Abstract
Pulmonary fibrosis is caused by excessive proliferation and accumulation of stromal cells. Fibrocytes are bone marrow (BM)-derived cells that contribute to pathologic stromal cell accumulation in human lung disease. However, the cellular source for these stromal cells and the degree of fibrocyte contribution to pulmonary fibrosis remain unclear. To determine the etiology of stromal cell excess during pulmonary fibrosis, we measured fibrocytes during the progression of fibrosis in the transforming growth factor (TGF)-α transgenic mouse model. Lung epithelial-specific overexpression of TGF-α led to progressive pulmonary fibrosis associated with increased accumulation of fibrocytes in the fibrotic lesions. Although reconstitution of BM cells into TGF-α mice demonstrated accumulation of these cells in fibrotic lesions, the majority of the cells did not express α-smooth muscle actin, suggesting that fibrocytes did not transform into myofibroblasts. To explore the mechanisms of fibrocytes in pulmonary fibrogenesis, adoptive cell-transfer experiments were performed. Purified fibrocytes were transferred intravenously into TGF-α transgenic mice, and fibrosis endpoints were compared with controls. Analysis of lung histology and hydroxyproline levels demonstrated that fibrocyte transfers augment TGF-α–induced lung fibrosis. A major subset of TGF-α–induced fibrocytes expressed CD44 and displayed excessive invasiveness, which is attenuated in the presence of anti-CD44 antibodies. Coculture experiments of resident fibroblasts with fibrocytes demonstrated that fibrocytes stimulate proliferation of resident fibroblasts. In summary, fibrocytes are increased in the progressive, fibrotic lesions of TGF-α–transgenic mice and activate resident fibroblasts to cause severe lung disease.
Keywords: pulmonary fibrosis, fibrocytes, myofibroblasts, TGF-α, fibroproliferation
Clinical Relevance
This study demonstrates the progressive accumulation of fibrocytes in the fibrotic lesions and their ability to alter the progression of fibrosis using a mouse model of TGF-α–induced fibrosis. Previous work has demonstrated increase in fibrocyte numbers in human fibrotic disease, and findings from this study provide mechanistic insights on fibrocyte-driven pulmonary fibrosis.
Pulmonary fibrosis represents a heterogeneous group of diseases in which progressive parenchymal lesions disrupt the structure and function of gas-exchanging regions of the lung (1–3). The treatment options for patients with progressive pulmonary fibrotic disease are limited because there are unknown factors involved in fibrogenesis (4, 5). Although accumulation of fibroblasts and extracellular matrix is central to the formation of these fibrotic lesions (3, 6), the origin of fibroblasts in lung fibrosis remains unclear. Current evidence suggests that multiple cellular sources contribute to fibrotic lesions, including proliferation of resident lung fibroblasts, transition of epithelial cells to a fibroblast phenotype (termed epithelial–mesenchymal transition [EMT]), and differentiation of bone marrow (BM)-derived circulating fibroblast precursors called “fibrocytes” (1, 7). Fibrocytes have been shown to be a component of hypertrophic scars and keloids (8), scleroderma, kidney lesions (9, 10), and thickened airways caused by asthma (11, 12) and are found in idiopathic pulmonary fibrosis (IPF) (13). The role of fibrocytes in lung fibrosis has been demonstrated using multiple mouse models, where inhibition of fibrocyte migration to the lung was sufficient to attenuate pulmonary fibrosis associated with bleomycin or fluorescein isothiocyanate–induced lung injury (7, 14). Despite the strong association of fibrocytes with human disease, the molecular pathways involved in fibrocyte accumulation in the lung and its functional contributions to the progressive expansion of fibrotic lung lesions remained elusive (7, 15, 16). In the setting of fibrogenic injury, BM-derived mesenchymal stem cells may be promoting repair and ameliorating fibrosis rather than causing persistent fibrotic lesions (17). Nonetheless, conflicting results using different stem cell pools and different animal models warranted mechanistic studies to determine the role of fibrocytes in animal models of progressive pulmonary fibrosis (for a review, see Ref. 18).
Overexpression of TGF-α in the lung using epithelial cell–specific promoters induces pronounced and progressive pulmonary fibrosis characterized by a number of features observed in human disease, including stromal cell and epithelial cell proliferation, extracellular matrix deposition and myofibroblast transformation, severe restrictive changes in lung mechanics, and secondary pulmonary hypertension (19, 20). Fibrosis in the transforming growth factor (TGF)-α transgenic mouse model is unique in that lesions are generated and progress in the absence of neutrophil infiltration or changes in inflammatory cytokines, thereby providing a model to assess the biological processes in fibrogenesis without the potentially confounding influences of acute lung injury (19). Previous work from our laboratory using epithelial cell fate mapping approaches failed to demonstrate conclusive evidence for EMT during lung fibrogenesis in TGF-α transgenic mice (21). In this study, we tested the hypothesis that fibrocytes contribute to the progression of fibrotic lesions after TGF-α overexpression. Using a combination of in vitro and in vivo approaches, including BM transfer of green fluorescent protein (GFP) and adoptive transfer of cultured fibrocytes into the TGF-α–transgenic mouse model, we demonstrated a profibrotic role for fibrocytes in the progression of pulmonary fibrosis.
Materials and Methods
Animals
The generation of TGF-α–overexpressing mice has been described previously (19). Mating homozygous Club cell (Clara cell) specific protein-rtTA+/− (CCSP) mice with heterozygous (TetO)7-cmv TGF-α mice produced bitransgenic TGF-α transgenic (CCSP/TGF-α) mice. To induce TGF-α expression, CCSP/TGF-α mice were administered food containing doxycycline (Dox) (62.5 mg/kg) (20). For BM transfer studies, transgenic mice expressing enhanced GFP (EGFP) in widespread tissues by the CMV-IE enhancer and chicken β-actin/rabbit β-globin hybrid promoter (stock no. 003516) were obtained from The Jackson Laboratory (Bar Harbor, ME). All mice were derived from the FVB/NJ inbred mouse strain (TGF-α transgenic mice) or backcrossed to FVB/NJ inbred mice for more than five generations (GFP transgenic mice). Animals were housed under specific pathogen–free conditions and handled in accordance with protocols approved by the Institutional Animal Care and Use Committee of the Cincinnati Children's Hospital Research Foundation.
GFP-Chimera Mice and Dox Treatments
GFP-chimera mice were generated by transplanting 3 × 106 BM cells from EGFP-transgenic mice into lethally irradiated (11.75 Gy) CCSP/− or CCSP/TGF-α recipients. BM cells were harvested from the tibia and femur of the EGFP donor mice and were obtained by flushing the bones with Dulbecco's PBS under aseptic conditions. BM cells were collected and washed by centrifugation (5 min at 1,000 × g, 4°C). Flow cytometry analysis of the total lung cells was used to determine the percentage of chimerism in recipient mice by calculating the total number of GFP-positive cells over the total number of CD45-positive cells (GFP+CD45+/CD45+), and lung cells of GFP-transgenic mice (donor mice) were used as controls (96 ± 2%; mean ± SEM). The extent of chimerism observed (71 ± 5%) was comparable to that previously reported (22). Nine weeks after reconstitution, CCSP/− and CCSP/TGF-α mice reconstituted with EGFP-BM were fed Dox-treated food for 4 or 6 weeks and were then killed to assess the role of BM-derived cells in fibrotic lesions.
Confocal Imaging
Lungs were embedded in optimum cutting temperature medium, and sections were prepared as previously described (23). Cells positive for CD45 or GFP were colocalized with desmin by immunofluorescence staining using a rabbit antidesmin antibody (clone ab15200; Abcam, Cambridge, MA), an anti–α−smooth muscle actin (SMA) antibody (clone 1A4; Sigma-Aldrich, St. Louis, MO), and a rat anti-CD45 antibody (clone 30-F11; BD Biosciences, San Jose, CA). Secondary antibodies were conjugated to Alexa-Fluor 488 and either Alexa-Fluor 594 or Alexa 647 (Invitrogen, Grand Island, NY). Confocal images were collected using an AIR-A1 laser-scanning confocal microscope (Nikon, Melville, NY). A z-stack of optical sections, 10 μm in total thickness, was captured from a lung tissue section, and five three-dimensional images were obtained per mouse. Three-dimensional volume rendering was performed to quantify the number of GFP-, desmin-, and α-SMA–positive cells in the lung lesions using the surface tool in Imaris (version 4.2.0; Bitplane, South Windsor, CT). The morphological criteria used to count cells positive for any two different fluorescent signals was with a maximum gap size of 2 μm between two fluorescence signals. Data were reported as ± SEM of cell number of experimental groups. The total five images per mouse were used to quantify stromal cells in CCSP/− and CCSP/TGF-α chimera mice on Dox for 4 or 6 weeks with four to six mice per group.
Histology and Hydroxyproline
Lungs were inflation fixed using 4% paraformaldehyde and stained with Masson trichrome as previously described (23). Lung fibrosis was assessed by measuring the total weight of the right lung, and hydroxyproline in the lung was quantified using a colorimetric assay as previously described (24).
Lung Function Measurements
Murine lung mechanics were assessed using a computerized Flexi Vent system (SCIREQ, Montreal, Canada) as previously described (23).
Statistics
All data were analyzed using Prism (version 5; GraphPad, La Jolla, CA). One-way ANOVA with Tukey’s multiple comparison post test was used to compare different experimental groups. Student’s t test was used to compare two experimental groups. Data were considered statistically significant at P < 0.05.
Results
Fibrocytes Accumulate in the Lung Lesions of TGF-α–Overexpressing Mice
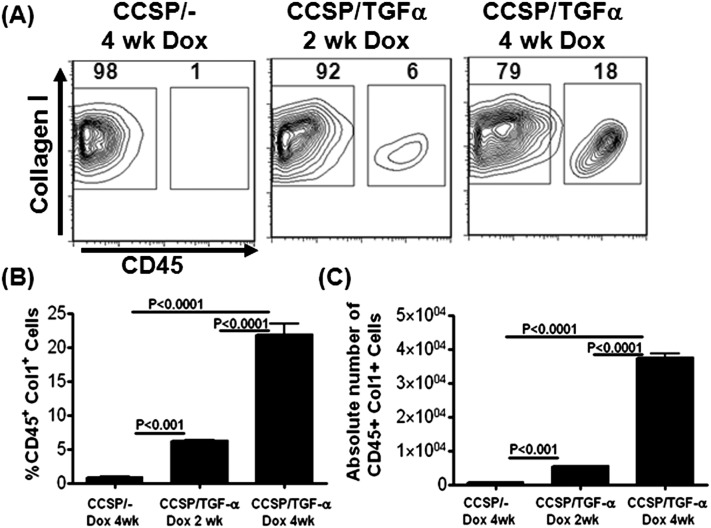
To determine if fibrocytes accumulate in fibrotic lung lesions of TGF-α mice, lung stromal cell cultures from minced whole lung homogenates were obtained from CCSP/− control and CCSP/TGF-α mice. Cells were cultured for 10 days and then analyzed by flow cytometry for the expression of CD45 and Col1. Fibrocytes were defined as cells expressing CD45 and Col1 markers. There was a significant increase in the percentage and absolute number of cells staining for CD45 and Col1 in CCSP/TGF-α mice on Dox for 2 and 4 weeks compared with control CCSP/− mice on Dox for 4 weeks (Figures 1A–1C). The majority of cells (> 95%) were collagen positive in lung stromal cell cultures. However, resident fibroblasts expressed more collagen than fibrocytes from TGF-α transgenic mice or control mice (Figure 1 and see Figure E1 in the online supplement). Collagen expression was similar in fibrocytes from the lung stromal cultures of CCSP/− and CCSP/TGF-α mice on Dox for 4 weeks (Figure E1). Immunostaining of lung sections for CCSP/TGF-α mice demonstrated an increase in costaining of CD45 with desmin at 2 and 4 weeks on Dox in fibrotic lesions, with little costaining found in the lung sections of controls (data not shown).
Figure 1.
Fibrocytes accumulate in the lungs of transforming growth factor (TGF)-α transgenic mice. Lungs from Club cell (Clara cell) specific protein-rtTA+/− (CCSP/−) and CCSP/TGF-α mice on doxycycline (Dox) for 2 or 4 weeks were minced, and stromal cells were cultured for 8 days. (A) Representative flow cytometry plots for CD45 and Col1 staining of cultured stromal cells. (B) Percentage of CD45+ and Col1+ cells. (C) Absolute number of CD45+ and Col1+ cells. One-way ANOVA was used to determine significant differences between groups. Data shown are means ± SEM (n = 3 mice per group). Results are representative of three independent experiments.
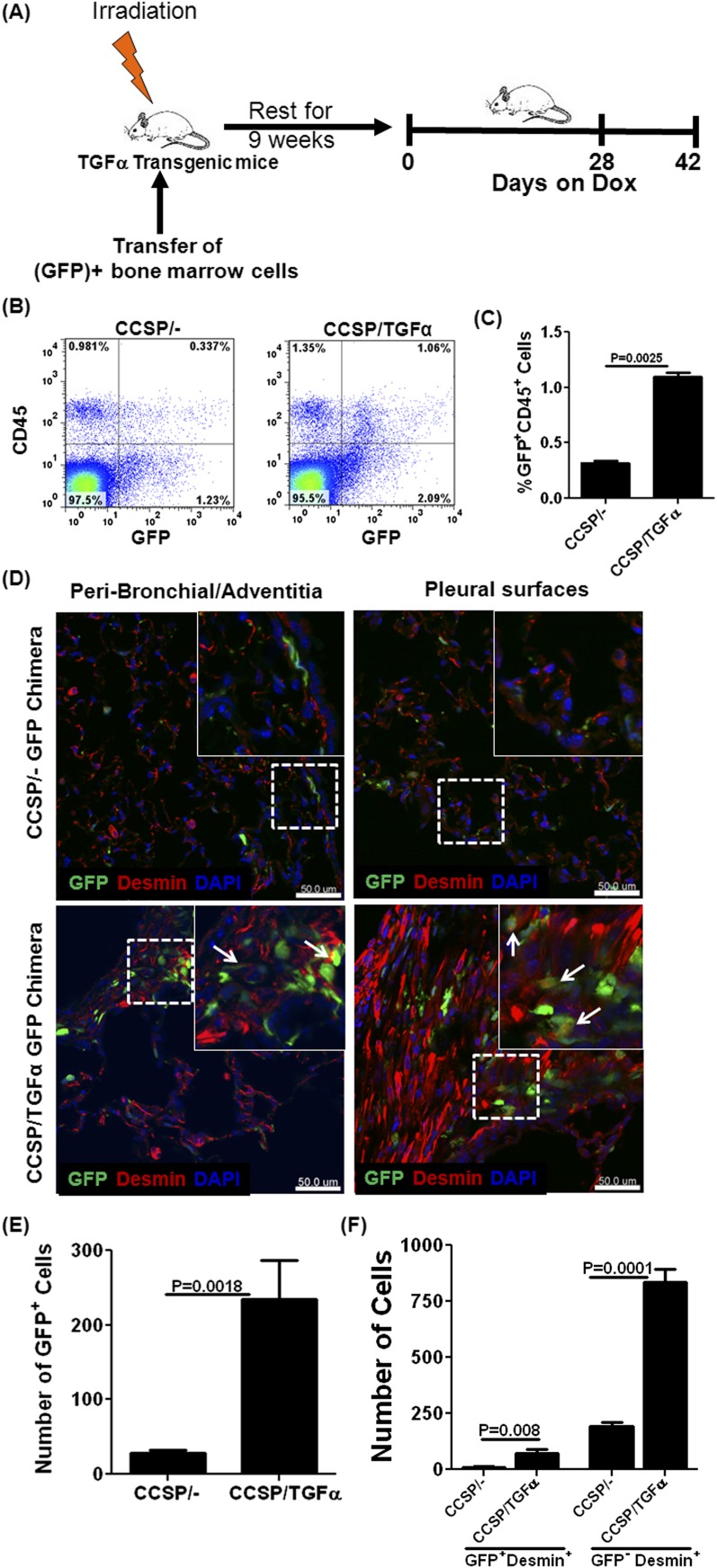
To further evaluate and quantify a role for fibrocytes in vivo in fibrotic lesions, GFP-expressing BM cells were transferred into CCSP/− and CCSP/TGF-α mice (Figure 2A). At 9 weeks after BM transplantation, the majority of CD45+ cells in the lungs of both strains of mice were GFP+ (data not shown). CCSP/− and CCSP/TGF-α chimera mice were then placed on Dox for 4 weeks. Flow cytometry of total lung cells from lung homogenates demonstrated a 3-fold increase in cells positive for GFP and CD45 in CCSP/TGF-α mice compared with controls (Figures 2B and 2C). Immunofluorescence staining demonstrated an increase in GFP+ and desmin+ cells in the fibrotic regions of CCSP/TGF-α chimera mice compared with controls (Figure 2D), with the majority of GFP+ cells localized in fibrotic lesions of the adventitia and pleura. Using confocal microscopy, z-stack images were obtained to construct three-dimensional images, and cells were counted based on GFP or desmin expression in chimera mice. CCSP/TGF-α chimera mice demonstrated a significant increase in GFP+ cells, fibrocytes (GFP+ desmin+), and resident fibroblasts (GFP− desmin+) compared with CCSP/− controls (Figures 2E and 2F). In the CCSP/TGF-α chimera mice, less than 10% of desmin-staining cells also stained for GFP, suggesting that fibrocytes make up a relatively small contribution to fibrotic lesions (Table 1). Similarly, flow cytometry analysis of the total lung stromal cell cultures demonstrated a significant increase in the percentage of GFP+ fibrocytes in CCSP/TGF-α–GFP chimera mice compared with CCSP/− GFP chimera mice on Dox for 4 weeks (Figure E2).
Figure 2.
(See figure legend on following page)
Green fluorescent protein (GFP)-tagged fibrocytes accumulate in lung fibrotic lesions during TGF-α–induced pulmonary fibrosis. (A) CCSP/− and CCSP/TGF-α recipient mice were irradiated and reconstituted with bone marrow cells from GFP transgenic mice. GFP chimeric mice were rested for 9 weeks to maximize bone marrow engraftment and later fed Dox for 4 or 6 weeks to induce TGF-α. (B) Representative flow cytometry plots of total lung cells from lung homogenates isolated from chimera mice on Dox for 4 weeks, correlating GFP intensity with number of CD45+ cells. (C) Quantitative analysis of flow cytometry demonstrating a significant increase in the percentage of cells positive for CD45 and GFP in the total lung cells of CCSP/TGF-α chimeric mice (n = 5 mice per group). Data shown are means ± SEM. Statistical significance between groups was determined using a t test. (D) Immunofluorescence of chimera mice after 4 weeks on Dox, demonstrating increased desmin staining (red) in the fibrotic lesions of CCSP/TGF-α mice compared with CCSP/− mice and, in overlaid and enlarged images, occasional colocalization of desmin with GFP in CCSP/TGF-α GFP-chimera mice (arrow). Scale bar, 50 μm. Immunofluorescence three-dimensional images, reconstructed from the z-series of confocal sections and the number of GFP+ cells (E) or desmin+ and GFP+ cells (F) counted in five lung-section images from CCSP/− (n = 4) and CCSP/TGF-α (n = 5) chimera mice, showing that CCSP/TGF-α chimera mice demonstrate an increase in GFP+ cells in fibrotic lesions and an increase in the total number of desmin+ cells and in desmin+ cells colocalizing with GFP compared with CCSP/− controls. Data shown are means ± SEM. An unpaired Student's t test was used to compare data between groups.
Table 1:
The Percentage of Green Fluorescent Protein– and Desmin-Positive Cells in Lung Sections from Chimera Mice, Determined Using Confocal Three-Dimensional Images
| Experimental Group (4 weeks on Dox) | GFP+ Desmin+ Cells |
|---|---|
| CCSP/− GFP chimera mice (n = 4) | 5.2 ± 0.5* |
| CCSP/TGF-α GFP chimera mice (N = 5) | 8.3 ± 0.7 |
Definition of abbreviations: CCSP, club cell (Clara cell)–specific protein-rtTA+/− mice; Dox, doxycycline; GFP, green fluorescent protein; TGF-α, transforming growth factor α.
Values are percentage ± SD.
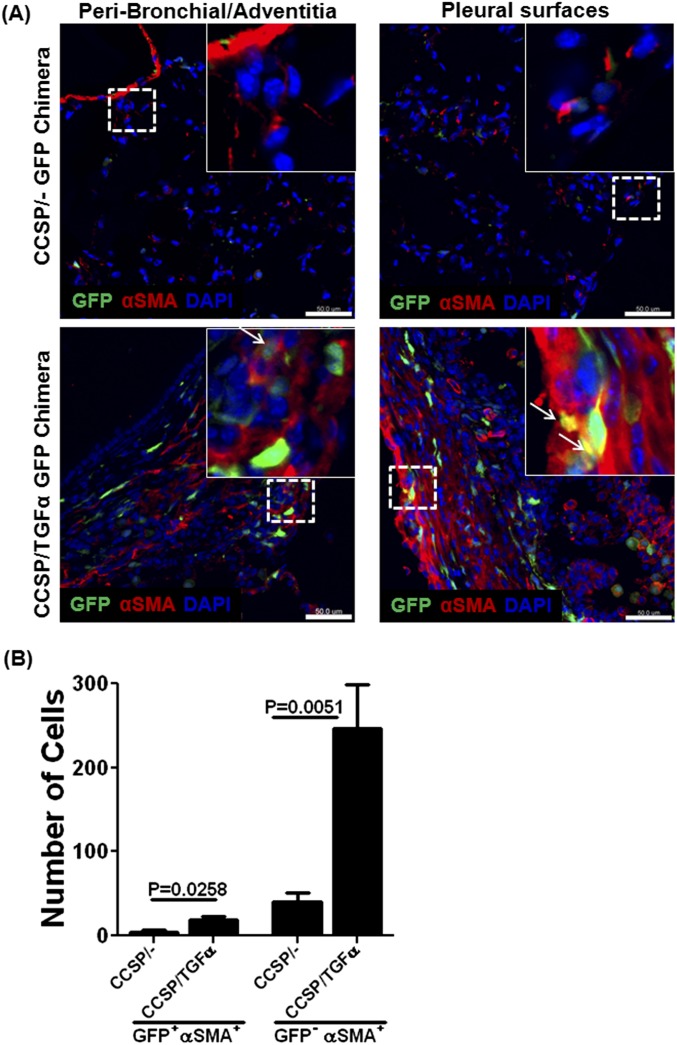
To determine whether fibrocytes that accumulate in lung lesions were myofibroblasts, CCSP/TGF-α chimera mice were placed on Dox for 6 weeks, a point at which fibrotic lesions have extensive myofibroblast accumulation (25). Lung sections from CCSP/− and CCSP/TGF-α chimera mice were immunostained with anti–α-SMA antibody, and confocal images were analyzed to count cells that coexpressed α-SMA and GFP. CCSP/TGF-α chimera mice demonstrated a 5-fold increase in the number of α-SMA+–staining cells compared with controls (Figures 3A and 3B). However, less than 10% of α-SMA–staining cells coexpressed GFP (Table 2). Together, these studies demonstrate a progressive increase in the number of fibrocytes that accumulate in the fibrotic lesions of TGF-α mice but suggest a limited contribution of fibrocytes transforming into myofibroblasts.
Figure 3.
Limited accumulation of cells positive for GFP and α-smooth muscle actin (SMA) in fibrotic lung lesions. (A) Lung sections from CCSP/− and CCSP/TGF-α GFP-chimera mice on Dox for 6 weeks were stained with antibody for α-SMA (red). GFP+ and α-SMA+ cells were increased in the fibrotic lesions of CCSP/TGF-α mice compared with CCSP/− mice. Overlaid and enlarged images demonstrate that the majority of α-SMA–positive cells were negative for GFP in adventitial tissue, with rare colocalization found in pleural fibrotic lesions of CCSP/TGF-α mice (arrow). Scale bar, 50 μm. (B) Immunofluorescence three-dimensional images, reconstructed from the z-series of confocal sections and the number of α-SMA and GFP cells counted in five lung-section images from CCSP/− (n = 5) and CCSP/TGF-α (n = 5) chimera mice, showing that CCSP/TGF-α chimera mice demonstrate an increase in the total number of α-SMA+ cells and GFP+ cells expressing α-SMA compared with CCSP/− control mice. Data shown are means ± SEM. Student's t test was used to compare data between groups.
Table 2:
The Percentage of Green Fluorescent Protein–Negative and α-Smooth Muscle Actin–Positive Cells in Lung Sections from Chimera Mice, Determined Using Confocal Three-Dimensional Images
| Experimental Group (6 weeks on Dox) | GFP+ α-SMA+ Cells* |
|---|---|
| CCSP/− GFP chimera nice (n = 5) | 2.9 ± 1.0* |
| CCSP/TGF-α GFP chimera mice (n = 5) | 7.2 ± 0.8 |
Definition of abbreviations: CCSP, club cell (Clara cell)–specific protein-rtTA+/− mice; Dox, doxycycline; GFP, green fluorescent protein; SMA, smooth muscle actin; TGF-α, transforming growth factor α.
Values are percentage ± SD.
The Role of Fibrocytes in TGF-α–Induced Fibrosis
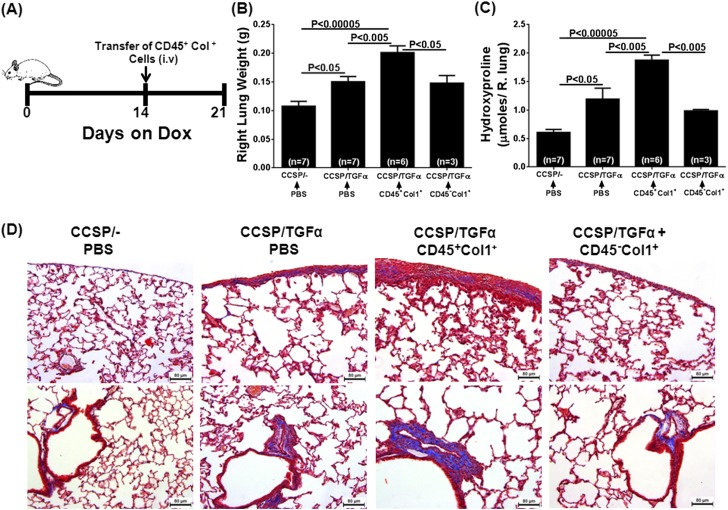
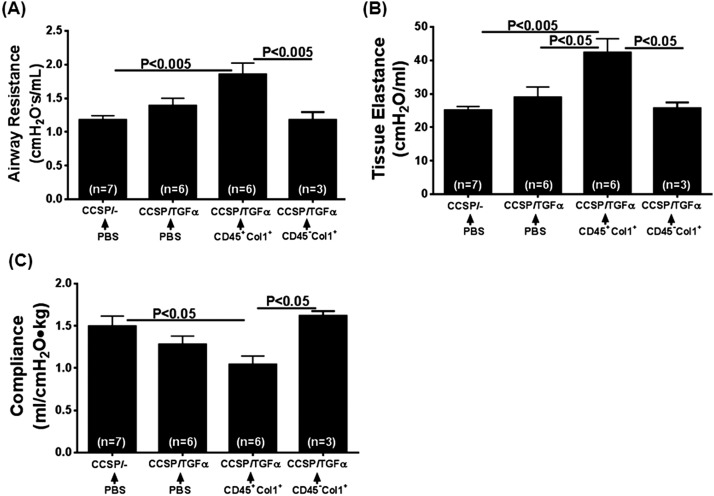
To determine whether fibrocytes influence the progressive expansion of fibrotic lung lesions, adoptive cell transfer experiments were performed. Previously, CCSP/TGF-α mice have been shown to begin progressively losing lung function after 2 to 4 weeks on Dox due to expansion of fibrotic lesions that, over time, become more organized in the pervascular, peribronchial, and pleural lung regions (19, 23). To study the effect of fibrocytes, purified populations of fibrocytes (CD45+Col1+) and resident fibroblasts (CD45−Col1+) were transferred intravenously into CCSP/TGF-α mice on Dox for 2 weeks, and fibrotic lesions of the lungs from these mice were assessed for fibrosis at Day 7 after cell transfer (3 wk total on Dox) (Figure 4A). To assess the purity and collagen expression of fibrocytes used in the adoptive transfers, a small fraction of purified fibrocytes were permeabilized and flow cytometry (FACS) stained for CD45 and Col1. Approximately 94% of cells used in adoptive transfers were positive for CD45 and Col1 (Figure E3). Transfer of CD45+ stromal cells, but not CD45− stromal cells, augmented the TGF-α–driven increase in lung weights and hydroxyproline levels in CCSP/TGF-α mice (Figures 4B and 4C). CCSP/TGF-α mice that received CD45+ cells demonstrated increased fibrotic lesions in the pleural and adventitial lung regions compared with CCSP/− and CCSP/TGF-α mice receiving PBS (Figure 4D). Observed pathological changes, in particular the lung weights, hydroxyproline, and collagen staining, in CCSP/TGF-α mice that received CD45+ cells are similar to CCSP/TGF-α mice on Dox for 4 weeks (Figure E4). Transfer of CD45+ stromal cells, but not CD45− stromal cells, accelerated TGF-α–induced declines in lung function, with significant increases in airway resistance and elastance and a decline in compliance measured in only CCSP/TGF-α mice that received CD45+ cells (Figure 5). Together, these results demonstrate that CD45+ cells directly augmented the expansion of fibrotic lesions and collagen deposition after TGF-α overexpression.
Figure 4.
Adoptive transfers of TGF-α–induced fibrocytes potentiate pulmonary fibrosis. (A) Experimental design in which CCSP/TGF-α recipient mice were placed on Dox for 2 weeks then infused with CD45+Col1+ or CD45−Col+ cells isolated from the stromal cell lung cultures of CCSP/TGF-α mice on Dox for 4 weeks. After the infusion, mice were continued on Dox for 1 week (3 wk total) before endpoints were recovered. Controls were CCSP/− and CCSP/TGF-α mice on Dox for 3 weeks and infused with PBS. The total lung weight (B) and total lung hydroxyproline (C) were increased in CCSP/TGF-α mice with CD45+Col1+ cell infusion compared with PBS-infused CCSP/− and CCSP/TGF-α mice or CCSP/TGF-α mice infused with CD45−Col+ cells. Data are presented as mean ± SEM (n = 3–7 per group). One-way ANOVA with Tukey’s multiple comparisons was used to compare data between groups. (D) Masson-trichrome stained lung sections demonstrating increased matrix staining (blue) in the pleural region (top) and adventitial region (bottom) of the lung from CCSP/TGF-α mice with CD45+Col1+ cell infusion, compared with CCSP/− and CCSP/TGF-α mice infused with PBS.
Figure 5.
Adoptive transfers of TGF-α–induced fibrocytes augment changes in lung mechanics during TGF-α–induced fibrosis. CCSP/TGF-α transgenic mice that received CD45+Col1+ cell infusion demonstrated increases in airway resistance (A) and elastance (B) and a decrease in compliance (C) compared with CCSP/− and CCSP/TGF-α mice infused with PBS or CCSP/TGF-α mice infused with CD45−Col+ cells. Data are presented as means ± SEM (n = 3–7 per group). One-way ANOVA with Tukey’s multiple comparisons was used to compare data between groups.
Characterization of TGF-α–Induced Fibrocytes
To examine the mechanisms by which CD45+ cells accumulate in fibrotic lung lesions and accelerate fibrosis, lung stromal cells from CCSP/TGF-α mice on Dox for 4 weeks were cultured and characterized using flow cytometry. TGF-α–induced fibrocytes displayed increased staining for monocyte-lineage markers CD14 and CD11b compared with CD45− stromal cells (Figure E5A). CD45+ and CD45− cells were isolated from total lung stromal cell cultures on Day 8 of culture using anti-CD45 beads, and Col1 protein was quantified using Western blots. TGF-α–induced fibrocytes expressed lower levels of Col1 compared with CD45− stromal cells (Figure E5B). These findings are in agreement with our data on collagen staining using FACS and with previous reports using a mouse model of bleomycin-induced fibrosis, in which fibrocytes were shown to express monocyte-lineage markers but lower levels of stromal cell markers than resident fibroblasts and myofibroblasts (7).
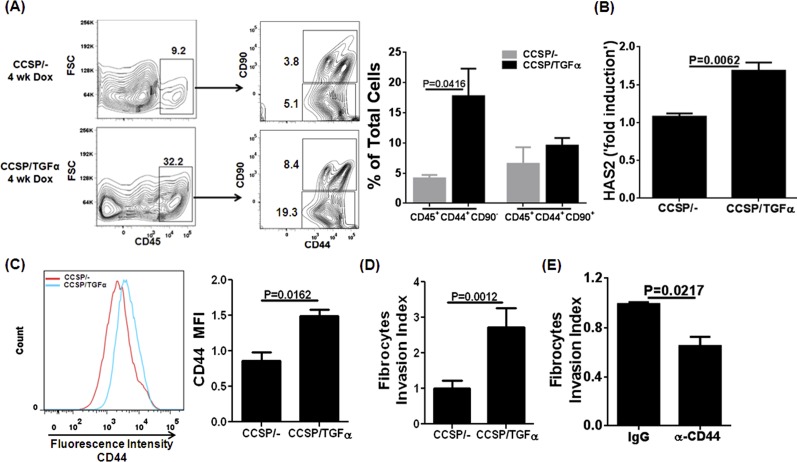
Further characterization of cultured stromal cells demonstrated that two major subsets of CD45+ cells exist in the lungs of CCSP/TGF-α mice based on their staining for CD44 and CD90. However, the large percentage of CD45+ cells that accumulate in the lungs of CCSP/TGF-α mice are positive for CD44 but negative for CD90 (Figure 6A). The enzyme hyaluronic acid synthase 2 (HAS2) has been shown to regulate the synthesis of hyaluronic acid (HA), which functions as a ligand to cause invasiveness of stromal cells expressing CD44 (26). We found that HAS2 was significantly elevated in lung homogenates from CCSP/TGF-α mice compared with controls (Figure 6B), and overexpression of TGF-α resulted in a significant increase in the expression of CD44 on the surface of fibrocytes (Figure 6C). Furthermore, cell invasion assays demonstrated that CD45+ cells from CCSP/TGF-α mice were significantly more invasive than CD45+ cells from CCSP/− mice (Figure 6D), and the invasive capacity of TGF-α–induced fibrocytes was attenuated with incubation of neutralizing CD44 antibodies compared with control IgG antibodies (Figure 6E). Together, these findings suggest that fibrocytes recruited after TGF-α overexpression are derived from monocytes and express less collagen than resident stromal cells but express higher amounts of CD44 and are more invasive, perhaps through increased induction of HAS2.
Figure 6.
A major subset of TGF-α–induced fibrocytes expresses CD44 and displays severe invasiveness. (A) Lungs of CCSP/− and CCSP/TGF-α mice on Dox for 4 weeks were cultured for 8 days and stained for CD45, CD44, and CD90. Representative flow cytometry plots demonstrate an increase in the percentage of a distinct subset of CD45+ cells that express CD44, but not CD90, in CCSP/TGF-α mice compared with CCSP/− controls. The flow cytometry gating was drawn to show the percentage of CD45+ cells in the total live cells (left panels). The total CD45+ cells were further gated (right panels) to indicate the percentage of CD44+CD90− and CD44+CD90+ in the total live cells. The bar graph quantifies the means ± SEM between groups, with statistical differences compared using Student’s t test (n = 3 per group). (B) The transcripts for HAS2 enzyme are significantly increased in the lung homogenates of CCSP/TGF-α mice compared with CCSP/− mice on Dox for 4 weeks. Student's t test was used to compare data between groups (n = 4 per group). (C) Representative histograms for CD44 staining demonstrate increased CD44 expression on CD45-gated cells from CCSP/TGF-α mice (blue) compared with CCSP/− mice (red). The bar graph quantifies the means ± SEM between groups with statistical differences compared using Student’s t test (n = 3 per group). (D) CD45+ cells from CCSP/TGF-α mice demonstrate significantly increased matrigel invasion compared with CCSP/− mice. The bar graph quantifies the means ± SEM between groups with statistical differences compared using Student’s t test (n = 3 per group). (E) Invasion of fibrocytes isolated from the lung stromal cell cultures of CCSP/TGF-α mice on Dox for 4 weeks and incubated with anti-CD44 or control IgG antibody for 22 hours (15 μg/ml). The bar graph quantifies the means ± SEM between groups with statistical differences compared using Student’s t test (n = 2 per group).
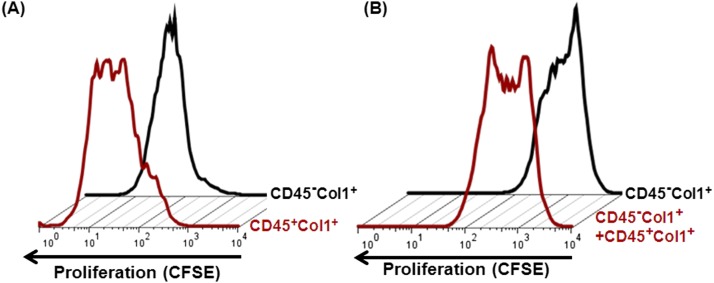
To determine if TGF-α–induced fibrocytes contributed to fibroproliferation in the lung lesions, we isolated fibrocytes (CD45+Col1+) and resident fibroblasts (CD45−Col1+) from lung stromal cell cultures using anti-CD45 magnetic beads to assess differences in proliferation. Using flow cytometry, changes in the fluorescence intensity of proliferating stromal cells labeled with CFSE dye were measured in these isolated cell subtypes. When compared with media-treated CD45− stromal cells, CD45+ cells displayed more dilution of CFSE dye, signifying increased proliferation (Figure 7A). To determine whether CD45+ cells influence proliferation of CD45− stromal cells, CD45− cells were cocultured with and without CD45+ cells. The addition of CD45+ cells increased the proliferation of CD45− stromal cells (Figure 7B). We also analyzed the average number of divisions for the dividing cell populations using the proliferation index based on CSFE-dye dilution (Figure E6). The proliferation index represents the total number of cell divisions divided by the number of cells that went into divisions. The data revealed that the proliferation index for fibrocytes is increased modestly compared with resident fibroblasts (Figure E6A). In cocultures, the proliferation index of resident fibroblasts was significantly increased in the presence of fibrocytes compared with resident fibroblasts cultured alone (Figure E6B). To confirm our findings using an additional dye, we monitored proliferation of PKH26-labeled CD45− cells in cocultures by flow cytometry. The addition of CD45+ cells increased PKH26-dye dilution and the proliferation index of CD45− stromal cells (Figure E7). These combined data suggest that molecular and cellular interactions exist in the proliferation of multiple stromal cells in fibrotic lung lesions.
Figure 7.
TGF-α–induced fibrocytes alter proliferation. (A) CD45+Col1+ and CD45−Col1+ cells were purified using anti-CD45 beads from minced lung cultures of CCSP/TGF-α mice on Dox for 4 weeks. Representative flow cytometry histograms show CFSE dilution in proliferating CD45−Col1+ cells (black) and CD45+Col1+ cells (red) in media for 3 days. (B) Representative flow cytometry histograms show CFSE-dye dilution in proliferating CD45−Col1+ cells alone (black) or cocultured with CD45+Col1+ cells (red) in media for 3 days. The data are representative of four independent experiments with similar results.
Discussion
Our study demonstrates that overexpression of TGF-α in the lung epithelium progressively increases the number of fibrocytes in fibrotic lung lesions. Although fibrocytes make up a relatively small percentage of cells in these lesions and produce less collagen than resident fibroblasts, fibrocytes accelerate fibrosis when infused directly into CCSP/TGF-α mice. Mechanistic studies determined that fibrocytes contribute to the expansion of fibrotic lesions by activating resident fibroblasts. These findings elucidate a direct role for fibrocytes in modifying fibrotic lung disease.
Our finding of progressive increases in the number of fibrocytes in expanding lung lesions is consistent with observations in human lung disease where fibrosis is a central, pathologic component of many medical conditions, including IPF, asthma, systemic sclerosis, and bronchiolitis obliterans (BO) after lung transplantation (10, 11, 13, 27, 28). A combination of specific hematopoietic (CD45, CD11a, CD11b, CD11c, CD13), stem cell (CD34), chemokine receptors (CXCR4, CCR2, CCR5, CCR7), and mesenchymal (prolyl 4-hydroxylase, Col1, desmin, α-SMA) markers have been used to identify fibrocytes in circulation, bronchoalveolar lavage preparations, and fibrotic lung lesions (29–31). Immunostaining for fibrocytes in the lung biopsies demonstrates that fibrocytes predominantly localize in areas of active fibrosis, such as thickened bronchial basement membranes in patients with asthma and fibrotic foci in patients with IPF (15, 32, 33). Recent studies demonstrated a strong correlation between the number of circulating fibrocytes (defined as CD45+ and Col1+) and poor outcomes in patients with IPF and BO (13, 28). Patients with IPF with acute exacerbations demonstrate markedly higher fibrocyte counts than patients with stable disease, and elevated circulating fibrocytes are a negative predictor of median survival (10, 13, 27). Increased circulating fibrocyte levels correlate with the development of BO after lung transplantation and positively correlate with advancing BO stage (28). Fibrocytes therefore have been proposed as a biomarker and predictor of disease severity. However, it is unclear whether elevated fibrocytes accelerated disease or merely were associated with it. In this study, we determined that pulmonary fibrosis was significantly amplified when purified fibrocytes were directly infused into the circulation of CCSP/TGF-α mice, thereby experimentally supporting a direct role for fibrocytes in advancing fibrotic disease. However, enlargement of alveoli was noted in mice receiving stromal cells but not PBS. Also, mice that received CD45− cells displayed moderate-to-severe immobility, and three out of six mice died in this group due to respiratory compromise unrelated to fibrosis; these events occurred within 24 hours of cell transfer, before significant fibrosis could develop. Future studies are warranted to determine possible differences in fibrocyte migration and invasion and the effect of aging, which can alter accumulation of fibrocytes and resident fibroblasts in the progression of fibrotic lesions. Nonetheless, our findings support the use of a mouse model of TGF-α–induced fibrosis to assess the role of fibrocytes in disease severity and possible therapeutic interventions (30).
To better understand mechanisms whereby fibrocytes promote fibrogenesis, we performed a series of experiments on fibrocytes and resident fibroblasts purified from the lung stromal cell cultures from TGF-α mice on Dox for 4 weeks. We determined that fibrocytes were more proliferative than resident stromal cells and stimulated the proliferation of resident cells when cocultured. Stromal cell proliferation in the lung is considered to be a major component in the pathobiology of pulmonary fibrosis, and our findings provide evidence that a primary role of fibrocytes is to activate resident lung stromal cell proliferation (1, 21). In support of this, clinical observations have demonstrated that EGFR-activated fibrocytes associate with remodeled lung in chronic asthma (32). In addition to proliferation, an increase in the migration of fibrocytes or their progenitor cells might contribute to the observed accumulations of fibrocytes in the fibrotic lung lesions of TGF-α mice. In support of this, we have observed a progressive increase in several chemokine levels in the lungs of TGF-α transgenic mice on Dox (unpublished data). Therefore, although it is tempting to speculate that migration and proliferative mechanisms contribute to fibrocyte accumulations, future studies are warranted to determine the relative contributions of migration and proliferation in causing fibrocyte accumulation in the lungs of TGF-α transgenic mice.
The phenotypes of human and mouse fibrocytes have been studied extensively in human tissues and in mouse models of bleomycin-induced pulmonary fibrosis (7, 31, 34). In agreement with these previous studies, we found that TGF-α–induced fibrocytes expressed lower amounts of Col1 than resident fibroblasts, indicating that fibrocytes are not primary contributors to collagen deposition in fibrotic lung lesions. Flow cytometry stainings demonstrated that the major subset of fibrocytes was negative for Thy-1 (CD90), which is known to be expressed on subsets of fibroblasts and myofibroblasts (35). However, TGF-α–induced fibrocytes expressed CD44, and this distinct subset of fibrocytes accumulated in the fibrotic lesions of TGF-α transgenic mice. In previous studies, overexpression of HAS2 selectively in stromal cells caused increased invasion of CD44-expressing stromal cells and was associated with more severe fibrosis after bleomycin-induced injury (26). We found that an increase in CD44 expression on TGF-α–induced fibrocytes correlated with a proportional increase in their matrigel invasiveness and that HAS2 transcripts were elevated in the fibrotic lung lesions, which supports a role for the TGF-α/EGFR pathway in regulating CD44/HA interactions and fibrocyte invasiveness.
In the current study, we quantified the number of fibrocytes in fibrotic lesions of TGF-α mice. Confocal three-dimensional images of GFP-labeled BM cells demonstrated that 8% of desmin-staining cells colocalized with GFP, indicating that fibrocytes make up a minority of cells in fibrotic lesions (Table 1). However, this percentage could be even lower because we could not exclude some of the GFP-staining cells that may have stained positive for desmin due to the phagocytic function of myeloid cells (e.g., macrophages) that are present in fibrotic lung lesions. An important question this study addressed was whether fibrocytes colocalize with myofibroblasts in the lung lesions of TGF-α transgenic mice. Confocal three-dimensional images determined that only 7% of the total α-SMA staining cells also stained for GFP (Table 2). To confirm whether fibrocytes differentiate into myofibroblasts, lineage tracing studies with α-SMA reporter mice need to be performed. Such studies would contribute to a determination of quantitative differences in the distribution and accumulation of fibrocytes in subpleural, peribronchial, and perivascular lesions of the lung that are responsible for pulmonary fibrosis and hypertension in TGF-α transgenic mice (20). However, the percentage of α-SMA staining GFP+ cells in the TGF-α mouse from this study is very similar to the percentage of circulating fibrocytes of patients with interstitial lung disease (< 10%), supporting a role for fibrocytes differentiating into myofibroblasts (13, 31, 36).
In summary, our results demonstrate that fibrocytes are a relatively small portion of the cells comprised in the dense mass of tissue in expanding fibrotic lesions in the TGF-α transgenic mouse model. Combined with our previous studies demonstrating a minimal role of EMT in the progression of lesions (21), our data suggest that the majority of cells comprising the lung fibrotic lesions arise from expansion of resident fibroblasts or from other mechanisms yet to be discovered. Although fibrocytes are relatively small in number, these cells augment the fibrotic response, likely through enhancing invasion and proliferation (Figure E8). Thus, multiple subsets of stromal cells play a collective role in causing the expansion of myofibroblasts that deposit collagen and other ECM proteins in the lung (Figure E8). Our results provide a basis for further determination of the molecules involved in multiple stromal cell interactions, which will inform the development of molecular therapies targeting resident stromal cells and fibrocyte-driven processes as a strategy to attenuate fibrotic lung diseases.
Acknowledgments
Acknowledgments
The authors thank Drs. Timothy D. LeCras and John P. Clancy for suggestions and helpful discussions, Angelica Schehr for technical assistance in measuring lung mechanics, the animal care technicians and irradiation core staff at the CCHMC animal facility for help with animal studies, and J. Denise Wetzel, CCHMC Medical Writer, for critical review of the manuscript.
Footnotes
This work was supported by National Institutes of Health grant HL8655598 (W.D.H.), by American Heart Association Scientist Development grants 12SDG9130040 and 1R03AR062832 (S.K.M.), and by a Parker B. Francis Fellowship (S.K.M.).
This article has an online supplement, which is accessible from this issue's table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1165/rcmb.2013-0042OC on November 7, 2013
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Duffield JS, Lupher M, Thannickal VJ, Wynn TA. Host responses in tissue repair and fibrosis. Annu Rev Pathol. 2013;8:241–276. doi: 10.1146/annurev-pathol-020712-163930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hardie WD, Glasser SW, Hagood JS. Emerging concepts in the pathogenesis of lung fibrosis. Am J Pathol. 2009;175:3–16. doi: 10.2353/ajpath.2009.081170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.White ES, Mantovani AR. Inflammation, wound repair, and fibrosis: reassessing the spectrum of tissue injury and resolution. J Pathol. 2013;229:141–144. doi: 10.1002/path.4126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wynn TA, Ramalingam TR. Mechanisms of fibrosis: therapeutic translation for fibrotic disease. Nat Med. 2012;18:1028–1040. doi: 10.1038/nm.2807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Raghu G, Collard HR, Egan JJ, Martinez FJ, Behr J, Brown KK, Colby TV, Cordier JF, Flaherty KR, Lasky JA, et al. ATS/ERS/JRS/ALAT Committee on Idiopathic Pulmonary Fibrosis. An official ATS/ERS/JRS/ALAT statement: idiopathic pulmonary fibrosis: evidence-based guidelines for diagnosis and management. Am J Respir Crit Care Med. 2011;183:788–824. doi: 10.1164/rccm.2009-040GL. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Friedman SL, Sheppard D, Duffield JS, Violette S. Therapy for fibrotic diseases: nearing the starting line. Sci Transl Med. 2013;5:167sr161. doi: 10.1126/scitranslmed.3004700. [DOI] [PubMed] [Google Scholar]
- 7.Moore BB, Kolodsick JE, Thannickal VJ, Cooke K, Moore TA, Hogaboam C, Wilke CA, Toews GB. CCR2-mediated recruitment of fibrocytes to the alveolar space after fibrotic injury. Am J Pathol. 2005;166:675–684. doi: 10.1016/S0002-9440(10)62289-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yang L, Scott PG, Dodd C, Medina A, Jiao H, Shankowsky HA, Ghahary A, Tredget EE. Identification of fibrocytes in postburn hypertrophic scar. Wound Repair Regen. 2005;13:398–404. doi: 10.1111/j.1067-1927.2005.130407.x. [DOI] [PubMed] [Google Scholar]
- 9.Sakai N, Wada T, Yokoyama H, Lipp M, Ueha S, Matsushima K, Kaneko S. Secondary lymphoid tissue chemokine (SLC/CCL21)/CCR7 signaling regulates fibrocytes in renal fibrosis. Proc Natl Acad Sci USA. 2006;103:14098–14103. doi: 10.1073/pnas.0511200103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mathai SK, Gulati M, Peng X, Russell TR, Shaw AC, Rubinowitz AN, Murray LA, Siner JM, Antin-Ozerkis DE, Montgomery RR, et al. Circulating monocytes from systemic sclerosis patients with interstitial lung disease show an enhanced profibrotic phenotype. Lab Invest. 2010;90:812–823. doi: 10.1038/labinvest.2010.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schmidt M, Sun G, Stacey MA, Mori L, Mattoli S. Identification of circulating fibrocytes as precursors of bronchial myofibroblasts in asthma. J Immunol. 2003;171:380–389. doi: 10.4049/jimmunol.171.1.380. [DOI] [PubMed] [Google Scholar]
- 12.Wang CH, Huang CD, Lin HC, Lee KY, Lin SM, Liu CY, Huang KH, Ko YS, Chung KF, Kuo HP. Increased circulating fibrocytes in asthma with chronic airflow obstruction. Am J Respir Crit Care Med. 2008;178:583–591. doi: 10.1164/rccm.200710-1557OC. [DOI] [PubMed] [Google Scholar]
- 13.Moeller A, Gilpin SE, Ask K, Cox G, Cook D, Gauldie J, Margetts PJ, Farkas L, Dobranowski J, Boylan C, et al. Circulating fibrocytes are an indicator of poor prognosis in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2009;179:588–594. doi: 10.1164/rccm.200810-1534OC. [DOI] [PubMed] [Google Scholar]
- 14.Hashimoto N, Jin H, Liu T, Chensue SW, Phan SH. Bone marrow-derived progenitor cells in pulmonary fibrosis. J Clin Invest. 2004;113:243–252. doi: 10.1172/JCI18847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Andersson-Sjöland A, de Alba CG, Nihlberg K, Becerril C, Ramírez R, Pardo A, Westergren-Thorsson G, Selman M. Fibrocytes are a potential source of lung fibroblasts in idiopathic pulmonary fibrosis. Int J Biochem Cell Biol. 2008;40:2129–2140. doi: 10.1016/j.biocel.2008.02.012. [DOI] [PubMed] [Google Scholar]
- 16.Rojas M, Xu J, Woods CR, Mora AL, Spears W, Roman J, Brigham KL. Bone marrow-derived mesenchymal stem cells in repair of the injured lung. Am J Respir Cell Mol Biol. 2005;33:145–152. doi: 10.1165/rcmb.2004-0330OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ortiz LA, Gambelli F, McBride C, Gaupp D, Baddoo M, Kaminski N, Phinney DG. Mesenchymal stem cell engraftment in lung is enhanced in response to bleomycin exposure and ameliorates its fibrotic effects. Proc Natl Acad Sci USA. 2003;100:8407–8411. doi: 10.1073/pnas.1432929100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Loebinger MR, Aguilar S, Janes SM. Therapeutic potential of stem cells in lung disease: progress and pitfalls. Clin Sci (Lond) 2008;114:99–108. doi: 10.1042/CS20070073. [DOI] [PubMed] [Google Scholar]
- 19.Hardie WD, Le Cras TD, Jiang K, Tichelaar JW, Azhar M, Korfhagen TR. Conditional expression of transforming growth factor-alpha in adult mouse lung causes pulmonary fibrosis. Am J Physiol Lung Cell Mol Physiol. 2004;286:L741–L749. doi: 10.1152/ajplung.00208.2003. [DOI] [PubMed] [Google Scholar]
- 20.Hardie WD, Korfhagen TR, Sartor MA, Prestridge A, Medvedovic M, Le Cras TD, Ikegami M, Wesselkamper SC, Davidson C, Dietsch M, et al. Genomic profile of matrix and vasculature remodeling in TGF-alpha induced pulmonary fibrosis. Am J Respir Cell Mol Biol. 2007;37:309–321. doi: 10.1165/rcmb.2006-0455OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hardie WD, Hagood JS, Dave V, Perl AK, Whitsett JA, Korfhagen TR, Glasser S. Signaling pathways in the epithelial origins of pulmonary fibrosis. Cell Cycle. 2010;9:2769–2776. doi: 10.4161/cc.9.14.12268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Abedi M, Greer DA, Foster BM, Colvin GA, Harpel JA, Demers DA, Pimentel J, Dooner MS, Quesenberry PJ. Critical variables in the conversion of marrow cells to skeletal muscle. Blood. 2005;106:1488–1494. doi: 10.1182/blood-2005-01-0264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Madala SK, Schmidt S, Davidson C, Ikegami M, Wert S, Hardie WD. MEK-ERK pathway modulation ameliorates pulmonary fibrosis associated with epidermal growth factor receptor activation. Am J Respir Cell Mol Biol. 2012;46:380–388. doi: 10.1165/rcmb.2011-0237OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wynn TA, Barron L, Thompson RW, Madala SK, Wilson MS, Cheever AW, Ramalingam T. Quantitative assessment of macrophage functions in repair and fibrosis. Curr Protoc Immunol. 2011;Chapter 14:Unit14.22. doi: 10.1002/0471142735.im1422s93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Le Cras TD, Korfhagen TR, Davidson C, Schmidt S, Fenchel M, Ikegami M, Whitsett JA, Hardie WD. Inhibition of PI3K by PX-866 prevents transforming growth factor-alpha-induced pulmonary fibrosis. Am J Pathol. 2010;176:679–686. doi: 10.2353/ajpath.2010.090123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li Y, Jiang D, Liang J, Meltzer EB, Gray A, Miura R, Wogensen L, Yamaguchi Y, Noble PW. Severe lung fibrosis requires an invasive fibroblast phenotype regulated by hyaluronan and CD44. J Exp Med. 2011;208:1459–1471. doi: 10.1084/jem.20102510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mehrad B, Burdick MD, Zisman DA, Keane MP, Belperio JA, Strieter RM. Circulating peripheral blood fibrocytes in human fibrotic interstitial lung disease. Biochem Biophys Res Commun. 2007;353:104–108. doi: 10.1016/j.bbrc.2006.11.149. [DOI] [PubMed] [Google Scholar]
- 28.LaPar DJ, Burdick MD, Emaminia A, Harris DA, Strieter BA, Liu L, Robbins M, Kron IL, Strieter RM, Lau CL.Circulating fibrocytes correlate with bronchiolitis obliterans syndrome development after lung transplantation: a novel clinical biomarker Ann Thorac Surg 201192470–477.discussion 477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pilling D, Fan T, Huang D, Kaul B, Gomer RH. Identification of markers that distinguish monocyte-derived fibrocytes from monocytes, macrophages, and fibroblasts. PLoS ONE. 2009;4:e7475. doi: 10.1371/journal.pone.0007475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gomperts BN, Strieter RM. Fibrocytes in lung disease. J Leukoc Biol. 2007;82:449–456. doi: 10.1189/jlb.0906587. [DOI] [PubMed] [Google Scholar]
- 31.Mehrad B, Strieter RM. Fibrocytes and the pathogenesis of diffuse parenchymal lung disease. Fibrogenesis Tissue Repair. 2012;5:S22. doi: 10.1186/1755-1536-5-S1-S22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang CH, Huang CD, Lin HC, Huang TT, Lee KY, Lo YL, Lin SM, Chung KF, Kuo HP. Increased activation of fibrocytes in patients with chronic obstructive asthma through an epidermal growth factor receptor-dependent pathway. J Allergy Clin Immunol. 2012;129:1367–1376. doi: 10.1016/j.jaci.2012.01.038. [DOI] [PubMed] [Google Scholar]
- 33.Keeley EC, Mehrad B, Strieter RM. The role of fibrocytes in fibrotic diseases of the lungs and heart. Fibrogenesis Tissue Repair. 2011;4:2. doi: 10.1186/1755-1536-4-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bellini A, Mattoli S. The role of the fibrocyte, a bone marrow-derived mesenchymal progenitor, in reactive and reparative fibroses. Lab Invest. 2007;87:858–870. doi: 10.1038/labinvest.3700654. [DOI] [PubMed] [Google Scholar]
- 35.Sanders YY, Kumbla P, Hagood JS. Enhanced myofibroblastic differentiation and survival in Thy-1(-) lung fibroblasts. Am J Respir Cell Mol Biol. 2007;36:226–235. doi: 10.1165/rcmb.2006-0178OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hong KM, Belperio JA, Keane MP, Burdick MD, Strieter RM. Differentiation of human circulating fibrocytes as mediated by transforming growth factor-beta and peroxisome proliferator-activated receptor gamma. J Biol Chem. 2007;282:22910–22920. doi: 10.1074/jbc.M703597200. [DOI] [PubMed] [Google Scholar]