Abstract
Cytokinesis is a critical step of airway smooth muscle cell division that plays an essential role in the development and homeostasis of the respiratory system, as well as the progression of airway remodeling. The mechanisms that regulate smooth muscle cytokinesis are not fully understood. c-Abl (c-Abelson tyrosine kinase) is a nonreceptor protein tyrosine kinase that has a role in regulating actin dynamics and smooth muscle contraction. The role of c-Abl in cytokinesis has not been investigated. Here, c-Abl was found in the contractile ring, as evidenced by immunofluorescent microscopy. In addition, cortactin is a phosphorylatable protein that has been implicated in actin filament assembly. In this report, phosphorylated cortactin was also found in the contractile ring. Knockdown of c-Abl by RNA interference attenuated cortactin phosphorylation in the midzone and contractile ring formation. c-Abl knockdown decreased the number of cells undergoing cytokinesis, but increased the quantity of cells in metaphase/anaphase and the number of multinucleate cells. Treatment with the c-Abl pharmacological inhibitors, imatinib and GNF-5, had similar effects. Furthermore, the expression of a nonphosphorylatable cortactin mutant diminished cytokinesis. Finally, inhibition of actin filament assembly by latrunculin A attenuated c-Abl recruitment to the midzone. Thus, we propose a novel mechanism that regulates smooth muscle cell cytokinesis. c-Abl is recruited to the equator during cytokinesis, which may mediate cortactin phosphorylation. Phosphorylated cortactin may promote actin filament assembly, which facilitates contractile ring formation and cytokinesis. In addition, actin filament polymerization may facilitate the positioning of c-Abl to the contractile ring.
Keywords: cytokinesis, tyrosine kinase, adapter protein, actin cytoskeleton, smooth muscle
Clinical Relevance
Cytokinesis is a critical step of airway smooth muscle cell division. The present study shows that c-Abl (c-Abelson tyrosine kinase) plays an essential role in regulating contractile ring formation and cytokinesis. This may lead to better understanding of the pathogenesis of lung diseases such as asthma.
Airway smooth muscle cell division plays an essential role in the development and homeostasis of the respiratory system. Abnormal smooth muscle cell division contributes to the progression of airway remodeling, a key characteristic of asthma (1, 2).
A critical step of cell division is cytokinesis, in which the cytoplasm of a mother cell is divided to form two daughter cells after karyokinesis (3–5). During cytokinesis, a contractile ring consisting of actin filaments and myosin II assembles equatorially at the cell cortex. Activated myosin promotes constriction of the contractile ring to induce cleavage furrow ingression and cytoplastic division of a mother cell (3). The small GTPase RhoA and actin-associated proteins (formins, profilin, and cofillin) may promote formation of the contractile ring and cytokinesis (3, 5). However, other molecules may also regulate cytokinesis.
c-Abl (c-Abelson tyrosine kinase) is a nonreceptor tyrosine kinase that has a role in the regulation of the actin cytoskeleton important for various cellular functions, including nonmuscle cell adhesion and migration, as well as cardiac growth and development (6, 7). In smooth muscle, c-Abl is necessary for force development in response to contractile activation (8–10). Furthermore, c-Abl gets phosphorylated and activated in smooth muscle cells in response to stimulation with growth factors, which has been implicated in regulating the extracellular signal–regulated kinase (ERK) 1/2 pathway (11, 12). Recent studies suggest that c-Abl expression is up-regulated in asthmatic smooth muscle cells/tissues (13). However, the role of c-Abl in cytokinesis has not been explored.
Cortactin is an adapter protein that has been implicated in regulating actin dynamics and cell motility (14, 15). Cortactin undergoes phosphorylation at Tyr-421 in response to external stimulation, which has been implicated in its activation (14, 15). Cortactin activation may interact with Abl related gene, the Nck adapter, neuronal Wiskott-Aldrich syndrome protein, caldesmon, and Alix, which, in turn, promotes actin dynamics (14–16). Nevertheless, the role of cortactin phosphorylation in contractile ring formation and cytokinesis has not been investigated.
The objective of this study was to evaluate the role of c-Abl in contractile ring formation and cytokinesis of airway smooth muscle cells. Furthermore, we evaluated the interactions of c-Abl with cortactin in these cellular processes.
Materials and Methods
Cell Culture
Normal human airway smooth muscle (HASM) cells were prepared and cultured using previously described methods (12, 17; see the online supplement). For synchronization, cells were seeded in 10-cm dishes for 24 hours. They were treated with 100 ng/ml nocodazole (Calbiochem/EMD Millipore USA, Billerica, MA) for 24 hours. Mitotic cells were then collected by tapping the dishes gently, followed by brief centrifugation. Cells were resuspended in Ham’s F12 medium supplemented with 10% (vol/vol) FBS, and were seeded to collagen-coated coverslips (BD Biosciences, San Jose, CA) for 1 hour. They were then immunostained to determine mitosis and cytokinesis. Additional cells were seeded to collagen-coated coverslips for 24 hours to evaluate multinucleation. To inhibit c-Abl, cells were treated with 5 μM imatinib (LC Laboratories, Woburn, MA) or GNF-5 (Calbiochem/EMD Millipore USA) for 1 hour (for mitosis or cytokinesis) or 24 hours (for multinucleation). DMSO was used as a control.
Immunofluorescent and fluorescent analysis was performed as previously described (11, 12, 18, 19). The cellular localization of fluorescently labeled molecules was viewed under a high-resolution digital fluorescent microscope (DMI600; Leica, Wetzlar, Germany). The time of image capturing, intensity gaining, and image black levels were optimally adjusted and kept constant for all experiments to standardize the fluorescence intensity measurements among experiments. To quantify cells in cytokinesis, metaphase/anaphase, and multinucleation, at least 100 cells were randomly selected for each group. The percent of cells in cytokinesis was calculated as follows: number of cells in cytokinesis/number of observed cells × 100. Similar methods were used to calculate cells in metaphase/anaphase and multinucleate cells. The fluorescent intensity of labeled proteins was evaluated by using ImageJ software (NIH, Bethesda, MD).
Immunoblot Analysis
Immunoblot analysis was performed as previously described (8, 11, 18, 20). Antibodies against c-Abl, cortactin, and α-tubulin were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). Phospho-cortactin (Tyr-421) antibody was purchased from Cell Signaling (Danvers, MA). Glyceraldehyde 3-phosphate dehydrogenase antibody was purchased from Fitzgerald (Acton, MA).
Viral Production, Infection, and Mutagenesis
Lentivirus and c-Abl knockdown (KD) cells were generated using previously described methods (9, 12). c-Abl KD cells and cells expressing control short hairpin RNA were stable at least eight passages after initial infection. Retroviral production and infection were performed as previously described (16, 21, 22). Cortactin nonphosphorylated mutant was produced as previously described (16, 18, 21–23).
Statistical Analysis
All statistical analysis was performed using Prism 6 software (GraphPad Software, San Diego, CA). Comparison among multiple groups was performed by one-way ANOVA followed by Tukey’s multiple comparison test. Differences between two groups were analyzed by Student-Newman-Keuls test or Dunn’s method. Values of n refer to the number of experiments used to obtain each value. A P value less than 0.05 was considered to be significant.
Results
c-Abl Is Localized in the Contractile Ring
During cytokinesis, F-actin assembles at the equatorial region, and is a key component of the contractile ring of nonmuscle cells (3). We used immunofluorescent microscopy to evaluate the spatial distribution of F-actin during HASM cell division. We also stained for α-tubulin to ensure identification of different phases of cell division. F-actin was distributed in the cell periphery and the nonchromosome area in metaphase and anaphase. In contrast, F-actin was largely localized in the equatorial region during cytokinesis (Figure 1A). These results verify that F-actin is a major component of the contractile ring during cytokinesis of smooth muscle cells.
Figure 1.
c-Abl (c-Abelson tyrosine kinase) is localized with the contractile ring during cytokinesis of human airway smooth muscle (HASM) cells. (A) The spatial distribution of F-actin and α-tubulin during different phases of cell division. After synchronization, mitotic HASM cells were seeded on collagen-coated coverslips for 1 hour. They were then stained with rhodamine-phalloidin for actin, α-tubulin antibody. Nuclei were stained using 4′,6-diamidino-2-phenylindole (Dapi). (B) Cellular localization of c-Abl and F-actin in different phases of cell division. Mitotic cells were cultured on collagen-coated coverslips for 1 hour. They were then stained with rhodamine-phalloidin for F-actin, c-Abl antibody, and Dapi (nuclei). Scale bars, 10 μm. Arrows point to the contractile ring. Images are representative of three experiments.
c-Abl is a nonreceptor tyrosine kinase that has a role in regulating cell adhesion, proliferation, growth, development, and smooth muscle contraction (6–12). The role of c-Abl in contractile ring formation has not been investigated. We hypothesized that c-Abl may be localized in the equator, which may facilitate F-actin assembly and contractile ring formation. To test this, cells were immunostained for c-Abl. c-Abl was distributed throughout the nonchromosome area in metaphase and anaphase. In contrast, c-Abl was primarily localized in the contractile ring during cytokinesis (Figure 1B). These results suggest that c-Abl may have a role in contractile ring formation.
Phospho-Cortactin Is Colocalized with c-Abl in the Contractile Ring
Cortactin is a phosphorylated protein that has been implicated in cell adhesion, spreading, and migration (14–16). Previous studies suggest that cortactin is a substrate of c-Abl tyrosine kinase in vitro (24). This raises a possibility that cortactin may also position in the contractile ring. To test this, we evaluated the spatial localization of cortactin by using immunofluorescent microscopy. As shown in Figure 2A, phospho-cortactin (Tyr-421) was primarily localized in the contractile ring during cytokinesis. However, total cortactin was not condensed in the contractile ring (Figure 2B). More importantly, phospho-cortactin was colocalized with c-Abl in the contractile ring (Figure 2C).
Figure 2.
Phosphorylated cortactin is predominantly colocalized with c-Abl in the contractile ring during cytokinesis. (A) Phospho-cortactin (Tyr-421) is primarily localized in the contractile ring. Cells were stained using rhodamine-phalloidin for F-actin (red), phospho-cortactin antibody (Tyr421; green), and Dapi (blue, nuclei). (B) Total cortactin is not predominantly positioned in the contractile ring. Cells were stained using rhodamine-phalloidin for F-actin (red) and cortactin antibody (green) and Dapi (blue). (C) Phospho-cortactin (red) is colocalized with c-Abl (green) in the midzone. Arrows indicate the contractile ring. Scale bars, 10 μm. Images are representative of three experiments.
Silencing and Rescue of c-Abl Attenuates Cortactin Phosphorylation and Contractile Ring Formation
To assess the role of c-Abl, we generated stable c-Abl KD HASM cells and rescue cells by using lentivirus-mediated RNA interference and expression, as previously described (11, 12, 17). Immunoblot analysis confirmed c-Abl KD and rescue in these cells (Figures 3A and 3B). We then determined the effects of c-Abl KD and rescue on cortactin phosphorylation by immunostaining. Cortactin phosphorylation in the equator was reduced in c-Abl KD cells compared with cells expressing scramble short hairpin RNA. However, localized cortactin phosphorylation was restored in rescue cells (Figures 3C and 3D). Moreover, F-actin staining in the midzone was also decreased in c-Abl KD cells, and recovered in rescue cells (Figures 3E and 3F).
Figure 3.
c-Abl silencing and rescue affect cortactin phosphorylation and contractile ring formation. (A) Representative immunoblots illustrating the knockdown (KD) and rescue of c-Abl by lentivirus-mediated RNA interference and expression. Blots of extracts of uninfected (UI) cells, cells expressing control short hairpin (sh) RNA (shCon), c-Abl shRNA (shAbl), or rescue (Res) cells were probed with antibodies against c-Abl and glyceraldehyde 3-phosphate dehydrogenase (GAPDH). (B) Protein ratios of c-Abl/GAPDH in cells expressing control shRNA or c-Abl shRNA, or rescue cells, are normalized to those in UI cells. *Significantly lower protein ratios in cells expressing c-Abl shRNA compared with UI cells or cells expressing control shRNA, or rescue cells (P < 0.05; n = 4). (C) Cells infected with lentivirus encoding shCon, cells expressing shAbl, or rescue cells were stained for phospho-cortactin (red) and Dapi for nuclei (blue). These cells also displayed fluorescence of green fluorescence protein (GFP; green), suggesting successful infection and shRNA expression. Arrows indicate the contractile ring. Scale bars, 10 μm. (D) Fluorescent intensity of the labeled protein was evaluated with NIH ImageJ software. Fluorescent intensity of phospho-cortactin in c-Abl KD cells and rescue cells is normalized to that in cells expressing control shRNA. Values are means ± SE (P < 0.05, n = 20). (E) Cells expressing control shRNA, c-Abl KD cells, or rescue cells were stained for F-actin (red) and Dapi for nuclei (blue). Cells with GFP (green) fluorescence indicates successful infection and shRNA expression. Arrows indicate the contractile ring. Scale bars, 10 μm. (F) Fluorescent intensity of F-actin in cells expressing c-Abl shRNA or rescue cells is normalized to that in cells expressing control shRNA. Values are means ± SE (P < 0.01; n = 20).
Cytokinesis Is Affected by c-Abl KD and Rescue
We also evaluated the role of c-Abl in cytokinesis by determining cell numbers in cytokinesis, metaphase, and anaphase in c-Abl KD cells and rescue cells. The number of cells in cytokinesis was reduced in c-Abl KD cells compared with control cells, but was restored in rescue cells (Figure 4A). Furthermore, the quantity of cells in metaphase/anaphase was affected by c-Abl KD and re-expression (Figure 4B). It is known that cell multinucleation is another index of cytokinesis (3–5). KD and rescue of c-Abl affected the number of multinucleate cells (Figure 4C).
Figure 4.
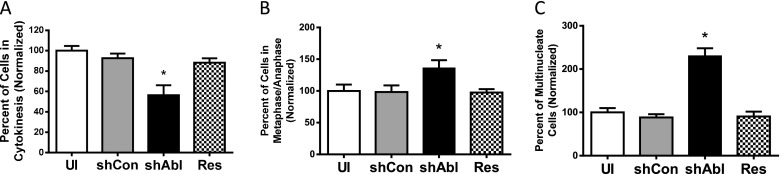
c-Abl KD and rescue regulate cytokinesis of HASM cells. Different phases of cell division were evaluated by immunofluorescent microscopy. Quantification analysis of cell numbers in different phases was determined as described in Materials and Methods. (A) c-Abl KD decreased the number of cells undergoing cytokinesis. In contrast, c-Abl rescue restored cytokinesis. (B) c-Abl silencing increased the quantity of cells in metaphase/anaphase, whereas c-Abl rescue restored metaphase/anaphase. (C) c-Abl silencing and rescue affect the percent of multinucleate cells. shAbl, cells expressing c-Abl shRNA; shCon, cells expressing control shRNA; UI, uninfected cells. Values are means ± SE. *Significantly different values in c-Abl KD cells compared with uninfected cells, cells expressing control shRNA, or rescue cells (*P < 0.05; n = 10).
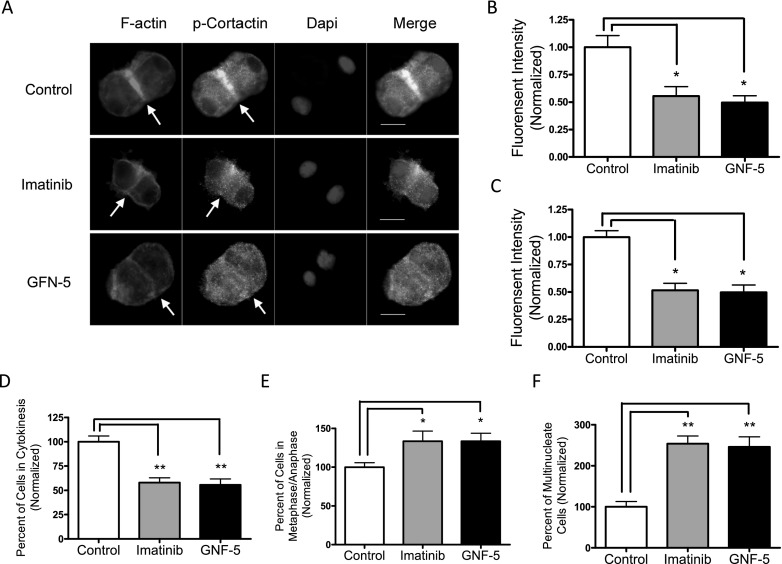
Inhibition of c-Abl Attenuates Cortactin Phosphorylation, Contractile Ring Formation, and Cytokinesis
We also evaluated the effects of the c-Abl inhibitors, imatinib (STI-571; Gleevec, Nvartis, Basel, Switzerland) (9) or GNF-5 (25). Cortactin phosphorylation in the equator was reduced in cells treated with the inhibitors compared with control cells (Figures 5A and 5B). Furthermore, F-actin staining in the equator was also decreased in cells treated with the c-Abl inhibitors (Figures 5A and 5C).
Figure 5.
Treatment with c-Abl inhibitors diminishes cortactin phosphorylation, contractile ring formation, and cytokinesis of HASM cells. Cells were treated with 5 μM imatinib or GNF-5 (dissolved in DMSO) for 1 hour (for mitosis or cytokinesis) or 24 hours (for multinucleation). Control cells were treated with DMSO. Immunofluorescent analysis was used to evaluate cortactin phosphorylation, contractile ring formation, and cytokinesis. (A) Representative images illustrating the effects of imatinib or GNF-5 on cortactin phosphorylation and F-actin staining in the equator. Arrows point to the equator. Scale bars, 10 μm. Treatment with imatinib or GNF-5 inhibits cortactin phosphorylation (B) (n = 20) and F-actin assembly (C) (n = 20) in the midzone. Moreover, treatment with imatinib or GNF-5 decreased the number of cells undergoing cytokinesis (D) (n = 10), but increased the quantity of cells in metaphase/anaphase (E) (n = 10) and multinucleate cells (F) (n = 10). Values are means ± SE (*P < 0.05, **P < 0.01).
Treatment with imatinib or GNF-5 inhibits cytokinesis of smooth muscle cells. The number of cells in cytokinesis was reduced in the presence of the inhibitors (Figure 5D). Consequently, the quantity of cells in metaphase/anaphase was higher when they were treated with the inhibitors (Figure 5E). Furthermore, treatment with the inhibitors increased the number of multinucleate cells (Figure 5F).
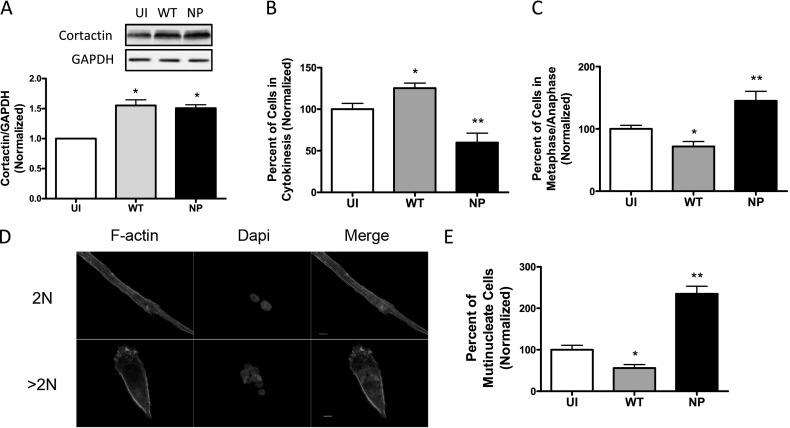
Cortactin Phosphorylation Promotes Cytokinesis
Thus far, our results suggest that c-Abl may regulate cytokinesis by controlling cortactin phosphorylation. To determine the role of cortactin phosphorylation, we expressed the nonphosphorylatable cortactin mutant in cells using recombinant retrovirus. Immunoblot analysis verified the expression of the recombinant proteins in HASM cells (Figure 6A). We then determined the effects of nonphosphorylatable cortactin mutant (Y421F) on cytokinesis. Expression of wild-type cortactin increased the number of cells undergoing cytokinesis. In contrast, expression of nonphosphorylatable cortactin decreased the number of cells undergoing cytokinesis (Figure 6B). Moreover, expression of wild-type cortactin decreased the quantity of cells in metaphase/anaphase, whereas the cortactin mutant increased the number of cells undergoing metaphase/anaphase (Figure 6C). Furthermore, the number of multinucleate cells was reduced by expression of wild-type cortactin. However, the quantity of multinucleate cells was increased in cells expressing mutant cortactin (Figures 6D and 6E).
Figure 6.
Cortactin phosphorylation at Tyr-421 promotes cytokinesis. (A) Blots of extracts from uninfected (UI) cells, cells infected with retrovirus encoding wild-type (WT) cortactin, and cells infected with virus for nonphosphorylated (NP) cortactin mutant were probed with use of antibodies against cortactin and GAPDH. Values are means ± SE (n = 5). *P < 0.05 compared with UI cells. (B) WT cortactin promotes cytokinesis, whereas NP cortactin inhibits cytokinesis. Percent of cells in cytokinesis was calculated as described in Materials and Methods. *P < 0.05, **P < 0.01 as compared with UI cells (n = 10). (C) WT cortactin decreases the number of cells in metaphase/anaphase. In contrast, NP cortactin increases the number of cells in metaphase/anaphase. *P < 0.05, **P < 0.01 compared with UI cells (n = 10). (D) Representative images illustrating cell multinucleation (two or more nuclei). 2N, cells with two nuclei; >2N, cells with more than two nuclei. Scale bars, 10 μm. (E) WT cortactin decreases number of multinucleate cells, whereas NP cortactin increases number of multinucleate cells. *P < 0.05, **P < 0.01 compared with UI cells (n = 10).
Actin Polymerization Affects Localization of c-Abl and Phospho-Cortactin during Cytokinesis
Actin polymerization has been shown to regulate glucose transporter type 4 translocation in lipocytes (26). To investigate whether actin polymerization affects c-Abl and cortactin, cells were treated with the actin polymerization inhibitor latrunculin A, and the spatial localization of c-Abl and phospho-cortactin was evaluated by immunofluorescent microscopy. Although karyokinesis was not affected, treatment with latrunculin A blocked the equatorial localization of c-Abl and phospho-cortactin (Figure 7). Consequently, treatment with latrunculin A blocked formation of the contractile ring and cleavage furrow (Figure 7).
Figure 7.
Actin assembly regulates recruitment of c-Abl and phospho-cortactin to the midzone. (A) Cells were treated with latrunculin A (Lat A) or DMSO (control). They were stained for F-actin, c-Abl, and nuclei. (B) After treatment with Lat A, cells were stained for F-actin, phospho-cortactin, and nuclei. Images are representative of four identical experiments. Arrows indicate the contractile ring. Scale bars, 10 μm.
Discussion
Cytokinesis plays a critical role in regulating cell division. Small GTPase RhoA and actin-associated proteins, profilin, formins, and cofilin, may be able to regulate cytokinesis (3). However, the role of a tyrosine kinase in the process is largely unknown. Our present studies suggest that the nonreceptor tyrosine c-Abl is required for cytokinesis. More importantly, we unveil a novel mechanism by which c-Abl mediates cortactin phosphorylation, which may facilitate actin filament assembly, contractile ring formation and cytokinesis. Intriguingly, actin filament assembly may promote positioning of c-Abl and cortactin in the contractile ring.
c-Abl has been implicated in regulating actin dynamics essential for cell adhesion, migration, growth, development, and smooth muscle contraction (6, 8–12). Nevertheless, the role of c-Abl in cytokinesis is not well understood. In this study, c-Abl was localized in the contractile ring. These results suggest that c-Abl may be associated with contractile ring formation.
Cortactin is a tyrosine-phosphorylated protein that has been implicated in the regulation of actin filament assembly (14, 15). Cell adhesion induces cortactin phosphorylation at Tyr-421 (an indication of cortactin activation) (14), which promotes cell edge protrusion (14, 15). Cortactin tyrosine phosphorylation may promote its interaction with the Src homology 2–containing proteins, such as Abl related gene and the Nck adapter, which may, in turn, activate neuronal Wiskott-Aldrich syndrome protein and actin dynamics during cell spreading and migration (14–16). Cortactin may also promote actin dynamics by affecting other proteins, such as caldesmon and Alix (14, 15).
In this study, phosphorylated cortactin (Tyr-421) was localized in the contractile ring. Furthermore, phospho-cortactin was colocalized with c-Abl. More importantly, KD and rescue of c-Abl affected cortactin phosphorylation and actin filament assembly in the midzone. Treatment with the c-Abl pharmacological agents had similar effects. Because cortactin is a known substrate of c-Abl (24), these findings suggest that c-Abl–mediated cortactin phosphorylation is critical for formation of the contractile ring.
In smooth muscle, c-Abl is necessary for force development in response to contractile activation (8–10). Furthermore, c-Abl activates the Raf-1/MEK/ERK1/2 pathway within minutes in response to stimulation with growth factors, which is pivotal for smooth muscle cell proliferation (11, 12). Here, KD or pharmacological inhibition of c-Abl diminished the percent of cells in cytokinesis, but increased the percent of cells undergoing metaphase/anaphase. Furthermore, silencing or inhibition of c-Abl led to an increase in multinucleate cells. To the best of our knowledge, this is the first evidence to suggest that c-Abl has a key role in the regulation of cytokinesis. Thus, c-Abl may have a dual role in smooth muscle cells in response to mitogenic stimulation. In the early stage of mitogenic response, c-Abl may mediate the activation of the Raf-1/MEK/ERK1/2 pathway. In the late stage, c-Abl may localize to the midzone, which regulates cytokinesis and cell division.
As described previously here, cortactin phosphorylation occurs in the contractile ring. To determine the role of cortactin phosphorylation in cytokinesis, we determined the effects of the expression of nonphosphorylatable cortactin mutant on cytokinesis. The expression of nonphosphorylatable cortactin mutant, but not wild-type cortactin, inhibited the process of cytokinesis. Thus, the results suggest that cortactin phosphorylation is necessary for cytokinesis of smooth muscle cells.
Airway remodeling is a cardinal feature of the pathological process in severe asthma. In addition to fibrosis, enhanced deposition of extracellular matrix protein, epithelial injury, and airway smooth muscle hypertrophy, airway smooth muscle cell division/proliferation significantly contributes to the pathogenesis of airway remodeling (27–30). Our recent studies suggest that c-Abl is up-regulated in asthmatic cells (13). In an animal model of asthma, conditional knockout of c-Abl in smooth muscle inhibits the development of smooth muscle mass in the airways. Moreover, treatment with the c-Abl pharmacological inhibitors imatinib and GNF-5 has similar effects (13). Thus, the increased expression of c-Abl in smooth muscle may contribute to the development of airway remodeling in chronic asthma. In addition to the ERK1/2 cascade (11, 12), it is likely that up-regulated c-Abl may promote contractile ring formation, cytokinesis, and cell division, which may contribute to the development of airway remodeling in asthma. Therefore, our present studies support the concept that c-Abl may be a novel biotarget for the development of a new therapy to treat airway remodeling in asthma (13).
Here, we propose a novel mechanism that regulates smooth muscle cytokinesis. During cytokinesis, c-Abl is recruited to the midzone, which may mediate cortactin phosphorylation, actin assembly, and contractile ring formation. c-Abl–mediated contractile ring formation facilitates cytokinesis and cell division. In addition, actin filament assembly promotes the localization of c-Abl to the contractile ring.
Footnotes
This work was supported by National Institutes of Health grants HL-110951 and HL-113208 (D.D.T.).
This article has an online supplement, which is accessible from this issue’s table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1165/rcmb.2013-0438OC on January 6, 2014
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Ammit AJ, Panettieri RA., Jr Airway smooth muscle cell hyperplasia: a therapeutic target in airway remodeling in asthma? Prog Cell Cycle Res. 2003;5:49–57. [PubMed] [Google Scholar]
- 2.Yan H, Deshpande DA, Misior AM, Miles MC, Saxena H, Riemer EC, Pascual RM, Panettieri RA, Penn RB. Anti-mitogenic effects of beta-agonists and PGE2 on airway smooth muscle are PKA dependent. FASEB J. 2011;25:389–397. doi: 10.1096/fj.10-164798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Glotzer M. The molecular requirements for cytokinesis. Science. 2005;307:1735–1739. doi: 10.1126/science.1096896. [DOI] [PubMed] [Google Scholar]
- 4.Bathe M, Chang F. Cytokinesis and the contractile ring in fission yeast: towards a systems-level understanding. Trends Microbiol. 2010;18:38–45. doi: 10.1016/j.tim.2009.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Green RA, Paluch E, Oegema K. Cytokinesis in animal cells. Annu Rev Cell Dev Biol. 2012;28:29–58. doi: 10.1146/annurev-cellbio-101011-155718. [DOI] [PubMed] [Google Scholar]
- 6.Qiu Z, Cang Y, Goff SP. c-Abl tyrosine kinase regulates cardiac growth and development. Proc Natl Acad Sci USA. 2010;107:1136–1141. doi: 10.1073/pnas.0913131107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hu H, Bliss JM, Wang Y, Colicelli J. RIN1 is an Abl tyrosine kinase activator and a regulator of epithelial-cell adhesion and migration. Curr Biol. 2005;15:815–823. doi: 10.1016/j.cub.2005.03.049. [DOI] [PubMed] [Google Scholar]
- 8.Anfinogenova Y, Wang R, Li QF, Spinelli AM, Tang DD. Abl silencing inhibits CAS-mediated process and constriction in resistance arteries. Circ Res. 2007;101:420–428. doi: 10.1161/CIRCRESAHA.107.156463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jia L, Tang DD. Abl activation regulates the dissociation of CAS from cytoskeletal vimentin by modulating CAS phosphorylation in smooth muscle. Am J Physiol Cell Physiol. 2010;299:C630–C637. doi: 10.1152/ajpcell.00095.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen S, Wang R, Li QF, Tang DD. Abl knockout differentially affects p130 CRK-associated substrate, vinculin, and paxillin in blood vessels of mice. Am J Physiol Heart Circ Physiol. 2009;297:H533–H539. doi: 10.1152/ajpheart.00237.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jia L, Wang R, Tang DD. Abl regulates smooth muscle cell proliferation by modulating actin dynamics and ERK1/2 activation. Am J Physiol Cell Physiol. 2012;302:C1026–C1034. doi: 10.1152/ajpcell.00373.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang R, Mercaitis OP, Jia L, Panettieri RA, Tang DD. Raf-1, actin dynamics and Abl in human airway smooth muscle cells. Am J Respir Cell Mol Biol. 2013;48:172–178. doi: 10.1165/rcmb.2012-0315OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cleary RA, Wang R, Wang T, Tang DD. Role of Abl in airway hyperresponsiveness and airway remodeling. Respir Res. 2013;14:105. doi: 10.1186/1465-9921-14-105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ammer AG, Weed SA. Cortactin branches out: roles in regulating protrusive actin dynamics. Cell Motil Cytoskeleton. 2008;65:687–707. doi: 10.1002/cm.20296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cosen-Binker LI, Kapus A. Cortactin: the gray eminence of the cytoskeleton. Physiology (Bethesda) 2006;21:352–361. doi: 10.1152/physiol.00012.2006. [DOI] [PubMed] [Google Scholar]
- 16.Lapetina S, Mader CC, Machida K, Mayer BJ, Koleske AJ. Arg interacts with cortactin to promote adhesion-dependent cell edge protrusion. J Cell Biol. 2009;185:503–519. doi: 10.1083/jcb.200809085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang T, Cleary RA, Wang R, Tang DD. Role of the adapter protein Abi1 in actin-associated signaling and smooth muscle contraction. J Biol Chem. 2013;288:20713–20722. doi: 10.1074/jbc.M112.439877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li QF, Spinelli AM, Wang R, Anfinogenova Y, Singer HA, Tang DD. Critical role of vimentin phosphorylation at Ser-56 by p21-activated kinase in vimentin cytoskeleton signaling. J Biol Chem. 2006;281:34716–34724. doi: 10.1074/jbc.M607715200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tang DD, Bai Y, Gunst SJ. Silencing of p21-activated kinase attenuates vimentin phosphorylation on Ser-56 and reorientation of the vimentin network during stimulation of smooth muscle cells by 5-hydroxytryptamine. Biochem J. 2005;388:773–783. doi: 10.1042/BJ20050065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang R, Li QF, Anfinogenova Y, Tang DD. Dissociation of CRK-associated substrate from the vimentin network is regulated by p21-activated kinase on ach activation of airway smooth muscle. Am J Physiol Lung Cell Mol Physiol. 2007;292:L240–L248. doi: 10.1152/ajplung.00199.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li QF, Spinelli AM, Tang DD. CDC42GAP (GTPase activating protein) regulates the activation of CDC42 and PAK (p21-activated kinase) in tracheal smooth muscle cells upon 5-ht stimulation. Am J Respir Crit Care Med. 2007;175:A348. [Google Scholar]
- 22.Li QF, Spinelli AM, Tang DD. Cdc42GAP, reactive oxygen species, and the vimentin network. Am J Physiol Cell Physiol. 2009;297:C299–C309. doi: 10.1152/ajpcell.00037.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li QF, Tang DD. Role of p47(phox) in regulating Cdc42GAP, vimentin, and contraction in smooth muscle cells. Am J Physiol Cell Physiol. 2009;297:C1424–C1433. doi: 10.1152/ajpcell.00324.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Boyle SN, Michaud GA, Schweitzer B, Predki PF, Koleske AJ. A critical role for cortactin phosphorylation by Abl-family kinases in PDGF-induced dorsal-wave formation. Curr Biol. 2007;17:445–451. doi: 10.1016/j.cub.2007.01.057. [DOI] [PubMed] [Google Scholar]
- 25.Zhang J, Adrian FJ, Jahnke W, Cowan-Jacob SW, Li AG, Iacob RE, Sim T, Powers J, Dierks C, Sun F, et al. Targeting Bcr-Abl by combining allosteric with ATP-binding-site inhibitors. Nature. 2010;463:501–506. doi: 10.1038/nature08675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Balamatsias D, Kong AM, Waters JE, Sriratana A, Gurung R, Bailey CG, Rasko JE, Tiganis T, Macaulay SL, Mitchell CA. Identification of P-Rex1 as a novel Rac1-guanine nucleotide exchange factor (GEF) that promotes actin remodeling and GLUT4 protein trafficking in adipocytes. J Biol Chem. 2011;286:43229–43240. doi: 10.1074/jbc.M111.306621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bentley JK, Deng H, Linn MJ, Lei J, Dokshin GA, Fingar DC, Bitar KN, Henderson WR, Jr, Hershenson MB. Airway smooth muscle hyperplasia and hypertrophy correlate with glycogen synthase kinase-3(beta) phosphorylation in a mouse model of asthma. Am J Physiol Lung Cell Mol Physiol. 2009;296:L176–L184. doi: 10.1152/ajplung.90376.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dekkers BG, Bos IS, Gosens R, Halayko AJ, Zaagsma J, Meurs H. The integrin-blocking peptide RGDS inhibits airway smooth muscle remodeling in a guinea pig model of allergic asthma. Am J Respir Crit Care Med. 2010;181:556–565. doi: 10.1164/rccm.200907-1065OC. [DOI] [PubMed] [Google Scholar]
- 29.Lazaar AL, Panettieri RA., Jr Airway smooth muscle: a modulator of airway remodeling in asthma. J Allergy Clin Immunol. 2005;116:488–495. doi: 10.1016/j.jaci.2005.06.030. [DOI] [PubMed] [Google Scholar]
- 30.Orsini MJ, Krymskaya VP, Eszterhas AJ, Benovic JL, Panettieri RA, Jr, Penn RB. MAPK superfamily activation in human airway smooth muscle: mitogenesis requires prolonged p42/p44 activation. Am J Physiol. 1999;277:L479–L488. doi: 10.1152/ajplung.1999.277.3.L479. [DOI] [PubMed] [Google Scholar]