Abstract
The first example of catalytic B–H activation of azaborines leading to a new family of stilbene derivatives through dehydrogenative borylation is reported. Ten 1,2-azaborine-based BN isosteres of stilbenes have been synthesized using this method, including a BN isostere of a biologically active stilbene. It is demonstrated that BN/CC isosterism in the context of stilbenes can lead to significant changes in the observed photophysical properties such as higher quantum yield and a larger Stokes shift. Direct comparative analysis of BN stilbene 3g and its carbonaceous counterpart 6g is consistent with a stronger charge-transfer character of the excited state exhibited by 3g in which the 1,2-azaborine heterocycle serves as a better electron donor than the corresponding arene.
Stilbene and its derivatives are ubiquitous in biologically active molecules1 and materials science applications. For instance, stilbenoid compounds such as resveratrol have demonstrated anti-inflammatory, anticancer, and cardioprotective activities.2 Similarly, the interaction of radiation with stilbenoid compounds has been intensively investigated3 for applications in nonlinear optics4 and as light-emitting diodes,5 scintillators,6 photoresists,7 and optical brighteners.8,9 Our group has been focusing on developing BN/CC isosterism10 as a strategy to expand the chemical space of compounds relevant to biomedical research11 and materials science.12−14 In view of the versatile applications exhibited by stilbene derivatives, we sought to develop a general method for the synthesis of BN isosteres of stilbene. In this paper, we report the first examples of 1,2-azaborine-based BN stilbenes including a BN isostere of 4-methoxy-trans-stilbene, a compound with in vitro activity against MCF-7/6 mammary carcinoma15 and human colon adenocarcinoma cancer (HT-29)16 cells. Furthermore, we demonstrate that 1,2-azaborine-based BN stilbenes exhibit photophysical properties that are distinct from their carbonaceous counterparts.
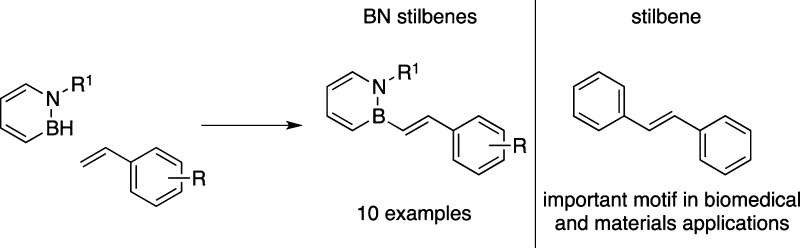
A retrosynthetic analysis revealed that B–H activation of 1,2-azaborines coupled with C–H activation of styrenes through dehydrogenative borylation could furnish the desired 1,2-azaborine-based BN stilbene compounds (Scheme 1). The use of styrenes as direct coupling partners appears particularly attractive due to their ready commercial availability.17 However, despite some recent progress in the late-stage functionalization of 1,2-azaborines,18 no systematic study of the B–H activation chemistry of azaborines has been performed.19 We reported previously that the B–H bond in 1,2-azaborines is relatively nonhydridic.20 Indeed, no reaction is observed between our model N-benzyl-B-H-1,2-azaborine 1 and p-methoxystyrene 2 in the absence of promoters even at elevated temperatures (up to 110 °C). We thus surveyed a number of commercially available catalysts known to promote B–H activation and dehydrogenative borylations21 to facilitate the formation of BN stilbene 3a.22
Scheme 1. BN Stilbene and Its Retrosynthetic Analysis.
As can be seen from Table 1, Wilkinson’s catalyst gave the desired stilbene as the major product in just 15% yield (Table 1, entry 1). Other phosphine-ligated rhodium complexes generally used in B–H activation reactions were ineffective for this reaction (Table 1, entries 2 and 3). On the other hand, Crabtree’s complex furnished the product in 51% yield (Table 1, entry 4). Gratifyingly, we found that phosphine-free rhodium alkene complexes such as [Rh(cod)2]BF4 or [Rh(nbd)(Cl)]2 are suitable catalysts for our model dehydrogenative borylation reaction (Table 1, entries 5 and 6). We thus chose those two complexes for further solvent optimization studies. We determined that methylene chloride was the best performing solvent for both rhodium complexes (Table 1, entries 7–12), providing the desired product in up to 98% HPLC yield (83% isolated yield) in the case of [Rh(nbd)(Cl)]2 (Table 1, entry 12). A reduction of the catalyst loading to 2.5 mol % [Rh(nbd)(Cl)]2 was not detrimental with respect to the isolated yield of 3a (Table 1, entry 13). Two equivalents of the styrene were necessary to achieve full conversion to the BN stilbene because one equivalent of styrene was hydrogenated during the catalytic cycle.
Table 1. Survey of Catalysts and Solvents for the Dehydrogenative Borylation Reaction between 1,2-Azaborine 1 and Styrene 2a.
| entry | catalyst | solvent | yieldb (%) |
|---|---|---|---|
| 1 | RhCl(PPh3)3 | THF | 15 |
| 2 | RhH(CO)(PPh3)3 | THF | 0 |
| 3 | Rh(dppb)(cod)BF4 | THF | 0 |
| 4 | Ir(cod)(py)(PCy3)PF6 | THF | 51 |
| 5 | [Rh(cod)2]BF4 | THF | 64 |
| 6 | [Rh(nbd)(Cl)]2 | THF | 80 |
| 7 | [Rh(cod)2]BF4 | toluene | 60 |
| 8 | [Rh(cod)2]BF4 | acetonitrile | 23 |
| 9 | [Rh(cod)2]BF4 | CH2Cl2 | 75 |
| 10 | [Rh(nbd)(Cl)]2 | toluene | 94 |
| 11 | [Rh(nbd)(Cl)]2 | acetonitrile | 52 |
| 12 | [Rh(nbd)(Cl)]2 | CH2Cl2 | 98 (83)c |
| 13 | [Rh(nbd)Cl]2 (2.5 mol %) | CH2Cl2 | (86)c,d |
Abbreviations: dppb (diphenylphosphinobutane), cod (cyclooctadiene), py (pyridine), nbd (norbornadiene).
Determined by HPLC versus a calibrated internal standard, average of two runs.
Isolated yields in parentheses, average of two runs.
20 h reaction time.
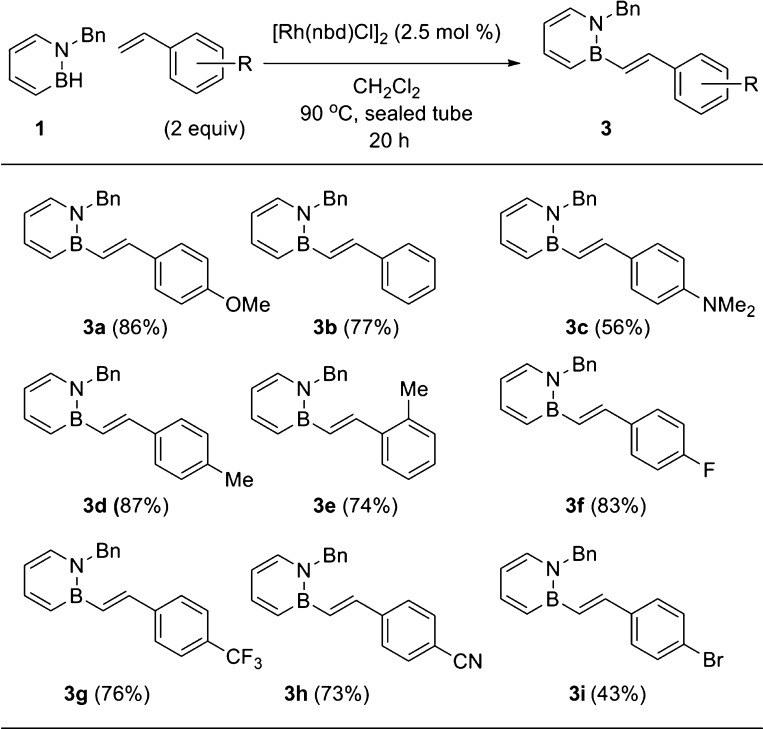
With the optimized conditions determined, we next turned our attention to the scope of the dehydrogenative borylation reaction (Scheme 2). The reaction with the parent styrene furnished 3b in 77% yield. While the electron-rich p-methoxystyrene was a suitable substrate for the reaction (3a, 86% yield), p-(dimethylamino)styrene produced the corresponding BN stilbene 3c in moderate 56% yield. Steric influences on styrene do not appear to affect the yield of BN stilbene products as both p- and o-tolylstyrene are suitable substrates (3d and 3e). Electron-withdrawing substituents are tolerated (3f, 3g, and 3h). Finally, p-bromostyrene couples with 1 to generate a BN stilbene that can be potentially further functionalized through cross-coupling (3i23).
Scheme 2. Synthesis of BN Stilbenes through Rh-Catalyzed Dehydrogenative Borylation.
The yields of isolated products are given as the average of two runs.
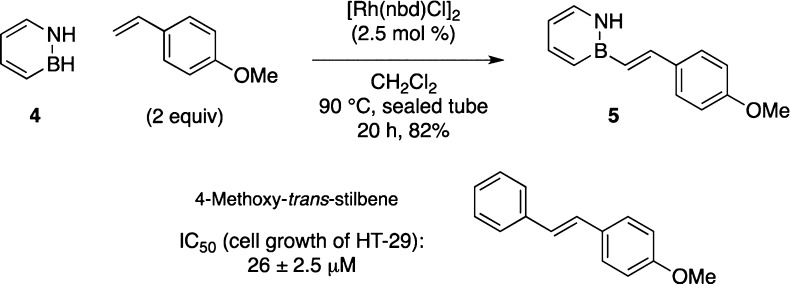
We sought to demonstrate the utility of this dehydrogenative coupling in the synthesis of a BN isostere of a biologically active molecule. We identified the BN isostere of 4-methoxy-trans-stilbene as a suitable target.15,16 Treatment of the parent 1,2-azaborine 4 with p-methoxystyrene under the optimized conditions results in the formation of the BN stilbene 5 in 82% isolated yield (Scheme 3). Thus, the reaction conditions are compatible with the protic N-H functional group in 4. We performed oxygen and water stability studies of compound 5 using published procedures.24 BN stilbene 5 showed <5% decomposition under an oxygen atmosphere in C6D6 at 50 °C for 4 h or at room temperature under ambient atmosphere for 16 h. The compound was also stable (<5% decomposition) to 8.5 equivalents of water in DMSO-d6 at room temperature for 2 h, then 50 °C for 2 h.
Scheme 3. Synthesis of a BN Isostere of 4-Methoxy-trans-stilbene.
We were able to isolate only the trans-BN stilbene isomers among possible reaction products under our optimized conditions (e.g., hydroboration adducts and cis-BN stilbene isomers).25 Single-crystal X-ray diffraction analysis unambiguously confirms our structural assignment for 3a, which is also consistent with the relatively large 3JHH coupling constant of 18 Hz observed by 1H NMR.26
Figure 1 illustrates the ORTEP of BN stilbene 3a along with selected bond distances. The molecule adopts a relatively planar structure in the solid state. The ∠N–B–C12–C13 and ∠C12–C13–C14–C15 dihedral angles are 170.4(1)° and 2.3(2)°, respectively. The heterocyclic intraring distances are consistent with a typical 1,2-azaborine motif.27 The C12–C13 distance of 1.333(2) Å is also consistent with typical distances observed in stilbenes.28 On the other hand, the B–C12 distance of 1.555(2) Å is significantly longer than a typical arene C(sp2)–alkene C(sp2) single-bond distance of ∼1.47 Å.29 On the other hand, the B–C12 distance observed in 5 is slightly shorter compared to a perpendicularly oriented 1,2-azaborine B–C(sp2)–arene single bond distance of ∼1.57 Å.18
Figure 1.

ORTEP illustration, with thermal ellipsoids drawn at the 35% probability level, of BN stilbene 3a. All hydrogens except for the alkenyl hydrogens at C12 and C13 have been omitted for clarity.
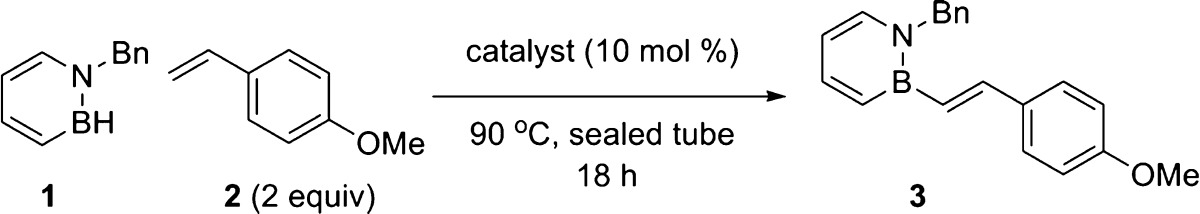
We then investigated the impact of BN/CC isosterism on the photophysical properties of stilbene derivatives. We chose a representative set of BN stilbenes containing an electron-withdrawing (3g, (−CF3) black in Figure 2), electron-neutral (3b, (−H) green in Figure 2), and electron-rich (3a, (−OMe) blue in Figure 2) arene for direct comparison with the corresponding carbonaceous stilbene analogues (6g (−CF3, pink), 6b (−H, orange), 6a (−OMe, red)). As can be seen from Figure 2, BN stilbenes exhibit a bathochromic shift in both the absorption and emission spectra. The substituent effect is more pronounced in the emission relative to the absorption. In particular, the effect of the substituents on the emission peak is significantly stronger in BN stilbenes (3a(λem): 390 nm; 3g(λem): 431 nm; Δ: 2439 cm–1) than in all-carbon series 6b(λem): 357 nm; 6a(λem): 380 nm; Δ: 1695 cm–1). The photophysical properties of BN stilbene 3g (−CF3, black trace) is particularly intriguing. Compound 3g exhibits a large Stokes shift (8340 cm–1) and the highest quantum yield (ΦPL: 0.29) among the stilbene compounds that we have investigated.
Figure 2.
Normalized absorption and emission spectra (in MeCN) of select BN stilbenes in direct comparison with their corresponding carbonaceous counterparts. Quantum yields were determined in EtOH at room temperature.
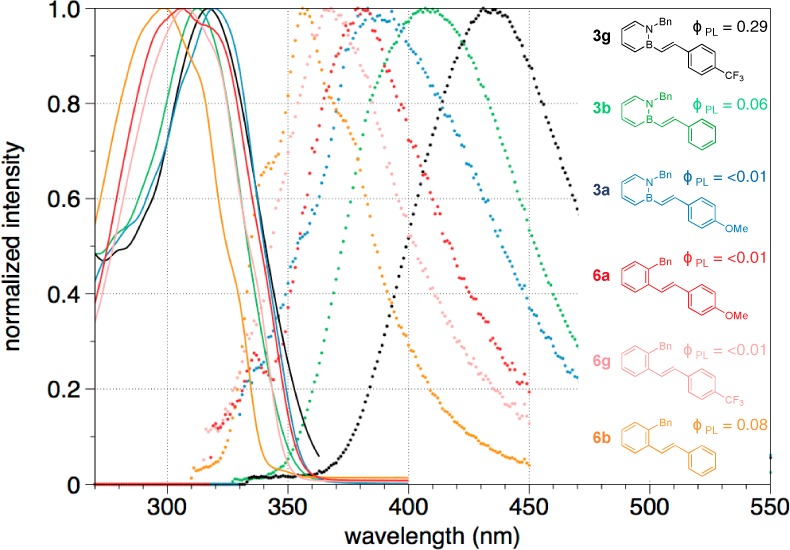
We performed emission solvatochromism studies on BN stilbene 3g and its carbonaceous analogue to elucidate the nature of the excited state. Figure 3 illustrates a significant positive solvatochromism on the emission maxima of BN stilbene 3g (a red shift of 2457 cm–1 going from cyclohexane (λem: 393 nm) to DMSO (λem: 435 nm)) as compared to the carbonaceous stilbene 6g (a red shift of 674 cm–1 from cyclohexane to DMSO).30 The solvatochromic study is consistent with a stronger charge transfer character of the excited state for 3g than for 6g, which also presumably involves a larger structural reorganization from ground state leading to the large observed Stokes shift for 3g. The relatively high photoluminescence quantum yield for BN stilbene 3g may be due to destabilization of biradical states relative to the charge-transfer state and subsequent deactivation of nonradiative decay pathways associated with the biradical states.31 Time-dependent density functional theory (TD-DFT) calculations for compound 3g reveal that the HOMO–LUMO transition is the predominant one with an oscillator strength of 0.57 (see Supporting Information for details of the calculations).
Figure 3.
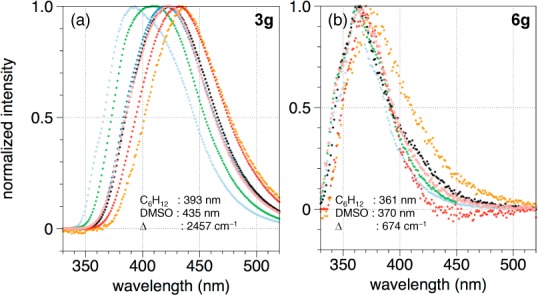
Normalized emission spectra of (a) BN stilbene 3g and (b) carbonaceous analogue 6g in various solvents (DMSO, orange; MeCN, red; black, CH2Cl2; pink, EtOH; blue, THF; green, Et2O; cyclohexane, pale blue). All measurements were taken at 1 × 10–5 M.
Figure 4 shows that the N-benzyl group does not significantly impact the HOMO–LUMO transition. The HOMO–LUMO diagrams are also consistent with 3g exhibiting a stronger charge transfer character than the carbonaceous 6g. Thus, our studies suggest that BN/CC isosterism in the context of stilbenes can promote charge transfer transitions in which the 1,2-azaborine heterocycle can be considered a better electron donor than the corresponding arene.
Figure 4.

HOMO and LUMO (B3LYP Kohn–Sham orbitals) of 3g and 6g.
In summary, we have established the first example of catalytic B–H activation of azaborines leading to a family of BN stilbenes through dehydrogenative borylation of styrenes. Ten examples of BN stilbenes have been synthesized using this method, including a BN isostere of a biologically active stilbene. Catalytic activation of the relatively unreactive aromatic B–H bond in these systems expands the basic synthetic utility of the 1,2-azaborines. This study has enabled the discovery that BN/CC isosterism in the context of stilbenes can lead to significant changes in observed photophysical properties such as higher quantum yield and a larger Stokes shift. Direct comparative analysis of BN stilbene 3g and its carbonaceous counterpart 6g is consistent with a stronger charge transfer character of the excited state exhibited by 3g in which the 1,2-azaborine heterocycle serves as a better electron donor than the corresponding arene.
Acknowledgments
Support has been provided by the National Institutes of Health (National Institute General Medical Sciences, R01-GM094541). Funding for the University of Oregon Chemistry Research and Instrumentation Services has been provided in part by the NSF (CHE-0923589). D.A.D thanks the Robert Ramsay Chair Fund of The University of Alabama for support.
Supporting Information Available
Experimental procedures, spectroscopic data, electronic structure calculations, and crystallographic data (CIF). This material is available free of charge via the Internet at http://pubs.acs.org.
The authors declare no competing financial interest.
Notes
Correspondence concerning electronic structure calculations should be directed to D.A.D. (dadixon@as.ua.edu). Correspondence concerning X-ray crystallography should be directed to L.N.Z. (lev@uoregon.edu).
Funding Statement
National Institutes of Health, United States
Supplementary Material
References
- Roupe K. A.; Remsberg C. M.; Yáñez J. A.; Davies N. M. Curr. Clin. Pharmacol. 2006, 1, 81–101. [DOI] [PubMed] [Google Scholar]
- For recent reviews on resveratrol, see:; a Baur J. A.; Sinclair D. A. Nat. Rev. Drug Discov 2006, 5, 493. [DOI] [PubMed] [Google Scholar]; b Cottart C.-H.; Nivet-Antoine V.; Beaudeux J.-L. Mol. Nutr. Food Res. 2014, 58, 7–21. [DOI] [PubMed] [Google Scholar]
- For pioneering mechanistic work on the photoisomerization of stilbenes, see:; a Saltiel J. J. Am. Chem. Soc. 1967, 89, 1036–1037. [Google Scholar]; b Saltiel J. J. Am. Chem. Soc. 1968, 90, 6394–6400. [Google Scholar]; c Saltiel J.; Waller A. S.; Sears D. F. Jr; Garrett C. Z. J. Phys. Chem. 1993, 97, 2516–2522. [Google Scholar]
- Cheng L. T.; Tam W.; Stevenson S. H.; Meredith G. R.; Rikken G.; Marder S. R. J. Phys. Chem. 1991, 95, 10631–10643. [Google Scholar]
- a Lo S.-C.; Burn P. L. Chem. Rev. 2007, 107, 1097–1116. [DOI] [PubMed] [Google Scholar]; b Likhtenshtein G.Stilbenes: Applications in Chemistry, Life Sciences, and Materials science; Wiley: New York, 2010. [Google Scholar]
- Zaitseva N. P.; Newby J.; Hamel S.; Carman L.; Faust M.; Lordi V.; Cherepy N. J.; Stoeffl W.; Payne S. A. Proc. SPIE 2009, 7449, 744911. [Google Scholar]
- For a leading reference, see:; a Stuber F. A.; Ulrich H.; Rao D. V.; Sayigh A. A. R. J. Appl. Polym. Sci. 1969, 13, 2247–2255. [Google Scholar]; b Soomro S. A.; Benmouna R.; Berger R.; Meier H. Eur. J. Org. Chem. 2005, 2005, 3586–3593. [Google Scholar]
- Dorlars A.; Schellhammer C.-W.; Schroeder J. Angew. Chem., Int. Ed. Engl. 1975, 14, 665–679. [Google Scholar]
- For an overview, see:Meier H. Angew. Chem., Int. Ed. Engl. 1992, 31, 1399–1420. [Google Scholar]
- For an overview of BN/CC isosterism, see:; a Liu Z.; Marder T. B. Angew. Chem., Int. Ed. 2008, 47, 242–244. [DOI] [PubMed] [Google Scholar]; b Bosdet M. J. D.; Piers W. E. Can. J. Chem. 2009, 87, 8–29. [Google Scholar]; c Campbell P. G.; Marwitz A. J.; Liu S. Y. Angew. Chem., Int. Ed. Engl. 2012, 51, 6074–6092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- a Liu L.; Marwitz A. J.; Matthews B. W.; Liu S.-Y. Angew. Chem., Int. Ed. 2009, 48, 6817–6819. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Abbey E. R.; Zakharov L. N.; Liu S.-Y. J. Am. Chem. Soc. 2010, 132, 16340–16342. [DOI] [PMC free article] [PubMed] [Google Scholar]; c Abbey E. R.; Zakharov L. N.; Liu S.-Y. J. Am. Chem. Soc. 2011, 133, 11508–11511. [DOI] [PMC free article] [PubMed] [Google Scholar]; d Knack D. H.; Marshall J. L.; Harlow G. P.; Dudzik A.; Szaleniec M.; Liu S.-Y.; Heider J. Angew. Chem., Int. Ed. 2013, 52, 2599–2601. [DOI] [PMC free article] [PubMed] [Google Scholar]; e Abbey E. R.; Liu S.-Y. Org. Biomol. Chem. 2013, 11, 2060–2069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- a Marwitz A. J.; Jenkins J. T.; Zakharov L. N.; Liu S.-Y. Angew. Chem., Int. Ed. 2010, 49, 7444–7447. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Marwitz A. J. V.; Lamm A. N.; Zakharov L. N.; Vasiliu M.; Dixon D. A.; Liu S.-Y. Chem. Sci. 2012, 3, 825–829. [Google Scholar]
- For pioneering work on monocyclic 1,2-azaborines, see:; a Dewar M. J. S.; Marr P. A. J. Am. Chem. Soc. 1962, 84, 3782. [Google Scholar]; b White D. G. J. Am. Chem. Soc. 1963, 85, 3634–3636. [Google Scholar]; c Ashe A. J.; Fang X. Org. Lett. 2000, 2, 2089–2091. [DOI] [PubMed] [Google Scholar]; d Ashe A. J. III; Fang X.; Fang X.; Kampf J. W. Organometallics 2001, 20, 5413–5418. [Google Scholar]
- For recent contributions to azaborine chemistry, see:; a Taniguchi T.; Yamaguchi S. Organometallics 2010, 29, 5732–5735. [Google Scholar]; b Lu J. S.; Ko S. B.; Walters N. R.; Kang Y.; Sauriol F.; Wang S. Angew. Chem., Int. Ed. 2013, 52, 4544–4548. [DOI] [PubMed] [Google Scholar]; c Neue B.; Araneda J. F.; Piers W. E.; Parvez M. Angew. Chem., Int. Ed. 2013, 52, 9966–9969. [DOI] [PubMed] [Google Scholar]; d Wang X. Y.; Lin H. R.; Lei T.; Yang D. C.; Zhuang F. D.; Wang J. Y.; Yuan S. C.; Pei J. Angew. Chem., Int. Ed. 2013, 52, 3117–3120. [DOI] [PubMed] [Google Scholar]; e Wisniewskim S. R.; Guenther C. L.; Argintaru O. A.; Molander G. A. J. Org. Chem. 2013, 79, 365. [DOI] [PMC free article] [PubMed] [Google Scholar]; f Bailey J. A.; Haddow M. F.; Pringle P. G. Chem. Commun. 2014, 50, 1432–1434. [DOI] [PubMed] [Google Scholar]; g Braunschweig H.; Geetharani K.; Jimenez-Halla J. O.; Schäfer M. Angew. Chem., Int. Ed. 2014, 53, 3500–4504. [DOI] [PubMed] [Google Scholar]
- Roman B. I.; De Coen L. M.; Thérèse F C Mortier S.; De Ryck T.; Vanhoecke B. W.; Katritzky A. R.; Bracke M. E.; Stevens C. V. Bioorg. Med. Chem. 2013, 21, 5054–5063. [DOI] [PubMed] [Google Scholar]
- Martí-Centelles R.; Cejudo-Marín R.; Falomir E.; Murga J.; Carda M.; Marco J. A. Bioorg. Med. Chem. 2013, 21, 3010–3015. [DOI] [PubMed] [Google Scholar]
- There are >50 commercially available monomeric styrene derivatives from Sigma-Aldrich.
- Rudebusch G. E.; Zakharov L. N.; Liu S.-Y. Angew. Chem., Int. Ed. 2013, 52, 9316–9319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- The synthesis of a B-vinyl 1,2-azaborine has been carried out in our laboratory through nucleophilic substitution of the B–Cl bond; however, generation of the corresponding styrene-based nucleophiles (e.g., Grignard or lithium reagents) was not attractive due to their low functional group tolerance and difficult synthesis.
- Marwitz A. J.; Matus M. H.; Zakharov L. N.; Dixon D. A.; Liu S.-Y. Angew. Chem., Int. Ed. Engl. 2009, 48, 973–977. [DOI] [PubMed] [Google Scholar]
- a Davan T.; Corcoran E. W. Jr; Sneddon L. G. Organometallics 1983, 2, 1693–1694. [Google Scholar]; b Kadlecek D. E.; Carroll P. J.; Sneddon L. G. J. Am. Chem. Soc. 2000, 122, 10868–10877. [Google Scholar]; c Burgess K.; Van der Donk W. A.; Westcott S. A.; Marder T. B.; Baker R. T.; Calabrese J. C. J. Am. Chem. Soc. 1992, 114, 9350–9359. [Google Scholar]; d Lynch A. T.; Sneddon L. G. J. Am. Chem. Soc. 1987, 109, 5867–5868. [Google Scholar]; e Mkhalid I. A. I.; Coapes R. B.; Edes S. N.; Coventry D. N.; Souza F. E. S.; Thomas R. L.; Hall J. J.; Bi S.-W.; Lin Z.; Marder T. B. Dalton Trans. 2008, 1055–1064. [DOI] [PubMed] [Google Scholar]; f Takaya J.; Kirai N.; Iwasawa N. J. Am. Chem. Soc. 2011, 133, 12980–12983. [DOI] [PubMed] [Google Scholar]; g Kuninobu Y.; Iwanaga T.; Omura T.; Takai K. Angew. Chem., Int. Ed. 2013, 52, 4431. [DOI] [PubMed] [Google Scholar]
- For an overview, see:Mkhalid I. A.; Barnard J. H.; Marder T. B.; Murphy J. M.; Hartwig J. F. Chem. Rev. 2010, 110, 890–899. [DOI] [PubMed] [Google Scholar]
- The low yield can be attributed to the aryl C–Br being partially reactive under our conditions.
- Lamm A. N.; Liu S.-Y. Mol. Biosyst. 2009, 5, 1303–1305See the Supporting Information for details. [DOI] [PubMed] [Google Scholar]
- A possible mechanism could involve an oxidative addition of the B–H bond to Rh followed by β-migratory insertion of the Rh–B bond into the styrene substrate to generate an internal Rh–benzyl intermediate. Subsequent β-hydride elimination would produce the desired trans BN stilbene product and a Rh dihydride complex which hydrogenates a second styrene substrate to restart the catalytic cycle. This proposed mechanism is analogous with ones that have been proposed for the dehydrogenative borylation reactions of pinacol borane; see:Brown J. M.; Lloyd-Jones G. C. J. Am. Chem. Soc. 1994, 116, 866–878. [Google Scholar]
- See the Supporting Information for details.
- For a structural study of 1,2-azaborines, see:Abbey E. R.; Zakharov L. N.; Liu S.-Y. J. Am. Chem. Soc. 2008, 130, 7250–7252. [DOI] [PubMed] [Google Scholar]
- Bis(p-methoxy)-trans-stilbene has a CC double bond length of 1.32 Å:Theocharis C. R.; Jones W.; Rao C. N. R. J. Chem. Soc., Chem. Commun. 1984, 1291–1293. [Google Scholar]
- This observation is consistent with the larger covalent radius of boron (0.85 Å) vs carbon (0.75 Å); see:Pyykkö P.; Atsumi M. Chem.—Eur. J. 2009, 15, 12770–12779. [DOI] [PubMed] [Google Scholar]
- Little perturbation of the absorbance λmax was observed in the solvatachromic study.
- Biradical states include singlet phantom state, triplet excited state, and triplet phantom state.Lewis F. D.; Weigel W. J. Phys. Chem. A 2000, 104, 8146–8153. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.