Abstract
Migraine is a chronic trigeminal pain condition that affects the existence of great part of our population. Its debilitating headache attacks, with increased sensitivity to multiple forms of stimuli, force many of the patients to frequently rely upon over the counter analgesics, and quite frequently resort to abuse of prescribed medications, particularly the opioid agonists. In the latter case, the indiscriminate medication-driven activation of the opioid system can lead to undesired side effects, such as the augmentation of hyperalgesia and allodynia, as well as the chronification of the attacks. However, we still lack information regarding the impact of migraine attacks, and its relief on the function of μ-opioid receptor (μOR) mediated neurotransmission, the primary target of opioid medications. This line of enquiry is of particular importance as this neurotransmitter system is arguably the endogenous brain mechanism most centrally involved in pain regulation, as well as the effectiveness of opioid medications. Recently, new advances in molecular neuroimaging and neuromodulation have provided important information that can elucidate, in vivo, the role of the endogenous opioid system in migraine suffering and relief.
Keywords: Migraine, headache, brain, opioid, neuroimaging, MRI, PET, neuromodulation, neurotransmission, tDCS, stimulation, μOR BPND, trigeminal, pain, allodynia, chronic, analgesia
Introduction
Opioid use in migraine is highly controversial. Recently, there has been an increased debate of its role in migraine treatment due to solid evidence that its frequent prescription can lead to augmented risk of headache chronification, allodynia, abuse/dependence, and depression/anxiety. For instance, health-care resource utilization is higher for migraine opioid-users compared to nonusers for emergency, primary, and specialty care visits. Nonetheless, the American Academy of Neurology Practice Parameter lists opioids as second- or third-tier treatments for migraine. This is not a surprise, since opioid analgesics are widely known for their ability to decrease experimental and, most important, clinical pain perception. Despite all the controversies regarding targeting medicinally the human opioid system, many questions regarding the impact of frequent headache suffering and the directly modulation of this system are still unanswered at the molecular level.
How is the Human Opioid System Affected by Acute and Chronic Pain?
The descending inhibitory system modulates pain perception in large part via μORs. They are found throughout the central and peripheral nervous system and play a crucial role in analgesia and in the successful action of exogenous opiate drugs frequently prescribed to address cancer and non-cancer pain treatment. Recent positron emission tomography (PET) studies have demonstrated that trigeminal pain activates endogenous μOR-mediated neurotrasmission in cortical and brainstem regions including, for example, the dorsolateral prefrontal cortex (DLPFC), anterior cingulate cortex (ACC), anterior and posterior insula, medial and lateral thalamus, hypothalamus, amygdala, periaqueductal gray matter (PAG) and nucleus accumbens. All of these regions have high concentrations of μORs, and the degree of their activations correlates with the ability to curb affective and sensory features of pain suffering [1]. Such developments in PET neuroreceptor labeling have permitted the investigation of valuable molecular mechanisms in the brain of other chronic pain disorders in vivo. For instance, μOR concentrations have been examined in patients diagnosed with fibromyalgia syndrome [2]. There were significant reductions in the μOR availability of those patients compared to healthy subjects in the basal ganglia, amygdala, and dorsal ACC. In a specific region of the basal ganglia, the nucleus accumbens, these results were further correlated with clinical pain. This region is part of the reward system and drug-taking behavior. Either by the loss of μORs or their elevated occupancy by pain-induced release of endogenous opioids, these reductions in μOR availability in vivo helps to elucidate why opioid therapy is anecdotally claimed as an ineffectual treatment for patients with fibromyalgia, which is also a divisive fact among clinicians in migraine therapy [3]. These changes in μOR availability (non-displaceable binding potential - BPND) may be also present in the brain of patients suffering with other forms of chronic pain disorders, but with slightly different patterns. For instance, in rheumatoid arthritis patients there are significant reductions in [11C]diprenorphine binding, a non-selective opioid radiotracer, in the frontal, cingulate and temporal cortices in association with the inflammatory-related pain levels. In neuropathic pain, reduced μOR BPND was demonstrated in both hemispheres. In contrast, in central post-stroke pain, reductions with [11C]diprenorphine binding decreased predominantly in the hemisphere contralateral to pain [4]. More recently, and in the opposite direction, increases in μOR availability and reductions in pain anticipation and pain-induced endogenous opioid release were observed in the thalamus and amygdala of patients diagnosed with non-neuropathic back pain, which were associated with clinical pain ratings. Such particularities indicate specific dysfunctional opioidergic central changes for each chronic pain disorder, and might underlie their different sensitivity to opiates. Hitherto, scarce information is available on the baseline and release of the endogenous μ-opioids in migraine pain, and how μOR availability and endogenous μ-opioid release relate to treatment responses.
What is the involvement of the Human μ-Opioid Receptor Mediated Neurotransmission in the Migraine Pathophysiology?
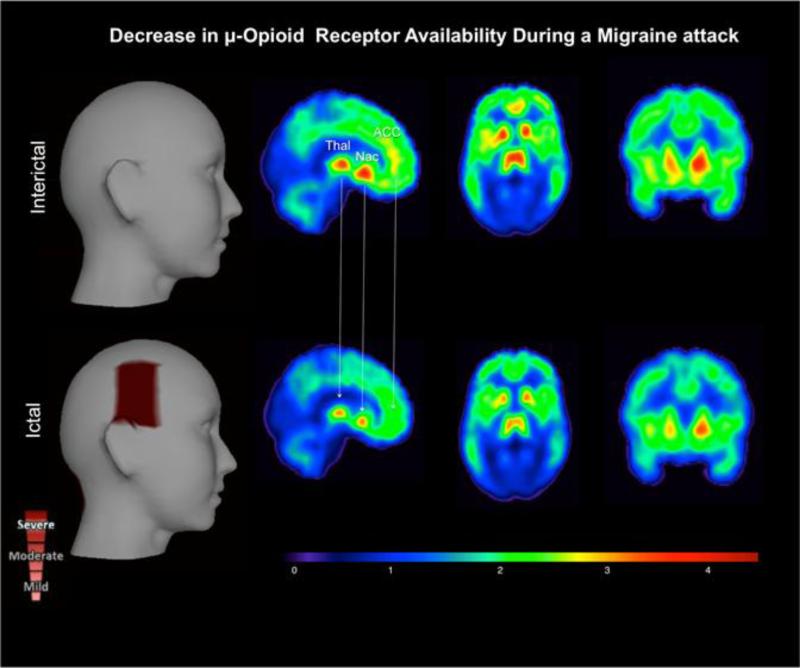
The pathophysiology of migraine is not completely understood, but MRI-based studies have reliably demonstrated neuroplastic changes along the trigeminal sensory system [5-7]. There is strong evidence of sensitization in the migraine brain attributable to abnormal trigeminal afferent traffic [8]. In contrast, there is a dysfunctional descending modulatory system that could also explain the headache and allodynic phenomenon in migraine. Under this scenario, descending projections from the dorsolateral prefrontal cortex and brainstem structures, such as the periaqueductal gray (PAG) and the red nucleus, where there is a high expression of μORs [9, 10], would be more inefficient in their inhibitory effects on ascending trigeminal sensory neurons [11]. In addition, the dural neurogenic vasodilation usually associated with the migraine pathophysiology can be prevented by the potent opiate analgesic morphine, and afterward be reversed by the opioid antagonist naloxone. These opposing effects of morphine and naloxone on neurogenic inflammation corroborate with the notion that this migraine-related process is mediated via activation of μORs. Recently, DaSilva and colleagues have developed a PET protocol that allows us to measure μOR BPND in migraine patients. They noticed reductions in μOR BPND during a spontaneous migraine attack compared to the baseline [••12]. There were reductions in μOR BPND in opioid-rich pain-modulatory regions, most notably the thalamus, ACC, NAcc and Insula. We also found such activation in the midbrain (PAG). This is the first evidence that changes in μOR BPND during a spontaneous migraine attack are reported. These results indicated the acute activation of the endogenous opioid neurotransmission interacting with μOR due to the pain of the migraine attack (Figure 1).
Figure 1. μ-Opioid Brain Profile of a Migraine Attack in vivo.
The ictal phase (lower row) – headache phase - shows a decrease in μ-opioid receptor availability (μOR BPND) in the pain-matrix regions. This result possibly represents an increase in endogenous μ-opioid release during the migraine attack, as a regulatory response to the ongoing severe headache. Key words: thalamus (Thal), nucleus accumbens (NAcc), and anterior cingular cortex (ACC). (DaSilva AF, Nascimento TD, Love TM, DosSantos MF, Martikainen IK, Cummiford CM et al. Impact of a Spontaneous Migraine Attack in the Endogenous μ-Opioid System In-Vivo. J Vis Exp. 2013;In Press)
What is the Neuroimaging evidence of endogenous opioid system involvement with Trigeminal Pain and its Relief?
Patients with trigeminal neuropathic pain (TNP) show reduced μOR BPND in the left nucleus accumbens, an area known to be involved in pain modulation and reward/aversive behaviors. In addition, μOR BPND in the NAcc was negatively correlated with the McGill sensory and total pain ratings in the TNP patients [13]. Nonetheless, we still lack information regarding the possible long-lasting effects of clinical persistent trigeminal pain upon this and other endogenous regulatory systems, including cases where there is intervention effect. In one of few studies available, Jones et al. [14, 15] utilized [11C]diprenorphine, again a non-selective opioid radiotracer, to examine the availability of opioid receptors in a small group of patients diagnosed with chronic pain including trigeminal neuralgia before and following treatment. The authors reported reductions in cortical and subcortical opioid receptor availability at baseline before treatment, which were reversed after the pain subsided. In another study with chronic neuropathic pain patients, surgical motor cortex stimulation decreased [11C]diprenorphine availability in the anterior MCC and PAG [•16]. Once more, those central opioid receptor changes following treatment were associated with pain relief. These findings are proof-of-concept that the decrease receptor availability as measured by radiotracer binding in the brain of chronic trigeminal pain patients is likely reflecting receptor downregulation in the context of persistent endogenous opioid system activation and chronic pain, while the acute changes associated with an intervention (e.g., motor cortex stimulation) reflect the capacity to active this neurotransmitter system by the stimulus employed. This endogenous opioid-mediated analgesic mechanism has been associated with M1 cortex stimulation, with repetitive transcranial magnetic stimulation (rTMS), as demonstrated by its blockade with naloxone [17]. The same did not occur when the target area was the DLPFC. The information above indicates that endogenous opioid mechanisms are highly influenced by the type of chronic pain disorder, therapeutic method, and cortical area targeted.
Can We Directly Target the Opioid-Regulated Regions in the Migrainous Brain? Evidence from Neuromodulation and Forward Neuroimaging-Analysis
Transcranial Direct Current Stimulation (tDCS) has potential advantages for the research of migraine in comparison to TMS, including small portable size, low cost and, most importantly a more reliable placebo control condition [18]. Several well-controlled studies have shown the efficacy of tDCS in pain alleviation [19, 20]. tDCS is based on the application of a weak direct current to the scalp that flows between two relatively large electrodes—anode and cathode. Its effects depend on polarity of stimulation, such as cathodal stimulation induces a decrease in cortical excitability, and anodal stimulation induces an increase in cortical excitability that may last beyond the duration of the stimulation. In fact, application of tDCS for 13 minutes to the motor cortex can modulate cortical excitability for several hours [21, 22]. Nonetheless, the efficacy of tDCS depends critically on parameters such as electrode position and current strength (http://www.jove.com/details.php?id=2744) [23-25]. For example, M1 is a reliable target to modulate the sensory and motor subthalamic activity associated with chronic pain, independent of the type of stimulation applied [26-29], which also affects other pain-related structures. This occurs directly and indirectly because of the multiple connections between corticospinal tract and thalamus.
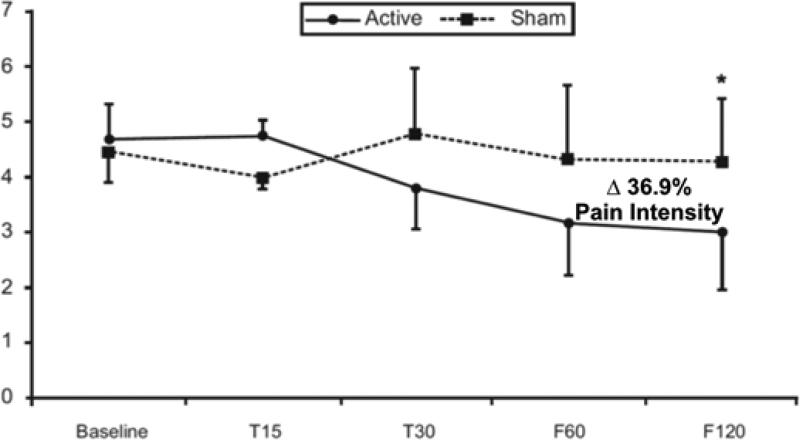
A preliminarily investigation, using a blind sham-controlled study, showed significant analgesic effects of tDCS over the primary motor (M1) cortex in chronic migraine (CM) [••30]. Patients were randomized to receive 10 sessions of active or sham tDCS for 20 minutes with 2 mA over a period of 4 weeks. The primary outcome measure was pain intensity (VAS ratings). There was a significant interaction term (time vs. group) for pain intensity ratings and also for length of migraine episodes. Post-hoc analysis showed a significant improvement in the follow-up period for both outcomes in the active tDCS group only. In addition, patients in the active group showed a significant improvement in the clinician global impression scale ratings when compared with the sham group (Figure 2). Using a finite element (FE) program, the authors analyzed the effect of their M1 cortex electrode montage on the current flow in the brain taking into consideration the electrical properties of cortical and subcortical structures. The human head model was based on a single high spatial resolution (1mm3) 3T MRI-derived FE from a healthy subject. The spatial focality of the analysis was restricted to 5x7 cm2 pads placed exactly as described: anode electrode over the motor cortex and the cathode electrode at the contralateral supraorbital area (SO).
Figure 2. Positive response of chronic migraine to M1-tDCS.
Mean pain levels (as assessed by VAS) at baseline, T15, T30, F60, and F120 in the 2 groups of stimulation (active & sham tDCS). Error bars indicate standard error of the mean [••30].
Afterward, the head was segmented into compartments representing the brain tissues, cerebrospinal fluid, skull, muscle, fatty tissue, eyes, blood vessels and the scalp respectively. The stimulation pads were imported as CAD models, and volumetric meshes were generated from the segmented data, and later exported to COMSOL 3.4 (MA, USA). One good analogy of our forward-tDCS analysis is the prediction of earthquake diffusion, taking into consideration a particular seismic strength, the terrain's geography and geology. On that study, instead, it was the current strength, size/location of electrodes, head/brain anatomy and white/gray matter constitution that dictated the flow of electricity. The results showed that significant electric fields are generated, not only in target cortical regions (M1) as was reported before, but also in the posterior thalamus (VPM) and other pain-matrix regions: Insula, ACC and even brainstem. Derived from the tDCS forward analysis, this direct modulation of the pain-related regions may explain the analgesic effects on migraine pain measures associated with their protocol [••30].
Can We Directly Modulate the Endogenous Opioid-System? Evidence from MRI and PET studies
When 20 minutes of M1-tDCS stimulation are administered during the PET imaging session, there is an immediate reduction in μOR availability in vivo in response to an acute motor cortex stimulation, consistent with the acute release of endogenous opioids interacting with μORs [••31]. Concentrations of μOR BPND in a chronic trigeminal pain patient during a single tDCS application induced a decrease in μOR availability in the thalamus and other pain-matrix structures including NAcc, ACC, and Insula, as predicted in the forward tDCS model, which were coupled with pain relief. Such analgesic effect of tDCS on pain measures are likely due to the acute release of endogenous opioids, activating μORs, by direct and indirect effect of M1 stimulation on the thalamus and other regions. tDCS over M1 induced immediate changes in thermal sensory perception, especially cold [32]. Lately, Polania and colleagues have additionally reported that tDCS regulates functional connectivity depending on the specific montage [33]. For instance, cathodal M1-tDCS diminishes functional coupling between ipsilateral M1 and contralateral putamen. On the other hand, anodal stimulation over M1 instantly increases functional coupling between ipsilateral M1 and thalamus.
The same active M1-tDCS montage significantly reduced μOR availability in the posterior thalamus compared to placebo tDCS [••31]. Interestingly, the single M1-tDCS session improved threshold for acute cold pain in the allodynic area, but the analgesic effects did not alter the patient's clinical pain. This result implies that the endogenous opioid system activating effects of a single tDCS session are subclinical, and multiple sessions are likely necessary to address neuroplastic cortical changes related to a chronic pain state, including migraine.
Conclusions
The μ-opioid system plays a pivotal role in migraine pain pathophysiology and relief. μOR BPND is an objective PET measurement in vivo of endogenous μ-opioid availability, and its acute reduction reflects the activation of this neurotransmitter system [1]. Clinical and experimental trigeminal pain suffering induces, at different levels, the release of endogenous opioids acting on μORs to suppress the ongoing headache and pain in general. Further activation of the endogenous μOR-mediated neurotransmission produces an analgesic effect, and changes toward control values in μOR BPND take place after continued treatment.
Acknowledgments
This work was supported by the following grants (DaSilva AF): National Institute of Health – National Institute of Neurological Disorders and Stroke – K23 NS062946, Dana Foundation's Brain and Immuno-Imaging Award, the Migraine Research Foundation Research Grant Award, and DaSilva's start-up fund, University of Michigan School of Dentistry.
Footnotes
Compliance with Ethics Guidelines
Conflict of Interest
Dr. Alexandre F. DaSilva, Dr. Thiago D. Nascimento, Dr. Marcos F. DosSantos, and Dr. Jon-Kar Zubieta each declare no potential conflicts of interest relevant to this article.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Contributor Information
A. F. DaSilva, Headache and Orofacial Pain Effort, Biologic and Materials Sciences Department, School of Dentistry, University of Michigan. 1011 N. University Ave., Room 1014A, Ann Arbor, MI 48109-1078, USA.
T. D. Nascimento, Headache and Orofacial Pain Effort, Biologic and Materials Sciences Department, School of Dentistry, University of Michigan. 1011 N. University Ave., Room 1014A, Ann Arbor, MI 48109-1078, USA Work Phone: 734 615-9390 nasci@umich.edu.
M. F. DosSantos, Headache and Orofacial Pain Effort, Biologic and Materials Sciences Department, School of Dentistry, University of Michigan. 1011 N. University Ave., Room 1014A, Ann Arbor, MI 48109-1078, USA Work Phone: 734 615-9390 santoshmfh@gmail.com.
JK Zubieta, Translational Neuroimaging Laboratory, Molecular and Behavioral Neuroscience Institute, Department of Psychiatry, University of Michigan, 205 Zina Pitcher Place Ann Arbor, MI 48109-5720, USA Work Phone: (734) 763-6843 Zubieta@umich.edu.
References
Papers of particular interest, published recently, have been highlighted as:
• Of importance
•• Of major importance
- 1.Zubieta JK, Smith YR, Bueller JA, Xu Y, Kilbourn MR, Jewett DM, et al. Regional mu opioid receptor regulation of sensory and affective dimensions of pain. Science. 2001;293(5528):311–5. doi: 10.1126/science.1060952. [DOI] [PubMed] [Google Scholar]
- 2.Harris RE, Clauw DJ, Scott DJ, McLean SA, Gracely RH, Zubieta JK. Decreased central muopioid receptor availability in fibromyalgia. J Neurosci. 2007;27(37):10000–6. doi: 10.1523/JNEUROSCI.2849-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lipton RB, Bigal ME. Opioid therapy and headache: a cause and a cure. Neurology. 2004;62(10):1662–3. doi: 10.1212/wnl.62.10.1662. [DOI] [PubMed] [Google Scholar]
- 4.Maarrawi J, Peyron R, Mertens P, Costes N, Magnin M, Sindou M, et al. Differential brain opioid receptor availability in central and peripheral neuropathic pain. Pain. 2007;127(1-2):183–94. doi: 10.1016/j.pain.2006.10.013. [DOI] [PubMed] [Google Scholar]
- 5.Granziera C, DaSilva AF, Snyder J, Tuch DS, Hadjikhani N. Anatomical alterations of the visual motion processing network in migraine with and without aura. PLoS Med. 2006;3(10):e402. doi: 10.1371/journal.pmed.0030402. doi:06-PLME-RA-0158R4 [pii] 10.1371/journal.pmed.0030402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.DaSilva AF, Granziera C, Snyder J, Hadjikhani N. Thickening in the somatosensory cortex of patients with migraine. Neurology. 2007;69(21):1990–5. doi: 10.1212/01.wnl.0000291618.32247.2d. doi:69/21/1990[pii]10.1212/01.wnl.0000291618.32247.2d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.DaSilva AF, Granziera C, Tuch DS, Snyder J, Vincent M, Hadjikhani N. Interictal alterations of the trigeminal somatosensory pathway and periaqueductal gray matter in migraine. Neuroreport. 2007;18(4):301–5. doi: 10.1097/WNR.0b013e32801776bb. doi:10.1097/WNR.0b013e32801776bb 00001756-200703050-00001 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Waeber C, Moskowitz MA. Migraine as an inflammatory disorder. Neurology. 2005;64(10 Suppl 2):S9–15. doi: 10.1212/wnl.64.10_suppl_2.s9. [DOI] [PubMed] [Google Scholar]
- 9.Bausch SB, Patterson TA, Ehrengruber MU, Lester HA, Davidson N, Chavkin C. Colocalization of mu opioid receptors with GIRK1 potassium channels in the rat brain: an immunocytochemical study. Receptors Channels. 1995;3(3):221–41. [PubMed] [Google Scholar]
- 10.Kruit MC, Launer LJ, Overbosch J, van Buchem MA, Ferrari MD. Iron accumulation in deep brain nuclei in migraine: a population-based magnetic resonance imaging study. Cephalalgia. 2009;29(3):351–9. doi: 10.1111/j.1468-2982.2008.01723.x. doi:CHA1723 [pii] 10.1111/j.1468-2982.2008.01723.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Goadsby PJ. Migraine, allodynia, sensitisation and all of that. Eur Neurol. 2005;53(Suppl 1):10–6. doi: 10.1159/000085060. [DOI] [PubMed] [Google Scholar]
- 12••.DaSilva AF, Nascimento TD, Love TM, DosSantos MF, Martikainen IK, Cummiford CM, et al. Impact of a Spontaneous Migraine Attack in the Endogenous μ-Opioid System In-Vivo. J Vis Exp. 2013 In Press. [This case-report shows for the first time that there are changes in μOR BPND during a spontaneous migraine attack.] [Google Scholar]
- 13.Dossantos MF, Martikainen IK, Nascimento TD, Love TM, Deboer MD, Maslowski EC, et al. Reduced basal ganglia mu-opioid receptor availability in trigeminal neuropathic pain: A pilot study. Mol Pain. 8(1):74. doi: 10.1186/1744-8069-8-74. doi:1744-8069-8-74 [pii] 10.1186/1744-8069-8-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jones AK, Cunningham VJ, Ha-Kawa S, Fujiwara T, Luthra SK, Silva S, et al. Changes in central opioid receptor binding in relation to inflammation and pain in patients with rheumatoid arthritis. Br J Rheumatol. 1994;33(10):909–16. doi: 10.1093/rheumatology/33.10.909. [DOI] [PubMed] [Google Scholar]
- 15.Jones AK, Kitchen ND, Watabe H, Cunningham VJ, Jones T, Luthra SK, et al. Measurement of changes in opioid receptor binding in vivo during trigeminal neuralgic pain using [11C] diprenorphine and positron emission tomography. J Cereb Blood Flow Metab. 1999;19(7):803–8. doi: 10.1097/00004647-199907000-00011. [DOI] [PubMed] [Google Scholar]
- 16•.Maarrawi J, Peyron R, Mertens P, Costes N, Magnin M, Sindou M, et al. Motor cortex stimulation for pain control induces changes in the endogenous opioid system. Neurology. 2007;69(9):827–34. doi: 10.1212/01.wnl.0000269783.86997.37. [This article demonstrates the contribution of the endogenous opioid system to invasive motor cortex stimulation-related pain relief.] [DOI] [PubMed] [Google Scholar]
- 17.de Andrade DC, Mhalla A, Adam F, Texeira MJ, Bouhassira D. Neuropharmacological basis of rTMS-induced analgesia: the role of endogenous opioids. Pain. 152(2):320–6. doi: 10.1016/j.pain.2010.10.032. doi:S0304-3959(10)00659-7 [pii] 10.1016/j.pain.2010.10.032. [DOI] [PubMed] [Google Scholar]
- 18.Gandiga PC, Hummel FC, Cohen LG. Transcranial DC stimulation (tDCS): a tool for double-blind sham-controlled clinical studies in brain stimulation. Clin Neurophysiol. 2006;117(4):845–50. doi: 10.1016/j.clinph.2005.12.003. [DOI] [PubMed] [Google Scholar]
- 19.Lima MC, Fregni F. Motor cortex stimulation for chronic pain: systematic review and meta-analysis of the literature. Neurology. 2008;70(24):2329–37. doi: 10.1212/01.wnl.0000314649.38527.93. [DOI] [PubMed] [Google Scholar]
- 20.Zaghi S, Thiele B, Pimentel D, Pimentel T, Fregni F. Assessment and treatment of pain with non-invasive cortical stimulation. Restor Neurol Neurosci. 29(6):439–51. doi: 10.3233/RNN-2011-0615. doi:426287160244Q72Q [pii] 10.3233/RNN-2011-0615. [DOI] [PubMed] [Google Scholar]
- 21.Nitsche MA, Paulus W. Excitability changes induced in the human motor cortex by weak transcranial direct current stimulation. J Physiol. 2000;527(Pt 3):633–9. doi: 10.1111/j.1469-7793.2000.t01-1-00633.x. doi:PHY_1055 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nitsche MA, Paulus W. Sustained excitability elevations induced by transcranial DC motor cortex stimulation in humans. Neurology. 2001;57(10):1899–901. doi: 10.1212/wnl.57.10.1899. [DOI] [PubMed] [Google Scholar]
- 23.DaSilva AF, Volz MS, Bikson M, Fregni F. Electrode positioning and montage in transcranial direct current stimulation. J Vis Exp. (51) doi: 10.3791/2744. doi:2744 [pii] 10.3791/2744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nitsche MA, Nitsche MS, Klein CC, Tergau F, Rothwell JC, Paulus W. Level of action of cathodal DC polarisation induced inhibition of the human motor cortex. Clin Neurophysiol. 2003;114(4):600–4. doi: 10.1016/s1388-2457(02)00412-1. doi:S1388245702004121 [pii] [DOI] [PubMed] [Google Scholar]
- 25.Nitsche MA, Liebetanz D, Lang N, Antal A, Tergau F, Paulus W. Safety criteria for transcranial direct current stimulation (tDCS) in humans. Clin Neurophysiol. 2003;114(11):2220–2. doi: 10.1016/s1388-2457(03)00235-9. author reply 2-3. doi:S1388245703002359 [pii] [DOI] [PubMed] [Google Scholar]
- 26.Lang N, Siebner HR, Ward NS, Lee L, Nitsche MA, Paulus W, et al. How does transcranial DC stimulation of the primary motor cortex alter regional neuronal activity in the human brain? Eur J Neurosci. 2005;22(2):495–504. doi: 10.1111/j.1460-9568.2005.04233.x. doi:EJN4233 [pii] 10.1111/j.1460-9568.2005.04233.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Strafella AP, Vanderwerf Y, Sadikot AF. Transcranial magnetic stimulation of the human motor cortex influences the neuronal activity of subthalamic nucleus. Eur J Neurosci. 2004;20(8):2245–9. doi: 10.1111/j.1460-9568.2004.03669.x. doi:10.1111/j.1460-9568.2004.03669.EJN3669 [pii] [DOI] [PubMed] [Google Scholar]
- 28.Garcia-Larrea L, Peyron R, Mertens P, Gregoire MC, Lavenne F, Le Bars D, et al. Electrical stimulation of motor cortex for pain control: a combined PET-scan and electrophysiological study. Pain. 1999;83(2):259–73. doi: 10.1016/s0304-3959(99)00114-1. doi:S0304395999001141 [pii] [DOI] [PubMed] [Google Scholar]
- 29.Garcia-Larrea L, Peyron R, Mertens P, Gregoire MC, Lavenne F, Bonnefoi F, et al. Positron emission tomography during motor cortex stimulation for pain control. Stereotact Funct Neurosurg. 1997;68(1-4 Pt 1):141–8. doi: 10.1159/000099915. [DOI] [PubMed] [Google Scholar]
- 30••.DaSilva AF, Datta A, Mendonca ME, Zaghi S, Lopes M, DosSantos MF, et al. Chronic Migraine Alleviation by tDCS Is Predicted To Be Associated with Current Flow through Pain-Related (Sub) Cortical Regions. Headache. 2011;51:48–9. [This study gives preliminary evidence that patients with chronic migraine have a positive response to anodal tDCS of the primary motor cortex.] [Google Scholar]
- 31••.DosSantos MF, Love TM, Martikainen IK, Nascimento TD, Fregni F, Cummiford C, et al. Immediate effects of tDCS on the μ-opioid system of a chronic pain patient. Front Psychiatry. 2012;3:93. doi: 10.3389/fpsyt.2012.00093. doi:10.3389. [This case-report highlights the involvement of the endogenous mu-opioid system to non-invasive motor cortex stimulation-related pain relief.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bachmann CG, Muschinsky S, Nitsche MA, Rolke R, Magerl W, Treede RD, et al. Transcranial direct current stimulation of the motor cortex induces distinct changes in thermal and mechanical sensory percepts. Clin Neurophysiol. 2010;121(12):2083–9. doi: 10.1016/j.clinph.2010.05.005. doi:S1388-2457(10)00423-2 [pii] 10.1016/j.clinph.2010.05.005. [DOI] [PubMed] [Google Scholar]
- 33.Polania R, Paulus W, Nitsche MA. Modulating cortico-striatal and thalamo-cortical functional connectivity with transcranial direct current stimulation. Hum Brain Mapp. 2011 doi: 10.1002/hbm.21380. doi:10.1002/hbm.21380. [DOI] [PMC free article] [PubMed] [Google Scholar]