Abstract
Purpose.
Acanthamoeba plasminogen activator (aPA) is a serine protease elaborated by Acanthamoeba trophozoites that facilitates the invasion of trophozoites to the host and contributes to the pathogenesis of Acanthamoeba keratitis (AK). The aim of this study was to explore if aPA stimulates proinflammatory cytokine in human corneal epithelial (HCE) cells via the protease-activated receptors (PARs) pathway.
Methods.
Acanthamoeba castellanii trophozoites were grown in peptone-yeast extract glucose for 7 days, and the supernatants were collected and centrifuged. The aPA was purified using the fast protein liquid chromatography system, and aPA activity was determined by zymography assays. Human corneal epithelial cells were incubated with or without aPA (100 μg/mL), PAR1 agonists (thrombin, 10 μM; TRAP-6, 10 μM), and PAR2 agonists (SLIGRL-NH2, 100 μM; AC 55541, 10 μM) for 24 and 48 hours. Inhibition of PAR1 and PAR2 involved preincubating the HCE cells for 1 hour with the antagonist of PAR1 (SCH 79797, 60 μM) and PAR2 (FSLLRY-NH2, 100 μM) with or without aPA. Human corneal epithelial cells also were preincubated with PAR1 and PAR2 antagonists and then incubated with or without PAR1 agonists (thrombin and TRAP-6) and PAR2 agonists (SLIGRL-NH2 and AC 55541). Expression of PAR1 and PAR2 was examined by quantitative RT-PCR (qRT-PCR), flow cytometry, and immunocytochemistry. Interleukin-8 expression was quantified by qRT-PCR and ELISA.
Results.
Human corneal epithelial cells constitutively expressed PAR1 and PAR2 mRNA. Acanthamoeba plasminogen activator and PAR2 agonists significantly upregulated PAR2 mRNA expression (1- and 2-fold, respectively) (P < 0.05). Protease-activated receptor 2 antagonist significantly inhibited aPA, and PAR2 agonists induced PAR2 mRNA expression in HCE cells (P < 0.05). Protease-activated receptor 1 agonists, but not aPA, significantly upregulated PAR1 mRNA expression, which was significantly inhibited by PAR1 antagonist in HCE cells. Acanthamoeba plasminogen activator and PAR2 agonists stimulated IL-8 mRNA expression and protein production, which is significantly diminished by PAR2 antagonist (P < 0.05). Protease-activated receptor 1 antagonist did not alter aPA-stimulated IL-8 mRNA expression and protein production in HCE cells. Flow cytometry and immunocytochemistry showed that aPA and SLIGRL-NH2 (PAR2 agonist) upregulated PAR2 surface protein as compared to that in unstimulated HCE cells. Thrombin, but not aPA, stimulated PAR1 surface protein in HCE cells.
Conclusions.
Acanthamoeba plasminogen activator specifically induces expression and production of IL-8 in HCE cells via PAR2 pathway, and PAR2 antagonists may be used as a therapeutic target in AK.
Keywords: Acanthamoeba, plasminogen activator, PAR2, PAR1, corneal epithelial cells, IL-8
This paper describes the pathogenicity of Acanthamoeba plasminogen activator that induces expression and production of IL-8 in HCE cells via PAR2 pathway, and PAR2 antagonists may be used as a therapeutic target in AK.
Introduction
Acanthamoeba keratitis (AK) is a sight-threatening corneal infection that is caused by the ubiquitous free-living species of pathogenic amoebae belonging to the genus Acanthamoeba.1 It can be found commonly in water, soil, air, cooling towers, heating/ventilating/air conditioning (HVAC) systems, and sewage systems.2,3 The first case of AK was reported by Naginton et al.4 in 1974 in the United Kingdom and shortly thereafter, in 1975, by Jones et al.5 in the United States. The incidence of this disease has been augmented with an increase in the number of contact lens (CL) wearers, with an estimated annual incidence of CL-related AK in the United States of 1 or 2 cases, in England 1.4 cases, in The Netherlands 3.06 cases, and in the west of Scotland 149 cases per million CL wearers.6 However, an increased incidence of AK has been recognized as an important cause of keratitis in non-CL lens wearers.7 It has been suggested that ocular exposure to Acanthamoeba species is more common than previously believed because trophozoites can produce mild corneal infections that escape diagnosis.8 More recently, the Centers for Disease Control and Prevention has reported that the incidence of AK has increased in several states in the United States.9 At present, diagnosis of AK is not straightforward, and therefore extreme disparities in the incidence of AK have been estimated.10,11 Treatment of AK is very demanding, consisting of hourly applications of brolene, polyhexamethylene biguanide, and chlorhexidine for several weeks. Even with such therapies, Acanthamoeba species can cause severe damage to the corneal epithelium and stroma, resulting in the need for corneal transplantation.12
Many studies have been conducted on the pathogenesis and treatment of AK; however, the pathogenesis, diagnosis, and treatment of AK are not fully explored.13–23 We have shown that Acanthamoeba trophozoites secrete a serine protease, Acanthamoeba plasminogen activator (aPA), that is involved in the pathogenesis of AK.17,18 The parasite-derived enzyme has a molecular mass of approximate 40 kDa and produces a single band of lysis on fibrinogen-agarose zymographs.17 Activity of this enzyme is completely inhibited by treatment with diisopropylfluorophosphate (DIFP), indicating that it is a serine protease; however, aPA activity is not inhibited by amiloride, which is a strong inhibitor of urokinase-type plasminogen activator. Additionally, the activity of this enzyme is not inhibited by plasminogen activator inhibitor-1, which is the primary physiological inhibitor of both urokinase and tissue-type plasminogen activator. It does not cross-react with antibodies specific for human urokinase or tissue-type plasminogen activator.17 Acanthamoeba plasminogen activator activates plasminogen from several mammalian species, including human, cow, and pig.17 Moreover, the aPA is a 40-kDa serine protease elaborated from the pathogenic, but not nonpathogenic, strains of Acanthamoeba.17,18 How aPA interacts with the corneal epithelial cells and stimulates inflammation in AK is unknown.
Protease-activated receptors belong to a unique family of G protein–coupled receptors that are cleaved at an activation site within the N-terminal exodomain by a variety of proteinases, essentially of the serine (Ser) proteinase family. After cleavage, the new N-terminal sequence functions as a tethered ligand that binds intramolecularly to activate the receptor and initiate signaling.24–28 To date, four members of the protease-activated receptors (PARs) family have been identified: PAR1, PAR2, PAR3, and PAR4.24–27 Among these, PAR1 has been implicated as a key mediator in cellular functions and is activated by the PAR1 agonist, thrombin, as well as by serine proteases, and PAR2 is activated by trypsin-like serine proteases.25–27 It has been demonstrated that PAR1 and PAR2 are involved in multiple biological as well as inflammatory and immune responses upon stimulation with thrombin and trypsin-like proteases, respectively.24–28 Recent studies have shown that human corneal and conjunctival epithelial cells express functional PAR1 and PAR2.28,29 Moreover, activation of PAR1 and PAR2 on human corneal epithelial (HCE) cells by thrombin and trypsin resulted in the secretion of proinflammatory cytokines such as IL-6, IL-8, and TNFα.28 It is not known whether the proteinase released from the microorganism can activate PARs and whether it triggers the inflammatory responses. Since serine protease, aPA, is produced by a pathogenic strain of Acanthamoeba, we hypothesized that aPA activates PAR1 or PAR2 on the corneal epithelial cells, resulting in signal transduction and production of proinflammatory cytokine IL-8 that modulates corneal inflammation in AK. In this study, we demonstrated that PAR2 mRNA and protein are upregulated by aPA and PAR2 agonists and induce IL-8 production in HCE cells.
Thus, disruption of PAR2 activity might have a major impact on preventing inflammatory responses in AK. The present study is the first to illustrate that proteinase released from the microorganism can activate PAR2 and that it triggers inflammatory responses in the corneal epithelial cells.
Materials and Methods
Amoebae and Cell Line
Acanthamoeba castellanii (ATCC 30868), isolated from a human cornea, was obtained from American Type Culture Collection (ATCC, Manassas, VA, USA). Amoebae were grown as axenic cultures in peptone-yeast extract glucose (PYG) at 35°C with constant agitation on a shaker incubator at 125 rpm.30 Human telomerase-immortalized corneal epithelial (HCE) cells31 were a generous gift from James Jester (University of California, Irvine). The HCE cells were cultured in keratinocyte medium (KGM-2 Bullet Kit; Lonza, Walkersville, MD, USA) containing 10% fetal bovine serum (HyClone, Logan, UT, USA) at 37°C in a humidified 5% CO2 atmosphere.
Acanthamoeba Plasminogen Activator
A. castellanii trophozoites were cultured for 7 days in PYG medium at 35°C, and the supernatants were collected and centrifuged as described previously.17 The aPA was purified using the fast protein liquid chromatography system (FPLC; ÄKTAFPLC, GE Healthcare Bio-Sciences AB, Uppsala, Sweden).17 Production of aPA was quantified by zymography assays,17,32 and the activity of aPA was determined by radial diffusion in fibrinogen-agarose clots.33 Protein concentrations were determined using the bicinchoninic acid (BCA) protein assay.34
HCE Cell Cultures and Treatment Experiments
Human corneal epithelial cells were cultured in 24-well plates at ∼90% confluence in KGM-2 medium and incubated with or without aPA (100 μg/mL), PAR1 agonists (thrombin, 10 μM; TRAP-6, 10 μM; Tocris Bioscience, Bristol, UK), and PAR2 agonists (SLIGRL-NH2, 100 μM; AC 55541, 10 μM; Tocris Bioscience) for 24 or 48 hours. Inhibition of PAR1 and PAR2 involved preincubating the HCE cells with the antagonist of PAR1 (SCH 79797, 60 mM; Tocris Bioscience) and PAR2 (FSLLRY-NH2, 100 μM; Tocris Bioscience). Human corneal epithelial cells were pretreated with either PAR1 or PAR2 antagonist for 1 hour and then incubated with or without aPA, thrombin, TRAP-6, SLIGRL-NH2, and AC 55541 for 24 or 48 hours. Human corneal epithelial cells without treatment with aPA and PAR1 and PAR2 agonists/antagonists served as the untreated control group.
Isolation of RNA
Human corneal epithelial cells were collected from 24-well plates at the indicated times after treatments. The total cellular RNA was extracted using TRIzol reagent (Invitrogen, Carlsbad, CA, USA) according to the manufacturer's instructions as reported previously.22 The quality of the extracted RNA was assessed spectrophotometrically using the A260/A280 ratio that in all samples was between 1.8 and 2.0, and the RNA integrity was confirmed by 1% agarose gel electrophoresis for the presence of 28S and 18S rRNA bands. Typical RNA concentrations from TRIzol RNA extraction protocol ranged between 250 and 1250 ng/μL with a 50-μL elution volume.
Real-Time Quantitative RT-PCR (qRT-PCR)
Complementary DNA was synthesized from 2 μg total RNA using the RT2 First Strand Kit (Qiagen, Valencia, CA, USA) by C1000 Thermal Cycler RT-PCR system (Bio-Rad Laboratories, Hercules, CA, USA) according to the manufacturer's protocol. Briefly, reactions were heated at 42°C for 5 minutes in a total volume of 10.0 μL in the presence of 2.0 μL 5× gDNA elimination buffer and placed on ice immediately for at least 1 minute. Then, a RT cocktail (Qiagen) consisting of 4 μL BC3 (5× first-strand buffer), 2 μL RE3 (Reverse Transcriptase Mix), 1 μL P2 (primer and external control mix), and 3 μL nuclease-free water was added. The mixture was then incubated at 42°C for exactly 15 minutes; then the reaction was immediately stopped by heating to 95°C for 5 minutes. RNase-free water (91 μL) was added to each 20 μL cDNA synthesis reaction.
Polymerase chain reaction was performed using the CFX Connect Real-Time System (Bio-Rad Laboratories) with SYBR green fluorescent dye. The reactions were conducted in a total volume of 25 μL containing 12.5 μL 2× RT2 SYBR Green Mastermix (Qiagen), 2.5 μL sense and 2.5 μL antisense primers of 0.5 μM, 5 μL cDNA, and 2.5 μL RNase-free water, all in Low Tube Strip, CLR (0.2 mL, Bio-Rad Laboratories). After PCR, melting curves were acquired stepwise from 65°C to 95°C to ensure that a single product was amplified in the reaction. Data were calculated using the 2−ΔΔCT method.35 The primers used in this study are listed in the Table.
Table.
Sequences of Oligonucleotide Primers
|
Primers |
|
Primer Sequence |
PCR Product |
| PAR1 | Sense: | 5′-CACCGGAGTGTTTGTAGTCA-3′ | 864 bp |
| Antisense: | 5′-TAACTGCTGGGATCGGAACT-3′ | ||
| PAR2 | Sense: | 5′-GTTGATGGCACATCCCACGTC-3′ | 865 bp |
| Antisense: | 5′-GTACAGGGCATAGACATGGC-3′ | ||
| IL-8 | Sense: | 5′-ATGACTTCCAAGCTGGCCGTGGCT-3′ | 289 bp |
| Antisense: | 5′-TCTCAGCCCTCTTCAAAAACTTCTC-3′ | ||
| GAPDH | Sense: | 5′-ACCACAGTCCATGCCATCAC-3′ | 450 bp |
| Antisense: | 5′-TCCACCACCCTGTTGCTGTA-3′ |
All primers were verified by BLAST (Basic Local Alignment Search Tool, available in the public domain at http://blast.ncbi.nlm.nih.gov/Blast.cgi). Search of the National Center for Biotechnology Information (NCBI) database demonstrated that these primers amplify human PAR1, PAR2, IL-8, and GAPDH gene products. All primers were from Integrated DNA Technologies, Inc., Commercial Park (Coralville, IA, USA).
Flow Cytometric Analysis
Human corneal epithelial cells were cultured in six-well plates (1 × 106 cells/well) with and without aPA (100 μg/mL), PAR1 agonist (thrombin, 10 μM), and PAR2 agonist (SLIGRL-NH2, 100 μM) for 24 hours. Human corneal epithelial cells without treatment with aPA, thrombin, and SLIGRL-NH2 served as control untreated group. Cells were processed for PAR1 and PAR2 surface protein expression at indicated times after treatments. Briefly, cells were incubated with Fc receptor blocking antibodies (Human TruStain FcX; BioLegend, San Diego, CA, USA) in 0.5% BSA at 4°C for 30 minutes. Cells were then immunostained for 30 minutes in the dark with the following antibodies: PE-labeled mouse IgG2b anti-human PAR1 and PE-labeled mouse IgG2a anti-human PAR2 (R&D Systems, Minneapolis, MN, USA). As a control, cells were stained with the appropriately matched antibodies, PE-labeled mouse IgG2a and PE-labeled mouse IgG2b (BioLegend). The samples were analyzed by Beckman Coulter Cytomics FC500, Flow Cytometry Analyzer (Beckman Coulter, Inc., Miami, FL, USA), and results were processed using CXP2.2 analysis software (Beckman Coulter). For each sample, 50,000 ungated events were acquired. Protease-activated receptor 1 and PAR2 expression in untreated HCE cells was compared with that in treated HCE cells. The results were expressed in normalized median fluorescence intensity (nMFI)36 of positively stained HCE cells with PE-labeled antibody subtracted from MFI of unstained HCE cells, nMFI = MFIPositive − MFINegative.
Immunocytochemistry Method
Human corneal epithelial cells were grown to confluence in four-well chamber slides (Thermo Fisher, Rochester, NY, USA). Human corneal epithelial cells were stimulated with aPA (100 μg/mL), PAR1 agonist (thrombin, 10 μM), and PAR2 agonist (SLIGRL-NH2, 100 μM) for 24 hours at 37°C. Human corneal epithelial control cells were left untreated for 24 hours at 37°C. After the incubation period, cells were fixed with 4% paraformaldehyde, washed with PBS, blocked with 5% goat serum (Vector Laboratories, Inc., Burlingame, CA, USA) in phosphate-buffered saline (PBS), washed, and then incubated with primary antibodies, including polyclonal rabbit anti-human PAR1 antibody and anti-PAR2 antibody (Alomone Labs, Jerusalem, Israel). Anti-PAR1 antibody was diluted 1:250 in blocking solution at 0.8 mg/mL, and anti-PAR2 antibody was diluted 1:500 in blocking solution at 0.6 mg/mL overnight at 4°C. Human PAR1 (61–76) peptide and rat PAR2 (368–382) peptide (antibody control antigen; Alomone Labs) were used as absorption control, to demonstrate that PAR1 and PAR2 antibodies were binding specifically to the antigen of interest. Primary antibodies (anti-PAR1 and anti-PAR2) were preincubated with the control antigen (10:1 [molar ratio] working dilution of antigen to antibody mixture) for 2 hours at 37°C. The preabsorbed antibody was then incubated with HCE cells in place of the primary antibody alone, overnight at 4°C. The slides were then washed three times with 1% goat serum in PBS. A secondary antibody (Alexa Fluor 488-conjugated goat anti-rabbit antibody; BioLegend) was diluted with blocking solution (1:500) and incubated on the slides for 2 hours at 37°C in a lightproof chamber. Cells without primary antibody incubation were used as a negative control. The sections were counterstained for 3 minutes in 300 ng 4,6-diamidino-2-phenylindole, dilactate (DAPI; Calbiochem, Darmstadt, Germany) for nuclei staining and washed with PBS. Three slides in each group were viewed using fluorescence microscopy. Images were captured with an Olympus AX70 Fluorescence Microscope (Olympus Optical Co. Ltd., Tokyo, Japan).
Enzyme-Linked Immunosorbent Assay (ELISA)
Interleukin-8 was quantified from cell supernatants using ELISA.22,37 Briefly, cell culture supernatants were collected at the indicated times after treatments and centrifuged at 2000g for 10 minutes at 4°C to remove cell debris. Level of IL-8 was determined by ELISA test kit (R&D Systems) according to the manufacturer's instructions. The absorbance was measured at 450 nm using a microplate reader (Gen51.10; BioTek Instruments, Inc., Winooski, VT, USA). The minimum detectable level of IL-8 by ELISA was 3.5 pg/mL. The results were expressed in pg/mg protein of IL-8.
Statistics
All experiments were performed in triplicate, and results are presented as mean ± SEM. Differences between two groups were determined by unpaired Student's t-test. P values < 0.05 were considered statistically significant.
Results
Protease-Activated Receptor PAR2, but Not PAR1, Is Upregulated by Acanthamoeba Plasminogen Activator in HCE Cells
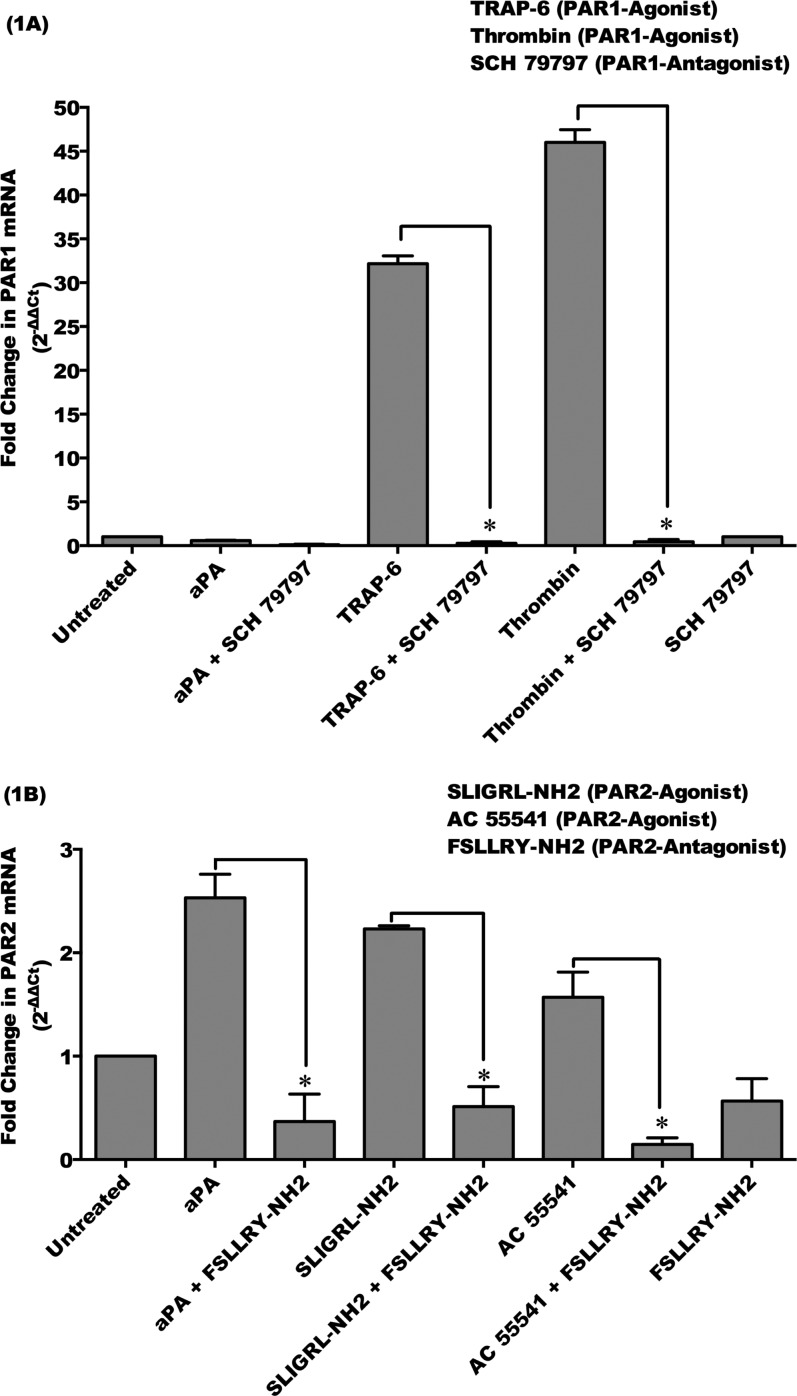
To determine whether PAR1 and PAR2 are involved in aPA-induced stimulation in corneal epithelial cells, HCE cells were incubated with or without aPA (100 μg/mL), PAR1 agonists (thrombin, 10 μM; TRAP-6, 10 μM), and PAR2 agonists (SLIGRL-NH2, 100 μM; AC 55541, 10 μM) for 24 hours. Inhibition of PAR1 and PAR2 involved preincubating the HCE cells for 1 hour with the antagonist of PAR1 (SCH 79797, 60 μM) or PAR2 (FSLLRY-NH2, 100 μM) with or without aPA. Human corneal epithelial cells also were preincubated with PAR1 or PAR2 antagonists and then incubated with or without PAR1 agonists (thrombin and TRAP-6) and PAR2 agonists (SLIGRL-NH2 and AC 55541) for 24 hours. Expression of PAR1 and PAR2 was examined by qRT-PCR (Figs. 1A, 1B).
Figure 1.
Protease-activated receptor (PAR) 2, but not PAR1, is upregulated by Acanthamoeba plasminogen activator in HCE cells. HCE cells were incubated with aPA (100 μg/mL), PAR1 agonists (thrombin, 10 μM; TRAP-6, 10 μM), and PAR2 agonists (SLIGRL-NH2, 100 μM; AC 55541, 10 μM) for 24 hours. Inhibition of PAR1 and PAR2 involved preincubating the HCE cells for 1 hour with the antagonist of PAR1 (SCH 79797, 60 μM) and PAR2 (FSLLRY-NH2, 100 μM) and then incubating with or without aPA, PAR1 agonists, and PAR2 agonists for 24 hours. Total RNA was isolated and assessed using qRT-PCR for mRNA expression of PAR1 (A) and PAR2 (B). Relative fold change of mRNA expression was quantified by 2−ΔΔCt method.35 The data are mean ± SEM of three independent experiments (*P < 0.05). P values were obtained by unpaired Student's t-test.
Acanthamoeba plasminogen activator significantly upregulated a PAR2 mRNA expression (2.5-fold) that was significantly inhibited by PAR2 antagonist, FSLLRY-NH2, in HCE cells (P < 0.05) (Fig. 1B). However, aPA did not stimulate PAR1 mRNA expression in HCE cells (Fig. 1A). Protease-activated receptor 1 and PAR2 mRNA were significantly upregulated by PAR1 agonists and PAR2 agonists, respectively, in HCE cells as compared to untreated HCE cells (P < 0.05). Upregulated transcripts of PAR1 and PAR2 by their specific agonists were significantly inhibited by PAR1 antagonist (SCH 79797) and PAR2 antagonist (FSLLRY-NH2), respectively (P < 0.05) (Figs. 1A, 1B). These results demonstrate that HCE cells express both PAR1 and PAR2 mRNA; however, serine aPA upregulates PAR2 mRNA in HCE cells that was inhibited by specific PAR2 inhibitor.
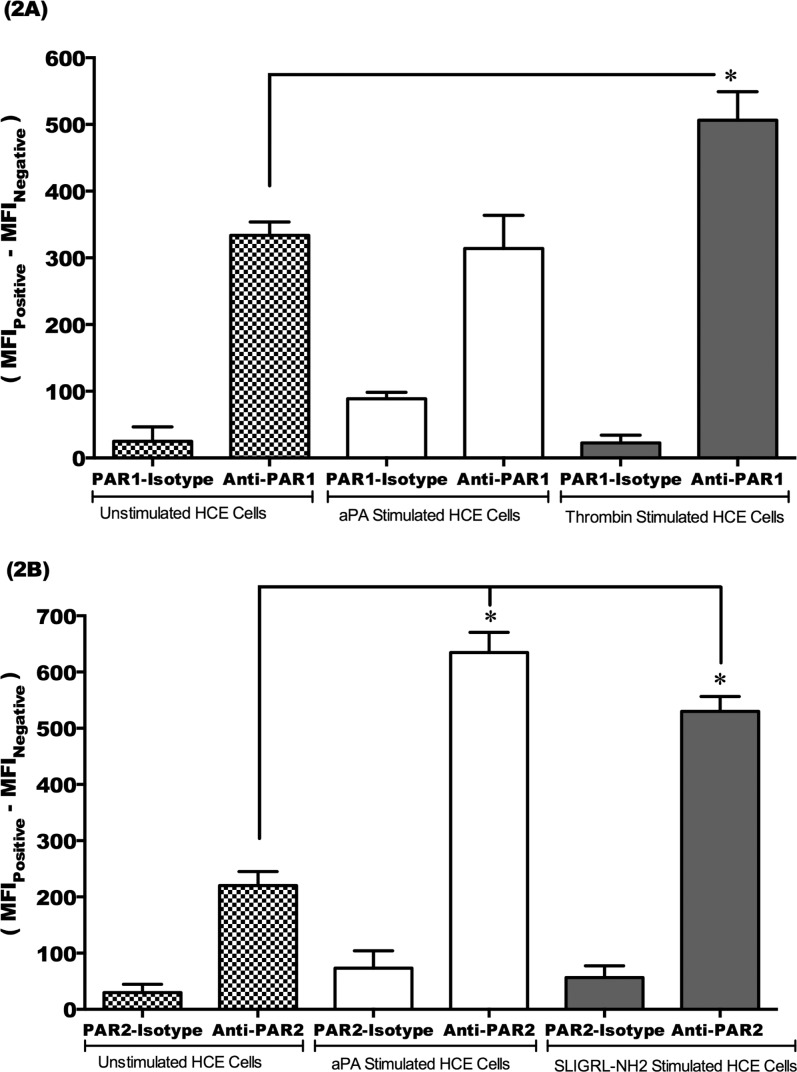
Acanthamoeba Plasminogen Activator Stimulates PAR2 Surface Protein Expression in HCE Cells
To confirm that aPA specifically activates PAR2, but not PAR1, in HCE cells, HCE cells were incubated with or without aPA, PAR1 agonist (thrombin), and PAR2 agonist (SLIGRL-NH2) for 24 hours. Surface protein expression of PAR1 and PAR2 in HCE cells was determined by flow cytometry (Fig. 2). Thrombin, but not aPA, stimulated PAR1 surface protein (1.5-fold change in nMFI) as compared to unstimulated HCE cells stained with an anti-PAR1 antibody (P < 0.05) (Fig. 2A). Flow cytometry results showed that aPA and SLIGRL-NH2 (PAR2 agonist) upregulated PAR2 surface protein (2.5–3.5-fold change in nMFI) as compared to unstimulated HCE cells stained with anti-PAR2 antibody (P < 0.05) (Fig. 2B). Protease-activated receptor 1 and PAR2 isotype control antibodies served as negative control for all experiments (Figs. 2A, 2B). These results suggest that aPA specifically stimulates PAR2 in HCE cells.
Figure 2.
PAR2 surface protein expression is upregulated by aPA in HCE cells. HCE cells were incubated with or without aPA (100 μg/mL), PAR1 agonist (thrombin, 10 μM), and PAR2 agonist (SLIGRL-NH2, 100 μM) for 24 hours. PAR1 (A) and PAR2 (B) surface protein expression in HCE cells was examined by flow cytometry. Cells were incubated with PE-labeled mouse IgG2b anti-human PAR1, PE-labeled mouse IgG2a anti-human PAR2, and isotype control (PE-labeled mouse IgG2a and IgG2b) antibody. PAR1 and PAR2 expression in untreated HCE cells was compared with that in treated HCE cells. The results were expressed as normalized median fluorescence intensity (nMFI)36 units of positively stained HCE cells with PE-labeled antibody subtracted from MFI of unstained HCE cells, MFIPositive − MFINegative. The data are mean ± SEM of three independent experiments (*P < 0.05). P values were obtained by unpaired Student's t-test.
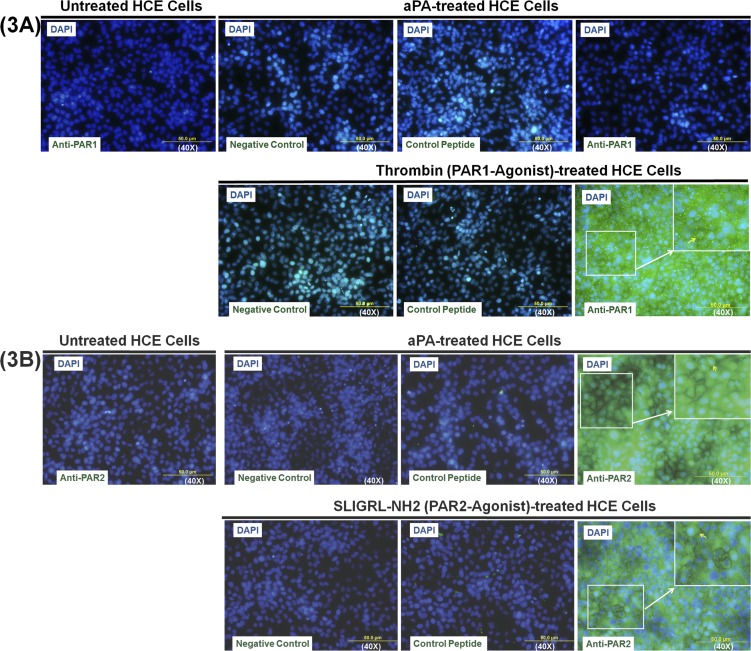
Acanthamoeba Plasminogen Activator–Induced Upregulation of PAR2 Surface Expression in HCE Cells
Immunocytochemistry was used to confirm the cell surface expression of PAR1 and PAR2 on HCE cells by aPA stimulation. To establish the positive distribution of PAR1 and PAR2 on HCE cell surfaces, HCE cells were treated with thrombin (PAR1 agonist) and SLIGRL-NH2 (PAR2 agonist). In control (untreated) HCE cells, few cells expressed PAR1 and PAR2 on the cell membrane (Fig. 3). Thrombin- but not aPA-treated HCE cells showed PAR1 staining on the cell surface; however, PAR1 control peptide (human PAR1 [61–76] peptide) neutralized the PAR1 cell surface expression on HCE cells treated with thrombin. These findings indicated that thrombin, but not aPA, stimulates PAR1 expression on HCE cell surface and demonstrated that aPA is not a PAR1 ligand like thrombin (Fig. 3A). Acanthamoeba plasminogen activator– or SLIGRL-NH2 (PAR2 agonist)–treated HCE cells showed PAR2 staining on cell surface as compared to negative control (staining without anti-PAR2 antibody) or untreated HCE cells. Protease-activated receptor 2 control peptide (rat PAR2 [368–382] peptide) neutralized the PAR2 cell surface expression on HCE cells treated with aPA or SLIGRL-NH2. These results reveal that aPA functions similarly to PAR2 agonist (Fig. 3B). Collectively, these results indicate that aPA stimulates HCE cells via PAR2 pathway (Fig. 3).
Figure 3.
Acanthamoeba plasminogen activator–induced upregulation of surface PAR2 expression in HCE cells. HCE cells were incubated with or without aPA (100 μg/mL), PAR1 agonist (thrombin, 10 μM), and PAR2 agonist (SLIGRL-NH2, 100 μM) for 24 hours. PAR1 (A) and PAR2 (B) surface protein expression in HCE cells was examined by immunocytochemistry using polyclonal rabbit anti-human PAR1 antibody, anti-PAR2 antibody, and Alexa Fluor 488-conjugated anti-rabbit antibody. Cells without primary antibody incubation were used as a negative control. Human PAR1 (61–76) peptide and rat PAR2 (368–382) peptide were used as absorption control (to demonstrate that PAR1 and PAR2 antibody are binding specifically to the antigen of interest). DAPI counterstaining was used to visualize cell location and morphology. Three slides in each group were viewed using fluorescence microscopy. Images were captured with an Olympus AX70 upright compound microscope.
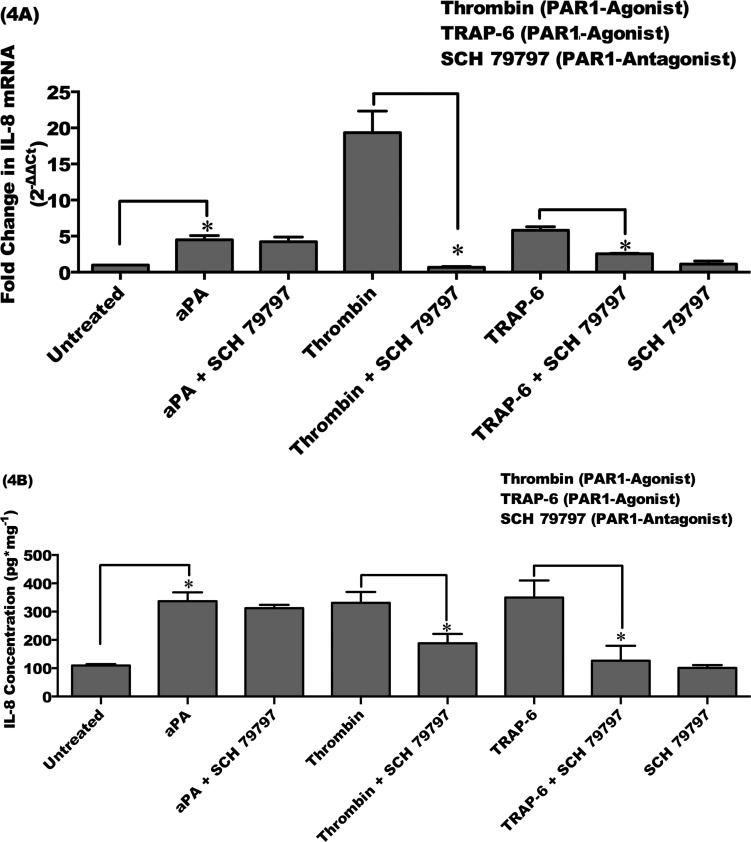
Acanthamoeba Plasminogen Activator Upregulates Proinflammatory Cytokine IL-8 Expression in HCE Cells by PAR2 Pathway
It has been shown that activation of PAR1 and PAR2 on HCE cells by thrombin and trypsin resulted in the secretion of proinflammatory cytokines such as IL-6, IL-8, and TNFα.28 We aimed to examine whether PAR1 or PAR2 is involved in the induction of IL-8 by aPA in HCE cells. Human corneal epithelial cells were incubated with or without aPA (100 μg/mL) and PAR1 agonists (thrombin, 10 μM; TRAP-6, 10 μM) for 48 hours. Human corneal epithelial cells were also incubated with or without aPA (100 μg/mL) and PAR2 agonists (SLIGRL-NH2, 100 μM; AC 55541, 10 μM) for 24 hours. For the inhibition assay, cells were preincubated with PAR1 or PAR2 antagonist for 1 hour as described in Materials and Methods. Human corneal epithelial cells also were preincubated with PAR1 and PAR2 antagonist and then incubated with or without aPA, PAR1, and PAR2 agonists for 24 or 48 hours. Interleukin-8 mRNA expression and protein production in HCE cells were measured by real-time qRT-PCR and ELISA, respectively.
Preincubation of HCE cells with SCH 79797 (PAR1 antagonist) did not abolish IL-8 mRNA expression and protein production, which is significantly induced by aPA as compared to that in untreated HCE cells (P < 0.05). However, PAR1 antagonist (SCH 79797) significantly inhibited IL-8 transcription and protein production induced by PAR1 agonists (thrombin and TRAP-6) (P < 0.05). Treatment with PAR1 antagonist alone had no significant effect on IL-8 expression in HCE cells (Figs. 4A, 4B).
Figure 4.
Acanthamoeba plasminogen activator–upregulated IL-8 is not diminished by PAR1 antagonist. HCE cells were incubated with aPA (100 μg/mL) and PAR1 agonists (thrombin, 10 μM; TRAP-6, 10 μM) for 48 hours. Inhibition of PAR1 involved preincubating the HCE cells for 1 hour with the PAR1 antagonist (SCH 79797, 60 μM) and then incubating with or without aPA or PAR1 agonists for 48 hours. (A) Total RNA was isolated and assessed using qRT-PCR for mRNA expression of IL-8. Relative fold change of mRNA expression was quantified by 2−ΔΔCt method.35 (B) Supernatants were collected from harvested cells and subjected to IL-8 ELISA. The data are mean ± SEM of three independent experiments (*P < 0.05). P values were obtained by unpaired Student's t-test.
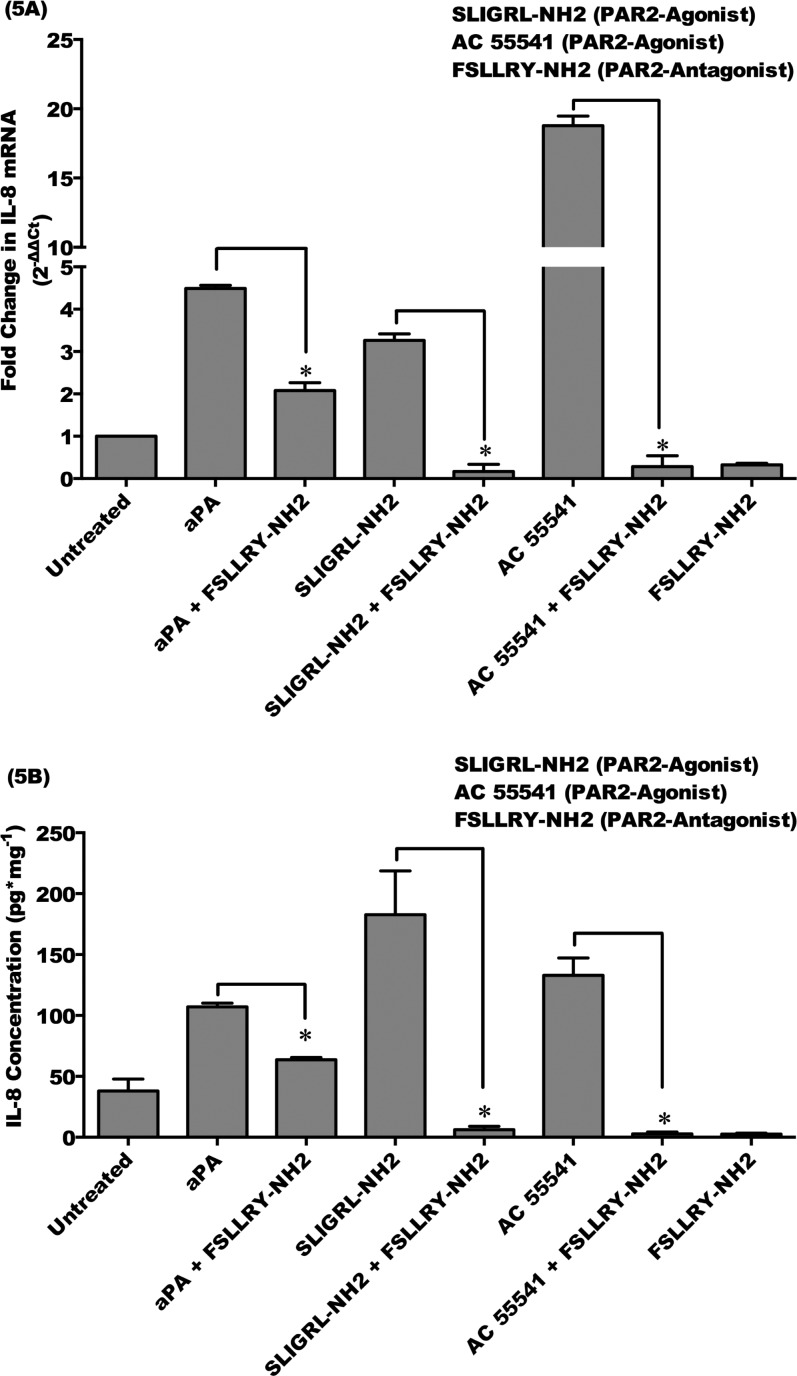
Acanthamoeba plasminogen activator and PAR2 agonist (SLIGRL-NH2 and AC 55541) treatment significantly upregulated IL-8 mRNA expression and protein production in HCE cells as compared to untreated HCE cells (P < 0.05). FSLLRY-NH2 (PAR2 antagonist) significantly attenuated IL-8 mRNA expression and protein production induced by aPA (P < 0.05). Moreover, pretreatment of HCE cells with FSLLRY-NH2 (PAR2 antagonist) significantly inhibited IL-8 mRNA expression and protein production induced by PAR2 agonist (SLIGRL-NH2 and AC 55541) (P < 0.05). Treatment with a PAR2 inhibitor alone had no significant effect on IL-8 expression in HCE cells (Figs. 5A, 5B). These results suggest that aPA induces expression and production of proinflammatory cytokine, IL-8, in HCE cells by PAR2 pathway, but not by PAR1 pathway.
Figure 5.
Acanthamoeba plasminogen activator upregulates IL-8 by PAR2 pathway in HCE cells. HCE cells were incubated with aPA (100 μg/mL) and PAR2 agonists (SLIGRL-NH2, 100 μM; AC 55541, 10 μM) for 24 hours. Inhibition of PAR2 involved preincubating the HCE cells for 1 hour with the PAR2 antagonist (FSLLRY-NH2, 100 μM) and then incubating with or without aPA and PAR2 agonists for 24 hours. (A) Total RNA was isolated and assessed using qRT-PCR for mRNA expression of IL-8. Relative fold change of mRNA expression was quantified by 2−ΔΔCt method.35 (B) Supernatants were collected from harvested cells and subjected to IL-8 ELISA. The data are mean ± SEM of three independent experiments (*P < 0.05). P values were obtained by unpaired Student's t-test.
Discussion
Acanthamoeba keratitis is the consequence of inflammation and tissue damage mediated by factors (preliminary proteases, MIP-133 and aPA) elaborated by pathogenic, but not nonpathogenic, species of Acanthamoeba.17–23 The role of MIP-133 in pathogenesis of AK has been extensively explored18–23; however, pathogenic strains of Acanthamoeba utilize aPA to facilitate invasion of the corneal cells.17 Acanthamoeba plasminogen activator has been characterized a serine protease that produces a single band of lysis on fibrinogen-agarose zymography and is capable of degrading human fibrinogen in the presence of plasminogen.17 Many proteases secreted by parasitic protozoan species have been known to play an important role in the pathogenesis of diseases; however, their precise mechanisms of action at the molecular level are only beginning to emerge.38 Pseudomonas aeruginosa virulence serine protease IV has also been demonstrated to play an important role in bacterial keratitis.39,40 We have demonstrated that the pathogenic potential of Acanthamoeba species is closely correlated with its secreted protease, aPA, which facilitates penetration of trophozoites through the basement membrane.18
It is known that plasminogen activators play an important role in corneal biology,41–45 and PARs are specific targets to initiate protease-mediated inflammation.24,26 Proteases cleave PARs molecules at a specific site on the extracellular N-terminal domain and release a new N-terminal domain for the receptor, which acts as a tethered ligand, binding to the second extracellular loop of the receptor to induce intracellular signaling.27 Importantly, pathogen-derived metalloproteinase, P. aeruginosa elastase (EPa), potentially silences the function of PAR2 in the respiratory tract and alters the host's innate defense mechanisms and respiratory functions, thus contributing to pathogenesis of a disease like cystic fibrosis.46 In this regard, we were particularly interested to determine if the Acanthamoeba-derived serine proteinase, aPA, contributes to the pathogenesis of AK by modulating the activity of PAR1 or PAR2.
Our results indicate that PAR1 and PAR2 transcripts were constitutively expressed in HCE cells and that aPA induced significant upregulation of PAR2 but not PAR1 transcript in the corneal epithelial cells. We have shown that HCE cells stimulated with PAR2 agonists (SLIGRL-NH2 and AC 55541) and PAR1 agonists (thrombin and TRAP-6) resulted in upregulation of PAR2 and PAR1 mRNA, respectively. Moreover, FSLLRY-NH2 (PAR2 antagonist), but not SCH 79797 (PAR1 antagonist), effectively inhibits PAR2 mRNA expression induced by aPA. These results indicate that aPA activates PAR2 similarly to PAR2 agonist. Likewise, upregulated transcripts of PAR1 and PAR2 by their specific agonists were significantly inhibited by PAR1 antagonist (SCH 79797) and PAR2 antagonist (FSLLRY-NH2), respectively. Our current studies are largely in agreement with those of Lang et al.,28 who reported that HCE cells express functional PAR1 and PAR2, and thrombin and trypsin activate PAR1 and PAR2 on HCE cells.
Furthermore, HCE cells stimulated with aPA and specific PAR1 and PAR2 agonist demonstrated that PAR1 and PAR2 are immunolocalized on cell surface. Thrombin (PAR1 agonist), but not aPA, stimulates PAR1 expression on HCE cell surface, which demonstrated that aPA is not a PAR1 ligand like thrombin. Acanthamoeba plasminogen activator– or SLIGRL-NH2 (PAR2 agonist)–treated HCE cells induced upregulation of PAR2 expression on cell surface as compared to negative control. These results indicate that aPA is recognized by PAR2 and acts like a PAR2 agonist. Immunolocalization of PAR1 and PAR2 in HCE cells agrees with surface protein expression of these receptors in whole human cornea28; however, in our study, receptors expression was observed upon stimulation by specific agonists while whole human cornea expressed PAR1 and PAR2 immunoreactivity on the apical surface of the most superficial corneal epithelial cells in normal condition.28
It is demonstrated that thrombin and trypsin are multifunctional serine proteinases and that they stimulate proinflammatory cytokines by PAR1 and PAR2 pathways, respectively.28,47–49 We determined the functional activity of Acanthamoeba proteinase, aPA, in HCE cells. Acanthamoeba plasminogen activator stimulated proinflammatory cytokine IL-8 by PAR2 pathway, but not by PAR1 pathway. Moreover, treatments with aPA, PAR2 agonists (SLIGRL-NH2 and AC 55541), and PAR1 agonists (thrombin and TRAP-6) significantly upregulated IL-8 mRNA expression and protein production in HCE cells; PAR2-specific antagonist, FSLLRY-NH2, significantly inhibited IL-8 mRNA expression and protein production stimulated by aPA and PAR2 agonists (SLIGRL-NH2 and AC 55541). In contrast, PAR1 antagonist, SCH 79797, inhibited IL-8 mRNA expression and protein production induced by PAR1 agonists, but not by aPA. These results suggest that aPA induces expression and production of proinflammatory cytokine IL-8 in HCE cells by PAR2 pathway, but not by PAR1 pathway. It is unlikely that the production of IL-8 induced by aPA is due to activation of TLR2 and TLR4 receptors on HCE cells. We have shown that PAR2-specific antagonist, FSLLRY-NH2, inhibited IL-8 production in HCE cells stimulated with aPA. Moreover, aPA-stimulated IL-8 mRNA expression is not inhibited by PAR1 antagonist, SCH 79797, in HCE cells. Our results are in agreement with those of Lang et al.,28 who demonstrated that trypsin (PAR1 agonist) and SLIGRL-NH2 (PAR2 agonist) specifically induced secretion of proinflammatory cytokines such as IL-6, IL-8, and TNFα protein in HCE cells. Other microorganisms such as Serratia marcescens (gram-negative enteric bacterium)– and Porphyromonas gingivalis–derived serine proteases induced activation of PAR2 and promoted inflammatory responses in vitro and in vivo.50,51 It is possible that other chemokines and cytokines are involved in inflammatory responses in AK. However, in this study we focused on IL-8 production since we have shown that CXCL2 (IL-8 equivalent in rodents) is the chemokine that plays a major role in attracting inflammatory cells such as neutrophils at the site of infection in a Chinese hamster model of AK.52
Our results also demonstrated that FSLLRY-NH2 (PAR2 antagonist) significantly attenuates IL-8 mRNA expression and protein secretion in HCE cells induced by aPA and PAR2 agonist (SLIGRL-NH2 and AC 55541); however, no significant inhibition was observed on IL-8 secretion by SCH 79797 (PAR1 antagonist) in HCE cells stimulated by aPA. These results suggest that aPA plays a role in inflammatory and pathogenesis of AK through a PAR2 pathway.
Trypsin or PAR2 agonists (SLIGRL-NH2 and AC 55541) appear to be the specific stimulator of PAR2 in many systems and activate PAR2 in a variety of cells including HCE cell lines28,53–55 as well as leading to proinflammatory cytokine production.28 In this regard, PAR1 and PAR2 may be important in inflammatory eye diseases. It is possible that PAR3 and PAR4 are involved in recognition of aPA on corneal epithelial cells; however, PAR3 and PAR4 have not been reported in HCE cells.28
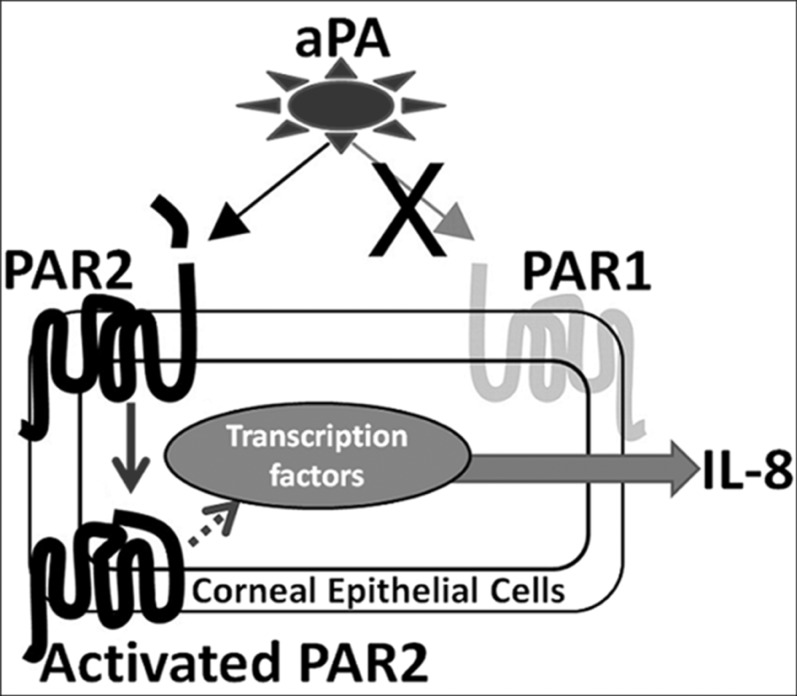
In summary (Fig. 6), our data indicate that PAR2 activation via aPA leads to upregulation of IL-8 and that aPA induces pathogenesis of AK by PAR2 pathway. Protease-activated receptor 2 antagonists may be used as a therapeutic target in AK.
Figure 6.
Schematic representation of aPA-induced proinflammatory cytokine IL-8 by PAR2 pathway in HCE cells. Acanthamoeba plasminogen activator activates PAR2, but not PAR1, in corneal epithelial cells, which upregulates IL-8 transcript and protein production. FSLLRY-NH2 (an antagonist of PAR2) inhibits aPA-induced PAR2 activation and diminishes IL-8 expression in HCE cells. PAR2 antagonists may be an important therapeutic target in Acanthamoeba keratitis.
Acknowledgments
Supported by Public Health Service Grant EY09756 from the National Institutes of Health.
Disclosure: T. Tripathi, None; M. Abdi, None; H. Alizadeh, None
References
- 1. Clarke DW, Niederkorn JY. The pathophysiology of Acanthamoeba keratitis. Trends Parasitol. 2006; 22: 175–180 [DOI] [PubMed] [Google Scholar]
- 2. Centers for Disease Control and Prevention. Acanthamoeba keratitis multiple states, 2005–2007. MMWR Morb Mortal Wkly Rep. 2007; 56: 532–534 [PubMed] [Google Scholar]
- 3. Kettesy B, Modis L Jr, Berta A, Kemeny-Beke A. Keratoplasty in contact lens related Acanthamoeba keratitis. Mosca L. ed Keratoplasties - Surgical Techniques and Complications. Rijeka, Croatia: InTech Europe; 2012: 31–52 [Google Scholar]
- 4. Naginton J, Watson PG, Playfair TJ, et al. Amoebic infection of the eye. Lancet. 1974; 2: 1537–1540 [DOI] [PubMed] [Google Scholar]
- 5. Jones BR, Visvesvara GS, Robinson NM. Acanthamoeba polyphaga keratitis and Acanthamoeba uveitis associated with fatal meningoencephalitis. Trans Ophthalmol Soc U K. 1975; 95: 221–232 [PubMed] [Google Scholar]
- 6. Radford CF, Minassian DC, Dart JK. Acanthamoeba keratitis in England and Wales: incidence, outcome, and risk factors. Br J Ophthalmol. 2002; 86: 536–542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bharathi MJ, Srinivasan M, Ramakrishnan R, et al. A study of the spectrum of AK: a three-year study at a tertiary eye care referral center in South India. Indian J Ophthalmol. 2007; 55: 37–42 [DOI] [PubMed] [Google Scholar]
- 8. Mathers WD, Sutphin JE, Folberg R, Meier PA, Wenzel RP, Elgin RG. Outbreak of keratitis presumed to be caused by Acanthamoeba. Am J Ophthalmol. 1996; 121: 129–142 [DOI] [PubMed] [Google Scholar]
- 9. Verani JR, Lorick SA, Yoder JS, et al. National outbreak of Acanthamoeba keratitis associated with use of a contact lens solution, United States. Emerg Infect Dis. 2009; 15: 1236–1242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Panjwani N. Pathogenesis of Acanthamoeba keratitis. Ocul Surf. 2010; 8: 70–79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Seal DV. Acanthamoeba keratitis update-incidence, molecular epidemiology and new drugs for treatment. Eye (Lond). 2003; 17: 893–905 [DOI] [PubMed] [Google Scholar]
- 12. Alizadeh H, Niederkorn JY, McCulley JP. Acanthamoeba keratitis. Pepose JS, Holland GN, Wilhelmus KR. eds Ocular Infection and Immunity. St. Louis, Missouri: Mosby; 1996: 1062–1071 [Google Scholar]
- 13. Yang Z, Cao Z, Panjwani N. Pathogenesis of Acanthamoeba keratitis: carbohydrate-mediated host-parasite interactions. Infect Immun. 1997; 65: 439–445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Vemuganti GK, Sharma S, Athmanathan S, Garg P. Keratocyte loss in Acanthamoeba keratitis: phagocytosis, necrosis or apoptosis? Indian J Ophthalmol. 2000; 48: 291–294 [PubMed] [Google Scholar]
- 15. Na BK, Kim JC, Song CY. Characterization and pathogenetic role of proteinase from Acanthamoeba castellanii. Microb Pathog. 2001; 30: 39–48 [DOI] [PubMed] [Google Scholar]
- 16. Garate M, Marchant J, Cubillos I, Cao Z, Kban AN, Panjwani N. In vitro pathogenicity of Acanthamoeba is associated with the expression of the mannose-binding protein. Invest Ophthalmol Vis Sci. 2006; 47: 1056–1062 [DOI] [PubMed] [Google Scholar]
- 17. Mitra MM, Alizadeh H, Gerard RD, Niederkorn JY. Characterization of a plasminogen activator produced by Acanthamoeba castellanii. Mol Biochem Parasitol. 1995; 73: 157–164 [DOI] [PubMed] [Google Scholar]
- 18. Alizadeh H, Neelam S, Niederkorn JY. Effect of immunization with the mannose-induced Acanthamoeba protein and Acanthamoeba plasminogen activator in mitigating Acanthamoeba keratitis. Invest Ophthalmol Vis Sci. 2007; 48: 5597–5604 [DOI] [PubMed] [Google Scholar]
- 19. Hurt M, Niederkorn J, Alizadeh H. Effects of mannose on Acanthamoeba castellanii proliferation and cytolytic ability to corneal epithelial cells. Invest Ophthalmol Vis Sci. 2003; 44: 3424–3431 [DOI] [PubMed] [Google Scholar]
- 20. Hurt M, Neelam S, Niederkorn J, Alizadeh H. Pathogenic Acanthamoeba spp secrete a mannose-induced cytolytic protein that correlates with the ability to cause disease. Infect Immun. 2003; 71: 6243–6255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Alizadeh H, Neelam S, Hurt M, Niederkorn JY. Role of contact lens wear, bacterial flora, and mannose-induced pathogenic protease in the pathogenesis of amoebic keratitis. Infect Immun. 2005; 73: 1061–1068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Tripathi T, Smith AD, Abdi M, Alizadeh H. Acanthamoeba-cytopathic protein induces apoptosis and proinflammatory cytokines in human corneal epithelial cells by cPLA2α activation. Invest Ophthalmol Vis Sci. 2012; 53: 7973–7982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Tripathi T, Abdi M, Alizadeh H. Role of phospholipase A2 (PLA2) inhibitors in attenuating apoptosis of the corneal epithelial cells and mitigation of Acanthamoeba keratitis. Exp Eye Res. 2013; 113: 182–191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Steinhoff M, Buddenkotte J, Shpacovitch V, et al. Proteinase-activated receptors: transducers of proteinase-mediated signaling in inflammation and immune response. Endocr Rev. 2005; 26: 1–43 [DOI] [PubMed] [Google Scholar]
- 25. Hollenberg MD, Compton SJ. International Union of Pharmacology. XXVIII. Proteinase-activated receptors. Pharmacol Rev. 2002; 54: 203–217 [DOI] [PubMed] [Google Scholar]
- 26. Ossovskaya VS, Bunnett NW. Protease-activated receptors: contribution to physiology and disease. Physiol Rev. 2003; 84: 579–621 [DOI] [PubMed] [Google Scholar]
- 27. Vergnolle N. Protease-activated receptors as drug targets in inflammation and pain. Pharmacol Ther. 2009; 123: 292–309 [DOI] [PubMed] [Google Scholar]
- 28. Lang R, Song PI, Legat FJ, et al. Human corneal epithelial cells express functional PAR-1 and PAR-2. Invest Ophthalmol Vis Sci. 2003; 44: 99–105 [DOI] [PubMed] [Google Scholar]
- 29. Nickel TJ, Kabir MH, Talreja J, Stechschulte DJ, Dileepan KN. Constitutive expression of functionally active protease-activated receptors 1 and 2 in human conjunctival epithelial cells. Mediators Inflamm. 2006; 2006: 61359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Visvesvara GS, Mirra SS, Brandt FH, et al. Isolation of 2 strains of Acanthamoeba castellanii from human tissue and their pathogenicity and isoenzyme profiles. J Clin Microbiol. 1983; 18: 1405–1412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Robertson DM, Li L, Fisher S, et al. Characterization of growth and differentiation in a telomerase-immortalized human corneal epithelial cell line. Invest Ophthalmol Vis Sci. 2005; 46: 470–478 [DOI] [PubMed] [Google Scholar]
- 32. Alizadeh H, Ma D, Berman M, et al. Tissue-type plasminogen activator-induced invasion and metastasis of murine melanomas. Curr Eye Res. 1995; 14: 449–458 [DOI] [PubMed] [Google Scholar]
- 33. Saksela O. Radial caseinolysis in agarose: a simple method for detection of plasminogen activator in the presence of inhibitory substances and serum. Anal Biochem. 1981; 111: 276–282 [DOI] [PubMed] [Google Scholar]
- 34. Smith PK, Krohn RI, Hermanson GT, et al. Measurement of protein using bicinchoninic acid. Anal Biochem. 1985; 150: 76–85 [DOI] [PubMed] [Google Scholar]
- 35. Schmittgen TD, Livak KJ. Analyzing real-time PCR data by the comparative CT method. Nat Protoc. 2008; 3: 1101–1108 [DOI] [PubMed] [Google Scholar]
- 36. Chan LY, Yim EK, Choo AB. Normalized median fluorescence: an alternative flow cytometry analysis method for tracking human embryonic stem cell states during differentiation. Tissue Eng Part C Methods. 2013; 19: 156–165 [DOI] [PubMed] [Google Scholar]
- 37. Alizadeh H, Tripathi T, Abdi M, Smith AD. Pathogenic strains of Acanthamoeba are recognized by TLR4 and initiated inflammatory responses in the cornea. PLoS ONE. 2014; 9: e92375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Piña-Vázquez C, Reyes-López M, Ortíz-Estrada G, de la Garza M, Serrano-Luna J. Host-parasite interaction: parasite-derived and -induced proteases that degrade human extracellular matrix. J Parasitol Res. 2012; 2012: 748206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Caballero A, Thibodeaux B, Marquart M, Traidej M, O'Callaghan R. Pseudomonas keratitis: protease IV gene conservation, distribution, and production relative to virulence and other Pseudomonas proteases. Invest Ophthalmol Vis Sci. 2004; 45: 522–530 [DOI] [PubMed] [Google Scholar]
- 40. Matsumoto K. Role of bacterial proteases in pseudomonal and serratial keratitis. Biol Chem. 2004; 385: 1007–1016 [DOI] [PubMed] [Google Scholar]
- 41. Geanon JD, Tripathi BJ, Tripathi RC, Barlow GH. Tissue plasminogen activator in avascular tissues of the eye: a quantitative study of its activity in the cornea, lens, and aqueous and vitreous humors of dog, calf, and monkey. Exp Eye Res. 1987; 44: 55–63 [DOI] [PubMed] [Google Scholar]
- 42. Wang HM, Berman M, Latent Law M. and active plasminogen activator in corneal ulceration. Invest Ophthalmol Vis Sci. 1985; 26: 511–524 [PubMed] [Google Scholar]
- 43. Berman M, Leary R, Gage J. Evidence for a role of the plasminogen activator--plasmin system in corneal ulceration. Invest Ophthalmol Vis Sci. 1980; 19: 1204–1221 [PubMed] [Google Scholar]
- 44. Tervo T, Tervo K, van Setten GB, Virtanen I, Tarkkanen A. Plasminogen activator and its inhibitor in the experimental corneal wound. Exp Eye Res. 1989; 48: 445–449 [DOI] [PubMed] [Google Scholar]
- 45. Mirshahi M, Mirshahi S, Soria C, et al. Production of proteases type plasminogen activator and their inhibitor in cornea. Biochem Biophys Res Commun. 1989; 160: 1021–1025 [DOI] [PubMed] [Google Scholar]
- 46. Dulon S, Leduc D, Cottrell GS, et al. Pseudomonas aeruginosa elastase disables proteinase-activated receptor 2 in respiratory epithelial cells. Am J Respir Cell Mol Biol. 2005; 32: 411–419 [DOI] [PubMed] [Google Scholar]
- 47. Scholz M, Vogel JU, Höver G, et al. Thrombin stimulates IL-6 and IL-8 expression in cytomegalovirus-infected human retinal pigment epithelial cells. Int J Mol Med. 2004; 13: 327–331 [PubMed] [Google Scholar]
- 48. Yoshida N, Katada K, Handa O, et al. Interleukin-8 production via protease-activated receptor 2 in human esophageal epithelial cells. Int J Mol Med. 2007; 19: 335–340 [DOI] [PubMed] [Google Scholar]
- 49. Ludwicka-Bradley A, Tourkina E, Suzuki S, et al. Thrombin upregulates interleukin-8 in lung fibroblasts via cleavage of proteolytically activated receptor-I and protein kinase C-gamma activation. Am J Respir Cell Mol Biol. 2000; 22: 235–243 [DOI] [PubMed] [Google Scholar]
- 50. Kida Y, Inoue H, Shimizu T, Kuwano K. Serratia marcescens serralysin induces inflammatory responses through protease-activated receptor 2. Infect Immun. 2007; 75: 164–174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Holzhausen M, Spolidorio LC, Ellen RP, et al. Protease-activated receptor-2 activation: a major role in the pathogenesis of Porphyromonas gingivalis infection. Am J Pathol. 2006; 168: 1189–1199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Hurt M, Apte S, Leher H, Howard K, Niederkorn J, Alizadeh H. Exacerbation of Acanthamoeba keratitis in animals treated with anti-macrophage inflammatory protein 2 or antineutrophil antibodies. Infect Immun. 2001; 69: 2988–2995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Ma JN, Burstein ES. The protease activated receptor 2 (PAR2) polymorphic variant F240S constitutively activates PAR2 receptors and potentiates responses to small-molecule PAR2 agonists. J Pharmacol Exp Ther. 2013; 347: 697–704 [DOI] [PubMed] [Google Scholar]
- 54. Molino M, Barnathan ES, Numerof R, et al. Interactions of mast cell tryptase with thrombin receptors and PAR-2. J Biol Chem. 1997; 272: 4043–4049 [DOI] [PubMed] [Google Scholar]
- 55. Steinhoff M, Corvera CU, Thoma MS, et al. Proteinase-activated receptor-2 in human skin: tissue distribution and activation of keratinocytes by mast cell tryptase. Exp Dermatol. 1999; 8: 282–294 [DOI] [PubMed] [Google Scholar]