Abstract
Myosin light chain kinase (MLCK; gene code, MYLK) is a multifunctional enzyme involved in isoform-specific nonmuscle (nm) and smooth muscle contraction, inflammation, and vascular permeability, processes directly relevant to asthma pathobiology. In this report, we highlight the contribution of the nm isoform (nmMLCK) to asthma susceptibility and severity, supported by studies in two lines of transgenic mice with knocking out nmMLCK or selectively overexpressing nmMLCK in endothelium. These mice were sensitized to exhibit ovalbumin-mediated allergic inflammation. Genetically engineered mice with targeted nmMLCK deletion (nmMLCK−/−) exhibited significant reductions in lung inflammation and airway hyperresponsiveness. Conversely, mice with overexpressed nmMLCK in endothelium (nmMLCKec/ec) exhibited elevated susceptibility and severity in asthmatic inflammation. In addition, reduction of nmMLCK expression in pulmonary endothelium by small interfering RNA results in reduced asthmatic inflammation in wild-type mice. These pathophysiological assessments demonstrate the positive contribution of nmMLCK to asthmatic inflammation, and a clear correlation of the level of nmMLCK with the degree of experimental allergic inflammation. This study confirms MYLK as an asthma candidate gene, and verifies nmMLCK as a novel molecular target in asthmatic pathobiology.
Keywords: asthma, endothelial permeability, nonmuscle myosin light chain kinase, transgenic mice
Clinical Relevance
This study demonstrates the contribution of nonmuscle myosin light chain kinase (nmMLCK) to asthmatic inflammation, and a clear correlation of the level of nmMLCK with the degree of experimental allergic inflammation. This study also verifies nmMLCK as a novel molecular target in asthmatic pathobiology.
Asthma is a complex inflammatory lung disorder, influenced by environmental and genetic factors, and characterized by airway hyperreactivity and airflow obstruction. Based on linkage of diverse atopic phenotypes (allergic asthma, allergic rhinitis, atopic dermatitis) to a genomic region within chromosome 3q21 (1–3), we previously explored the gene encoding the Ca2+/calmodulin–dependent myosin light chain kinase (MLCK; gene code, Mylk) as a candidate gene involved in asthma susceptibility (4). The human MYLK gene spans 272 kilobases (kb) on chromosome 3q21.1, and encodes a 210-kD nonmuscle (nm) MLCK isoform (nmMLCK) that we previously identified via cloning from a human endothelium–derived cDNA library (5). In addition, MYLK encodes a 108-kD smooth muscle (sm) isoform (smMLCK) (6, 7). Both smMLCK and nmMLCK are central regulators of cellular contraction (8) via robust MLC phosphorylation; however, conventional concepts of asthma pathogenesis have implicated only the smMLCK isoform as a potentially key contributor to the asthmatic phenotype, with increased smMLCK expression noted in sm tissues (9) and in bronchial biopsies (10) from subjects with asthma, contributing to bronchial sm proliferation and increased bronchial contractility. Consistent with this concept, we recently identified an MYLK variant residing within the putative smMLCK promoter (11) that is significantly associated with asthma susceptibility (12).
In contrast, information on the role of the nmMLCK isoform in asthma pathobiology is severely limited. Leveraging extensive MYLK resequencing (13), we evaluated the association of 17 MYLK variants (reflecting specific smMLCK and nmMLCK genomic locales) in two ethnically diverse case–control samples (European and African descent) collected as part of a Chicago-based asthma cohort (14). These studies revealed the association of an African-specific, nonsynonymous variant in MYLK (Pro147Ser) with severe asthma (4) with replication in a family-based African descent asthma cohort in Barbados (12). This severe asthma single-nucleotide polymorphism was associated with total IgE levels and resides within the unique N terminus of nmMLCK at significant distance from the smMLCK start site (4). In addition, nmMLCK is consistently up-regulated upon contact with asthmatic stimuli (see Figure E1 in the online supplement). Human lung biopsy with existing asthma exhibited higher expression of nmMLCK, as did the lung tissue from mice with experimental asthma (Figure E1). Human lung endothelial cells challenged with T helper (Th) 2 cytokines, IL-4 and IL-13, also exhibit higher expression of nmMLCK (Figure E1). These findings with human asthma, murine model of experimental asthma, and cellular response to asthmatic stimuli strongly suggest a potential role for nmMLCK in asthma pathobiology, and are potentially consistent with the observed gate-keeper function of nmMLCK in leukocyte trafficking into gastrointestinal and lung tissues (15–17) (Figure E2), and in regulation of endothelial/epithelial barrier integrity (18, 19). Complementary in vitro studies have highlighted the multifunctional nature of the nmMLCK isoform and activation by proinflammatory stimuli, leading to apoptotic regulation (20), leukocyte diapedesis (17), and endothelial and epithelial monolayer integrity, events potentially contributing to airway obstruction and airway hyperreactivity (21).
In this report, we confirm the contribution of nmMLCK to asthma susceptibility and pathobiology using transgenic mice with altered expression of nmMLCK regulating the severity of ovalbumin (OVA)-mediated lung inflammation and airway hyperreactivity. In addition, we verify nmMLCK as a novel molecular therapeutic target in asthmatic pathobiology.
Materials and Methods
Animals
All mice were housed in an environmentally controlled animal facility. All animal procedures were approved by the Institutional Animal Care and Use Committee (University of Illinois, Chicago, IL). Two genetically engineered murine lines (nmMLCK−/− and nmMLCKec/ec mice) were used to assess the influence of the nmMLCK isoform on asthma responsiveness. Details of the murine nmMLCK−/− knockout mice (22, 23) and nmMLCKec/ec mice (endothelium-specific overexpression) (24) have been described previously. Experimental murine asthma was induced by OVA administration as previously described (25). Briefly, 6- to 8-month-old mice (25–30 g) received 20 μg OVA mixed with 1 mg alum (intraperitoneal, Days 0 and 7), followed by an intratracheal OVA challenge (30 mg/kg, Day 14). On Day 17 (3 d after OVA challenge), airway hyperresponsiveness (AHR) was determined. Animals were killed for bronchoalveolar lavage (BAL) fluid extraction and tissue harvesting.
Airway Responsiveness Measurements
Airway responsiveness to intravenously administered airway constrictors was assessed in anesthetized, tracheostomized, mechanically ventilated mice via two previously described methods: airway pressure time index (APTI) (25) and respiratory system resistance (R) (26).
BAL Fluid
At the termination of each experiment, animals were killed in accordance with institutional guidelines for animal care and use. BAL was performed as previously described (19) through the tracheal cannula (after airway hyperreactivity measurements) with cold Hank’s balanced salt solution. BAL cells were counted. Protein concentrations in BAL fluid supernatant were measured using an RC DC Protein Assay (Bio-Rad, Hercules, CA). IL-5, IL-13, and eotaxin levels were measured in unconcentrated BAL fluid (supernatant) via a mouse BioPlex cytokine panel system (Bio-Rad, Hercules, CA), as previously described (25).
Lung Histopathology
Murine lungs were excised and the left lung immersed in 10% formalin for 48 hours, washed with 70% ethanol, dehydrated, and embedded in glycol methacrylate. In addition, paraffin-embedded sections were stained for several markers of airway inflammation and remodeling: hematoxylin and eosin for evidence of inflammatory injury in peribronchial and perivascular; periodic acid-Schiff (PAS) for identification of mucus-containing goblet cells; and antisera (EMBP [S-16]: sc-33938; Santa Cruz Biotechnology, Santa Cruz, CA) to major basic protein for determination of lung eosinophil content. Two sections (5–6 μm) from each experimental animal were stained and quantified.
Statistical Analysis
For all data reported, values are reported as means (± SD). We performed statistical comparisons among treatment groups by randomized-design, two-way ANOVA, followed by the Newman-Keuls post hoc test for more than two groups, or by an unpaired Student’s t test for two groups. In all cases, we defined statistical significance as a P value less than 0.05. The statistical analysis was done using OriginPro 8.1 software (OriginLab Corp., Northampton, MA).
Results
Influence of Altered nmMLCK Expression on Murine Airway Reactivity
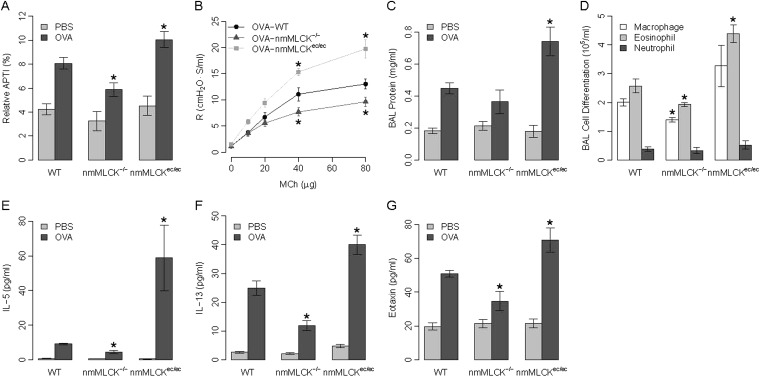
Initial studies were designed to assess the contribution of the nmMLCK isoform to the pathobiology of asthmatic inflammation using the well accepted model of OVA-induced murine asthma and airway hyperreactivity. We employed OVA-sensitized, genetically engineered nmMLCK−/− knockout mice (23) and nmMLCKec/ec transgenic mice (selectively expressing nmMLCK in endothelium) (24) to define the influence of nmMLCK on the susceptibility and severity of a murine asthmatic phenotype. OVA-induced AHR was dually assessed by measurements of acetylcholine-induced airway pressures and quantified as the relative APTI (25) and by R after methacholine challenge (26). OVA challenge significantly increased airway responsiveness (elevated AHR) in wild-type (WT) mice (Figure 1A). Targeted deletion of the nmMLCK isoform (nmMLCK−/− mice) attenuated the development of OVA-mediated elevations in APTI or R, whereas nmMLCKec/ec mice, with overexpression of nmMLCK restricted to the vasculature, exhibited exaggerated airway reactivity responses (Figures 1A–1B). Mice with either nmMLCK deletion (nmMLCK−/−) or nmMLCK overexpression (nmMLCKec/ec) failed to exhibit altered airway responsiveness in the absence of prior OVA sensitization.
Figure 1.
Effect of nonmuscle myosin light chain kinase (nmMLCK) expression on airway reactivity and inflammation in a murine asthma model. (A) Airway hyperreactivity was assessed in wild-type (WT), nmMLCK−/−, and nmMLCKec/ec mice using relative airway pressure time index (APTI), a measurement of acetylcholine-induced changes in airway pressures. (B) Airway reactivity to methacholine in ovalbumin (OVA)-challenged mice. (C) Effects of nmMLCK expression on bronchoalveolar lavage (BAL) protein leakage. (D) Effects of nmMLCK expression on inflammatory leukocyte infiltration. (E–G) Effects of nmMLCK expression on Th2 cytokine secretion: IL-5 (E), IL-13 (F), and eotaxin (G) into the alveolar space (BAL) in all murine groups. *P < 0.05 compared with OVA-challenged WT mice; n ≥ 5 for all groups.
Effects of Expression Levels of nmMLCK on Protein Leakage and Leukocyte Infiltration
The nmMLCK isoform is also a critical participant in epithelial and endothelial barrier regulation controlling leukocyte infiltrations into tissues and the extent of vascular permeability during inflammatory injury (15, 18, 22, 24). In prior studies, we demonstrated that nmMLCK−/− mice are significantly protected from LPS-mediated inflammatory lung injury (23, 24), whereas nmMLCKec/ec mice with overexpression of nmMLCK restricted to the vascular endothelium exhibit augmented LPS-mediated lung vascular permeability, which is linearly correlated to gene dose (23), compared with WT mice (23, 24). Assessment of the influence of nmMLCK expression on murine lung inflammatory indices revealed no differences in baseline BAL protein levels in OVA-sensitized WT, nmMLCK−/−, and nmMLCKec/ec mice; however, OVA challenge induced a marked increase in BAL protein levels in each group of sensitized mice. Compared with WT mice, nmMLCK−/− knockout mice exhibited reduced BAL protein content, whereas nmMLCKec/ec mice demonstrated significantly greater BAL protein levels after OVA challenge (Figure 1C). Similar results were observed with regard to leukocyte counts, with nmMLCK−/− mice demonstrating the lowest OVA-induced increases in total BAL leukocytes (25–30% reduction in BAL macrophages and eosinophils) compared with OVA-challenged WT mice (Figure 1D). Conversely, nmMLCKec/ec mice exhibited significantly greater numbers of BAL leukocytes (60–70% increase in macrophages and eosinophils; Figure 1D).
Influence of Altered nmMLCK Expression on Th2 Cytokine Release
We next assessed BAL levels of Th2 cytokines (IL-5, IL-13, eotaxin) in the three murine groups with varying levels and sites of nmMLCK expression. Consistent with this established preclinical model of asthma, OVA challenge resulted in increased levels of Th2 cytokines in the alveolar space in all three experimental groups (Figures 1E–1G), with nmMLCK−/− mice demonstrating reduced BAL levels of Th2 cytokines, and nmMLCKec/ec mice exhibiting greater BAL levels of each Th2 cytokine compared with OVA-challenged WT mice.
Effects of Altered nmMLCK Expression Levels on Lung Asthmatic Histology
The above indices of lung inflammation were confirmed by histologic evaluation of lung sections obtained from the three groups of OVA-sensitized mice varying in the level of nmMLCK expression (WT, nmMLCK−/−, and nmMLCKec/ec; Figures 2A and 2B). OVA-challenged WT mice displayed increased leukocyte recruitment to peribronchial and perivascular areas, with substantially reduced recruitment in OVA-challenged nmMLCK−/− mice and markedly increased levels of infiltrated leukocytes, particularly in perivascular sites, in OVA-sensitized nmMLCKec/ec mice. PAS staining to identify mucus-producing goblet cells, a marker of asthmatic airway inflammation and remodeling (27), was reduced in the airways of nmMLCK−/− mice, whereas nmMLCKec/ec mice exhibited greater numbers of PAS-staining goblet cells compared with OVA-challenged WT mice (Figure 2C). Similar to PAS staining, staining of major basic protein, an eosinophilic marker, was markedly reduced in OVA-challenged nmMLCK−/− mice (Figure 2D), but, strikingly, increased in nmMLCKec/ec mice, which is consistent with the BAL cellularity (Figure 1D).
Figure 2.
Histological Evaluation of the influence of nmMLCK expression on lung inflammation and airway remodeling. Histology (hematoxylin and eosin [H&E]) sections in WT, nmMLCK−/−, and nmMLCKec/ec mice demonstrating leukocyte infiltration and recruitment to the (A) peribronchial and (B) perivascular areas, respectively, after OVA challenge. (C) Lung sections stained with periodic acid-Schiff (PAS) to detect goblet cell (arrows) presence and activity in PBS or OVA-challenged mice. (D) Major basic protein (MBP) staining in tissue microarray samples derived from the OVA-challenged murine lines. All histological evaluations were quantified and summarized in the bar graphs accompanying each analysis shown in the last column. *P < 0.05; n = 5–6. OD, optical density.
Evaluation of Lung Endothelial nmMLCK as a Novel Therapeutic Target for Murine Asthmatic Inflammation
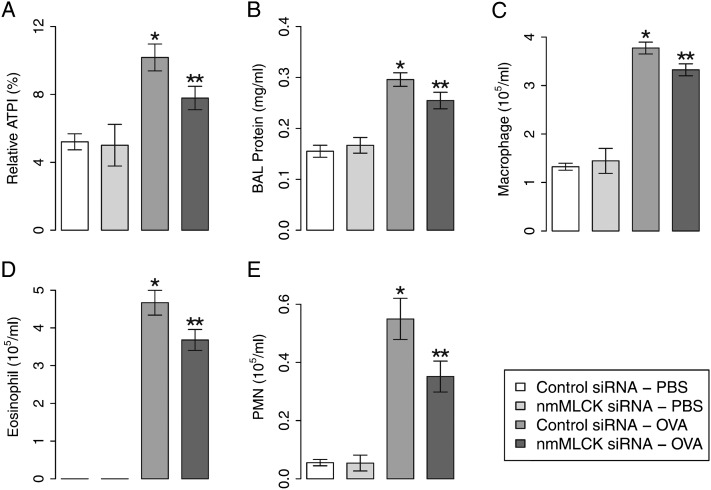
Finally, to further validate the critical role of nmMLCK in asthmatic susceptibility, nmMLCK small interfering RNAs (siRNAs) were delivered intravenously to OVA-sensitized asthmatic mice via angiotensin-converting enzyme antibody–tagged liposome delivery to target pulmonary endothelium (5 mg/kg, intravenous) (19) before OVA challenge. With this delivery system, nmMLCK expression is selectively reduced (Figure E3) in pulmonary endothelium in mouse receiving siRNA, but not in endothelium in other organs or other pulmonary tissues (19). Mice receiving nmMLCK siRNAs have exhibited significant reductions in OVA-mediated AHR, BAL protein leakage, and inflammatory leukocyte infiltration (Figures 3A–3E). WT mice challenged with nmMCLK siRNAs failed to alter inflammatory indices in the absence of OVA sensitization. Together, these findings indicate that availability of the nmMLCK isoform, especially when expressed in lung endothelium, facilitates the migration of inflammatory eosinophils into OVA-challenged murine airways and parenchyma, and is a key determinant of AHR, mucus production, and eosinophilic inflammation. These studies, confirming the involvement of the nmMLCK isoform in asthma pathobiology, support MYLK as a legitimate and novel therapeutic target in severe asthma.
Figure 3.
Effect of nmMLCK silencing on asthmatic inflammation in mice. WT mice received nmMLCK small interfering RNA (siRNA) to reduce nmMLCK expression in the lungs, and were challenged by OVA to activate asthmatic inflammation. (A) Effects of nmMLCK siRNA on airway hyperreactivity reflected by acetylcholine-induced APTI. (B) Effects of nmMLCK siRNA on BAL protein leakage. (C–E) Effects of nmMLCK expression on inflammatory leukocyte infiltration (macrophage, eosinophil, and neutrophil). *P < 0.05 compared with control siRNA–PBS group; **P < 0.05 compared with control siRNA–OVA group; n = 5 or 6.
Discussion
Asthma is an enigmatic inflammatory lung disorder with a strong genetic influence and associated with staggering health disparities across racial groups (28, 29). Our prior studies exploring the genetic basis for acute inflammatory lung disorders (13) identified common genetic variation in several innate immunity genes (MIF, PBEF, and MYLK) contributing to the risk of sepsis and acute inflammatory lung injury (12, 13, 30). Encouraged by supportive linkage evidence to chromosome 3q21 (1–3), we recently demonstrated that MYLK, a gene encoding the critical cytoskeletal effector, MLCK, is a candidate gene for asthma susceptibility with particular relevance in subjects of African descent (4, 12). Several studies have suggested involvement of smMLCK in airway sm cell hypertrophy and in development of the severe asthma phenotype (9). Our studies now clearly demonstrate that nmMLCK isoform should be considered a relevant and equally important participant in asthma susceptibility. The most important finding of the current study is to validate nmMLCK as a novel therapeutic target for asthmatic inflammation.
Three distinct lines of evidence support the impactful role of nmMLCK in asthma pathobiology. The initial line of evidence relates to the strong protection from the development of asthma-like phenotypes (airway inflammation and hyperreactivity) exhibited by OVA-sensitized nmMLCK−/− knockout mice compared with WT littermates. We previously demonstrated that the nmMLCK−/− murine line is also protected from sepsis lethality and ventilator-associated inflammatory lung injury (23), highlighting the essential contribution of nmMLCK to lung inflammatory responses and development of allergic responses in OVA-challenged mice. In addition, nmMLCK expression altered secretion of Th2-cytokines, such as IL-5, IL-13, and eotaxin, mediators involved in the production, differentiation, maturation, recruitment, and activation of eosinophils, a key asthma effector (31, 32).
A second line of evidence strongly supporting involvement of the nmMLCK isoform in asthma pathobiology is the markedly exaggerated responses of OVA-sensitized nmMLCKec/ec transgenic mice to OVA challenge with increased vascular permeability, increased leukocyte infiltration, increased Th2 cytokine secretion, and augmented AHR. Given that nmMLCKec/ec mice exhibit targeted nmMLCK overexpression only in the vascular endothelium (24), these studies unequivocally highlight not only the essential role of nmMLCK in lung inflammatory regulatory processes, but the gate-keeper function of nmMLCK in lung endothelial responses to inflammation and modulation of innate immunity.
The third line of evidence supports the therapeutic strategy targeting pulmonary nmMLCK, with a novel delivery technology for siRNA to target pulmonary endothelium (19). These data from our siRNA study further indicate the essential role of nmMLCK in acute allergic inflammation, including airway remodeling, cytokine production, and endothelium/epithelium integrity, which might still be explained by the classical mechanism of nmMLCK in cytoskeleton rearrangement regulation upon stimulation, especially in endothelium.
The potential mechanisms by which the nmMLCK isoform contributes to asthma pathogenesis and severity have not been precisely identified. However, based on immunohistochemical studies of human lung tissues in subjects with asthma, human genetic studies, and studies in genetically engineered transgenic mice, the expression of nmMLCK in the vascular endothelium (as noted previously here) should be considered as an essential contributor to the asthma phenotype. The dynamic role of the nmMLCK-driven cytoskeleton in endothelial cell barrier regulation is well documented, as is the known gate-keeper function of nmMLCK in vascular regulation of fluid flux, and trafficking of inflammatory leukocytes into the lung parenchyma and airways (8, 17). In addition, we have further validated this gate-keeper function of nmMLCK in lung inflammation with a distinct approach. High tidal volume ventilation induces ventilation-induced lung injury, a similar but different type of lung inflammation. Leukocyte transendothelial migration pathway is significantly dysregulated by ventilation-induced lung injury in WT mouse lungs, but not in nmMLCK knockout lungs (GEO dataset: GSE14525 [19]; Figure E2), suggesting that nmMLCK plays a central role of this cellular process mediating lung inflammation. This is further supported by results in nmMLCK−/− mice receiving OVA challenge in which marked down-regulation of the OVA-induced leukocyte infiltration was noted. Strategies that reduce MLCK activity serve to eliminate edemagenic agonist–induced endothelial cell contraction and reduce inflammation-mediated permeability in vitro as well as in isolated lung preparations and in vivo murine models (22). We previously demonstrated that neutrophil–endothelial interaction directly increases nmMLCK activity and modulates neutrophil transmigration in response to leukotriene B4 (17). The elegant selective nmMLCK−/− knockout results in protection from combined injury induced by LPS, as well as by excessive mechanical stress via mechanical ventilation in murine models (23).
Consistent with endothelial expression of nmMLCK in asthma pathobiology, the profound phenotypic changes observed in both genetically engineered nmMLCKec/ec transgenic mice and nmMLCKec/ec mice overexpressing the nmMLCK isoform in the vascular endothelium also support the lung endothelium as an asthma-relevant target tissue. The detection of vascular endothelial growth factor and other endothelial-specific biomarkers in peripheral blood of subjects with moderate and severe asthma (33) are consistent with angiogenic and microvascular remodeling processes associated with asthma (34). Furthermore, in addition to serving as an active element regulating cardinal features of airway inflammation, the vascular endothelial cell barrier is a key element in determining protein leakiness of remodeled blood vessels in inflamed sites (35), and represents a potential therapeutic target (36).
In summary, our studies underscore the contributory role of nmMLCK expression, especially in lung endothelium, to asthma pathogenesis. The expression level of nmMLCK is correlated with susceptibility and severity of murine asthmatic inflammation. More importantly, this study has validated that lung endothelial nmMLCK is a novel therapeutic target for asthma.
Acknowledgments
Acknowledgments
The authors thank Drs. Julian Solway and Bohao Chen (University of Chicago) for their thoughtful discussions and assistance on the airway pressure measurements.
Footnotes
This work was supported by National Institutes of Health grants HL 91899 (J.G.N.G.), HL 58064 (J.G.N.G.), and HL 88144 (S.M.D.).
This article has an online supplement, which is accessible from this issue’s table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1165/rcmb.2013-0434OC on January 15, 2014
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Dizier MH, Besse-Schmittler C, Guilloud-Bataille M, Annesi-Maesano I, Boussaha M, Bousquet J, Charpin D, Degioanni A, Gormand F, Grimfeld A, et al. Genome screen for asthma and related phenotypes in the French EGEA study. Am J Respir Crit Care Med. 2000;162:1812–1818. doi: 10.1164/ajrccm.162.5.2002113. [DOI] [PubMed] [Google Scholar]
- 2.Kurz T, Altmueller J, Strauch K, Ruschendorf F, Heinzmann A, Moffatt MF, Cookson WO, Inacio F, Nurnberg P, Stassen HH, et al. A genome-wide screen on the genetics of atopy in a multiethnic European population reveals a major atopy locus on chromosome 3q21.3. Allergy. 2005;60:192–199. doi: 10.1111/j.1398-9995.2005.00646.x. [DOI] [PubMed] [Google Scholar]
- 3.Lee YA, Wahn U, Kehrt R, Tarani L, Businco L, Gustafsson D, Andersson F, Oranje AP, Wolkertstorfer A, v Berg A, et al. A major susceptibility locus for atopic dermatitis maps to chromosome 3q21. Nat Genet. 2000;26:470–473. doi: 10.1038/82625. [DOI] [PubMed] [Google Scholar]
- 4.Flores C, Ma SF, Maresso K, Ober C, Garcia JG. A variant of the myosin light chain kinase gene is associated with severe asthma in African Americans. Genet Epidemiol. 2007;31:296–305. doi: 10.1002/gepi.20210. [DOI] [PubMed] [Google Scholar]
- 5.Lazar V, Garcia JG. A single human myosin light chain kinase gene (MLCK; MYLK) Genomics. 1999;57:256–267. doi: 10.1006/geno.1999.5774. [DOI] [PubMed] [Google Scholar]
- 6.Gallagher PJ, Herring BP, Griffin SA, Stull JT. Molecular characterization of a mammalian smooth muscle myosin light chain kinase. J Biol Chem. 1991;266:23936–23944. [PMC free article] [PubMed] [Google Scholar]
- 7.Garcia JG, Lazar V, Gilbert-McClain LI, Gallagher PJ, Verin AD. Myosin light chain kinase in endothelium: molecular cloning and regulation. Am J Respir Cell Mol Biol. 1997;16:489–494. doi: 10.1165/ajrcmb.16.5.9160829. [DOI] [PubMed] [Google Scholar]
- 8.Dudek SM, Garcia JG. Cytoskeletal regulation of pulmonary vascular permeability. J Appl Physiol. 2001;91:1487–1500. doi: 10.1152/jappl.2001.91.4.1487. [DOI] [PubMed] [Google Scholar]
- 9.Benayoun L, Druilhe A, Dombret MC, Aubier M, Pretolani M. Airway structural alterations selectively associated with severe asthma. Am J Respir Crit Care Med. 2003;167:1360–1368. doi: 10.1164/rccm.200209-1030OC. [DOI] [PubMed] [Google Scholar]
- 10.Ma X, Cheng Z, Kong H, Wang Y, Unruh H, Stephens NL, Laviolette M. Changes in biophysical and biochemical properties of single bronchial smooth muscle cells from asthmatic subjects. Am J Physiol Lung Cell Mol Physiol. 2002;283:L1181–L1189. doi: 10.1152/ajplung.00389.2001. [DOI] [PubMed] [Google Scholar]
- 11.Han YJ, Ma SF, Wade MS, Flores C, Garcia JG. An intronic MYLK variant associated with inflammatory lung disease regulates promoter activity of the smooth muscle myosin light chain kinase isoform. J Mol Med (Berl) 2012;90:299–308. doi: 10.1007/s00109-011-0820-9. [DOI] [PubMed] [Google Scholar]
- 12.Gao L, Grant AV, Rafaels N, Stockton-Porter M, Watkins T, Gao P, Chi P, Munoz M, Watson H, Dunston G, et al. Polymorphisms in the myosin light chain kinase gene that confer risk of severe sepsis are associated with a lower risk of asthma. J Allergy Clin Immunol. 2007;119:1111–1118. doi: 10.1016/j.jaci.2007.03.019. [DOI] [PubMed] [Google Scholar]
- 13.Gao L, Grant A, Halder I, Brower R, Sevransky J, Maloney JP, Moss M, Shanholtz C, Yates CR, Meduri GU, et al. Novel polymorphisms in the myosin light chain kinase gene confer risk for acute lung injury. Am J Respir Cell Mol Biol. 2006;34:487–495. doi: 10.1165/rcmb.2005-0404OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.CSGA. A genome-wide search for asthma susceptibility loci in ethnically diverse populations. The Collaborative Study on the Genetics of Asthma (CSGA) Nat Genet. 1997;15:389–392. doi: 10.1038/ng0497-389. [DOI] [PubMed] [Google Scholar]
- 15.Clayburgh DR, Rosen S, Witkowski ED, Wang F, Blair S, Dudek S, Garcia JG, Alverdy JC, Turner JR. A differentiation-dependent splice variant of myosin light chain kinase, MLCK1, regulates epithelial tight junction permeability. J Biol Chem. 2004;279:55506–55513. doi: 10.1074/jbc.M408822200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kawkitinarong K, Linz-McGillem L, Birukov KG, Garcia JG. Differential regulation of human lung epithelial and endothelial barrier function by thrombin. Am J Respir Cell Mol Biol. 2004;31:517–527. doi: 10.1165/rcmb.2003-0432OC. [DOI] [PubMed] [Google Scholar]
- 17.Garcia JG, Verin AD, Herenyiova M, English D. Adherent neutrophils activate endothelial myosin light chain kinase: role in transendothelial migration. J Appl Physiol. 1998;84:1817–1821. doi: 10.1152/jappl.1998.84.5.1817. [DOI] [PubMed] [Google Scholar]
- 18.Clayburgh DR, Barrett TA, Tang Y, Meddings JB, Van Eldik LJ, Watterson DM, Clarke LL, Mrsny RJ, Turner JR. Epithelial myosin light chain kinase–dependent barrier dysfunction mediates T cell activation–induced diarrhea in vivo. J Clin Invest. 2005;115:2702–2715. doi: 10.1172/JCI24970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mirzapoiazova T, Moitra J, Moreno-Vinasco L, Sammani S, Turner JR, Chiang ET, Evenoski C, Wang T, Singleton PA, Huang Y, et al. Non-muscle myosin light chain kinase isoform is a viable molecular target in acute inflammatory lung injury. Am J Respir Cell Mol Biol. 2011;44:40–52. doi: 10.1165/rcmb.2009-0197OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Petrache I, Verin AD, Crow MT, Birukova A, Liu F, Garcia JG. Differential effect of MLC kinase in TNF-alpha–induced endothelial cell apoptosis and barrier dysfunction. Am J Physiol Lung Cell Mol Physiol. 2001;280:L1168–L1178. doi: 10.1152/ajplung.2001.280.6.L1168. [DOI] [PubMed] [Google Scholar]
- 21.Lee KS, Park SJ, Kim SR, Min KH, Jin SM, Puri KD, Lee YC. Phosphoinositide 3-kinase-delta inhibitor reduces vascular permeability in a murine model of asthma. J Allergy Clin Immunol. 2006;118:403–409. doi: 10.1016/j.jaci.2006.04.041. [DOI] [PubMed] [Google Scholar]
- 22.Rossi JL, Velentza AV, Steinhorn DM, Watterson DM, Wainwright MS. MLCK210 gene knockout or kinase inhibition preserves lung function following endotoxin-induced lung injury in mice. Am J Physiol Lung Cell Mol Physiol. 2007;292:L1327–L1334. doi: 10.1152/ajplung.00380.2006. [DOI] [PubMed] [Google Scholar]
- 23.Wainwright MS, Rossi J, Schavocky J, Crawford S, Steinhorn D, Velentza AV, Zasadzki M, Shirinsky V, Jia Y, Haiech J, et al. Protein kinase involved in lung injury susceptibility: evidence from enzyme isoform genetic knockout and in vivo inhibitor treatment. Proc Natl Acad Sci USA. 2003;100:6233–6238. doi: 10.1073/pnas.1031595100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Moitra J, Evenoski C, Sammani S, Wadgaonkar R, Turner JR, Ma SF, Garcia JG. A transgenic mouse with vascular endothelial over-expression of the non-muscle myosin light chain kinase-2 isoform is susceptible to inflammatory lung injury: role of sexual dimorphism and age. Transl Res. 2008;151:141–153. doi: 10.1016/j.trsl.2007.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang T, Moreno-Vinasco L, Huang Y, Lang GD, Linares JD, Goonewardena SN, Grabavoy A, Samet JM, Geyh AS, Breysse PN, et al. Murine lung responses to ambient particulate matter: genomic analysis and influence on airway hyperresponsiveness. Environ Health Perspect. 2008;116:1500–1508. doi: 10.1289/ehp.11229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tong J, Bandulwala HS, Clay BS, Anders RA, Shilling RA, Balachandran DD, Chen B, Weinstock JV, Solway J, Hamann KJ, et al. Fas-positive T cells regulate the resolution of airway inflammation in a murine model of asthma. J Exp Med. 2006;203:1173–1184. doi: 10.1084/jem.20051680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Walter MJ, Morton JD, Kajiwara N, Agapov E, Holtzman MJ. Viral induction of a chronic asthma phenotype and genetic segregation from the acute response. J Clin Invest. 2002;110:165–175. doi: 10.1172/JCI14345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.El-Ekiaby A, Brianas L, Skowronski ME, Coreno AJ, Galan G, Kaeberlein FJ, Seitz RE, Villaba KD, Dickey-White H, McFadden ER., Jr Impact of race on the severity of acute episodes of asthma and adrenergic responsiveness. Am J Respir Crit Care Med. 2006;174:508–513. doi: 10.1164/rccm.200603-431OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lester LA, Rich SS, Blumenthal MN, Togias A, Murphy S, Malveaux F, Miller ME, Dunston GM, Solway J, Wolf RL, et al. Ethnic differences in asthma and associated phenotypes: collaborative study on the genetics of asthma. J Allergy Clin Immunol. 2001;108:357–362. doi: 10.1067/mai.2001.117796. [DOI] [PubMed] [Google Scholar]
- 30.Meyer NJ, Garcia JG. Wading into the genomic pool to unravel acute lung injury genetics. Proc Am Thorac Soc. 2007;4:69–76. doi: 10.1513/pats.200609-157JG. [DOI] [PubMed] [Google Scholar]
- 31.Hogan SP, Mould AW, Young JM, Rothenberg ME, Ramsay AJ, Matthaei K, Young IG, Foster PS. Cellular and molecular regulation of eosinophil trafficking to the lung. Immunol Cell Biol. 1998;76:454–460. doi: 10.1046/j.1440-1711.1998.00766.x. [DOI] [PubMed] [Google Scholar]
- 32.Conroy DM, Williams TJ. Eotaxin and the attraction of eosinophils to the asthmatic lung. Respir Res. 2001;2:150–156. doi: 10.1186/rr52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Holgate ST, Polosa R. The mechanisms, diagnosis, and management of severe asthma in adults. Lancet. 2006;368:780–793. doi: 10.1016/S0140-6736(06)69288-X. [DOI] [PubMed] [Google Scholar]
- 34.McDonald DM. Angiogenesis and remodeling of airway vasculature in chronic inflammation. Am J Respir Crit Care Med. 2001;164:S39–S45. doi: 10.1164/ajrccm.164.supplement_2.2106065. [DOI] [PubMed] [Google Scholar]
- 35.Jeffery PK. Remodeling in asthma and chronic obstructive lung disease. Am J Respir Crit Care Med. 2001;164:S28–S38. doi: 10.1164/ajrccm.164.supplement_2.2106061. [DOI] [PubMed] [Google Scholar]
- 36.Harlan JM, Winn RK. Leukocyte–endothelial interactions: clinical trials of anti-adhesion therapy. Crit Care Med. 2002;30(5) Suppl:S214–S219. doi: 10.1097/00003246-200205001-00007. [DOI] [PubMed] [Google Scholar]