Abstract
Protein Misfolfing Cyclic amplification (PMCA) is a technique that take advantage of the nucleation-dependent prion replication process to accelerate the conversion of PrPC into PrPSc in the test tube. PMCA uses ultrasound waves to fragment the PrPSc polymers, increasing the amount of seeds present in the infected sample without affecting their ability to act as conversion nucleus. Over the past 5 years PMCA has became an invaluable technique to study diverse aspects of prions. The PMCA technology has been used by several groups to understand the molecular mechanism of prion replication, the cellular factors involved in prion propagation, the intriguing phenomena of prion strains and species barriers, to detect PrPSc in tissues and biological fluids and to screen for inhibitors against prion replication. In this article we describe a detailed protocol of the PMCA technique, highlighting some of the important technical aspects to obtain a successful and reproducible application of the technology.
Keywords: Prions, Creutzfeldt-Jakob disease, Bovine Spongiform Encephalopathy, Scrapie, Protein Misfolding Cyclic Amplification (PMCA)
1. Introduction
1.1. Prion Diseases and the Infectious Agent
Transmissible spongiform encephalopathies (TSEs), also known as prion diseases, fatal and infectious neurodegenerative disorders that affect both human and animals (1). In these diseases a long incubation period, in which the infectious agent starts its replication in the target organs, is followed by a brief and fatal clinical phase (2). Creutzfeldt Jakob disease (CJD) in humans, and scrapie, bovine spongiform encephalopathy (BSE) and chronic wasting disease (CWD) in animals are the most common forms of TSE (3). Although TSE are rare, their unique mechanism of transmission and the concerns generated by the transmission of the cattle disease into humans has put prions in the spotlight (4). In addition, due to the lack of early pre-symptomatic diagnosis, there is a tremendous risk of iatrogenic transmission of vCJD from human to human, a route that has already produced thousands of deaths in other human prion diseases, such as kuru and iatrogenic CJD (5). Finally, it is not yet clear whether the disease in sheep (scrapie) and in deer (CWD) can be transmitted to human beings. Although, there seem to be a species barrier protecting humans from these animal infections, this phenomenon is not yet completely understood, especially in the case of CWD. The latter is worrisome, because the number of CWD cases has been dramatically increasing recently in the USA (6).
The nature of the infectious agent in TSE has been the center of passionate controversy (7, 8). The most accepted hypothesis proposes that PrPSc is the sole component of the infectious agent and that its propagation does not involve nucleic acid (9). PrPSc is a post-translationally modified version of the normal host protein, PrPC. Chemical differences have not been detected to distinguish these two PrP isoforms and the conversion seems to involve a conformational change whereby the misfolded protein is organized in β-sheet rich aggregates. This structural change confers new biochemical properties to the misfolded protein: insolubility in non-denaturing detergents and partial resistance against digestion with proteinase K (10, 11). The hypothesis that PrPSc is the active component of the infectious agent associated to TSE remained controversial for decades, but recent exciting studies demonstrating the generation of infectious material from purified prion protein in vitro by PMCA have settled all doubts regarding the validity of the prion hypothesis (12).
1.2 The mechanism of prion replication
According to the protein-only hypothesis, PrPSc propagates itself by an autocatalytic reaction (10). The mechanism by which PrPSc propagates the disease in vivo has been a subject of intense investigation. At the time of infection the proportion of PrPSc inoculated is very low compared to endogenous PrPC. However, by the time animals develop the clinical signs, the amount of PrPSc in brain is very high. This finding suggests that PrPSc replicates in vivo at expenses of the normal protein. The notion that endogenous PrPC is involved in the development of infection is supported by the findings that PrP knockout animals are resistant to infection (13). These findings suggest that infectious PrPSc is capable of inducing the conversion of host PrPC, resulting in the autocatalytic formation of PrPSc.
PrPSc has been described as an oligomer of variable size that can even form large fibrillar aggregates, resembling amyloid fibrils observed in Alzheimer's disease and other amyloid-related disorders (14, 15). The comparison of prion conversion with the process of amyloidosis provides a model for the transition of PrPC to PrPSc, in which the pathological protein may act as a seed to recruit molecules of partially misfolded PrPC, stabilizing the misfolding by incorporation into the oligomer (14, 16). Thus, the PrPSc oligomer is elongated at the ends as new molecules of PrPC are converted and incorporated. The kinetics of such nucleated conformational conversion is limited by the number of seeds present in the sample (14, 16). This rate-limiting process might explain in part the long period of time needed in vivo to generate a concentration of PrPSc high enough to trigger neurodegeneration.
1.3. Protein Misfolding Cyclic Amplification: Rationale and Applications
Based on the nucleation/polymerization model, we have described a strategy that mimics in vitro the PrPSc conversion process that takes place in vivo, and that amplifies in an exponential fashion minute quantities of PrPSc present in a sample (17). This system is called Protein Misfolding Cyclic Amplification (PMCA), and consists of cycles of accelerated prion replication. Each cycle is composed of two phases. During the first phase the sample containing minute amounts of PrPSc and an excess of PrPC are incubated to induce growing of PrPSc polymers. In the second phase the sample is subjected to sonication to break down the polymers, multiplying the number of nuclei (18). After each cycle the number of seeds increases in an exponential fashion. PMCA is conceptually analogous to DNA amplification by PCR. In both systems a template grows at expenses of a substrate in a cyclic reaction, combining growing and multiplication of the template units.
We have previously reported proof-of-concept experiments in which the technology was applied to replicate the misfolded protein from diverse species (17, 19). The newly generated protein exhibits the same biochemical, biological, and structural properties as brain-derived PrPSc and strikingly it is infectious to wild-type animals, producing a disease with similar characteristics as the illness produced by brain-isolated prions (20). The technology has been automated, leading to a dramatic increase on efficiency of amplification. Indeed, one round of 144 PMCA cycles results in a 6000-fold increase of sensitivity of detection (21), whereas 2 and 7 rounds of successive PMCA result in 10 million and 3 billion folds amplification (22). Moreover, our results demonstrate that PMCA is capable to detect as little as a single molecule of oligomeric infectious PrPSc (22). PMCA enables to generate millions infectious units starting with the equivalent to one PrPSc oligomer, that is way below the infectivity threshold (22). This data demonstrates that PMCA has a similar power of amplification as PCR techniques used to amplify DNA and open a great promise for development of a highly sensitive detection of PrPSc and for understanding the molecular basis of prion replication. Indeed, we have demonstrated that after amplification we can detect PrPSc in blood of hamsters experimentally infected with scrapie during both the symptomatic (21) and pre-symptomatic phases (23) as well as in urine.
The PMCA technology has been used by several groups to understand the molecular mechanism of prion replication, the effect of cellular components, to detect PrPSc in tissues and biological fluids and to screen for inhibitors against prion replication (24–58). There are now more than 50 publications successfully using the technology from more than 10 different groups. Of particular interest are the studies by Supattapone and colleagues who have been able to produce prion replication in vitro by PMCA using purified PrPC and PrPSc with the sole addition of synthetic polyanions (25, 59). Recently, Caughey and colleagues were able to optimize PMCA using recombinant bacterial PrPC (42). This advance is important since it provides a much easier source for PrPC than mammalian brain homogenate and enables labeling of the protein in several ways for structural and biochemical studies. Finally, in a recent milestone study Ma and colleagues were able to generate bona-fide infectious material from recombinant PrP with the sole addition of RNA and lipids as catalyzers (57). This study constitutes one of the strongest evidences in favor of the prion hypothesis.
PMCA allows the faithful replication of prion strains in many different species of prions, indicating that all the elements required for strain determination are enciphered in the folding of PrPSc (60). Furthermore, the related phenomena of species barrier, strain adaptation, and molecular memory were also reproduced in vitro by PMCA (61–63), suggesting again that they are dependent purely on PrPSc replication. When PrPC from one species is used to replicate prions from a different species, new strains are generated, pointing to an extremely high flexibility of PrP. Finally, PMCA allows to reproduce the spontaneous generation of prions, which happen in the sporadic forms of prion disease (59, 64).
2. Materials
PMCA have been applied successfully to a variety of brain samples from different species, including human, cow, sheep, cervid, mink, mouse and hamster (19). In the following sections we describe the equipment required, the technical considerations, and the standard parameters that afford an optimal amplification of PrPSc.
2.1 Equipment
Sonicator Misonix S3000 or S4000
Tube holder and cover for PMCA. Part # 444 Misonix
Potter-Elvehjem Tissue Grinders 15 or 30 ml
Refrigerated centrifuge (Eppendorf model 5810R)
Ultra low freezer So-Low Model u-8525
Pipettes (serological and micropipttes)
Incubator (Shel Lab)
Thermomixer Eppendorf (Cat No 022670107)
Eppendorf Adaptor plate for 96 × 02 ml PCR tubes (Cat No 022670581)
2.2. Biological Samples
Normal brain homogenate (NBH)
Infectious material homogenate (IMH)
2.3. Solutions, reagents and buffers
Triton X-100, Sigma, Cat No T8787-100ML
EDTA 0.5M, Promega, Cat No V4231
Complete Protease Inhibitor Cocktail Tablets (PI), Roche, Cat No 11697498001.
Proteinase K (PK), Sigma-Aldrich, Cat No P2308-25mg
PBS 1X, Cellgro, Cat No. 21-040-CV
Sampler buffer, Invitrogen
Conversion Buffer: 150mM NaCl, 1% Triton X-100, 1X Protease Inhibitor Cocktail in PBS 1X. (see Note 1)
Solution PK 1 μg/μl in PBS.
Phenylmethanesulfonyl fluoride (PMSF) 50 mM, Sigma, Cat No P7626.
3. Methods
3.1 Preparation of Normal brain homogenate (NBH)
Extract brain immediately after death (see Note 2). Using the tissue grinder homogenate 1.0 g of normal brain in chilled conversion buffer to finally obtain a solution 10% W/V. Work with the tissue grinder buried on ice to keep the homogenate at low temperature.
Centrifuge the homogenate sample at 2000 rpm for 40 seconds in a refrigerated centrifuge (4°C) (see Note 3). This step is necessary to remove tissue debris.
Carefully remove the tube from the centrifuge. Avoid mixing supernatant and pellet.
Save the supernatant and discard the pellet. Make aliquots of the supernatant in 1.5 ml micro-centrifuge tubes. Quick-freeze the aliquots in liquid nitrogen and store at −80 until use (see Note 4).
3.2 Preparation of Infectious material homogenate (IMH)
It is possible to use as infectious material any tissue homogenate or biological fluid suspected to contain prions. Prepare infectious material following the same protocol used for the preparation of NBH, except that quick freezing is not so critical for the IMH. For some tissues or fluids, especially those containing large amounts of blood, it is necessary to perform a step to remove inhibitors of PMCA reaction (65). For this purpose we recommend to add 1 volume of 20% sarkosyl, incubate for 10 min at room temperature and centrifuge at 100,000 × g for 1h at 4°C. Supernatants are discarded and pellets resuspended into 2 volumes of 10% sarkosyl. The centrifugation process is repeated and pellets resuspended directly in 10% normal brain homogenate prepared in conversion buffer. Following this protocol, PrPSc is recovered with a yield higher than 90%. We do not recommend using precipitation with phosphotungstic acid or immunoprecipitation with anti-PrP antibodies, since both of these procedures interfere with PMCA.
3.3 Preparations of serially diluted samples for PMCA
Take and thaw an aliquot of NBH and IMH.
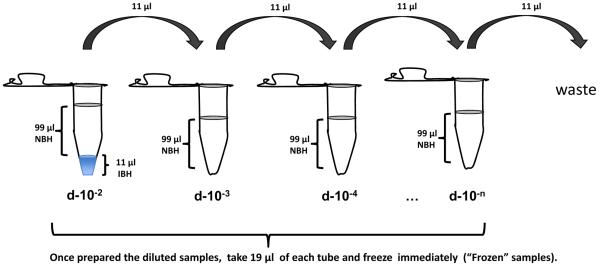
Mix carefully 11 μl of infectious brain homogenate with 99 μl of normal brain homogenate in 0.2 ml PCR tubes (Nunc, Cat No 265643) (see Note 5) to prepare a standard PMCA dilution (Fig. 1).
Take 11 μl from the first PMCA dilution and transfer to the second tube (d-10−3) (Fig 1). Pipette up and down to mix both homogenates.
Continue with the serial dilution (1/10 up to the dilution of interest) using normal brain homogenate and the previous seeded dilution d-10−4, d-10−5, etc. as an inoculum (Fig 1).
Take 19 μl from the serially diluted samples (called “Frozen” samples) and freeze immediately. This is the control for non-amplified reaction.
Mix carefully the samples before to incubate/sonicate in the sonicator.
Figure 1. Diagram depicting serial dilution of infected brain in normal brain homogenate.
The first dilution (1:100 with respect to the brain) will contain 1 volume of 10% infected brain homogenate (source of PrPSc seeds) and 9 volumes of normal brain (source of PrPC substrate); the second tube is a 10x dilution of the previous mix (1:1000 dilution with respect to the brain); the third tube is a 100x dilution of the first tube; etc. Note that all tubes should end up with the same final volume (99 ul). From each tube 19 ul are removed and kept frozen for the non-amplified control. Thus the final reaction volume is 80 ul.
3.4 Automatic Cyclic Amplification
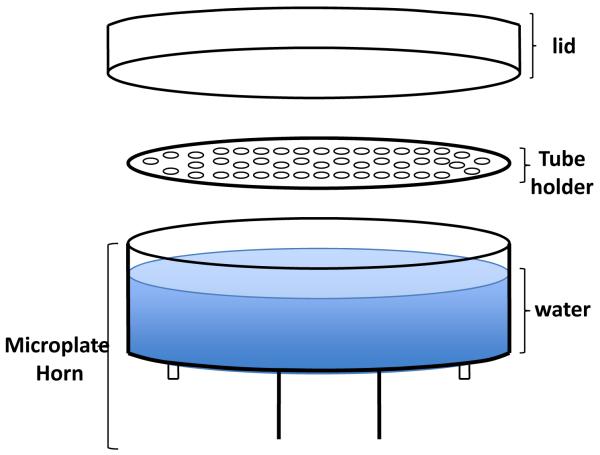
The sonication step in the PMCA technique is a key element to increase the efficiency and decrease the time used in the conversion of the PrPC into the abnormal form of the prion protein. The aim of the sonication is to fragment the PrPSc aggregates to increase the amount of seeds present in the infected sample without affecting their ability to act as conversion nucleus. The microplate horn and the tube holder (Fig 2) offer an alternative of indirect sonication and function as a high intensity ultrasound water baths capable to process a group of samples at the same time (see Note 6).
Figure 2. Diagram of tube-adapted for misonix sonicator.
PMCA-tubes are hanged from the tube holder allowing their partial immersion in water. An acrylic cover is put on top of the tubes to keep them on place during the sonication.
Connect the Misonix generator S3000 or 4000 to the convertor/microplate horn.
Put the convertor/microplate horn in the sound enclosure box provided by Misonix (see Note 7).
Put the system (convertor/microplate horn/sound enclosure box) inside of an incubator previously set at 37° C. Kept the temperature constant during the whole process.
Set carefully the parameters of sonication for the Cyclic Amplification. For the sonicator S3000 or S4000 use the amplitude necessary to obtain potency between 200–300 watts (see Note 8).
Use 20 seconds as a pulse ON time and 29 min 40sec a pulse OFF time for the setting of the sonicator. The efficiency of the NBH used as substrate decreases over time and the decrease is not the same for distinct strains. The optimal duration of the PMCA rounds are usually between 24 to 72 h of incubation/sonication (see Note 9).
Add between 180 to 200 ml of clear tap water to the microplate horn. (see Fig. 2). In our experience it is not necessary to re-circulate the water, but it is important to check that the temperature does not increase over 45C at any point during PMCA.
Put the samples in the PMCA tube holder and dipped into the water. Check carefully that the level of water is not in touch with the tube holder.
Keep the samples amplifying for 24 to 72 h (see Note 9).
3.3 PrPSc Detection
To detect the PrPSc product after amplification it is possible to use any assay designed for this purpose, including Western blot, ELISA, conformational-dependent immunoassay (CDI), etc. In our studies we traditionally use Western blots after proteinase K (PK) digestion to remove the remaining PrPC from the samples. Western blot is a widely used methodology in the field and has the advantage over some of the other assays that it is possible to distinguish PrPC from PrPSc by the molecular weight of the product after proteolytic digestion (see Note 10). Following is the protocol we use.
Take 19 μl of each sample and incubate with 1 μl of 1μg/μl Proteinase K for 1h at 45°C and 450 rpm in a Thermomixer (see Notes 10 and 11).
After PK treatment centrifuge the samples at 2000 rpm for 10 sec.
Stop the reaction adding 2 μl of 50 mM PMSF and 8 μl of 4X Sampler buffer, Invitrogen.
Heat the tubes for 10min at 100° C.
Centrifuge the heated samples at 2000 rpm for 10 sec to bring down the condensate.
Load the samples into an 4–12% acrylamide gel for SDS-PAGE
Run the electrophoresis at 70V for 30 minutes until the samples enter to the staking gel and then continue at 135V for 1 h and 30 minutes.
Proteins are electro-transfered to a Hybond-ECL nitrocellulose membrane (GE Healthcare) using 800 mA constant for 1 h.
Block the membrane with blocking solution for 1 h.
Incubate the blocked membrane for 1 h with the mAb 3F4 (Covance) diluted in 1X PBS with 0.05% Tween 20 (PBST)
Rinse 3 times (10 min each time) with 1X PBST (washing buffer)
Add Sheep Anti-Mouse IgG Alkaline Phosphatase (GE Healthcare) and incubate for 1 h.
Rinse the membrane 3 times for 10 min each with washing buffer to eliminate excess of secondary antibody.
Develop the membrane with a luminescent peroxidase substrate like the ECL-Plus from GE.
Expose the membrane to a photographic film or to other gel documentation system. Digitalize the image for future analysis.
Amplification rate should be calculated by comparing the last dilutions giving similar reactivity (intermediate intensity) between frozen and amplified samples (Figure 3). Do not compare signal intensities to estimate amplification rate, because this can produce highly misleading results.
Figure 3. Example of PrPSc amplification by PMCA.
Serial dilutions of infected brain homogenate were frozen (panel A) or subjected to our round of 96 PMCA cycles (panel B). Samples were PK-digested (except lane 8 the NBH control), western blotted and detected with an anti-PrP antibody. An initial dilution of 1×10−2 (lane 1, panel A) has an equivalent reactivity to a dilution 1×10−6 after PMCA (lane 5, panel B). Therefore, the estimated amplification rate in this experiment is 10,000-fold. Lanes 1 to 6: Serial dilution of infected sample. Lane 7: molecular weight marker. Line 8: non-digested normal brain homogenate.
3.4. Recommendations to avoid the possibility of cross-contamination
Because of the high power of amplification of PMCA, special care should be taken to prevent as much as possible cross-contamination. In addition, because PMCA enables the de novo generation of PrPSc (without pre-existing PrPSc) under defined set of conditions (64), it is very important to reduce any potential source of contamination that could complicate interpretation of these results. Following is a list of precautions recommended to minimize the possibility of cross-contamination.
Work under similar conditions used to manipulate sterile material (double gloves, biosafety cabinet, change tips everytime, avoid spills, keep neatly clean bench, clean frequently bench, pipettes and other materials with NaOH)
Use only recommended tubes. Discard tubes that open during the experiment or have higher or lower volume of buffer (see Note 5).
Change water and clean sonicator cup with NaOH after each experiment.
Do not mix prions from different sources in one sonicator.
If attempting to detect PrPSc with very high sensitivity (e.g. in blood samples) do not manipulate simultaneously samples with high quantity of infectious material.
Do not do more than 7 rounds of serial PMCA, except when propagating large quantities of infectious material.
Always include a large number of controls (NBH without PrPSc) to assess false positives in each experiment. These samples should be amplified and serially diluted in parallel with the experimental samples. In case a control sample result positive using standard PMCA conditions (i.e. not set for de novo formation of PrPSc) (64), discard the entire experiment and clean the machine with NaOH.
For safety conditions, filter tips should be used for liquid handling, and tissue homogenization should be performed in a closed container inside a biosafety hood to avoid the spread of infectious material.
Avoid working with easily convertible PrPC substrates (e.g. Bank voles).
4. Important Notes
-
1:
Composition of the conversion buffer has been established and optimized following exhaustive studies and we have found that even small changes may dramatically affect the efficiency of the amplification process. Particular attention has to be put in adding the right amount of Triton, which owing to it viscosity is difficult to pipette accurately.
-
2:
For the preparation of NBH animals should be perfused with PBS plus 5 mM EDTA to remove as much as possible the amount of blood that can interfere with PMCA. Brains have to be taken in the shortest possible time after death. In case animal perfusion is not possible, we recommend washing the fresh tissue immediately with cold PBS + 5mM EDTA to remove as much blood as possible and add EDTA to the conversion buffer.
-
3:
A high centrifugal force is not recommended because it might remove important membrane components implicated in PrPC → PrPSc conversion.
-
4:
Frequent freezing-thawing of the NBH reduces significantly the amplification. Store the stock in aliquots and use them once. Discard the remaining of the unused material.
-
5:
It is very important to use thin-walled 0.2 mL PCR tubes in order to obtain the most effective penetration of ultrasound waves. However, care should be taken in that tubes should not crack or open during the procedure. This happens with tubes from some manufacturers. In case the tube is open or the volume is significantly higher or smaller than expected, these samples should be discarded.
-
6:
The Misonix sonicator was not designed to hold tubes, so a tube holder was manufactured (Fig. 2). Although the holder can be devised to hold around 100 tubes or more, we have found that PMCA efficiency is best if the holder is not filled more than 50% capacity with tubes. This is probably because each tube attenuates to some extent the effect of the ultrasound waves.
-
7:
Do not close the sound enclosure box. This will increase substantially the temperature of the microplate horn.
-
8:
Different strains require different settings for optimal amplification. In our hands, for example, the 263K strain has optimal amplification using potency around 215–230 watts.
-
9:
Different strains also require distinct time for re-freshing the substrate, because the decrease in the efficiency of the NBH substrate decreases differently for distinct strains. For optimal amplification of 263K we use 48h and for variant CJD 24 h as a total time in each round of cyclic amplification.
-
10:
The conditions for proteinase K digestion (concentration and temperature) are different for each prion strain, and have to be carefully chosen. The critical issue is to make sure that no PrPC remains undigested after PK treatment, because it is a common mistake to confuse incomplete digestion of PrPC with false positive PrPSc formation. When PrP is detected by western blotting, it is easy to distinguish incomplete PrPC digestion from bona-fide PrPSc, because the latter exhibit a switch on molecular weight due to the removal of the first ~90 amino acids. To assure complete digestion, especially following extended incubations, a higher concentration of PK may be required in order to digest increasingly larger aggregates. Addition of up to 0.05% SDS in the buffer used for the PK treatment may also help. Digestions using temperatures of between 42° C and 64° C and with shaking at 350–450 rpm are also recommended.
-
11:
Aliquots of PK are stored frozen at −20°C and in the interests of reproducibility any thawed, unused enzyme is discarded at the end of the experiment.
5. References
- 1.Prusiner SB. Molecular biology of prion diseases. Science. 1991;252:1515–1522. doi: 10.1126/science.1675487. [DOI] [PubMed] [Google Scholar]
- 2.Roos R, Gajdusek DC, Gibbs CJ., Jr. The clinical characteristics of transmissible Creutzfeldt-Jakob disease. Brain. 1973;96:1–20. doi: 10.1093/brain/96.1.1. [DOI] [PubMed] [Google Scholar]
- 3.Collinge J. Prion diseases of humans and animals: their causes and molecular basis. Annu Rev Neurosci. 2001;24:519–550. doi: 10.1146/annurev.neuro.24.1.519. [DOI] [PubMed] [Google Scholar]
- 4.Soto C, Saborio GP. Prions: Disease propagation and disease therapy by conformational transmission. Trends Mol Med. 2001;7:109–114. doi: 10.1016/s1471-4914(01)01931-1. [DOI] [PubMed] [Google Scholar]
- 5.Brown P, Preece M, Brandel JP, Sato T, McShane L, Zerr I. Iatrogenic Creutzfeldt-Jakob disease at the millenium. Neurol. 2000;55:1075–1081. doi: 10.1212/wnl.55.8.1075. [DOI] [PubMed] [Google Scholar]
- 6.Sigurdson CJ, Aguzzi A. Chronic wasting disease. Biochim Biophys Acta. 2006;1772:610–618. doi: 10.1016/j.bbadis.2006.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Aguzzi A, Polymenidou M. Mammalian prion biology: one century of evolving concepts. Cell. 2004;116:313–327. doi: 10.1016/s0092-8674(03)01031-6. [DOI] [PubMed] [Google Scholar]
- 8.Soto C, Castilla J. The controversial protein-only hypothesis of prion propagation. Nat Med. 2004;10:S63–S67. doi: 10.1038/nm1069. [DOI] [PubMed] [Google Scholar]
- 9.Prusiner SB. Prions. Proc Natl Acad Sci U S A. 1998;95:13363–13383. doi: 10.1073/pnas.95.23.13363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cohen FE, Prusiner SB. Pathologic conformations of prion proteins. Annu Rev Biochem. 1998;67:793–819. doi: 10.1146/annurev.biochem.67.1.793. [DOI] [PubMed] [Google Scholar]
- 11.Baldwin MA, Cohen FE, Prusiner SB. Prion protein isoforms, a convergence of biological and structural investigations. J Biol Chem. 1995;270:19197–19200. doi: 10.1074/jbc.270.33.19197. [DOI] [PubMed] [Google Scholar]
- 12.Soto C. Prion Hypothesis: The end of the Controversy? Trends Biochem Sci. 2011 doi: 10.1016/j.tibs.2010.11.001. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bueler H, Aguzzi A, Sailer A, Greiner RA, Autenried P, Aguet M, et al. Mice devoid of PrP are resistant to Scrapie. Cell. 1993;73:1339–1347. doi: 10.1016/0092-8674(93)90360-3. [DOI] [PubMed] [Google Scholar]
- 14.Caughey B, Kocisko DA, Raymond GJ, Lansbury PT., Jr. Aggregates of scrapie-associated prion protein induce the cell-free conversion of protease-sensitive prion protein to the protease-resistance state. Chem Biol. 1995;2:807–817. doi: 10.1016/1074-5521(95)90087-x. [DOI] [PubMed] [Google Scholar]
- 15.Ghetti B, Piccardo P, Frangione B, Bugiani O, Giaccone G, Young K, et al. Prion protein amyloidosis. Brain Pathol. 1996;6:127–145. doi: 10.1111/j.1750-3639.1996.tb00796.x. [DOI] [PubMed] [Google Scholar]
- 16.Jarrett JT, Lansbury PT., Jr. Seeding “one-dimensional crystallization” of amyloid: a pathogenic mechanism in Alzheimer's disease and scrapie? Cell. 1993;73:1055–1058. doi: 10.1016/0092-8674(93)90635-4. [DOI] [PubMed] [Google Scholar]
- 17.Saborio GP, Permanne B, Soto C. Sensitive detection of pathological prion protein by cyclic amplification of protein misfolding. Nature. 2001;411:810–813. doi: 10.1038/35081095. [DOI] [PubMed] [Google Scholar]
- 18.Soto C, Saborio GP, Anderes L. Cyclic amplification of protein misfolding: application to prion- related disorders and beyond. Trends Neurosci. 2002;25:390–394. doi: 10.1016/s0166-2236(02)02195-1. [DOI] [PubMed] [Google Scholar]
- 19.Soto C, Anderes L, Suardi S, Cardone F, Castilla J, Frossard MJ, et al. Pre-symptomatic detection of prions by cyclic amplification of protein misfolding. FEBS Lett. 2005;579:638–642. doi: 10.1016/j.febslet.2004.12.035. [DOI] [PubMed] [Google Scholar]
- 20.Castilla J, Saá P, Hetz C, Soto C. In vitro generation of infectious scrapie prions. Cell. 2005;121:195–206. doi: 10.1016/j.cell.2005.02.011. [DOI] [PubMed] [Google Scholar]
- 21.Castilla J, Saa P, Soto C. Detection of prions in blood. Nat Med. 2005;11:982–985. doi: 10.1038/nm1286. [DOI] [PubMed] [Google Scholar]
- 22.Saa P, Castilla J, Soto C. Ultra-efficient replication of infectious prions by automated protein misfolding cyclic amplification. J Biol Chem. 2006;281:35245–35252. doi: 10.1074/jbc.M603964200. [DOI] [PubMed] [Google Scholar]
- 23.Saa P, Castilla J, Soto C. Presymptomatic detection of prions in blood. Science. 2006;313:92–94. doi: 10.1126/science.1129051. [DOI] [PubMed] [Google Scholar]
- 24.Lucassen R, Nishina K, Supattapone S. In vitro amplification of protease-resistant prion protein requires free sulfhydryl groups. Biochemistry. 2003;42:4127–4135. doi: 10.1021/bi027218d. [DOI] [PubMed] [Google Scholar]
- 25.Deleault NR, Lucassen RW, Supattapone S. RNA molecules stimulate prion protein conversion. Nature. 2003;425:717–720. doi: 10.1038/nature01979. [DOI] [PubMed] [Google Scholar]
- 26.Nishina K, Deleault NR, Lucassen RW, Supattapone S. In vitro prion protein conversion in detergent-solubilized membranes. Biochemistry. 2004;43:2613–2621. doi: 10.1021/bi035889l. [DOI] [PubMed] [Google Scholar]
- 27.Nishina K, Jenks S, Supattapone S. Ionic strength and transition metals control PrPSc protease resistance and conversion-inducing activity. J Biol Chem. 2004;279:40788–40794. doi: 10.1074/jbc.M406548200. [DOI] [PubMed] [Google Scholar]
- 28.Deleault NR, Geoghegan JC, Nishina K, Kascsak R, Williamson RA, Supattapone S. Protease-resistant prion protein amplification reconstituted with partially purified substrates and synthetic polyanions. J Biol Chem. 2005;280:26873–26879. doi: 10.1074/jbc.M503973200. [DOI] [PubMed] [Google Scholar]
- 29.Orem NR, Geoghegan JC, Deleault NR, Kascsak R, Supattapone S. Copper (II) ions potently inhibit purified PrPres amplification. J Neurochem. 2006;96:1409–1415. doi: 10.1111/j.1471-4159.2006.03650.x. [DOI] [PubMed] [Google Scholar]
- 30.Bieschke J, Weber P, Sarafoff N, Beekes M, Giese A, Kretzschmar H. Autocatalytic self-propagation of misfolded prion protein. Proc Natl Acad Sci USA. 2004;101:12207–12211. doi: 10.1073/pnas.0404650101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Piening N, Weber P, Giese A, Kretzschmar H. Breakage of PrP aggregates is essential for efficient autocatalytic propagation of misfolded prion protein. Biochem Biophys Res Commun. 2005;326:339–343. doi: 10.1016/j.bbrc.2004.11.039. [DOI] [PubMed] [Google Scholar]
- 32.Sarafoff NI, Bieschke J, Giese A, Weber P, Bertsch U, Kretzschmar HA. Automated PrPres amplification using indirect sonication. J Biochem Biophys Methods. 2005;63:213–221. doi: 10.1016/j.jbbm.2005.05.004. [DOI] [PubMed] [Google Scholar]
- 33.Barret A, Tagliavini F, Forloni G, Bate C, Salmona M, Colombo L, et al. Evaluation of quinacrine treatment for prion diseases. J Virol. 2003;77:8462–8469. doi: 10.1128/JVI.77.15.8462-8469.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jones M, Peden AH, Prowse CV, Groner A, Manson JC, Turner ML, et al. In vitro amplification and detection of variant Creutzfeldt-Jakob disease PrPSc. J Pathol. 2007;213:21–26. doi: 10.1002/path.2204. [DOI] [PubMed] [Google Scholar]
- 35.Kim NH, Choi JK, Jeong BH, Kim JI, Kwon MS, Carp RI, et al. Effect of transition metals (Mn, Cu, Fe) and deoxycholic acid (DA) on the conversion of PrPC to PrPres. FASEB J. 2005;19:783–785. doi: 10.1096/fj.04-2117fje. [DOI] [PubMed] [Google Scholar]
- 36.Kurt TD, Perrott MR, Wilusz CJ, Wilusz J, Supattapone S, Telling GC, et al. Efficient in vitro amplification of chronic wasting disease PrPres. J Virol. 2007;81:9605–9608. doi: 10.1128/JVI.00635-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Murayama Y, Yoshioka M, Yokoyama T, Iwamaru Y, Imamura M, Masujin K, et al. Efficient in vitro amplification of a mouse-adapted scrapie prion protein. Neurosci Lett. 2006;413:270–273. doi: 10.1016/j.neulet.2006.11.056. [DOI] [PubMed] [Google Scholar]
- 38.Seidel B, Thomzig A, Buschmann A, Groschup MH, Peters R, Beekes M, et al. Scrapie Agent (Strain 263K) can transmit disease via the oral route after persistence in soil over years. PLoS ONE. 2007;2:e435. doi: 10.1371/journal.pone.0000435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Haley NJ, Seelig DM, Zabel MD, Telling GC, Hoover EA. Detection of CWD prions in urine and saliva of deer by transgenic mouse bioassay. PLoS ONE. 2009;4:e4848. doi: 10.1371/journal.pone.0004848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mays CE, Titlow W, Seward T, Telling GC, Ryou C. Enhancement of protein misfolding cyclic amplification by using concentrated cellular prion protein source. Biochem Biophys Res Commun. 2009;388:306–310. doi: 10.1016/j.bbrc.2009.07.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shi S, Dong CF, Wang GR, Wang X, An R, Chen JM, et al. PrP(Sc) of scrapie 263K propagates efficiently in spleen and muscle tissues with protein misfolding cyclic amplification. Virus Res. 2009;141:26–33. doi: 10.1016/j.virusres.2008.12.010. [DOI] [PubMed] [Google Scholar]
- 42.Atarashi R, Moore RA, Sim VL, Hughson AG, Dorward DW, Onwubiko HA, et al. Ultrasensitive detection of scrapie prion protein using seeded conversion of recombinant prion protein. Nat Methods. 2007;4:645–650. doi: 10.1038/nmeth1066. [DOI] [PubMed] [Google Scholar]
- 43.Geoghegan JC, Valdes PA, Orem NR, Deleault NR, Williamson RA, Harris BT, et al. Selective incorporation of polyanionic molecules into hamster prions. J Biol Chem. 2007;282:36341–36353. doi: 10.1074/jbc.M704447200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Haley NJ, Mathiason CK, Zabel MD, Telling GC, Hoover EA. Detection of sub-clinical CWD infection in conventional test-negative deer long after oral exposure to urine and feces from CWD+ deer. PLoS ONE. 2009;4:e7990. doi: 10.1371/journal.pone.0007990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jones M, Peden AH, Yull H, Wight D, Bishop MT, Prowse CV, et al. Human platelets as a substrate source for the in vitro amplification of the abnormal prion protein (PrP) associated with variant Creutzfeldt-Jakob disease. Transfusion. 2009;49:376–384. doi: 10.1111/j.1537-2995.2008.01954.x. [DOI] [PubMed] [Google Scholar]
- 46.Kim JI, Surewicz K, Gambetti P, Surewicz WK. The role of glycophosphatidylinositol anchor in the amplification of the scrapie isoform of prion protein in vitro. FEBS Lett. 2009;583:3671–3675. doi: 10.1016/j.febslet.2009.10.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kurt TD, Telling GC, Zabel MD, Hoover EA. Trans-species amplification of PrP(CWD) and correlation with rigid loop 170N. Virology. 2009;387:235–243. doi: 10.1016/j.virol.2009.02.025. [DOI] [PubMed] [Google Scholar]
- 48.Mays CE, Ryou C. Plasminogen stimulates propagation of protease-resistant prion protein in vitro. FASEB J. 2010 doi: 10.1096/fj.10-163600. in press. [DOI] [PubMed] [Google Scholar]
- 49.Murayama Y, Yoshioka M, Horii H, Takata M, Yokoyama T, Sudo T, et al. Protein misfolding cyclic amplification as a rapid test for assessment of prion inactivation. Biochem Biophys Res Commun. 2006;348:758–762. doi: 10.1016/j.bbrc.2006.07.130. [DOI] [PubMed] [Google Scholar]
- 50.Murayama Y, Yoshioka M, Masujin K, Okada H, Iwamaru Y, Imamura M, et al. Sulfated dextrans enhance in vitro amplification of bovine spongiform encephalopathy PrP(Sc) and enable ultrasensitive detection of bovine PrP(Sc) PLoS One. 2010 doi: 10.1371/journal.pone.0013152. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Pastrana MA, Sajnani G, Onisko B, Castilla J, Morales R, Soto C, et al. Isolation and Characterization of a Proteinase K-Sensitive PrP(Sc) Fraction. Biochemistry. 2006;45:15710–15717. doi: 10.1021/bi0615442. [DOI] [PubMed] [Google Scholar]
- 52.Shi S, Dong CF, Tian C, Zhou RM, Xu K, Zhang BY, et al. The propagation of hamster-adapted scrapie PrPSc can be enhanced by reduced pyridine nucleotide in vitro. FEBS J. 2009;276:1536–1545. doi: 10.1111/j.1742-4658.2009.06871.x. [DOI] [PubMed] [Google Scholar]
- 53.Shikiya RA, Ayers JI, Schutt CR, Kincaid AE, Bartz JC. Coinfecting prion strains compete for a limiting cellular resource. J Virol. 2010;84:5706–5714. doi: 10.1128/JVI.00243-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Suyama K, Yoshioka M, Akagawa M, Murayama Y, Horii H, Takata M, et al. Prion inactivation by the Maillard reaction. Biochem Biophys Res Commun. 2007;356:245–248. doi: 10.1016/j.bbrc.2007.02.113. [DOI] [PubMed] [Google Scholar]
- 55.Tattum MH, Jones S, Pal S, Collinge J, Jackson GS. Discrimination between prion-infected and normal blood samples by protein misfolding cyclic amplification. Transfusion. 2010;50:996–1002. doi: 10.1111/j.1537-2995.2010.02595.x. [DOI] [PubMed] [Google Scholar]
- 56.Thorne L, Terry LA. In vitro amplification of PrPSc derived from the brain and blood of sheep infected with scrapie. J Gen Virol. 2008;89:3177–3184. doi: 10.1099/vir.0.2008/004226-0. [DOI] [PubMed] [Google Scholar]
- 57.Wang F, Wang X, Yuan C-G, Ma J. Generating a Prion with Bacterially Expressed Recombinant Prion Protein. Science. 2010;327:1132–1135. doi: 10.1126/science.1183748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Weber P, Giese A, Piening N, Mitteregger G, Thomzig A, Beekes M, et al. Generation of genuine prion infectivity by serial PMCA. Vet Microbiol. 2007;123:346–357. doi: 10.1016/j.vetmic.2007.04.004. [DOI] [PubMed] [Google Scholar]
- 59.Deleault NR, Harris BT, Rees JR, Supattapone S. Formation of native prions from minimal components in vitro. Proc Natl Acad Sci U S A. 2007;104:9741–9746. doi: 10.1073/pnas.0702662104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Castilla J, Morales R, Saa P, Barria M, Gambetti P, Soto C. Cell-free propagation of prion strains. EMBO J. 2008;27:2557–2566. doi: 10.1038/emboj.2008.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Castilla J, Gonzalez-Romero D, Saa P, Morales R, De Castro J, Soto C. Crossing the species barrier by PrP(Sc) replication in vitro generates unique infectious prions. Cell. 2008;134:757–768. doi: 10.1016/j.cell.2008.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Green KM, Castilla J, Seward TS, Napier DL, Jewell JE, Soto C, et al. Accelerated high fidelity prion amplification within and across prion species barriers. PLoS Pathog. 2008;4:e1000139. doi: 10.1371/journal.ppat.1000139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Meyerett C, Michel B, Pulford B, Spraker TR, Nichols TA, Johnson T, et al. In vitro strain adaptation of CWD prions by serial protein misfolding cyclic amplification. Virology. 2008;382:267–276. doi: 10.1016/j.virol.2008.09.023. [DOI] [PubMed] [Google Scholar]
- 64.Barria MA, Mukherjee A, Gonzalez-Romero D, Morales R, Soto C. De novo generation of infectious prions in vitro produces a new disease phenotype. PLoS Pathog. 2009;5:e1000421. doi: 10.1371/journal.ppat.1000421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Chen B, Morales R, Barria MA, Soto C. Estimating prion concentration in fluids and tissues by quantitative PMCA. Nat Methods. 2010;7:519–520. doi: 10.1038/nmeth.1465. [DOI] [PMC free article] [PubMed] [Google Scholar]