Abstract
Disturbances in respiration are common and debilitating features of Rett syndrome (RTT). A previous study showed that the 5-HT1a receptor agonist (R)-(+)-8-hydroxy-dipropyl-2-aminotetralin hydrobromide (8-OH-DPAT) significantly reduced the incidence of apnea and the irregular breathing pattern in a mouse model of the disorder. 8-OH-DPAT, however, is not available for clinical practice. Sarizotan, a full 5-HT1a agonist and a dopamine D2–like agonist/partial agonist, has been used in clinical trials for the treatment of l-dopa–induced dyskinesia. The purpose of this study was to evaluate the effects of sarizotan on respiration and locomotion in mouse models of RTT. Studies were performed in Bird and Jaenisch strains of methyl-CpG–binding protein 2-–deficient heterozygous female and Jaenisch strain Mecp2 null male mice and in knock-in heterozygous female mice of a common nonsense mutation (R168X). Respiratory pattern was determined with body plethysmography, and locomotion was determined with open-field recording. Sarizotan or vehicle was administered 20 minutes before a 30-minute recording of respiratory pattern or motor behavior. In separate studies, a crossover design was used to administer the drug for 7 and for 14 days. Sarizotan reduced the incidence of apnea in all three RTT mouse models to approximately 15% of their pretreatment levels. The irregular breathing pattern was corrected to that of wild-type littermates. When administered for 7 or 14 days, apnea decreased to 25 to 33% of the incidence seen with vehicle. This study indicates that the clinically approved drug sarizotan is an effective treatment for respiratory disorders in mouse models of RTT.
Keywords: apnea, locomotion, respiratory arrhythmia
Clinical Relevance
These studies show that sarizotan, a phase III 5-HT1a receptor agonist, decreases the incidence of apnea and corrects irregular breathing in mouse models of Rett syndrome. The results may lead to clinical trials of 5-HT1a receptor agonists.
Rett syndrome (RTT) is an autism spectrum disorder caused by mutations in the gene that encodes the nuclear protein methyl-CpG-binding protein 2 (MeCP2). Because MeCP2 is X-linked, the syndrome is seen primarily in female subjects (1, 2). In RTT there are disturbances in respiration characterized by an irregular breathing pattern and frequent apnea, which are common and debilitating (3–5). Various studies in mouse models of the disorder have demonstrated that restoration of MeCP2 in symptomatic animals markedly reverses the phenotype (6, 7). These observations indicate that the syndrome does not result in degeneration of neurons or irreversible developmental impairment of neuronal networks. Therefore, pharmacological treatment of symptoms is a valid approach.
In a mouse model, recent studies demonstrated that excess excitation in expiratory neurons characterizes the respiratory abnormality (8). Building on earlier reports showing that 5-HT1a agonists inhibit expiratory neurons (9, 10), we showed that (R)-(+)-8-hydroxy-dipropyl-2-aminotetralin hydrobromide (8-OH-DPAT) significantly reduced the incidence of apnea and restored regularity to the breath cycle (8). 8-OH-DPAT, however, is not clinically available. Sarizotan, a full 5-HT1a agonist and a dopamine D2-like agonist/partial agonist (11), has been used in clinical trials for the treatment of l-dopa–induced dyskinesias (12, 13). The goal of the present study was to evaluate the effects of sarizotan on respiration in mouse models of RTT. To determine possible side effects, locomotion was also studied. Because the Kölliker-Fuse (KF) is a key region for control of expiratory neuron activity and expiratory cycle regularity (14) in this study, we also explored it as a potential 5-HT1a agonist site of action for rescuing breathing in RTT.
Materials and Methods
The protocols were approved by the Oregon Health and Science University Animal Care and Use Committee, were in agreement with the National Institutes of Health “Guide for the Care and Use of Laboratory Animals,” and were performed according to the UK Home Office (Scientific Procedures) Act (1986). Studies were performed in heterozygous female mice with deletion of the third Mecp2 exon (15), designated Mecp2Jae/+, and wild-type (WT) littermates at 6.7 to 12.3 months of age; in null male mice of the same strain designated Mecp2Jae/y at 1.6 to 2.9 months of age; in heterozygous female mice with deletion of the third and fourth exons of Mecp2 (16), designated Mecp2Bird/+, at 6.8 to 14.2 months of age; and in heterozygous female mice carrying a common nonsense mutation seen in patients with RTT (R168X) (17, 18), designated Mecp2R168X/+, at 8.5 to 14 months of age.
Respiratory pattern was determined with body plethysmography as previously described (19, 20). Briefly, individual unanesthetized animals were placed in a 65-ml chamber with their heads exposed through a close fitting hole in Parafilm (Pechiney, Chicago, IL). A pneumotachograph (19) was connected to the chamber and a differential pressure transducer (Model PT5A; Grass Instrument Co., West Warick, RI). Each study was 30 minutes in duration, and the traces were analyzed in 5-minute blocks. Apnea was defined as an expiratory time of 1.0 second or longer. At the frequency of Mecp2-deficient mice, approximately 140 breaths per minute (bpm) (Table 1), 1.0 seconds is equal to 2.4 breath cycles. The irregularity score was calculated from: absolute (TTOTn-TTOTn+1)/(TTOTn+1) (21) and reported as the variance.
Table 1:
Baseline Respiratory Pattern in Mouse Models of Rett Syndrome
| Strain | Frequency (bpm) | Irregularity (variance) | Apnea (no./h) | Apnea (range) |
|---|---|---|---|---|
| Mecp2Jae/y (n = 6) | 193 ± 13* | 0.62 ± 0.21 | 215 ± 40 | 80–382 |
| WT | 259 ± 10 | 0.052 ± 0.008 | 2.3 ± 1.2 | 0–9.0 |
| Mecp2Jae/+ (n = 4) | 141 ± 12 | 0.29 ± 0.05 | 162 ± 10 | 140–190 |
| WT | 207 ± 16 | 0.057 ± 0.015 | 2.1 ± 0.9 | 0–6.0 |
| Mecp2Bird/+ (n = 6) | 158 ± 20 | 0.41 ± 0.11 | 158 ± 52 | 42–390 |
| WT | 224 ± 11 | 0.096 ± 0.009 | 5.8 ± 3.1 | 0–16.8 |
| Mecp2R168X/+ (n = 8) | 132 ± 4 | 0.18 ± 0.028 | 168 ± 42 | 64–421 |
| WT | 190 ± 6 | 0.02 ± 0.003 | 3.8 ± 1.5 | 0–9.6 |
Definition of abbreviations: bpm, breaths per minute; WT, wild type.
Values are mean ± SEM.
The acute effects of sarizotan were examined in Mecp2-deficient heterozygous female mice and in null male mice. For each sarizotan dose, a vehicle (2.5% DMSO in water, intraperitoneally) 30-minute study preceded the drug study. After this, sarizotan was administered intraperitoneally, and 20 minutes later the 30-minute respiratory monitoring was repeated. Sarizotan injections were separated by a minimum of 48 hours.
A crossover design was used for the long-term studies so that half of the Mecp2Bird/+ female mice received vehicle (1.25% DMSO) in their drinking water and half received sarizotan (0.0625 mg/ml). At the end of 7 days, the treatment was reversed so that the mice that were receiving vehicle were changed to sarizotan and those on sarizotan were changed to water. Vehicle and drug solutions contained 0.1% saccharin. Water intake with sarizotan approached that with vehicle (86 ± 1.9%), and the difference was not significant (P = 0.37; paired t test). The dose of sarizotan ingested averaged 13.8 ± 1.9 mg/kg/d. Respiratory pattern was studied on Days 3, 5, and 7 of treatment. An identical design was used for a long-term study in MecpR168X/+ mice except that treatment was for 14 days. Water intake and daily dose of sarizotan were similar to those in the 7-day study. The mice were studied with plethysmography on Days 4, 7, 10, and 14 after receiving sarizotan or vehicle.
Motor activity studies were performed at the same time of day (12:00–18:00) and in the same dedicated observation room. Mice were placed singly into a standard open field box with side-viewing and top-viewing cameras (Clever Sys, Reston, VA). Mice were recorded for 20 minutes after an approximate 1-minute delay from initial placement in the center of the open field. Activity traces were acquired in real time using StereoScan Software (Clever Sys).
To determine the location of 5-HT1a receptor agonist effects in the respiratory network, we used an in situ decerebrated “working heart-brainstem preparation” of the mouse (22), which is a modified version of cardiopulmonary bypass and allows direct recordings to be made from the phrenic nerve (PN) and the central vagus nerve (cVN) and the ability to manipulate precisely arterial CO2 concentration. This preparation is not confounded by the depressant action of anesthetic agents or changes in sleep/arousal states. Briefly, female heterozygous Mecp2Bird/+ mice (n = 4) were heparinized (1,500 U intraperitoneally) and anesthetized deeply with halothane until loss of their paw withdrawal reflex. Mice were decerebrated and exsanguinated and bisected subdiaphragmatically, and the head and thorax were immersed in ice-cold carbogenated artificial cerebrospinal fluid. The thoracic PN and cVN were cut distally. Preparations were moved to a recording chamber. A double-lumen catheter (Braintree Scientific, Braintree, MA) was inserted into the descending aorta for retrograde perfusion by a peristaltic roller pump (505Du; Watson Marlow, Falmouth, UK). The perfusion solution consisted of carbogenated modified Ringer’s at 32°C with an oncotic agent replacing serum albumin (Ficoll 1.25%). The second lumen of the catheter was used to monitor aortic perfusion pressure. The baseline perfusate flow was preset between 18 and 20 ml/min. The composition of the Ringer’s solution was 125 mM NaCl, 24 mM NaHCO3, 3 mM KCl, 2.5 mM CaCl2, 1.25 mM MgSO4, 1.24 mM KH2PO4, and 10 mM glucose; pH ranged from 7.35 to 7.4 after carbogenation (6% CO2 and 94% O2). Osmolality was 290 ± 5 mOsm per kg H2O. Ficoll (1.25%; type 70) was added as an oncotic agent. Unless stated, all chemicals were from Sigma-Aldrich (St. Louis, MO). Vecuronium bromide (4 μg/ml) (Organon Teknica, Durham, NC) was added to the perfusion solution to block neuromuscular transmission.
Simultaneous recordings of the left PN and cVN motor activities were obtained with bipolar suction electrodes mounted on individual 3D micromanipulators. Nerve activities were AC amplified (10 k), band-pass filter (100 Hz to 5 kHz) rectified, and integrated (50 ms time constant) online (Spike 2 software; Cambridge Electronic Design, Cambridge, UK). All electrophysiological data were digitized (10 kHz) with an A-D converter (Cambridge Electronic Design) with Spike 2 software and analyzed offline.
Custom–built, three-barreled micropipettes (∼ 20 μm ø) were used for brainstem microinjections. For that, three glass-calibrated micropipettes (0.4 mm inner diameter) (Drummond Scientific, Broomall, PA) were glued together, twisted to form a single tip, and pulled on a Narishige Pipette Puller (Model PE 2; Narishige, East Meadow, NY). Micropipettes contained 10 mM l-glutamate (Sigma-Aldrich), 100 μM 8-OH-DPAT (Tocris Bioscience, Minneapolis, MN), and 2% Evans Blue dye (Sigma-Aldrich), all dissolved in aCSF (Harvard Apparatus, Holliston, MA) at pH 7.4.
Micropipettes were placed just caudally to the left inferior colliculus, 1.7 to 1.8 mm lateral of the midline and 1 to 1.5 mm ventral of the dorsal surface. After functional identification of an effective KF injection site (i.e., a prolonged PN apnea with prolonged postinspiratory activity on cVN) with l-glutamate (30 nl), 8-OH-DPAT was microinjected (60 nl). At the end of the experiment, Evans blue (30 nl) was injected to mark the injection site. The injected volumes were measured through a microscope fitted with a calibrated ocular graticule. Repeated microinjections into the same site, performed at least 15 minutes apart (for washout and recovery), yielded reproducible responses. In all four mice, microinjections were delivered on the left side; in two mice, after an appropriate washout interval of at least 15 minutes, an additional contralateral microinjection was performed. There was no difference between right and left side injections, so they were grouped. At the end of each experiment, the brainstem was removed and fixed in 4% paraformaldehyde for 48 hours (Sigma-Aldrich) and cryoprotected in 20% sucrose in PBS for 24 hours (Sigma-Aldrich). Brainstems were then cut in a series of 30-μm-thick coronal sections through the pons with a cryostat. The sections were counterstained with DAPI and examined under and fluorescence microscope (Evans blue emission peak = 680 nm; DAPI emission peak = 461 nm).
Data are reported as mean ± SEM. Comparisons between WT and Mecp2-deficient groups were made with unpaired t tests. The effect of a single dose of sarizotan was determined with a paired t test. The effects of long-term treatment in Mecp2-deficient female mice were determined with two-way repeated measures ANOVA with treatment and days as the two factors. Post hoc testing used the Tukey method. The effects of KF microinjections were compared using one-way repeated measures ANOVA followed by a comparison versus baseline using the Dunnett method. P < 0.05 was taken as significant. Sigma Stat 3.1 (Systat, Chicago, IL) was used for statistical evaluation.
Results
Baseline Respiratory Patterns
Respiratory frequency was decreased by approximately 25 to 30% in Mecp2-deficient mice compared with WT in all four strains (Table 1). The interbreath period was very irregular and the incidence of apnea (Table 1) much greater in the Mecp2-deficient animals.
Acute Effect of Sarizotan on Respiration
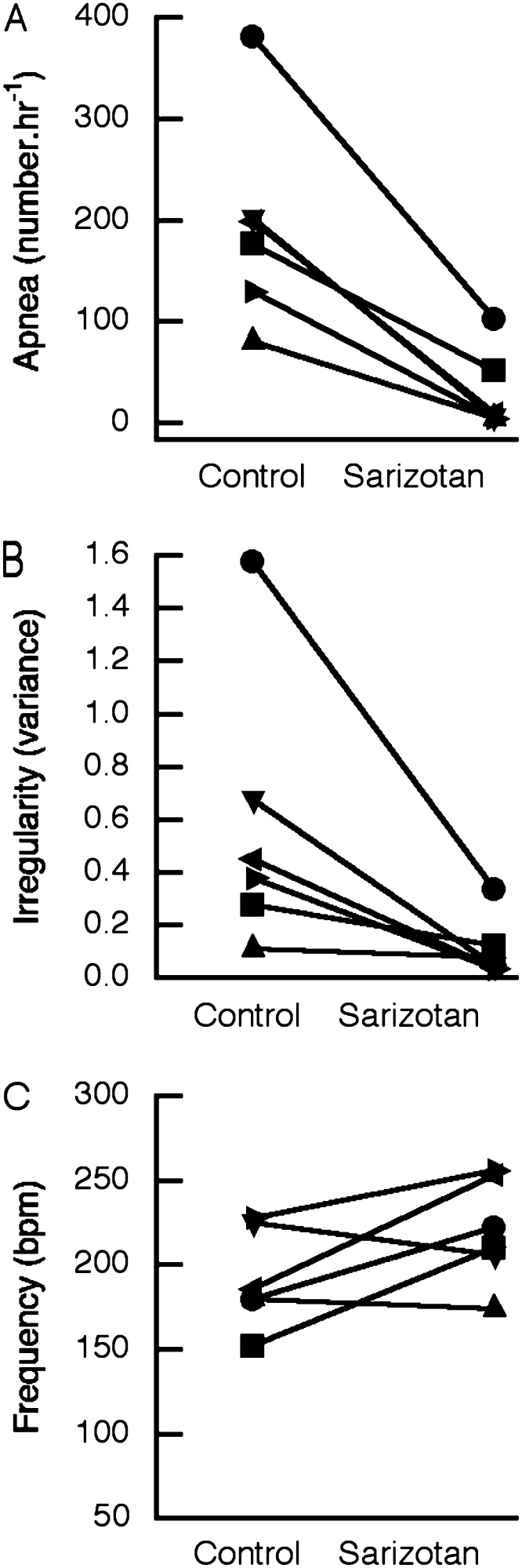
Preliminary studies were performed to estimate the effective dose of sarizotan. A dose of 1.0 mg/kg reduced the incidence of apnea in Mecp2Jae/+ mice to 47.5 ± 10.4% (P = 0.089) of that seen after vehicle (n = 4). This dose reduced apnea to 36.9 ± 14.5% (P = 0.091) in Mecp2Jae/y male mice (n = 4). Subsequently, 5.0 mg/kg was used in heterozygous female mice, and 10.0 mg/kg was used in null male mice. The effects of sarizotan (5.0 mg/kg) in MeCP2Jae/+ and Mecp2Bird/+ mice were similar, so the results have been combined (Figure 1). This compound significantly reduced the incidence of apnea in MeCP2-deficient heterozygous female mice (n = 10) from 143 ± 31/h to 20 ± 8/h (P = 0.001; paired t test) (Figure 1A). In 5 of 10 studies, apnea was decreased to levels that were within the range seen in WT mice (Figure 1A; Table 1). Sarizotan resulted in a decrease in the irregularity score from 0.34 ± 0.07 to 0.06 ± 0.01 (P < 0.0001). In 8 of 10 experiments, the irregularity score was corrected to that of WT littermates (Figure 1B). Sarizotan in heterozygous female mice significantly increased respiratory frequency from 153 ± 12 bpm to 177 ± 10 bpm (P = 0.012) (Figure 1C). After a dose of 5.0 mg/kg, the drug did not affect core body temperature in MeCP2-deficient female mice (36.2 ± 0.3 versus 35.2 ± 0.4; P = 0.093; n = 6). The effects of sarizotan on improving respiration did not depend on the age of female mice at the time of study. The heterozygous female mice were grouped into younger (6.7–10.7 mo; n = 5) and older animals (12.0–14.2 mo; n = 5). Apnea was reduced to 5.4 ± 2.3% of baseline in the younger mice and to 15.6 ± 7% in the older animals (P = 0.248; unpaired t test). Irregularity fell to 24.3 ± 5.8% of baseline in younger female mice and to 12.7 ± 5.7% in older female mice (P = 0.319). Respiratory frequency increased to 109.9 ± 6.4% of baseline in younger mice and to 128.0 ± 5.9% in older mice (P = 0.071).
Figure 1.
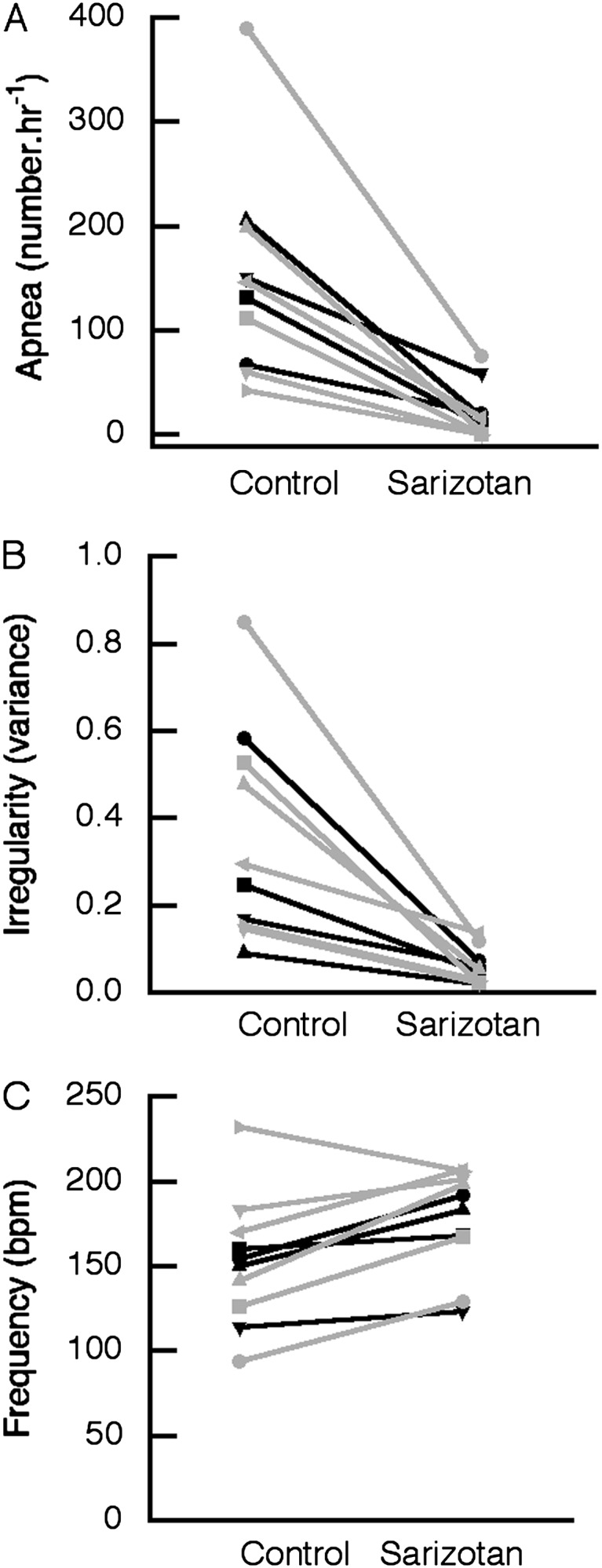
Acute effect of sarizotan (5 mg/kg) on respiratory pattern in Mecp2-deficient heterozygous female mice. Data shown for individual mice (black lines = Mecp2Jae/+ mice; gray lines = MeCP2Bird/+ mice). (A) Number of apneas per hour. (B) Respiratory irregularity given as the variance in absolute (TTOTn − TTOTn + 1)/(TTOTn + 1). (C) Respiratory frequency in breaths per minute (bpm).
Sarizotan (10.0 mg/kg) was equally effective in improving respiration in null male mice. The incidence of apnea fell from 200 ± 42 to 30 ± 16/h (n = 6) (P = 0.003; paired test). In three of six male mice, apnea was reduced to that seen in WT animals (Figure 2A; Table 1). Irregularity declined from 0.55 ± 0.21 to 0.11 ± 0.05 (P = 0.048) (Figure 2B). Sarizotan did not affect frequency in null male mice (220 ± 13 versus 192 ± 12; P = 0.094) (Figure 2C).
Figure 2.
Acute effect of sarizotan (10 mg/kg) on respiratory pattern in Mecp2Jae/y null male mice. Data are shown for individual mice. (A) Number of apneas per hour. (B) Respiratory irregularity given as the variance in absolute (TTOTn − TTOTn + 1)/(TTOTn + 1). (C) Respiratory frequency in breaths per minute (bpm).
Long-Term Effect of Sarizotan on Respiration
When added to drinking water, sarizotan reduced the incidence of apnea in Mecp2Bird/+ mice by 63.3% on Day 5 of treatment (P = 0.009) and by 66.1% on Day 7 (n = 8) (P = <0.001; two-way repeated measures ANOVA) (Figure 3A). In addition to its effects in reducing the incidence of apnea, sarizotan corrected the irregular breathing pattern that is characteristic of RTT. On Days 5 and 7 of treatment, the irregularity score reverted to a level similar to that of WT mice (0.096 ± 0.009) (Figure 3B). Sarizotan slightly increased respiratory frequency from 156 ± 10 while receiving vehicle to 174 ± 5 on Day 5 of drug treatment and to 166 ± 8 on the Day 7, but not to a significant level (P = 0.093 and 0.087).
Figure 3.
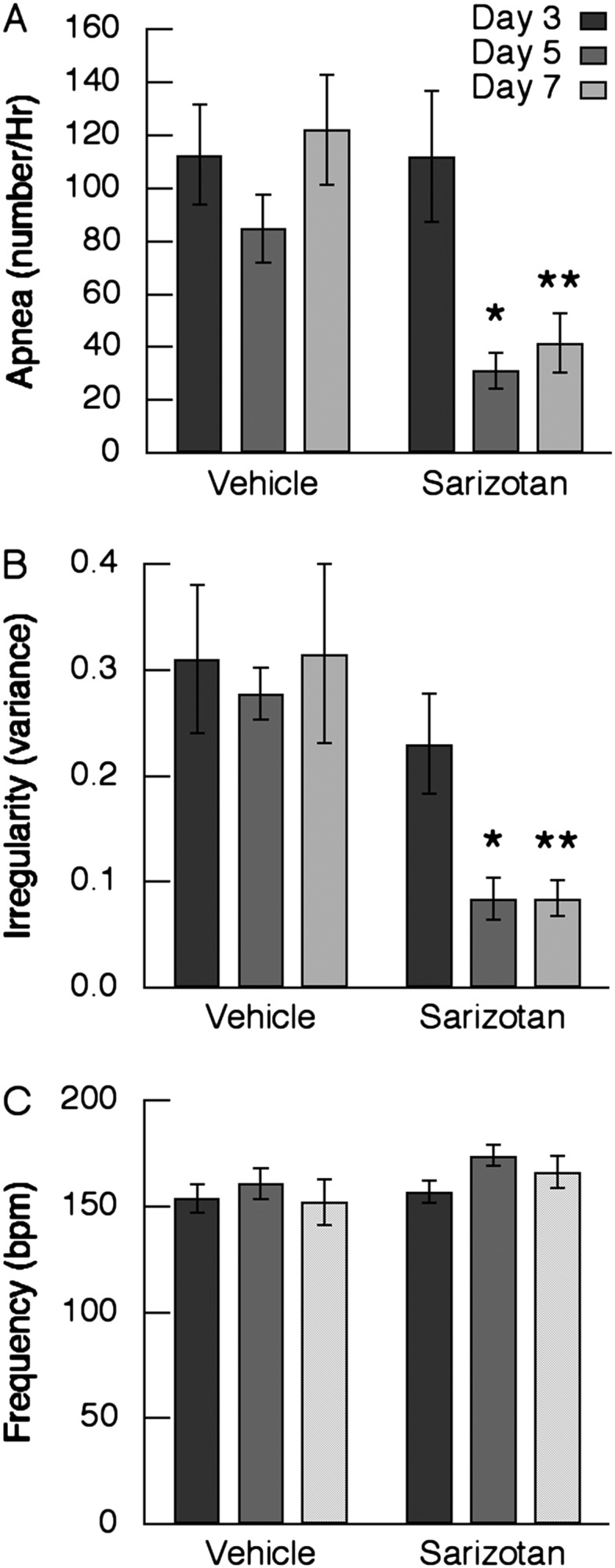
Effect of 7-day treatment with sarizotan on respiration in Mecp2Bird/+ female mice (n = 8). Thirty-minute monitoring of respiratory pattern with plethysmography was performed on the Days 3, 5, and 7 of vehicle or sarizotan administered in drinking water. (A) Incidence of apnea. (B) Irregularity score expressed as the variance. (C) Respiratory frequency. *P < 0.05 versus corresponding day receiving vehicle. **P < 0.01.
Sarizotan was also effective in improving respiration in Mecp2R168X/+ mice (n = 8) in a 2-week trial. Treatment with this drug reduced the incidence of apnea by 73.9, 75, and 75.6% compared with vehicle on Days 7, 10, and 14 of treatment, respectively (P = 0.008–0.022) (Figure 4A). On Days 7 (P = 0.006), 10 (P = 0.049), and 14 (P = 0.05), irregularity was significantly below that of animals receiving vehicle but did not reach WT levels (0.02 ± 0.003) (Figure 4B; Table 1). Respiratory frequency was not affected by sarizotan during the 14-day treatment (Figure 4C).
Figure 4.
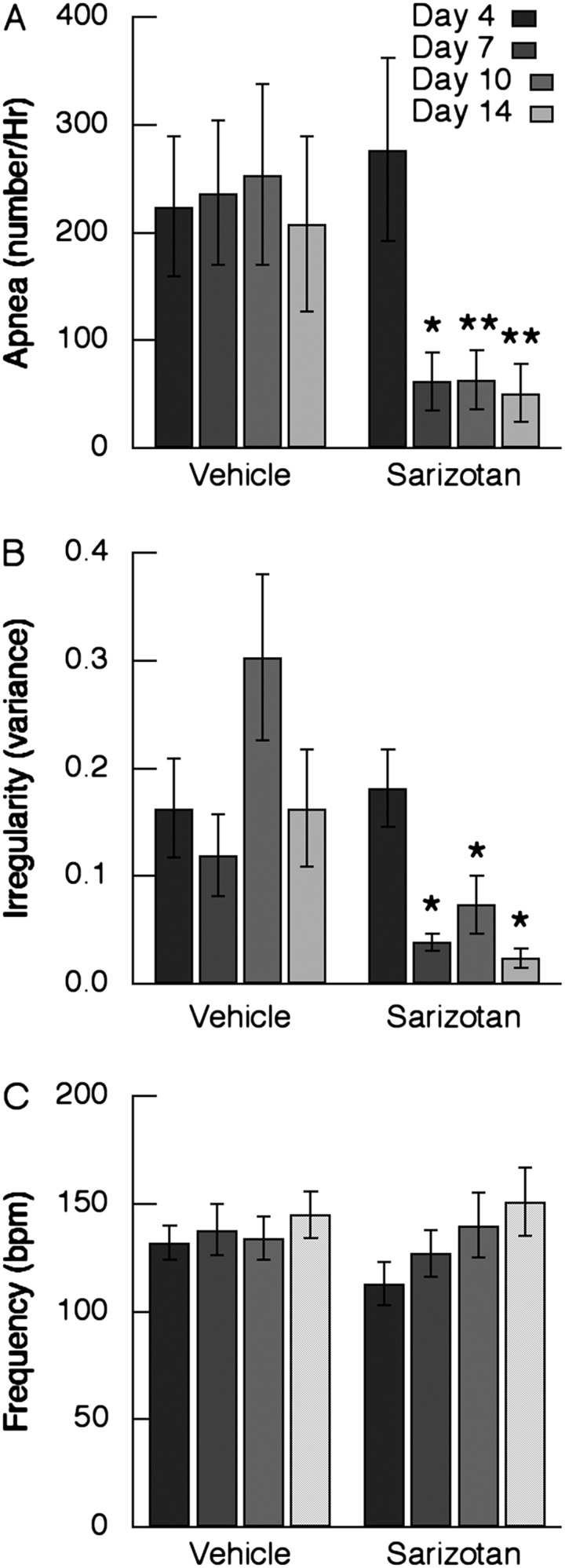
Effect of 14-day treatment with sarizotan on respiration in Mecp2R168X/+ mice (n = 8). Monitoring of respiratory pattern with plethysmography was performed at 0 minutes on Days 4, 7, 10, and 14 of vehicle or sarizotan administered in drinking water. (A) Incidence of apnea. (B) Irregularity score expressed as the variance. (C) Respiratory frequency. *P < 0.05 versus corresponding day receiving vehicle. **P < 0.01.
Effect of Sarizotan on Locomotion
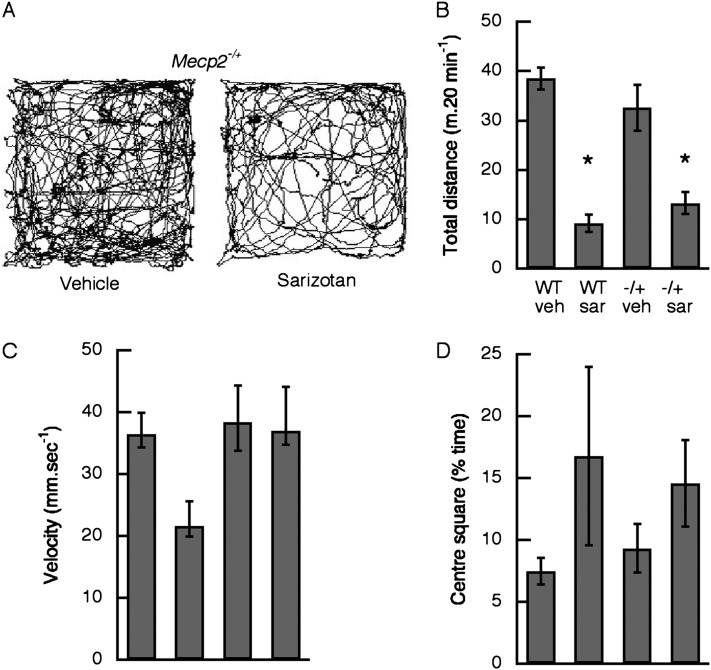
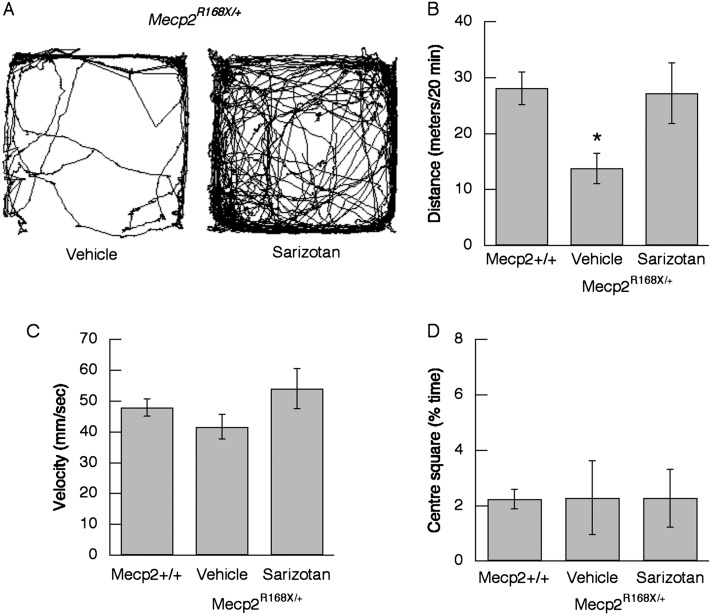
Open-field behavioral studies were performed with Mecp2Jae/+ and Mecp2Bird/+ heterozygous female mice. There were no differences in the effects of sarizotan, and the data were combined. Acute administration of sarizotan (5.0 mg/kg) significantly reduced the total distance traveled in WT (P <0.0001) and Mecp2-deficient animals (P = 0.0004) (Figure 5B). The drug had no effect on the velocity of movement (P = 0.135) or on the percentage of time that mice spent in the center square (P = 0.091). In the 7-day long-term study, locomotion was examined on Day 6 of treatment. Total distance traveled (31.3 ± 4.6 m in 20 min) while receiving sarizotan was not different than that with vehicle (32.6 ± 6.8 m) (see Figure E1A in the online supplement). In addition, average velocity (58.1 ± 4.1 versus 53.9 ± 4.9 mm/s) was not affected by the drug (Figure E1B). Mecp2−/+ mice receiving vehicle spent less time in the center square than WT animals (Figure E1C) (P = 0.032). The Mecp2R168X/+ mice treated with sarizotan for 14 days were examined in open field on Day 13. Total distance traveled in mice receiving vehicle was less than the total distance traveled by WT mice and animals receiving sarizotan (P = 0.031) (Figure 6B).
Figure 5.
Acute effect of sarizotan (5 mg/kg) on motor activity in Mecp2-deficient heterozygous and wild-type (WT) female mice. (A) Representative trace of locomotor activity in a Mecp2−/+ mouse after receiving intraperitoneal injection of vehicle (left panel) and after sarizotan (right panel). (B) Total distance traveled in a 20 minute period. (C) Average velocity for all movements that exceed 10 mm/second−1. (D) Percent time mouse spent in the centre square of the nine square grid. Total distance traveled was significantly decreased after sarizotan compared with after vehicle injections. *P < 0.0001 for WT; P = 0.0004 for Mecp2−/+. Effects on velocity and time spent in center square were not significant. −/+, heterozygous Mecp2 deficient; sar, sarizotan; veh, vehicle.
Figure 6.
Effect of 13-day treatment with sarizotan on motor activity in Mecp2R168/X mice (n = 8). (A) Representative trace of locomotor activity in a Mecp2R168X/+ mouse on Day 13 of vehicle in drinking water (left panel) and on Day 13 of sarizotan (right panel). (B) Total distance traveled in a 20 minute period. (C) Average velocity for all movements that exceed 10 mm/second−1. (D) Percent time mouse spent in the centre square of the nine square grid. *P = 0.031.
Effect of 5-HT1a Agonist Injected into the KF Nucleus on Respiratory Pattern
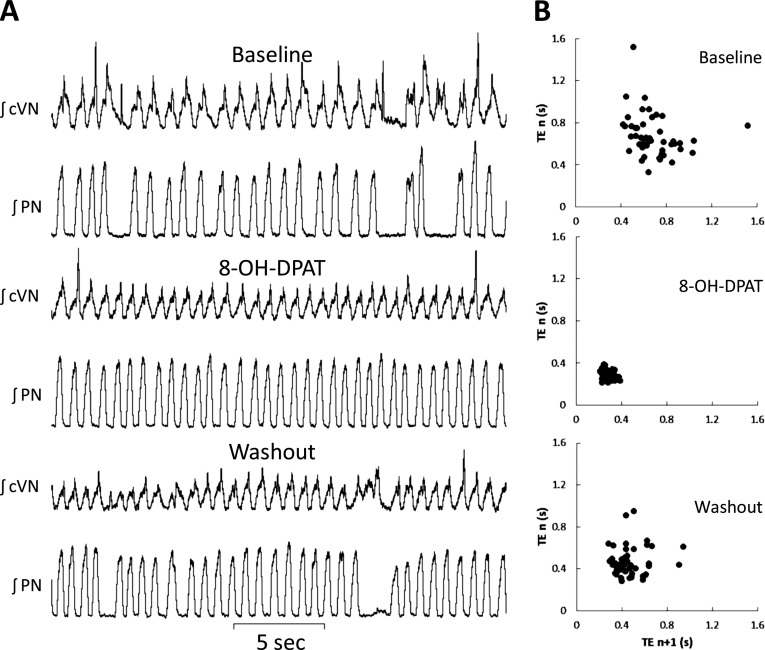
At the concentration needed for intraparenchymal administration, sarizotan could only be solubilized in 5% DMSO, thus precluding its use in direct instillation in the brainstem. Diluted DMSO solutions (5% and lower) applied directly to neuron soma and axon projections were shown to reduce action potential velocity and to block sodium, potassium, and calcium currents (23, 24). This could lead us to under- or overestimate the effect of the sarizotan even if DMSO did not show any acute respiratory effects. Therefore, to localize the site of action of 5-HT1a receptor agonists, we used 8-OH-DPAT for the in situ experiments. These studies used Mecp2Bird/+ mice that were 4 to 10 months of age. The KF was identified by a prior microinjection of glutamate that evoked PN apnea and prolonged postinspiratory activity in the cVN. Figure 7 shows the effect of a unilateral 8-OH-DPAT microinjection into the KF nucleus. Unilateral injections of dipropyl-2-aminotetralin in the KF reduced apnea in Mecp2Bird/+ heterozygote mice studied in situ from 188.6 ± 21 to 102.9 ± 30/h (n = 6; P < 0.01). In addition, the 5-HT1a agonist lowered the coefficient of variation for breath intervals from 32 ± 5 to 20 ± 2 (P < 0.01). When the agonist was washed out, apnea and irregularity returned to their preinjection levels. Ineffective 8-OH-DPAT injections proved to be outside the KF region on post hoc histological analysis (Figure E2). Vehicle and glutamate injections had no effect on the number of apneas or on breath interval variation (P > 0.05).
Figure 7.
Effect of serotonin 1a agonist injection unilateral into a Kölliker-Fuse nucleus. (A) Integrated nerve activity of central vagus (cVN) and phrenic (PN) nerves. The top two traces were made during baseline recording. The frequent phrenic apneas are characterized by prolonged cVN activity. The middle two traces were made after injection of (R)-(+)-8-hydroxy-dipropyl-2-aminotetralin hydrobromide (8-OH-DPAT) (60 nl, 500 μM) into a Kölliker-Fuse nucleus that had been identified by an earlier injection of glutamate. The bottom two traces were made after washout of dipropyl-2-aminotetralin. (B) Poincaré plots for periods that correspond to the adjacent traces.
Discussion
This study shows, to the best of our knowledge, for the first time, that the class III drug sarizotan reduces the incidence of apnea and corrects the irregular interbreath pattern in three mouse models of RTT. Sarizotan is formulated into an oral medication (25) and has been used in two clinical trials in adults (12, 13). The similar results in Mecp2Jae/+, Mecp2Bird/+, and Mecp2R168X/+ heterozygous female mice indicate that the effects are not strain specific or confined to animal models with null mutations of the DNA binding protein. R168X retains the methyl-DNA binding domain but lacks the large C-terminus of the protein including the transcription repression domain (17, 18). This mutation is seen in approximately 10 to 15% of patients with RTT (26, 27). The results extend the initial observation by demonstrating that sarizotan, a phase III drug, is equally as effective as 8-OH-DPAT (8). In addition, the beneficial effects on respiratory disturbances occurred without a significant alteration in breathing frequency. As with 5-HT1a receptor agonists, augmenting GABA inhibition in heterozygous Mecp2-deficient female mice ameliorated respiratory symptoms (8). These treatments reduced respiratory rate by approximately 35%. In a separate study, the GABA-A allosteric modulator midazolam was shown to correct posthypoxic apnea and irregularity in Mecp2Bird/y null male mice (28). This compound caused a 28% reduction in frequency.
The baseline respiratory pattern for mice lacking the third Mecp2 exon in the present studies differed from some previous reports. Mecp2Jae/y null male mice on a mixed (129v, C57BL/6, BALB/c) background studied at postnatal day (P)35 were found to have a respiratory frequency that was greater than that seen in WT mice (29). In contrast, null male mice of this same strain on a C57BL/6 at P42 had a longer total breath cycle (30). Mecp2Jae/y mice on C57BL/6 or BALB/c studied between P48 and P88, reported herein, had a respiratory frequency that was less than WT mice (Table 1). The differences may be due to background and/or age at which the studies were conducted. A recent study found that respiratory frequency in Mecp2Jae/y male mice declined in older animals (31). Fifty percent of Mecp2Jae/+ female mice on a mixed background studied under urethane anesthesia at 18 to 29 months of age showed a respiratory frequency that exceeded that of WT mice (32). These mice did not exhibit frequent apnea, in contrast to those reported here. The differences may be due to background and/or the effects of anesthesia. Recently Mecp2Jae/+ female mice on a mixed background have been found to have an increased respiratory frequency at 10 and 12 weeks of age (33). Age at time of observation and/or background strain may account for the differences from the results found in the present studies, as might anesthetic agents, which is why we chose to study conscious animals.
The serotonin metabolite 5-hydroxyindoleacetic acid is reduced in cerebrospinal fluid of individuals with RTT (34). 5-HT concentrations are reduced in pons and brainstem of mature (P42–P60) Mecp2 null male mice (21, 34–36). 5-Hydroxyindoleacetic acid was found to be reduced in one of these studies (36) but not in the other two (21, 35). Selective removal of Mecp2 from serotonergic neurons caused a marked reduction in brain 5-HT and its rate-limiting enzyme tryptophan hydroxylase (34). The respiratory pattern in these conditional Mecp2-deficient mice was not different from controls that carried a floxed Mecp2. The latter are hypomorphic for Mecp2, and their brain levels of the protein are only one half that of WT (37). In addition, mice that had deletion of LIM homeobox transcription factor 1β confined to pheochromocytoma 12 ETS factor-1–expressing 5-HT neurons displayed normal baseline breathing (38). More recently, a genetic strategy was used to acutely inhibit serotonergic neurons (39). These mice did not have significant baseline respiratory disturbances. Thus, it is unlikely that treatment with 5-HT1a agonists modify respiratory disturbances in RTT mice by overcoming a defect in 5-HT content. Given that apneas are associated with excessive expiratory discharges in vagal and abdominal motor outflows (8, 40), the relative inhibition of expiratory neurons secondary to activation of G-protein–coupled inward rectifying potassium channel by the 5-HT1a agonist is most likely the mechanism for sarizotan’s effects (9, 10).
Previous studies indicate that 5-HT1a receptors in the KF region play a role in respiratory rhythm regularity. The KF is a key region for controlling postinspiration and expiratory cycle length, it suppresses inspiration, and it can reset the respiratory oscillator phase (41). Furthermore, KF microinjections of a 5-HT1a receptor antagonist (WAY100635) increased respiratory irregularity in WT mice during normal breathing, suggesting a role for endogenous 5-HT1a activity in rhythm regularity (42). Other work also suggested hyperexcitability of respiratory reflexes mediated in this region in MeCP2-deficient mice (40). In the present study, we showed that local microinjection of the 5-HT1a into the KF reduced the number of apneas and irregularity scores in MeCP2Bird/+ female mice, suggesting this is a key target site for sarizotan’s beneficial effects on breathing rhythm in RTT.
A number of regimens have been used to treat the respiratory disorders in mouse models of RTT. Catecholaminergic defects have been demonstrated in the brain of human subjects with RTT (34) and in RTT mouse models (35). They have also been documented in a number of respiratory-related regions, including pons, medulla (21), locus coeruleus (43), carotid body, and petrosal ganglion (44). These observations prompted acute and long-term examination of the effects of augmenting norepinephrine (NE) systemically. In juvenile Mecp2 null mice (P14–P21), NE eliminated cycle irregularity (21). Chronic treatment with the NE reuptake blocker desipramine given intraperitoneally beginning when Mecp2−/y animals apneas reached 100/h stabilized apnea at approximately 100/h and rose to approximately 300/h in mice that received a placebo (45). Oral treatment beginning at P30 with the reuptake blocker reduced the incidence of apnea and the irregularity score at P51 and P65 (46). Unlike the results shown herein, these long-term treatments did not reduce apnea to levels approaching that in WT no correct irregularity.
Brain-derived neurotrophic factor (BDNF) is depleted in brain (47) and nodose ganglia (48) of Mecp2Jae/y mice. The respiratory phenotype of these mice on a mixed background was characterized by a rapid frequency and irregular pattern. Ampakine therapy beginning at P35 increased nodose BDNF content and reduced respiratory frequency to that of WT mice (29). The authors suggested and later demonstrated that the increased BDNF inhibits glutamatergic excitation in the nucleus of the solitary tract (49). Ampakine treatment, unlike 5-HT1a receptor agonists, did not restore a regular breathing pattern. Two recent papers have examined the effects of small molecules that activate TrkB, the BDNF receptor. In Mecp2Jae/y on a C57BL6 background, 7,8-dihydroxyflavone shortened their prolonged breath cycle and reduced irregularity, but it remained above that of WT mice (30). LM22A-4 reduced the elevated frequency seen in Mecp2Jae/+ heterozygous female mice on a mixed background to that of WT at 12 weeks of age (33). This compound at the dose used did not affect apnea. Irregularity was not reported in this study.
More recently, the effects of a tripeptide derivative of insulin-like growth factor 1 have been examined (50). Long-term treatment in null male mice started at P15. There was a modest reduction in breath irregularity when the animals were studied at P56. The incidence of apnea was not mentioned. Motor activity was improved to that of WT mice with this treatment. Another recent study showed in Mecp2Jae/y male mice that inducing stress using movement restraint or threatening odorant (trimethylthiazoline) increased the incidence of apnea (31). In older male mice, however, the corticotropin-releasing hormone receptor 1 antagonist antalarmin did not reduce the incidence of apnea.
The effects of sarizotan mediated through dopamine D2–like receptors should not detract from its beneficial modulation on respiration. Indeed, activation of these receptors has been shown to depress expiratory neurons, an effect that would be beneficial (51) given the excessive expiratory motor activity that is associated with the apneas (8, 40). The depression of motor activity seen with the acute intraperitoneal administration of sarizotan (Figure 5) is most likely due to D2 receptor activation of the indirect basal ganglia motor pathway (52, 53), and not 5-HT1a receptor activation.
In summary, the 5-HT1a agonist sarizotan is an effective pharmacologic approach for the respiratory disturbances in mouse models of RTT. When used orally over a 7- or 14-day period, there was no indication of desensitization, and the locomotor inhibitory effects seen with an acute administration were not observed. We support the use of a 5-HT1a receptor agonist in a new trial for treating RTT.
Acknowledgments
Acknowledgments
Sarizotan was a kind gift from Gerd D Bartoszyk, Merck, Darmstadt, Germany. The R168X mice were a generous gift from Joanne Berger-Sweeney, Tufts University.
Footnotes
NIH HD056503 grants to Gail Mandel; This work was supported by an International Rett Syndrome Foundation (A.P.A.); by the Oregon Brain Institute (D.T.L.); by a Rett Syndrome Research Trust (D.T.L. and S.K.G.); by a NewLife Foundation for Disabled Children (UK) grant (J.F.R.P.); and by grants from the International Rett Syndrome Foundation, Northwest Rett Syndrome Foundation and the Rett Syndrome Research Trust (J.M.B.).
Author Contributions: A.P.A., D.T.L., J.F.R.P., and J.M.B. conceived and designed the studies. A.P.A., D.T.L., S.K.G., S.J.K., and J.M.B. performed and analyzed the experiments. A.P.A. and J.M.B. wrote the manuscript. All authors read and approved the final manuscript.
This article has an online supplement, which is accessible from this issue's table of contents at www.atsjournals.org.
Originally Published in Press as DOI: 10.1165/rcmb.2013-0372OC on December 18, 2013
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Bird A. The methyl-CpG-binding protein MeCP2 and neurological disease. Biochem Soc Trans. 2008;36:575–583. doi: 10.1042/BST0360575. [DOI] [PubMed] [Google Scholar]
- 2.Chahrour M, Zoghbi HY. The story of Rett syndrome: from clinic to neurobiology. Neuron. 2007;56:422–437. doi: 10.1016/j.neuron.2007.10.001. [DOI] [PubMed] [Google Scholar]
- 3.Julu PO, Kerr AM, Apartopoulos F, Al-Rawas S, Engerström IW, Engerström L, Jamal GA, Hansen S. Characterisation of breathing and associated central autonomic dysfunction in the Rett disorder. Arch Dis Child. 2001;85:29–37. doi: 10.1136/adc.85.1.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Southall DP, Kerr AM, Tirosh E, Amos P, Lang MH, Stephenson JB. Hyperventilation in the awake state: potentially treatable component of Rett syndrome. Arch Dis Child. 1988;63:1039–1048. doi: 10.1136/adc.63.9.1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Weese-Mayer DE, Lieske SP, Boothby CM, Kenny AS, Bennett HL, Silvestri JM, Ramirez JM. Autonomic nervous system dysregulation: breathing and heart rate perturbation during wakefulness in young girls with Rett syndrome. Pediatr Res. 2006;60:443–449. doi: 10.1203/01.pdr.0000238302.84552.d0. [DOI] [PubMed] [Google Scholar]
- 6.Lioy DT, Garg SK, Monaghan CE, Raber J, Foust KD, Kaspar BK, Hirrlinger PG, Kirchhoff F, Bissonnette JM, Ballas N, et al. A role for glia in the progression of Rett’s syndrome. Nature. 2011;475:497–500. doi: 10.1038/nature10214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Robinson L, Guy J, McKay L, Brockett E, Spike RC, Selfridge J, De Sousa D, Merusi C, Riedel G, Bird A, et al. Morphological and functional reversal of phenotypes in a mouse model of Rett syndrome. Brain. 2012;135:2699–2710. doi: 10.1093/brain/aws096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Abdala AP, Dutschmann M, Bissonnette JM, Paton JF. Correction of respiratory disorders in a mouse model of Rett syndrome. Proc Natl Acad Sci USA. 2010;107:18208–18213. doi: 10.1073/pnas.1012104107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lalley PM, Bischoff AM, Richter DW. 5-HT-1A receptor-mediated modulation of medullary expiratory neurones in the cat. J Physiol. 1994;476:117–130. [PMC free article] [PubMed] [Google Scholar]
- 10.Richter DW, Manzke T, Wilken B, Ponimaskin E. Serotonin receptors: guardians of stable breathing. Trends Mol Med. 2003;9:542–548. doi: 10.1016/j.molmed.2003.10.010. [DOI] [PubMed] [Google Scholar]
- 11.Bartoszyk GD, Van Amsterdam C, Greiner HE, Rautenberg W, Russ H, Seyfried CA. Sarizotan, a serotonin 5-HT1A receptor agonist and dopamine receptor ligand. 1. Neurochemical profile. J Neural Transm. 2004;111:113–126. doi: 10.1007/s00702-003-0094-7. [DOI] [PubMed] [Google Scholar]
- 12.Goetz CG, Damier P, Hicking C, Laska E, Müller T, Olanow CW, Rascol O, Russ H. Sarizotan as a treatment for dyskinesias in Parkinson’s disease: a double-blind placebo-controlled trial. Mov Disord. 2007;22:179–186. doi: 10.1002/mds.21226. [DOI] [PubMed] [Google Scholar]
- 13.Olanow CW, Damier P, Goetz CG, Mueller T, Nutt J, Rascol O, Serbanescu A, Deckers F, Russ H. Multicenter, open-label, trial of sarizotan in Parkinson disease patients with levodopa-induced dyskinesias (the SPLENDID Study) Clin Neuropharmacol. 2004;27:58–62. doi: 10.1097/00002826-200403000-00003. [DOI] [PubMed] [Google Scholar]
- 14.Morschel M, Dutschmann M. Pontine respiratory activity involved in inspiratory/expiratory phase transition. Philo Trans R Soc Lond B Biol Sci. 2009;364:2517–2526. doi: 10.1098/rstb.2009.0074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen RZ, Akbarian S, Tudor M, Jaenisch R. Deficiency of methyl-CpG binding protein-2 in CNS neurons results in a Rett-like phenotype in mice. Nat Genet. 2001;27:327–331. doi: 10.1038/85906. [DOI] [PubMed] [Google Scholar]
- 16.Guy J, Hendrich B, Holmes M, Martin JE, Bird A. A mouse Mecp2-null mutation causes neurological symptoms that mimic Rett syndrome. Nat Genet. 2001;27:322–326. doi: 10.1038/85899. [DOI] [PubMed] [Google Scholar]
- 17.Brendel C, Belakhov V, Werner H, Wegener E, Gärtner J, Nudelman I, Baasov T, Huppke P. Readthrough of nonsense mutations in Rett syndrome: evaluation of novel aminoglycosides and generation of a new mouse model. J Mol Med (Berl) 2011;89:389–398. doi: 10.1007/s00109-010-0704-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lawson-Yuen A, Liu D, Han L, Jiang ZI, Tsai GE, Basu AC, Picker J, Feng J, Coyle JT. Ube3a mRNA and protein expression are not decreased in Mecp2R168X mutant mice. Brain Res. 2007;1180:1–6. doi: 10.1016/j.brainres.2007.08.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mortola JP, Noworaj A. Two-sidearm tracheal cannula for respiratory airflow measurements in small animals. J Appl Physiol. 1983;55:250–253. doi: 10.1152/jappl.1983.55.1.250. [DOI] [PubMed] [Google Scholar]
- 20.Bissonnette JM, Knopp SJ. Separate respiratory phenotypes in methyl-CpG-binding protein 2 (Mecp2) deficient mice. Pediatr Res. 2006;59:513–518. doi: 10.1203/01.pdr.0000203157.31924.4a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Viemari JC, Roux JC, Tryba AK, Saywell V, Burnet H, Peña F, Zanella S, Bévengut M, Barthelemy-Requin M, Herzing LB, et al. Mecp2 deficiency disrupts norepinephrine and respiratory systems in mice. J Neurosci. 2005;25:11521–11530. doi: 10.1523/JNEUROSCI.4373-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Paton JF. A working heart-brainstem preparation of the mouse. J Neurosci Methods. 1996;65:63–68. doi: 10.1016/0165-0270(95)00147-6. [DOI] [PubMed] [Google Scholar]
- 23.Larsen J, Gasser K, Hahin R. An analysis of dimethylsulfoxide-induced action potential block: a comparative study of DMSO and other aliphatic water soluble solutes. Toxicol Appl Pharmacol. 1996;140:296–314. doi: 10.1006/taap.1996.0225. [DOI] [PubMed] [Google Scholar]
- 24.Jourdon P, Berwald-Netter Y, Dubois JM. Effects of dimethylsulfoxide on membrane currents of neuroblastoma x glioma hybrid cell. Biochim Biophys Acta. 1986;856:399–402. doi: 10.1016/0005-2736(86)90053-2. [DOI] [PubMed] [Google Scholar]
- 25.Krösser S, Tillner J, Fluck M, Ungethüm W, Wolna P, Kovar A. Pharmacokinetics of sarizotan after oral administration of single and repeat doses in healthy subjects. Int J Clin Pharmacol Ther. 2007;45:271–280. doi: 10.5414/cpp45271. [DOI] [PubMed] [Google Scholar]
- 26.Archer H, Evans J, Leonard H, Colvin L, Ravine D, Christodoulou J, Williamson S, Charman T, Bailey ME, Sampson J, et al. Correlation between clinical severity in patients with Rett syndrome with a p.R168X or p.T158M MECP2 mutation, and the direction and degree of skewing of X-chromosome inactivation. J Med Genet. 2007;44:148–152. doi: 10.1136/jmg.2006.045260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Neul JL, Fang P, Barrish J, Lane J, Caeg EB, Smith EO, Zoghbi H, Percy A, Glaze DG. Specific mutations in methyl-CpG-binding protein 2 confer different severity in Rett syndrome. Neurology. 2008;70:1313–1321. doi: 10.1212/01.wnl.0000291011.54508.aa. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Voituron N, Hilaire G. The benzodiazepine midazolam mitigates the breathing defects of Mecp2-deficient mice. Respir Physiol Neurobiol. 2011;177:56–60. doi: 10.1016/j.resp.2011.02.002. [DOI] [PubMed] [Google Scholar]
- 29.Ogier M, Wang H, Hong E, Wang Q, Greenberg ME, Katz DM. Brain-derived neurotrophic factor expression and respiratory function improve after ampakine treatment in a mouse model of Rett syndrome. J Neurosci. 2007;27:10912–10917. doi: 10.1523/JNEUROSCI.1869-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Johnson RA, Lam M, Punzo AM, Li H, Lin BR, Ye K, Mitchell GS, Chang Q. 7,8-dihydroxyflavone (7,8-dhf) exhibits therapeutic efficacy in a mouse model of rett syndrome. J Appl Physiol. 2012;112:704–710. doi: 10.1152/japplphysiol.01361.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ren J, Ding X, Funk GD, Greer JJ. Anxiety-related mechanisms of respiratory dysfunction in a mouse model of Rett syndrome. J Neurosci. 2012;32:17230–17240. doi: 10.1523/JNEUROSCI.2951-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Song G, Tin C, Giacometti E, Poon CS. Habituation without nmda receptor-dependent desensitization of hering-breuer apnea reflex in a mecp2 mutant mouse model of rett syndrome. Front Integr Neurosci. 2011;5:6. doi: 10.3389/fnint.2011.00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schmid DA, Yang T, Ogier M, Adams I, Mirakhur Y, Wang Q, Massa SM, Longo FM, Katz DM. A TrkB small molecule partial agonist rescues TrkB phosphorylation deficits and improves respiratory function in a mouse model of Rett syndrome. J Neurosci. 2012;32:1803–1810. doi: 10.1523/JNEUROSCI.0865-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Samaco RC, Mandel-Brehm C, Chao HT, Ward CS, Fyffe-Maricich SL, Ren J, Hyland K, Thaller C, Maricich SM, Humphreys P, et al. Loss of MeCP2 in aminergic neurons causes cell-autonomous defects in neurotransmitter synthesis and specific behavioral abnormalities. Proc Natl Acad Sci USA. 2009;106:21966–21971. doi: 10.1073/pnas.0912257106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ide S, Itoh M, Goto Y. Defect in normal developmental increase of the brain biogenic amine concentrations in the mecp2-null mouse. Neurosci Lett. 2005;386:14–17. doi: 10.1016/j.neulet.2005.05.056. [DOI] [PubMed] [Google Scholar]
- 36.Panayotis N, Ghata A, Villard L, Roux JC. Biogenic amines and their metabolites are differentially affected in the Mecp2-deficient mouse brain. BMC Neurosci. 2011;12:47. doi: 10.1186/1471-2202-12-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Samaco RC, Fryer JD, Ren J, Fyffe S, Chao HT, Sun Y, Greer JJ, Zoghbi HY, Neul JL. A partial loss of function allele of methyl-CpG-binding protein 2 predicts a human neurodevelopmental syndrome. Hum Mol Genet. 2008;17:1718–1727. doi: 10.1093/hmg/ddn062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hodges MR, Tattersall GJ, Harris MB, McEvoy SD, Richerson DN, Deneris ES, Johnson RL, Chen ZF, Richerson GB. Defects in breathing and thermoregulation in mice with near-complete absence of central serotonin neurons. J Neurosci. 2008;28:2495–2505. doi: 10.1523/JNEUROSCI.4729-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ray RS, Corcoran AE, Brust RD, Kim JC, Richerson GB, Nattie E, Dymecki SM. Impaired respiratory and body temperature control upon acute serotonergic neuron inhibition. Science. 2011;333:637–642. doi: 10.1126/science.1205295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Stettner GM, Huppke P, Brendel C, Richter DW, Gärtner J, Dutschmann M. Breathing dysfunctions associated with impaired control of postinspiratory activity in Mecp2-/y knockout mice. J Physiol. 2007;579:863–876. doi: 10.1113/jphysiol.2006.119966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dutschmann M, Herbert H. The Kölliker-Fuse nucleus gates the postinspiratory phase of the respiratory cycle to control inspiratory off-switch and upper airway resistance in rat. Eur J Neurosci. 2006;24:1071–1084. doi: 10.1111/j.1460-9568.2006.04981.x. [DOI] [PubMed] [Google Scholar]
- 42.Dhingra RRBD, Hsieh YH, Jacono FJ, Dusschmann M, Katz DM, Dick TE.Blockade of dorsolateral pontine 5ht1a receptors destabilizes the respiratory rhythm in wild-type mice. Program No 29815 2010 Neuroscience Meeting Planner San Diego, CA: Society for Neuroscience, 2010 Online 2010
- 43.Roux JC, Panayotis N, Dura E, Villard L. Progressive noradrenergic deficits in the locus coeruleus of Mecp2 deficient mice. J Neurosci Res. 2010;88:1500–1509. doi: 10.1002/jnr.22312. [DOI] [PubMed] [Google Scholar]
- 44.Roux JC, Dura E, Villard L. Tyrosine hydroxylase deficit in the chemoafferent and the sympathoadrenergic pathways of the Mecp2 deficient mouse. Neurosci Lett. 2008;447:82–86. doi: 10.1016/j.neulet.2008.09.045. [DOI] [PubMed] [Google Scholar]
- 45.Roux JC, Dura E, Moncla A, Mancini J, Villard L. Treatment with desipramine improves breathing and survival in a mouse model for Rett syndrome. Eur J Neurosci. 2007;25:1915–1922. doi: 10.1111/j.1460-9568.2007.05466.x. [DOI] [PubMed] [Google Scholar]
- 46.Zanella S, Mebarek S, Lajard AM, Picard N, Dutschmann M, Hilaire G. Oral treatment with desipramine improves breathing and life span in rett syndrome mouse model. Respir Physiol Neurobiol. 2008;60:116–121. doi: 10.1016/j.resp.2007.08.009. [DOI] [PubMed] [Google Scholar]
- 47.Chang Q, Khare G, Dani V, Nelson S, Jaenisch R. The disease progression of Mecp2 mutant mice is affected by the level of BDNF expression. Neuron. 2006;49:341–348. doi: 10.1016/j.neuron.2005.12.027. [DOI] [PubMed] [Google Scholar]
- 48.Wang H, Chan SA, Ogier M, Hellard D, Wang Q, Smith C, Katz DM. Dysregulation of brain-derived neurotrophic factor expression and neurosecretory function in Mecp2 null mice. J Neurosci. 2006;26:10911–10915. doi: 10.1523/JNEUROSCI.1810-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kline DD, Ogier M, Kunze DL, Katz DM. Exogenous brain-derived neurotrophic factor rescues synaptic dysfunction in Mecp2-null mice. J Neurosci. 2010;30:5303–5310. doi: 10.1523/JNEUROSCI.5503-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tropea D, Giacometti E, Wilson NR, Beard C, McCurry C, Fu DD, Flannery R, Jaenisch R, Sur M. Partial reversal of Rett syndrome-like symptoms in MeCP2 mutant mice. Proc Natl Acad Sci USA. 2009;106:2029–2034. doi: 10.1073/pnas.0812394106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lalley PM. D1/D2-dopamine receptor agonist dihydrexidine stimulates inspiratory motor output and depresses medullary expiratory neurons. Am J Physiol Regul Integr Comp Physiol. 2009;296:R1829–R1836. doi: 10.1152/ajpregu.00057.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Smith Y, Bevan MD, Shink E, Bolam JP. Microcircuitry of the direct and indirect pathways of the basal ganglia. Neuroscience. 1998;86:353–387. doi: 10.1016/s0306-4522(98)00004-9. [DOI] [PubMed] [Google Scholar]
- 53.Kravitz AV, Freeze BS, Parker PR, Kay K, Thwin MT, Deisseroth K, Kreitzer AC. Regulation of parkinsonian motor behaviours by optogenetic control of basal ganglia circuitry. Nature. 2010;466:622–626. doi: 10.1038/nature09159. [DOI] [PMC free article] [PubMed] [Google Scholar]