Abstract
Aim of the study
To present the characteristics and clinical outcomes in 94 patients with mucinous breast cancer treated at the Oncology Centre in Krakow between 1952 and 2002.
Material and methods
Stage I or II carcinomas were found in 66 patients (69.4%) of the presented group and in the remaining 28 patients (29.8%) stage III disease was diagnosed. In 27 cases regional lymph nodes were involved. All patients had been treated with surgery: mastectomy (90 patients) or breast-conserving treatment (4 patients). Radiotherapy was administered in 14 patients, adjuvant chemo-therapy in 14 patients, and endocrine therapy in 39 patients.
Results
The maximum follow-up was 257 months. Ten-year survival was as follows: 75.7% (overall survival), 82.5% (disease-free survival). During the follow-up, 4 patients developed local recurrence, 5 patients developed metastases. Second primary cancer was found in 8 patients.
Conclusions
The presented results confirm the good prognosis in patients treated for mucinous breast cancer. The diagnosis of early-stage breast cancer based on mammography can allow breast-conserving treatment.
Keywords: mucinous breast cancer, mastectomy, breast-conserving treatment, second primary cancer
Introduction
Pure mucinous breast cancer represents approximately 1–4% of all malignant breast carcinomas. This type of cancer is most commonly diagnosed in women aged 55–60 and above [1–9].
The microscopic assessment of the cancer presents cell clusters floating in mucus lakes, separated by thin epithelial membranes containing capillaries. The clusters differ in quantity and in some cases tend to form tubular structures. The cancer cells indicate low nuclear atypia. The above characteristics are dominant in 90% of pure mucinous cancer cases. In mixed form, the ductal carcinoma component is present additionally. Mucinous breast cancer has been defined as low grade cancer with the presence of hormone receptors (oestrogen in 91–94%, progesterone in 79–81%) and lack of HER2 amplification. Lymph node metastases are rarely seen clinically in this form of cancer. All the factors are linked with favourable prognosis [1, 2, 4, 5, 9, 10].
Mucinous breast cancer is usually diagnosed in relatively early stages of the disease [2, 6]. In approximately 50% of patients, physical examinations indicate the presence of a well-circumscribed tumour in the breast. For the remaining 50% of patients, in whom clinical symptoms are not present, the form of cancer is diagnosed during screening mammography. Its image resembles benign lesions. In such cases histopathological examinations are decisive [5, 11, 12].
In comparison to the infiltrating ductal carcinoma of the breast, pure mucinous breast carcinoma portends a less aggressive clinical course. Ten-year disease-free survival rates are over 90% [13–15].
Aim of the study
The purpose of this study is to present the characteristics and the results of the treatment of patients suffering from pure mucinous breast carcinoma treated over a 50-year period at the Oncology Centre in Kraków.
Material and methods
In the period 1952 to 2002, 94 patients were diagnosed with pure mucinous breast carcinoma at the Oncology Centre in Kraków. These cases represented 0.7% of all (13,571) patients treated for breast cancer in this period.
Table 1 presents the clinical characteristics of the discussed group of patients.
Table 1.
The clinical characteristics of 94 patients with pure mucinous breast cancer treated at the Oncology Centre in Krakow between 1952 and 2002
| Clinical feature | No. of patients | % |
|---|---|---|
| primary tumour in breast (T): | ||
| T1 | 14 | 14.9 |
| T2 | 60 | 63.8 |
| T3 | 20 | 21.3 |
| stage (TNM): | ||
| I | 15 | 16.0 |
| II | 51 | 54.3 |
| III | 28 | 29.8 |
| nodal status (pN): | ||
| pN0 | 67 | 71.3 |
| pN+ | 27 | 28.7 |
| 1–3 | 15 | 16.0 |
| ≥ 4 | 12 | 12.8 |
The age ranged from 32 up to 85 years and was on average 64 years (median age: 67). Cancer symptoms (presence of a tumour in the breast) in 83 patients (88.3%) preceded recognition by 1–24 months and lasted for approximately 6 months. In 11 patients (11.7%) breast cancer was recognised by prophylactic exams.
The extent of cancer presented in the study was based on the presently adopted TNM classification of breast cancer at the time of treatment planning.
The majority of patients (60 patients – 63.8%) were diagnosed with stage T2 cancer.
In 67 (71.3%) patients, no lymph node metastases were noted. For the remaining 27 patients (28.7%), metastases of lymph nodes were indicated: 1–3 lymph nodes in 15 patients, 4 or more in 12 patients.
Clinical staging was defined as follows: stage I – 15 patients (16%), stage II – 51 patients (53.4%), stage III – 28 patients (29.8%).
Table 2 presents the treatment methods.
Table 2.
Treatment methods in 94 patients with pure mucinous breast cancer treated at the Oncology Centre in Krakow between 1952 and 2002
| Treatment methods | No. of patients | % |
|---|---|---|
| surgery | 94 | 100.0 |
| mastectomy | 90 | 95.7 |
| Patey's method | 60 | 63.8 |
| Halsted's method | 30 | 31.9 |
| breast-conserving surgery | 4 | 4.3 |
| radiotherapy | 25 | 26.6 |
| chemotherapy | 14 | 14.9 |
| endocrine therapy | 39 | 41.5 |
All the patients suffering from mucinous carcinoma received primary surgical treatment. In 90 patients (95.7%) a mastectomy was performed, using either Patey's method (60 patients) or Halsted's method (30 patients). In the remaining 4 patients (4.3%), breast-conserving treatment was introduced (tumour resection and axillary lymphadenectomy). In this group of patients post-operative, adjuvant radiation treatment was provided. Radiotherapy was performed in 25 patients (26.6%).
Fourteen patients (14.9%) were treated with adjuvant chemotherapy, whereas 39 patients (41.5%) received endocrine therapy with tamoxifen.
In the follow-up period the incidence of complications and treatment failures was assessed. The presence of second primary cancers was also investigated. The 10-year overall and disease-fee survival rates were assessed. The Kaplan-Meier estimation was used for this purpose.
Results
In the studied group the follow-up lasted a maximum of 257 months (median: 106 months).
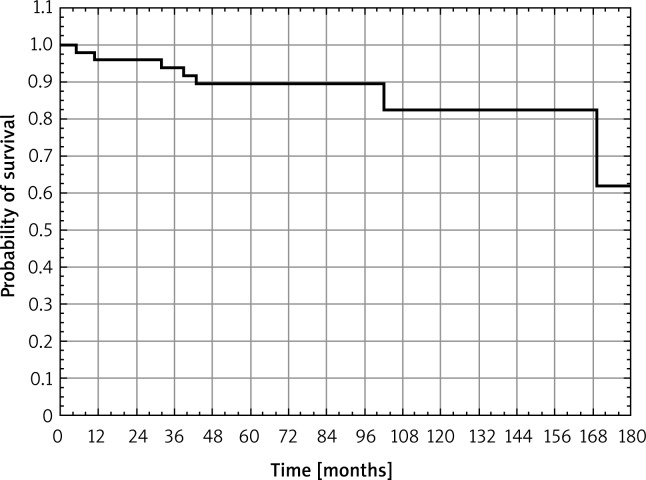
The actuarial 10-year survival rates were as follows: overall – 75.7%, disease-free survival – 82.5%.
Figure 1 presents the disease-free survival rates.
Fig. 1.
The disease-freee survival in 94 patients with mucinous breast cancer
In the follow-up period, complications after treatment were observed in 15 patients (16%). In 11 patients upper limb oedema on the operated side was observed. One patient (1.1%) suffered from venous thrombosis, 3 patients (3.2%) from endometrial hyperplasia of the uterus. All patients received systemic adjuvant treatment.
Table 3 presents the causes of treatment failures.
Table 3.
Failures in 94 patients with pure mucinous breast cancer treated at the Oncology Centre in Krakow between 1952 and 2002
| Failures | No. of patients | % |
|---|---|---|
| local recurrence | 4 | 4.3 |
| distant metastases | 5 | 5.3 |
| lung | 4 | 4.3 |
| bones | 1 | 1.1 |
| lymph nodes | 1 | 1.1 |
| brain | 1 | 1.1 |
In 4 patients (4.3%) local recurrences were noted within 18–43 months (approximately: 33 months) after breast cancer treatment.
The development of distant metastases was observed in 5 (5.3%). It occurred within 5–169 months (approximately 77.8 months) after treatment. Metastases were most frequently localized in lungs (4 patients).
In 8 patients (8.5%) second primary tumours were noted, among which 3 were localised in the opposite breast. The other localisations were: uterus (2 patients), cervix (1 patient), lung (1 patient) and non-Hodgkin's lymphoma (1 patient). The above neoplasms developed 24–126 months (approximately 76 months) after the treatment of mucinous breast carcinoma.
Discussion
Pure mucinous breast cancer has positive prognostic features. According to the literature, 5-year and 10-year survival rates are: 77–92% and 75–89% (overall), and 76–91% and 74–93% (disease-free) [2, 4, 12, 16, 17].
The obtained results are comparable to the above. Ten-year survival rates were: 75.7% (overall) and 82.5% (disease-free survival).
In approximately 50% of the patients, mucinous breast carcinoma is diagnosed by palpation, whereas in asymptomatic cases the cancer is diagnosed after mammographic screenings [11, 12].
In the studied material, for the majority of patients (83 from 94 – 88.3%) the main symptom of the cancer was an examined tumour in the breast. In the remaining 11.7% of the patients the neoplasm was diagnosed in a mammography examination. Differences between the studied and the previously published data exist due to the fact that the studied material comes from a period of 50 years, i.e. 1952–2002. Over this period diagnosis and treatment rules changed due to the development of medicine. This can be confirmed by the observations made of the patients suffering from mucinous breast cancer treated at the Oncology Centre in Krakow in the 1952–2010 period, presented in Table 4. The period 1952–2002 is the subject of the study. In more recent years, i.e. in the period 2003–2010, mucinous breast carcinoma was detected in mammography screenings (34% of patients).
Table 4.
Frequency of breast cancer diagnosis during screening mammography and frequency of breast-conserving surgery in patients with pure mucinous breast cancer in material of the Oncology Centre in Krakow
| No. of patients | Period of therapy | ||
|---|---|---|---|
|
| |||
| 1952–2002 (present paper) | 2003–2010 | total 1952–2010 | |
| mucinous breast cancer | 94 | 53 | 147 |
| diagnosis during screening mammography | 11 (11.7%) | 18 (34%) | 29 (19.7%) |
| breast-conserving surgery | 4 (4.3%) | 19 (35.8%) | 23 (15.6%) |
Mucinous breast carcinoma is most often diagnosed in older patients, aged 55–60 and above [2–9, 16]. The characteristic feature of this type of cancer is that it can be diagnosed in relatively early stages of the disease. A stage T1–2 tumour is diagnosed in 93–97% of patients, whereas the lack of metastases to lymph nodes is observed in 62-88% of patients [4, 13, 14, 16–19].
In the studied material stage T1–2 was present in 74.7% of patients, whereas the lack of metastases to lymph nodes (pN0) was observed in 71.3% of patients. In case of the presence of metastases to lymph nodes 16% of patients suffered from metastases of 1–3 lymph nodes. According to data from the relevant literature, the presence of metastases in 1–3 lymph nodes is diagnosed in 10–11% of patients suffering from mucinous breast cancer [2, 19].
The results of multivariate analysis presented in the literature by Di Saverio et al. and Vo et al. indicate that particularly good prognosis in patients suffering from mucinous breast cancer is associated with the following independent factors: age, tumour size, status of lymph nodes and oestrogen receptor [2, 4, 14]. The significant influence of the status of the lymph nodes on the treatment outcome was indicated in a previous publication published by our institute, in which the results of a group of patients treated in the years 1952–1979 were presented. The cancer-free survival rate drops by two-thirds with recognition of the incidence of lymph node metastases (26.7% vs. 83.3%) [20].
Diab et al. observed convergence between the size of the primary tumour and the status of lymph nodes in a group of 111 patients with mucinous breast cancer. In the case of tumours below 2 cm in size, in 90% of the patients metastases to lymph nodes were not indicated. Increase in tumour size corresponds with a decrease in the percentage of negative lymph nodes, to the extent that in the case of tumours over 5 cm in size it equals 56% [2]. As a result, the number of performed lymphadenectomies and the application of systemic treatment in early mucinous cancer deteriorates. The primary method of treatment in patients suffering from the studied diagnosis is surgical treatment with post-operative adjuvant treatment: radiotherapy, chemotherapy, endocrine therapy.
In the presented material 90 out of 94 patients (95.7%) were treated with a mastectomy (Patey's method in 60 patients, Halsted's method in 30 patients). In the remaining 4 patients (4.3%) breast-conserving treatment was introduced.
According to the data from the literature breast-conserving treatment is employed in over 60% of patients suffering from mucinous breast cancer [13, 18]. The difference with the studied material, in which this procedure involved only 4.5% of patients, could reflect the period of the study, i.e. 1952–2002. Over this period both the methods and the spectra of treatment, along with the indications, changed. This conclusion was indicated in a prior publication of the Oncology Centre, in which a mastectomy had been performed in all patients [20]. In contrast, Table 4 presents a significant increase in the employment of breast-conserving treatment in patients with mucinous breast carcinoma during the years 2003–2010, which was 35.8%.
The data concerning the incidence of radiotherapy indicate that it was performed in 31–90% of patients [4, 13, 14, 17]. This could be the result of the frequency of breast-conserving treatment, during which radiotherapy was an integral part. In the studied material, in which mastectomy was a dominant procedure, 26.6% of patients were treated with radiotherapy.
According to the literature, the use of adjuvant, systemic treatment is comparable with the studied material, with frequencies of 10–37% and 14.9% (chemotherapy), and 33–84% (endocrine therapy) [2, 4, 18, 19].
Vo et al., Li et al. and Bae et al., when comparing various histological cancer types, indicated that the involvement of systemic treatment in patients with mucinous breast cancer is less frequent than in patients with ductal breast cancer [4, 13, 17].
In the presented group of patients, 4 out of 94 (4.3%) indicated local recurrence, whereas in 5 patients (5.3%) the cancer spread. According to the data, local and distant failures in patients with mucinous breast cancer are rare and concern less than 5% of the patients [4, 18]. Diab et al. and Vo et al. claim that the decisive risk factor for the development of the failures is the presence of lymph node metastases [2, 4]. Furthermore, as was indicated by Vo et al., the failures are less frequently noted in patients with mucinous breast cancer in comparison with other histological types of breast cancer [4].
The studied observations and data from the literature indicate the good prognosis in case of mucinous breast carcinoma. Mammography screenings enable cancer to be detected at an early stage, which leaves the possibility of introducing breast-conserving treatment.
The authors declare no conflict of interest.
References
- 1.Lakhani SR, Ellis IO, Schnitt SJ, et al. World Health Organization Classification of Tumours. 4th ed. Lyon: International Agency for Research on Cancer; 2012. WHO Classification of tumours of the breast. [Google Scholar]
- 2.Diab SG, Clark GM, Osborne CK, Libby A, Allred DC, Elledge RM. Tumor characteristics and clinical outcome of tubular and mucinous breast carcinomas. J Clin Oncol. 1999;17:1442–8. doi: 10.1200/JCO.1999.17.5.1442. [DOI] [PubMed] [Google Scholar]
- 3.Thurman SA, Schnitt SJ, Connolly JL, Gelman R, Silver B, Harris JR, Recht A. Outcome after breast-conserving therapy for patients with stage I or II mucinous, medullary, or tubular breast carcinoma. Int J Radiat Oncol Biol Phys. 2004;59:152–9. doi: 10.1016/j.ijrobp.2003.10.029. [DOI] [PubMed] [Google Scholar]
- 4.Vo T, Xing Y, Meric-Bernstam F, et al. Long-term outcomes in patients with mucinous, medullary, tubular, and invasive ductal carcinomas after lumpectomy. Am J Surg. 2007;194:527–31. doi: 10.1016/j.amjsurg.2007.06.012. [DOI] [PubMed] [Google Scholar]
- 5.Reimer T. Management of rare histological types of breast. Breast Care (Basel) 2008;3:190–6. doi: 10.1159/000136826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Li CI, Uribe DJ, Daling JR. Clinical characteristic of different histologic types of breast cancer. Br J Cancer. 2005;93:1046–52. doi: 10.1038/sj.bjc.6602787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Karan B, Pourbagher A, Bolat FA. Unusual malignant breast lesion: imaging-pathological correlations. Diagn Interv Radiol. 2012;18:270–6. doi: 10.4261/1305-3825.DIR.4454-11.2. [DOI] [PubMed] [Google Scholar]
- 8.Cyrta J, Andreiuolo F, Azoulay S, et al. Pure and mixed mucinous carcinoma of the breast: fine needle aspiration cytology findings and review of the literature. Cytopathology. 2013;24:377–84. doi: 10.1111/cyt.12016. [DOI] [PubMed] [Google Scholar]
- 9.Laucirica R, Bentz JS, Khalbuss WE, Clayton AC, Souers RJ, Moriarty AT. Performance characteristics of mucinous (colloid) carcinoma of the breast in fine-needle aspirates: observations from the College of American Pathologists Interlaboratory Comparison Program in Nongynecologic Cytopathology. Arch Pathol Lab Med. 2011;135:1533–8. doi: 10.5858/arpa.2010-0652-CP. [DOI] [PubMed] [Google Scholar]
- 10.Work ME, Andrulis IL, John EM, et al. Risk factors for uncommon histologic subtypes of breast cancer using centralized pathology review in the Breast Cancer Family Registry. Breast Cancer Res Treat. 2012;134:1209–20. doi: 10.1007/s10549-012-2056-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dhillon R, Depree P, Metcalf C, Wylie E. Screen-detected mucinous breast carcinoma: potential for delayed diagnosis. Clin Radiol. 2006;61:423–30. doi: 10.1016/j.crad.2005.10.008. [DOI] [PubMed] [Google Scholar]
- 12.Tan JZ, Waugh J, Kumar B, Evans J. Mucinous carcinomas of the breast: Imaging features and potential for misdiagnosis. J Med Imaging Radiat Oncol. 2013;57:25–31. doi: 10.1111/1754-9485.12006. [DOI] [PubMed] [Google Scholar]
- 13.Li CI. Risk of mortality by histologic type of breast cancer in the United States. Horm Cancer. 2010;1:156–65. doi: 10.1007/s12672-010-0016-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Di Saverio S, Gutierrez J, Avisar E. A retrospective review with long term follow up of 11,400 cases of pure mucinous breast carcinoma. Breast Cancer Res Treat. 2008;111:541–7. doi: 10.1007/s10549-007-9809-z. [DOI] [PubMed] [Google Scholar]
- 15.Komaki K, Sakamoto G, Sugano H, Morimoto T, Monden Y. Mucinous carcinoma of the breast in Japan: a prognostic analysis based on morphologic features. Cancer. 1988;61:989–96. doi: 10.1002/1097-0142(19880301)61:5<989::aid-cncr2820610522>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 16.Morand C, Verrièle V, Valo I, Remoue P, Paillocher N, Chassevent A. Pure mucinous carcinomas of the breast: prognostic study including DNA flow cytometry. Clin Cytom. 2009;76B:56–62. doi: 10.1002/cyto.b.20436. [DOI] [PubMed] [Google Scholar]
- 17.Bae SY, Choi MY, Cho DH, Lee JE, Nam SJ, Yang JH. Mucinous carcinoma of the breast in comparison with invasive ductal carcinoma: clinicopathologic characteristics and prognosis. J Breast Cancer. 2011;14:308–13. doi: 10.4048/jbc.2011.14.4.308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Barkley CR, Ligibel JA, Wong JS, Lipsitz S, Smith BL, Golshan M. Mucinous breast carcinoma: a large contemporary series. Am J Surg. 2008;196:549–51. doi: 10.1016/j.amjsurg.2008.06.013. [DOI] [PubMed] [Google Scholar]
- 19.Cao AY, He M, Liu ZB, et al. Outcome of pure mucinous breast carcinoma compared to infiltrating ductal carcinoma: a population- based study from China. Ann Surg Oncol. 2012;19:3019–27. doi: 10.1245/s10434-012-2322-6. [DOI] [PubMed] [Google Scholar]
- 20.Stelmach A, Reinfuss M, Smolak K, Mitus J. Śluzowaty rak sutka. Obraz kliniczny, leczenie i rokowanie. Nowotwory. 1992;42:151–5. [Google Scholar]