Abstract
We examined the association of nitrate-nitrogen exposure from rural private drinking water and incidence of age-related macular degeneration (AMD). Participants of the Beaver Dam Eye Study living in rural areas within the 53916 zone improvement plan code but outside the city limits of Beaver Dam, Wisconsin (Beaver Dam Township) completed a questionnaire and ocular examination including standardized, graded fundus photographs at five examinations. Data from an environmental monitoring study with probabilistic-based agro-chemical sampling, including nitrate-nitrogen, of rural private drinking water were available. Incidence of early AMD was associated with elevated nitrate-nitrogen levels in rural private drinking water supply (10.0% for low, 19.2% for medium, and 26.1% for high nitrate-nitrogen level in the right eye). The odds ratios (ORs) were 1.77 (95% confidence interval [CI]: 1.12–2.78) for medium and 2.88 (95% CI: 1.59–5.23) for high nitrate-nitrogen level. Incidence of late AMD was increased for those with medium or high levels of nitrate-nitrogen compared to low levels (2.3% for low and 5.1% for the medium or high nitrate-nitrogen level, for the right eye). The OR for medium or high nitrate-nitrogen groups was 2.80 (95% CI: 1.07–7.31) compared to the low nitrate-nitrogen group.
Keywords: Disease, Epidemiology, Personal exposure, Population based studies
INTRODUCTION
Nitrate-nitrogen is the most common chemical contaminant in groundwater, [1] and groundwater is the usual source of rural drinking water. In the US, nitrate-nitrogen in groundwater is highest in agricultural areas compared to urban areas. [2] Dietary intake, particularly leafy green vegetables, is considered a significant contributor to nitrate-nitrogen exposure in humans. [3] However, with 10 ppm or more of nitrate-nitrogen in groundwater found in Wisconsin private well water, [4] drinking water becomes an important contributor in humans as well. [5,6]
Methemoglobin has been associated with intake of nitrates in ground water and drinking water. [7] Methemoglobin has also been associated with lipid peroxidation in the retina which is hypothesized to cause age-related macular degeneration (AMD). [8] Nitrates/nitrites have been associated with a variety of complaints and illnesses although the possibility of an association with AMD has not been examined. [9] Ecologic studies have shown a positive correlation of nitrate-nitrogen with stomach cancer [10] and non-Hodgkin’s lymphoma. [10] In a female colorectal case-control study, nitrate-nitrogen exposure from rural private drinking water was associated with increased colon cancer risk. [11] AMD pathogenesis is largely unknown, [12] although neuroinflammatory response including immune cells reacting to persistent stressful stimuli generating low-grade chronic inflammation or compromised autoimmunity related to oxidative stress may provide insight into the mechanism leading to AMD. [13] Similarly, mechanisms associated with nitrate exposure and inflammatory response in humans has not been well described but a few recent studies are suggestive of an association. [14] Due to the potential relationship between nitrate-nitrogen exposure in rural drinking water to AMD, we examined the cumulative incidence of this endpoint in a population-based cohort study in Wisconsin.
MATERIALS AND METHODS
A private census of Beaver Dam, Wisconsin, was performed in 1987–1988 to identify all eligible residents within the 53916 ZIP code. [15] Of the 5924 eligible, 4926 (83%) persons 43–86 years of age participated in the baseline examination in 1988–1990. Ninety-nine percent of participants were white and 56% were female. The cohort was re-examined at 5- (n=3722), 10- (n=2962), 15- (n=2375), and 20-year (n=1913) follow-up examinations. There was greater than 80% participation among survivors at each examination. [15–18] Differences between participants and nonparticipants have been presented elsewhere. [15–18] In general, participants at each examination phase were younger, had lower blood pressure, and had fewer co-morbid conditions than nonparticipants. Written informed consent was obtained from each participant. The investigations were performed according to the guidelines of the Declaration of Helsinki and approval from the Institutional Review Board of the University of Wisconsin was obtained.
Geocodes were assigned based on the residential address at the baseline examination, obtained from March 1988 to September 1990, and were used to assign residence location. Participants residing within the 53916 ZIP code boundary but not within the Beaver Dam city limits were categorized as rural residents, and were eligible for inclusion in these analyses.
Publicly available data on nitrate-nitrogen contamination of groundwater were obtained from the Wisconsin Department of Agriculture, Trade and Consumer Protection (WDATCP) from the time of the first examination. [19] As part of the Atrazine Rule Evaluation Study, WDATCP randomly sampled 289 wells to analyze private well water for various herbicides and nitrate-nitrogen, beginning in 1995. This study’s purpose was to quantify agricultural chemical levels, including nitrate-nitrogen, in Wisconsin groundwater servicing the rural population and to compare these levels over time through repeat measurements. The chlorotriazine herbicides (e.g., atrazine) and nitrogen-nitrate were agricultural chemicals consistently tested over the fifteen years. Additional agricultural chemicals, such as chloroacetanilide herbicides, analyzed for any one study period, varied as new methods became available. [4] Only nitrate-nitrogen values were considered for evaluation due to the consistent levels of nitrate-nitrogen in well water over time determined from sequential WDATCP studies. [4,20] A natural neighbor interpolation [21] from the WDATCP well water samples’ nitrate-nitrogen levels was used to estimate nitrate-nitrogen levels in groundwater across the entire state. Natural neighbor interpolation uses a weighted moving average of concentrations of nitrate-nitrogen residues in residential drinking water in surrounding or neighboring observed wells. Neighboring points and the corresponding weights are based on the Voronoi diagram of the data points. [22] The Voronoi diagram of a set of points is a partitioning of the plane into regions associated with each point such that every point in a given partition is closer to the generation point than any other point. This interpolation was performed using ArcGIS 9.3 (Environmental Systems Research Institute, Redwood, CA). The rural study participants were categorized into three groups of nitrate-nitrogen in their residential drinking water; low (0–4 ppm), medium (5–9 ppm), and high (≥10 ppm).
Severity of AMD was determined based on standardized fundus photographs that were graded according to protocol with stringent quality control procedures. [23] For purposes of this analysis, AMD severity was grouped into categories of early (defined as the presence of any drusen and retinal pigmentary abnormalities or soft indistinct drusen) and late AMD (defined as the presence of pure geographic atrophy or exudative macular degeneration). These analyses evaluate incidence of early or late AMD in a given eye, over the course of 20 years of follow-up, by baseline nitrate-nitrogen level. To be included in incidence analyses, an eye must be at risk of developing early or late AMD (i.e., free from prevalence at baseline) and must have gradable photographs for early or late AMD, respectively, for successive follow-up visits until incidence of AMD, death, or loss to follow-up (censoring) occurs. Cumulative incidences of early and late AMD over 20 years of follow-up are presented for each eye separately using a competing risk approach, an adaptation of the Kaplan-Meier product limit method, where death is the competing outcome (Table 2).
Table 2.
Cumulative Incidence of Age-Related Macular Degeneration by Nitrate-Nitrogen Levelsa in the Beaver Dam Eye Study
| Right Eye | Left Eye | Age and Sex Adjusted Models |
Age, Sex, Education, Heavy Drinking and Smoking Adjusted Models |
|||||
|---|---|---|---|---|---|---|---|---|
| Outcome | N at Risk (N Incident) |
Cumulative Incidenceb |
N at Risk (N Incident) |
Cumulative Incidenceb |
OR (95% CI) | P valuec |
OR (95% CI) | P valuec |
| Early AMD | ||||||||
| Low | 264 (22) | 10.0% | 266 (26) | 11.3% | Referent | Referent | ||
| Medium | 254 (40) | 19.2% | 242 (33) | 16.2% | 1.77 (1.12, 2.78) | 0.01 | 1.84 (1.16, 2.91) | 0.01 |
| High | 72 (15) | 26.1% | 68 (18) | 31.4% | 2.88 (1.59, 5.23) | <.001 | 3.03 (1.65, 5.60) | <.001 |
| Late AMD | ||||||||
| Low | 289 (5) | 2.3% | 290 (3) | 1.3% | Referent | Referent | ||
| Medium/High | 361 (14) | 5.1% | 359 (14) | 5.3% | 2.80 (1.07, 7.31) | 0.04 | 3.08 (1.13, 8.41) | 0.03 |
Abbreviations: AMD, age related macular degeneration; CI, confidence interval; OR, odds ratio.
Low (0–4 ppm), medium (5–9 ppm), and high (≥10 ppm).
Cumulative incidence accounts for competing risk of death.
P values compared to low nitrate group.
Several baseline covariates were considered for the analyses in this paper. They included age at interview, educational status as a marker of socioeconomic status (4 categories: less than high school graduate, high school graduate or GED, some college not including a baccalaureate degree, and baccalaureate degree, graduate or professional school [e.g., law, medicine]), hypertension (dichotomous: defined as blood pressure reading greater than or equal to 140/90 mmHg or taking medications), cardiovascular disease history (dichotomous: defined as reported history of angina, myocardial infarction or stroke), any type of cancer diagnosis including skin cancer (dichotomous), current or past heavy alcohol drinker (dichotomous: defined as drinking at least 28 drinks per week). Smoking status was stratified into three categories as follows: never smoker (smoked less than 100 cigarettes in lifetime), past smoker (smoked at least 100 cigarettes in lifetime, but not currently smoking), and current smoker (smoked at least 100 cigarettes in lifetime and currently smoking). Sun exposure was estimated from residential history that permitted calculation of Wisconsin sun-years (WISYs) at the baseline examination and was dichotomized into those with <1.01 WISYs vs. ≥1.01 WISYs. [24] Total glycosylated hemoglobin level was determined using affinity chromatography (Isolab, Inc., Akron, OH, USA). [25] Elevated glycosylated hemoglobin level was defined as having a measurement greater than two standard deviations above the mean for that participant's age and sex group. Glucose level was determined using the hexokinase method. [26] Elevated glucose level was defined as greater than 200 mg/dL. Diabetes was defined as a self-report confirmed by treatment, diet, or hyperglycemia. Differences in distributions of baseline covariates between participants in the low, medium, and high nitrate-nitrogen groups are presented by person, rather than by eye (Table 1). Categorical regression models are used to test for differences between low and medium nitrate groups, and low and high nitrate groups, adjusting for age and sex. Tests for significant differences in age between nitrate groups are adjusted only for sex, and tests for differences in sex are adjusted only for age.
Table 1.
Characteristics of the Rural Population Included in Analyses by Low/Medium/High Nitrate-Nitrogen Levelsa in the Beaver Dam Eye Study
| Nitrate-Nitrogen Levels |
|||||
|---|---|---|---|---|---|
| Low N=299 |
Medium N=287 |
High N=82 |
|||
| Characteristic | Crude % | Crude % | P valueb | Crude % | P valueb |
| Age, years | |||||
| 43–54 | 51.51 | 41.11 | 0.07 | 35.37 | 0.01 |
| 55–64 | 27.42 | 36.93 | 34.15 | ||
| 65–74 | 17.06 | 16.03 | 24.39 | ||
| ≥75 | 4.01 | 5.92 | 6.10 | ||
| Cancer self-report | |||||
| No | 90.64 | 91.99 | 0.44 | 89.02 | 0.98 |
| Yes | 9.36 | 8.01 | 10.98 | ||
| History of CVDc | |||||
| No | 90.20 | 89.32 | 0.93 | 87.50 | 0.81 |
| Yes | 9.80 | 10.68 | 12.50 | ||
| History of diabetesd | |||||
| No | 94.61 | 94.06 | 0.88 | 90.12 | 0.27 |
| Yes | 5.39 | 5.94 | 9.88 | ||
| Comorbidities of cancer/CVD/diabetes | |||||
| No | 76.43 | 74.47 | 0.85 | 69.23 | 0.47 |
| Yes | 23.57 | 25.53 | 30.77 | ||
| Sunlight exposuree | |||||
| <1.01 WISY | 75.76 | 78.6 | 0.38 | 82.93 | 0.24 |
| ≥1.01 WISY | 24.24 | 21.4 | 17.07 | ||
| Ever heavy drinker (≥ 28 drinks/week) | |||||
| No | 86.91 | 81.82 | 0.06 | 75.61 | 0.002 |
| Yes | 13.09 | 18.18 | 24.39 | ||
| Hypertensionf | |||||
| No | 62.54 | 57.14 | 0.35 | 52.44 | 0.42 |
| Yes | 37.46 | 42.86 | 47.56 | ||
| Education status | |||||
| Not a high school graduate | 21.74 | 21.25 | 0.43 | 37.80 | <.001 |
| High school graduate or GED | 44.15 | 51.57 | 52.44 | ||
| Some college but not including baccalaureate degree | 19.40 | 15.33 | 6.10 | ||
| Baccalaureate degree or beyond | 14.72 | 11.85 | 3.66 | ||
| Sex | |||||
| Women | 49.50 | 50.87 | 0.72 | 57.32 | 0.18 |
| Men | 50.50 | 49.13 | 42.68 | ||
| Smoking statusg | |||||
| Never | 51.51 | 45.30 | 0.09 | 40.24 | 0.003 |
| Past | 32.11 | 36.24 | 31.71 | ||
| Current | 16.39 | 18.47 | 28.05 | ||
Abbreviations: CVD, cardiovascular disease; GED, general education diploma; WISY, Wisconsin sun years.
Low (0–4 ppm), medium (5–9 ppm), and high (≥10 ppm).
Compared to low nitrate group; age adjusted for sex, sex adjusted for age, all others adjusted for age and sex.
Angina, myocardial infarction, or stroke.
Estimated from residential history permitting calculation of WISY at the baseline examination.13
Blood pressure ≥140/90 mmHg or on antihypertensive medication.
Never smoker: <100 cigarettes in his/her lifetime; Past smoker: ≥100 cigarettes in his/her lifetime but not currently smoking; Current smoker: ≥100 cigarettes in lifetime and currently smoking.
The effect of nitrate-nitrogen level at baseline on incidence of early and late AMD was evaluated by eye. Odds ratios for incidence were estimated using logistic regression with generalized estimating equations account for the correlation between the eyes and within each participant over the study intervals. Odds ratios, confidence intervals, and P values reported use the low nitrate-nitrogen category as the reference group. All models were adjusted for age and sex. Further adjustment for education, heavy drinking, and smoking did not significantly alter the findings. SAS version 9 (SAS Institute, Cary, North Carolina) was used for all analyses.
RESULTS AND DISCUSSION
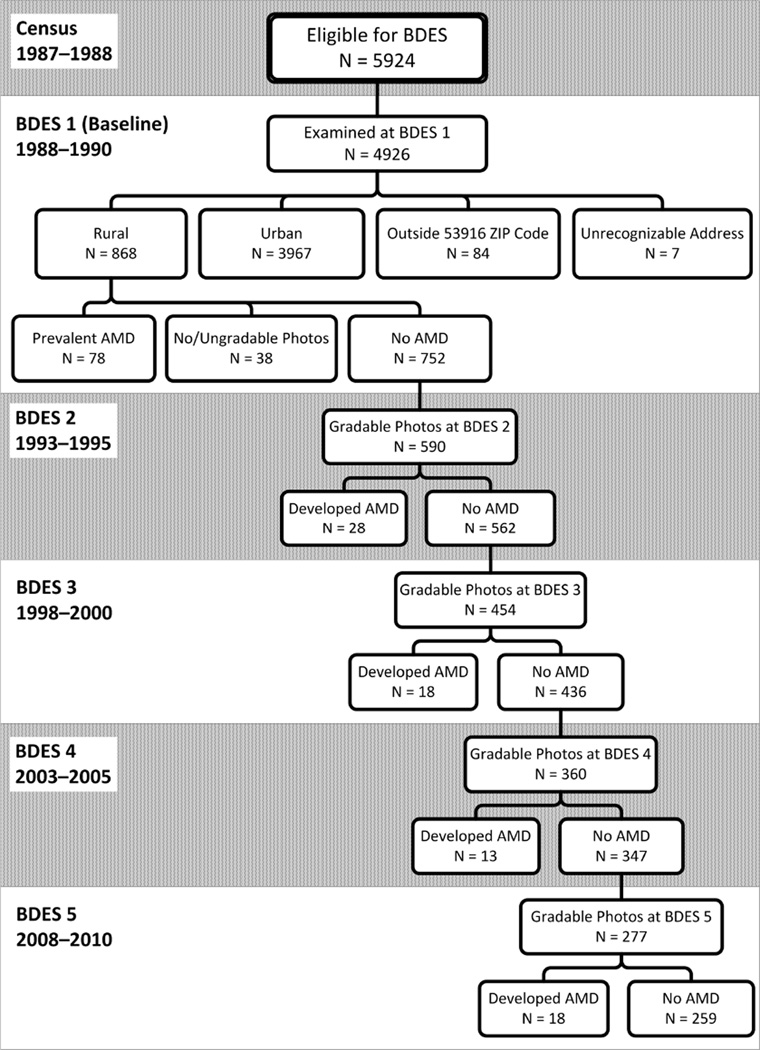
Of the 4926 persons seen at baseline, 868 lived in rural areas, of which 853 had gradable retinal photos for at least one outcome (early or late AMD) for at least one eye. In reference to the figure, focusing specifically on the incidence of early AMD in the right eye, there were 752 participants with gradable photos who were free of early or late AMD at baseline (at risk for incidence of early AMD). Of these, 590 had gradable retinal photos at the first follow-up visit, of which 28 had developed early AMD and 562 had remained free of early AMD. This pattern continues through the fifth examination phase, with a total of 77 right eyes developing early AMD at some point over the next 20 years (Figure). There were 650 rural residents at risk for incident late AMD in the right eye, and a total of 19 developed late AMD over the next 20 years. Similar numbers exist for the left eye for incidence of both early and late AMD. The main reason for participants not returning for follow-up was death.
Figure.
Incident Cases of Early Age-Related Macular Degeneration (AMD) in the Right Eye in the Rural Population of the Beaver Dam Eye Study (BDES; 1988–2010).
Characteristics of the rural population included in analyses by low, medium, and high nitrate-nitrogen levels are given in Table 1. Age, heavy drinking, education, and smoking were significantly different between the groups. Early AMD risk increased significantly with increasing nitrate-nitrogen levels (Table 2). The odds were minimally changed when further adjusting for smoking, heavy drinking, and education. Due to the small number of incident cases of late AMD, the medium and high nitrate-nitrogen categories were combined to calculate the odds ratio. The odds of developing late AMD in persons living in areas with medium or high levels of nitrate-nitrogen in their drinking water were significantly elevated compared to those with low levels (Table 2).
As the first study to evaluate environmental exposure of nitrate-nitrogen and incidence of AMD, our results suggest an association. The pathogenesis of AMD is a multifactorial disease with numerous established risk factors, including light damage, [27] oxidative stress, [28] inflammation, [29] disturbance in the choroidal blood vessels, [30] smoking, [31] and genetic predisposition. [32,33]
Research to identify the mechanisms underlying AMD development is ongoing. For example, N-nitrosoamines and N-nitrosoamides have been shown to affect pathways dealing with oxidative stress and inflammation, [34] and both of these states have been postulated to be involved in the etiology of AMD. [13] Specifically, Hebels et al. [34] reported nitrosamines influencing transcriptomic profiles through modification of pathways of oxidative stress and inflammation and Yoshizawa et al. evaluated the role of p53 in response to stressors to the retina. [35] Our study is the first to extend some of these preliminary findings in basic research to a population study, though we were unable to assess whether these pathways affect the retina of older adult humans or whether other pathways in the causal chain may be affected.
The design of our study has potential limitations. First, we used well water samples from randomly selected wells in an environmental monitoring study conducted in 1994. An exposure surface was estimated and these surface values were assigned to participants’ residences. Nitrate-nitrogen application has a long history in Wisconsin, with roughly 10% of the total nitrate application leaching into groundwater [36] where its half-life ranges from approximately 500 days in zones where organic substances are present to 2750 days in zones where organic substances are absent. [37] In the sequential evaluation of well water from 1994 to 2009, nitrate levels have statistically significantly increased at 0.37 parts per million per year [4] which increases our confidence in the use of well data from 1994 to assign exposure to participants in this study. Second, the quality of the data gathered from study participants about use of home filtration on their kitchen tap water is questionable since filtration systems’ ability to extract contaminants varies widely. However, our results did not change after adding this information into the model (data not shown). Third, there may be other contaminants of the water source that may be more important than nitrate-nitrogen in influencing the progression of AMD, and because these chemicals occur in the company of nitrate-nitrogen, it is possible they could be responsible for these findings. Because data are only available on nitrate-nitrogen, the possibility that other compounds are more strongly (or more directly) associated with AMD cannot be tested. Additionally, for this analysis, the exposure classification system may not accurately reflect the participant’s actual exposure to nitrate-nitrogen and, as described by Vineis, [38] non-differential misclassification of exposure may attenuate the estimation of risk. For example, only home water supply was considered as the potential source of nitrate-nitrogen exposure. However, an exploratory analysis of the amount of water consumed at home did not materially change our findings. Furthermore, the models have been adjusted for age, a major risk factor for AMD. If age is associated with water source, then this is likely to have been accounted for by including age in the model. The models controlled for several known risk factors, but were unable to control for other suspected AMD risk factors, such as diet or abdominal obesity; however, adjusting for body mass index and sedentary lifestyle did not affect our model estimates. The environmental exposure assessment was an estimation of exposure and not a biomarker of exposure. These are provocative findings that need further investigation with special attention to adjustment for important confounders and, ideally, obtainment of an appropriate biomarker of exposure to nitrate-nitrogen.
CONCLUSION
The Beaver Dam Eye Study was not designed to examine the association of nitrate-nitrogen exposure to age-related eye diseases. Therefore, there are potential confounding factors that we did not measure, such as other water contaminants, specific occupational exposures, and exact amount and source of water actually imbibed. Also, there is a limited number of cumulative cases of AMD. Nevertheless, the data may provide clues to possible effects of nitrogen-nitrate intake that may be associated with causes and pathways leading to AMD.
ACKNOWLEDGEMENT
Technical editing and writing assistance was provided by Mary Kay Aprison, BS and Heidi M. G. Christian, BA.
FINANCIAL SUPPORT
This research was supported by National Institutes of Health grant EY06594 (Drs B. E. K. Klein, R. Klein). The National Eye Institute provided funding for entire study including collection and analyses of data. Additional support was provided by Research to Prevent Blindness. The content of this report is solely the responsibility of the authors and does not necessarily reflect the official views of the National Eye Institute or the National Institutes of Health.
Footnotes
CONFLICT OF INTEREST STATEMENT
None of the authors have any proprietary interests or conflicts of interest related to this submission.
REFERENCES
- 1.Spalding RF, Exner ME. Occurrence of nitrate in groundwater--a review. J Environ Qual. 1993;22(3):392–402. [Google Scholar]
- 2.Ward MH, deKok TM, Levallois P, Brender J, Gulis G, Nolan BT, VanDerslice J. Workgroup report: Drinking-water nitrate and health--recent findings and research needs. Environ Health Perspect. 2005;113(11):1607–1614. doi: 10.1289/ehp.8043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.IARC Monographs on the Evaluation of Carcinogenic Risks to Humans. Volume 94. Ingested Nitrate and Nitrite and Cyanobacterial Peptide Toxins. 2010 43-325. [PMC free article] [PubMed] [Google Scholar]
- 4.Fifteen Years of the DATCP Exceedence Well Survey. Wisconsin Department of Agriculture, Trade and Consumer Protection; 2010. [Google Scholar]
- 5.Chilvers C, Inskip H, Caygill C, Bartholomew B, Fraser P, Hill M. A survey of dietary nitrate in well-water users. Int. J Epidemiol. 1984;13(3):324–331. doi: 10.1093/ije/13.3.324. [DOI] [PubMed] [Google Scholar]
- 6.Moller H, Landt J, Jensen P, Pedersen E, Autrup H, Jensen OM. Nitrate exposure from drinking water and diet in a Danish rural population. Int. J Epidemiol. 1989;18(1):206–212. doi: 10.1093/ije/18.1.206. [DOI] [PubMed] [Google Scholar]
- 7.Johnson CJ, Kross BC. Continuing importance of nitrate contamination of groundwater and wells in rural areas. Am J Ind. Med. 1990;18(4):449–456. doi: 10.1002/ajim.4700180416. [DOI] [PubMed] [Google Scholar]
- 8.Ito T, Nakano M, Yamamoto Y, Hiramitsu T, Mizuno Y. Hemoglobin-induced lipid peroxidation in the retina: a possible mechanism for macular degeneration. Arch Biochem. Biophys. 1995;316(2):864–872. doi: 10.1006/abbi.1995.1116. [DOI] [PubMed] [Google Scholar]
- 9.Zeman C, Beltz L, Linda M, Maddux J, Depken D, Orr J, Theran P. New questions and insights into nitrate/nitrite and human health effects: a retrospective cohort study of private well users' immunological and wellness status. J Environ. Health. 2011;74(4):8–18. [PubMed] [Google Scholar]
- 10.Gulis G, Czompolyova M, Cerhan JR. An ecologic study of nitrate in municipal drinking water and cancer incidence in Trnava District, Slovakia. Environ Res. 2002;88(3):182–187. doi: 10.1006/enrs.2002.4331. [DOI] [PubMed] [Google Scholar]
- 11.McElroy JA, Trentham-Dietz A, Gangnon RE, Hampton JM, Bersch AJ, Kanarek MS, Newcomb PA. Nitrogen-nitrate exposure from drinking water and colorectal cancer risk for rural women in Wisconsin, USA. J Water Health. 2008;6(3):399–409. doi: 10.2166/wh.2008.048. [DOI] [PubMed] [Google Scholar]
- 12.Nowak JZ. Age-related macular degeneration (AMD): pathogenesis and therapy. Pharmacol. Rep. 2006;58(3):353–363. [PubMed] [Google Scholar]
- 13.Buschini E, Piras A, Nuzzi R, Vercelli A. Age related macular degeneration and drusen: neuroinflammation in the retina. Prog. Neurobiol. 2011;95(1):14–25. doi: 10.1016/j.pneurobio.2011.05.011. [DOI] [PubMed] [Google Scholar]
- 14.Winyard PG, Ryan B, Eggleton P, Nissim A, Taylor E, Lo Faro ML, Burkholz T, Szabo-Taylor KE, Fox B, Viner N, Haigh RC, Benjamin N, Jones AM, Whiteman M. Measurement and meaning of markers of reactive species of oxygen, nitrogen and sulfur in healthy human subjects and patients with inflammatory joint disease. Biochem. Soc. Trans. 2011;39(5):1226–1232. doi: 10.1042/BST0391226. [DOI] [PubMed] [Google Scholar]
- 15.Klein R, Klein BE, Linton KL, De Mets DL. The Beaver Dam Eye Study: visual acuity. Ophthalmology. 1991;98(8):1310–1315. doi: 10.1016/s0161-6420(91)32137-7. [DOI] [PubMed] [Google Scholar]
- 16.Klein R, Klein BE, Lee KE. Changes in visual acuity in a population. The Beaver Dam Eye Study. Ophthalmology. 1996;103(8):1169–1178. doi: 10.1016/s0161-6420(96)30526-5. [DOI] [PubMed] [Google Scholar]
- 17.Klein R, Klein BE, Lee KE, Cruickshanks KJ, Chappell RJ. Changes in visual acuity in a population over a 10-year period: The Beaver Dam Eye Study. Ophthalmology. 2001;108(10):1757–1766. doi: 10.1016/s0161-6420(01)00769-2. [DOI] [PubMed] [Google Scholar]
- 18.Klein R, Klein BE, Lee KE, Cruickshanks KJ, Gangnon RE. Changes in visual acuity in a population over a 15-year period: the Beaver Dam Eye Study. Am J Ophthalmol. 2006;142(4):539–549. doi: 10.1016/j.ajo.2006.06.015. [DOI] [PubMed] [Google Scholar]
- 19.LeMasters G, Baldock J. A survey of atrazine in Wisconsin groundwater, Report Number 26a. Madison, WI: Wisconsin Department of Agriculture, Trade and Consumer Protection, Agricultural Resource Management Division; 1997. pp. 1–26. [Google Scholar]
- 20.Vanden Brook J, Rheineck B, Postle J, Zogbaum R, Funk J, et al. Agricultural chemicals in Wisconsin groundwater, final report. Madison, WI: Wisconsin Department of Agriculture, Trade, and Consumer Protection; 2002. pp. 1–21. [Google Scholar]
- 21.Sibson R. A brief description of natural neighbor interpolation. In: Barnett V, editor. Interpreting Multivariate Data. New York, NY: John Wiley & Sons; 1981. pp. 21–36. [Google Scholar]
- 22.Okabe A, Boots B, Sugihara K, Chin SN. Spatial tessellations: concepts and applications of Voronoi diagrams. 2nd ed. Chichester: John Wiley & Sons; 2000. [Google Scholar]
- 23.Klein R, Davis MD, Magli YL, Segal P, Klein BE, Hubbard L. The Wisconsin age-related maculopathy grading system. Ophthalmology. 1991;98(7):1128–1134. doi: 10.1016/s0161-6420(91)32186-9. [DOI] [PubMed] [Google Scholar]
- 24.Cruickshanks KJ, Klein BE, Klein R. Ultraviolet light exposure and lens opacities: the Beaver Dam Eye Study. Am. J. Public Health. 1992;82(12):1658–1662. doi: 10.2105/ajph.82.12.1658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Klenk DC, Hermanson GT, Krohn RI, Fujimoto EK, Mallia AK, Smith PK, England JD, Wiedmeyer HM, Little RR, Goldstein DE. Determination of glycosylated hemoglobin by affinity chromatography: comparison with colorimetric and ion-exchange methods, and effects of common interferences. Clin. Chem. 1982;28(10):2088–2094. [PubMed] [Google Scholar]
- 26.Stein MW. D-glucose determination with hexokinase and glucose-6-phosphate dehydrogenase. In: Bergmeyer HC, editor. Methods of Enzymatic Analysis. New York: Academic Press; 1963. p. 117. [Google Scholar]
- 27.Shaban H, Richter C. A2E and blue light in the retina: the paradigm of age-related macular degeneration. Biol. Chem. 2002;383(3–4):537–545. doi: 10.1515/BC.2002.054. [DOI] [PubMed] [Google Scholar]
- 28.Beatty S, Koh H, Phil M, Henson D, Boulton M. The role of oxidative stress in the pathogenesis of age-related macular degeneration. Surv. Ophthalmol. 2000;45(2):115–134. doi: 10.1016/s0039-6257(00)00140-5. [DOI] [PubMed] [Google Scholar]
- 29.Hageman GS, Luthert PJ, Victor Chong NH, Johnson LV, Anderson DH, Mullins RF. An integrated hypothesis that considers drusen as biomarkers of immune-mediated processes at the RPE-Bruch's membrane interface in aging and age-related macular degeneration. Prog. Retin. Eye Res. 2001;20(6):705–732. doi: 10.1016/s1350-9462(01)00010-6. [DOI] [PubMed] [Google Scholar]
- 30.Feigl B. Age-related maculopathy - linking aetiology and pathophysiological changes to the ischaemia hypothesis. Prog. Retin. Eye Res. 2009;28(1):63–86. doi: 10.1016/j.preteyeres.2008.11.004. [DOI] [PubMed] [Google Scholar]
- 31.Seddon JM, Reynolds R, Maller J, Fagerness JA, Daly MJ, Rosner B. Prediction model for prevalence and incidence of advanced age-related macular degeneration based on genetic, demographic, and environmental variables. Invest Ophthalmol Vis. Sci. 2009;50(5):2044–2053. doi: 10.1167/iovs.08-3064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ting AY, Lee TK, MacDonald IM. Genetics of age-related macular degeneration. Curr Opin. Ophthalmol. 2009;20(5):369–376. doi: 10.1097/ICU.0b013e32832f8016. [DOI] [PubMed] [Google Scholar]
- 33.Hageman GS, Anderson DH, Johnson LV, Hancox LS, Taiber AJ, Hardisty LI, Hageman JL, Stockman HA, Borchardt JD, Gehrs KM, Smith RJ, Silvestri G, Russell SR, Klaver CC, Barbazetto I, Chang S, Yannuzzi LA, Barile GR, Merriam JC, Smith RT, Olsh AK, Bergeron J, Zernant J, Merriam JE, Gold B, Dean M, Allikmets R. A common haplotype in the complement regulatory gene factor H (HF1/CFH) predisposes individuals to age-related macular degeneration. Proc Natl Acad Sci U. S. A. 2005;102(20):7227–7232. doi: 10.1073/pnas.0501536102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hebels DG, Jennen DG, Kleinjans JC, de Kok TM. Molecular signatures of N-nitroso compounds in Caco-2 cells: implications for colon carcinogenesis. Toxicol Sci. 2009;108(2):290–300. doi: 10.1093/toxsci/kfp035. [DOI] [PubMed] [Google Scholar]
- 35.Yoshizawa K, Kuwata M, Kawanaka A, Uehara N, Yuri T, Tsubura A. N-methyl-N-nitrosourea-induced retinal degeneration in mice is independent of the p53 gene. Mol Vis. 2009;15:2919–2925. [PMC free article] [PubMed] [Google Scholar]
- 36.Chern L, Kraft G, Postle G. Nitrate in Groundwater-A continuing issue for Wisconsin citizens. Madison WI USA: Dept of Natural Resources; 1999. [Google Scholar]
- 37.Uffink GJM. Determination of denitrification parameters in deep groundwater. A pilot study for several pumping stations in the Netherlands. RIVM Report 703717011. 2003:1–76. [Google Scholar]
- 38.Vineis P. A self-fulfilling prophecy: are we underestimating the role of the environment in gene-environment interaction research? Int. J Epidemiol. 2004;33(5):945–946. doi: 10.1093/ije/dyh277. [DOI] [PubMed] [Google Scholar]