcheselli VL, Bodker M, Botkjaer A, Gotlinger K, Serhan CN, Bazan NG. A role for docosahexaenoic acid-derived neuroprotectin D1 in neural cell survival and Alzheimer disease. The Journal of clinical investigation. 2005;115:2774–2783. doi: 10.1172/JCI25420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcheselli VL, Hong S, Lukiw WJ, et al. Novel docosanoids inhibit brain ischemia-reperfusion-mediated leukocyte infiltration and pro-inflammatory gene expression. The Journal of biological chemistry. 2003;278:43807–43817. doi: 10.1074/jbc.M305841200. [DOI] [PubMed] [Google Scholar]
- Mingam R, Moranis A, Bluthe RM, De Smedt-Peyrusse V, Kelley KW, Guesnet P, Lavialle M, Dantzer R, Laye S. Uncoupling of interleukin-6 from its signalling pathway by dietary n-3-polyunsaturated fatty acid deprivation alters sickness behaviour in mice. Eur J Neurosci. 2008;28:1877–1886. doi: 10.1111/j.1460-9568.2008.06470.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukherjee PK, Marcheselli VL, Serhan CN, Bazan NG. Neuroprotectin D1: a docosahexaenoic acid-derived docosatriene protects human retinal pigment epithelial cells from oxidative stress. Proceedings of the National Academy of Sciences of the United States of America. 2004;101:8491–8496. doi: 10.1073/pnas.0402531101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy EJ. Brain fixation for analysis of brain lipid-mediators of signal transduction and brain eicosanoids requires head-focused microwave irradiation: an historical perspective. Prostaglandins & other lipid mediators. 2010;91:63–67. doi: 10.1016/j.prostaglandins.2009.07.005. [DOI] [PubMed] [Google Scholar]
- Orr SK, Tong JY, Kang JX, Ma DW, Bazinet RP. The fat-1 mouse has brain docosahexaenoic acid levels achievable through fish oil feeding. Neurochemical research. 2010;35:811–819. doi: 10.1007/s11064-010-0139-x. [DOI] [PubMed] [Google Scholar]
- Orr SK, Trepanier MO, Bazinet RP. n-3 Polyunsaturated fatty acids in animal models with neuroinflammation. Prostaglandins, leukotrienes, and essential fatty acids. 2012 doi: 10.1016/j.plefa.2012.05.008. [DOI] [PubMed] [Google Scholar]
- Rao JS, Ertley RN, DeMar JC, Jr., Rapoport SI, Bazinet RP, Lee HJ. Dietary n-3 PUFA deprivation alters expression of enzymes of the arachidonic and docosahexaenoic acid cascades in rat frontal cortex. Molecular psychiatry. 2007a;12:151–157. doi: 10.1038/sj.mp.4001887. [DOI] [PubMed] [Google Scholar]
- Rao JS, Ertley RN, Lee HJ, DeMar JC, Jr., Arnold JT, Rapoport SI, Bazinet RP. n-3 polyunsaturated fatty acid deprivation in rats decreases frontal cortex BDNF via a p38 MAPK-dependent mechanism. Molecular psychiatry. 2007b;12:36–46. doi: 10.1038/sj.mp.4001888. [DOI] [PubMed] [Google Scholar]
- Rosenberger TA, Villacreses NE, Hovda JT, Bosetti F, Weerasinghe G, Wine RN, Harry GJ, Rapoport SI. Rat brain arachidonic acid metabolism is increased by a 6-day intracerebral ventricular infusion of bacterial lipopolysaccharide. J Neurochem. 2004;88:1168–1178. doi: 10.1046/j.1471-4159.2003.02246.x. [DOI] [PubMed] [Google Scholar]
- Rudaya AY, Steiner AA, Robbins JR, Dragic AS, Romanovsky AA. Thermoregulatory responses to lipopolysaccharide in the mouse: dependence on the dose and ambient temperature. Am J Physiol Regul Integr Comp Physiol. 2005;289:R1244–1252. doi: 10.1152/ajpregu.00370.2005. [DOI] [PubMed] [Google Scholar]
- Serhan CN, Chiang N, Van Dyke TE. Resolving inflammation: dual anti-inflammatory and pro-resolution lipid mediators. Nat Rev Immunol. 2008;8:349–361. doi: 10.1038/nri2294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serhan CN, Dalli J, Karamnov S, Choi A, Park CK, Xu ZZ, Ji RR, Zhu M, Petasis NA. Macrophage proresolving mediator maresin 1 stimulates tissue regeneration and controls pain. Faseb J. 2012 doi: 10.1096/fj.11-201442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serhan CN, Hong S, Gronert K, Colgan SP, Devchand PR, Mirick G, Moussignac RL. Resolvins: a family of bioactive products of omega-3 fatty acid transformation circuits initiated by aspirin treatment that counter proinflammation signals. The Journal of experimental medicine. 2002;196:1025–1037. doi: 10.1084/jem.20020760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serhan CN, Petasis NA. Resolvins and protectins in inflammation resolution. Chem Rev. 2011;111:5922–5943. doi: 10.1021/cr100396c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serhan CN, Yang R, Martinod K, Kasuga K, Pillai PS, Porter TF, Oh SF, Spite M. Maresins: novel macrophage mediators with potent antiinflammatory and proresolving actions. The Journal of experimental medicine. 2009;206:15–23. doi: 10.1084/jem.20081880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheets KG, Zhou Y, Ertel MK, Knott EJ, Regan CE, Jr., Elison JR, Gordon WC, Gjorstrup P, Bazan NG. Neuroprotectin D1 attenuates laser-induced choroidal neovascularization in mouse. Mol Vis. 2010;16:320–329. [PMC free article] [PubMed] [Google Scholar]
- Smith BK, Holloway GP, Reza-Lopez S, Jeram SM, Kang JX, Ma DW. A decreased n-6/n-3 ratio in the fat-1 mouse is associated with improved glucose tolerance. Appl Physiol Nutr Metab. 2010;35:699–706. doi: 10.1139/H10-066. [DOI] [PubMed] [Google Scholar]
- Spite M, Norling LV, Summers L, Yang R, Cooper D, Petasis NA, Flower RJ, Perretti M, Serhan CN. Resolvin D2 is a potent regulator of leukocytes and controls microbial sepsis. Nature. 2009a;461:1287–1291. doi: 10.1038/nature08541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spite M, Summers L, Porter TF, Srivastava S, Bhatnagar A, Serhan CN. Resolvin D1 controls inflammation initiated by glutathione-lipid conjugates formed during oxidative stress. British journal of pharmacology. 2009b;158:1062–1073. doi: 10.1111/j.1476-5381.2009.00234.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun YP, Oh SF, Uddin J, Yang R, Gotlinger K, Campbell E, Colgan SP, Petasis NA, Serhan CN. Resolvin D1 and its aspirin-triggered 17R epimer. Stereochemical assignments, anti-inflammatory properties, and enzymatic inactivation. The Journal of biological chemistry. 2007;282:9323–9334. doi: 10.1074/jbc.M609212200. [DOI] [PubMed] [Google Scholar]
- Xu ZZ, Zhang L, Liu T, Park JY, Berta T, Yang R, Serhan CN, Ji RR. Resolvins RvE1 and RvD1 attenuate inflammatory pain via central and peripheral actions. Nature medicine. 2010;16:592–597. doi: 10.1038/nm.2123. 591p following 597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang R, Chiang N, Oh SF, Serhan CN. Metabolomics-lipidomics of eicosanoids and docosanoids generated by phagocytes. Curr Protoc Immunol. 2011;Chapter 14(Unit 14):26. doi: 10.1002/0471142735.im1426s95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yong VW, Rivest S. Taking advantage of the systemic immune system to cure brain diseases. Neuron. 2009;64:55–60. doi: 10.1016/j.neuron.2009.09.035. [DOI] [PubMed] [Google Scholar]
- Zhao Y, Calon F, Julien C, Winkler JW, Petasis NA, Lukiw WJ, Bazan NG. Docosahexaenoic acid-derived neuroprotectin D1 induces neuronal survival via secretase- and PPARgamma-mediated mechanisms in Alzheimer's disease models. PLoS One. 2011;6:e15816. doi: 10.1371/journal.pone.0015816. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.