Abstract
Background
Echinacea plant preparations (family Asteraceae) are widely used in Europe and North America for common colds. Most consumers and physicians are not aware that products available under the term Echinacea differ appreciably in their composition, mainly due to the use of variable plant material, extraction methods and the addition of other components.
Objectives
To assess whether there is evidence that Echinacea preparations are effective and safe compared to placebo in the prevention and treatment of the common cold.
Search methods
We searched CENTRAL 2013, Issue 5, MEDLINE (1946 to May week 5, 2013), EMBASE (1991 to June 2013), CINAHL (1981 to June 2013), AMED (1985 to February 2012), LILACS (1981 to June 2013), Web of Science (1955 to June 2013), CAMBASE (no time limits), the Centre for Complementary Medicine Research (1988 to September 2007), WHO ICTRP and clinicaltrials.gov (last searched 5 June 2013), screened references and asked experts in the field about published and unpublished studies.
Selection criteria
Randomized controlled trials (RCTs) comparing mono‐preparations of Echinacea with placebo.
Data collection and analysis
At least two review authors independently assessed eligibility and trial quality and extracted data. The primary efficacy outcome was the number of individuals with at least one cold in prevention trials and the duration of colds in treatment trials. For all included trials the primary safety and acceptability outcome was the number of participants dropping out due to adverse events. We assessed trial quality using the Cochrane 'Risk of bias' tool.
Main results
Twenty‐four double‐blind trials with 4631 participants including a total of 33 comparisons of Echinacea preparations and placebo met the inclusion criteria. A variety of different Echinacea preparations based on different species and parts of plant were used. Evidence from seven trials was available for preparations based on the aerial parts of Echinacea purpurea.
Ten trials were considered to have a low risk of bias, six to have an unclear risk of bias and eight to have a high risk of bias. Ten trials with 13 comparisons investigated prevention and 15 trials with 20 comparisons investigated treatment of colds (one trial addressed both prevention and treatment).
Due to the strong clinical heterogeneity of the studies we refrained from pooling for the main analysis. None of the 12 prevention comparisons reporting the number of patients with at least one cold episode found a statistically significant difference. However a post hoc pooling of their results, suggests a relative risk reduction of 10% to 20%. Of the six treatment trials reporting data on the duration of colds, only two showed a significant effect of Echinacea over placebo. The number of patients dropping out or reporting adverse effects did not differ significantly between treatment and control groups in prevention and treatment trials. However, in prevention trials there was a trend towards a larger number of patients dropping out due to adverse events in the treatment groups.
Authors' conclusions
Echinacea products have not here been shown to provide benefits for treating colds, although, it is possible there is a weak benefit from some Echinacea products: the results of individual prophylaxis trials consistently show positive (if non‐significant) trends, although potential effects are of questionable clinical relevance.
Plain language summary
Echinacea for preventing and treating the common cold
Preparations of the plant Echinacea are widely used in some European countries and in North America for common colds. Echinacea preparations available on the market differ greatly as different types (species) and parts (herb, root or both) of the plant are used, different manufacturing methods (drying, alcoholic extraction or pressing out the juice from fresh plants) are used and sometimes also other herbs are added.
We reviewed 24 controlled clinical trials with 4631 participants investigating the effectiveness of several different Echinacea preparations for preventing and treating common colds or induced rhinovirus infections. Our review shows that a variety of products prepared from different Echinacea species, different plant parts and in a different form have been compared to placebo in randomized trials. Due to the significant differences in the preparations tested, it was difficult to draw strong conclusions. Five trials were rated as having a low risk of bias in all five categories of the Cochrane 'Risk of bias' tool. Five more trials were rated as low risk of bias, having an unclear risk of bias in only one category. Eight trials were rated as having a high risk of bias in at least one category and the remaining six as having an unclear risk of bias.
The majority of trials investigated whether taking Echinacea preparations after the onset of cold symptoms shortens the duration, compared with placebo. Although it seems possible that some Echinacea products are more effective than a placebo for treating colds, the overall evidence for clinically relevant treatment effects is weak. In general, trials investigating Echinacea for preventing colds did not show statistically significant reductions in illness occurrence. However, nearly all prevention trials pointed in the direction of small preventive effects. The number of patients dropping out or reporting adverse effects did not differ significantly between treatment and control groups in prevention and treatment trials. However, in prevention trials there was a trend towards a larger number of patients dropping out due to adverse events in the treatment groups.
The evidence is current to July 2013.
Background
Description of the condition
Common cold is the most frequent disease in humans. A large US‐American survey showed that over 70% of the population annually was suffering from at least one viral respiratory tract infection. The authors concluded that the economic burden in the USA was almost USD 40 billion annually (Fendrick 2003). Viral agents causing common colds are mostly picornaviruses (rhinoviruses and enteroviruses), coronaviruses, adenoviruses, parainfluenza viruses and respiratory syncytial viruses (Denny 1995; Monto 1987). The incidence of the common cold has a peak in the winter months. There are several different hypotheses and explanations for this. One is that cooling of the nasal airway decreases the effectiveness of local respiratory defenses such as mucociliary clearance and leucocyte phagocytosis (Eccles 2002).
Description of the intervention
Extracts of the plant Echinacea (of the family Asteraceae) are widely used by consumers and practitioners in some European countries and in the US for preventing and treating upper respiratory tract infections (Barrett 2003). In the US mainstream market, Echinacea preparations are among the second top‐selling herbal products (Blumenthal 2005).
Assessment of the effectiveness of Echinacea preparations is complicated by the limited comparability of the available preparations for the following reasons.
Three different species are in medical use: Echinacea purpurea (E. purpurea),Echinacea pallida (E. pallida) and Echinacea angustifolia (E. angustifolia).
Different parts of the plant are used (root, herb, flower or whole plant).
Different methods of extraction are used.
In some preparations other plant extracts or homeopathic components are added.
The evidence available from clinical trials on its effectiveness has been considered inconsistent in several reviews (Barrett 1999; Caruso 2005; Linde 2006; Melchart 1994; Melchart 1999). Two meta‐analyses pooling trials using different heterogeneous Echinacea preparations for the treatment of induced rhinovirus infections (Schoop 2006b) or the common cold (Shah 2007) found more positive results for the effect of Echinacea. These results have to be interpreted with caution, as the great heterogeneity of tested Echinacea preparations makes comparison and pooling of data methodologically questionable.
How the intervention might work
The exact mechanisms of action for the immunomodulating effects of Echinacea preparations are unclear. Four classes of compounds are known to contribute to the immunomodulatory activity of Echinacea extracts: alkamides, glycoproteins, polysaccharides and caffeic acid derivatives (CADs). Phenolic compounds include caffeic, cichoric, caftaric and chlorogenic acid, as well as cynarin and echinacoside and are found in differing concentrations in the roots of both E. angustifolia and E. purpurea but also in the aerial parts of E. purpurea. Alkamides (alkylamides; fatty acid amides) are characteristic constituents of E. angustifolia roots, but are also found in roots and aerial parts of E. purpurea. Flavonoids, essential oils, polyacetylenes, ketones and pyrrolizidine alkaloids have also been isolated from Echinacea species. It is important to note that the pharmacologic effects associated with the constituents of Echinacea may result from independent or synergistic interactions with single or multiple constituents.
E. purpurea extracts rich in glycoproteins, polysaccharides and CADs have long been reported to demonstrate immunoactivity. Research in mice more than two decades ago demonstrated activation of macrophages and natural killer cells (Bauer 1989). Since then, numerous studies have supported these findings and have reported a variety of additional effects on adaptive and innate immune mechanisms (Chavez 2007; Gurbuz 2010; Hall 2007; Ramasahayam 2011; Ritchie 2011; Sadigh‐Eteghad 2011; Yamada 2011; Zhai 2007).
In contrast, early research on alkamide‐rich extracts of E. angustifolia and E. purpurea suggested anti‐inflammatory activity (Müller‐Jakic 1994). Since then, however, studies have reported immunoactivity attributable to alkamides (Lalone 2009; Matthias 2008) and have suggested that influences on inflammatory pathways are complex, with both pro‐ and anti‐inflammatory effects reported (Birt 2008; Qiang 2013; Yu 2013). Echinacea alkamides have been shown to be absorbed into the blood (Matthias 2005; Woelkart 2005b; Woelkart 2006; Woelkart 2008) and appear to exert a variety of effects through the endocannabinoid system (Chicca 2009; Woelkart 2005a; Woelkart 2007). Research on the effects of gene expression and signaling pathways is well underway (Altamirano‐Dimas 2007; Gertsch 2004; Uluisik 2012).
In addition to research on immune and inflammatory pathways, indications of antiviral activity have been reported (Bodinet 2002; Ghaemi 2009; Sharma 2006). Finally, recent research suggests potential anti‐anxiety properties (Haller 2013), potentially due to neuro‐synaptic modulation in the hippocampus (Hajos 2012).
In summary, while it is clear that variousEchinacea extracts and constituents have demonstrated pharmacological activities in a variety of biological assays, there is as yet no evidence‐based conceptual framework to explain howEchinacea might effectively prevent or treat acute respiratory infections.
Why it is important to do this review
The common cold has a high prevalence and although it is a self limiting condition effective treatment options which lessen the severity and duration of symptoms would be of major importance. Echinacea products are widely used but their effectiveness is uncertain. We completed a first version of this review in 1998 (Melchart 1999), updated it in 2006 (Linde 2006) and again in 2008 (Linde 2008). The last literature search was conducted in 2007 and did not detect new publications on the issue. Now, six years later, several new trials have been published and evidence may have changed. Therefore, a major update of this review was necessary.
Objectives
To assess whether there is evidence that Echinacea preparations are effective and safe compared to placebo in the prevention and treatment of the common cold.
Methods
Criteria for considering studies for this review
Types of studies
Randomized controlled trials (RCTs).
Types of participants
We included studies if participants were:
individuals with non‐specific viral upper respiratory tract infections (URTIs) with a clinical diagnoses of common cold, influenza‐like syndrome or viral URTI (it was not possible to apply a standard definition of common cold across all trials);
volunteers without acute URTIs but treated for preventative purposes (prevention studies);
volunteers without acute URTIs but challenged with rhinovirus treated for preventative or therapeutic purposes (or both).
We did not include studies of individuals suffering from other URTIs with a defined etiology (for example influenza) or a more specific symptomatology (for example acute sinusitis, angina tonsillaris).
Types of interventions
We included trials of oral Echinacea mono‐preparations versus placebo. We excluded trials on combinations of Echinacea and other herbs and trials comparing Echinacea with no treatment or another treatment than placebo.
Types of outcome measures
Selected trials had to include clinical outcome measures related to occurrence (prevention studies) and severity or duration of infections (prevention and treatment studies). We excluded trials focusing solely on physiological parameters (such as phagocytosis activity). We did not include or exclude studies based on their primary outcome measure if at least one clinically relevant outcome measure listed above was reported.
Primary outcomes
The primary efficacy outcome measure for prevention trials was the number of participants experiencing at least one cold episode.
The primary efficacy outcome measure for treatment trials was duration in days.
The primary outcome for safety and acceptability for both prevention and treatment trials was the number of participants dropping out due to side effects or adverse events.
Secondary outcomes
Secondary efficacy outcome measures for prevention trials were the number of participants experiencing more than one cold episode; cold duration in days; severity scores.
Secondary efficacy outcome measures for treatment trials were total severity and duration measures (e.g. area under the curve); severity of symptoms at days two to four and at days 5 to 10; in trials with very early onset of treatment also the number of participants who developed the 'full picture of a cold'.
Secondary safety and acceptability outcome measures for both prevention and treatment trials were the total number of drop‐outs and the number of participants reporting side effects or adverse events.
Search methods for identification of studies
Electronic searches
For this 2013 update we searched for new studies published since the last publication of our review and also searched for older trials. This was done as search methods have evolved over time and the inclusion criteria of our reviews have changed considerably.
We searched the Cochrane Central Register of Controlled Trials (CENTRAL) 2013, Issue 5, part of The Cochrane Library, www.thecochranelibrary.com (accessed 5 June 2013) which contains the Acute Respiratory Infections Group's Specialized Register, MEDLINE (1946 to May week 4, 2013), EMBASE (1991 to June 2013), CINAHL (1981 to June 2013), AMED (1985 to February 2012), LILACS (1981 to June 2013) and Web of Science (1955 to June 2013).
We used the following search strategy to search CENTRAL and MEDLINE. We combined the MEDLINE search strategy with the Cochrane Highly Sensitive Search Strategy for identifying randomized trials in MEDLINE: sensitivity‐maximizing version (2008 revision); Ovid format (Lefebvre 2011).
MEDLINE (Ovid)
1 Echinacea/ 2 echinac*.tw. 3 coneflower*.tw. 4 ("E. purpurea" or "E. angustifolia" or "E. pallida").tw. 5 1 or 2 or 3 or 4
We adapted the search strategy to search EMBASE (Appendix 1), CINAHL (Appendix 2), AMED (Appendix 3), LILACS (Appendix 4) and Web of Science (Appendix 5). Searches for the first review (published in 1999) and the 2007 update are described in Appendix 6.
Searching other resources
We searched WHO ICTRP and clinicaltrials.gov (latest search 8 October 2012), the Centre for Complementary Medicine Research (1988 to September 2007) and CAMBASE (latest search 5 June 2013). We screened bibliographies of identified trials and review articles for further potentially relevant publications. We contacted experts in the field and asked about further published and unpublished studies.
Data collection and analysis
Selection of studies
One review author (MKV) screened the titles and abstracts, where available, of all identified references and eliminated non‐human studies and trials without a control group. We obtained and checked further copies of all other references for eligibility. At least two review authors independently checked all potentially relevant publications or reports identified by the screening process for fulfillment of the selection criteria. We resolved disagreements by discussion. We assessed eligibility of trials in which one of the review authors was involved by review authors not involved in the trial.
Data extraction and management
At least two authors independently extracted descriptive information on patients, interventions, outcomes, results, drop‐outs and side effects using a standard data extraction form. Details on extraction of outcomes used for analyses are described below. Trials in which review authors were involved were extracted and assessed by review authors not involved in the trial. We contacted trial authors or manufacturers and sponsors and asked them to provide lacking or additional data if the information in the available publications or reports was incomplete. A pharmacist with specific expertise on Echinacea (KAW) extracted information on the Echinacea preparations.
Assessment of risk of bias in included studies
At least two authors independently assessed the methodological quality of the included trials using the Cochrane 'Risk of bias' tool (Higgins 2011b). We assessed the generation of the random sequence, allocation concealment, blinding, incomplete outcome data and selective reporting. For an overall assessment we considered as low risk of bias only trials in which at least four of the five items were rated as low risk and none high risk. Any trial with one or more items rated as high risk was considered high risk in the overall assessment. The remaining trials were considered as unclear risk.
Measures of treatment effect
For dichotomous efficacy outcomes we calculated risk ratios (RR) and for safety/adverse event outcomes we calculated odds ratios (OR). For continuous efficacy outcomes we calculated mean differences (MD) if the same scale of measure was used (e.g. number of days) and standardized MD if measurement tools or scales varied. For all effect estimates we calculated 95% confidence intervals (CI).
Unit of analysis issues
In all trials individual patients were randomized. However, in one trial (Taylor 2003) investigating early self treatment more than one cold could be treated by participants (on average participants treated about 1.5 cold episodes) and the results for duration and severity presented in the publication were based on the number of cold episodes. For effect size calculation we used the number of cold episodes, because using the number of patients would only lead to a small change of the weight of this trial.
Dealing with missing data
In case of missing outcome data we tried to obtain additional information from study authors. If, in case of continuous outcomes, means were presented but standard deviations were missing we calculated standard deviations from standard errors, P values or confidence intervals as described in the Cochrane Handbook for Systematic Reviews of Interventions Section 7.7.3 (Higgins 2011a). In case of missing dichotomous data we assumed that no event occurred.
Data synthesis
We expected a priori that meaningful quantitative meta‐analyses of the studies would not be possible for the following reasons: 1) a variety of Echinacea preparations are used in trials (phytochemical comparability is unclear); 2) the approach of studies differs (some investigate prevention, some self treatment, some treatment, some treatment and/or prevention of experimentally induced colds); 3) outcome measures differ in the trials; and 4) the presentation of results in available reports often includes insufficient detail to allow effect size estimation. However, we aimed to calculate effect size estimates for relevant outcome measures in single trials whenever possible. If an outcome was probably measured but data for effect size calculation were not reported we documented this.
For our main analysis we had predefined criteria to consider pooling (fixed‐effect model) of data from different trials: 1) treatment given for the same purpose (prevention or treatment); 2) use of the same or a very similar (regarding plant species, part and extraction mode) preparation and in similar dosage; and 3) at least two trials that met the criteria 1 and 2. Because of these criteria we ended up with multiple subgroups and most subgroups consisted of only one trial (with a maximum of two trials when criteria were met for pooling) we decided to run additional exploratory random‐effects meta‐analyses including all available trials regardless of the type of Echinacea product tested. For these meta‐analyses studies with more than one Echinacea group were entered only once (pooling data from the Echinacea groups) to avoid duplicate use of placebo data. The meta‐analyses serve to provide a crude overview of the overall direction and magnitude of the available study results and to investigate consistency and heterogeneity of the findings. We considered pooled effect sizes as clinically interpretable – at least with caution – when a) at least two‐thirds of trials measuring an outcome actually could be included in the meta‐analysis; b) at least five trials could be pooled; c) the I² statistic was ≤ 40%; and d) the P value of the Chi² test for heterogeneity was ≥ 0.25. All other pooled effect sizes were not considered clinically interpretable and only used to check whether results differed between studies.
Duration of colds was analyzed and reported in highly variable manner in the primary studies. While only two presented MD with some measure of variability (the measure we would have preferred for meta‐analysis), some provided median duration and P values from log rank text, Cox regression or a Wilcoxon rank sum test. According to our protocol we included only the two trials reporting mean duration. To provide at least a crude summary of study findings in a post hoc secondary analysis we used an overall estimate of effect for each study rather than summary data for each intervention group for an inverse variance analysis. For this exploratory analysis we interpreted medians as means for calculating the MD and calculated standard errors as if P values were derived from a t‐test. We did not pool findings from individual studies due these liberal assumptions and the heterogeneity of the study findings but included the resulting forest plot only for giving a graphical impression of the overall evidence.
In general we used the number of patients randomized when calculating effect estimates for dichotomous outcome and the number of patients analyzed for continuous measures. However, for some study approaches this was considered inappropriate. For example, in five trials of self treatment, participants were randomized to receive an Echinacea product or placebo medication to take at home but told to take their medication only in case a cold occurred. In these cases we used the number of patients in whom a cold actually occurred for analyses.
In the case of pooling we examined heterogeneity between trials by calculating a Chi² test, the I² statistic and the Tau² statistic. An I² statistic value of 0% to 40% was not considered to be important heterogeneity; 40% to 60% was considered moderate heterogeneity; 60% to 90% was considered substantial and an I2 statistic value greater than 90% indicated considerable heterogeneity (Higgins 2003). We generated funnel plots for meta‐analyses including at least four studies. We carried out all calculations in RevMan 2012, version 5.2.
Results
Description of studies
Results of the search
The database searches identified a total of 556 hits (Figure 1). Three additional records were identified through other sources. We identified a total of 82 full‐text articles describing trials which tested preparations of Echinacea in humans (alone or in combination with other plant extracts).
1.
study flow diagram.
Included studies
Twenty‐four studies (in 29 publications) met the inclusion criteria (Characteristics of included studies table). Of these, 15 had been included in the previous version of the review (Barrett 2002; Brinkeborn 1999; Bräunig 1992; Dorn 1997; Galea 1996; Goel 2004; Goel 2005; Grimm 1999; Hoheisel 1997; Kim 2002a; Lindenmuth 2000; Melchart 1998; Schulten 2001; Taylor 2003; Yale 2004), two of the now included studies on induced colds had initially been excluded in the previous version of the review (Sperber 2004; Turner 2000) and seven studies have been newly included (Barrett 2010; Hall 2007; Jawad 2012; O'Neill 2008; Tiralongo 2012; Turner 2005; Zhang 2003). One study (Taylor 2003) was in children, the other 23 in adults. Three trials (Brinkeborn 1999; Bräunig 1992; Melchart 1998; Turner 2005) had more than one experimental group receiving an Echinacea product (different dosage in one and different extracts in three) so there were a total of 33 comparisons of an Echinacea preparation with placebo. One study had both a placebo and a no treatment control group (Kim 2002a) and one study had a no treatment and an unblinded Echinacea group in addition to the blinded Echinacea and placebo groups (Barrett 2010).
Twelve trials originated from the USA, five from Germany, three from Canada, two from Sweden, one from the United Kingdom and one from Australia. Two trials from the USA and one from Canada were only available as unpublished manuscripts (Galea 1996; Kim 2002a; Zhang 2003).
Excluded studies
Forty‐nine studies (in 53 publications) did not meet the inclusion criteria (Characteristics of excluded studies table; Figure 1). In 19 studies Echinacea in combination with other remedies was used. Eleven studies examined other conditions than common cold. Six studies examined other conditions than common cold using Echinacea in combination with other remedies. Ten studies were not randomized controlled trials, five of which also examined other conditions than common cold. Four studies only examined laboratory measurement results, without clinical outcomes and three of them examined other conditions than common cold.
Among the 49 excluded studies there was one which was included in the previous version of this review (Spasov 2004). It was an unblinded study without placebo control.
Study approaches
As described in the methods section, we separated prevention trials (treatment of healthy volunteers without cold symptoms to avoid occurrence of colds or reduce severity and duration of occurring cold) and treatment trials (treatment of individuals with colds or of early cold symptoms).
Ten studies were prevention trials (with a total of 13 Echinacea groups). In four of these trials 431 healthy volunteers (Hall 2007) or persons being challenged by inoculation with rhinovirus (Sperber 2004; Turner 2000; Turner 2005) were treated over a shorter period (two to four weeks). Six trials including 1391 participants (Grimm 1999; Jawad 2012; Melchart 1998; O'Neill 2008; Tiralongo 2012; Zhang 2003) treated healthy volunteers over a longer period (6 to 16 weeks) for preventative purposes.
Fifteen studies (with 20 comparisons) were categorized as treatment trials, but among these studies two different approaches were used: five placebo‐controlled trials with a total of seven Echinacea groups (Brinkeborn 1999; Galea 1996; Goel 2004; Goel 2005; Taylor 2003) investigated self treatment. Healthy volunteers were randomized and instructed to start treatment only if they caught a cold. These trials randomized a total of 1910 participants (range 150 to 559). However, 846 did not start treatment as they did not catch a cold during the study period; therefore only 1064 (62 to 436) actually started treatment. In 10 trials with a total of 13 Echinacea groups (Barrett 2002; Barrett 2010; Bräunig 1992; Dorn 1997; Hoheisel 1997; Kim 2002a; Lindenmuth 2000; Schulten 2001; Turner 2005; Yale 2004) individuals with cold symptoms were randomized and treated. These 10 trials included a total of 1538 participants (range 57 to 359 in each). Typically, they tried to start treatment as early as possible. Three trials (Hoheisel 1997; Schulten 2001; Turner 2005) explicitly not only investigated duration and severity of symptoms but also tried to prevent the development of a 'full cold' by treatment of first symptoms. Two of these trials were performed in industrial plants where employees could access treatment very fast (Hoheisel 1997; Schulten 2001). Development of a "full cold" was also tested by one trial examining experimental rhinovirus colds (Turner 2005).
Echinacea preparations tested
In the 33 experimental groups of the 24 included trials, widely different Echinacea preparations were used (Table 1). A large proportion of the preparations used in the trials were pressed juices (stabilized with alcohol), alcohol tinctures or tablets made from dried extracts. In six trials preparations from the pressed juice of the aerial parts of E. purpurea were used (Grimm 1999; Hoheisel 1997; Schulten 2001; Sperber 2004Taylor 2003; Yale 2004). In five of these six trials (Grimm 1999; Hoheisel 1997; Schulten 2001; Sperber 2004; Taylor 2003) the same product was used. Preparations based on E. purpurea root alone were used in two trials, with three experimental groups (Bräunig 1992; Zhang 2003). An identical product based on a mixture of E. purpurea root (5%) and herb (95%) was used in two trials with three experimental groups (Brinkeborn 1999; Jawad 2012). One of these trials (Brinkeborn 1999) tested two different concentrations of the same product. Two trials by the same study group (Goel 2004; Goel 2005) investigated the effectiveness of an extract of 'various' parts of E. purpurea. The particular aspect of this preparation was that it was standardized for its content of three bioactive components (alkamides, cichoric acid and polysaccharides).
1. Details of the preparations used in the included trials.
| Reference | Preparation | Manufacturer | Species, part | Extraction | Content details | Galenic form | Dosage | Treatment period | Remarks |
| Barrett 2002 | Not reported | Shaklee Tecninca, Pleasanton, California | Unrefined E. ang. root (50%), E. purp. herb (25%) and root (25%) | Not applicable (dried Echinacea) | 0.20% to 0.26% echinacoside, 0.77% to 0.84% dichroic acid, 0.82% alkamides, 0.03% chlorogenic acid, 0.33% cafeolytartaric acid | Capsules | 6 x 4 capsules (6 g) during first 24 hours, then 3 x 4 capsules (3 g/day) | 10 days | — |
| Barrett 2010 |
Not reported | MediHerb, Warwick, Queensland, Australia | E. purp. root, E.ang. root | Not reported | 2.1 mg of alkamides per tablet | Tablets | 4 x 2 tablet during first 24 hours, then 4 x 1 tablet per day for the next 4 days; that means 10.2 g of dried Echinacea root during first 24 hours, 5.1 g during each of the next days | 5 days | — |
| Brinkeborn 1999, Group 1 | Echinaforce | Bioforce, Roggwil, Switzerland | E. purp. herb (95%) and root (5%) | Alcoholic aqueous extract | 6.78 mg crude extract | Tablets | 2 x 3 (40.68 mg/day) | Max. 7 days | — |
| Brinkeborn 1999, Group 2 | No brand name | Bioforce, Roggwil, Switzerland | E. purp. herb (95%) and root (5%) | Alcoholic aqueous extract | 48.27 mg crude extract | Tablets | 2 x 3 (289.62 mg/day) | Max. 7 days | — |
| Brinkeborn 1999, Group 3 | No brand name | Bioforce, Roggwil, Switzerland | E. purp. root | Alcoholic aqueous extract | 29.60 mg crude extract | Tablets | 2 x 3 (177.6 mg/day) | Max. 7 days | — |
| Bräunig 1992, group 1 | Not reported | Not reported | E. purp. root | 55% v/v ethanolic extract, DER 1:5 | Not reported | Tincture | 180 drops (900 mg/day) | Probably 8 to 10 days | — |
| Bräunig 1992, group 2 | Not reported | Not reported | E. purp. root | 55% v/v ethanolic extract, DER 1:5 | Not reported | Tincture | 90 drops (450 mg/day) | Probably 8 to 10 days | — |
| Dorn 1997 | Not reported | Not reported | E. pallida root | Not reported | Not reported | Tincture | 90 drops (900 mg/day) | 8 to 10 days | — |
| Galea (unpublished) | Not reported | Local pharmacist | E. ang. (part not specified) | Not reported | Powder standardized at 4% content of echinacoside | Capsules | 3 x 1 (750 mg/day) | 10 days | — |
| Goel 2004 | Echinilin | Natural Factors Nutritional Products, Inc., Vancouver, BC, Canada | E. purp. (various parts) | Aqueous and alcoholic extract combined to a 40% ethanolic formulation | Standardized for 0.25 mg/ml alkamides, 2.5 mg/ml cichoric acid, 25 mg/ml polysaccharides | Liquid | 10 x 4 ml day 1, then 6 days 4 x 4 ml | 7 days | Extract standardized on the basis of 3 known active components |
| Goel 2005 | Echinilin | Natural Factors Nutritional Products, Inc., Vancouver, BC, Canada | E. purp. (various parts) | Aqueous and alcoholic extract combined to a 40% ethanolic formulation | Standardized for 0.25 mg/ml alkamides, 2.5 mg/ml cichoric acid, 25 mg/ml polysaccharides | Liquid | 8 x 5 ml day 1, then 6 days 3 x 5 ml | 7 days | Extract standardized on the basis of 3 known active components |
| Grimm 1999 | Echinacin | Madaus AG, Cologne, Germany | E. purp. aerial parts | Fresh expressed juice of whole flowering plants harvested without roots, containing 22% alcohol | Not reported | Liquid | 2 x 4 ml day | 8 weeks | — |
| Hall 2007 | Echinacea Standardized | Nature´s Way, Springville, UT (USA) | E. purp. (part not specified) | Not reported | Not reported | Capsules | 8 capsules/day (3 x 2 with each meal and 2 at bedtime) | 4 weeks | — |
| Hoheisel 1997 | Echinagard (Echinacin) | Madaus AG, Cologne, Germany | E. purp. aerial parts | Fresh expressed juice of whole flowering plants harvested without roots, stabilized with 20% ethanol | Not reported | Liquid | 20 drops every 2 hours day 1, then 3 x 20 drops/day | Max. 10 days | — |
| Jawad 2012 | Echinaforce drops | A. Vogel, Bioforce, Switzerland | E. purp. (95% herb, 5% roots) | Alcohol (57%) extract from freshly harvested E. purp. | 5 mg/100g of dodecatetraenoic acid isobutylamide | Liquid | 3 x 0.9 ml/day (2400 mg of extract/day); in case of cold: 5 x 0.9ml/day (4000 mg of extract/day) | 4 months | Each single dose was diluted in water and retained in mouth for 10 seconds |
| Kim (unpublished) | Not reported | Nature's Way products, Inc. R/O America's Natural healthcare Company, Springville, Utah | E. purp. herb (80%), E. ang. roots (20%) | No detailed information; final tincture with 25% to 35% alcohol | Not reported | Liquid | 101 ml (1000 mg dry plant) per day | At least 5 days | — |
| Lindenmuth 2000 |
Echinacea Plus herbal tea | Dry extract ingredient by Emil Flachsmann AG, Zurich, Switzerland | E. purp. and E. ang. aerial parts; E. purp. roots | E. purp. root water soluble dry extract DER 6:1 | 1.275 mg per tea bag serving | Tea bag | 5 to 6 cups day 1, titration to one cup on day 5 | 5 days | — |
| Melchart 1998 Group 1 | No brand name | Plantapharmazie, Göttingen, Germany | E. ang. root | 30% ethanolic extract, DER 1:11 | Extract contained 1007.9 µg/ml glycoproteins/polysaccharides and echinacoside | Tincture | 2 x 50 drops | 12 weeks (intake on 5 days per week) | — |
| Melchart 1998 Group 2 | No brand name | Plantapharmazie, Göttingen, Germany | E. purp. root | 30% ethanolic extract, DER 1:11 | Extract contained 1026.2 µg/ml glycoproteins/polysaccharides and cichoric acid | Tincture | 2 x 50 drops | 12 weeks (intake on 5 days per week) | — |
| O'Neil 2008 | No brand name | Natures Resource, Mission Hill, California | E. purp. (part not specified) | Not reported | Not reported | Capsules | 3 x 2 capsules/day (1 capsule containing 300 mg E. purp.) | 8 weeks | — |
| Schulten 2001 | Echinacin | Madaus AG, Cologne, Germany | E. purp. aerial parts | Fresh expressed juice of whole flowering plants harvested without roots, stabilized with 20% ethanol, DER 1.7‐2.5:1 | Not reported | Liquid | 2 x 5 ml/day | 10 days | — |
| Sperber 2004 | EchinaGuard | Madaus AG, Cologne, Germany | E. purp. aerial parts | Pressed juice in 22% alcohol base | Not reported | Liquid | 3 x 2.5 ml/day | 14 days | — |
| Taylor 2003 | Echinacin | Madaus AG, Cologne, Germany | E. purp. aerial parts | Pressed juice, combined with syrup | Not reported | Liquid (dried pressed juice dissolved in syrup) | 2 x 3.75 ml/day (children 2 to 5 years), 2 x 5 ml/day (6 to 11 years) | Max. 10 days | — |
| Tiralongo 2012 | MediHerb | Integria Healthcare Pty Ltd., Australia | E. purp. root, E. ang. root | Extract, details not reported | Tablets standardized for a content of 4.4 mg alkylamides with 112.5 mg E. purp. 6:1 extract and 150 mg E. ang. 4:1 extract (detailed alkamide composition is summarized in a table with e.g. a content of 1.504 mg/tablet dodecatetraenoic acid isobutyl amides) | Tablets | Priming dose 2 x 1 tablet/day, flying dose 2 x 2 tablets/day, overseas dose 2 x 1 tablet/day, after‐travel dose 2 x 1 tablet/day, sick dose 2 x 3 tablets/day | 35 days if 1 week of travel (14 days primary dose, 7 days overseas, 14 days after‐travel dose) or 63 days if 5 weeks of travel (11 days with priming dose, 10 days flying dose, 25 days overseas dose, 10 days flying dose and 7 days after travel dose) | — |
| Turner 2005 Group 1 and 4 | No brand name | Not reported | E. ang. root | Extraction with supercritical carbon dioxide | No polysaccharides, 73.8% alkamides, no echinacosides | Liquid | 3 x 1.5 ml/day (3 x equivalent of 300 mg Echinacea root) | 13 days (virus challenge on day 8) | — |
| Turner 2005 Group 2 and 5 | No brand name | Not reported | E. ang. root | Extraction with 60% ethanol | 48.9% polysaccharides, 2.3% alkamides, no echinacosides, 0.16 mg/ml cynarine) | Liquid | 3 x 1.5 ml/day (3 x equivalent of 300 mg Echinacea root) | 13 days (virus challenge on day 8) | — |
| Turner 2005 Group 3 and 6 | No brand name | Not reported | E. ang. root | Extraction with 20% ethanol | 42.1% polysaccharides, 0.1% alkamides, no echinacosides | Liquid | 3 x 1.5 ml/day (3 x equivalent of 300 mg Echinacea root) | 13 days (virus challenge on day 8) | — |
| Turner 2000 | No brand name | Not reported | E. purp. and E. ang. | 4% phenolic extract | 0.16% cichoric acid, almost no echinacosides or alkamides | Capsules with powder | 3 x 300 mg/day | 19 days (virus challenge on day 14) | — |
| Yale 2004 | EchinaFresh | Enzymatic Therapy, Green Bay, Wisconsin | E. purpurea aerial parts | Pressed juice | Standardized for a content of 2.4% soluble beta‐1,2‐D‐fructofuranosides | Capsules with freeze dried juice | 3 x 1 capsule/day | 7 days, if symptoms not resolved max. 14 days | — |
| Zhang 2003 | No brand name | Not reported | E. purp. root | Root powder | 4.4 mg cichoric acid | Capsules with 294 mg powder | 1 capsule/day | 8 weeks | — |
E. ang: Echinacea angustifolia E. pallida: Echinacea pallida E. purp: Echinacea purpurea v/v: volume/volume
The 14 remaining Echinacea preparations were only used in one trial each: tinctures or extracts prepared from E. pallida root (Dorn 1997), E. angustifolia root (Melchart 1998) or E. angustifolia, without information on the plant parts used (Galea 1996); a dried plant preparation based on 50% E. angustifolia root, 25% E. purpurea root and 25% E. purpurea herb (Barrett 2002); a preparation based on E. purpurea root and E. angustofolia root, without information on extraction details (Barrett 2010); a tincture from 80% E. purpurea herb and 20% E. angustifolia root (Kim 2002a); a preparation based on an extract of E. purpurea root and E. angustofolia root (extraction details not reported) (Tiralongo 2012); a 4% phenolic extract of E. purpurea and E. angustofolia (Turner 2000); three preparations based on E. angustofolia root using three different extraction methods (20% alcohol; 60% alcohol and CO2) (Turner 2005); two kinds of E. purpurea capsules (parts and extraction details not reported (Hall 2007; O'Neill 2008); and a tea preparation based on dry extracts from the aerial parts of E. purpurea and E. angustifolia (Lindenmuth 2000).
Outcome measurement
All 10 prevention trials investigated the occurrence of cold. Other outcomes, like the number of people with more than one cold episode, duration and severity scores were only measured in some of the prevention trials. Among the self treatment and treatment trials, methods for outcome measurements and the results actually presented varied greatly (there were differences regarding instruments used, timing of measurements, type of analysis and descriptive statistics).
Risk of bias in included studies
'Risk of bias' judgements are given in the Characteristics of included studies table and in Figure 2 and Figure 3. We rated five trials (Barrett 2002; Barrett 2010; Brinkeborn 1999; Goel 2004; Taylor 2003) as having a low risk of bias in all five categories of the Cochrane 'Risk of bias' tool (Higgins 2011b). We rated five more trials as low risk of bias, having an unclear risk of bias in only one category. We rated eight trials as having a high risk of bias (Bräunig 1992; Dorn 1997; Galea 1996; Hoheisel 1997; Jawad 2012; Lindenmuth 2000; O'Neill 2008; Zhang 2003) and six as having an unclear risk of bias.
2.
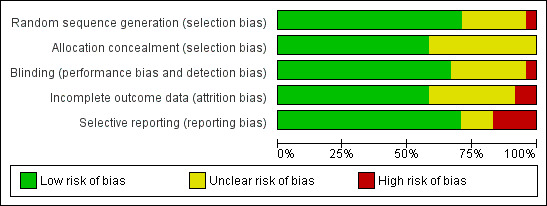
'Risk of bias' graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
3.
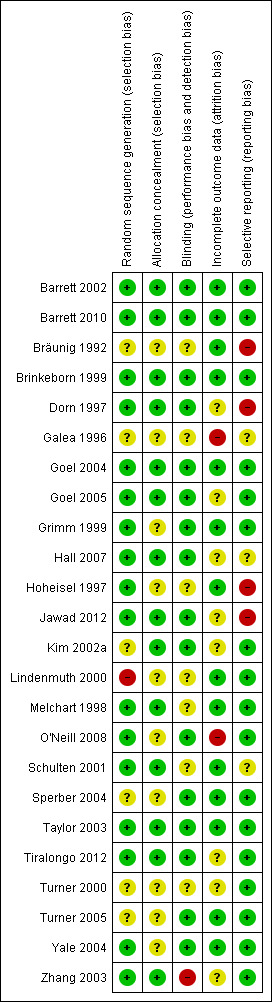
'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Random sequence generation was performed appropriately in at least 17 studies. Additional information by authors and sponsors was taken into account. In one study (Lindenmuth 2000), allocation to groups appeared to follow an alternating sequence rather than true randomization. However, the allocation process was handled by an independent and blinded secretary after inclusion of participants in the trial. This study was already included in the previous version of the review. An adequate method of concealment was used in at least 14 studies (taking into account additional information received from authors and sponsors). In 10 studies sufficiently detailed information on allocation concealment was not reported.
Blinding
All 24 trials were described as blinded. In 16 trials we considered the risk of performance and detection bias as low. One older trial was not adequately blinded (Bräunig 1992). In this three‐armed trial, one Echinacea group received 180 drops daily while the other Echinacea group and the placebo group received 90 drops. One trial was performed among employees of the manufacturer (Schulten 2001) who may have recognized the taste of their Echinacea product; the success of blinding was not tested. In one trial capsules filled with vegetable oil were used as placebo and may have been distinguishable from Echinacea capsules by taste (Galea 1996). In two more trials (Hoheisel 1997; Lindenmuth 2000) Echinacea preparation and placebo could possibly have been distinguishable by taste. Thirteen trials reported a test for the success of blinding. In 11 trials blinding seemed to have been successful while in two there was evidence of some unblinding (Melchart 1998) or major unblinding (Zhang 2003).
Incomplete outcome data
The risk of attrition bias was considered low in 14 trials, having reported less than 20% attrition and performed an intention‐to‐treat analysis or reported generally less than 5% attrition. The risk of attrition bias was unclear in three and high in seven trials.
Selective reporting
We assessed 17 trials as having a low risk of reporting bias. In four trials important relevant outcomes are not reported/examined (Bräunig 1992; Hall 2007; Hoheisel 1997; Jawad 2012). In two trials the reports are not systematically biased, but outcomes have been reported insufficiently to allow effect size calculation (Galea 1996; Schulten 2001).
Effects of interventions
Primary outcomes for prevention trials and for treatment or self treatment trials
Primary efficacy outcome measure for prevention trials: number of participants experiencing at least one cold episode
Nine of the 10 prevention trials reported the number of patients experiencing at least one cold. None of the 12 comparisons of Echinacea preparations and placebo in these nine trials (Grimm 1999; Hall 2007; Melchart 1998; O'Neill 2008; Sperber 2004; Tiralongo 2012; Turner 2000; Turner 2005; Zhang 2003) demonstrated statistically significant results in comparison to placebo (Figure 4; Analysis 1.1).
4.
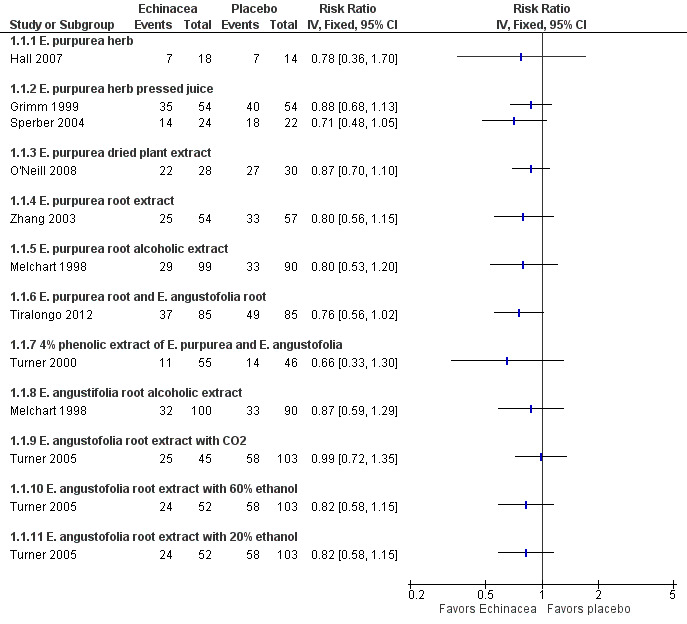
Forest plot of comparison: 1 Echinacea versus placebo to prevent common cold, outcome: 1.1 Number of participants with at least 1 cold episode.
1.1. Analysis.
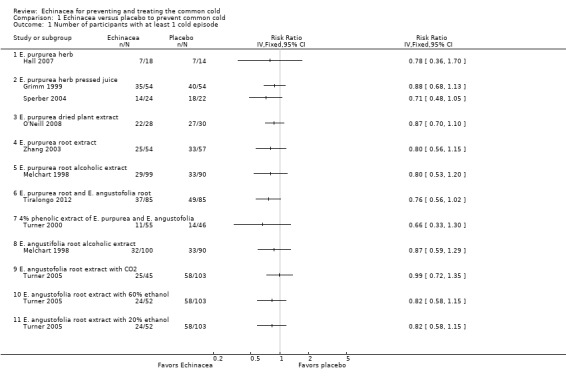
Comparison 1 Echinacea versus placebo to prevent common cold, Outcome 1 Number of participants with at least 1 cold episode.
Only two trials investigated the same Echinacea product; the pooled risk ratio (RR) also did not show significant effects over placebo (RR 0.82, 95% confidence interval (CI) 0.67 to 1.02; P = 0.07). However, in our exploratory meta‐analysis pooling all trials (1167 patients totally), regardless of the product used, prophylactic treatment with Echinacea products was associated with a reduced risk of experiencing a cold (RR 0.83, 95% CI 0.75 to 0.92; P < 0.001). Study findings were highly consistent across studies with an I² statistic of 0%, a Tau² of 0.00 and a P value of 0.98 in the Chi² test for heterogeneity. The funnel plot showed some asymmetry (Eggers test p = 0.03) but point estimates in the single trials were similar and including only the four most precise trials in meta‐analysis reduced the pooled estimate only marginally (RR 0.85, 95% CI 0.75 to 0.96). We could not include the largest prevention study (Jawad 2012) in the quantitative analyses as it did not report the number of patients with at least one cold but only the total number of cold episodes. There were 149 cold episodes in the Echinacea group versus 188 in the placebo group; this finding seems very compatible with the reduced RR suggested by our meta‐analysis.
The observed risk ratio of 0.83 in our meta‐analysis corresponds to an absolute risk reduction of 10% (95% CI 5% to 16%) and a number needed to treat of 10 (95% CI 6 to 20). As more participants in the control groups experienced a cold the absolute risk reduction in the four most precise trials was 11% (94% CI 4% to 19%) and the number needed to treat 9 (95% CI 5 to 25) in spite of the slightly larger risk ratio of 0.85.
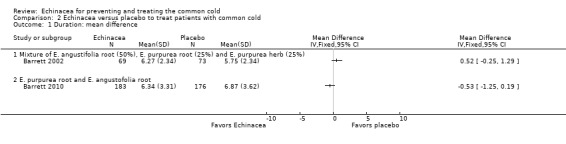
Primary efficacy outcome measure for treatment or self treatment trials: duration in days
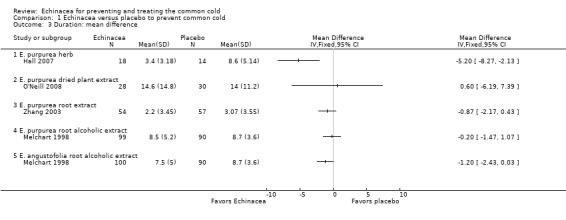
Only one trial of a mixture of 50% E. angustifolia root, 25% E. purpurea root and 25% E. purpurea herb (Barrett 2002) and one trial of a mixture of E. purpurea root and E. angustifolia root (Barrett 2010) reported the mean duration of colds (Figure 5; Analysis 2.1). Neither trial found a significant difference compared to placebo. A total of six trials could be included in our post hoc secondary inverse variance analysis also using other data on duration (Analysis 2.2). Study findings were heterogeneous (I² statistic = 77%, Tau² = 0.88, P = 0.0002 in Chi² test) with two trials (Lindenmuth 2000; Schulten 2001) finding a significantly shorter duration in the Echinacea group.
5.

Forest plot of comparison: 2 Echinacea versus placebo to treat patients with common cold, outcome: 2.1 Duration: mean difference.
2.1. Analysis.
Comparison 2 Echinacea versus placebo to treat patients with common cold, Outcome 1 Duration: mean difference.
2.2. Analysis.
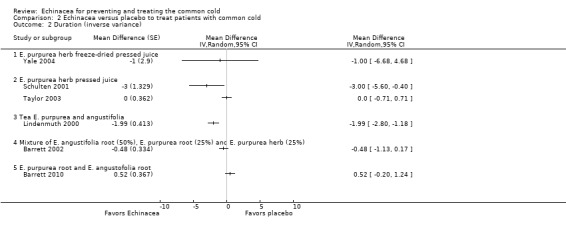
Comparison 2 Echinacea versus placebo to treat patients with common cold, Outcome 2 Duration (inverse variance).
Primary safety and acceptability outcome for prevention trials: number of participants dropping out due to side effects or adverse events
Our main outcome measure for the safety and acceptability analysis, the number of patients dropping out due to adverse effects, was reported in eight comparisons in seven studies (Grimm 1999; Jawad 2012; Melchart 1998; O'Neill 2008; Sperber 2004; Tiralongo 2012; Turner 2000; see Figure 6; Analysis 1.5). There were no significant differences in the single trials but the confidence intervals are wide as the number of patients dropping out was generally low. If study findings were pooled regardless of the Echinacea product used (heterogeneity indicators I² statistic = 0%; Tau² = 0.00; P = 0.87 in Chi² test) 2.4% in Echinacea groups dropped out from the studies due to side effects compared to 0.8% from the placebo groups (odds ratio (OR) 2.17, 95% CI 0.85 to 5.53; P = 0.10).
6.
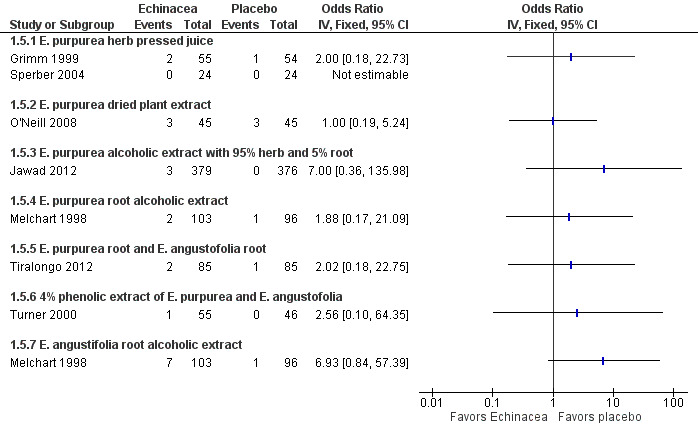
Forest plot of comparison: 1 Echinacea versus placebo to prevent common cold, outcome: 1.5 Number of patients dropping out due to adverse effects.
1.5. Analysis.
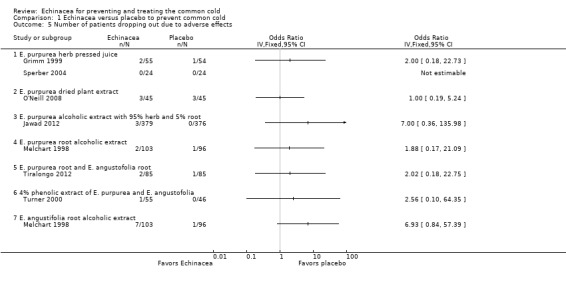
Comparison 1 Echinacea versus placebo to prevent common cold, Outcome 5 Number of patients dropping out due to adverse effects.
Primary safety and acceptability outcome for treatment or self treatment trials: number of participants dropping out due to side effects or adverse events
For 11 trials (14 comparisons) the number of patients dropping out due to side or adverse effects could be extracted. Only three of 1088 patients who received an Echinacea product and none of the 930 patients who received placebo dropped out for these reasons (Analysis 2.7).
2.7. Analysis.
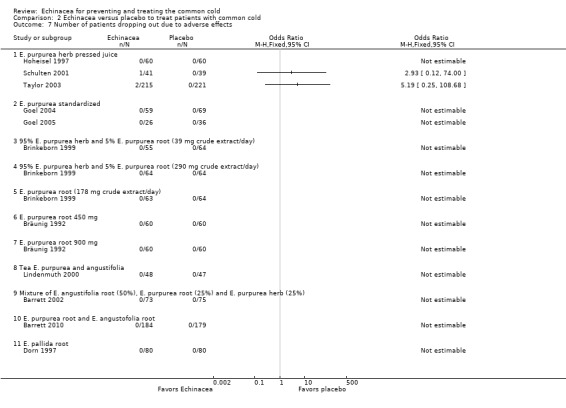
Comparison 2 Echinacea versus placebo to treat patients with common cold, Outcome 7 Number of patients dropping out due to adverse effects.
Secondary outcomes for prevention trials and for treatment or self treatment trials
Secondary efficacy outcome measures for prevention trials: number of participants experiencing more than one cold episode; cold duration in days; severity scores
Three trials (with four comparisons) reported the number of patients with more than one cold episode (Analysis 1.2). None of the trials found significant differences but confidence intervals were very wide. Four trials (with five comparisons) reported the duration of cold episode (Analysis 1.3). Only one small trial (Hall 2007) found a very large (5.2 days on average) statistically significant effect over placebo. Point estimates in the other comparisons vary between 1.2 days shorter to 0.6 days longer duration in the Echinacea groups. Five trials (with seven comparisons) presented sufficient data for calculations on effect sizes for severity of cold episodes (Analysis 1.4). Again, none of the single trials found a significant effect over placebo. However, as study findings were similar across trials (I² statistic = 0% and Tau² = 0.00 and P = 0.86 in Chi² test) we report the pooled standardized mean difference (SMD) for this outcome which is ‐0.24 (95% CI ‐0.07 to ‐0.40; P = 0.005), indicating a small effect over placebo.
1.2. Analysis.
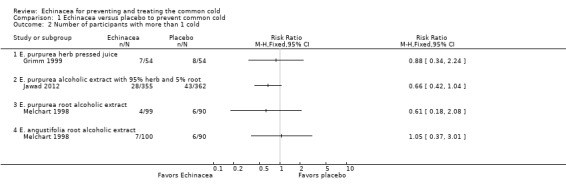
Comparison 1 Echinacea versus placebo to prevent common cold, Outcome 2 Number of participants with more than 1 cold.
1.3. Analysis.
Comparison 1 Echinacea versus placebo to prevent common cold, Outcome 3 Duration: mean difference.
1.4. Analysis.
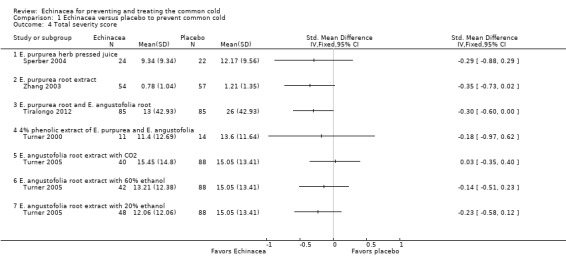
Comparison 1 Echinacea versus placebo to prevent common cold, Outcome 4 Total severity score.
Secondary efficacy outcome measures for treatment or self treatment trials: total severity and duration measures; severity of symptoms at days two to four and at days 5 to 10; in trials with very early onset of treatment also the number of participants who developed the 'full picture of a cold'
In nine trials (nine comparisons) measures integrating both severity and duration were presented in sufficient detail, or were provided by the authors, to calculate effect sizes (Analysis 2.2). The two trials on E. purpurea herb pressed juice preparations also reporting duration again reported conflicting results (Schulten 2001; Taylor 2003). The pooled SMD was not significant (SMD ‐0.26, 95% CI ‐0.75 to 0.23; P = 0.30; I² statistic = 76%). This applies also to two trials testing a standardized extract of E. purpurea (SMD ‐0.18, 95% CI ‐0.57 to 0.21; P = 0.20; I² = 40%). Trials of other extracts did not find any significant differences. While heterogeneity seems limited (I² statistic = 17%; Tau² 0.00; P = 0.29 in Chi² test) our SMD from meta‐analysis of all available studies has to be interpreted with great caution (SMD ‐0.09, 95% CI ‐0.20 to 0.02; P = 0.10). Data on severity scores after two to four days (Analysis 2.4) and five to 10 days of treatment (Analysis 2.5) were reported in seven trials (eight comparisons) and eight trials (11 comparisons), respectively. Significant differences were found in two comparisons after two to four days and four comparisons after five to 10 days. As study findings were heterogeneous (I² statistic = 76% and 90%, respectively) we do not report a pooled effect estimate of all pooled data. For the standardized E. purpurea extract tested in two trials pooled SMDs were non‐significant (after two to four days: SMD ‐0.20, 95% CI ‐0.88 to 0.48; P = 0.56 and after five to 10 days SMD ‐0.31, 95% CI ‐0.75 to ‐1.00; P = 0.18). Only three trials reported the number of patients developing a "full" cold after the early treatment of prodromes (self treatment) (Analysis 2.6). For the two studies using the same Echinacea product (E. purpurea herb pressed juice) the pooled RR was non‐significant (RR 0.79, 95% CI 0.54 to 1.14; P = 0.21).
2.4. Analysis.
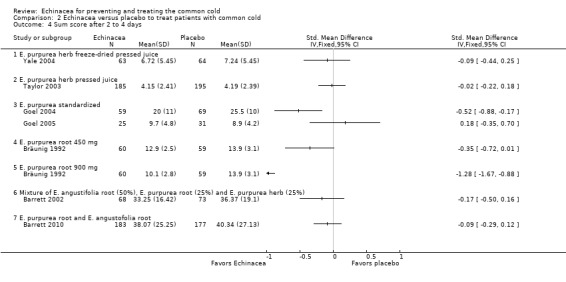
Comparison 2 Echinacea versus placebo to treat patients with common cold, Outcome 4 Sum score after 2 to 4 days.
2.5. Analysis.
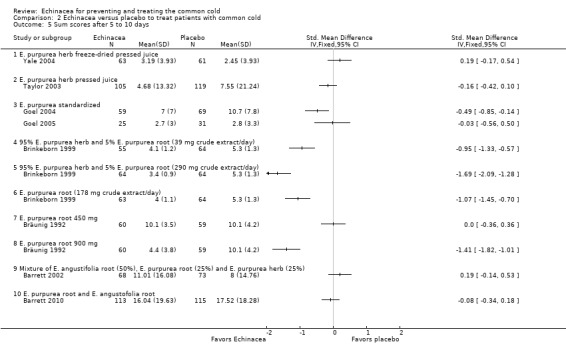
Comparison 2 Echinacea versus placebo to treat patients with common cold, Outcome 5 Sum scores after 5 to 10 days.
2.6. Analysis.
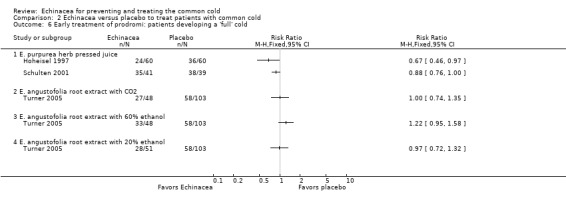
Comparison 2 Echinacea versus placebo to treat patients with common cold, Outcome 6 Early treatment of prodromi: patients developing a 'full' cold.
Secondary safety and acceptability outcomes for prevention trials: total number of drop‐outs and the number of participants reporting side effects or adverse events
For 12 comparisons in nine trials the number of patients dropping out (Analysis 1.6) has been reported. Two studies reported that significantly more patients were dropping out in the Echinacea group than in the placebo group (Jawad 2012 and the comparison using E. angustofolia root extracted with CO2 in Turner 2005). The other trials found no significant differences in the number of drop‐outs. As study results seem broadly consistent (I² statistic = 8%, Tau² = 0.02; P = 0.37 in Chi² test) we also report pooled results. The percentage of participants in the Echinacea groups terminating studies early was 12.7% compared to 9.0% in the placebo groups (OR 1.37, 95% CI 0.98 to 1.91; P = 0.06). For nine comparisons in eight trials the number of persons reporting adverse effects (Analysis 1.7) has been reported. Results were non‐significant in all trials except for one trial which found significantly fewer persons reporting adverse effects in the placebo group (Zhang 2003). In most of the trials there was a trend towards fewer adverse effects in the placebo groups. As some heterogeneity cannot be ruled out (I² statistic = 25%, Tau² = 0.10; P = 0.23 in Chi² test) our pooled findings are hard to interpret. 11.8% versus 8.6% of patients reported side or adverse effects (RR 1.49, 95% CI 0.95 to 2.35; P = 0.09).
1.6. Analysis.
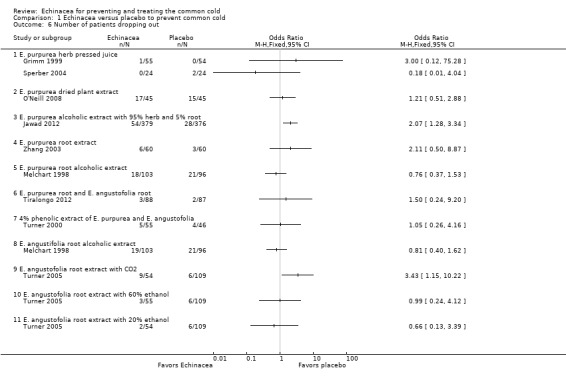
Comparison 1 Echinacea versus placebo to prevent common cold, Outcome 6 Number of patients dropping out.
1.7. Analysis.
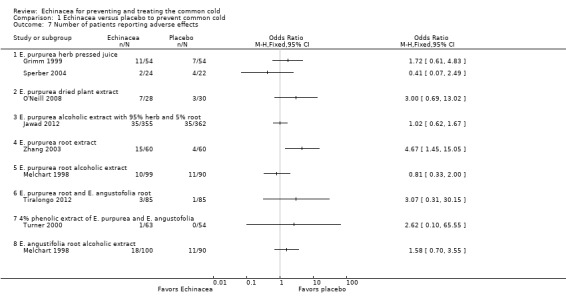
Comparison 1 Echinacea versus placebo to prevent common cold, Outcome 7 Number of patients reporting adverse effects.
Secondary safety and acceptability outcomes for treatment or self treatment trials: total number of drop‐outs and the number of participants reporting side effects or adverse events
Numbers of participants dropping out were similar in Echinacea and placebo groups in the trials presenting these data (Analysis 2.8 to Analysis 2.9), except for E. angustofolia root extracted with 60% ethanol which led to more drop‐outs in the Echinacea group (Turner 2005). The number of patients reporting adverse effects did not differ significantly between treatment and control groups in the single trials. Heterogeneity was low (I² statistic = 0%, Tau² = 0.00, P = 0.84 Chi² test). Meta‐analysis showed a significant difference in the number of patients reporting side effects in the placebo groups (32.6%) and in the treatment groups (34.1%) (OR 1.28, 95% CI 1.02 to 1.60, P = 0.03). One trial in children using a preparation made from pressed juice of E. purpurea herb found an increased frequency of rash in the experimental group (Taylor 2003).
2.8. Analysis.
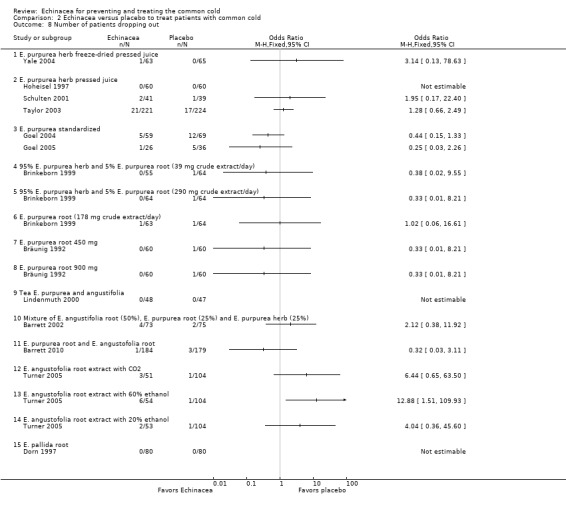
Comparison 2 Echinacea versus placebo to treat patients with common cold, Outcome 8 Number of patients dropping out.
2.9. Analysis.
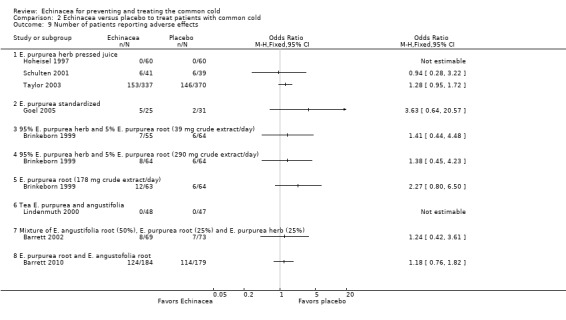
Comparison 2 Echinacea versus placebo to treat patients with common cold, Outcome 9 Number of patients reporting adverse effects.
Discussion
Summary of main results
Our review shows that a variety of products prepared from different Echinacea species, different plant parts and in different forms have been compared to placebo in randomized trials. These preparations contain quite different amounts of bioactive components and hence are not biochemically comparable. Furthermore, trial approaches and methods for cold assessment were highly variable. Taken together, results from prevention trials suggest that a number of Echinacea products slightly reduce the risk of getting a cold in healthy individuals. If this conclusion is true, the lack of significance in individual trials could be due to a lack of statistical power (too few patients included in single studies). Although it seems possible that some Echinacea products also have effects over placebo for treating colds, the overall evidence for clinically relevant treatment effects over placebo is weak.
Overall completeness and applicability of evidence
For our review we could identify and include three unpublished trials which did not find significant effects over placebo (Galea 1996; Kim 2002a; Zhang 2003). We are also aware of at least one further unpublished, negative trial from the USA. During the search for an earlier review on Echinacea (Melchart 1994) one of the authors was informed by an expert in the field through personal communication that there were several negative trials from Germany (however, possibly partly of combinations). It seems possible that there are additional unpublished trials, though we think that it is unlikely that the conclusions of our review would change substantially; the evidence regarding treatment effects is weak and cannot justify recommendations to take Echinacea or a specific product. All single prevention trials included in our review yielded non‐significant results. Particularly in smaller trials, P values were far from statistically significant.
Quality of the evidence
The great heterogeneity of preparations tested makes conclusions difficult. Several of the newer trials tested products which were standardized on the content of a bioactive ingredient. However, the available research indicates that the clinical effects of (some) Echinacea preparations are likely to be due to several components which may have synergistic effects. Two of the tested products were standardized for known bioactive components, namely alkamides, cichoric acid and polysaccharides (Goel 2004; Goel 2005; Tiralongo 2012). Components (or one component) of the study medication have been analyzed and documented for several trials (Barrett 2002; Barrett 2010; Galea 1996; Jawad 2012; Melchart 1998; Turner 2000; Turner 2005; Yale 2004; Zhang 2003). Testing preparations that have been standardized to specific components seems like a desirable way to move forward. The quality of the included trials was heterogeneous as we considered 38% of the trials to have a high risk of bias while we considered 42% of the trials to have a low risk of bias.
In 2005, a further systematic review on the effectiveness of Echinacea for the treatment (not prevention) of colds was published (Caruso 2005). The authors concluded that the possible therapeutic effectiveness of Echinacea had not been established. A major criticism was that most studies (apart from two negative trials) lacked a proof of blinding. While we agree that successful blinding is crucial for the validity of a trial, we find it problematic to overemphasize this criterion when assessing the available evidence. First, the vast majority (93%) of placebo‐controlled RCTs provide no evidence of blinding success and, of those that do, the majority report less than satisfactory results (Fergusson 2004). Second, participant guesses at the end of a trial are also influenced by the perceived outcome and are not necessarily evidence of bias. Nevertheless, we agree with the authors of this review that the available evidence is far from convincing and that a lack of blinding can be a relevant problem in trials of Echinacea products.
Potential biases in the review process
Study selection and data extraction were performed by at least two review authors independently. Studies in which one of the authors were involved were handled by another review author. We checked study findings entered for effect size calculation against the original publications. However, it was a major challenge for the authors of this review to summarize the results of the included studies in a manner that is both concise and reflects the heterogeneity adequately. In our main analysis we did not pool studies unless they clearly investigated comparable Echinacea products. Yet, deviating from our protocol, we included some pooled estimates from meta‐analysis across different Echinacea preparations in the text. We believe that this decision is justified as a) it allows us to check whether study findings are consistent across studies and products and b) it provides a crude idea of the possible size of potential effects. However, we urge that these results have to be interpreted with caution and should not be interpreted as 'average' effects of Echinacea products.
Agreements and disagreements with other studies or reviews
A meta‐analysis (Schoop 2006b) of the three trials on induced rhinovirus infections included in our review (Sperber 2004; Turner 2000; Turner 2005) found that the likelihood of participants experiencing a clinical cold was significantly lower in the Echinacea groups. The results were pooled, although different Echinacea preparations were examined in the trials. These findings could indicate that the trials were too small to detect a small effect of the tested preparations. This conclusion is consistent with our results.
In 2007 another meta‐analysis of RCTs investigating the effectiveness of Echinacea products for preventing and treating common colds was published (Shah 2007). This review drew more favorable conclusions, especially on the effect of Echinacea on the cold duration, than we do. Shah 2007 used different inclusion criteria and also included trials investigating combinations of Echinacea. These authors heavily relied on meta‐analysis, pooling findings from studies investigating very different Echinacea preparations and from treatment and prevention trials in one analysis. If all these trials are interpreted as investigating the same treatment for the same purpose, then the evidence can be considered as more positive and the conclusions reasonable. For our main analysis we refrained from pooling studies testing different Echinacea preparations.
Other complementary and alternative medicine (CAM) interventions for prevention and treatment have been investigated in systematic reviews. The evidence that vitamin C supplementation or probiotics used for prevention of the common cold, and zinc used for treatment of the common cold, are effective (Hao 2011; Hemilä 2013; Singh 2013a) is stronger than the evidence for Echinacea. Evidence for the effects of other CAM interventions is similarly limited (Pelargonium sidoides, Timmer 2009) or even weaker (saline nasal irrigation, Kassel 2010; increased fluid intake Guppy 2011; heated humidified air, Singh 2013b; garlic, Lissiman 2012; and Chinese medical herbs, Zhang 2010).
Authors' conclusions
Implications for practice.
The most important recommendation for consumers and clinicians is to be aware that the available Echinacea products differ greatly. The overwhelming majority of these products have not been tested in clinical trials. It has been shown that labeling of products marketed in health food stores can be incorrect (Gilroy 2003). Our exploratory meta‐analyses suggest that at least some Echinacea preparations may reduce the relative risk of catching a cold by 10% to 20%. A risk reduction of 15% would mean that if 500 out of 1000 persons receiving a placebo would catch a cold this figure would be 425 of 1000 persons with an Echinacea product. This is clearly a small effect of unclear clinical relevance. Furthermore, we cannot say which Echinacea products have an effect of this size, or a greater or lesser effect. While there are some hints that both alcoholic extracts and pressed juices that are based primarily on the aerial parts of E. purpurea have beneficial effects on cold symptoms in adults, the evidence for clinically relevant treatment effects is weak. There are still many remaining doubts due to the fact that not all trials using such preparations show even a trend towards an effect.
As randomized controlled trials include limited numbers of participants and often exclude persons with relevant co‐morbidity, a review of such trials can only contribute limited knowledge on safety issues. The number of patients dropping out or reporting adverse effects did not differ significantly between treatment and control groups in prevention and treatment trials. However, in prevention trials there was a trend towards a larger number of patients dropping out due to side effects or reporting side effects in the treatment groups.The most relevant potential adverse effects of Echinacea preparations are probably allergic reactions (Huntley 2005; Mullins 2002). One trial suggested an absolute 5% increase in rash in children (Taylor 2003). Parenteral application of Echinacea preparation should be discouraged, as there is no evidence of either safety or effectiveness.
Implications for research.
In principle, further research is clearly desirable given the widespread use of Echinacea products. However, given the multiplicity and diversity of products on the market applying the knowledge gained from such studies will remain a challenge to persons without in‐depth knowledge of herbal preparations. The use of chemically well‐defined preparations is recommended to improve comparability of results from different studies. It would be desirable if experts in research on common colds could develop recommendations for a core set of outcome measures to be used and reported in randomized clinical trials. Trials investigating the prevention of colds need large sample sizes as the potential effects of Echinacea products are likely to be small.
Feedback
Duration of Echinacea dosage
Summary
I wish to comment on the Cochrane review 'Echinacea for preventing and treating the common cold'. It is claimed in the Hot Topic of the Month (Relief from coughs and colds), August 2001, p. 5, para. 6.1, that "the German drug regulatory authority recommends that it be used for no longer than eight weeks at a time". I have asked the Consumer Network about the evidence for this and been told that it is not available. Nevertheless, I think that if it is indeed a recommendation of the German drug regulatory authority, it should be mentioned in both the review and the abstract.
I certify that I have no affiliations with or involvement in any organisation or entity with a direct financial interest in the subject matter of my criticisms.
Reply
We appreciate this comment. It is correct that the German drug regulatory authority recommends that Echinacea preparations should not be taken for longer than eight weeks at a time. While there is no evidence that longer intake can be harmful, such a precaution seems justified in the absence of data on long‐term use. We included a statement on this issue in the review conclusion section 'Implications for practice'.
Klaus Linde
Contributors
David Potter Comment posted 02/06/2005
What's new
| Date | Event | Description |
|---|---|---|
| 2 July 2014 | Amended | A mistake in the Abstract regarding treatment trials reporting data on the duration of colds has been corrected. |
History
Protocol first published: Issue 4, 1997 Review first published: Issue 1, 1999
| Date | Event | Description |
|---|---|---|
| 5 June 2013 | New citation required and conclusions have changed | Change to authorship byline: new first author Marlies Karsch‐Völk. Dieter Melchart was not involved in this update. Inclusion criteria changed: now only randomized controlled trials and also studies on induced rhinovirus infections included. Outcome measures changed: duration of cold is now a primary outcome of treatment trials. Conclusions changed: possibly slight effect of Echinacea in the prevention of colds. Evidence for treatment effectiveness is weak. |
| 5 June 2013 | New search has been performed | Searches updated. Seven new studies were included (Barrett 2010; Hall 2007; Jawad 2012; O'Neill 2008; Tiralongo 2012; Turner 2005; Zhang 2003). Two formerly excluded studies are now included (Sperber 2004; Turner 2000). One formerly included study is now excluded (Spasov 2004) and 12 new trials were excluded (Di Pierro 2012; Hauke 2002; Heinen‐Kammerer 2005; Isbaniah 2011; Minetti 2011; Narimanian 2005; Naser 2005; Saunders 2007; Schapowal 2009; Schoop 2006a; Wahl 2008; Yakoot 2011). |
| 15 March 2010 | New search has been performed | Searches conducted |
| 6 August 2009 | Amended | Contact details updated. |
| 8 May 2009 | Amended | Contact details updated. |
| 16 January 2008 | Amended | Converted to new review format. |
| 5 October 2007 | New search has been performed | Conclusions remain unchanged. |
| 12 September 2005 | New search has been performed | Searches conducted. |
| 1 June 2005 | Feedback has been incorporated | Feedback and reply added. |
| 16 November 1998 | New search has been performed | Review first published. |
Acknowledgements
We would like to thank Sarah Thorning (Trials Search Co‐ordinator of the Cochrane ARI Group) for her help with the literature search and Liz Dooley (Managing Editor of the Cochrane ARI Group) for her patience when waiting for this update to be completed. Furthermore we would like to thank Dieter Melchart for his contribution to the previous versions of the review. The authors would also like to thank the following people for commenting on drafts of this review: Janet Wale, Ann Fonfa, Ajima Olaghere, Steven Yale, Zaina AlBalawi, Sree Nair, Mark Jones and Meenu Singh. We are indebted to the authors of primary studies who provided additional information, often to a considerable extent.
Bruce Barrett is supported by a K24 Mid‐Career Investigator Award from the National Center for Complementary and Alternative Medicine at the U.S. National Institutes of Health. David Kiefer is a post‐doctoral research fellow, supported by a T32 National Research Service Award from the National Center for Complementary and Alternative Medicine at the U.S. National Institutes of Health.
Appendices
Appendix 1. Embase.com search strategy
#10 #1 AND #9 #9 #4 NOT #8 #8 #5 NOT #7 #7 #5 AND #6 #6 'human'/de #5 'animal'/de OR 'nonhuman'/de OR 'animal experiment'/de #4 #2 OR #3 #3 random*:ab,ti OR placebo*:ab,ti OR crossover*:ab,ti OR 'cross‐over':ab,ti OR (doubl* NEXT/1 blind*):ab,ti OR allocat*:ab,ti OR trial:ti #2 'randomized controlled trial'/exp OR 'single blind procedure'/exp OR 'double blind procedure'/exp OR 'crossover procedure'/exp #1 1922 #1.6 #1.1 OR #1.2 OR #1.3 OR #1.4 OR #1.5 #1.5 coneflower*:ab,ti #1.4 'e. purpurea':ab,ti OR 'e. pallida':ab,ti OR 'e.angustifolia':ab,ti #1.3 'echinacea purpurea'/de OR 'echinacea extract'/de OR 'echinacea purpurea extract'/de OR 'echinacea pallida extract'/de OR 'echinacea angustiflora extract'/de #1.2 echinac*:ab,ti #1.1 'echinacea'/exp
Appendix 2. CINAHL (EBSCO) search strategy
S15 S5 and S14 S14 S6 or S7 or S8 or S9 or S10 or S11 or S12 or S13 S13 TI placebo* OR AB placebo* S S12 TI clinic* W1 trial* OR AB clinic* W1 trial* S11 (MH "Quantitative Studies") S10 (MH "Placebos") S9 TI random* OR AB random* S8 TI ((singl* or doubl* or tripl* or trebl*) W1 (blind* or mask*)) OR AB ((singl* or doubl* or tripl* or trebl*) W1 (blind* or mask*)) S7 PT clinical trial S6 (MH "Clinical Trials+") S5 S1 or S2 or S3 or S4 S4 TI coneflower* OR AB coneflower* S3 TI ("E. purpurea" or "E. pallida" or "E. angustifolia") OR AB ("E. purpurea" or "E. pallida" or "E. angustifolia") S2 TI echinac* OR AB echinac* S1 (MH "Echinacea")
Appendix 3. AMED (Ovid) search strategy
1 exp echinacea/ 2 echinac*.tw. 3 1 or 2 4 randomized controlled trials/ 5 exp clinical trials/ 6 random allocation/ 7 double blind method/ 8 (clin* adj25 trial*).tw. 9 ((singl* or doubl* or trebl* or tripl*) adj25 (blind* or mask*)).tw. 10 placebos/ 11 placebo*.tw. 12 random*.tw. 13 or/4‐12 14 3 and 13
Appendix 4. LILACS (BIREME) search strategy
> Search > MH:"Echinacea angustifolia" OR MH:"Echinacea purpurea" OR MH:echinacea OR MH:HP4.018.251.105 OR MH:HP4.018.251.116 OR MH:B01.650.940.800.575.100.100.310 OR echinac$ OR "E. angustifolia" OR "E. pallida" OR "E. purpurea"
Appendix 5. Web of Science (Thomson Reuters) search strategy
| # 3 | 209 | #2 AND #1 Databases=SCI‐EXPANDED, CPCI‐S Timespan=All Years Lemmatization=On |
|
| # 2 | 1,292,302 | Topic=(random* or placebo* or ((singl* or doubl*) NEAR/1 blind*) or allocat* or crossover* or "cross over") OR Title=(trial) Databases=SCI‐EXPANDED, CPCI‐S Timespan=All Years Lemmatization=On |
|
| # 1 | 1,605 | Topic=(echinac* OR "E. purpurea" OR "E. angustifolia" OR "E. pallida" OR coneflower*) Databases=SCI‐EXPANDED, CPCI‐S Timespan=All Years Lemmatization=On |
Appendix 6. Previous searches
For the first publication of this review in The Cochrane Library, 1999, Issue 1 (Melchart 1999) the following sources were searched:
MEDLINE (1966 to 1998): all hits for Echinac* screened;
EMBASE (1991 to 1998): all hits for Echinac* screened;
the Cochrane Acute Respiratory Infections Group Specialized Register: all hits for Echinac* screened;
the database of the Cochrane Field Complementary Medicine: all hits for Echinac* screened;
the database Phytodok (Munich, specialized on Phytomedicine) screening all clinical studies for Echinac*;
bibliographies of identified articles;
existing reviews;
manufacturers and researchers in the field (who were contacted to identify published and unpublished trials);
proceedings of phytomedicine congresses (International Congresses on Phytomedicine and Congresses of the German Society of Phytotherapy) (screened).
For the 2007 update we searched the Cochrane Central Register of Controlled Trials (CENTRAL) (The Cochrane Library 2007, Issue 3); PubMed (1997 to September 2007); EMBASE (1998 to April 2007); AMED (to August 2005) and the Centre for Complementary Medicine Research (in Munich) (1988 to September 2007). We also contacted experts and screened references of reviews.
We searched PubMed and CENTRAL using the following terms combined with the highly sensitive search strategy devised by Dickersin (Dickersin 1994). 1 exp ECHINACEA/ 2 Echinacea 3 or/1‐2
We searched EMBASE and AMED using adapted terms. We searched the database of the Centre of Complementary Medicine Research in Munich for controlled trials of Echinacea.
We screened bibliographies of identified trials and review articles for further potentially relevant publications. We contacted experts in the field and asked about further published and unpublished studies.
Data and analyses
Comparison 1. Echinacea versus placebo to prevent common cold.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Number of participants with at least 1 cold episode | 9 | Risk Ratio (IV, Fixed, 95% CI) | Totals not selected | |
| 1.1 E. purpurea herb | 1 | Risk Ratio (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 E. purpurea herb pressed juice | 2 | Risk Ratio (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.3 E. purpurea dried plant extract | 1 | Risk Ratio (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.4 E. purpurea root extract | 1 | Risk Ratio (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.5 E. purpurea root alcoholic extract | 1 | Risk Ratio (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.6 E. purpurea root and E. angustofolia root | 1 | Risk Ratio (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.7 4% phenolic extract of E. purpurea and E. angustofolia | 1 | Risk Ratio (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.8 E. angustifolia root alcoholic extract | 1 | Risk Ratio (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.9 E. angustofolia root extract with CO2 | 1 | Risk Ratio (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.10 E. angustofolia root extract with 60% ethanol | 1 | Risk Ratio (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.11 E. angustofolia root extract with 20% ethanol | 1 | Risk Ratio (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Number of participants with more than 1 cold | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2.1 E. purpurea herb pressed juice | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.2 E. purpurea alcoholic extract with 95% herb and 5% root | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.3 E. purpurea root alcoholic extract | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.4 E. angustifolia root alcoholic extract | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Duration: mean difference | 4 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 3.1 E. purpurea herb | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.2 E. purpurea dried plant extract | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.3 E. purpurea root extract | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.4 E. purpurea root alcoholic extract | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.5 E. angustofolia root alcoholic extract | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4 Total severity score | 5 | Std. Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 4.1 E. purpurea herb pressed juice | 1 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.2 E. purpurea root extract | 1 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.3 E. purpurea root and E. angustofolia root | 1 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.4 4% phenolic extract of E. purpurea and E. angustofolia | 1 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.5 E. angustofolia root extract with CO2 | 1 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.6 E. angustofolia root extract with 60% ethanol | 1 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.7 E. angustofolia root extract with 20% ethanol | 1 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5 Number of patients dropping out due to adverse effects | 7 | Odds Ratio (IV, Fixed, 95% CI) | Totals not selected | |
| 5.1 E. purpurea herb pressed juice | 2 | Odds Ratio (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.2 E. purpurea dried plant extract | 1 | Odds Ratio (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.3 E. purpurea alcoholic extract with 95% herb and 5% root | 1 | Odds Ratio (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.4 E. purpurea root alcoholic extract | 1 | Odds Ratio (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.5 E. purpurea root and E. angustofolia root | 1 | Odds Ratio (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.6 4% phenolic extract of E. purpurea and E. angustofolia | 1 | Odds Ratio (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.7 E. angustifolia root alcoholic extract | 1 | Odds Ratio (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6 Number of patients dropping out | 9 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 6.1 E. purpurea herb pressed juice | 2 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6.2 E. purpurea dried plant extract | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6.3 E. purpurea alcoholic extract with 95% herb and 5% root | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6.4 E. purpurea root extract | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6.5 E. purpurea root alcoholic extract | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6.6 E. purpurea root and E. angustofolia root | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6.7 4% phenolic extract of E. purpurea and E. angustofolia | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6.8 E. angustifolia root alcoholic extract | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6.9 E. angustofolia root extract with CO2 | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6.10 E. angustofolia root extract with 60% ethanol | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6.11 E. angustofolia root extract with 20% ethanol | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 7 Number of patients reporting adverse effects | 8 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 7.1 E. purpurea herb pressed juice | 2 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 7.2 E. purpurea dried plant extract | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 7.3 E. purpurea alcoholic extract with 95% herb and 5% root | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 7.4 E. purpurea root extract | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 7.5 E. purpurea root alcoholic extract | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 7.6 E. purpurea root and E. angustofolia root | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 7.7 4% phenolic extract of E. purpurea and E. angustofolia | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 7.8 E. angustifolia root alcoholic extract | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
Comparison 2. Echinacea versus placebo to treat patients with common cold.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Duration: mean difference | 2 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 1.1 Mixture of E. angustifolia root (50%), E. purpurea root (25%) and E. purpurea herb (25%) | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 E. purpurea root and E. angustofolia root | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Duration (inverse variance) | 6 | Mean Difference (Random, 95% CI) | Totals not selected | |
| 2.1 E. purpurea herb freeze‐dried pressed juice | 1 | Mean Difference (Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.2 E. purpurea herb pressed juice | 2 | Mean Difference (Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.3 Tea E. purpurea and angustifolia | 1 | Mean Difference (Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.4 Mixture of E. angustifolia root (50%), E. purpurea root (25%) and E. purpurea herb (25%) | 1 | Mean Difference (Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.5 E. purpurea root and E. angustofolia root | 1 | Mean Difference (Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Total severity and duration measures (for example, area under the curve) | 9 | Std. Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 3.1 E. purpurea pressed juice | 2 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.2 E. purpurea standardized | 2 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.3 Mixture of 80% E. purpurea herb and 20% E. angustifolia roots | 1 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.4 Mixture of E. angustifolia root (50%), E. purpurea root (25%) and E. purpurea herb (25%) | 1 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.5 E. purpurea root and E. angustofolia root | 1 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.6 E. angustifolia | 1 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.7 E. angustofolia root extract with CO2 | 1 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.8 E. angustofolia root extract with 60% ethanol | 1 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.9 E. angustofolia root extract with 20% ethanol | 1 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4 Sum score after 2 to 4 days | 7 | Std. Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 4.1 E. purpurea herb freeze‐dried pressed juice | 1 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.2 E. purpurea herb pressed juice | 1 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.3 E. purpurea standardized | 2 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.4 E. purpurea root 450 mg | 1 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.5 E. purpurea root 900 mg | 1 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.6 Mixture of E. angustifolia root (50%), E. purpurea root (25%) and E. purpurea herb (25%) | 1 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.7 E. purpurea root and E. angustofolia root | 1 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5 Sum scores after 5 to 10 days | 8 | Std. Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 5.1 E. purpurea herb freeze‐dried pressed juice | 1 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.2 E. purpurea herb pressed juice | 1 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.3 E. purpurea standardized | 2 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.4 95% E. purpurea herb and 5% E. purpurea root (39 mg crude extract/day) | 1 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.5 95% E. purpurea herb and 5% E. purpurea root (290 mg crude extract/day) | 1 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.6 E. purpurea root (178 mg crude extract/day) | 1 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.7 E. purpurea root 450 mg | 1 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.8 E. purpurea root 900 mg | 1 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.9 Mixture of E. angustifolia root (50%), E. purpurea root (25%) and E. purpurea herb (25%) | 1 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.10 E. purpurea root and E. angustofolia root | 1 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6 Early treatment of prodromi: patients developing a 'full' cold | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 6.1 E. purpurea herb pressed juice | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6.2 E. angustofolia root extract with CO2 | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6.3 E. angustofolia root extract with 60% ethanol | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6.4 E. angustofolia root extract with 20% ethanol | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 7 Number of patients dropping out due to adverse effects | 11 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 7.1 E. purpurea herb pressed juice | 3 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 7.2 E. purpurea standardized | 2 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 7.3 95% E. purpurea herb and 5% E. purpurea root (39 mg crude extract/day) | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 7.4 95% E. purpurea herb and 5% E. purpurea root (290 mg crude extract/day) | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 7.5 E. purpurea root (178 mg crude extract/day) | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 7.6 E. purpurea root 450 mg | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 7.7 E. purpurea root 900 mg | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 7.8 Tea E. purpurea and angustifolia | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 7.9 Mixture of E. angustifolia root (50%), E. purpurea root (25%) and E. purpurea herb (25%) | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 7.10 E. purpurea root and E. angustofolia root | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 7.11 E. pallida root | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 8 Number of patients dropping out | 13 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 8.1 E. purpurea herb freeze‐dried pressed juice | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 8.2 E. purpurea herb pressed juice | 3 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 8.3 E. purpurea standardized | 2 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 8.4 95% E. purpurea herb and 5% E. purpurea root (39 mg crude extract/day) | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 8.5 95% E. purpurea herb and 5% E. purpurea root (290 mg crude extract/day) | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 8.6 E. purpurea root (178 mg crude extract/day) | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 8.7 E. purpurea root 450 mg | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 8.8 E. purpurea root 900 mg | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 8.9 Tea E. purpurea and angustifolia | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 8.10 Mixture of E. angustifolia root (50%), E. purpurea root (25%) and E. purpurea herb (25%) | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 8.11 E. purpurea root and E. angustofolia root | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 8.12 E. angustofolia root extract with CO2 | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 8.13 E. angustofolia root extract with 60% ethanol | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 8.14 E. angustofolia root extract with 20% ethanol | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 8.15 E. pallida root | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 9 Number of patients reporting adverse effects | 8 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 9.1 E. purpurea herb pressed juice | 3 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 9.2 E. purpurea standardized | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 9.3 95% E. purpurea herb and 5% E. purpurea root (39 mg crude extract/day) | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 9.4 95% E. purpurea herb and 5% E. purpurea root (290 mg crude extract/day) | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 9.5 E. purpurea root (178 mg crude extract/day) | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 9.6 Tea E. purpurea and angustifolia | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 9.7 Mixture of E. angustifolia root (50%), E. purpurea root (25%) and E. purpurea herb (25%) | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 9.8 E. purpurea root and E. angustofolia root | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
2.3. Analysis.
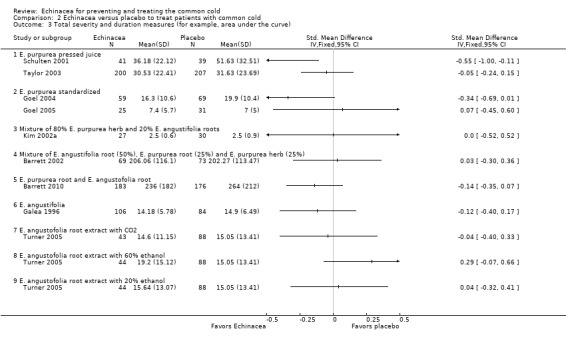
Comparison 2 Echinacea versus placebo to treat patients with common cold, Outcome 3 Total severity and duration measures (for example, area under the curve).
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Barrett 2002.
| Methods | Approach: treatment trial Design: randomized; placebo‐controlled, double‐blind |
|
| Participants | Numbers: 73 randomized to Echinacea, 75 to placebo; 69 analyzed in the Echinacea group and 73 in the placebo group
Setting: University Family Medicine Dept., USA Participants: university students Demographics: mean age 21 years, 69% female Main selection criteria: at least 2 of 15 cold symptoms for less than 36 hours |
|
| Interventions |
Echinacea: capsules containing 50% E. angustifolia root (123 mg per capsule), 25% E. purpurea root (62 mg), 25% E. purpurea herb (62 mg) and thyme and peppermint to disguise taste and flavor
Placebo: capsules containing 333 mg alfalfa
Dosage and treatment duration: 6 x 4 capsules in the first 24 hours, then 3 x 4 capsules up to 10 days Concurrent medication: "Patients using antibiotics, antihistamines or decongestants were excluded." No further information on the actual intake of concurrent medication is reported |
|
| Outcomes | Primary: severity score, duration, severity of single symptoms (daily reporting) Secondary: not defined |
|
| Notes | Funding source: U.S. Department of Health and Human Services and the National Institutes of health; Shaklee Tecnica Conflict of interest: none disclosed Additional information provided by author |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "...random number‐generator (MS Excel) and a balanced blocks‐of‐four design." |
| Allocation concealment (selection bias) | Low risk | Sequentially labeled bottles, allocation concealed until data had been collected, entered and cleaned |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Exit interview: blinding successful Placebo and Echinacea indistinguishable |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | < 5% drop‐outs, available case analysis considered appropriate |
| Selective reporting (reporting bias) | Low risk | Relevant outcomes presented in the manuscript Additional information provided from author |
Barrett 2010.
| Methods | Approach: treatment trial Design: randomized; placebo‐controlled, double‐blind |
|
| Participants | Numbers: 184 randomized to the blinded Echinacea group, 179 to the blinded placebo group, 183 analyzed in the blinded Echinacea group and 176 in the blinded placebo group Setting: 2 centers in Wisconsin Participants: recruited via newspaper advertisements, posters community talks, targeted mailings, e‐mails and word of mouth Demographics: mean age 34 years, 65% female Main selection criteria: cold symptoms since up to 36 hours |
|
| Interventions |
Echinacea: tablets containing 675 mg of E. purpurea root and 600 mg of E. angustifolia root, each standardized to 2.1 mg of alkamides. Manufacturer MediHerb (Australia) Placebo: "Tablet excipients included calcium acid phosphate, cellulose, silica, sodium starch glycolate, hypromellose and magnesium stearate. Placebo and Echinacea tablets contained the same proportion of inert ingredients and were covered with identical digestible coatings." Dosage and treatment duration: 2 tablets doses 4 times within the first 24 hours of enrolment, then 1 tablet 4 times daily for 4 days; that means 10.2 g of dried Echinacea root during first 24 hours, 5.1 g during each of the next days Concurrent medication: "Patients receiving antibiotics, antivirals, nasal steroids, decongestants, antihistamines, combination of cold formulas, Echinacea, zinc or vitamin C were excluded." No further information on the actual intake of concurrent medication is provided |
|
| Outcomes | Primary: area under the curve for global severity, with duration and severity assessed twice daily by self report, duration, severity score (Wisconsin Upper Respiratory Symptom Survey, short version; WURSS‐21) Secondary: self report on psycho‐social questionnaires and biomarker of immune response and inflammation |
|
| Notes | Funding source: National Center for Complementary and Alternative Medicine at the National Institute of Health, Robert Wood Johnson Foundation Generalist Physician Faculty Scholars Program; MediHerb (Queensland , Australia) provided the placebo and Echinacea tablets and conducted the phytochemical assays; financial support facilitated by Deans Robert Golden and Paul DeLuca of the University of Wisconsin Medical School Conflict of interest: no relevant conflict of interest reported The trial had 2 additional arms (open Echinacea and no treatment) to investigate placebo and expectation effects in colds. These 2 arms were not included in this review |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "We used SAS (SAS Institute, Cary, North Carolina) to generate a single block of 804 unique identification numbers so that each of 12 cells (3 clinician groups by 4 pill groups) was represented equally." |
| Allocation concealment (selection bias) | Low risk | "Using these codes, the UW Hospitals Pharmaceutical Research Center Investigational Drug Service prepared consecutively numbered, sealed envelopes to direct allocation. An envelope‐within envelope strategy was used, so that group assignment would be revealed as soon as the participant gave consent and the research assistant opened the larger outer envelope. Allocation concealment for the 2 blinded pill groups was accomplished by using identical coated tablets and plastic pill bottles. For the 2 thirds of the sample who would see a clinician, a second, smaller envelope that directed allocation to a standard or enhanced visit group was opened by the study clinician before entering the examination room. The randomized allocation key was not shared with investigators until after all data were collected, entered and cleaned and analysis strategies were determined." |
| Blinding (performance bias and detection bias) All outcomes | Low risk | "Blinding was tested at the exit interview by asking participants which group they thought they had been assigned to." Group differences were not statistically significant. "Placebo and echinacea tablets were covered with identical digestible coatings." (There were also an open‐label group and a no treatment group examined in the study, which are not taken into account for this review) |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | "... 719 were enrolled and randomly assigned. Retention was high. 2 participants were lost to follow‐up and 4 withdrew before primary outcome data could be gathered;..." |
| Selective reporting (reporting bias) | Low risk | All predefined main outcome measures and secondary outcomes are well reported |
Brinkeborn 1999.
| Methods | Approach: treatment trial Design: randomized; placebo‐controlled, double‐blind |
|
| Participants | Numbers: 3 Echinacea groups and 1 placebo group, 559 randomized, 246 got a cold and started treatment, 2 dropped out, 181 in per protocol analysis (41/49/44/46), 246 in intent‐to‐treat analysis (55/64/63/64)
Setting: infectious disease center in Sweden Demographics: 74% female, mean age 41 years Main selection criteria: healthy volunteers, prone to common cold |
|
| Interventions |
Echinacea 1: Echinaforce tablets (6.78 mg E. purpurea crude extract based on 95% herb and 5% roots)
Echinacea 2: concentrate preparation (48.27 mg of the same extract)
Echinacea 3: 29.6 mg crude extract based on root only Placebo: not described Dosage and treatment duration: 3 x 2 tablets daily up to a maximum of 7 days Concurrent medication: patients were excluded if they were taking "other medications which may affect the immune system like immunostimulants and antibiotics or may influence the symptoms like nose‐drops or anticoughs". No further information on the actual intake of concurrent medication is reported |
|
| Outcomes | Primary: relative reduction of the complaint index (= sum score) based on 12 symptoms according to doctor's record Secondary: relative reduction of the complaint index according to the patient's diary, assessment of efficacy and tolerance by the investigator and the patients, the frequency and severity of adverse events |
|
| Notes | Funding source: Bioforce AG, Switzerland Conflict of interest: not stated Additional information provided by sponsor |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "computer‐generated randomization list in blocks of four" |
| Allocation concealment (selection bias) | Low risk | "consecutively numbered drug bottles" |
| Blinding (performance bias and detection bias) All outcomes | Low risk | "The trial doses of the four treatments were presented in identical vials with identical labels and could almost not be distinguished from one another by their smell or taste." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Only 2 drop‐outs and ITT‐analysis available (although methods for replacing data not clear) |
| Selective reporting (reporting bias) | Low risk | Predefined relevant outcomes adequately reported |
Bräunig 1992.
| Methods | Approach: treatment trial Design: randomized; placebo‐controlled. Comparison of the lower dosage of Echinacea and placebo probably double‐blind, group receiving the higher dosage probably not blinded (it seems that all patients received 90 drops placebo) |
|
| Participants | Numbers: 60 persons were randomized to treatment group 1, 60 to treatment group 2 and 60 to placebo group, only 1 drop‐out in the placebo group
Setting: 1 general practice, Germany Demographics: not reported Main selection criteria: influenza‐like URTI |
|
| Interventions |
Echinacea 1: Echinacea purpurea root extract 90 drops (450 mg) daily for 8 to 10 days
Echinacea 2: Echinacea purpurea root extract 180 drops (900 mg) daily for 8 to 10 days
Placebo: mixture of ethanol and water (50 vol. %) with caramel color Dosage and treatment duration: Echinacea (see above), for placebo not mentioned explicitly Concurrent medication: patients were excluded, if they were using antihistamines, antibiotics or other relevant medication influencing the clinical picture. No further information on the actual intake of concurrent medication is provided |
|
| Outcomes | Duration of disease was described as a main outcome measure but results were not reported 2 scores on 5 medical findings (assessed by the physician after 3 to 4 and 8 to 10 days) and 8 symptoms (assessed by the patient) |
|
| Notes | Funding source: Fink GmbH Herrenberg, Firma Salus‐Haus Bruckmühl Conflict of interest: not stated Study not truly double‐blind Additional information provided by author |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "randomized", not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | For treatment group 1: low For treatment group 2: high Double‐blinding stated but 1 group received more medication (180 drops instead of 90 drops in the first Echinacea group and in the placebo group) and was therefore not truly double‐blind |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 1 drop‐out in the placebo group is mentioned in the publication, but it is not stated if the results of this participant were included in the analysis. (Author confirmed in personal communication that there were no drop‐outs and withdrawals) |
| Selective reporting (reporting bias) | High risk | Duration of disease was described as a main outcome measure but results were not reported |
Dorn 1997.
| Methods | Approach: treatment trial Design: randomized; placebo‐controlled, double‐blind |
|
| Participants | Numbers: 160 randomized to Echinacea group and analyzed, 160 randomized to placebo group and analyzed
Setting: 1 general practice, Germany Demographics: 48% female, age not reported Man inclusion criteria: upper respiratory tract infection (viral origin was suspected in 114 patients (70 Echinacea versus 44 placebo), bacterial in 46 (10 Echinacea versus 36 placebo)) |
|
| Interventions |
Echinacea:Echinacea pallida root extract
Placebo: "coloured aqueous alcoholic solution" Dosage and treatment duration: 90 drops (900 mg Echinacea) daily for 8 to 10 days Concurrent medication: patients were excluded if they "were being treated with other drugs that might interact with a herbal preparation". No detailed information on the actual intake of concurrent medication is provided |
|
| Outcomes | Length of illness, symptoms, clinical findings (assessed after 3 to 4 and 8 to 10 days), lymphocytes and granulocytes, effect of the frequency of URTI during the previous 3 years on the treatment | |
| Notes | Funding source: not stated, but probably funding by Pascoe GmbH, Germany Conflict of interest: not stated Additional information provided by author |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomization list (provided by author) |
| Allocation concealment (selection bias) | Low risk | "neutral packaging with appropriate code numbers" (author confirmed "consecutively numbered") |
| Blinding (performance bias and detection bias) All outcomes | Low risk | "The study was double blind: ...received a coloured aqueous alcoholic solution that mimicked and was indistinguishable..." |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Report suggests that there were no drop‐outs or withdrawals |
| Selective reporting (reporting bias) | High risk | Insufficiently reported trial; relevant group differences regarding suspected cause (more bacterial infections in the placebo group than in Echinacea group: 36 versus 10) |
Galea 1996.
| Methods | Approach: treatment trial Design: randomized; placebo‐controlled, double‐blind |
|
| Participants | Numbers: 395 randomized, 235 returned symptom checklist, 192 had a cold, 2 incomplete checklists due to drop‐out, 106 participants in the Echinacea group and 84 in the placebo group completed the trial
Setting: Family Medicine Department, Canada Demographics: no information Main selection criteria: healthy students |
|
| Interventions |
Echinacea: capsules with 250 mg E. angustifolia (standardized on 4% content of echinacoside)
Placebo: placebo capsules (filled with vegetable oil)
Dosage and treatment duration: 3 x 1 capsule for 10 days Concurrent medication: not reported |
|
| Outcomes | Number of symptoms (4 major and 4 minor; single and summed) over 10‐day period | |
| Notes | Funding source: the authors thank C.E. Jamieson and Co. Ltd. and R. P. Scherer Canada Inc (Windsor, Ontario) for supplying the Echinacea, placebo and remuneration for the trial participants. No direct financial support stated Conflict of interest: not stated Simple, unpublished trial; only short report available |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not mentioned |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Placebo capsules were filled with vegetable oil. They may have been distinguishable from Echinacea capsules by taste |
| Incomplete outcome data (attrition bias) All outcomes | High risk | > 20% attrition for unclear reasons, not partitioned between treatment and placebo groups |
| Selective reporting (reporting bias) | Unclear risk | Reporting does not seem systematically biased but several outcomes are reported insufficiently to allow effect size calculation |
Goel 2004.
| Methods | Approach: treatment trial Design: randomized; placebo‐controlled, double‐blind |
|
| Participants | Numbers: 282 randomized, 128 contracted a cold and were included in the analysis (ITT: 59 Echinacea, 69 placebo; PP: 54 Echinacea, 57 placebo)
Demographics: 54% in Echinacea and 75% in placebo group female, mean age 32 years Setting: University of Alberta, Canada Main selection criteria: healthy adults with at least 2 colds last year |
|
| Interventions |
Echinacea: Echinacea purpurea extract containing 0.25 mg/ml alkamides, 2.5 mg/ml cichoric acid, 25.5 mg/ml polysaccharides
Placebo: "Placebo made to look, taste and smell like the echinacea extract"
Dosage and treatment duration: 10 x 4 ml the first day, then 4 x 4 ml for 6 days Concurrent medication: "Volunteers were excluded if they...were on immunosuppressive drugs such as corticosteroids or cyclosporine...The subjects were instructed not to take any other medication during the treatment." Subjects using "concomitant relief medications on a regular basis during the treatment period" were excluded from the PP population |
|
| Outcomes | Total daily symptom score, 13 symptoms (reported daily by patients and on day 3 and 8 by nurse) | |
| Notes | Funding source: treatment provided by Factors R&D Technologies, Canada Conflict of interest: not stated 5 (Echinacea) versus 12 (placebo) drop‐outs/excluded patients Additional information provided by author |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "The randomization list was generated using a computer program." |
| Allocation concealment (selection bias) | Low risk | Numbered drugs (information from author) |
| Blinding (performance bias and detection bias) All outcomes | Low risk | "Placebo made to look, taste and smell like the echinacea extract." Blinding tested after completion of the study and was maintained adequately during the treatment period |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Treatment group with 9% attrition, placebo group with 17% attrition, ITT analysis done. Plausible method for replacing missing data described |
| Selective reporting (reporting bias) | Low risk | The published report includes all expected outcomes, including those that were pre‐specified |
Goel 2005.
| Methods | Approach: treatment trial Design: randomized; placebo‐controlled, double‐blind |
|
| Participants | Numbers: 150 randomized, 62 contracted a cold (26 Echinacea, 36 placebo), 56 were analyzed (25 Echinacea, 31 placebo)
Setting: University of Alberta, Canada Demographics: 59% female, mean age 40 years Main selection criteria: volunteers between 18 and 65 years with 2 or more colds previous year |
|
| Interventions |
Echinacea: Echinacea purpurea extract containing 0.25 mg/ml alkamides, 2.5 mg/ml cichoric acid, 25.5 mg/ml polysaccharides
Placebo: "The placebo contained similar ingredients without the echinacea." (40% ethanol)
Dosage and treatment duration: 8 x 5 ml the first day, then 3 x 5 ml for 6 days Concurrent medication: "Volunteers were excluded if they...were on immunosuppressive drugs such as corticosteroids or cyclosporine...Participants reporting the concomitant use of other relief medications on a regular basis during their cold were excluded from the study." |
|
| Outcomes | Diary with 8 cold symptoms, multiple physiological measures | |
| Notes | Funding source: Echinacea and placebo "were provided by Factors R & D Technologies, Burnaby, BC, Canada." No direct financial support stated Conflict of interest: not stated Subsequent trial after the Goel 2004 trial focusing on physiological outcomes and reporting clinical outcomes only briefly. Author (T. K. Basu) provided additional information. Patients in the Echinacea group had higher symptom scores at baseline. Analyses of % change from day 1 showed significant differences in favor of the Echinacea group, however, absolute values (provided for us by the author) at defined time points were similar in both groups |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "The randomization codes were generated using an Excel 97 computer program." |
| Allocation concealment (selection bias) | Low risk | Numbered bottles |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double‐blind ("indistinguishable as to appearance, color, or flavor") |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Almost 10% of the participants excluded from analysis, no ITT analysis performed |
| Selective reporting (reporting bias) | Low risk | Publication includes all expected outcomes, including those that were pre‐specified |
Grimm 1999.
| Methods | Approach: prevention trial Design: randomized; placebo‐controlled, double‐blind |
|
| Participants | Numbers: 109 randomized, 108 analyzed (54 Echinacea, 54 placebo)
Setting: 1 general practice, Germany Demographics: 62% female, mean age 40 years Main selection criteria: volunteers with more than 3 URTIs in the previous 6 months of winter |
|
| Interventions |
Echinacea: pressed juice of Echinacea purpurea herb in 22% alcohol Placebo: alcohol/water solution with artificial color Dosage and treatment duration: 2 x 4 ml daily for 8 weeks Concurrent medication: "Use of immunostimulating drugs within 4 weeks before study entry" was an exclusion criterion. No further information on the actual intake of concurrent medication reported |
|
| Outcomes | Primary: incidence, number, severity and duration of infections; time to first infection, CD4/CD8‐ratio Secondary: side effects |
|
| Notes | Funding source: "The study was sponsored by Madaus AG, Cologne, Germany." Conflict of interest: not stated No additional information sought |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "computer‐generated randomization list" |
| Allocation concealment (selection bias) | Unclear risk | Probably numbered drug containers, not clearly described |
| Blinding (performance bias and detection bias) All outcomes | Low risk | "According to the manufacturer, verum and placebo were indistinguishable as to appearance, color, flavor." Adequacy of blinding assessed at the end of follow‐up: "27 (50%) of 54 patients in the echinacea group stated that they would like to continue to take the allocated medicine compared to 23 (43%) of 54 patients in the placebo group (P=0.54)" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Drop‐outs: 1 of 109 (before starting treatment) |
| Selective reporting (reporting bias) | Low risk | Extractable data provided |
Hall 2007.
| Methods | Approach: prevention trial Design: randomized; placebo‐controlled, double‐blind | |
| Participants | Numbers: 32 analyzed (Echinacea: 18; placebo: 14); number randomized not reported Setting: not reported, probably Dept. of Kinesiology, Elmhurst College (USA) Demographics: mean age 26 years, gender not reported Main selection criteria: active, non‐smoking adults aged 19 to 46 years |
|
| Interventions |
Echinacea: E. purpurea herb 1.2 g in capsules Placebo: gelatin capsules containing sugar mixture Dosage and treatment duration: 8 capsules/day for 4 weeks Concurrent medication: "Only those subjects that were...not taking any medications and / or dietary supplements...were included in the study." No further information on the actual intake of concurrent medication is provided |
|
| Outcomes | Number and duration of URTI additionally to salival IgA, secretion rate of IgA and salival flow rate | |
| Notes | Funding source: study medication was provided by Nature's Way, Springville (USA), no financial support reported Conflict of interest: not stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "...randomly assigned to a group based on a computer generated table of random numbers..." |
| Allocation concealment (selection bias) | Low risk | "The placebo and echinacea capsules were placed into identical containers... and were randomly assigned and coded, by a third party,to assure allocation concealment" |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Placebo: "same weight, shape and color as the echinacea capsules", identical containers |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Number randomized and drop‐outs not mentioned |
| Selective reporting (reporting bias) | Unclear risk | Gender not reported, side effects not examined/reported, relevant results without numerical standard deviation or not shown numerically, unclear if other outcomes have been examined |
Hoheisel 1997.
| Methods | Approach: treatment trial Design: randomized; placebo‐controlled, double‐blind |
|
| Participants | Numbers: 120 randomized and analyzed (60 Echinacea, 60 placebo)
Setting: company physician of a large furniture‐making factory Demographics: 10% female, mean age 36.5 years Main inclusion criteria: patients presenting with first symptoms of an URTI and having a history of recurrent URTI (> 3 episodes in the previous 12 months) (employees of the furniture‐making factory) |
|
| Interventions |
Echinacea: pressed juice of Echinacea purpurea herb
Placebo: "identical in color and ethanol concentration"
Dosage and treatment duration: on day 1 every 2 hours 20 drops, then up to maximally 10 days 3 x 20 drops daily Concurrent medication: "Nine patients in the Echinagard group and 4 in the placebo group reported taking concomitant medication, mainly analgesics and anti‐ulcer agents." These patients were at least included in the ITT‐analysis |
|
| Outcomes | Primary: number of patients who developed a 'full' common cold and days until improvement Secondary: symptom diary, global assessments |
|
| Notes | Funding source: not stated
Conflict of interest: not stated
No diary data presented, subjective patient definition what was considered a 'full' cold (could be a major problem if patients should have been unblinded) No additional information received |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Patients were randomized to treatment groups (using the programme Random V5.0)..." |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | "Placebo identical in colour and ethanol concentration; identical bottles". Taste not mentioned, no test of blinding, no description of development/testing of liquid placebo |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All 120 patients randomized completed the study and were analyzed |
| Selective reporting (reporting bias) | High risk | "Recorded subjective symptoms daily in diary card" not shown in tables. Subsample record only of those who report "real cold" may introduce bias |
Jawad 2012.
| Methods | Approach: prevention trial Design: randomized; placebo‐controlled, double‐blind |
|
| Participants | Numbers: Echinacea: 379 randomized, 325 analyzed; placebo: 376 randomized, 348 analyzed Setting: Common Cold Center in Cardiff University (United Kingdom) Demographics: Echinacea: 68.7% female, mean age 23.6. Placebo: 62.7% female, mean age 23.2 Main selection criteria: healthy adults with 2 or more colds per year |
|
| Interventions |
Echinacea: alcoholic extraction from freshly harvested E. purpurea with 95% herb and 5% roots Placebo: "Placebo drops were similar in shape, color, consistency, odor, flavor and they contained the same amount of alcohol." Dosage and treatment duration: 3 x 0.9 ml per day for illness prevention (2400 mg of extract per day), during acute stages of cold dose was increased to 5 x 0.9 ml per day (4000 mg of extract per day). Treatment duration was 4 months Concurrent medication: "The exclusion criteria were...currently taking antimicrobial or antiviral medication...corticosteroid treated asthma, medicinal treated atopy or allergy...In the Echinacea and placebo groups, 58 and 88 episodes, respectively, were treated with aspirin, paracetamol, or ibuprofen. Thus, significantly more (+52%) cold episodes in the placebo group were additionally treated with pain medication..." |
|
| Outcomes | Adverse effects, multiple physiological measures, prevalence of colds, symptom scores | |
| Notes | Funding source: not stated in the publication. Sponsoring by A. Vogel Bioforce AG, Switzerland stated in additional information by the authors Conflict of interest: no conflicts of interest of first and last author, conflicts of interest of the other authors not stated Some information relevant for this review is missing in the publication. Additional information was requested from the authors and has partly been provided |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "The randomization code was prepared in block‐sizes of 6 with "RANCODE Professional 3.6" program." |
| Allocation concealment (selection bias) | Low risk | "Each participant received treatment based on his/her identification number, which was allocated according to the time point of inclusion." |
| Blinding (performance bias and detection bias) All outcomes | Low risk | "...double blind...", "Placebo drops similar in shape, colour, consistency, odor, flavor and they contained the same amount of alcohol.", "Primary and secondary packaging was identical..." Blinding was tested in 79 persons and was found to be adequate |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Population not clearly described. Drop‐outs relatively few, but distribution disproportionate: 54/379 versus 28/376 |
| Selective reporting (reporting bias) | High risk | Most results adequately reported but N of patients with at least 1 infection not reported. No P value for accumulated number of cold episodes reported. Severity and duration mentioned, but not reported |
Kim 2002a.
| Methods | Approach: treatment trial Design: randomized; placebo‐controlled, double‐blind |
|
| Participants | Numbers: Echinacea: 27; placebo: 30 (no treatment: 25)
Setting: Cornell University, Ithaca, NY, USA Demographics: 55% female, mean age 20 years Main outcome criteria: healthy students |
|
| Interventions |
Echinacea: extract from 80% E. purpurea herb, 20% E. angustifolia root
Control 1: placebo: parsley juice and orange extract.
Control 2: no treatment
Dosage and treatment duration: 101 ml containing 1000 mg (dry plant) per day for at least 5 days Concurrent medication: "Screening criteria included...those who were taking medication for seasonal allergies and those who had taken Echinacea." Further information on the actual intake of concurrent medication is not provided |
|
| Outcomes | Duration, retrospective assessment of 11 cold symptoms | |
| Notes | Funding source: not stated Conflict of interest: not stated Unpublished 3‐armed study |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomization not described |
| Allocation concealment (selection bias) | Low risk | Identically labeled bottles |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Post cold survey to test success of blinding: blinding effective |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Loss to follow‐up and missing data not described |
| Selective reporting (reporting bias) | Low risk | Study focuses on single cold symptoms; no evidence of selective reporting |
Lindenmuth 2000.
| Methods | Approach: treatment trial Design: placebo‐controlled, double‐blind; quasi‐randomized but adequate concealment likely |
|
| Participants | Numbers: 95 allocated, 95 analyzed (Echinacea: 48; placebo: 47)
Setting: nursing and rehabilitation center in York, PN, USA Demographics: 85% women, mean age 40 years Main inclusion criteria: employees of a nursing and rehabilitation center with earliest symptoms of a cold |
|
| Interventions |
Echinacea: tea preparation from aerial parts of E. purpurea and E. angustifolia and E. purpurea root. The Echinacea tea contained small amounts of 2 flavoring components (lemon grass leaf and spearmint leaf)
Placebo: placebo tea (cinnamon bark, ginger rhizome, peppermint leaf, sweet fennel seed, rose hip, papaya leaf, alfalfa leaf)
Dosage and treatment duration: 5 to 6 cups on day 1, titration to 1 cup on day 5 Concurrent medication: "Subjects who were excluded included...those who...were already taking antibiotics." No further information on the actual intake of concurrent medication is reported |
|
| Outcomes | Global assessment of effectiveness and duration | |
| Notes | Funding source: donation of treatment and placebo teas from Traditional Medicinals Inc., no direct financial funding stated Conflict of interest: not stated Additional information sought but no answer received Simple study with insufficient outcome measurement. Not truly randomized but adequately concealed alternate allocation. Duration of symptoms has been coded reciprocally ("1" for "more then 5 days" and "5" for "immediately", "4" for "2 days" etc.). Thus the numeric results in the publication dissemble an effect favoring placebo. Therefore, the group results were exchanged between Echinacea and placebo group for the analysis with regard to the fact that the text of the publication describes results favoring Echinacea |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Not truly randomized: "...allocation was accomplished by utilization of alternation for assignment..." |
| Allocation concealment (selection bias) | Unclear risk | "The random assignment was accomplished by specific trained secretary personnel not associated with the study and having no prior knowledge of the groups or which of the 2boxes of tea bags contained the packets ofEchinacea Plus tea bags and that contained the packets of placebo tea bags. These personnel used a numbered system..." |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | "....each subject was given 21 tea bags of the same appearance..." "...this tea... does not have any obvious or easily recognizable flavor characteristics that would make it easily distinguishable from the Echinacea Plus tea by an untrained palate." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All included patients remained in the study and were analyzed |
| Selective reporting (reporting bias) | Low risk | Results for all measured outcomes reported |
Melchart 1998.
| Methods | Approach: prevention trial Design: randomized; placebo‐controlled, double‐blind |
|
| Participants | Numbers: 302 were randomized; 289 participants were analyzed by intention‐to‐treat, 214 complied fully with the protocol. (Echinacea 1: 103/99/85; Echinacea 2: 103/100/84; placebo: 96/90/75)
Setting: 5 centers (4 military centers, 1 industrial plant, Munich, Germany) Demographics: 29% female, mean age 29.5 years Main inclusion criteria: healthy volunteers |
|
| Interventions |
Echinacea 1: Echinacea angustifolia root extract (plant extract ratio 1:11 in 30% alcohol)
Echinacea 2: Echinacea purpurea root extract (plant extract ratio 1:11 in 30% alcohol)
Placebo: colored ethanolic solution
Dosage and treatment duration: 2 x 50 drops daily from Monday to Friday for 12 weeks Concurrent medication: exclusion criteria were: "systemic intake of corticosteroids, antibiotics, or immunostimulants in the previous 2 weeks." Further information on the actual intake of concurrent medication is not provided |
|
| Outcomes | Predefined main outcome measure: time until occurrence of first URTI Secondary outcomes: proportions with at least 1 URTI; number, severity and duration of episodes, global assessment. Predefined subgroup analysis on patients with more than 3 infections in the previous 12 months | |
| Notes | Funding source: Bavarian Parliament, Plantapharmazie (Göttingen, Germany) Conflict of interest: not stated Treatment preparations characterized by the content of glycoproteins/polysaccharides (additional information from manufacturer) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "stratified for participants with up to 3 and more than 3 colds in the last year; computer‐generated randomization list, block size 15..." |
| Allocation concealment (selection bias) | Low risk | "concealment by consecutively numbered medication" |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | More volunteers in treatment groups correctly guessed that they had received a true treatment (P < 0.001; some unblinding likely) |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Drop‐outs: 58/302, intention‐to‐treat analysis performed |
| Selective reporting (reporting bias) | Low risk | All relevant outcomes reported |
O'Neill 2008.
| Methods | Approach: prevention trial | |
| Participants | Numbers: 90 randomized, 58 analyzed (Echinacea: 45 randomized, 28 analyzed; placebo: 45 randomized, 30 analyzed) Setting: University Medical Center and Family Health Center at the University of California San Francisco‐Fresno (USA) Demographics: mean age 40 years, sex not reported Main selection criteria: healthy adults recruited from hospital personnel |
|
| Interventions |
Echinacea: E. purpurea dried plant extract in capsules Placebo: parsley capsules Dosage and treatment duration: 3 capsules 2 times daily for 8 weeks (300 mg per capsule) Concurrent medication: "Persons with...immunosuppressive therapy...were excluded...Individuals currently using Echinacea were not considered for the study, whereas those using other upper respiratory tract infection preventive measures were allowed to continue their use...Participants with symptoms were asked about...any medications used to treat symptoms (e.g., aspirin, acetaminophen, vitamins and cold formulas)" |
|
| Outcomes | Days with symptoms, days missed from work, medications used to treat symptoms, adverse effects | |
| Notes | Funding source: grant from Health Resources and Services Administration Border Health Education and Training Center, Medication used was donated by Natures Resource, Mission Hills, California (USA) Conflict of interest: none |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "...list generated using the random‐number generator in a spreadsheet program (Microsoft Excel....)" |
| Allocation concealment (selection bias) | Unclear risk | The method of concealment is not described in sufficient detail to allow a definite judgement |
| Blinding (performance bias and detection bias) All outcomes | Low risk | "...indistinguishable in size, color and smell...Participants, the main investigator and all persons involved in the study remained blinded to the identity of each group until data analyses were completed." |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Large number of drop‐outs (32/90), no ITT analysis |
| Selective reporting (reporting bias) | Low risk | All mentioned outcomes reported |
Schulten 2001.
| Methods | Approach: treatment trial Design: randomized; placebo‐controlled, double‐blind |
|
| Participants | Numbers: 80 randomized and analyzed in ITT‐analysis (ITT: Echinacea: 41; placebo: 39; per protocol: Echinacea: 37; placebo: 33)
Setting: industrial plant in Germany Demographics: 49% female, mean age 39 years Main inclusion criteria: patients (employees of the manufacturer) with subjective sensation of a cold and at least 1 of 8 symptoms |
|
| Interventions |
Echinacea: E. purpurea herb (pressed juice from fresh flowering plants)
Placebo: not described
Dosage and treatment duration: 2 x 5 ml for 10 days Concurrent medication: "Therapy with immunosuppressants in the week prior to the trial and therapy with immunostimulants (herbal immunostimulants, cytokines, thymus fractions), zinc or antibiotics during 2 weeks before commencement of the trial were not allowed." No further information on the actual intake of concurrent medication is provided |
|
| Outcomes | Primary: number of days with illness, number of patients with a "full" cold, area under the curve for the daily symptom score (modified Jackson score) and single symptoms, symptom score Secondary: subjective efficacy assessment by patients |
|
| Notes | Funding source: Madaus AG, Cologne, Germany Conflict of interest: not stated, but study was performed by and with Madaus employees Additional information sought but not received Rigorous and well‐reported study, however, performed in employees of the manufacturer without testing the success of blinding. Adaptive design with early stopping after 80 patients as a significant difference was detected |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "computer generated randomization list" |
| Allocation concealment (selection bias) | Low risk | Consecutive randomization, identical packaging |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | "indistinguishable in terms of appearance, taste, smell, colour and packaging", but we are uncertain: Can Madaus employees recognize the taste of their Echinacea product? |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 3/80, ITT analysis performed |
| Selective reporting (reporting bias) | Unclear risk | Median duration versus mean (not reported); were URIs defined a priori? |
Sperber 2004.
| Methods | Approach: prevention trial (experimental rhinovirus colds) Design: randomized; placebo‐controlled, double‐blind |
|
| Participants | Numbers: 48 randomized, 46 analyzed (Echinacea: 24; placebo: 22) Setting: not clearly described, probably Department of Internal Medicine, Hackensack University Center, Hackensack (USA) Demographics: mean age 33 years, Echinacea: 50% female, placebo: 58% female Main selection criteria: healthy adults, aged 18 to 65 years, antibody titers of =< 1:2 to Rhinovirus type 39 |
|
| Interventions |
Echinacea: pressed juice of E. purpurea in 22% alcohol Placebo: "matching placebo", not described Dosage and treatment duration: 2.5 ml 3 times daily for 14 days (intranasal virus inoculation with RV‐39 after 7 days) Concurrent medication: "Individuals who had received medication known to affect rhinorrhea, cough, or nasal congestion within 7 days (4 weeks for cromolyn sodium and long‐acting antihistamines) before study initiation were excluded." Further information on actual intake of concurrent medication is not reported |
|
| Outcomes | Primary: occurrence and severity of symptoms Secondary: increase in RV‐39 neutralizing antibody titer and/or recovery of rhinovirus on viral culture |
|
| Notes | Funding source: Madaus AG, Cologne, Germany Conflict of interest: not stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomization only stated |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double‐blinding stated. "The active medication and placebo were identical in appearance, taste and smell and were packaged in identical 100ml bottles." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Only 2/48 excluded (< 5%) |
| Selective reporting (reporting bias) | Low risk | Measured outcomes well reported |
Taylor 2003.
| Methods | Approach: treatment trial Design: randomized; placebo‐controlled, double‐blind | |
| Participants | Numbers: 524 randomized, probably 436 with infection, 407 analyzed (Echinacea: 200; placebo: 207)
Setting: 7 private practices and 1 inner‐city clinic, USA Demographics: 50% female, mean age 5.5 years Main inclusion criteria: healthy children between 2 and 11 years |
|
| Interventions |
Echinacea: E. purpurea herb harvested at flowering, in syrup
Placebo: syrup
Dosage and treatment duration: 7.5 ml/day in children 2 to 5 years and 10 ml/day in those 6 to 11 years up to a maximum of 10 days Concurrent medication: "...and those receiving chronic medications of any kind or herbal, mineral, or specific vitamin supplements were excluded...parents were asked to not give their child any medication other than the study medication and acetaminophen (if desired) unless prescribed by a physician. However, if another medication was administered, the parent was requested to record the name." Use of concomitant medication was reported (secondary outcome) |
|
| Outcomes | Primary: duration, severity, adverse events Secondary: peak severity, number of days with fever, parental global assessment of severity, concomitant medication |
|
| Notes | Funding source: grant from the National Center for Complementary and Alternative Medicine. Study medication and placebo provided by Madaus AG, Cologne, Germany
Conflict of interest: not stated
Additional information on results in the first cold episode was provided from authors. Additional publication: Weber 2005. In the "Data and analyses" section of this review the outcome "the number of adverse effects per URI" reported in this trial is used as an equivalent to the outcome "number of patients reporting adverse effects per number of participants" Rigorous and well‐reported study; complex analysis as several infections per child were monitored |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Randomization was performed using a computer‐generated randomization list and was stratified by site and in blocks of 10." |
| Allocation concealment (selection bias) | Low risk | "...unique study number corresponding to the numbers on the bottles of study medication" |
| Blinding (performance bias and detection bias) All outcomes | Low risk | "Placebo identical in appearance and similar in taste and smell..." Blinding tested: only 35.1%/22.7% correct guesses |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Drop‐outs: 36/436. Missing outcome data balanced in numbers across intervention groups, with similar reasons for missing data across groups |
| Selective reporting (reporting bias) | Low risk | All mentioned outcomes have been reported |
Tiralongo 2012.
| Methods | Approach: prevention trial Design: randomized; placebo‐controlled, double‐blind |
|
| Participants | Numbers: 175 randomized, 170 analyzed (ITT; Echinacea: 85; placebo: 85) Setting: not clearly described, probably School of Pharmacy, Griffith Health Institute, Griffith University, Gold Coast Australia Demographics: mean age 43 years, 67% female Main inclusion criteria: healthy adults, traveling on intercontinental flights, 18 to 65 years |
|
| Interventions |
Echinacea: tablets containing 112.5 mg E. purpurea root 6:1 extract (equivalent to 675 mg dry root) and 150 mg E. angustofolia root 4:1 extract (equivalent to 600 mg dry root) Placebo: not clearly described, tablets covered identically Dosage and treatment duration: days ‐14 to ‐3: 1 tablet. twice a day; days ‐2 to +7: 2 tablets twice a day; +8 to +32 1 tablet twice a day; +33 to +42 2 tablets twice a day; +43 to +49 1 tablet twice a day. For shorter travels the dose between day 8 and 32 was shortened, reflecting the time the patient was spending abroad Concurrent medication: "Volunteers were excluded if they...were on regular treatment with Echinacea, antibiotics, corticosteroids, antihistamines and immunosuppressants." Further details on the actual intake of concurrent medication are not provided |
|
| Outcomes | Primary: symptom score, URI symptom‐related quality of life Secondary: occurrence and duration of jet lag, headache, sleep pattern, herpes simplex scores |
|
| Notes | Funding source: Integria Healthcare Pty. Ltd. Conflict of interest: not stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "The random allocation sequence provided by the sponsor was computer generated using a randomization plan from http://www.randomization.com/ with randomization in blocks of 10." |
| Allocation concealment (selection bias) | Low risk | "...allocation was concealed by providing each participant with a number...Labelling only identified the patient number." |
| Blinding (performance bias and detection bias) All outcomes | Low risk | "Placebo tablets were manufactured to match the Echinacea tablets in size, excipients and colour. Both sets of tablets were coated with a brown colour and hypromellose to make them indistinguishable. Tablets were packed in identical amber glass bottles with identical labelling." Blinding tested and successful |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 27 patients lost to follow‐up (16% drop‐outs, no real ITT) |
| Selective reporting (reporting bias) | Low risk | All outcomes reported |
Turner 2000.
| Methods | Approach: prevention trial (experimental rhinovirus colds) Design: randomized; placebo‐controlled, double‐blind |
|
| Participants | Numbers: 117 randomized, 92 challenged with rhinovirus type 23, infection occurred in 22 of 50 in Echinacea group and in 24 of 42 in placebo group Setting: not clearly stated, probably Department of Pediatrics and Medicine, Medical University of South Carolina (USA) Demographics: not reported Main selection criteria: healthy young adults with a titer of neutralizing antibody of ≤ 1:4 to rhinovirus type 23 |
|
| Interventions |
Echinacea: 4% phenolic extract of a mixture of E. purpurea and E. angustofolia formulated as powder, containing 0.16% cichoric acid with almost no echinacosides or alkamides Placebo: not described Dosage and treatment duration: 1 capsule containing 900 mg Echinacea of placebo once a day, for 14 days prior to virus challenge and 5 days after virus challenge Concurrent medication: according to additional information provided by author exclusion criteria were "use of any anti‐inflammatory (steroids or NSAIDs) or couch/cold preparation in the 2 weeks prior to the study, use of astemizole in the month prior to the study..." No detailed information on the actual intake of concurrent medication is reported |
|
| Outcomes | Number of rhinovirus infections, incidence of clinical colds, severity of symptoms | |
| Notes | Funding source: grant from the Procter & Gamble Company, Cincinnati, Ohio (USA) Conflict of interest: not stated, but the second author is a Procter & Gamble employee Publication is a short report. Some additional information (study protocol) was provided by author |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Unpublished study protocol provided by author states that allocation is randomized but does not report further details |
| Allocation concealment (selection bias) | Unclear risk | See above |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Blinding not mentioned; assessment of blinding with some (non‐significant) differences in correct guesses ‐ therefore blinding of participants done; blinding of providers unclear |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 24 drop‐outs before virus challenge |
| Selective reporting (reporting bias) | Low risk | Relevant outcomes seem to be reported |
Turner 2005.
| Methods | Approach: prevention and treatment trial (experimental rhinovirus colds) Design: randomized; placebo‐controlled, double‐blind |
|
| Participants | Numbers: the trial consisted of a prophylaxis phase of 7 days, then a challenge with rhinovirus inoculation, followed by a 5‐day treatment phase. Participants were allocated to 7 study groups. 3 groups received 1 of 3 different Echinacea preparations in the prophylaxis phase and in the treatment phase. 3 groups received placebo in the prophylaxis phase and 1 of the 3 different Echinacea preparations in the treatment phase. The control group received placebo in both the prophylaxis and the treatment phase. 437 were randomized and 399 analyzed. E1/E1: 52; E2/E2: 52; E3/E3: 45; placebo/E1: 48; placebo/E2: 51; placebo/E3: 48; placebo/placebo: 103 (Echinacea = E) Setting: University of Virginia School of Medicine, Charlottesville (USA) Demographics: mean age 21 years, 60% female Main selection criteria: healthy young adults, serum‐neutralizing antibody titer =< 1:4 to rhinovirus type 39 |
|
| Interventions |
Echinacea: E. angustofolia root extract with CO2, with 60% ethanol or with 20% ethanol Placebo: mixture of alcoholic beverages, denatonium benzoate and tap water Dosage and treatment duration: 1.5 ml (equivalent of 300 mg Echinacea root) tincture 3 times daily for 13 days (prophylaxis groups) or placebo for 7 days and Echinacea for 5 days (treatment groups), the control group received placebo for 13 days Concurrent medication: not reported |
|
| Outcomes | Primary: symptom score Secondary: testing of infection with rhinovirus type 39, nasal secretion weight, assessment of inflammation in nasal lavage specimens |
|
| Notes | Funding source: grant from the National Center for Complementary and Alternative Medicine (NCCAM) of the National Institutes of Health Conflict of interest: first author has served as a consultant for Wyeth Consumer Healthcare, Schering‐Plough Research Institute, Nordic Phytopharma A7S, the Dial Corporation and Procter & Gamble and having received grant support from Biopolymer Engineering, the Dial Corporation and Procter & Gamble. The last author has received grant support from the U.S. Department of Defense |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "randomly assigned in blocks" no further description of the sequence generation |
| Allocation concealment (selection bias) | Unclear risk | Not clearly described |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Blinding tested and successful |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Missing outcome data balanced in numbers across intervention groups, with similar reasons for missing data across groups |
| Selective reporting (reporting bias) | Low risk | Relevant outcomes reported |
Yale 2004.
| Methods | Aproach: treatment trial Design: randomized; placebo‐controlled, double‐blind |
|
| Participants | Numbers: 128 (Echinacea: 63, placebo: 65)
Setting: clinic network, USA Demographics: 86% female, mean age 38 years Main inclusion criteria: adult patients presenting with acute sneezing and nasal discharge for 6 to 24 hours |
|
| Interventions |
Echinacea: Echinacea fresh capsule (freeze‐dried pressed juice from E. purpurea herb standardized on a content of 2.4% soluble beta‐1,2‐D‐fructofuranosides)
Placebo: capsules with lactose
Dosage and treatment duration: 3 x 100 mg up to a maximum of 14 days Concurrent medication: "Exclusion from the study occurred if thee patient...received antibiotics, antihistamines, decongestants, nasal sprays, or corticosteroids in the 48 hours before enrolment,...used corticosteroids during the 8 weeks before enrolment...Patients were encouraged not to take any other cold remedies, including antihistamines, decongestants, cough suppressants, corticosteroids, or isotonic sodium chloride solution during the study. Acetaminophen was allowed, if needed." Actual intake is reported |
|
| Outcomes | Severity score, single symptoms (daily reporting), duration, global assessment, adverse effects | |
| Notes | Funding source: Marshfield Clinic Research Foundation, Wisconsin (USA), Enzymatic Therapy provided study materials Conflict of interest: not stated Some additional information received from author | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "...randomization list sent from the Biostatistics and Bioinformatics core to the Investigational Drug Pharmacy..." (information from author) |
| Allocation concealment (selection bias) | Unclear risk | No exact description but all persons were blinded except the clinical pharmacist |
| Blinding (performance bias and detection bias) All outcomes | Low risk | "...identical‐appearing lactose placebo capsule...", "treatments and group assignments remained blinded to patients and investigators, with the exception of the clinical pharmacist." Blinding tested and successful |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | According to information provided by author only 1 non‐completer |
| Selective reporting (reporting bias) | Low risk | Additional results provided by author |
Zhang 2003.
| Methods | Approach: prevention trial Design: randomized; placebo‐controlled, double‐blind |
|
| Participants | Numbers: 120 randomized, 111 analyzed (Echinacea: 54; placebo: 57) Setting: University of Wolverhampton (USA) Demographics: 57% female, median age 25 Main inclusion criteria: healthy participants, 18 to 65 years old |
|
| Interventions |
Echinacea: E. purpurea root extract (capsules containing 294 mg E. purpurea root extract, 4.4 mg cichoric acid and 500 mg of non‐specified herb powder) Placebo: capsules containing 500 mg herb powder Dosage and treatment duration: 1 capsule daily for 8 weeks Concurrent medication: "The general exclusion criteria were...use of antibiotics / corticosteroids within 2 weeks or immunostimulating drugs within 4 weeks of study entry..." No detailed information on the actual intake of concurrent medication is provided |
|
| Outcomes | Symptom and severity score, total episodes of URI, missed doses, experience of side effects | |
| Notes | Funding source: not stated Conflict of interest: not stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "...stratified randomization procedure...", "In order of their recruitment subjects in each pre‐treatment URI score group were grouped into blocks of 4 and 2 subjects in each block of 4 were allocated randomly to Echinacea and the other 2 to placebo by using separate sets of randomized numbers provided for each of the 2 pre‐treatment URI categories. The randomized number sequences were generated by a statistician..." |
| Allocation concealment (selection bias) | Low risk | "The Echinacea and placebo dose‐pots were then labeled with the numbers that had been allocated to individual subjects..." |
| Blinding (performance bias and detection bias) All outcomes | High risk | " Echinacea and the identical placebo doses were enclosed in cellulose capsules...This enabled concealment of colour differences.", "Subjects were advised not to open the capsules since the taste/smell/colour of the contents might lead to identification of the doses." By testing of blinding 82% in the placebo group correctly identified their group |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Drop‐outs: 9/120, no ITT |
| Selective reporting (reporting bias) | Low risk | All outcomes reported |
Abbreviations:
URTI = upper respiratory tract infection URI = upper respiratory infection ITT = intention‐to‐treat PP = per protocol
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Baetgen 1984 | Not randomized Not common cold (pertussis) |
| Baetgen 1988 | Not randomized Not common cold (acute bronchitis) |
| Bendel 1989 | Not common cold (reduction of immunosuppressive side effects of combined radio‐chemotherapy in patients with breast cancer), combination of Echinacea with other herbal extracts |
| Bendel 1990 | Not common cold (reduction of immunosuppressive side effects of radiotherapy in patients with breast cancer), combination of Echinacea with other herbal extracts |
| Blumröder 1985 | Not common cold (angina lacunaris), combination of Echinacea with other herbal extracts |
| Coeugniet 1986 | Not randomized Not common cold (recurrent vaginal candida infection) |
| Cohen 2004 | Randomized trial of a combination |
| Cubasch 1992 | Only measurement of laboratory and psychological parameters |
| Di Pierro 2012 | Randomized trial about enhancement of immune response to influenza vaccines, not common cold |
| Dorn 1989 | Randomized trial of a combination of Echinacea with other herbal extracts |
| Ehringer 1968 | Not common cold (venous insufficiency) |
| Engel 1988 | Not randomized |
| Forth 1981 | Randomized trial of a combination of Echinacea with other herbal extracts |
| Freitag 1984 | Not common cold (pertussis) |
| Freyer 1974 | Trial of a combination of Echinacea with other herbal extracts, with alternate allocation |
| Hauke 2002 | Trial of Esberitox® as supportive therapy to antibiotics, not common cold (acute exacerbation of chronic bronchitis) |
| Heinen‐Kammerer 2005 | Open study, not a randomized controlled trial |
| Helbig 1961 | Trial of a combination of Echinacea with other herbal extracts, with alternate allocation |
| Henneicke 1999 | Randomized trial of a combination of Echinacea with other herbal extracts |
| Hill 1993 | Not common cold (topical treatment of insect bites) |
| Hill 1995 | Not common cold (topical treatment of insect bites) |
| Hill 1996 | Not common cold (topical treatment of insect bites) |
| Isbaniah 2011 | Trial of a combination of Echinacea with other agents, not common cold (chronic obstructive pulmonary disease) |
| Kim 2002b | Measurement of physiological outcomes in healthy volunteers |
| Kleinschmidt 1965 | Trial of a combination of Echinacea with other herbal extracts with alternate allocation |
| Melchart 1995a | Measurement of immunological parameters in healthy volunteers |
| Melchart 1995b | Measurement of immunological parameters in healthy volunteers |
| Melchart 1995c | Measurement of immunological parameters in healthy volunteers |
| Melchart 1995d | Measurement of immunological parameters in healthy volunteers |
| Melchart 1995e | Measurement of immunological parameters in healthy volunteers |
| Minetti 2011 | Trial of a combination of Echinacea with other herbal extracts |
| Narimanian 2005 | Randomized trial of a combination of Echinacea with other herbal extracts |
| Naser 2005 | Randomized trial of a combination of Echinacea with other herbal extracts |
| Pohl 1970 | Not randomized Not common cold (reduction of radiotherapy‐induced leukopenia) |
| Quadripur 1976 | Not common cold (skin infections) |
| Reitz 1990 | Randomized trial of a combination of Echinacea with other herbal extracts |
| Sartor 1972 | Not common cold (reduction of radiotherapy‐induced leukopenia) |
| Saunders 2007 | Open‐label study, not a randomized controlled trial |
| Scaglione 1995 | Randomized trial of a combination of Echinacea with other herbal extracts |
| Schapowal 2009 | Randomized trial of a combination of Echinacea with other herbal extracts |
| Schmidt 1990 | Randomized trial of a combination of Echinacea with other herbal extracts |
| Schoop 2006a | Open study, not a randomized controlled trial |
| Schwarz 2002 | Measurement of immunological parameters in healthy volunteers |
| Spasov 2004 | Unblinded study without placebo control |
| Stolze 1986 | Not randomized Not common cold (variety of respiratory tract infections treated with antibiotics) |
| Thom 1997 | Randomized trial of a combination |
| Timmermanns 1990 | Not common cold (urinary dysfunction) |
| Vonau 2001 | Randomized trial of Echinacea purpurea for recurrent genital herpes |
| Vorberg 1984 | Randomized trial of a combination of Echinacea with other herbal extracts |
| Vorberg 1989 | Randomized trial of a combination of Echinacea with other herbal extracts |
| Wahl 2008 | Randomized trial of Echinacea in combination with osteopathic treatment in children with recurrent otitis media |
| Yakoot 2011 | Randomized trial of a combination of Echinacea with other herbal extracts |
| Zimmer 1985 | Not common cold (acute sinusitis) |
Characteristics of studies awaiting assessment [ordered by study ID]
Rahmati 2012.
| Methods | Approach: treatment trial Design: randomized; placebo‐controlled, double‐blind |
| Participants | 100 children aged 5 to 11 years with upper respiratory tract infection |
| Interventions | Echinacea root extract or placebo |
| Outcomes | Duration and severity of symptoms |
| Notes | Study in Persian language identified in the update search |
Differences between protocol and review
Change to authorship byline: New first author is Marlies Karsch‐Völk. Dieter Melchart was not involved in this update. Inclusion criteria changed: Now only randomized controlled trials and also studies on induced rhinovirus infections have been included. Outcome measures changed: Duration of cold is now a primary outcome of treatment trials.
Contributions of authors
Co‐ordination of the review update: MKV Planning of updates: MKV, KL, BB, DK Searches in addition to searches done by the Cochrane ARI Group: MKV Study selection, data extraction and quality assessment: MKV, KL, BB, DK Extraction of pharmaceutical data/pharmaceutical expertise: KAW, RB Statistical analyses: MKV, KL Drafting of the manuscript: MKV, KL Interpretation of results, critical feedback on draft versions: BB, DK, KAW, RB
Sources of support
Internal sources
Centre for Complementary Medicine Research, Technische Universität München, Germany.
Institute of Pharmaceutical Sciences, Department of Pharmacognosy, Karl‐Franzens‐University, Graz, Austria.
Department of Family Medicine, University of Wisconsin, USA.
External sources
National Center for Complementary and Alternative Medicine at the U.S. National Institutes of Health (RO1 AT001428), USA.
Robert Wood Johnson Foundation Generalist Physician Scholars Program, USA.
Declarations of interest
Klaus Linde was involved in one, Karin Ardjomand‐Woelkart in one, Bruce Barrett in two and Rudolf Bauer in four of the studies included in this review. Marlies Karsch‐Völk and David Kiefer have no conflict of interest. Authors did not carry out data extraction or quality assessment of studies they were involved in.
Edited (no change to conclusions)
References
References to studies included in this review
Barrett 2002 {published and unpublished data}
- Barrett BP, Brown RL, Locken K, Maberry R, Bobula JA, D'Alessio D. Treatment of the common cold with unrefined Echinacea. A randomized, double‐blind, placebo‐controlled trial. Annals of Internal Medicine 2002;137:939‐46. [DOI] [PubMed] [Google Scholar]
Barrett 2010 {published data only}
- Barrett B, Brown R, Rakel D, Mundt M, Bone K, Barlow S. Echinacea for treating the common cold. Annals of Internal Medicine 2010;153:769‐77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett P, Brown R, Rakel D, Rabago D, Marchand L, Scheder J, et al. Placebo effects and the common cold: a randomized controlled trial. Annals of Family Medicine 2011;9:312‐22. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bräunig 1992 {published and unpublished data}
- Bräunig B, Dorn M, Knick E. [Echinaceae purpureae radix: zur Stärkung der körpereigenen Abwehr bei grippalen Infekten]. Zeitschrift für Phytotherapie 1992;13:7‐13. [Google Scholar]
Brinkeborn 1999 {published and unpublished data}
- Brinkeborn R, Shah D, Geissbühler S, Degenring FH. Echinaforce for the treatment of the common cold. Results of a placebo‐controlled double‐blind trial in Sweden [Echinaforce zur Behandlung von akuten Erkältungen. Ergebnisse einer placebokontrollierten Doppelblindstudie in Schweden]. Schweizerische Zeitschrift für Ganzheitsmedizin 1998;10:26‐9. [Google Scholar]
- Brinkeborn RM, Shah DV, Degenring FH. Echinaforce and other Echinacea fresh plant preparations in the treatment of the common cold. A randomized, placebo‐controlled, double‐blind clinical trial. Phytomedicine 1999;6:1‐5. [DOI] [PubMed] [Google Scholar]
Dorn 1997 {published and unpublished data}
- Bräunig B, Knick E. [Therapeutische Erfahrungen mit Echinacea pallida bei grippalen Infekten]. Naturheilpraxis mit Naturmedizin 1993;1:72‐5. [Google Scholar]
- Dorn M, Knick E, Lewith G. Placebo‐controlled, double‐blind study of Echinaceae pallidae radix in upper respiratory tract infections. Complementary Therapies in Medicine 1997;5:40‐2. [Google Scholar]
Galea 1996 {unpublished data only}
- Galea S, Thacker K. Double blind prospective trial investigating the effectiveness of a commonly prescribed herbal remedy in altering duration, severity and symptoms of the common cold. Unpublished report 1996.
Goel 2004 {published and unpublished data}
- Goel V, Lovlin R, Barton R, Lyon MR, Bauer R, Lee TDG, et al. Efficacy of a standardized echinacea preparation (Echinilin) for the treatment of the common cold: a randomized, double‐blind, placebo‐controlled trial. Journal of Clinical Pharmacy and Therapeutics 2004;29:75‐83. [DOI] [PubMed] [Google Scholar]
Goel 2005 {published and unpublished data}
- Goel V, Lovlin R, Chang C, Slama JV, Barton R, Gahler R, et al. A proprietary extract from the Echinacea plant (Echinacea purpurea) enhances systemic immune response during a common cold. Phytotherapy Research 2005;19:689‐94. [DOI] [PubMed] [Google Scholar]
Grimm 1999 {published data only}
- Grimm W, Müller HH. A randomized controlled trial of the effect of fluid extract of Echinacea purpurea on the incidence and severity of colds and respiratory infections. American Journal of Medicine 1999;106:138‐43. [DOI] [PubMed] [Google Scholar]
- Schöneberger D. [Einfluß der immunstimulierenden Wirkung von Preßsaft aus Herba Echinaceae purpureae auf Verlauf und Schweregrad von Erkältungskrankheiten]. Forum Immunologie 1992;2(8):18‐22. [Google Scholar]
Hall 2007 {published data only}
- Hall H, Fahlmann MM, Engels HJ. Echinacea purpurea and mucosal immunity. International Journal of Sports Medicine 2007;28:792‐7. [DOI] [PubMed] [Google Scholar]
Hoheisel 1997 {published data only}
- Hoheisel O, Sandberg M, Bertram S, Bulitta M, Schäfer M. Echinagard treatment shortens the course of the common cold: a double‐blind, placebo‐controlled clinical trial. European Journal of Clinical Research 1997;9:261‐9. [Google Scholar]
Jawad 2012 {published and unpublished data}
- Jawad M, Schoop R, Suter A, Klein P, Eccles R. Safety and efficacy profile of Echinacea purpurea to prevent common cold episodes: A randomized, double‐blind, placebo‐controlled trial. Evidence‐Based Complementary and Alternative Medicine 2012;2012:1‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kim 2002a {unpublished data only}
- Kim KH, Levitsky DA. Echinacea's effect on the common cold: a double‐blind, placebo controlled, clinical trial. Unpublished report 2002.
Lindenmuth 2000 {published data only}
- Lindenmuth GF, Lindenmuth EB. The efficacy of Echinacea compound herbal tea preparation on the severity and duration of upper respiratory and flu symptoms: a randomized, double‐blind placebo‐controlled study. Journal of Alternative and Complementary Medicine 2000;6:327‐34. [DOI] [PubMed] [Google Scholar]
Melchart 1998 {published and unpublished data}
- Melchart D, Walther E, Linde K, Brandmaier R, Lersch C. Echinacea root extracts for the prevention of upper respiratory tract infections ‐ a double‐blind, placebo‐controlled randomized trial. Archives of Family Medicine 1998;7:541‐5. [DOI] [PubMed] [Google Scholar]
O'Neill 2008 {published data only}
- O'Neil J, Hughes S, Lourie A, Zweifler J. Effects of echinacea on the frequency of upper respiratory tract symptoms: a randomized, double‐blind, placebo‐controlled trial. Annals of Allergy, Asthma & Immunology 2008;100:384‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Schulten 2001 {published data only}
- Schulten B, Bulitta M, Ballering‐Brühl B, Köster U, Schäfer M. Efficacy of Echinacea purpurea in patients with a common cold. A placebo‐controlled, randomised, double‐blind clinical trial. Arzneimittel Forschung 2001;51(ii):563‐8. [DOI] [PubMed] [Google Scholar]
Sperber 2004 {published data only}
- Sperber SJ, Shah LP, Gilbert RD, Ritchey TW, Monto AS. Echinacea purpurea for prevention of experimental rhinovirus colds. Clinical Infectious Diseases 2004;38:1367‐71. [DOI] [PMC free article] [PubMed] [Google Scholar]
Taylor 2003 {published data only}
- Taylor JA, Weber W, Standish L, Quinn H, Goesling J, McGann M. Efficacy and safety of Echinacea in treating upper respiratory tract infections in children. A randomized controlled trial. JAMA 2003;290:2824‐30. [DOI] [PubMed] [Google Scholar]
- Weber W, Taylor JA, Stoep A, Weiss NS, Standish LJ, Calabrese C. Echinacea purpurea for prevention of upper respiratory tract infections in children. Journal of Alternative and Complementary Medicine 2005;11(6):1021‐6. [DOI] [PubMed] [Google Scholar]
Tiralongo 2012 {published data only}
- Tiralongo E, Lea RA, Wee SS, Hanna MM, Griffiths LR. Randomised, double blind, placebo‐controlled trial of Echinacea supplementation in air travellers. Evidence‐Based Complementary and Alternative Medicine 2012;2012:1‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Turner 2000 {published and unpublished data}
- Turner RB, Riker DK, Gangemi JD. Ineffectiveness of Echinacea for prevention of experimental rhinovirus colds. Antimicrobial Agents and Chemotherapy 2000;44:1708‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Turner 2005 {published data only}
- Turner RB, Bauer R, Woelkart K, Hulsey TC, Gangemi D. An evaluation of Echinacea angustofolia in experimental rhinovirus infections. New England Journal of Medicine 2005;252:341‐8. [DOI] [PubMed] [Google Scholar]
Yale 2004 {published and unpublished data}
- Yale SH, Liu K. Echinacea purpurea therapy for the treatment of the common cold. A randomized, double‐blind, placebo‐controlled clinical trial. Archives of Internal Medicine 2004;164:1237‐41. [DOI] [PubMed] [Google Scholar]
Zhang 2003 {unpublished data only}
- Zhang X, Lowe D, Badesha G, Ishaq S, Rai H, Williams P, et al. A double‐blinded trial evaluating the effectiveness of Echinacea in countering upper respiratory tract infections. Unpublished report 2003.
References to studies excluded from this review
Baetgen 1984 {published data only}
- Baetgen D. [Erfolge in der Keuchhusten‐Behandlung mit Echinacin]. Therapiewoche 1984;34:5115‐9. [Google Scholar]
Baetgen 1988 {published data only}
- Baetgen D. [Behandlung der akuten Bronchitis im Kindesalter]. Therapiewoche Pädiatrie 1988;1:66‐70. [Google Scholar]
Bendel 1989 {published data only}
- Bendel R, Bendel V, Renner K, Carstens V, Stolze K. [Zusatzbehandlung mit Esberitox N bei Patientinnen mit chemo‐strahlentherapeutischer Behandlung eines fortgeschrittenen Mammakarzinoms]. Onkologie 1989;12(Suppl 3):32‐8. [DOI] [PubMed] [Google Scholar]
Bendel 1990 {published data only}
- Bendel R, Renner K, Stolze K. [Zusatzbehandlung mit Esberitox bei Patientinnen mit kurativer adjuvanter Bestrahlung nach Mammakarzinom]. Strahlentherapie und Onkologie 1988;164(5):278‐83. [PubMed] [Google Scholar]
Blumröder 1985 {published data only}
- Blumröder WOv. [Angina lacunaris ‐ eine Untersuchung zum Thema "Steigerung der körpereigenen Abwehr"]. Zeitschrift für Allgemeinmedizin 1985;61(8):271‐3. [Google Scholar]
Coeugniet 1986 {published data only}
- Coeugniet E, Kühnast R. [Rezidivierende Candidiasis. Adjuvante Immuntherapie mit verschiedenen Echinacea‐Darreichungsformen]. Therapiewoche 1986;36:3352‐8. [Google Scholar]
Cohen 2004 {published data only}
- Cohen HA, Varsano I, Kahan E, Sarrell EM, Uziel Y. Effectiveness of an herbal preparation containing Echinacea, Propolis, and vitamin C in preventing respiratory tract infections in children. A randomized, double‐blind, placebo‐controlled, multicenter study. Archives of Pediatrics and Adolescent Medicine 2004;158:217‐21. [DOI] [PubMed] [Google Scholar]
Cubasch 1992 {published data only}
- Cubasch H, Stocksmeier U. [Deutliche Zunahme der T‐Helferzellen]. Therapiewoche 1992;42:990‐1000. [Google Scholar]
Di Pierro 2012 {published data only}
- Pierro F, Rapacioli G, Ferrara T, Togni S. Use of a standardized extract from Echinacea angustofolia (Polinacea) for the prevention of respiratory tract infections. Alternative Medicine Review 2012;17:36‐41. [PubMed] [Google Scholar]
Dorn 1989 {published and unpublished data}
- Dorn M. [Milderung grippaler Infekte durch ein pflanzliches Immunstimulans]. Natur‐ und Ganzheitsmedizin 1989;2:314‐9. [Google Scholar]
Ehringer 1968 {published data only}
- Ehringer H. [Objektivierbare Venentonisierung nach oraler Gabe eines Kombinationspräparates mit Roßkastanienextrakt]. Arzmeimittel‐Forschung 1968;18:432‐4. [Google Scholar]
Engel 1988 {published data only}
- Engel G. [Behandlung grippaler Infekte mit einem homöopathischen Komplexarzneimittel]. Therapiewoche 1988;38:3586‐9. [Google Scholar]
Forth 1981 {published data only}
- Forth H, Beuscher N. [Beeinflussung der Häufigkeit banaler Erkältungsinfekte durch Esberitox]. Zeitschrift für Allgemeinmedizin 1981;57(32):2272‐5. [PubMed] [Google Scholar]
Freitag 1984 {published data only}
- Freitag U, Stammwitz U. [Reduzierte Krankheitsdauer bei Pertussis durch unspezifisches Immunstimulans]. Der Kinderarzt 1984;15:1068‐71. [Google Scholar]
Freyer 1974 {published data only}
- Freyer HU. [Häufigkeit banaler Infekte im Kindesalter und Möglichkeiten der Prophylaxe]. Fortschritte der Medizin 1974;92:165‐8. [PubMed] [Google Scholar]
Hauke 2002 {published data only}
- Hauke W, Kohler G, Henneicke‐von Zepelin HH, Freudenstein J. Esberitox N as supportive therapy when providing standard antibiotic treatment in subjects with a severe bacterial infection (acute exacerbation of chronic bronchitis) ‐ A multicentric, prospective, double‐blind, placebo‐controlled study. Chemotherapy 2002;48(5):259‐66. [DOI] [PubMed] [Google Scholar]
Heinen‐Kammerer 2005 {published data only}
- Heinen‐Kammerer T, Holtmannspötter C, Schnabel S, Motzkat K, Kencke P, Rychlik R. Effectiveness of Echinacin in therapy of chronic recurrent respiratory disease [Nutzenbewertung der Therapie chronisch rezidivierender Atemwegsinfekte mit Echinacin]. Das Gesundheitswesen 2005;67:296‐301. [DOI] [PubMed] [Google Scholar]
Helbig 1961 {published data only}
- Helbig G. [Unspezifische Reizkörpertherapie zur Infektionsprophylaxe]. Medizinische Klinik 1961;56:1512‐4. [PubMed] [Google Scholar]
Henneicke 1999 {published data only}
- Henneicke‐von Zepelin HH, Hentschel C, Schnitker J, Kohnen R, Köhler G, Wüstenberg P. Efficacy and safety of a fixed combination phytomedicine in the treatment of the common cold (acute viral respiratory tract infection): results of a randomised, double blind, placebo controlled, multicentre study. Current Medical Research and Opinion 1999;15:214‐27. [DOI] [PubMed] [Google Scholar]
- Henneicke‐von‐Zepelin HH, Hentschel C, Kohnen R, Köhler G, Wüstenberg P. [Plazebokontrollierte, randomisierte, doppelblinde, multizentrische klinische Prüfung zur therapeutischen Wirksamkeit und Sicherheit von Esberitox N Tabletten bei Patienten mit akutem viralem Atemwegsinfekt (Erkältung)]. Abstractband, 8. Kongreß der Gesellschaft für Phytotherapie. 1997:27.
Hill 1993 {published data only}
- Hill N, Haselen RA. Clinical trial of a homeopathic insect after‐bite treatment. HomInt R&D Newsletter 1993;3/4:4‐5. [Google Scholar]
Hill 1995 {published data only}
- Hill N, Stam C, Tuinder S, Haselen RA. A placebo‐controlled trial investigating the efficacy of a homeopathic after‐bite gel in reducing mosquito‐bite induced erythema. European Journal of Clinical Pharmacology 1995;49:103‐8. [DOI] [PubMed] [Google Scholar]
Hill 1996 {published data only}
- Hill N, Stam C, Haselen RA. The efficacy of Prrrigweg gel in the treatment of insect bites: a double‐blind, placebo‐controlled clinical trial. Pharmacy World and Science 1996;18:35‐41. [DOI] [PubMed] [Google Scholar]
Isbaniah 2011 {published data only}
- Isbaniah F, Wiyono WH, Yunus F, Setiawati A, Totzke U, Verbruggen MA. Echinacea purpurea along with zinc, selenium and vitamin C to alleviate exacerbations of chronic obstructive pulmonary disease: Results from a randomized controlled trial. Journal of Clinical Pharmacy and Therapeutics 2011;36(5):568‐76. [DOI] [PubMed] [Google Scholar]
Kim 2002b {published data only}
- Kim LS, Waters RF, Burkholder PM. Immunological activity of larch arabinogalactan and Echinacea: a preliminary, randomized, double‐blind, placebo‐controlled trial. Alternative Medicine Review 2002;7:138‐49. [PubMed] [Google Scholar]
Kleinschmidt 1965 {published data only}
- Kleinschmidt H. [Versuche zur Herabsetzung der Infektneigung bei Kleinkindern mit Esberitox]. Therapie der Gegenwart 1965;104(9):1258‐62. [PubMed] [Google Scholar]
Melchart 1995a {published data only}
- Jurcic K, Melchart D, Holzmann M, et al. Zwei Probandenstudien zur Stimulierung der Granulozytenphagozytose durch Echinacea‐Extrakt‐haltige Präparate. Zeitschrift für Phytotherapie 1989;10:67‐70. [Google Scholar]
- Melchart D, Linde K, Worku F, Sarkady L, Holzmann M, et al. Results of five randomized studies on the immunomodulatory activity of preparations of Echinacea. Journal of Alternative and Complementary Medicine 1995;1:145‐60. [DOI] [PubMed] [Google Scholar]
- Wagner H, Jurcic K, Doenicke A, Rosenhuber E, Behrens N. [Die Beeinflussung der Phagozytosefähigkeit von Granulozyten durch homöopathische Arzneipräparate. In‐vitro‐Tests und kontrollierte Einfachblindstudien]. Arzneimittel Forschung 1986;36(II):1421‐5. [PubMed] [Google Scholar]
Melchart 1995b {published data only}
- Jurcic K, Melchart D, Holzmann M, et al. [Zwei Probandenstudien zur Stimulierung der Granulozytenphagozytose durch Echinacea‐Extrakt‐haltige Präparate]. Zeitschrift für Phytotherapie 1989;10:67‐70. [Google Scholar]
- Melchart D, Linde K, Worku F, Sarkady L, Holzmann M, et al. Results of five randomized studies on the immunomodulatory activity of preparations of Echinacea. Journal of Alternative and Complementary Medicine 1995;1:145‐60. [DOI] [PubMed] [Google Scholar]
Melchart 1995c {published data only}
- Melchart D, Linde K, Worku F, Sarkady L, Holzmann M, et al. Results of five randomized studies on the immunomodulatory activity of preparations of Echinacea. Journal of Alternative and Complementary Medicine 1995;1:145‐60. [DOI] [PubMed] [Google Scholar]
Melchart 1995d {published data only}
- Melchart D, Linde K, Worku F, Sarkady L, Holzmann M, et al. Results of five randomized studies on the immunomodulatory activity of preparations of Echinacea. Journal of Alternative and Complementary Medicine 1995;1:145‐60. [DOI] [PubMed] [Google Scholar]
Melchart 1995e {published data only}
- Melchart D, Linde K, Worku F, Sarkady L, Holzmann M, et al. Results of five randomized studies on the immunomodulatory activity of preparations of Echinacea. Journal of Alternative and Complementary Medicine 1995;1:145‐60. [DOI] [PubMed] [Google Scholar]
Minetti 2011 {published data only}
- Minetti AM, Forti S, Tassone G, Torretta S, Pignataro L. Efficacy of complex herbal compound of Echinacea angustofolia (ImoviralTM Junior) in recurrent upper respiratory tract infections during pediatric age: preliminary results. Minerva Pediatrica 2011;63:177‐82. [PubMed] [Google Scholar]
Narimanian 2005 {published data only}
- Narimanian M, Badalyan M, Panosyan V, Gabrielyan E, Panossian A, Wikmann A, et al. Randomized trial of a fixed combination (KanJang) of herbal extracts containing Adhatoda vasica, Echinacea purpurea and Eleutherococcus senticosus in patients with upper respiratory tract infections. Phytomedicine 2005;12:539‐47. [DOI] [PubMed] [Google Scholar]
Naser 2005 {published data only}
- Naser B, Lund B, Henneicke‐von Zepelin HH, Köhler G, Lehmacher W, Scaglione F. A randomized, double‐blind, placebo controlled clinical dose‐response trial of an extract of Baptista, Echinacea and Thuja for the treatment of patients with common cold. Phytomedicine 2005;12:715‐22. [DOI] [PubMed] [Google Scholar]
Pohl 1970 {published data only}
- Pohl P. Zur Therapie der strahlenbedingten Leukopenie mit Esberitox. Therapie der Gegenwart 1970;109(6):902‐6. [PubMed] [Google Scholar]
Quadripur 1976 {published data only}
- Quadripur SA. [Medikamentöse Beeinflussung der Phagozytosefähigkeit der Granulozyten]. Therapie der Gegenwart 1976;115(6):1072‐8. [PubMed] [Google Scholar]
Reitz 1990 {published and unpublished data}
- Reitz HD. [Immunmodulatoren mit pflanzlichen Wirkstoffen. 2. Teil: eine wissenschaftliche Studie am Beispiel Esberitox N]. Notabene Medici 1990;20:362‐6. [Google Scholar]
Sartor 1972 {published data only}
- Sartor KJ. [Zur Wirksamkeit von Esberitox in der Behandlung strahlenbedingter Leukopenien]. Therapie der Gegenwart 1972;111(8):1147‐50. [PubMed] [Google Scholar]
Saunders 2007 {published data only}
- Saunders PR, Smith F, Schusky RW. Echinacea purpurea L. in children: safety, tolerability, compliance, and clinical effectiveness in upper respiratory tract infections. Canadian Journal of Physiology and Pharmacology 2007;85:1195‐9. [DOI] [PubMed] [Google Scholar]
Scaglione 1995 {published data only}
- Scaglione F, Lund B. Efficacy in the treatment of the common cold of a preparation containing an Echinacea extract. International Journal of Immunotherapy 1995;6:163‐6. [Google Scholar]
Schapowal 2009 {published data only}
- Schapowal A, Berger D, Klein P, Suter A. Echinacea/sage or chlorhexidine/lidocaine for treating acute sore throats: a randomized double‐blind trial. European Journal of Medical Research 2009;14:406‐12. [DOI] [PMC free article] [PubMed] [Google Scholar]
Schmidt 1990 {published data only}
- Schmidt U, Albrecht M, Schenk N. [Pflanzliches Immunstimulans senkt Häufigkeit grippaler Infekte. Plazebokontrollierte Doppelblindstudie mit einem kombinierten Echinacea‐Präparat mit 646 Studenten der Kölner Universität]. Natur‐ und Ganzheitsmedizin 1990;3(9):277‐81. [Google Scholar]
Schoop 2006a {published data only}
- Schoop R, Büechi S, Suter A. Open, multicenter study to evaluate the tolerability and efficacy of Echinaforce forte tablets in athletes. Advances in Therapy 2006;23:823‐33. [DOI] [PubMed] [Google Scholar]
Schwarz 2002 {published data only}
- Schwarz E, Metzler J, Diedrich JP, Freudenstein J, Bode C, Bode JC. Oral administration of freshly expressed juice of Echinacea purpurea herbs fail to stimulate the non‐specific immune response in healthy young men: results of a double‐blind, placebo‐controlled crossover study. Journal of Immunology 2002;25:413‐20. [DOI] [PubMed] [Google Scholar]
Spasov 2004 {published data only}
- Spasov AA, Ostrovskij OV, Chernikov MV, Wikman G. Comparative controlled study of Andrographis paniculata fixed combination, Kan Jang and an Echinacea preparation as adjuvant, in the treatment of uncomplicated respiratory disease in children. Phytotherapy Research 2004;18:47‐53. [DOI] [PubMed] [Google Scholar]
Stolze 1986 {published data only}
- Stolze H, Forth H. [Eine Antibiotikabehandlung kann durch zusätzliche Immunstimulierung optimiert werden]. Der Kassenarzt 1983;23:43‐8. [Google Scholar]
Thom 1997 {published data only}
- Thom E, Wollan T. A controlled clinical study of Kanjang mixture in the treatment of uncomplicated upper respiratory tract infections. Phytotherapy Research 1997;11:207‐10. [Google Scholar]
Timmermanns 1990 {published data only}
- Timmermanns LM, Timmermanns LG. [Mesure de l'activité des extraits d'Echinaceae et de Sabal dans le traitement des mégavessies idiopathiques chez la femme]. Acta Urologica Belgica 1990;58(2):43‐59. [PubMed] [Google Scholar]
Vonau 2001 {published data only}
- Vonau B, Chard S, Mandalia S, Wilkinson D, Barton SE. Does the extract of the plant Echinacea purpurea influence the clinical course of recurrent genital herpes. International Journal of STD and AIDS 2001;12:154‐8. [DOI] [PubMed] [Google Scholar]
Vorberg 1984 {published data only}
- Vorberg G. [Bei Erkältung unspezifische Immunabwehr stimulieren]. Ärztliche Praxis 1984;36:97‐8. [Google Scholar]
Vorberg 1989 {published and unpublished data}
- Vorberg G, Schneider B. [Pflanzliches Immunstimulans verkürzt grippalen Infekt. Doppelblindstudie belegt die Steigerung der unspezifischen Infektabwehr]. Arzmeimittel‐Forschung 1989;36:3‐8. [Google Scholar]
Wahl 2008 {published data only}
- Wahl RA, Aldous MB, Worden KA, Grant KL. Echinacea purpurea and osteopathic manipulative treatment in children with recurrent otitis media: a randomized controlled trial. BMC Complementary and Alternative Medicine 2008;8:1‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Yakoot 2011 {published data only}
- Yakoot M, Salem A. Efficacy and safety of a multiherbal formula with vitamin C and zinc (Immumax) in the management of the common cold. International Journal of General Medicine 2011;4:45‐51. [DOI] [PMC free article] [PubMed] [Google Scholar]
Zimmer 1985 {published data only}
- Zimmer M. [Gezielte konservative Therapie der akuten Sinusitis in der HNO‐Praxis]. Therapiewoche 1985;35(36):4024‐8. [Google Scholar]
References to studies awaiting assessment
Rahmati 2012 {published data only}
- Rahmati MB, Fatemeh S, Hamedi Y, Khadem AA, Rezai MS. Efficacy and safety of echinacea root extracts in the treatment ot pediatric common cold: a randomized clinical trial [ﯽﮔدرﻮﺧﺎﻣﺮﺳ نﺎﻣرد رد ﻪﺳﺎﻨﯿﮐا ﻪﺸﯾر هﺮﯿﺷ ضراﻮﻋ و ﯽﺸﺨﺑ ﺮﺛارد نﺎﮐدﻮﮐ : هﺪﺷ ﯽﻓدﺎﺼﺗ ﯽﻨﯿﻟﺎﺑ ﯽﺋﺎﻣزآرﺎﮐ ﻪﻌﻟﺎﻄﻣ ﮏﯾ]. Journal of Mazandaran University of Medical Sciences 2012;22(93):12‐8. [Google Scholar]
Additional references
Altamirano‐Dimas 2007
- Altamirano‐Dimas M, Hudson JB, Cochrane D, Nelson C, Arnason JT. Modulation of immune response gene expression by echinacea extracts: results of a gene array analysis. Canadian Journal of Physiology and Pharmacology 2007;85(11):1091‐8. [DOI] [PubMed] [Google Scholar]
Barrett 1999
- Barrett B, Vohmann M, Calabrese C. Echinacea for upper respiratory tract infection. Journal of Family Practice 1999;48:628‐35. [PubMed] [Google Scholar]
Barrett 2003
- Barrett B. Medicinal properties of Echinacea. Phytomedicine 2003;10:66‐86. [DOI] [PubMed] [Google Scholar]
Bauer 1989
- Bauer R, Remiger P, Jurcic K, Wagner H. Effect of extracts of Echinacea on phagocytic activity [Beeinflussung der Phagozytoseaktivität durch Echinacea‐Extrakte]. Zeitschrift für Phytotherapie 1989;10:43‐8. [Google Scholar]
Birt 2008
- Birt DF, Widrlechner MP, Lalone CA, Wu L, Bae J, Solco AKS, et al. Echinacea in infection. American Journal of Clinical Nutrition 2008;87(Suppl 2):488‐92. [DOI] [PMC free article] [PubMed] [Google Scholar]
Blumenthal 2005
- Blumenthal M. Herb sales down 6 percent in mainstream market. Herbal Gram 2005;66:63. [Google Scholar]
Bodinet 2002
- Bodinet C, Mentel R, Wegner U, Lindequist U, Teuscher E, Freudenstein J. Effect of oral application of an immunomodulating plant extract on influenza virus type A infection in mice. Planta Medica 2002;68:896‐900. [DOI] [PubMed] [Google Scholar]
Caruso 2005
- Caruso TJ, Gwaltney JM Jr. Treatment of the common cold with Echinacea: a structured review. Clinical Infectious Diseases 2005;40:807‐10. [DOI] [PubMed] [Google Scholar]
Chavez 2007
- Chavez M, Chacon M, Badilla B, Arevalo C. Effect of Echinacea purpurea (Asteraceae) aqueous extract on antibody response to Bothrops asper venom and immune cell response.. Revista de Biologia Tropical 2007;55(1):113‐9. [DOI] [PubMed] [Google Scholar]
Chicca 2009
- Chicca A, Raduner S, Pellati F, Strompen T, Altmann KH, Schoop R, et al. Synergistic immunopharmacological effects of N‐alkamides in Echinacea purpurea herbal extracts. International Immunopharmacology 2009;9(7‐8):850‐8. [DOI] [PubMed] [Google Scholar]
Denny 1995
- Denny FW Jr. The clinical impact of human respiratory virus infections. American Journal of Respiratory Critical Care Medicine 1995;152(4 Pt 2):S4‐12. [DOI] [PubMed] [Google Scholar]
Dickersin 1994
- Dickersin K, Scherer R, Lefebvre C. Identifying relevant studies for systematic reviews. BMJ 1994;309:1286‐91. [DOI] [PMC free article] [PubMed] [Google Scholar]
Eccles 2002
- Eccles R. An explanation for the seasonality of acute respiratory tract viral infections. Acta Oto‐Laryngologica 2002;122:183‐91. [DOI] [PubMed] [Google Scholar]
Fendrick 2003
- Fendrick AM, Monto AS, Nightengale B, Sarnes M. The economic burden of non‐influenza‐related viral respiratory tract infection in the United States. Archives of Internal Medicine 2003;163:487‐94. [DOI] [PubMed] [Google Scholar]
Fergusson 2004
- Fergusson D, Glass KC, Waring D, Shapiro S. Turning a blind eye: The success of blinding reported in a random sample of randomised, placebo controlled trials. BMJ 2004;328:432‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Gertsch 2004
- Gertsch J, Schoop R, Kuenzle U, Suter A. Echinacea alkylamides modulate TNF‐alpha gene expression via cannabinoid receptor CB2 and multiple signal transduction pathways. FEBS Letters 2004;577:563‐9. [DOI] [PubMed] [Google Scholar]
Ghaemi 2009
- Ghaemi A, Soleimanjahi H, Gill P, Arefian E, Soudi S, Hassan Z. Echinacea purpurea polysaccharide reduces the latency rate in herpes simples virus type‐1 infections. Intervirology 2009;52(1):29‐34. [DOI] [PubMed] [Google Scholar]
Gilroy 2003
- Gilroy CM, Steiner JF, Byers T, Shapiro H, Georgian W. Echinacea and truth in labeling. Archives of Internal Medicine 2003;163:699‐704. [DOI] [PubMed] [Google Scholar]
Guppy 2011
- Guppy MPB, Mickan SM, Mar CB, Thorning S, Rack A. Advising patients to increase fluid intake for treating acute respiratory infections. Cochrane Database of Systematic Reviews 2011, Issue 2. [DOI: 10.1002/14651858.CD004419.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Gurbuz 2010
- Gurbuz E, Balevi T, Kurtoglu V, Coskun B, Oznurlu Y, Kan Y, et al. Effects of Echinacea extract on the performance, antibody titres, and intestinal histology of layer chicks. British Poultry Science 2010;51(6):805‐10. [DOI] [PubMed] [Google Scholar]
Hajos 2012
- Hajos N, Holderith N, Nemeth B, Papp OI, Szabo GG, Zemankovics R, et al. The effects of an Echinacea preparation on synaptic transmission and the firing properties of CA1 pyramidal cells in the hippocampus. Phytotherapy Research 2012;26(3):354‐62. [DOI] [PubMed] [Google Scholar]
Haller 2013
- Haller J, Freund TF, Pelczer KG, Furedi J, Krecsak L, Zambori J. The anxiolytic potential and psychotropic side effects of an echinacea preparation in laboratory animals and healthy volunteers. Phytotherapy Research 2013;27(1):54‐61. [DOI] [PubMed] [Google Scholar]
Hao 2011
- Hao Q, Lu Z, Dong BR, Huang CQ, Wu T. Probiotics for preventing acute upper respiratory tract infections. Cochrane Database of Systematic Reviews 2011, Issue 9. [DOI: 10.1002/14651858.CD006895.pub2] [DOI] [PubMed] [Google Scholar]
Hemilä 2013
- Hemilä H, Chalker E. Vitamin C for preventing and treating the common cold. Cochrane Database of Systematic Reviews 2013, Issue 1. [DOI: 10.1002/14651858.CD000980.pub4] [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2003
- Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analyses. BMJ 2003;327:557‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011a
- Higgins JPT, Deeks JJ (editors). Chapter 7: Selecting studies and collecting data. In: Higgins JPT, Green S editor(s). Cochrane Handbook for Systematic Reviews of Interventions. 5.0.1. Chichester, UK: Wiley‐Blackwell, 2011. [Google Scholar]
Higgins 2011b
- Higgins JPT, Altman DG, Gotzsche PC, Jüni P, Moher D Oxman AD, et al. The Cochrane Collaboration' stool for assessing risk of bias in randomised trials. BMJ 2011;343(d5928):1‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Huntley 2005
- Huntley A, Coon JT, Ernst E. The safety of herbal medicine products derived from Echinacea species. A systematic review. Drug Safety 2005;28:387‐400. [DOI] [PubMed] [Google Scholar]
Kassel 2010
- Kassel JC, King D, Spurling GKP. Saline nasal irrigation for acute upper respiratory tract infections. Cochrane Database of Systematic Reviews 2010, Issue 10. [DOI: 10.1002/14651858.CD006821.pub2] [DOI] [PubMed] [Google Scholar]
Lalone 2009
- Lalone CA, Rizshsky L, Hammer KD, Wu L, Solco AK, Yum M, et al. Endogeneous levels of Echinacea alkylamides and ketones are important contributors to the inhibition of prostaglandin E2 and nitric oxide production in cultured macrophages. Journal of Agricultural and Food Chemistry 2009;57(19):8820‐30. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lefebvre 2011
- Lefebvre C, Manheimer E, Glanville J. Chapter 6: Searching for studies. In: Higgins JPT, Green S editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. Available from www.cochrane‐handbook.org. Chichester, UK: Wiley‐Blackwell, 2011. [Google Scholar]
Lissiman 2012
- Lissiman E, Bhasale AL, Cohen M. Garlic for the common cold. Cochrane Database of Systematic Reviews 2012, Issue 3. [DOI: 10.1002/14651858.CD006206.pub3] [DOI] [PubMed] [Google Scholar]
Matthias 2005
- Matthias A, Addison RS, Penman KG, Dickinson RG, Bone KM, Lehmann RP. Echinacea alkamide disposition and pharmacokinetics in humans after tablet ingestion. Life Sciences 2005;77(16):2018‐29. [DOI] [PubMed] [Google Scholar]
Matthias 2008
- Matthias A, Banbury L, Bone KM, Leach DN, Lehman RP. Echinacea alkamides modulate induced immune responses in T‐cells. Fitoterapia 2008;79(1):53‐8. [DOI] [PubMed] [Google Scholar]
Melchart 1994
- Melchart D, Linde K, Worku F, Bauer R, Wagner H. Immunomodulation with Echinacea ‐ a systematic review of controlled clinical trials. Phytomedicine 1994;1:245‐54. [DOI] [PubMed] [Google Scholar]
Monto 1987
- Monto AS, Bryan ER, Ohmit S. Rhinovirus infections in Tecimseh Michigan: frequency of illness and number of serotypes. Journal of Infectious Diseases 1987;156:43‐9. [DOI] [PubMed] [Google Scholar]
Mullins 2002
- Mullins RJ, Heddle R. Adverse reactions associated with Echinacea: the Australian experience. Annals of Allergy, Asthma & Immunology 2002;88:42‐51. [DOI] [PubMed] [Google Scholar]
Müller‐Jakic 1994
- Müller‐Jakic B, Breu W, Probstle A, Redl K, Greger H, Bauer R. In vitro inhibition of cyclooxygenase and 5‐lipoxygenase by alkamides from Echinacea and Achillea species. Planta Medica 1994;60:37‐40. [DOI] [PubMed] [Google Scholar]
Qiang 2013
- Qiang Z, Hauck C, McCoy JA, Widrlechner MP, Reddy MB, Murphy PA, et al. Echinacea sanguinea and Echinacea pallida extracts stimulate glucuronidation and basolateral transfer of bauer alkamides 8 and 10 and ketone 24 and inhibit P‐glycoprotein transporter in caco‐2 cells. Planta Medica 2013;79(3‐4):266‐74. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ramasahayam 2011
- Ramasahayam S, Baraka HN, Abdel Bar FM, Abuasal BS, Widrlechner MP, Sayed KA, et al. Effects of chemically characterized fractions from aerial parts of Echinacea purpurea and Echinacea angustofolia on myelopoiesis in rats. Planta Medica 2011;77(17):1883‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
RevMan 2012 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.2. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2012.
Ritchie 2011
- Ritchie MR, Gertsch J, Klein P, Schoop R. Effects of Echinaforce ® treatment on ex vivo stimulated blood cells. Phytomedicine 2011;18(10):826‐31. [DOI] [PubMed] [Google Scholar]
Sadigh‐Eteghad 2011
- Sadigh‐Eteghad S, Khayat‐Nuri H, Abadi N, Ghavami S, Golabi M, Shanebandi D. Synergetic effects of oral administration of levamisole and Echinacea purpurea on immune response in Wistar rat. Research in Veterinary Science 2011;91(1):82‐5. [DOI] [PubMed] [Google Scholar]
Schoop 2006b
- Schoop R, Klein P, Suter A, Johnston SL. Echinacea in the prevention of induced rhinovirus colds: a meta‐analysis. Clinical Therapeutics 2006;28:174‐83. [DOI] [PubMed] [Google Scholar]
Shah 2007
- Shah SA, Sander S, White CM, Rinaldi M, Coleman CI. Evaluation of echinacea for the prevention and treatment of the common cold: a meta‐analysis. Lancet Infectious Diseases 2007;7:473‐80. [DOI] [PMC free article] [PubMed] [Google Scholar]
Sharma 2006
- Sharma M, Anderson SA, Schoop R, Hudson JB. Introduction of multiple pro‐inflammatory cytokines by respiratory viruses and reversal by standardized Echinacea, a potent antiviral herbal extract. Antiviral Research 2006;83(2):165‐70. [DOI] [PubMed] [Google Scholar]
Singh 2013a
- Singh M, Das RR. Zinc for the common cold. Cochrane Database of Systematic Reviews 2013, Issue 6. [DOI: 10.1002/14651858.CD001364.pub4] [DOI] [PubMed] [Google Scholar]
Singh 2013b
- Singh M, Singh M. Heated, humidified air for the common cold. Cochrane Database of Systematic Reviews 2013, Issue 6. [DOI: 10.1002/14651858.CD001728.pub5] [DOI] [PubMed] [Google Scholar]
Timmer 2009
- Timmer A, Günther J, Rücker G, Motschall E, Antes G, Kern WV. Pelargonium sidoides extract for acute respiratory tract infections. Cochrane Database of Systematic Reviews 2009, Issue 3. [DOI: 10.1002/14651858.CD006323.pub2] [DOI] [PubMed] [Google Scholar]
Uluisik 2012
- Uluisik D, Keskin E. Effects of ginseng and echinacea on cytokine mRNA expression in rats. Scientific World Journal 2012;942025:1‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Woelkart 2005a
- Woelkart K, Xu W, Pei Y, Makriyannis A, Picone RP, Bauer R. The endocannabinoid system as a target for alkamides from Echinacea angustifolia roots. Planta Medica 2005;71:701‐5. [DOI] [PubMed] [Google Scholar]
Woelkart 2005b
- Woelkart K, Koidl C, Grisold A, Gangemi JD, Turner RB, Marth E, et al. Bioavailability and pharmacokinetics of alkamides from the roots of Echinacea angustifolia in humans. Journal of Clinical Pharmacology 2005;45(6):683‐9. [DOI] [PubMed] [Google Scholar]
Woelkart 2006
- Woelkart K, Marth E, Suter A, Schoop R, Raggam RB, Koidl C, et al. Bioavailability and pharmacokinetics of Echinacea purpurea preparations and their interaction with the immune system. International Journal of Clinical Pharmacology and Therapeutics 2006;44(9):401‐8. [DOI] [PubMed] [Google Scholar]
Woelkart 2007
- Woelkart K, Bauer R. The role of alkamides as an active principle of Echinacea. Planta Medica 2007;73:615‐23. [DOI] [PubMed] [Google Scholar]
Woelkart 2008
- Woelkart K, Dittrich P, Beubler E, Pinl F, Schoop R, Suter A, et al. Pharmacokinetics of the main alkamides after administration of three different Echinacea purpurea preparations in humans. Planta Medica 2008;74(6):651‐6. [DOI] [PubMed] [Google Scholar]
Yamada 2011
- Yamada K, Hung P, Park TK, Park PJ, Lim BO. A comparison of the immunostimulatory effects of the medical herbs Echinacea, Ashwagandha and Brahmi. Journal of Ethnopharmacology 2011;137(1):231‐5. [DOI] [PubMed] [Google Scholar]
Yu 2013
- Yu D, Yuan Y, Jiang L, Tai Y, Yang X, Hu F, et al. Anti‐inflammatory effects of essential oil in Echinacea purpurea L. Pakistan Journal of Pharmaceutical Sciences 2013;26(2):403‐8. [PubMed] [Google Scholar]
Zhai 2007
- Zhai Z, Liu Y, Wu L, Senchina DS, Wurtele ES, Murphy PA, et al. Enhancement of innate and adaptive immune functions by multiple Echinacea species. Journal of Medical Food 2007;10(3):423‐34. [DOI] [PMC free article] [PubMed] [Google Scholar]
Zhang 2010
- Zhang X, Wu T, Zhang J, Yan Q, Xie L, Liu GJ. Chinese medicinal herbs for the common cold. Cochrane Database of Systematic Reviews 2010, Issue 10. [DOI: 10.1002/14651858.CD004782.pub2] [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Linde 2006
- Linde K, Barrett B, Wölkart K, Bauer R, Melchart D. Echinacea for preventing and treating the common cold. Cochrane Database of Systematic Reviews 2006, Issue 1. [DOI: 10.1002/14651858.CD000530.pub2] [DOI] [PubMed] [Google Scholar]
Linde 2008
- Linde K, Barrett B, Bauer R, Melchart D, Wölkart K. Echinacea for preventing and treating the common cold. Cochrane Database of Systematic Reviews 2008, Issue 3. [DOI: 10.1002/14651858.CD000530.pub2] [DOI] [PubMed] [Google Scholar]
Melchart 1999
- Melchart D, Linde K, Fischer P, Kaesmayr J. Echinacea for preventing and treating the common cold. Cochrane Database of Systematic Reviews 1999, Issue 1. [DOI: 10.1002/14651858.CD000530.pub2] [DOI] [PubMed] [Google Scholar]


