Abstract
Peroxisome proliferator activated receptor γ coactivator 1α (PGC-1α) is a transcriptional coactivator known to regulate gene programs in a cell-specific manner in energy-demanding tissues, and its dysfunction has been implicated in numerous neurological and psychiatric disorders. Previous work from the Cowell laboratory indicates that PGC-1α is concentrated in inhibitory interneurons and required for the expression of the calcium buffer parvalbumin (PV) in the cortex; however, the impact of PGC-1α deficiency on inhibitory neurotransmission in the motor cortex is not known. Here, we show that mice lacking PGC-1α exhibit increased amplitudes and decreased frequency of spontaneous inhibitory postsynaptic currents in layer V pyramidal neurons. Upon repetitive train stimulation at the gamma frequency, decreased GABA release is observed. Furthermore, PV-positive interneurons in PGC-1α −/− mice display reductions in intrinsic excitability and excitatory input without changes in gross interneuron morphology. Taken together, these data show that PGC-1α is required for normal inhibitory neurotransmission and cortical PV-positive interneuron function. Given the pronounced motor dysfunction in PGC-1α −/− mice and the essential role of PV-positive interneurons in maintenance of cortical excitatory:inhibitory balance, it is possible that deficiencies in PGC-1α expression could contribute to cortical hyperexcitability and motor abnormalities in multiple neurological disorders.
Introduction
Peroxisome proliferated-activated receptor γ coactivator 1α (PGC-1α) is a transcriptional coactivator which, by interacting with different transcription factors, initiates cell and tissue-specific gene programs. Since the discovery of PGC-1α in 1998 (Puigserver, Wu et al. 1998), many studies have suggested that a reduction in its levels and/or activity plays a role in neurological disorders including Parkinson Disease (Zheng, Liao et al. 2010), Alzheimer Disease (Qin, Haroutunian et al. 2009, Sheng, Wang et al. 2012), Huntington Disease (Cui, Jeong et al. 2006, Taherzadeh-Fard, Saft et al. 2009, Chaturvedi, Calingasan et al. 2010), schizophrenia (Christoforou, Le Hellard et al. 2007, Jiang, Rompala et al. 2013), anxiety disorders (Hettema, Webb et al. 2011) and multiple sclerosis (Witte, Nijland et al. 2013). Studies with whole body and neuron-specific PGC-1α −/− mice indicate that PGC-1α is required for the expression of a subset of metabolic and neuronal transcripts (Lin, Wu et al. 2004, Lucas, Markwardt et al. 2010, Ma, Li et al. 2010, Lucas, Dougherty et al. 2012), but the physiological consequences of these transcriptional changes are not clear. Elucidating the impact of PGC-1α deficiency on neuronal function will give us insight into its contribution to neuronal dysfunction in various disorders.
The PGC-1α protein is highly concentrated in GABAergic cell populations throughout the brain (Cowell, Blake et al. 2007, Jiang, Rompala et al. 2013), and PGC-1α −/− mice exhibit deficiencies in the expression of the calcium buffer protein parvalbumin (PV) in forebrain regions including the cortex, hippocampus, and striatum (Lucas, Markwardt et al. 2010). In these regions, PV is expressed by a subset of GABAergic interneurons that exhibit fast-spiking and non-adapting properties (Kawaguchi 1993, Kawaguchi and Kondo 2002, Tepper and Bolam 2004) and entrain local pyramidal neurons to generate gamma oscillations (Wang and Buzsaki 1996, Bartos, Vida et al. 2002, Vreugdenhil, Jefferys et al. 2003, Sohal, Zhang et al. 2009). Interestingly, mice lacking PGC-1α show pronounced motor abnormalities and decreased PV protein expression in the motor cortex by 4 weeks of age (Lucas, Dougherty et al. 2012), suggesting that the motor cortex may be particularly dependent on PGC-1α for proper function. Previous investigations of inhibitory neurotransmission in the hippocampus of PGC-1α −/− mice (Lucas, Markwardt et al. 2010) suggest that inhibition is enhanced in this region, similar to what is observed in PV −/− mice (Vreugdenhil, Jefferys et al. 2003). However, it is possible that inhibition in the cortex is affected differentially by a lack of PGC-1α; it is therefore important to evaluate the impact of PGC-1α deficiency in the cortex, with relevance for disorders in which cortical PGC-1α deficits have been reported, including Parkinson Disease (Zheng, Liao et al. 2010) and Alzheimer Disease (Qin, Haroutunian et al. 2009).
In light of the deficiency in PV expression in the cortex of PGC-1α −/− mice and the profound motor dysfunction in these animals, we sought to determine the physiological impact of PGC-1α deletion on inhibitory neurotransmission in the motor cortex. We hypothesized that mice lacking PGC-1α would exhibit altered inhibitory transmission onto cortical pyramidal neurons and that PV+ interneurons would be especially affected. In order to investigate the potential role of PGC-1α in cortical inhibitory neurotransmission, we utilized motor cortex acute slices from a PGC-1α −/− mouse model (Lin, Wu et al. 2004). Our data show that, in contrast to results from the PGC-1α −/− hippocampus, a loss of PGC-1α leads to alterations in basal GABA release in the cortex, concurrent with reduced GABA release upon gamma frequency stimulation. Furthermore, in PGC-1α −/− mice expressing EGFP specifically in PV+ cells, we found that fast-spiking interneurons from PGC-1α −/− mice have a reduced firing rate upon current injection, suggesting that PGC-1α functions in a cell-autonomous manner to regulate interneuron excitability. Additionally, PV+ cells showed reduced synaptic excitatory activity, suggesting that PV+ cells could be less active in the motor cortex of PGC-1α −/− mice. Taken together these data suggest that reductions in PGC-1α expression are associated with deficiencies in inhibitory neurotransmission and synaptic function in the cortex. These results have implications for understanding the impact of PGC-1α alterations on cortical network signaling in disease, as synchronization of firing by PV+ interneurons is critical for normal cortical output and higher cognitive processing (Sohal, Zhang et al. 2009).
Methods
Animals
All experimental protocols were approved by the Institutional Animal Care and Use Committee of the University of Alabama at Birmingham. PGC-1α −/− mice (generous gift of Jiandie Lin, University of Michigan, (Lin, Wu et al. 2004)) were maintained on a C57BL/6J genetic background and housed two to five in a cage at 26±2°C room temperature with food and water ad libitum. All experiments were conducted with 4 week old male and female PGC-1α +/+ and −/− littermates generated by breeding PGC-1α +/− mice.
For pyramidal neuron recordings, PGC-1α +/+ and −/− mice were used. For targeted interneuron recordings, mice from the PGC-1α −/− line were crossed with mice expressing EGFP under the control of the GAD67 promoter (G42 line; JAX#7677) to generate EGFP-positive PGC-1α +/+ and EGFP-positive PGC-1α −/− littermates. This mouse line was chosen for two reasons: 1) EGFP is only expressed in the PV+ subset of inhibitory interneurons, primarily in the cortex (Chattopadhyaya, Di Cristo et al. 2004, Bartley, Huang et al. 2008) and 2) EGFP expression is not dependent on the activity of the PV promoter (which would be expected to be with the reduced in the absence of PGC-1α). All experiments were conducted in accordance adopted by the U.S. National Institutes of Guide for the Care and Use of Laboratory Animals Health.
For Pyramidal Neuron Recordings
Whole Cell Recording
Mice aged postnatal day (P) 27 to P33 were anesthetized with isoflourane and then decapitated. Brains were placed in ice-cold artificial CSF (ACSF) containing the following (in mM): 125 NaCl, 3.5 KCl, 0.5 CaCl2, 3.5 MgCl2, 26 NaHCO3 and 10 D-glucose. The ACSF was bubbled with 95%O2/5%CO2. Coronal brain slices (300 μm thick) containing motor cortex were cut using a Vibratome (Ted Pella, Inc., Riverside, CA). The slices were kept for 30 minutes at 37±1°C and then stored at room temperature (22±1°C). Slices were perfused continuously with oxygenated recording ACSF containing the following (in mM): 125 NaCl, 3.5 KCl, 2.0 CaCl2, 2.0 MgCl2, 26 NaHCO3 and 10 D-glucose at room temperature. Whole-cell patch clamp recordings were acquired from visually identified pyramidal neurons in layer five of the motor cortex. Position in cortex was verified through inclusion of 0.4% biocytin in the internal solution followed by streptavidin staining (Invitrogen, s32355). Recordings were conducted on a Zeiss AxioExaminer microscope (Carl Zeiss, Thornwood, NY). Cells were voltage clamped at -70 mV, using internal solution containing the following (in mM): 129 CsCl, 2 MgATP, 10 EGTA, 10 HEPES, 0.2 GTP and 2 QX-314, pH 7.2. Pipette tip resistance was 2–5 MΩ. Voltage clamp recordings were obtained using a PC505A amplifier (Warner Instruments, Hamden, CT) controlled by Clampex 8.0 software via a Digidata 1322A interface (Molecular Devices), filtered at 5 kHz and digitized at 10 kHz. Input resistance and series resistance were monitored by applying a 10 mV voltage step. Spontaneous IPSCs (sIPSCs) and evoked IPSCs were pharmacologically isolated with CNQX (6-cyano-7-nitroquinoxaline-2,3-dione) (10 μM) and DL-APV(DL-2-amino-5-phosphonoovaleric acid) (50 μM). Miniature IPSCs (mIPSCs) were recorded, on a separate cohort of animals, in the presence of CNQX, D-APV, and TTX (tetrodotoxin) (1 μM).
Stimulation
Synaptic responses were evoked with a bipolar stimulating electrode consisting of a twisted pair of 25 μm Formvar insulated nichrome wires. The electrode was positioned in layer V of the motor cortex. For each cell the stimulation threshold for evoking IPSCs was determined, and the stimulus intensity was set at twice the threshold intensity. A series of paired stimulations at 20, 30, and 100 ms intervals were applied to elucidate paired-pulse ratios. For gamma train recordings, 34 stimulations were applied in 500 ms to evoke a response in patched pyramidal cells (66 Hz). Recordings were taken at 32±1°C.
Data Analyses
Analyses of sIPSCs and mIPSCs were performed using the EVAN event analysis software (generously provided by Istvan Mody, UCLA, CA) and Clampfit 8.0 software which focused on amplitude, inter-event interval, rise time, and decay time of events. T50, which is 50% of the peak decay time, was used as a measure of decay time. All sIPSCs/mIPSCs that fit the template and passed visual inspection were included in the analysis. Analyses of all other electrophysiological experiments were performed using Clampfit 8.0, GraphPad Prism, and Microsoft Excel. To calculate the paired-pulse ratio, the amplitude of the second IPSC was measured after subtracting the first IPSC, and divided by the amplitude of the first IPSC relative to the baseline set immediately before the first stimulus. Train stimulation was analyzed by measuring the charge transfer (area under the curve) for 1 second after stimulation. The amplitude of the first pulse was also measured, as was the ratio of the last to first response (IPSC34/IPSC1). Response amplitudes were measured by taking the peak value minus the value immediately before stimulation. For figure 3E, we calculated IPSCn/IPSC1 for each pulse in the train and determined the standard deviations within the train for each cell. A two-tailed student t-test assuming unequal variance was utilized to assess statistical significance. Values were considered statistically significant when the p value was less than 0.05.
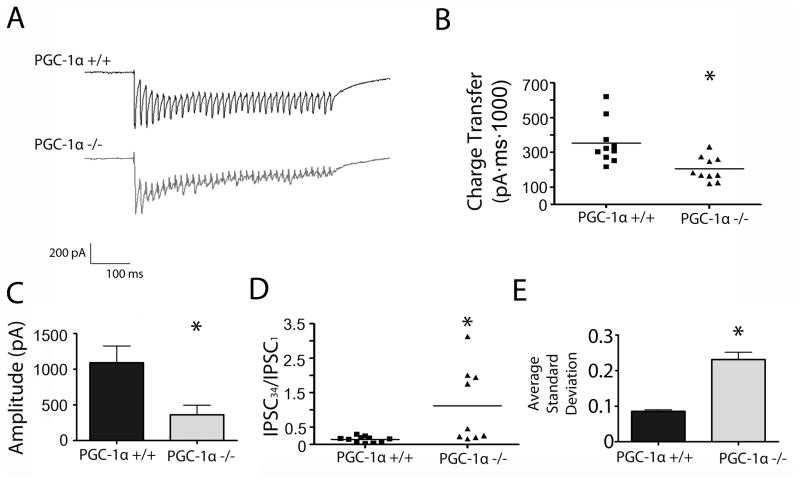
Figure 3. Absence of PGC-1a alters evoked IPSC activity in the motor cortex.
A. Representative traces of train stimulation and response in both PGC-1α +/+ and PGC-1α −/− animals (average of 10 sweeps per trace). B. Total charge transfer during the train (area under curve) was significantly reduced in PGC-1α −/− mice as compared to PGC-1α +/+ mice. C. The initial response amplitude is reduced in PGC-1α −/− mice as compared to PGC-1α +/+ mice. D. Alterations in short-term plasticity over the length of the train were also evident. E. The average standard deviation of IPSCn/IPSC1 across events of cells reveals much higher variation in the PGC-1α −/− as compared to the events from PGC-1α +/+ cells. Student’s t-test *p<0.05 n=3 animals/group, approx. 10 cells per condition.
For Interneuron Recordings
Slice Preparation
Experiments were conducted in 300 μm acute brain slices prepared from of P27 to P33 mice. The animals used were obtained from crossing the PGC1α heterozygous (Lucas et al., 2010) line with the G42 mouse line. In the G42 mouse line, GFP was only expressed in a subset of neocortical PV+ inhibitory neurons, the fast-spiking basket cells (Bartley et al, 2008; Chattopadhyaya et al., 2004). The mice were anesthetized with isoflurane and decapitated, and their brains were removed rapidly. Coronal slices of the brain were cut using a vibrating microtome (VT1000S; Leica, Bannockburn, IL). Slicing and dissection of the cortex was done in ice-cold (1–3°C) dissecting solution containing the following (in mM): 87 NaCl, 3 KCl, 0.5 CaCl2, 7.0 MgCl2, 1.25 NaH2PO4, 26 NaHCO3, 75 sucrose and 20 glucose, bubbled with 95% O2-5% CO2, pH 7.35–7.45. Slices were stored in a holding chamber containing a slightly modified artificial cerebral spinal fluid (ACSF, see below) for approximately 30 minutes at 30° to 32° C and then transferred to room temperature. Modified ACSF contained (in mM) 1 CaCl2 and 2 MgCl2. Slices were bubbled with 95% O2-5% CO2 for ≥1 h before recording.
Intrinsic Firing Assessment
During the experiment, slices were held in a submersion recording chamber perfused (3–4 mLs/min) with ACSF composed of (in mM): 126 NaCl, 3 KCl, 2 CaCl2, 1 MgCl2, 1.25 NaH2PO4, 26 NaHCO3, and 20 glucose. The solution was bubbled with 95% O2/5% CO2, and the pH was between 7.35 to 7.45. Picrotoxin (100 μM) was added to the external solution to block inhibitory synaptic responses mediated by GABAA receptors; 50 μM D-APV was added to prevent NMDA receptor mediated currents. All intrinsic firing experiments were performed at 28° to 30° C. Interneurons expressing EGFP were identified visually using infrared differential inference contrast optics and epifluorescent optics on a Nikon (New York) E600FN upright microscope. Targeted layer IV interneurons were patched in the voltage-clamp configuration and recorded in current-clamp configuration while maintaining a holding potential of −60 ± 1mV using an Axopatch 200B amplifier (Molecular Devices, Union City, CA). Patch electrodes (4–6MΩ) were filled with internal solution composed of the following (in mM): 150 K-gluconate, 0.1 EGTA, 3 NaCl, 6 KCl, 10 HEPES, 10 Na-ATP, and 0.3 GTP. pH was adjusted to 7.3 with KOH. The resting potential was measured immediately after break-in, and input resistance was measured in voltage-clamp with a 400-ms, −8-mV step from a −60-mV holding potential. Firing frequency versus injected current plots (F–I plots) were made by measuring the initial firing frequency of a spike train evoked by a series of incremental 600-ms current steps at intervals of 50 or 100pA. The spike threshold potential was defined as the membrane potential, in a 5-ms window preceding spike peak, at which the third derivative was a maximum (an inflection point). The action potential (AP) amplitude was calculated from the spike threshold to the peak of the AP. The AP half-width is the duration of the AP by the 500 pA current step. Afterhyperpolarization (AHP) was calculated from the spike threshold to the peak of the AHP. The access resistance and holding current (<200 pA) were monitored continuously. Recordings were rejected if either access resistance or holding current increased >20% during the experiment.
Electrophysiological Interneuron Classification
To distinguish fast-spiking (FS) interneurons from non-FS (NFS) interneurons, depolarizing current steps were used to analyze the firing response of each interneuron. Single spike properties were determined on spikes elicited by near threshold current injection. Spike-frequency adaptation was quantified by the ratio between the last and first interspike intervals in spike trains evoked by 600 ms depolarizing steps. Cells were classified as FS if they had the following (Rotaru, Yoshino et al. 2011): (1) by the 500 pA current step the firing frequency reached at least 150 Hz; (2) narrow spikes (duration at half peak amplitude ≤ 0.6 ms); (3) large afterhyperpolarizing potentials (amplitude ≥ 14 mV); and (4) absence of significant spike-frequency adaptation (adaptation ratio ≤ 1.5). These criteria may exclude some FS neurons. Spontaneous AMPA mediated EPSCs (sEPSCs) were pharmacologically isolated with 100 μM picrotoxin and 50 μM D-APV.
Biocytin filling of interneurons
Brain slices were prepared as described above. Interneurons expressing eGFP were identified visually using epifluorescent optics on a Nikon E600FN upright microscope (Melville, New York, USA). Patch electrodes (4–6MΩ) were filled with internal solution containing 0.4% biocytin. Cells were patched for 15–25 minutes to allow for adequate filling of the processes. After filling, slices were immediately placed in 4% PFA for 24–72 hours and stored in PBS until processing. Slices were washed in PBS, incubated in 10% methanol and 3.5% H2O2 in PBS for 10 minutes, washed in PBS, and incubated in TRITC conjugated steptavidin (Jackson Immunoresearch, West Grove, PA) in 0.3% PBST for two hours. After washes with PBS, slices were mounted onto charged microscope slides and coverslipped with Prolong Antifade Gold (Invitrogen). Neurolucida software (MBF Bioscience, Williston, VT, USA) was used to trace biocytin-labeled interneurons. As axons were not reliably labeled, only soma and dendrite characteristics were measured. Variables of interest included soma size, dendrite length and volume, and number of dendrites, branch points, and branches.
Data Analyses
All statistics were performed using Origin software (Origin Lab Corporation, 2002) and statistical significance was p < 0.05. Data are presented as means ± SE and sample number (n) refers to cell number for electrophysiological experiments. Statistical comparisons for electrophysiological data were made using the Student’s t-test or one-way ANOVA followed by Tukey’s posthoc analysis. In figures and table, * indicates a statistically significant difference.
Results
Basal GABA Release is Reduced in the Motor Cortex of PGC-1α −/− Mice
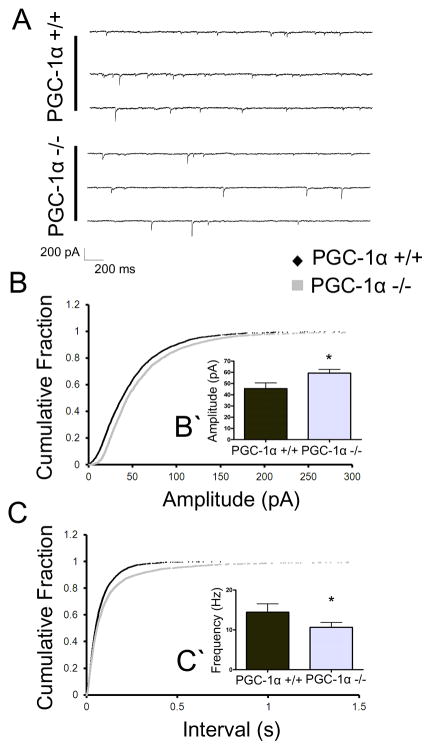
To assess basal inhibitory neurotransmission, we initially evaluated both spontaneous and miniature IPSCs in the motor cortex from PGC-1α −/− mice around postnatal day 30. This age was chosen based on evidence that these mice exhibit structural changes in the brain at older ages (Lucas, Dougherty et al. 2012) and that PGC-1α expression in the cortex peaks around postnatal day 14–21 (Cowell, Blake et al. 2007). The motor cortex is of particular interest because decreases in PV protein expression in PGC-1α −/− mice are very robust in this region (Lucas et al. 2010), and these mice have pronounced motor deficits (Lucas, Dougherty et al. 2012). Whole cell voltage clamp recordings were performed on layer V pyramidal neurons in the motor cortex in the presence of APV and CNQX. The observed sIPSCs exhibited increased amplitudes (fig 1B&B′, p = 0.0363, t(19) = 2.252) and decreased frequency (fig 1C&C′, p = 0.0265, t(14) = 1.993) in PGC-1α −/− mice as compared to PGC-1α +/+ littermates. There were no significant differences in event kinetics as measured by rise time (2.12 ± 0.51ms in PGC-1α +/+ mice and 2.38 ± 0.24ms in PGC-1α −/− mice; p = 0.627) or time to decay to 50% of peak value (T50 2.69 ± 0.35ms in PGC-1α +/+ mice and 3.54 ± 0.23ms in PGC-1α −/− mice; p = 0.07).
Figure 1. Global ablation of PGC-1α results in alterations in basal GABA release.
A. Representative traces of sIPSCs from PGC-1α +/+ and PGC-1α −/− animals B. Cumulative probability plot of sIPSC amplitudes for PGC-1α +/+ and PGC-1α −/− groups. Curves are shifted to the right in the PGC-1α −/− group, indicating an increase in amplitude. Insert (B′) shows histogram summarizing effects on mean amplitude. C. Similar to B but showing cumulative probability plots of inter-event intervals. C′ sIPSC frequency (extrapolated from inter-event interval) was reduced in the PGC-1α −/− animals as evidenced by a lengthening of the inter-event interval. The observed changes in sIPSCs are indicative of alterations in basal GABA release from the cortical interneurons. Student’s t-test *p<0.05 n=3 animals/group, approx. 10 cells per condition.
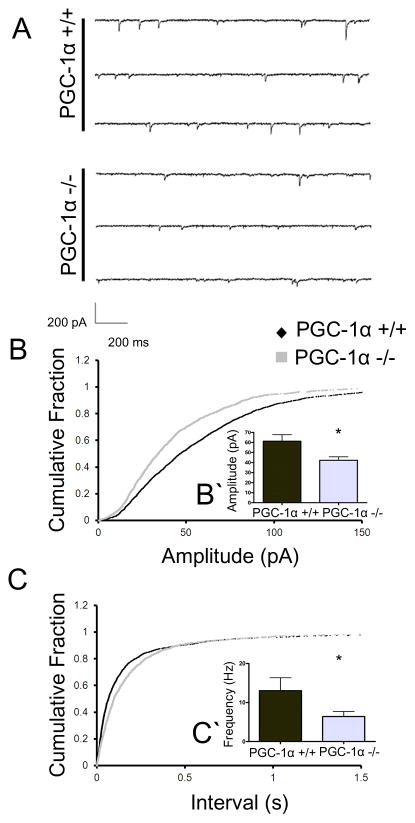
Action potential-mediated activity and spontaneous vesicle fusion events (miniature IPSCs, mIPSCs) can be differentially regulated (Ramirez and Kavalali 2011). Therefore, we pharmacologically isolated mIPSCs with bath application of TTX to eliminate action potential-mediated activity. The observed mIPSCs exhibited decreased amplitudes (fig 2B&B′, p = 0.0231, t(14) = 2.551) and decreased frequencies (fig 2C&C′, p = 0.0426, t(14) = 1.853) in PGC-1α −/− mice as compared to those measured in +/+ littermates. There were no significant differences in event kinetics as measured by rise time (1.82 ± 0.23ms in PGC-1α +/+ mice and 2.18 ± 0.16ms in PGC-1α −/− mice; p = 0.19) or time to decay to 50% of peak value (T50 2.86 ± 0.36ms in PGC-1α +/+ mice and 3.14 ± 0.30ms in PGC-1α −/− mice; p = 0.8). These alterations suggest that PGC-1α differentially affects spontaneous vesicle release and action potential-mediated activity; mIPSCs are smaller and less frequent whereas action potential-regulated events are larger in magnitude but still less frequent.
Figure 2. Global ablation of PGC-1α results in alterations in spontaneous inhibitory vesicle fusion events.
A. Representative traces of mIPSCs from PGC-1α +/+ and PGC-1α −/− animals. B, C. Cumulative probability plots of amplitude and interval, respectively. B′. Quantification revealed that the mIPSC amplitude was reduced in the PGC-1α −/− animals as compared to PGC-1α +/+ mice. C′. mIPSC frequency (extrapolated from inter-event interval) was reduced in the PGC-1α −/− animals as evidenced by a lengthening of the inter-event interval. Student’s t-test *p<0.05 n=3 animals/group, approx. 10 cells per condition.
Global Ablation of PGC-1α Is Associated with Reduced Evoked Cortical GABA Release with Gamma Frequency Stimulation
In order to further investigate the presynaptic contribution to the altered GABA neurotransmission in these mice, we recorded evoked IPSCs at a stimulus intensity set to be twice the intensity threshold for producing detectible IPSCs, and measured short-term plasticity in response to paired stimuli and short stimulus trains. In response to paired-pulse stimulation, which has been shown to indirectly measure presynaptic release (Dobrunz, Huang et al. 1997, Dobrunz and Stevens 1997), we saw no change in PGC-1α −/− mice in the paired-pulse ratio of inhibitory responses onto pyramidal neurons in motor cortex (data not shown). To evaluate how a lack of PGC-1α affects evoked GABA release during stimulation trains, we applied repetitive trains of stimuli in the gamma frequency range, which mimics the frequency at which PV+ interneurons entrain the cortical network (Traub, Whittington et al. 1996, Wang and Buzsaki 1996). Example traces are shown in Figure 3A; while responses from PGC-1α +/+ mice consistently showed short-term depression during the train, responses from PGC-1α −/− mice were more variable. Some cells in PGC-1α −/− mice showed even larger short-term depression whereas others showed a mixture of facilitation and depression. We observed a reduction in overall charge transfer in the PGC-1α −/− mice as compared to PGC-1α +/+ mice (fig 3B, p = 0.0044, t(18) = 3.257). This was caused in part by a reduction in the initial response size (fig 3C, p = 0.025, t(7) = 2.838). When we compared the amplitude of the final response to the amplitude of the first response to evaluate short-term plasticity over the stimulation period, we found that the ratio of the last response to the first response was consistently <1 in PGC-1α +/+ mice, while the ratio was larger and more variable in PGC-1α −/− mice (fig 3D, p = 0.0082, t(18) = 2.967). The decrease in short-term depression (increase in IPSC34/IPSC1) is consistent with a decrease in the initial release probability, which could also contribute to the observed reduction in the initial response size. Further, the stimulation to stimulation variability across events, measured as the standard deviation of IPSCn/IPSC1 across all stimuli in the train, is significantly larger in cells from the PGC-1α −/− mice (fig 3E, p < 0.0001, t(64) = 6.996). There is no change in event decay kinetics as measured by decay slope (p = 0.158). These observations suggest altered regulation of synchronous release in response to gamma frequency stimulation. This could potentially result from the exhaustion of actively docked vesicles in the PGC-1α −/− mice, or from the stimulated axons firing less reliably in the PGC-1α −/− mice compared to PGC-1α +/+ mice.
Global Ablation of PGC-1α Causes Reductions in Intrinsic Firing Rate and Excitability of Fast-Spiking Interneurons
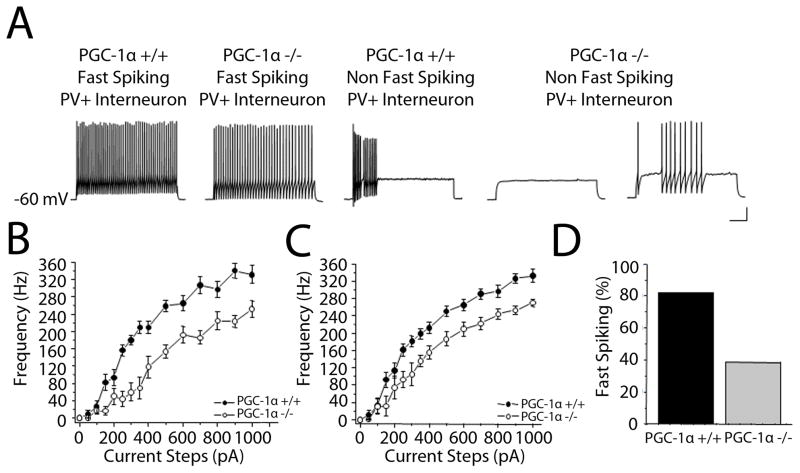
Two interneuron subtypes that contribute significantly to perisomatic inhibition of layer V pyramidal neurons are PV-positive and cholecystokinin (CCK)-positive interneurons. Based on our previous findings of PV deficiency in PGC-1α −/− mice and the high concentration of PGC-1α in PV-positive interneurons (Jiang, Rompala et al. 2013), we sought to determine whether the intrinsic firing properties of PV-positive interneurons are disrupted in PGC-1α −/− mice. It is possible that reductions in any number of PGC-1α’s putative targets, including PV and metabolic genes (St-Pierre, Drori et al. 2006, Cowell, Talati et al. 2009), could contribute to alterations in calcium concentrations and depletion of metabolic substrates for the maintenance of normal intrinsic firing. To allow for visualization of PV+ neurons, whose somas are concentrated in cortical layer IV, we generated PGC-1α +/+ and −/− mice with EGFP-positive PV+ interneurons by crossing PGC-1α −/− mice with the G42 GAD-EGFP mouse line (Chattopadhyaya, Di Cristo et al. 2004, Bartley, Huang et al. 2008) to use for whole cell current clamp recordings. Upon current injection, PV+ interneurons, in layer IV, of EGFP-PGC-1α −/− mice showed a reduction in excitability as compared to +/+ littermates (fig 4A&B, ANOVA, p < 0.02). The frequency is still reduced in PGC-1α −/− mice when comparing only fast-spiking PV+ interneurons (fig 4C, Table 1). Furthermore, fast-spiking PV interneurons in EGFP-PGC-1α −/− mice showed an increase in afterhyperpolarization and a reduction in frequency at the maximum current step with no alteration in resting membrane potential or input resistance (Table 1). Interestingly, there was a large reduction in the percentage of PV+ interneurons that met the classification criteria for fast-spiking in the EGFP-PGC-1α −/− mice (fig 4D). This reduction in interneuron excitability could contribute to the observed reduction in action potential-mediated GABA release in the cortex during gamma stimulation.
Figure 4. Absence of PGC-1α alters interneuron intrinsic excitability.
A. Representative traces of the intrinsic firing due to an injection of a 200 pA current step in fast-spiking and non-fast-spiking PV+ interneurons from PGC-1α +/+ and PGC-1α −/− animals. B. Data are plotted as firing frequency as a function of injected current (F–I). The PGC-1α −/− fast-spiking PV+ interneurons overall have decreased excitability compared to PGC-1α +/+ fast-spiking PV+ interneurons (n=11 PGC-1α +/+, n=13 PGC-1α −/−). C. The firing frequency is still decreased in PGC-1α −/− mice when comparing only the fast-spiking interneurons (n= 9, 5). D. The percentage of PV+ interneurons that met the criteria for classification as fast spiking interneurons was also reduced.
Table 1. Intrinsic properties of PV interneurons in motor cortex from PGC-1α +/+ and PGC-1α −/− mice.
The values displayed in bold show statistically significant differences. The majority of the intrinsic properties between fast-spiking and non fast spiking PV interneurons are not significantly altered between genotypes.
| WT (9) All PV INs |
PGC1a KO (13) All PV INs |
p-value | WT (8) Fast-Spiking |
PGC1a KO (5) Fast-Spiking |
p-value | WT (2) Non Fast-Spiking |
PGC1a KO (8) Non Fast-Spiking |
p-value | |
|---|---|---|---|---|---|---|---|---|---|
| Resting Potential (mV) | −58.56 | −55.92 | 0.610 | −56.00 | −65.60 | 0.163 | −56.00 | −48.63 | 0.317 |
| Input Resistance (MΩ) | 172.89 | 145.15 | 0.369 | 160.13 | 206.20 | 0.252 | 200.00 | 107.00 | 0.096 |
| Spiking Threshold (mV) | −44.09 | −39.79 | 0.037 | −43.63 | −44.01 | 0.666 | −43.04 | −37.77 | 0.149 |
| Action Potential Amplitude (mV) | 59.66 | 51.74 | 0.073 | 54.27 | 54.39 | 0.979 | 64.60 | 46.29 | 0.019 |
| Action Potential Half-Width (ms) | 0.54 | 0.59 | 0.196 | 0.51 | 0.54 | 0.222 | 0.69 | 0.63 | 0.530 |
| AHP (mV) | 17.87 | 17.06 | 0.063 | 17.41 | 21.68 | 0.007 | 19.87 | 14.31 | 0.334 |
| Frequency at Maximum Current Step (Hz) | 314.21 | 251.57 | 0.040 | 327.98 | 268.46 | 0.020 | 185.37 | 236.70 | 0.428 |
Global Ablation of PGC-1α Results in Alterations in the Excitatory Synaptic Activity onto Fast-Spiking Interneurons without altering Interneuron Morphology
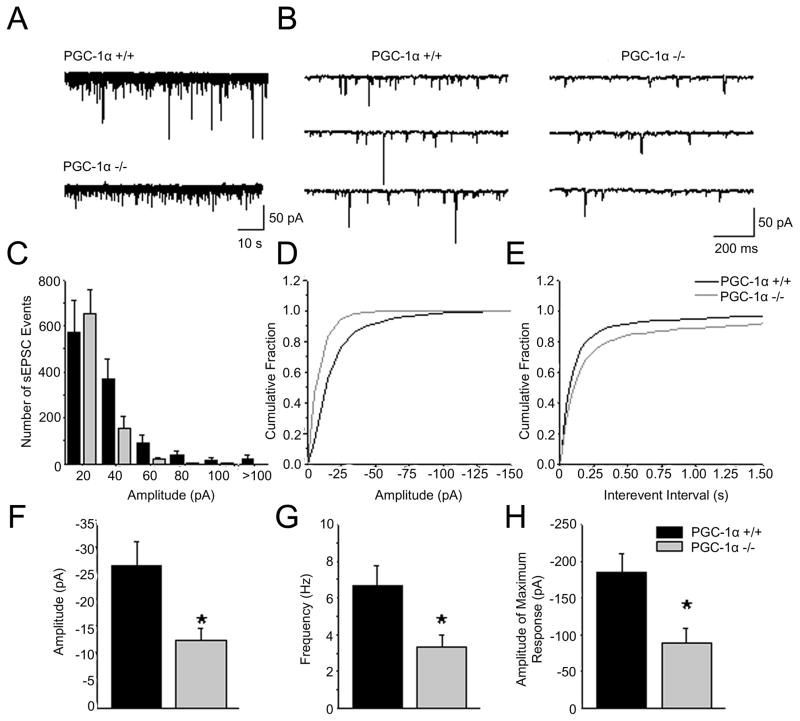
The decrease in the frequency of sIPSCs onto pyramidal cells in PGC-1α −/− mice could be caused by reduced activation of PV+ interneurons. In order to determine if activation of these cells is affected by the absence of PGC-1α, whole cell voltage clamp recordings were performed on EGFP-positive PV+ interneurons in layer IV of the motor cortex in the presence of picrotoxin and APV to isolate AMPA mediated sEPSCs. The observed sEPSCs exhibited decreased amplitudes (fig 5D&F, p = 0.0098, t(11)= 3.120) and decreased frequency (fig 5E&G, p = 0.00381, t(11) = 3.651) in PGC-1α −/− mice as compared to PGC-1α +/+ littermates. Interestingly, the decrease in sEPSC amplitudes is due to a loss of the large sEPSCs events (fig 5C&H, p = 0.04094, t(11) = 2.315). These large sEPSCs events are normally caused by glutamate release in response to action potentials. Additionally, it is possible that the large events observed are multiquantal and that the extent of multiquantal release is reduced in the PGC-1α −/− mice. The number of smaller sEPSCs events (≤ 20 pAs) is unaltered between PGC-1α −/− and PGC-1α +/+ mice. These results suggest that a possible consequence of a lack of PGC-1α is decreased excitatory drive onto PV+ interneurons, potentially leading to a decrease in spontaneous inhibition and a blunted response to gamma frequency stimulation.
Figure 5. Global ablation of PGC-1α results in decreased spontaneous excitatory activity onto parvalbumin interneurons in the motor cortex.
A. Representative traces of sEPSCs onto PV+ interneurons in PGC-1α +/+ and PGC-1α −/− animals. B. Representative traces of sEPSCs in PGC-1α +/+ and PGC-1α −/− animals on an expanded time scale. C. Histogram plot of sEPSCs onto PV+ interneurons from PGC-1α +/+ (black) and PGC-1α −/− (grey) animals. The larger amplitude sEPSCs are diminished in the PGC-1α −/− animals D, E. Cumulative probability plots of amplitude and inter-event interval, respectively. F. Quantification revealed that the sEPSC amplitude was reduced in the PGC-1α −/− animals compared to PGC-1α +/+. G. sEPSC frequency was reduced in the PGC-1α −/− animals. H. The maximum amplitude of sEPSCs is reduced on the PV+ interneurons in the PGC-1α −/− animals. Student’s t-test *p<0.05 (PGC-1α +/+ n=6; PGC-1α −/− n= 8)
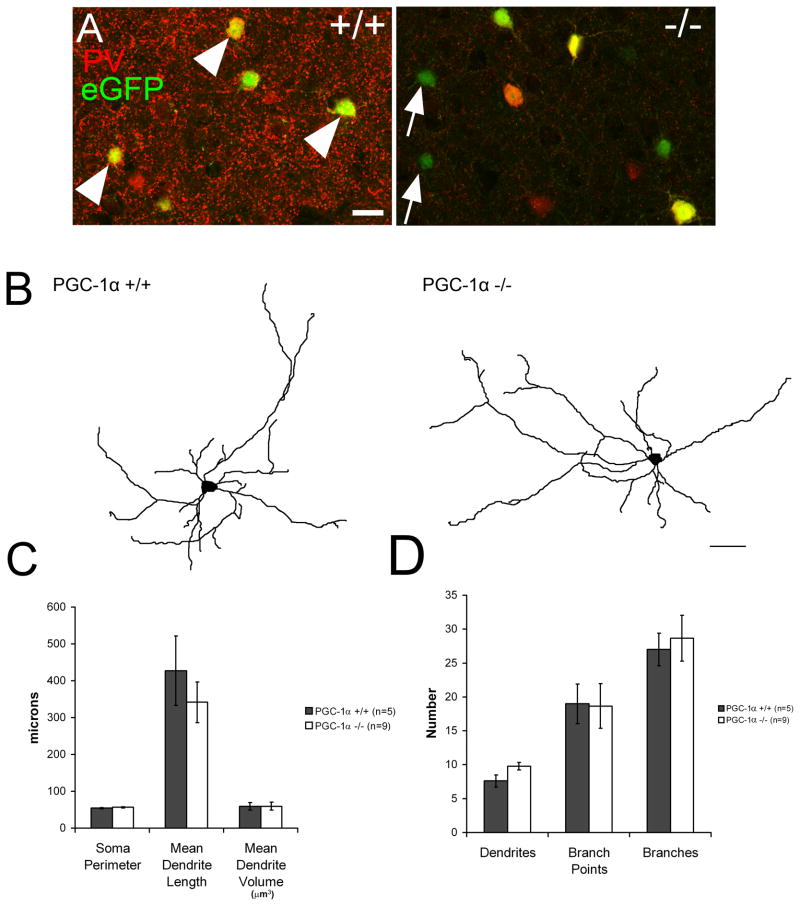
In order to assess the potential contribution of interneuron morphological changes in the observed physiological abnormalities of the PGC-1α −/− mice we performed structural analysis on EGFP+ PV+ interneurons. While PV immunoreactivity is strong in the processes and cell bodies of PGC-1α +/+ EGFP+ interneurons (fig 6A) EGFP+ PV-negative cells are observed (6A, arrows) in PGC-1α −/− cortex, demonstrating that the interneurons are still present but lack PV expression. To determine if loss of PGC-1α affects interneuron morphology, interneurons patched in the previously described experiments were filled with biocytin, counterstained with streptavidin-TRITC, imaged and traced with Neurolucida software (sample traces, fig 6B). Cell soma size and dendrite characteristics were measured (fig 6C&D, n= 5–9 cells/group). No significant differences in interneuron morphology were observed between PGC-1α +/+ and −/− mice, with no changes in the size and/or number of dendrites, branches, or branch points.
Figure 6. Unaltered PV interneuron morphology in PGC-1α −/− mice.
A. Immunohistochemistry reveals that PV and EGFP colocalize as expected in PGC-1α +/+ animals (arrowheads). In PGC-1α −/− mice, EGFP+ PV-negative cells are observed (arrows). B. Sample traces of PV+ interneurons with morphological quantification of size (C) and number (D) of soma perimeter, dendrites, branches, and branch points. No differences are observed between PGC-1α +/+ and PGC-1α −/− measurements. (n=5–9 cells/group) Scale bars A = 25 μm, B = 50 μm.
Discussion
Despite reports of PGC-1α deficiency in multiple neurological disorders and the documented ability of PGC-1α to drive transcriptional programs in energy-demanding tissues, little is known about the impact of PGC-1α deficiency on neurotransmission and neuronal function in the cortex. To determine the functional consequences of a lack of PGC-1α expression, we performed electrophysiological recordings in acute slices from the motor cortex of PGC-1α −/− mice. Considering that PGC-1α is most highly concentrated in GABAergic cell populations (Cowell, Blake et al. 2007), we postulated that alterations in PGC-1α would result in dysfunction of inhibitory neurotransmission. Here we show that PGC-1α −/− mice exhibit dysfunctional inhibitory transmission in the motor cortex characterized by alterations in basal GABA release. Upon repetitive stimulation at the gamma frequency, there is a marked reduction in overall charge transfer. Finally, PV+ interneurons displayed reduced intrinsic excitability and decreased excitatory drive. As these mice display impairments in motor function, including reductions in coordination and an increased occurrence of resting tremor (Lucas, Dougherty et al. 2012), it is intriguing to postulate that the physiological alterations in cortical function contribute to hyperactivity via overexcitation of downstream neuronal targets.
In the absence of PGC-1α, PV expression is reduced by approximately 80% in the cortex (Lucas, Markwardt et al. 2010); as such, it is possible that a loss of PV could be contributing to the synaptic deficits in these mice. While the electrophysiological responses to gamma frequency stimulation are similar in the hippocampus of PGC-1α −/− and PV −/− mice (Schwaller, Dick et al. 1999, Caillard, Moreno et al. 2000, Vreugdenhil, Jefferys et al. 2003, Lucas, Markwardt et al. 2010), motor impairment is much more severe in PGC-1α −/− mice than PV −/− mice (Schwaller, Dick et al. 1999, Farre-Castany, Schwaller et al. 2007, Lucas, Dougherty et al. 2012). Therefore it is possible that alterations in other PGC-1α-dependent transcripts in PV+ interneurons, such as metabolic regulators (Lin, Wu et al. 2004, Finck and Kelly 2006, St-Pierre, Drori et al. 2006) or synaptic proteins (St-Pierre, Drori et al. 2006, Cowell, Talati et al. 2009) could be causing changes in inhibitory neurotransmission. Considering the reduced excitatory drive onto PV+ interneurons in PGC-1α −/− mice, it is possible that while PGC-1α is not highly concentrated in pyramidal neurons, its loss could have an effect on pyramidal neuron function. In fact, previous work suggests that deletion of PGC-1α in excitatory neurons can cause structural abnormalities (vacuolizations) in the cortex (Ma, Li et al. 2010), although the functional consequences of PGC-1α deletion from pyramidal neurons have not been evaluated. Additionally, we previously suggested that the observed higher facilitation in the hippocampus was due to a deficiency of PV at presynaptic terminals and reduced PV-mediated calcium buffering. In contrast, we observed a reduction in IPSC amplitude and charge transfer in the cortex; we believe that changes in the expression of transcripts, in addition to PV, could be occurring in the cortex, contributing to the observed changes in cortical physiology.
PV interneurons are described as primarily fast-spiking and non-adapting (Kawaguchi 1993, Kawaguchi and Kondo 2002, Tepper and Bolam 2004). These intrinsic properties, together with their perisomatic targeting of pyramidal neurons (Freund and Katona 2007), make PV+ interneurons ideal modulators of network oscillations in cortex, particularly gamma oscillations (Freund 2003, Bartos, Vida et al. 2007, Sohal 2012). PV−/− mice have an enhancement of kainate induced gamma oscillations in hippocampal CA3 (Vreugdenhil, Jefferys et al. 2003). However, mice in which NMDA receptors onto PV interneurons have been ablated exhibit a loss of regional synchrony in the hippocampus and deficits in working memory (Korotkova, Fuchs et al. 2010). Further studies could provide additional insight into how the absence of PGC-1α, which is associated with both a reduction in PV levels and an impairment in excitatory input onto PV neurons, affects gamma oscillations and cognition.
There are multiple ways in which decreases in inhibitory neurotransmission could arise. A loss of GABAergic neurons, for example, could lead to the observed decreases in sIPSC and mIPSC frequency. However, Glutamic Acid Decarboxylase 67 immunostaining is normal in the cortex (Lucas, Markwardt et al. 2010), no appreciable difference in the number of EGFP+ interneurons in PGC-1α −/− mice was noted when performing recordings of PV+ interneurons, and dendritic morphology and soma size are not affected. It is possible, though, that a subtle reduction in perisomatic innervation of pyramidal neurons by PV+ interneurons could contribute to this result, as neurons from knockout mice have reduced complexity of axonal arbors in culture (Lin, Wu et al. 2004). Additionally, there is a small reduction in mIPSC amplitude in the PGC-1α −/− mice. Since PV+ interneurons primarily innervate the soma, the reduced mIPSC amplitude could potentially be caused by a reduction in the number of synaptic contacts from PV+ interneurons. This would result in a relatively greater contribution of dendritic targeting interneurons in PGC-1α −/− mice compared to PGC-1α +/+ mice, which would also be expected to cause mIPSCs to have slower kinetics (Magee 2000). However, there were no significant changes in mIPSC kinetics, making this unlikely.
A lack of PGC-1α −/− is also accompanied by changes in the intrinsic properties of PV+ interneurons, including a decrease in firing rate in response to current injection and a reduction in the percentage of PV+ interneurons that met the criteria to be classified as fast spiking. These changes in PV+ cell firing rate could be caused by changes in metabolic genes regulated by PGC-1α (Finck and Kelly 2006); there is abundant evidence for a high metabolic requirement for cortical PV+ interneurons (reviewed in (Jiang, Cowell et al. 2013)). A loss of PV itself could contribute to the decreased firing rate, as loss of the calcium buffer in PV knockout mice has been shown to alter intrinsic excitability of GABAergic neurons in the reticular thalamic nucleus (Alberi, Lintas et al. 2013). However it is also possible that other factors contribute to this reduction. In addition, there was a reduction in the frequency and amplitude of sEPSCs onto PV+ interneurons in the PGC-1α −/− mice. Altogether, these changes could cause a decrease in spontaneous action potential activity in PV+ cells, which could contribute to the decrease in sIPSC frequency observed in layer V pyramidal cells. The observed increase in sIPSC amplitude seems contrary to this line of reasoning; however, it is possible that the increase in sIPSC amplitude could be an indirect consequence of the reduced mIPSC frequency. The loss of the smallest events would bias the sIPSC measurements in favor of larger events.
The change in intrinsic excitability could also contribute to the decrease in IPSCs onto pyramidal cells at gamma frequency, if the presynaptic axons from PV+ interneurons are not able to fire as reliably at 66 Hz in the PGC-1α −/− mice. The observed reduction in initial response size could contribute to the reduced charge transfer observed. Though when given in train stimulation if the first event is small we observed that the size of the subsequent responses can still increase over time. Alternatively, the decrease in GABA release during gamma frequency stimulation could be caused by the depletion of vesicles in the PGC-1α −/− mice. Because short-term depression during trains of stimuli is governed in part by the size of the readily releasable vesicle pool (Dobrunz 2002), this could be caused by a reduction in the number of readily releasable vesicles (Pozzo-Miller, Gottschalk et al. 1999), which are thought to equal the number of docked vesicles (Schikorski and Stevens 2001). A change in the number of readily releasable vesicles might be expected to also alter the paired pulse ratio (Walters, Hallengren et al. 2014), which was seen at dentate gyrus synapses from PGC-1α−/− mice (Lucas, Markwardt et al. 2010) but not the cortical synapses studied here. However, other examples have shown no difference in the paired pulse ratio between two types of synapses but differences in short-term plasticity during longer physiological trains (Speed and Dobrunz 2009).
Our studies of evoked inhibitory transmission utilized perisomatic (inter-layer V) stimulation in efforts to primarily activate axons from GABAergic cell populations that target the soma of pyramidal neurons. As previously discussed, PGC-1α is concentrated primarily in GABAergic neurons and tightly regulates PV; therefore, it is tempting to postulate that the main subtype of interneuron contributing to the observed physiological changes is the PV+ interneuron. In fact, we found that the excitatory input onto and the firing rate of PV+ interneurons are reduced in the absence of PGC-1α. However, it is possible that other interneuron populations also contribute to alterations in inhibitory neurotransmission. For example, cholecystokinin (CCK)+ interneurons also target the perisomatic regions of pyramidal neurons and are thought to work in concert with PV+ cells to regulate oscillations (reviewed in (Freund and Katona 2007)). It is possible that PGC-1α is playing a regulatory role on transcription within the CCK+ interneuron subtype. Cell-specific PGC-1α ablation studies could shed light on the role of these individual populations in the observed synaptic dysfunction and allow for differentiation of interneuron susceptibility in response to loss of PGC-1α.
Here we show that genetic ablation of the coactivator PGC-1α results in overt alterations in GABAergic transmission in the motor cortex that are different from what was previously seen in hippocampus. The reduction in GABAergic transmission during high frequency stimulation could result in hyperexcitability and contribute to the observed motor dysfunction in these animals. Although further studies are required to identify additional PGC-1α-regulated transcripts that could influence interneuron function and definitively link deficits in inhibitory neurotransmission to the motor abnormalities, these studies highlight the critical role for PGC-1α in the maintenance of normal cortical inhibition.
Mice lacking PGC-1α exhibit alterations in basal inhibition in the motor cortex
Absence of PGC-1α causes attenuation of GABA release upon repetitive stimulation
PGC-1α is critical for the maintenance of intrinsic properties of PV+ interneurons
Deficiencies in PGC-1α could contribute to cortical and motor dysfunction in disease
Acknowledgments
This work was funded by National Institutes of Health (NIH) Grant 1R01NS070009-04 (R.M.C.), 1R01MH098534-02 (L.E.D.), 5P30NS047466-09 (J.J.H.), and the Civitan McNulty Scientist (RMC) and Emerging Scholar (EKL) Awards. We would like to acknowledge the work of Grace Nix in performing immunuohistochemistry for PV/EGFP images. We would like to thank the members of the Hablitz laboratory for additional instruction and aid in electrophysiological training.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alberi L, Lintas A, Kretz R, Schwaller B, Villa AE. The calcium-binding protein parvalbumin modulates the firing 1 properties of the reticular thalamic nucleus bursting neurons. J Neurophysiol. 2013;109(11):2827–2841. doi: 10.1152/jn.00375.2012. [DOI] [PubMed] [Google Scholar]
- Bartley AF, Huang ZJ, Huber KM, Gibson JR. Differential activity-dependent, homeostatic plasticity of two neocortical inhibitory circuits. J Neurophysiol. 2008;100(4):1983–1994. doi: 10.1152/jn.90635.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartos M, Vida I, Frotscher M, Meyer A, Monyer H, Geiger JR, Jonas P. Fast synaptic inhibition promotes synchronized gamma oscillations in hippocampal interneuron networks. Proc Natl Acad Sci U S A. 2002;99(20):13222–13227. doi: 10.1073/pnas.192233099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartos M, Vida I, Jonas P. Synaptic mechanisms of synchronized gamma oscillations in inhibitory interneuron networks. Nat Rev Neurosci. 2007;8(1):45–56. doi: 10.1038/nrn2044. [DOI] [PubMed] [Google Scholar]
- Caillard O, Moreno H, Schwaller B, Llano I, Celio MR, Marty A. Role of the calcium-binding protein parvalbumin in short-term synaptic plasticity. Proc Natl Acad Sci U S A. 2000;97(24):13372–13377. doi: 10.1073/pnas.230362997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chattopadhyaya B, Di Cristo G, Higashiyama H, Knott GW, Kuhlman SJ, Welker E, Huang ZJ. Experience and activity-dependent maturation of perisomatic GABAergic innervation in primary visual cortex during a postnatal critical period. J Neurosci. 2004;24(43):9598–9611. doi: 10.1523/JNEUROSCI.1851-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaturvedi RK, Calingasan NY, Yang L, Hennessey T, Johri A, Beal MF. Impairment of PGC-1alpha expression, neuropathology and hepatic steatosis in a transgenic mouse model of Huntington’s disease following chronic energy deprivation. Hum Mol Genet. 2010;19(16):3190–3205. doi: 10.1093/hmg/ddq229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christoforou A, Le Hellard S, Thomson PA, Morris SW, Tenesa A, Pickard BS, Wray NR, Muir WJ, Blackwood DH, Porteous DJ, Evans KL. Association analysis of the chromosome 4p15-p16 candidate region for bipolar disorder and schizophrenia. Mol Psychiatry. 2007;12(11):1011–1025. doi: 10.1038/sj.mp.4002003. [DOI] [PubMed] [Google Scholar]
- Cowell RM, Blake KR, Russell JW. Localization of the transcriptional coactivator PGC-1alpha to GABAergic neurons during maturation of the rat brain. J Comp Neurol. 2007;502(1):1–18. doi: 10.1002/cne.21211. [DOI] [PubMed] [Google Scholar]
- Cowell RM, Talati P, Blake KR, Meador-Woodruff JH, Russell JW. Identification of novel targets for PGC-1alpha and histone deacetylase inhibitors in neuroblastoma cells. Biochem Biophys Res Commun. 2009;379(2):578–582. doi: 10.1016/j.bbrc.2008.12.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui L, Jeong H, Borovecki F, Parkhurst CN, Tanese N, Krainc D. Transcriptional repression of PGC-1alpha by mutant huntingtin leads to mitochondrial dysfunction and neurodegeneration. Cell. 2006;127(1):59–69. doi: 10.1016/j.cell.2006.09.015. [DOI] [PubMed] [Google Scholar]
- Dobrunz LE. Release probability is regulated by the size of the readily releasable vesicle pool at excitatory synapses in hippocampus. Int J Dev Neurosci. 2002;20(3–5):225–236. doi: 10.1016/s0736-5748(02)00015-1. [DOI] [PubMed] [Google Scholar]
- Dobrunz LE, Huang EP, Stevens CF. Very short-term plasticity in hippocampal synapses. Proc Natl Acad Sci U S A. 1997;94(26):14843–14847. doi: 10.1073/pnas.94.26.14843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobrunz LE, Stevens CF. Heterogeneity of release probability, facilitation, and depletion at central synapses. Neuron. 1997;18(6):995–1008. doi: 10.1016/s0896-6273(00)80338-4. [DOI] [PubMed] [Google Scholar]
- Farre-Castany MA, Schwaller B, Gregory P, Barski J, Mariethoz C, Eriksson JL, Tetko IV, Wolfer D, Celio MR, Schmutz I, Albrecht U, Villa AE. Differences in locomotor behavior revealed in mice deficient for the calcium-binding proteins parvalbumin, calbindin D-28k or both. Behav Brain Res. 2007;178(2):250–261. doi: 10.1016/j.bbr.2007.01.002. [DOI] [PubMed] [Google Scholar]
- Finck BN, Kelly DP. PGC-1 coactivators: inducible regulators of energy metabolism in health and disease. J Clin Invest. 2006;116(3):615–622. doi: 10.1172/JCI27794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freund TF. Interneuron Diversity series: Rhythm and mood in perisomatic inhibition. Trends Neurosci. 2003;26(9):489–495. doi: 10.1016/S0166-2236(03)00227-3. [DOI] [PubMed] [Google Scholar]
- Freund TF, Katona I. Perisomatic inhibition. Neuron. 2007;56(1):33–42. doi: 10.1016/j.neuron.2007.09.012. [DOI] [PubMed] [Google Scholar]
- Hettema JM, Webb BT, Guo AY, Zhao Z, Maher BS, Chen X, An SS, Sun C, Aggen SH, Kendler KS, Kuo PH, Otowa T, Flint J, van den Oord EJ. Prioritization and association analysis of murine-derived candidate genes in anxiety-spectrum disorders. Biol Psychiatry. 2011;70(9):888–896. doi: 10.1016/j.biopsych.2011.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang Z, Cowell RM, Nakazawa K. Convergence of genetic and environmental factors on parvalbumin-positive interneurons in schizophrenia. Front Behav Neurosci. 2013;7:116. doi: 10.3389/fnbeh.2013.00116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang Z, Rompala GR, Zhang S, Cowell RM, Nakazawa K. Social Isolation Exacerbates Schizophrenia-Like Phenotypes via Oxidative Stress in Cortical Interneurons. Biol Psychiatry. 2013 doi: 10.1016/j.biopsych.2012.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawaguchi Y. Physiological, morphological, and histochemical characterization of three classes of interneurons in rat neostriatum. J Neurosci. 1993;13(11):4908–4923. doi: 10.1523/JNEUROSCI.13-11-04908.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawaguchi Y, Kondo S. Parvalbumin, somatostatin and cholecystokinin as chemical markers for specific GABAergic interneuron types in the rat frontal cortex. J Neurocytol. 2002;31(3–5):277–287. doi: 10.1023/a:1024126110356. [DOI] [PubMed] [Google Scholar]
- Korotkova T, Fuchs EC, Ponomarenko A, von Engelhardt J, Monyer H. NMDA receptor ablation on parvalbumin-positive interneurons impairs hippocampal synchrony, spatial representations, and working memory. Neuron. 2010;68(3):557–569. doi: 10.1016/j.neuron.2010.09.017. [DOI] [PubMed] [Google Scholar]
- Lin J, Wu PH, Tarr PT, Lindenberg KS, St-Pierre J, Zhang CY, Mootha VK, Jager S, Vianna CR, Reznick RM, Cui L, Manieri M, Donovan MX, Wu Z, Cooper MP, Fan MC, Rohas LM, Zavacki AM, Cinti S, Shulman GI, Lowell BB, Krainc D, Spiegelman BM. Defects in adaptive energy metabolism with CNS-linked hyperactivity in PGC-1alpha null mice. Cell. 2004;119(1):121–135. doi: 10.1016/j.cell.2004.09.013. [DOI] [PubMed] [Google Scholar]
- Lucas EK, Dougherty SE, McMeekin LJ, Trinh AT, Reid CS, Cowell RM. Developmental alterations in motor coordination and medium spiny neuron markers in mice lacking pgc-1alpha. PLoS One. 2012;7(8):e42878. doi: 10.1371/journal.pone.0042878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucas EK, Markwardt SJ, Gupta S, Meador-Woodruff JH, Lin JD, Overstreet-Wadiche L, Cowell RM. Parvalbumin deficiency and GABAergic dysfunction in mice lacking PGC-1alpha. J Neurosci. 2010;30(21):7227–7235. doi: 10.1523/JNEUROSCI.0698-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma D, Li S, Lucas EK, Cowell RM, Lin JD. Neuronal inactivation of peroxisome proliferator-activated receptor gamma coactivator 1alpha (PGC-1alpha) protects mice from diet-induced obesity and leads to degenerative lesions. J Biol Chem. 2010;285(50):39087–39095. doi: 10.1074/jbc.M110.151688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magee JC. Dendritic integration of excitatory synaptic input. Nat Rev Neurosci. 2000;1(3):181–190. doi: 10.1038/35044552. [DOI] [PubMed] [Google Scholar]
- Pozzo-Miller LD, Gottschalk W, Zhang L, McDermott K, Du J, Gopalakrishnan R, Oho C, Sheng ZH, Lu B. Impairments in high-frequency transmission, synaptic vesicle docking, and synaptic protein distribution in the hippocampus of BDNF knockout mice. J Neurosci. 1999;19(12):4972–4983. doi: 10.1523/JNEUROSCI.19-12-04972.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puigserver P, Wu Z, Park CW, Graves R, Wright M, Spiegelman BM. A cold-inducible coactivator of nuclear receptors linked to adaptive thermogenesis. Cell. 1998;92(6):829–839. doi: 10.1016/s0092-8674(00)81410-5. [DOI] [PubMed] [Google Scholar]
- Qin W, Haroutunian V, Katsel P, Cardozo CP, Ho L, Buxbaum JD, Pasinetti GM. PGC-1alpha expression decreases in the Alzheimer disease brain as a function of dementia. Arch Neurol. 2009;66(3):352–361. doi: 10.1001/archneurol.2008.588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramirez DM, Kavalali ET. Differential regulation of spontaneous and evoked neurotransmitter release at central synapses. Curr Opin Neurobiol. 2011;21(2):275–282. doi: 10.1016/j.conb.2011.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rotaru DC, Yoshino H, Lewis DA, Ermentrout GB, Gonzalez-Burgos G. Glutamate receptor subtypes mediating synaptic activation of prefrontal cortex neurons: relevance for schizophrenia. J Neurosci. 2011;31(1):142–156. doi: 10.1523/JNEUROSCI.1970-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schikorski T, Stevens CF. Morphological correlates of functionally defined synaptic vesicle populations. Nat Neurosci. 2001;4(4):391–395. doi: 10.1038/86042. [DOI] [PubMed] [Google Scholar]
- Schwaller B, Dick J, Dhoot G, Carroll S, Vrbova G, Nicotera P, Pette D, Wyss A, Bluethmann H, Hunziker W, Celio MR. Prolonged contraction-relaxation cycle of fast-twitch muscles in parvalbumin knockout mice. Am J Physiol. 1999;276(2 Pt 1):C395–403. doi: 10.1152/ajpcell.1999.276.2.C395. [DOI] [PubMed] [Google Scholar]
- Sheng B, Wang X, Su B, Lee HG, Casadesus G, Perry G, Zhu X. Impaired mitochondrial biogenesis contributes to mitochondrial dysfunction in Alzheimer’s disease. J Neurochem. 2012;120(3):419–429. doi: 10.1111/j.1471-4159.2011.07581.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sohal VS. Insights into cortical oscillations arising from optogenetic studies. Biol Psychiatry. 2012;71(12):1039–1045. doi: 10.1016/j.biopsych.2012.01.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sohal VS, Zhang F, Yizhar O, Deisseroth K. Parvalbumin neurons and gamma rhythms enhance cortical circuit performance. Nature. 2009;459(7247):698–702. doi: 10.1038/nature07991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Speed HE, Dobrunz LE. Developmental changes in short-term facilitation are opposite at temporoammonic synapses compared to Schaffer collateral synapses onto CA1 pyramidal cells. Hippocampus. 2009;19(2):187–204. doi: 10.1002/hipo.20496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- St-Pierre J, Drori S, Uldry M, Silvaggi JM, Rhee J, Jager S, Handschin C, Zheng K, Lin J, Yang W, Simon DK, Bachoo R, Spiegelman BM. Suppression of reactive oxygen species and neurodegeneration by the PGC-1 transcriptional coactivators. Cell. 2006;127(2):397–408. doi: 10.1016/j.cell.2006.09.024. [DOI] [PubMed] [Google Scholar]
- Taherzadeh-Fard E, Saft C, Andrich J, Wieczorek S, Arning L. PGC-1alpha as modifier of onset age in Huntington disease. Mol Neurodegener. 2009;4:10. doi: 10.1186/1750-1326-4-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tepper JM, Bolam JP. Functional diversity and specificity of neostriatal interneurons. Curr Opin Neurobiol. 2004;14(6):685–692. doi: 10.1016/j.conb.2004.10.003. [DOI] [PubMed] [Google Scholar]
- Traub RD, Whittington MA, Colling SB, Buzsaki G, Jefferys JG. Analysis of gamma rhythms in the rat hippocampus in vitro and in vivo. J Physiol. 1996;493 (Pt 2):471–484. doi: 10.1113/jphysiol.1996.sp021397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vreugdenhil M, Jefferys JG, Celio MR, Schwaller B. Parvalbumin-deficiency facilitates repetitive IPSCs and gamma oscillations in the hippocampus. J Neurophysiol. 2003;89(3):1414–1422. doi: 10.1152/jn.00576.2002. [DOI] [PubMed] [Google Scholar]
- Walters BJ, Hallengren JJ, Theile CS, Ploegh HL, Wilson SM, Dobrunz LE. A catalytic independent function of the deubiquitinating enzyme USP14 regulates hippocampal synaptic short-term plasticity and vesicle number. J Physiol. 2014;592(Pt 4):571–586. doi: 10.1113/jphysiol.2013.266015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang XJ, Buzsaki G. Gamma oscillation by synaptic inhibition in a hippocampal interneuronal network model. J Neurosci. 1996;16(20):6402–6413. doi: 10.1523/JNEUROSCI.16-20-06402.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witte ME, Nijland PG, Drexhage JA, Gerritsen W, Geerts D, vanHet Hof B, Reijerkerk A, de Vries HE, van der Valk P, van Horssen J. Reduced expression of PGC-1alpha partly underlies mitochondrial changes and correlates with neuronal loss in multiple sclerosis cortex. Acta Neuropathol. 2013;125(2):231–243. doi: 10.1007/s00401-012-1052-y. [DOI] [PubMed] [Google Scholar]
- Zheng B, Liao Z, Locascio JJ, Lesniak KA, Roderick SS, Watt ML, Eklund AC, Zhang-James Y, Kim PD, Hauser MA, Grunblatt E, Moran LB, Mandel SA, Riederer P, Miller RM, Federoff HJ, Wullner U, Papapetropoulos S, Youdim MB, Cantuti-Castelvetri I, Young AB, Vance JM, Davis RL, Hedreen JC, Adler CH, Beach TG, Graeber MB, Middleton FA, Rochet JC, Scherzer CR. PGC-1alpha, a potential therapeutic target for early intervention in Parkinson’s disease. Sci Transl Med. 2010;2(52):52ra73. doi: 10.1126/scitranslmed.3001059. [DOI] [PMC free article] [PubMed] [Google Scholar]