Abstract
Purpose
To describe change in spherical equivalent (M) in a longitudinal sample of Tohono O'odham students ages 3 to 18 years and to test the hypothesis that astigmatism creates complex cues to emmetropization, resulting in increased change in M in the direction of increasing myopia and increased occurrence of myopia.
Methods
Subjects were 777 Tohono O'odham Native American children on whom cycloplegic right eye autorefraction was measured on at least two study encounters between ages 3 and 18 years (first encounter prior to age 5.5 years, final encounter ≥ 3 years later). Regression lines were fit to individual subjects’ longitudinal M data to estimate rate of change in M (regression slope, D/year). Regression was also used to predict if a subject would be myopic (≤−0.75D M) by age 18 years. ANCOVA was used to assess the relation between M slope and magnitude of baseline M and astigmatism. Chi-square analyses were used to assess the relation between predicted myopia onset and magnitude of baseline M and astigmatism.
Results
Mean M slope was significantly more negative for hyperopes (M ≥ +2.00) than for myopes (M ≤ −0.75) or for subjects neither hyperopic nor myopic (NHM, M > −0.75 and < +2.00), but there was no significant difference between the myopic and NHM groups. Chi-square analysis indicated that final myopia status varied across level of baseline astigmatism. Subjects with high astigmatism were more likely to be predicted to have significant myopia by age 18 years.
Conclusions
The association between greater shift in M towards myopia with age in subjects who were hyperopic at baseline is consistent with continued emmetropization in the school years. Results regarding predicted myopia development imply that degradation of image quality due to refractive astigmatism creates complex cues to emmetropization, resulting in increased occurrence of myopia.
Keywords: astigmatism, myopia, refractive development, emmetropization
Corneal astigmatism occurs as a result of unequal curvature of the anterior cornea, with contributions from the posterior cornea.1 The combination of corneal astigmatism and internal astigmatism results in the eye's total refractive astigmatism, which can create a blurred retinal image. Pujol et al.2 analyzed the influence of astigmatism and changes in axis of astigmatism on the eye's optical performance, and showed significant degradation of image quality. Deprivation of a focused retinal image can cause high myopia in primates and chicks.3,4 It is this line of reasoning, along with reports of an association between astigmatism and the onset of myopia (reviewed in Grosvenor and Goss5) that led researchers to further investigate the matter.
Fulton et al.6 found that in 3-year-old children and younger, myopia progressed in eyes with ≥ 1 diopter (D) of refractive astigmatism and tended to increase through age 8 years in those with ≥ 3D. They suggested that uncorrected astigmatism may be a causative factor in the development of myopia. Gwiazda et al.7 analyzed manifest refractions from 245 infant subjects with 6-23 years of regular follow-up. Results showed that infantile astigmatism was associated with increased astigmatism and myopia during the school years. Tong et al.8 examined the epidemiological risk factors for astigmatism in Singapore school children and found that a family history of myopia was associated with the severity of oblique astigmatism. Fan et al.,9 in a study of 522 Chinese preschool children, found that the presence of astigmatism appeared to predispose the children to progressive myopia.
Some studies have concentrated their efforts on with-the-rule (WTR) astigmatism, where the steepest corneal meridian is oriented vertically. Farbrother et al.10 in a cross sectional analysis of 19 optometric practices in the north of England, found an association between WTR astigmatism and high myopia. Heidary et al.11 completed a retrospective study of 217 severely myopic patients. They found that the degree of myopic spherical refractive error is correlated with WTR astigmatism severity.
Not all studies have shown an association between the presence of astigmatism and the progression of myopic refractive errors.12 Pärssinen13 measured the degree of astigmatism at the beginning of the study and, controlling for the spherical equivalent, found no association with myopic progression.
Many members of a Native American tribe, the Tohono O'odham, show moderate to high levels of WTR corneal astigmatism in infancy. While there are sometimes minor fluctuations in astigmatic power, anterior corneal astigmatism typically becomes stable by 3 years of age and most astigmatic Tohono O'odham children remain astigmatic throughout childhood.14 We have assembled a large longitudinal database of refractive error in Tohono O'odham children ranging in age from 3 to 18 years. In the present study, we investigate the hypothesis that degradation of image quality due to refractive astigmatism creates complex cues to emmetropization, resulting in increased rate of spherical equivalent shift towards myopia and increased occurrence of myopia. Specifically, we predict that change in spherical equivalent with age will show an elevated rate of shift toward myopia with age in high astigmats, and that high astigmats will be more likely to become myopic during development (prior to age19 years) than children with little or no astigmatism.
METHODS
Subjects
Subjects were participants in at least one of six studies of refractive error and visual development in Tohono O'odham children, funded by the National Institutes of Health National Eye Institute. Assent was obtained from minor subjects, with permission from the parent/guardian prior to examination. Written informed consent for participation was obtained from the subject if he or she was age 18 or older. This research followed the tenets of the Declaration of Helsinki and was approved by the Tohono O'odham Nation and by the University of Arizona Institutional Review Board.
Procedures
Once recruited for participation, a baseline examination was conducted and follow-up examinations were attempted yearly (with the exception of Phase I, in which examinations were conducted twice per year for preschool children) until the end of the study or until the subject reached the oldest grade of eligibility for a given study. At each study encounter, subjects participated in a cycloplegic eye examination and autorefraction. Cycloplegia was accomplished through one of 3 protocols, each of which included 3 drops: Proparacaine 0.5% followed by 2% cyclopentolate followed by 1% cyclopentolate; Proparacaine 0.5% followed by two drops of 1% cyclopentolate; Proparacaine 0.5% followed by 1% tropicamide followed by 1% cyclopentolate. At least 30 minutes after dispensing of eye drops, autorefraction was conducted with the Retinomax (KPlus or KPlus2 models, Nikon Inc., Tokyo Japan). A previous report has shown that the Retinomax provides reliable and valid measures of refractive error in this population.15
Statistical Analysis
Data from subject encounters were included if the subject's age was 3 to < 19 years on the date of encounter, and right eye cycloplegic Retinomax autorefraction showed a confidence of 8 or higher (per manufacturer recommendations).16 Only data from subjects with at least 2 study encounters were included in longitudinal analyses. Retinomax right eye measurements of spherical equivalent (M) and refractive astigmatism were included in analyses. Astigmatism data were analyzed in terms of clinical notation (Cyl).
Preliminary Analysis of Linear Association Between M and Age
We anticipated that M would show a shift towards myopia with age, but it was not known if the change with age would be consistent across the full age range (3 through 18 years). A LMM was used to model the longitudinal data and determine if the tested association between age and M was a straight line or if it instead was best fit by two line segments that have different slopes (i.e., different rates of change with age) and thus a significant inflection point (i.e., an age after which progression towards myopia increased or decreased). This statistical model removes the serial correlation due to multiple measures on the same subjects by simultaneously fitting a regression line to each individual's data (called the random component) and a linear model to data that included all subjects (called the fixed component), hence the name LMM since it has both random and fixed components. To determine if an inflection point existed, we fit a piece-wise linear term as the fixed component and compared the goodness of fit with that obtained by fitting a straight line. Additional variables included in the model were gender, baseline astigmatism magnitude, years of follow-up, and number of follow-up points. Once an inflection point was identified using this method, subsequent analyses of change in M (D/year) and final myopia status (myopic vs. non-myopic) were conducted for data from subjects who met the following criteria: at least one data point prior to the age at which an inflection in the regression line was identified, and at least 3 years of follow-up (so that in all instances subjects had at least one data point after the inflection point age).
Analysis of the Relation Between Change in M with Age and Magnitude of Baseline M and Baseline Astigmatism
Individual regression lines were calculated for each subject's M data, and regression slopes were used in analyses as an estimate of rate of change in M with age (D/year). To test the hypothesis that rate of progression towards myopia (evidenced by negative slope values) differs by magnitude of baseline M and baseline astigmatism, mean M slopes (i.e., mean change in M per year) were compared using an Analysis of Covariance (ANCOVA) across baseline M and astigmatism groups. Baseline M was categorized as myopic (M ≤ −0.75), neither hyperopic nor myopic, abbreviated as NHM (> −0.75 and < +2.00), or hyperopic (M ≥ +2.00). Baseline astigmatism was categorized as no/low astigmatism (< 1.00 D), moderate astigmatism (1.00 to < 3.00 D), or high astigmatism (≥ 3.00 D). ANCOVA allows comparisons between categories while adjusting for covariates. Years of follow-up and number of encounters were included in the ANCOVA as covariates.
Analysis of the Relation Between Final Myopia Status and Magnitude of Baseline M and Baseline Astigmatism
For each subject, final myopia status was determined using a prediction method which classified subjects as becoming myopic if, based on the regression line generated from the subject's longitudinal data, the subject became or would be predicted to become myopic prior to age 18, with myopia defined as M of ≤ −0.75D. We utilized this prediction method because there was variability across subjects in final age of follow-up, and thus any subjects who might have become myopic between their final study encounter and age 18 would have been incorrectly classified as not having developed myopia. We repeated the analysis using an occurrence method that classified subjects with M of ≤ −0.75D at the final study visit as becoming myopic, and the results were similar.
Since one of the hypotheses we are testing is that astigmatism-related blur contributes to myopia onset and change, glasses wear in our subjects is a significant confounding variable, particularly because subjects were provided spectacles through the studies. Subjects who consistently wore their glasses might not experience enough blur for astigmatism to influence myopia progression. Therefore, we conducted secondary analyses to determine if the M slope and predicted myopia occurrence results differed when the sample was limited only to subjects who did not consistently wear spectacle correction. At each study encounter (including screenings, eyeglass dispensing, and examinations), spectacle wear was recorded. We assembled these data for all subjects in our longitudinal database, and determined the number of encounters for each subject at which spectacle wear was recorded and the percentage of those encounters on which they were wearing spectacle correction. A minimum of 3 data points were required for inclusion in the secondary analysis, and subjects who were wearing spectacles on less than one-third of study visits were not considered “consistent wearers” and were therefore included in the secondary analyses.
RESULTS
A total of 10,799 subject encounters were conducted (3,601 different subjects), and data from 9,472 encounters (88%) met inclusion criteria. Reasons for exclusion of study encounters were right eye Retinomax reliability score < 8 or not recorded (1214), > 18 years old (25), < 3 years old (19), refused drops (37), poor cooperation (15), incomplete examinations (14), right eye prosthesis (1), and right eye patch graft (4), with some encounters meeting more than one criterion. The full longitudinal dataset included 2,522 subjects who had at least two study encounters that met these inclusion criteria. The number of encounters ranged from 2 to 12. A demographic and baseline refractive error summary is provided in Table 1.
Table 1.
Summary of sample characteristics.
| Variable | Longitudinal Sample | Reduced Sample (baseline age ≤ 5.5, FU years ≥ 3) |
|---|---|---|
| N | 2522 | 777 |
| Sex (Female/Male) | 1290/1232 | 401/376 |
| Mean Baseline Age (SD) | 6.52 (3.10) | 4.17 (0.62) |
| Mean FU Years (SD) | 3.85 (3.30) | 7.15 (3.15) |
| Mean Number of encounters (SD) | 3.33 (1.63) | 4.75 (1.81) |
| Mean Baseline Cyl (SD), Refractive | 1.19 (1.27) | 1.35 (1.21) |
| Mean Baseline J0 (SD), Refractive | +0.54 (0.64) | +0.63 (0.61) |
| Mean Baseline J45 (SD), Refractive | +0.03 (0.23) | +0.03 (0.23) |
| Mean Baseline M (SD) | +0.71 (1.34) | +1.03 (1.19) |
| Mean M Slope | −0.16 (0.36) | −0.16 (0.17) |
| Mean M Offset | 0.75 (1.33) | 1.14 (1.16) |
Preliminary Analysis of Linear Association Between M and Age
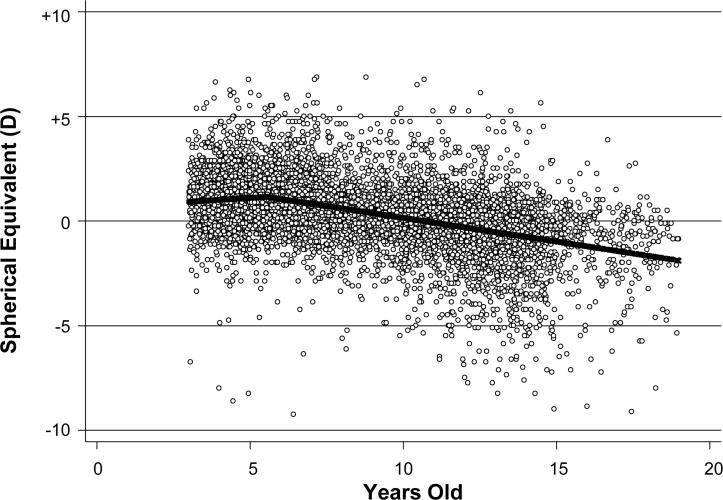
The best fitting LMM had a single inflection point at 5.5 years of age (see Figure 1). The slope for M up to age 5.5 years was +0.09, and the slope after age 5.5 years was −0.23 (p < 0.001). The amount of baseline astigmatism and gender were not statistically significant predictors, but number of observations (Beta=0.06, p <0.005) and length of follow-up (Beta=−0.03, p < 0.003) were both significant.
Figure 1.
Scatterplot of the longitudinal spherical equivalent (M) data across age with the best-fit line. The inflection point occurs at age 5.5 years.
Analysis of the Relation Between Change in M with Age and Baseline M and Baseline Astigmatism
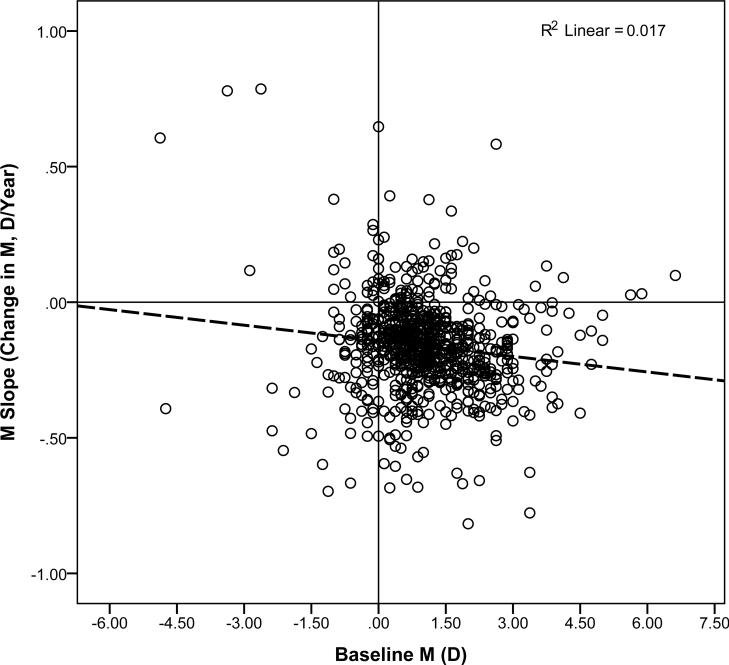
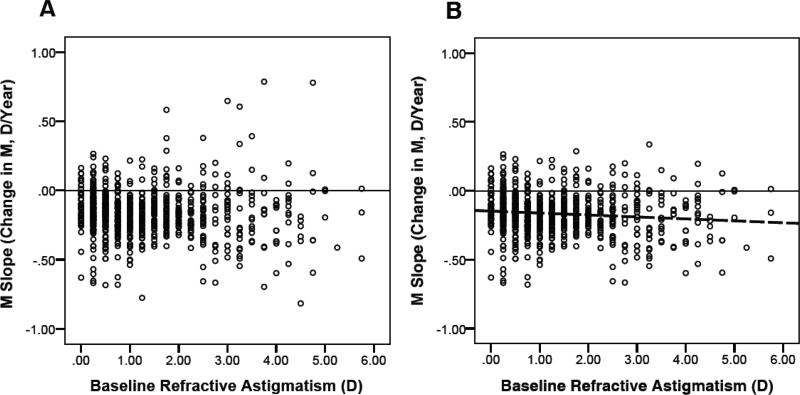
Analyses included all encounters from 777 subjects who had a baseline measurement at age ≤ 5.5 years (prior to the inflection point at which slope changed) and who had ≥ 3 years of follow-up (from baseline to final measurement). ANCOVA compared mean M slope for subjects across levels of baseline M (hyperopic, NHM, and myopic) and baseline astigmatism (no/low astigmatism, moderate astigmatism, and high astigmatism), including years of follow-up and number of encounters as covariates. Results are summarized in Table 2. Mean M slope (D/year) did differ depending on baseline M (p < 0.004), but did not significantly differ based on magnitude of baseline astigmatism. Post hoc analyses with Bonferroni correction for multiple comparisons indicated that mean slope was significantly more negative for hyperopic than for myopic or NHM subjects, but there was no significant difference between the myopic and NHM groups. The interaction between baseline astigmatism and baseline M was not significant.Years of follow-up was a significant covariate (p < 0.001), but number of encounters was not. A post hoc analysis indicated that years of follow-up did not vary significantly across subjects who were hyperopic, NHM, or myopic at baseline. Figure 2 presents the scatterplot of slope (D/year) by baseline M and Figure 3a presents the slope (D/year) by level of baseline astigmatism. The pattern of significance of the ANCOVA results did not differ when the analysis was limited to subjects who never or rarely wore spectacles (see Table 2, right side).
Table 2.
Relation between baseline M, baseline astigmatism, and progression towards myopia (M slope, D/year).
| Baseline M | Baseline Astigmatism | All subjects in reduced sample | Never or rarely wore spectacles | ||||
|---|---|---|---|---|---|---|---|
| Mean | SD | N | Mean | SD | N | ||
| Myopic (M </= −0.75) | < 1.00 D | −0.04 | 0.23 | 8 | −0.01 | 0.23 | 6 |
| 1 to < 3 D | −0.13 | 0.20 | 13 | −0.13 | 0.11 | 8 | |
| 3 D or more | −0.12 | 0.49 | 15 | −0.04 | 0.49 | 9 | |
| Total | −0.11* | 0.35 | 36 | −0.16 | 0.32 | 23 | |
| NHM (M > −0.75 and < +2.00) | < 1.00 D | −0.15 | 0.14 | 336 | −0.14 | 0.12 | 319 |
| 1 to < 3 D | −0.15 | 0.15 | 194 | −0.14 | 0.15 | 137 | |
| 3 D or more | −0.16 | 0.22 | 59 | −0.15 | 0.23 | 32 | |
| Total | −0.15* | 0.16 | 589 | −0.14 | 0.14 | 488 | |
| Hyperopic (M >/= +2.00) | < 1.00 D | −0.20 | 0.14 | 31 | −0.21 | 0.13 | 29 |
| 1 to < 3 D | −0.22 | 0.17 | 87 | −0.21 | 0.17 | 74 | |
| 3 D or more | −0.21 | 0.19 | 34 | −0.22 | 0.21 | 17 | |
| Total | −0.21* | 0.17 | 152 | −0.21 | 0.17 | 120 | |
| All | < 1.00 D | −0.15 | 0.15 | 375 | −0.14 | 0.13 | 354 |
| 1 to < 3 D | −0.17 | 0.16 | 294 | −0.16 | 0.16 | 219 | |
| 3 D or more | −0.17 | 0.27 | 108 | −0.15 | 0.28 | 58 | |
| Total | −0.16 | 0.17 | 777 | −0.15 | 0.16 | 631 | |
Statistically significant difference between slopes for myopes, NHM, and hyperopes (p < 0.004).
Figure 2.
Scatterplot of M Slope by Baseline M. Dashed line represents the regression line.
Figure 3.
Scatterplot of M Slope by Refractive Astigmatism, (A) with outliers and (B) with outliers removed.
Analysis of the Relation Between Final Myopia Status and Baseline M and Baseline Astigmatism
Table 3 (left side) presents a summary of predicted final myopia status (myopia defined as M ≤ −0.75 D) by baseline M and baseline astigmatism. Chi-square analysis indicated that final myopia status varied across level of baseline astigmatism for subjects who were myopic at baseline (p < 0.05) and for subjects who were NHM at baseline (p < 0.005), but was not significant at the 95% confidence level for subjects who were hyperopic at baseline (p=0.10).
Table 3.
Relation between baseline M, baseline astigmatism, and development of myopia.
| All Subjects in Reduced Sample (n=777) | Only Subjects who never or rarely wore spectacles (n=631) | ||||
|---|---|---|---|---|---|
| Baseline M | Baseline Astigmatism | Predicted Not Myopic Count | Predicted Myopic Count (%) | Predicted Not Myopic Count | Predicted Myopic Count (%) |
| Myopic (M <= −0.75)* | < 1.00 D | 4 | 4 (50) | 4 | 2 (33) |
| 1 to <3 D | 1 | 12 (92) | 0 | 8 (100) | |
| 3 D or more | 2 | 13 (87) | 2 | 7 (78) | |
| Total | 7 | 29 (81) | 5 | 23 (74) | |
| NHM (M > −0.75 and < +2.00)* | < 1.00 D | 241 | 95 (28) | 238 | 81 (25) |
| 1 to <3 D | 115 | 79 (41) | 90 | 47 (34) | |
| 3 D or more | 32 | 27 (46) | 19 | 13 (41) | |
| Total | 388 | 201 (34) | 347 | 141 (29) | |
| Hyperopic (M >= +2.00)** | < 1.00 D | 31 | 0 (0) | 29 | 0 (0) |
| 1 to <3 D | 79 | 8 (9) | 71 | 3 (4) | |
| 3 D or more | 29 | 5(15) | 14 | 3 (18) | |
| Total | 139 | 13 (9) | 114 | 6 (5) | |
| All * | < 1.00 D | 276 | 99 (26) | 271 | 83 (23) |
| 1 to <3 D | 195 | 99 (34) | 161 | 58 (26) | |
| 3 D or more | 63 | 45 (42) | 35 | 23 (40) | |
| Total | 534 | 243 (31) | 467 | 164 (26) | |
Statistically significant difference (0.05 confidence level) in predicted final myopia status across levels of baseline astigmatism for both samples.
Statistically significant difference (95% confidence level) in predicted final myopia status across levels of baseline astigmatism for sample of subjects who never/rarely wore spectacles.
The right side of Table 3 summarizes results when the sample was limited to subjects who never or rarely wore spectacle correction. Chi-square analyses indicated significant effect of the amount of astigmatism on myopia outcome status for subjects who were myopic (p <0.02) and NHM (p = 0.05) at baseline and for subjects who were hyperopic (p< 0.03).
The estimated age of onset was 10.52 years (SD = 3.23) for the subjects who were predicted to become myopic. Age of onset did not significantly differ based on level of baseline astigmatism (mean age =10.37 years [SD = 3.31], 10.88 years [SD = 3.07], and 9.93 years [SD = 3.30] for low, moderate and high baseline astigmats, respectively). There was very little change to this pattern of results when analyses were limited to subjects who never/rarely wore spectacle correction.
DISCUSSION
The strengths of this study are the relatively large study sample, followed from preschool and into elementary school and often into the teen years, with a high occurrence of moderate to high corneal astigmatism leading to moderate to high refractive astigmatism. The astigmatism is relatively stable from a young age14 and clearly present before the onset of myopia in most cases.
The LMM (Figure 1) shows a slope of +0.09 D/year up to age 5.5 years, with a single inflection point, followed by a slope of −0.23 (p < 0.001). We can only speculate on the reasons for this inflection point, but one possibility is that the children typically enter school at the age of 5 or 6 with an increasingly large near work demand. While some studies have shown associations between near work and myopia onset,17,18 other studies have shown weak associations or none at all.19,20 An increase in overall body growth could be associated with ocular growth. Yip et al.21 found that variations in the onset and peak progression of myopia may be associated with height spurts, but we did not measure height at all study visits and are unable to assess this association in our sample.
The data indicate that baseline M is associated with the rate of change in M (Table 2, left hand columns). Hyperopes change at a greater rate than those NHM, and those NHM more than myopes, demonstrating evidence of continuing emmetropization in the school years.
It appeared that the lack of association between M slope and level of baseline astigmatism was due to the strong effect of a relatively limited number of outliers (see Figure 3a). To test this assumption, we removed the M slope outliers (slopes > 3 SD from the mean M slope), and reanalyzed the regression (see Figure 3b). We found that relation between M slope and level of baseline astigmatism was statistically significant (p<0.02). The outliers represented extreme M scores at baseline that over time lessened in magnitude, either due to emmetropization, measurement error at baseline, or regression to the mean.
Table 3 presents a summary of final myopia status (myopia defined as M ≤ −0.75 D) by baseline M and baseline astigmatism. The strongest evidence of astigmatism influencing the onset of myopia is seen in subjects who were NHM at baseline. Percent occurrence of myopia increases with level of baseline astigmatism from 28, to 41, to 46 percent occurrence for low, moderate, and high astigmats, respectively, (p < 0.005). The trend was similar for the 81 percent of NHM subjects who rarely or never wore spectacles (either due to noncompliance or because they were not prescribed) with 25, 34, and 41 percent occurrence of myopia for low, moderate, and high astigmats, respectively. There was no significant difference in final study encounter age for NHM subjects across astigmatism groups (low = 11.22 years, moderate = 11.38 years, high = 11.16 years). Thus it is not likely that this factor contributed to the effects of astigmatism on predicted final myopia status shown in Table 3.
The main limitation of the study is that we did not utilize a randomized clinical trial study design to assign subjects to astigmatic correction (no blur) or no correction (astigmatic blur) to assess the blur hypothesis of myopia development. Spectacle correction for moderate to high levels of astigmatism is the standard of care, and it would have been unethical to randomize children into a non-treatment group. This means that confounding is a concern. However, secondary analyses in which we included subjects who never or rarely wore spectacles yielded similar results, suggesting that spectacle wear had a minimal, if any, influence on the results, perhaps because spectacle compliance tends to be poor. However, we must acknowledge that our estimates of spectacle wearing compliance may have been limited in reliability, as compliance was not a primary measure in our studies and they were based on the limited data we had available.
It is possible that other factors, besides the effects of blur induced by astigmatism, could be the cause of the association we observed between astigmatism and myopia. Many factors influence image degradation on the retina, including level of accommodation. Increased levels of accommodative lag have been shown in models to affect the progression of myopia. 22 There could be an interaction between level of astigmatism and accommodative lag, and we are currently studying this possibility.
A genetic factor with a common association between astigmatism and myopia could also play a role in refractive development in our sample. A susceptibility locus for astigmatism has been identified,23 although it was in a study sample of persons of European descent. Genetic factors play a significant role in refractive error development.24, 25
Smith et al.26 found that high levels of ambient lighting retard the development of form-deprivation myopia in monkeys. Several studies have examined the relation between time spent outdoors and myopia, with mixed results (as reviewed by Sherwin et al.27). We did not measure time spent outdoors or exposure to light levels in our sample. However, the Tohono O'odham reservation is located in the high desert of southern Arizona which is one of the sunniest places in the United States.28 This has implications for the hypothesis that myopia development may be associated with blur. High light levels decrease pupil size which results in lower levels of retinal blur from uncorrected refractive error including astigmatism and myopia. Thus, the retinal blur experienced by our astigmatic subjects may have been reduced due to this effect, and therefore the association between astigmatic blur and myopia may be underestimated in our sample.
In summary, we have shown an association between spherical equivalent refractive status before the age of 5.5 years and refractive change over time. As a group, hyperopic subjects at baseline showed the greatest refractive change, followed by NHM subjects, and myopic subjects showed the least refractive change. This implies continuing emmetropization into the school-aged years. Higher levels of refractive astigmatism were associated with predicted myopia onset in this sample of Native American children. This supports the hypothesis that degradation of image quality due to refractive astigmatism created complex cues to emmetropization, resulting in increased rates of myopia onset.
ACKNOWLEDGEMENTS
The authors thank the Tohono O'odham Nation, the Tohono O'odham Early Childhood Head Start Program, the Baboquivari School District, the Bureau of Indian Affairs Office of Indian Education Programs (BIA OIEP), the San Xavier Mission School, and the parents and children who participated in the study. This study is overseen by an NIH/NEI Data Monitoring and Oversight Committee [Robert Hardy, PhD (chair), Tina Aguilar Donald Everett, MA, Jeannette Francisco Jonathan Holmes, MD, Ronald Johnson, MD, Rosemary Lopez, and Karla Zadnik, OD, PhD].
Supported by the National Eye Institute/National Institutes of Health grants U10-EY13153, U10-EY08893, and Research to Prevent Blindness.
Footnotes
The authors declare no conflicts of interest.
These data were presented in part at the Association for Research in Vision and Ophthalmology Annual Meeting, Seattle WA, May 2013.
REFERENCES
- 1.Read SA, Collins MJ, Carney LG. A review of astigmatism and its possible genesis. Clin Exp Optom. 2007;90:5–19. doi: 10.1111/j.1444-0938.2007.00112.x. [DOI] [PubMed] [Google Scholar]
- 2.Pujol J, Arjona M, Arasa J, Badia V. Influence of amount and changes in axis of astigmatism on retinal image quality. J Opt Soc Am A Opt Image Sci Vis. 1998;15:2514–21. doi: 10.1364/josaa.15.002514. [DOI] [PubMed] [Google Scholar]
- 3.Raviola E, Wiesel TN. Effect of dark-rearing on experimental myopia in monkeys. Invest Ophthalmol Vis Sci. 1978;17:485–8. [PubMed] [Google Scholar]
- 4.Wallman J, Turkel J, Trachtman J. Extreme myopia produced by modest change in early visual experience. Science. 1978;201:1249–51. doi: 10.1126/science.694514. [DOI] [PubMed] [Google Scholar]
- 5.Grosvenor T, Goss DA. Role of the cornea in emmetropia and myopia. Optom Vis Sci. 1998;75:132–45. doi: 10.1097/00006324-199802000-00017. [DOI] [PubMed] [Google Scholar]
- 6.Fulton AB, Hansen RM, Petersen RA. The relation of myopia and astigmatism in developing eyes. Ophthalmology. 1982;89:298–302. doi: 10.1016/s0161-6420(82)34788-0. [DOI] [PubMed] [Google Scholar]
- 7.Gwiazda J, Grice K, Held R, McLellan J, Thorn F. Astigmatism and the development of myopia in children. Vision Res. 2000;40:1019–26. doi: 10.1016/s0042-6989(99)00237-0. [DOI] [PubMed] [Google Scholar]
- 8.Tong L, Saw SM, Carkeet A, Chan WY, Wu HM, Tan D. Prevalence rates and epidemiological risk factors for astigmatism in Singapore school children. Optom Vis Sci. 2002;79:606–13. doi: 10.1097/00006324-200209000-00012. [DOI] [PubMed] [Google Scholar]
- 9.Fan DS, Rao SK, Cheung EY, Islam M, Chew S, Lam DS. Astigmatism in Chinese preschool children: prevalence, change, and effect on refractive development. Br J Ophthalmol. 2004;88:938–41. doi: 10.1136/bjo.2003.030338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Farbrother JE, Welsby JW, Guggenheim JA. Astigmatic axis is related to the level of spherical ametropia. Optom Vis Sci. 2004;81:18–26. doi: 10.1097/00006324-200401000-00006. [DOI] [PubMed] [Google Scholar]
- 11.Heidary G, Ying GS, Maguire MG, Young TL. The association of astigmatism and spherical refractive error in a high myopia cohort. Optom Vis Sci. 2005;82:244–7. doi: 10.1097/01.opx.0000159361.17876.96. [DOI] [PubMed] [Google Scholar]
- 12.Goss DA, Shewey WB. Rates of childhood myopia progression as a function of type of astigmatism. Clin Experiment Optom. 1990;73:159–63. [Google Scholar]
- 13.Pärssinen O. Astigmatism and school myopia. Acta Ophthalmol (Copenh) 1991;69:786–90. doi: 10.1111/j.1755-3768.1991.tb02061.x. [DOI] [PubMed] [Google Scholar]
- 14.Harvey EM, Miller JM, Schwiegerling J, Sherrill D, Messer DH, Dobson V. Developmental changes in anterior corneal astigmatism in Tohono O'odham Native American infants and children. Ophthalmic Epidemiol. 2013;20:102–8. doi: 10.3109/09286586.2013.767355. [DOI] [PubMed] [Google Scholar]
- 15.Harvey EM, Miller JM, Dobson V, Tyszko R, Davis AL. Measurement of refractive error in Native American preschoolers: validity and reproducibility of autorefraction. Optom Vis Sci. 2000;77:140–9. doi: 10.1097/00006324-200003000-00013. [DOI] [PubMed] [Google Scholar]
- 16.Vision in Preschoolers Study Group. Impact of confidence number on the screening accuracy of the Retinomax autorefractor. Optom Vis Sci. 2007;84:181–8. doi: 10.1097/OPX.0b013e3180339f5a. [DOI] [PubMed] [Google Scholar]
- 17.Saw SM, Carkeet A, Chia KS, Stone RA, Tan DT. Component dependent risk factors for ocular parameters in Singapore Chinese children. Ophthalmology. 2002;109:2065–71. doi: 10.1016/s0161-6420(02)01220-4. [DOI] [PubMed] [Google Scholar]
- 18.Pan CW, Ramamurthy D, Saw SM. Worldwide prevalence and risk factors for myopia. Ophthalmic Physiol Opt. 2012;32:3–16. doi: 10.1111/j.1475-1313.2011.00884.x. [DOI] [PubMed] [Google Scholar]
- 19.Mutti DO, Mitchell GL, Moeschberger ML, Jones LA, Zadnik K. Parental myopia, near work, school achievement, and children's refractive error. Invest Ophthalmol Vis Sci. 2002;43:3633–40. [PubMed] [Google Scholar]
- 20.Ip JM, Saw SM, Rose KA, Morgan IG, Kifley A, Wang JJ, Mitchell P. Role of near work in myopia: findings in a sample of Australian school children. Invest Ophthalmol Vis Sci. 2008;49:2903–10. doi: 10.1167/iovs.07-0804. [DOI] [PubMed] [Google Scholar]
- 21.Yip VC, Pan CW, Lin XY, Lee YS, Gazzard G, Wong TY, Saw SM. The relationship between growth spurts and myopia in Singapore children. Invest Ophthalmol Vis Sci. 2012;53:7961–6. doi: 10.1167/iovs.12-10402. [DOI] [PubMed] [Google Scholar]
- 22.Schor C. The influence of interactions between accommodation and convergence on the lag of accommodation. Ophthalmic Physiol Opt. 1999;19:134–50. doi: 10.1046/j.1475-1313.1999.00409.x. [DOI] [PubMed] [Google Scholar]
- 23.Lopes MC, Hysi PG, Verhoeven VJ, Macgregor S, Hewitt AW, Montgomery GW, Cumberland P, Vingerling JR, Young TL, van Duijn CM, Oostra B, Uitterlinden AG, Rahi JS, Mackey DA, Klaver CC, Andrew T, Hammond CJ. Identification of a candidate gene for astigmatism. Invest Ophthalmol Vis Sci. 2013;54:1260–7. doi: 10.1167/iovs.12-10463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dirani M, Chamberlain M, Shekar SN, Islam AF, Garoufalis P, Chen CY, Guymer RH, Baird PN. Heritability of refractive error and ocular biometrics: the Genes in Myopia (GEM) twin study. Invest Ophthalmol Vis Sci. 2006;47:4756–61. doi: 10.1167/iovs.06-0270. [DOI] [PubMed] [Google Scholar]
- 25.He M, Hur YM, Zhang J, Ding X, Huang W, Wang D. Shared genetic determinant of axial length, anterior chamber depth, and angle opening distance: the Guangzhou Twin Eye Study. Invest Ophthalmol Vis Sci. 2008;49:4790–4. doi: 10.1167/iovs.08-2130. [DOI] [PubMed] [Google Scholar]
- 26.Smith EL, 3rd, Hung LF, Huang J. Protective effects of high ambient lighting on the development of form-deprivation myopia in rhesus monkeys. Invest Ophthalmol Vis Sci. 2012;53:421–8. doi: 10.1167/iovs.11-8652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sherwin JC, Reacher MH, Keogh RH, Khawaja AP, Mackey DA, Foster PJ. The association between time spent outdoors and myopia in children and adolescents: a systematic review and meta-analysis. Ophthalmology. 2012;119:2141–51. doi: 10.1016/j.ophtha.2012.04.020. [DOI] [PubMed] [Google Scholar]
- 28.Ranking of cities based on percent annual possible sunshine in descending order from most to least average possible sunshine. NOAA; Available at: http://www1.ncdc.noaa.gov/pub/data/ccd-data/pctposrank.txt. Accessed July 20, 2013. [Google Scholar]