Abstract
There is a need to identify novel agents that elicit small airway relaxation when β2-adrenoceptor agonists become ineffective in difficult-to-treat asthma. Because chronic treatment with the synthetic peroxisome proliferator activated receptor (PPAR)γ agonist rosiglitazone (RGZ) inhibits airway hyperresponsiveness in mouse models of allergic airways disease, we tested the hypothesis that RGZ causes acute airway relaxation by measuring changes in small airway size in mouse lung slices. Whereas the β-adrenoceptor agonists albuterol (ALB) and isoproterenol induced partial airway relaxation, RGZ reversed submaximal and maximal contraction to methacholine (MCh) and was similarly effective after precontraction with serotonin or endothelin-1. Concentration-dependent relaxation to RGZ was not altered by the β-adrenoceptor antagonist propranolol and was enhanced by ALB. RGZ-induced relaxation was mimicked by other synthetic PPARγ agonists but not by the putative endogenous agonist 15-deoxy-PGJ2 and was not prevented by the PPARγ antagonist GW9662. To induce airway relaxation, RGZ inhibited the amplitude and frequency of MCh-induced Ca2+ oscillations of airway smooth muscle cells (ASMCs). In addition, RGZ reduced MCh-induced Ca2+ sensitivity of the ASMCs. Collectively, these findings demonstrate that acute bronchodilator responses induced by RGZ are PPARγ independent, additive with ALB, and occur by the inhibition of ASMC Ca2+ signaling and Ca2+ sensitivity. Because RGZ continues to elicit relaxation when β-adrenoceptor agonists have a limited effect, RGZ or related compounds may have potential as bronchodilators for the treatment of difficult asthma.
Keywords: airway smooth muscle, asthma, bronchodilator, lung slices, rosiglitazone
Clinical Relevance
Contraction of small airways contributes significantly to the airflow limitation in asthma and is an important, but relatively neglected, target for treatment. This research reports novel dilator responses to rosiglitazone in mouse small airways under conditions where responsiveness to the β2-adrenoceptor agonist, albuterol is limited. This suggests that rosiglitazone may offer an alternative or additional therapeutic option to target small airway contraction in asthma.
Contraction of small airways contributes significantly to the airflow limitation in asthma (1, 2) and is an important but relatively neglected target for treatment (3, 4). Although β2-adrenoceptor agonists such as albuterol (ALB) are an effective therapy for acute asthma symptoms, isolated small airways from humans (usually defined as < 2 mm diameter) have been shown to be less sensitive to their bronchodilator effects than larger airways (5, 6). This may contribute to an impaired ability of β2-adrenoceptor agonists to reverse small airway contraction in severe asthma (1, 7). Furthermore, the continual or excessive use of β2-adrenoceptor agonists can lead to tachyphylaxis and a loss of response to this class of drugs (8). Therefore, there is a pressing need for novel agents that can elicit relaxation of small airways via alternative pathways to β2-adrenoceptor agonists.
A possible candidate is rosiglitazone (RGZ), a potent synthetic agonist of the nuclear peroxisome proliferator activated receptor (PPAR)γ that is expressed at increased levels in airway smooth muscle cells (ASMCs) in asthma (9). RGZ and ciglitazone (CGZ) regulate ASMC proliferation and the production of inflammatory mediators such as GM-CSF in vitro (10). Consistent with these actions, chronic treatment with PPARγ agonists inhibits inflammatory cell influx, airway wall remodeling, and the development of airways hyperresponsiveness to the bronchoconstrictor methacholine (MCh) in mice with ovalbumin-induced airway disease (11–15) (reviewed in Reference 10).
RGZ has also been shown to improve lung function in the absence of detectable antiinflammatory actions in a mouse ovalbumin model (13) and in a small clinical trial that studied smokers with asthma (16). In the latter study, oral treatment with RGZ over 4 weeks produced improvements in forced expiratory flow values as compared with treatment with the inhaled steroid beclometasone dipropionate; this result may reflect a direct effect of RGZ to reduce small airway obstruction (16). In another study, RGZ was shown to relax mouse isolated tracheal preparations precontracted with carbachol (17), but its acute effects on small airways were not assessed.
The major aim of this study was to assess if RGZ and other PPARγ agonists can serve as novel bronchodilators of small airways. To evaluate this potential, mouse lung slices were prepared containing airways with a diameter down to 50 μm, which is comparable to human airways with a diameter of 1 to 2 mm (18). This technique maintains the interdependency of the small airways with surrounding tissue and allows the visualization of airway contraction and relaxation with phase-contrast microscopy and image analysis. Calcium signaling in ASMCs within lung slices was also measured using confocal microscopy, with agonist-induced intracellular Ca2+ release seen as Ca2+ oscillations within ASMCs loaded with Ca2+-sensitive fluorescent dyes (19). A pharmacological approach was used to abrogate these oscillations by simultaneous treatment of slices with caffeine and ryanodine to lock ryanodine receptors in an open state and to release Ca2+ from the sarcoplasmic reticulum. This has been shown to clamp ASMC [Ca2+]i at extracellular levels (20, 21). Subsequent changes in airway lumen area induced by agonists are thus solely the result of altered Ca2+ sensitivity.
We report here that RGZ and related drugs caused relaxation of small airways independently of PPARγ activation irrespective of the contractile agent used. In addition, RGZ inhibited MCh-induced increases in [Ca2+]i and Ca2+ sensitivity of ASMCs. The maintained efficacy of RGZ when responses to β-adrenoceptor agonists were limited suggests that RGZ may offer an alternative or additional therapeutic option to target small airway contraction in asthma.
Materials and Methods
Solutions and Chemicals
Pentobarbitone sodium was from Cenvet (Sydney, Australia). Ultrapure, low-melting-point agarose, buffers, and media were from GIBCO/Invitrogen (Carlsbad, CA). CGZ, 15 d-PGJ2, GW9662, RGZ, and troglitazone were from Cayman (Ann Arbor, MI). PGZ was from 21CEC PX Pharma (East Sussex, UK). Ryanodine was from Calbiochem (Sydney, Australia). All other drugs were from Sigma-Aldrich (Sydney, Australia). PPARγ agonist stocks (100 mM) were prepared in DMSO with other stocks prepared in water.
Preparation of Lung Slices
All procedures were approved by the Ethics Committees of Melbourne University and the Massachusetts Medical School. Male Balb/C mice (6–12 wk) were killed by an overdose of sodium pentobarbitone. Their lungs were inflated with ∼ 1.4 ml of 2% agarose gel dissolved in HBSS supplemented with 20 mM HEPES (sHBSS) followed by < 0.5 ml air to push the gel into the alveolar sacs. After solidifying the agarose in sHBSS (4°C, 20 min), lung slices (150 μm thick) were sectioned using a vibratome (VT 1000S, Leica) and cultured in supplemented DMEM (1% penicillin-streptomycin, 37°C, 5% CO2) as described previously (22, 23).
Mounting and Microscopy
Slices were mounted in a custom-made chamber (∼ 100 μl volume) and observed under phase contrast on an inverted microscope (Diaphot 300; Nikon, Melville, NY) using a 10× objective lens, a zoom adaptor, a reducing lens, and a CCD camera (TM-62EX; Pulnix, JAI Inc., San Jose, CA). Single airways (200–400 μm) with an intact epithelium displaying ciliary activity were selected for experimentation.
Image Capture and Analysis
Digital images (744 × 572 pixels) recorded in time lapse (0.5 Hz) using imaging software (Video Savant; IO Industries, London, ON, Canada) were analyzed using NIH/Scion (Scion Corp., Torrance, CA). An appropriate gray-scale threshold distinguished between the airway lumen and surrounding tissue and changes in area with time were calculated by pixel summation.
Lung Slice Perfusion
Individual solutions were delivered over timed intervals using a perfusion control system (Warner Instruments, Hamden, CT) and gravity-powered flow (∼ 3 ml/min) (22). Dilator responses were assessed in airways precontracted with MCh, serotonin (5HT), or endothelin (ET)-1. The effects of RGZ on the development of contraction to MCh were also assessed. RGZ responses were measured in the presence of PPARγ antagonist GW9662 (10 μM), propranolol (10 nM), SQ22536 (100 μM), indomethacin (10 μM), the nitric oxide synthase inhibitor Nω-nitro-L-arginine (NOLA) (100 μM), the guanylate cyclase inhibitor 1H-[1,2,4]Oxadiazolo[4,3-a]quinoxalin-1-one (10 μM), and tetraethylammonium (TEA) (1 mM).
Measurement of Intracellular Ca2+
Slices were loaded with 20 μM Oregon Green-AM in sHBSS (∼ 10 slices/ml) containing 0.1% Pluronic F-127 (Sigma-Aldrich, St. Louis, MI) and 100 μM sulfobromophthalein (1 h; 30°C), followed by sHBSS containing 100 μM sulfobromophthalein (30 min) (19, 24). Fluorescence images of ASMCs were observed using confocal or two-photon microscopy as described previously (19, 24) and recorded by VideoSavant (IO Industries, London, ON, Canada) (30 Hz). The average fluorescence intensity of a region of interest (∼ 5 × 5 pixels) inside a single SMC was analyzed with Scion image software and custom-written macros. Final fluorescence values (Ft) in response to 300 nM MCh and increasing RGZ were expressed as the ratio (Ft/F0) normalized to the initial fluorescence (F0).
Preparation of Ca2+-Permeabilized Slices
After control responses to 300 nM MCh in the absence and presence of RGZ were obtained, slices were exposed to 20 mM caffeine/50 μM ryanodine (5 min) to clamp intracellular calcium ([Ca2+]i) at a sustained high level (19). Ca2+ permeabilization was confirmed by the absence of caffeine-mediated contraction. Responses due to alterations in Ca2+ sensitivity alone were then determined.
Statistics
All data were expressed as mean ± SEM, with each n representing one slice per mouse, for analysis using Graph Pad Prism, with P < 0.05 considered statistically significant. All responses were averaged over the final minute of perfusion. To correct for differences in airway size, contractions were normalized to % initial area. Relaxation responses were expressed as % initial precontraction, with concentration-response curves fitted to obtain half maximal effective concentration (EC50) and maxima. RGZ responses in the absence and presence of inhibitors were compared by two-way ANOVA or one-way ANOVA with Dunnett’s post hoc test. Responses to single concentrations of agonists were compared by paired or unpaired t tests as appropriate.
Results
Comparison of RGZ and β-Adrenoceptor Agonists
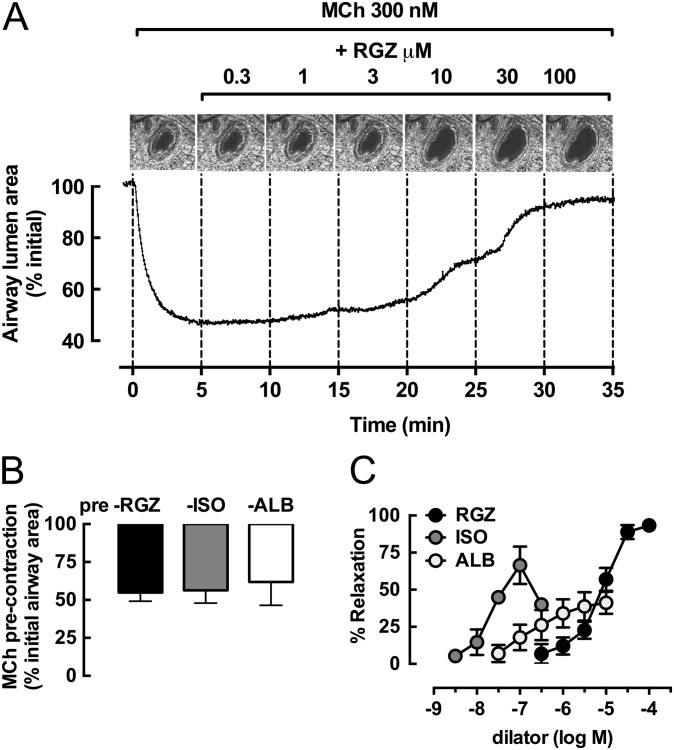
To assess the ability of RGZ to elicit relaxation relative to β-adrenoceptor agonists, we first precontracted small airways with 300 nM MCh, a concentration that causes submaximal contraction (25). Representative images of a single small airway and frame-by-frame analysis of changes in airway lumen area show that cumulative additions of RGZ reversed the MCh-induced precontraction (Figure 1A). In separate airways precontracted to a similar approximately 40% reduction in lumen area in response to MCh (Figure 1B), RGZ, isoproterenol (ISO), and ALB elicited relaxation (Figure 1C). Their relative potencies were ISO > ALB > RGZ, with RGZ being approximately 100-fold less potent than ISO. However, only RGZ caused near-complete relaxation, with neither ISO nor ALB being able to fully reverse the contraction to MCh (maximum relaxation, 100 μM RGZ, 93.4 ± 2.1%; 100 nM ISO, 66.7 ± 12.6%; 10 μM ALB, 41.2 ± 7.5%; one-way ANOVA, P < 0.05) (Figure 1C).
Figure 1.
Rosiglitazone (RGZ), but not β-adrenoceptor agonists, elicits full relaxation of mouse small airways in lung slices. Airways in lung slices were precontracted with 300 nM methacholine (MCh) to achieve ∼ 70% of the maximum MCh response before perfusion with increasing concentrations of RGZ, albuterol (ALB), or isoproterenol (ISO). (A) Representative phase-contrast images of the last frame of each 5-minute perfusion period and frame-by-frame analysis (0.5 Hz) show changes in lumen area with time in the presence of MCh and RGZ. Responses (mean ± SEM) to precontraction with MCh (B) before relaxation with RGZ (n = 4), ALB (n = 8), and ISO (n = 7) (C) are expressed as % initial airway lumen area and % relaxation of the submaximal MCh precontraction, respectively.
RGZ was also able to inhibit the development of MCh-induced contraction. The reduction in airway lumen area in response to MCh (42.4 ± 4%; n = 4) was prevented in a concentration-dependent manner by RGZ, with no contraction to MCh evident in small airways in the presence of 100 μM RGZ (see Figure E1 in the online supplement).
To confirm that RGZ was not acting via β-adrenoceptors, we assessed RGZ-mediated relaxation in the presence of an effective concentration of propranolol, a β-adrenoceptor antagonist. Propranolol significantly decreased the potency of ISO (EC50: control, 17.9 ± 5.1 nM; +propranolol, 270 ± 115 nM; unpaired t test, P < 0.05) but not RGZ (EC50: 3.5 ± 1.3 μM) (Figure E2).
Additive Effects of RGZ and ALB
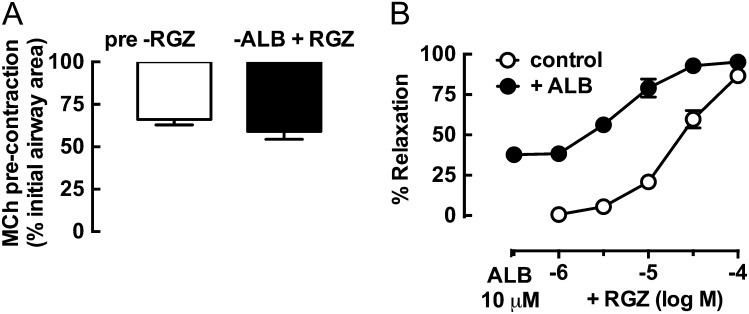
Because RGZ was able to cause relaxation independently of β-adrenoceptor activation, we wanted to determine whether it was able to overcome the residual contraction that could not be reversed by ALB (Figure 2). In airways matched for precontraction (Figure 2A), treatment with 10 μM ALB alone elicited 37.7 ± 2.0% relaxation (Figure 2B). In these partially relaxed airways, the effects of RGZ and ALB were additive, and the potency of RGZ was increased approximately 3-fold (EC50: RGZ control, 0.39 ± 0.13 μM) (Figure 2B).
Figure 2.
Relaxation of mouse small airways to RGZ is additive with ALB. Airways in lung slices were precontracted with 300 nM MCh before perfusion with RGZ in the absence (n = 4) and presence of 10 μM ALB (n = 5). Responses (mean ± SEM) are expressed as % initial airway lumen area after MCh (A) and % relaxation of the submaximal MCh precontraction (B).
Effects of Increasing Contraction on Responses to RGZ and ALB
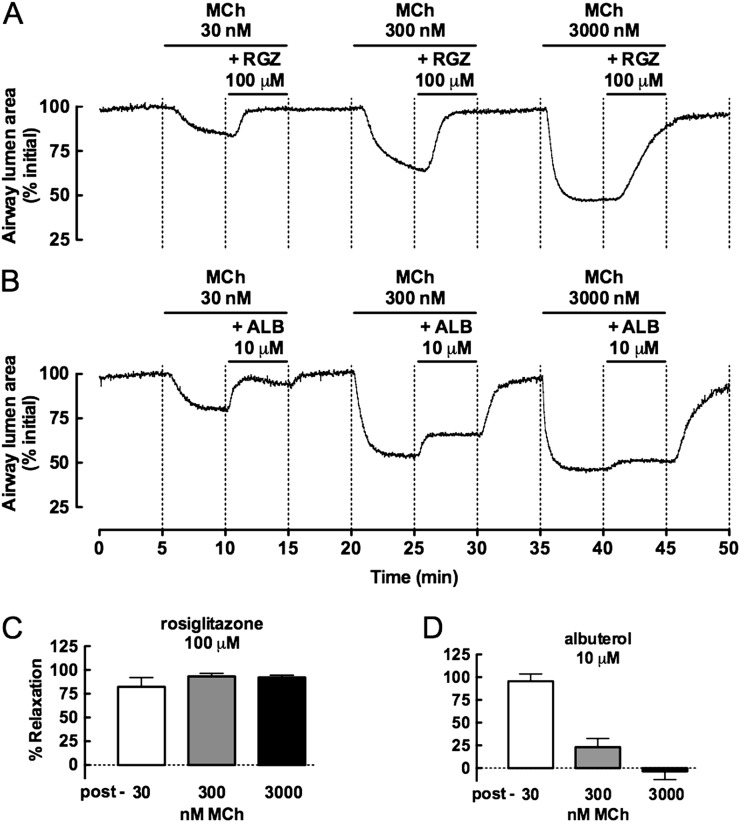
We then wanted to determine the effect of varying the level of precontraction with MCh on the relative dilator capacities of RGZ and ALB (Figure 3). Before assessment of RGZ-mediated relaxation, MCh at 30, 300, and 3,000 nM caused increasing reductions in airway lumen area by 11.6 ± 4.9%, 39.1 ± 3.0%, and 53.4 ± 1.1%, respectively. Although the maximum relaxation with RGZ was delayed with increasing MCh concentrations, its efficacy was maintained (Figures 3A and 3C). In contrast, ALB became progressively less effective and did not elicit relaxation in maximally contracted airways (Figures 3B and 3D).
Figure 3.
Relaxation to RGZ, but not ALB, is maintained with increasing MCh contraction. Airways in lung slices were precontracted with 30, 300, and 3,000 nM MCh before perfusion with 100 μM RGZ (n = 4) or 10 μM ALB (n = 4). Representative frame-by-frame analysis (0.5 Hz) of changes in lumen area with time in the presence of each concentration of MCh alone and with RGZ (A) or ALB (C). Responses (mean ± SEM) to RGZ (C) or ALB (D) are expressed as % relaxation of the sequential precontractions.
Relaxation to RGZ against Different Contractile Agonists
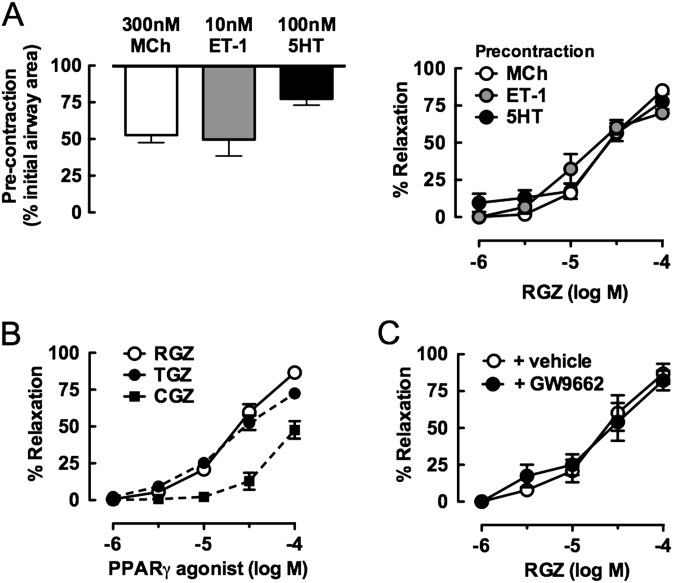
Although MCh is most commonly used to assess bronchodilator responses, Et-1 and 5HT also elicit contraction of mouse airways (26). We tested the ability of RGZ to reverse contraction to these contractile agonists using concentrations previously defined to produce submaximal precontraction in mouse small airways (22, 25) (Figure 4A). The potency and efficacy of RGZ was similar irrespective of the contractile agonist used, with 50% relaxation to RGZ occurring at 45 ± 15, 49 ± 17, and 14 ± 4 μM against MCh, 5-HT, and Et-1, respectively (Figure 4A).
Figure 4.
RGZ elicits relaxation of mouse small airways independently of peroxisome proliferator activated receptor (PPAR)γ activation. Airways in lung slices were precontracted with various constrictors before perfusion with PPARγ agonists including RGZ. Responses to RGZ after precontraction with 300 nM MCh (n = 4), 10 nM Et-1 (n = 4), or 100 nM 5HT (n = 4) (A); RGZ, troglitazone (TGZ), and ciglitazone (CGZ) after precontraction with 300 nM MCh (all n = 4) (B); and RGZ in the absence and presence of 10 μM GW9662 (PPARγ antagonist) after precontraction with 300 nM MCh (n = 9 and n = 5, respectively) (C). Responses (mean ± SEM) are expressed as % initial airway lumen area after precontraction with MCh, endothelin (Et)-1, or 5HT (A, left panel) or as % relaxation of the submaximal precontraction (A [right panel], B, C).
Comparison of RGZ with other PPARγ Agonists
To explore the mechanism underlying the acute bronchodilator response to RGZ, other structurally related PPARγ agonists were assessed. In airways matched for precontraction to 300 nM MCh, RGZ and troglitazone elicited complete relaxation with similar potency (Figure 4B). CGZ was less effective, with only partial relaxation evident up to 100 μM CGZ (Figure 4B). Pioglitazone (PGZ) (30 μM) elicited similar relaxation to 30 μM CGZ (21.9 ± 3 0.6%; n = 3), but higher concentrations of PGZ were not tested due to its limited solubility.
Because all these glitazones are known agonists of PPARγ, we wanted to determine whether relaxation to RGZ was mediated through PPARγ activation. We used a concentration of the selective PPARγ antagonist GW9662 (2-chloro-5-nitrobenzanilide) that prevented the antiproliferative effects of RGZ in human ASMCs (27). RGZ-mediated relaxation was maintained in the presence of GW9662, suggesting a PPARγ-independent mechanism (Figure 4C). In support of this finding, small airway contraction was not reversed by 100 μM 15-deoxy-Δ12,14-prostaglandin-J2 (15 d-PGJ2), a putative endogenous PPARγ agonist that is structurally unrelated to the glitazones (% reduction in lumen area to 300 nM MCh: control, 37.4 ± 4.7%; +15 d-PGJ2, 38.1 ± 4.3% [n = 3]; unpaired t test, NS)
Effect of Inhibitors on Bronchodilator Responses to RGZ
Given that RGZ appeared to cause airway relaxation independently of activation of β-adrenoceptors or PPARγ, we used a variety of pharmacological inhibitors to implicate other mechanisms of action. We focused on the potential contributions of intracellular signaling pathways, of the production of epithelial-derived factors, and of various K+ channels known to contribute to responses to other bronchodilators (e.g., Substance P, bitter taste receptor agonists) by using effective concentrations of various pharmacological inhibitors (28–32) (Table 1). Neither the adenylate cyclase inhibitor SQ22536, the guanylate cyclase inhibitor 1H-[1,2,4]oxadiazolo[4,3-a]quinoxalin-1-one, the cyclooxygenase inhibitor indomethacin, or NOLA altered the decrease in lumen area to MCh or the subsequent relaxation responses to 100 μM RGZ. Treatment with the nonselective K+-channel inhibitor TEA alone partially reversed the contraction to MCh. Subsequent perfusion with RGZ in the continued presence of TEA was still able to elicit full relaxation of the residual MCh contraction (Table 1).
Table 1:
Effect of Inhibitors on Relaxation to Rosiglitazone in Mouse Small Airways*
| Inhibitor | Target | n | % Relaxation |
|
|---|---|---|---|---|
| + Inhibitor | + Inhibitor + RGZ | |||
| Control | 4 | — | 86.1 ± 1.1 | |
| SQ22536 (100 μM) | Adenylate cyclase | 4 | 7.3 ± 8.4 | 79.7 ± 7.7 |
| ODQ (10 μM) | Soluble guanylate cyclase | 5 | 8.9 ± 4.2 | 63.9 ± 16.0 |
| INDO (10 μM) | Cyclooxygenase (nonselective) | 3 | 9.7 ± 10.5 | 79.1 ± 0.7 |
| NOLA (100 μM) | Nitric oxide synthase | 3 | −2.4 ± 4.0 | 68.8 ± 13.2 |
| TEA (1 mM) | K+ channels (nonselective) | 4 | 42.3 ± 6.6† | 90.9 ± 3.1 |
Definition of abbreviations: INDO, indomethacin; NOLA, Nω-nitro-L-arginine; ODQ, 1H-[1,2,4]Oxadiazolo[4,3-a]quinoxalin-1-one; RGZ, rosiglitazone; TEA, tetraethylammonium.
Airways in lung slices were continuously perfused with 300 nM methacholine to elicit a precontraction before addition of inhibitor alone followed by inhibitor and 100 μM RGZ. Responses (mean ± SEM) are expressed as % relaxation of the submaximal precontraction averaged over the last minute of perfusion as described in Materials and Methods.
P < 0.05 (paired t test methacholine precontraction alone).
Regulation of Calcium Signaling and Sensitivity by RGZ
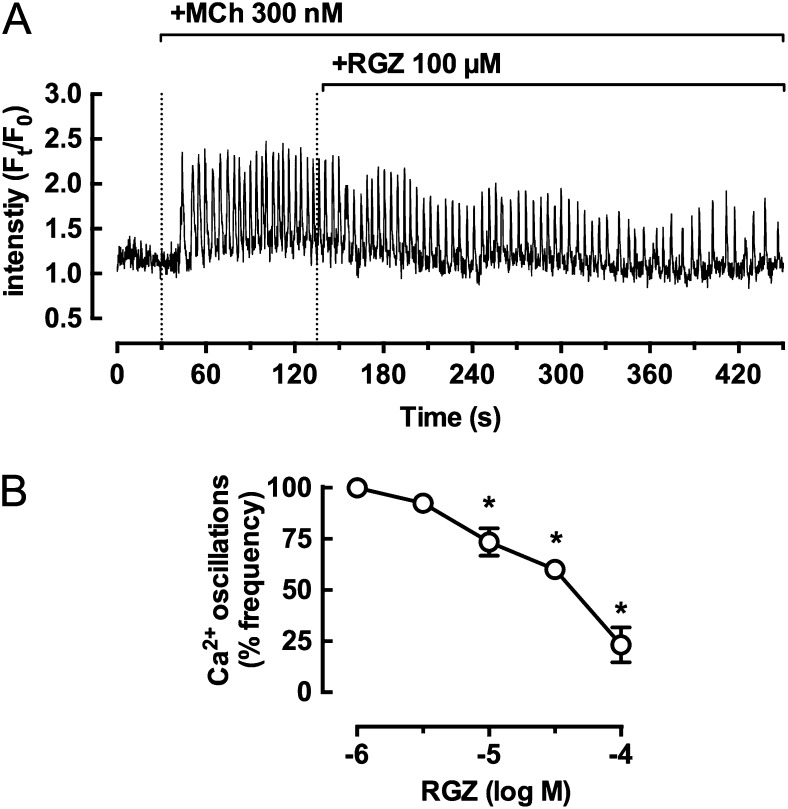
We then directed our attention to the ability of RGZ to regulate ASMC Ca2+ signaling and sensitivity. MCh-induced contraction is associated with Ca2+ oscillations within ASMCs due to cycles of Ca2+ release and reuptake from the sarcoplasmic reticulum. We measured the effects of RGZ on Ca2+ oscillations in the presence of 300 nM MCh. The increases in Ca2+ oscillation frequency and amplitude in response to MCh were slightly reduced by 10 μM RGZ, whereas a representative trace shows a marked reduction in the presence of 100 μM RGZ (Figure 5A). Ca2+ oscillations in the absence of RGZ (28.0 ± 5.1 Hz; n = 4) were inhibited by 76.8 ± 8.5% in the presence of 100 μM RGZ (Figure 5B).
Figure 5.
RGZ inhibits MCh-induced calcium oscillations in airway smooth muscle cells (ASMCs) in mouse small airways. Ca2+ oscillations were measured in ASMCs within airways in lung slices incubated with 20 μM Oregon Green-AM. (A) Representative trace showing Ca2+ oscillations induced by 300 nM MCh and the inhibitory effect of 100 μM RGZ. (B) Ca2+ oscillation frequency of ASMCs after 4 minutes of RGZ exposure. Responses (mean ± SEM) are expressed as % of the Ca2+ oscillation frequency in the presence of MCh in four to eight slices from four mice. *P < 0.05 one-way ANOVA, methacholine precontraction alone. Ft/F0 = ratio of final fluorescence normalized to the initial fluorescence.
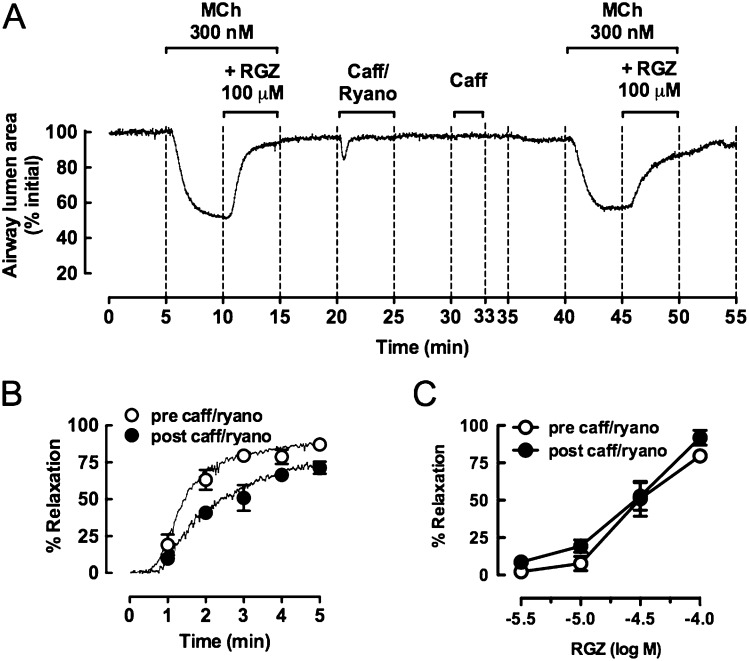
MCh-induced contraction is also associated with increased Ca2+ sensitivity, mediated through Rho kinase signaling. This can be studied in isolation using Ca2+–permeabilized slices in which MCh-induced Ca2+ oscillations are abolished pharmacologically. Before Ca2+ permeabilization, a normal contractile response to 300 nM MCh and the expected near-complete relaxation in response to 100 μM RGZ were observed (Figure 6A). Ca2+ permeabilization with caffeine/ryanodine to lock ryanodine receptors in an open state and clamp [Ca2+]i levels to a steady state elicited a transient contraction. Subsequent exposure to caffeine did not cause contraction, confirming that [Ca2+]i levels had been clamped and that subsequent responses could be attributed to altered Ca2+ sensitivity alone.
Figure 6.
RGZ inhibits MCh-induced increases in calcium sensitivity in mouse small airways. Airways in lung slices were precontracted with 300 nM MCh before the addition of 100 μM RGZ. After washout, slices were perfused with 20 mM caffeine (caff)/50 μM ryanodine (ryano), opening intracellular Ca2+ stores (a transient contraction) and clamping [Ca2+]i levels. A second caffeine application did not evoke contraction, confirming the Ca2+–permeabilized status of the lung slice. Subsequent exposure to 300 nM MCh induced similar contraction due to increased Ca2+–sensitivity alone before assessment of dilator responses to RGZ. (A) Representative trace of responses to 300 nM MCh and 100 μM RGZ under control and Ca2+–permeabilized conditions. (B) Frame-by-frame analysis of responses to 100 μM RGZ in the same airways under control and Ca2+–permeabilized conditions. Data are expressed as mean ± SEM at minute intervals, with data points in between expressed as median only (n = 4). (C) Concentration-response curves to RGZ before and after caffeine ryanodine treatment (n = 6). Responses (mean ± SEM) are expressed as % initial airway lumen area (A) and % relaxation of the submaximal precontraction with MCh (B and C).
The magnitude of contraction to MCh was similar within the same airway before and after Ca2+ permeabilization, with reductions in airway lumen area of 47.0 ± 5.7% and 43.2 ± 6.3%, respectively (n = 4). When a single addition of 100 μM RGZ was repeated after caffeine/ryanodine treatment, relaxation occurred at a slower rate without reaching a plateau response within 5 minutes (Figures 6A and 6B), and the average % relaxation over the last minute of perfusion decreased from 86.1 ± 1.1% to 70.4 ± 2.9% under Ca2+–permeabilized conditions (P < 0.05, paired t test). However, there was no difference in the potency or maximum relaxation to RGZ derived from concentration-relaxation curves prepared under control conditions or after caffeine/ryanodine treatment (not significant, unpaired t tests) (Figure 6C).
Discussion
Although β2-adrenoceptor agonists are commonly used for the relief of acute asthma symptoms, there is a need for alternative bronchodilators for patients with poorly controlled asthma (33, 34). With repeated use, tolerance to β2-adrenoceptor agonists can occur. In addition, these drugs may have only limited efficacy in small airways (5, 6), where there is evidence of increased inflammation (35) and airway wall remodeling (36) and higher airway resistance in patients with mild asthma compared with healthy subjects (1, 37). In vitro studies also indicate that small airways are relatively more sensitive to contractile mediators (38).
PPARγ is a widely expressed ligand-activated transcription factor implicated in human lung diseases associated with inflammation and remodeling (10, 14). PPARγ is the target for the thiazolidinedione class of synthetic antidiabetic agents, including RGZ (39), PGZ, and CGZ as well as the proposed endogenous agonist 15 d-PGJ2. Activation of PPARγ results in suppression of cytokine production from activated macrophages and monocytes, T cells, airway epithelial cells, and ASMCs (10). In human ASMC cultures, we and others have shown that PPARγ ligands also decrease proliferation (14, 27, 40), with inhibition prevented by the PPARγ antagonist GW9662 (27).
In bronchial biopsies from asthmatic human airways, increased PPARγ expression occurs in inflammatory and epithelial cells (9). Consequently, the role of PPARγ has been explored in models of allergic airways disease in mice. Chronic treatment with RGZ and CGZ inhibited the influx of eosinophils and lymphocytes (15), the deposition of collagen and mucus in the airways (11), and inhibition of AHR (13, 14). Here, we explored the potential direct bronchodilator effects of RGZ and other PPARγ agonists in small airways of mouse lung slices. To achieve this, we used the lung slice technique, which offers many advantages for evaluating small airway reactivity in vitro. Critically, a quantitative analysis of changes in airway lumen area and Ca2+ signaling within ASMCs can be simultaneously performed during assessment of responses to constrictor and dilator agents (41, 42).
First, we demonstrated acute relaxation to RGZ and the β-adrenoceptor agonists ISO and ALB after submaximal contraction with MCh. RGZ was able to elicit concentration-dependent and near-complete relaxation within minutes, whereas only partial relaxation was evident for either of the β-adrenoceptor agonists. The nonselective β1/β2-adrenoceptor agonist ISO was more potent and elicited greater relaxation than the selective β2-adrenoceptor agonist ALB. This finding can be attributed to ISO being a full agonist and ALB being a partial agonist and is consistent with the contribution of β1- and β2-adrenoceptors to mouse airway relaxation (43). Using the nonselective β-adrenoceptor competitive antagonist propranolol, we confirmed that ISO was eliciting relaxation via β-adrenoceptors but that responses to RGZ were unaltered, implicating an alternative mechanism for RGZ.
Given the importance of combination therapy in asthma patients, we assessed the bronchodilator effects of RGZ in the presence of ALB. We found that the effects of RGZ were additive, with the partial relaxation achieved in the presence of a maximally effective concentration of ALB. This finding contrasts with a single study in mouse trachea, where ISO-mediated relaxation was not increased in the presence of RGZ despite a modest dilation seen with RGZ alone (17). However, these contrasting results have been obtained under different experimental conditions, measuring either changes in area of small airways in perfused lung slices or changes in force in trachea under static conditions. Differences in airway size, in the potential for accumulation of endogenous relaxing and/or constricting factors depending on incubation time and the technique used, and in the order in which the drugs are added may be important factors in assessing whether there is additivity and/or synergy between RGZ and β-adrenoceptor agonists.
A major limitation of the current therapy in severe asthma is the failure of β-adrenoceptor agonists to fully reverse symptoms. The effectiveness of ALB has been shown to be less effective with increasing levels of MCh-induced tone (44). We wanted to determine if the higher efficacy of RGZ relative to β-adrenoceptor agonists under conditions of submaximal contraction was maintained in maximally contracted airways. Our results clearly demonstrate that RGZ, but not ALB, was able to overcome the increasing functional antagonism in the presence of high concentrations of MCh. This finding suggests that RGZ may be able to reverse severe bronchoconstriction when β-adrenoceptor responsiveness is limited.
Further evidence of the potential of RGZ as a novel bronchodilator was its ability to overcome small airway contraction induced by 5HT or Et-1. Although 5HT is not thought to play a significant role in human asthma, it is a key mast cell–derived mediator in rodents (45). The reversal of Et-1–mediated contraction is of particular interest because it is among the most potent bronchoconstrictors described in human airways (46) and because its levels are increased in asthmatic airways (47).
Because many actions of RGZ are mediated by the nuclear receptor PPARγ, we explored its potential involvement in RGZ-mediated relaxation. Even though other synthetic PPARγ agonists relaxed small airways, the failure of the endogenous PPARγ agonist 15 d-PGJ2 to oppose MCh-induced contraction suggests that an alternative mechanism is more likely to be involved. PPARγ independence was also supported by the finding that relaxation to RGZ was maintained in the presence of GW9662, a PPARγ antagonist (27). Because all glitazones tested were not equally potent, it would be of interest to conduct structure-activity studies to identify key elements associated with dilator potency.
RGZ-induced relaxation was unaffected by the adenylate cyclase inhibitor SQ22536 at a concentration that inhibits relaxation to β-adrenoceptor agonists (28). In addition, dilator responses to RGZ were maintained in the presence of validated effective concentrations of indomethacin or NOLA (17, 31). These findings allowed us to exclude the possible contributions of cAMP or epithelial-derived relaxing factors such as PGE2 or NO to RGZ-mediated relaxation of small airways in perfused lung slices.
A previous study in mouse trachea had shown indomethacin-sensitive relaxation in response to RGZ in a static organ bath under isometric conditions (17). This relaxation was attributed to inhibition of endogenous PGE2 metabolism (48), with PGE2 accumulating at high enough levels to elicit ASMC relaxation. However, this mechanism is unlikely to have contributed to RGZ-mediated dilation in the current study because perfusion of lung slices is likely to preclude accumulation of sufficient PGE2 to contribute to small airway relaxation.
We then explored a role for regulation of K+-channel activity in RGZ-induced relaxation. Membrane hyperpolarization resulting from activation of store-operated Ca2+ channels has been proposed as a mechanism for ASMC relaxation in response to the recently described novel bronchodilators, the bitter taste agonists (49). However, because relaxation to RGZ was maintained in the presence of TEA, it remains to be determined whether changes in ion conductance contribute to RGZ-induced relaxation.
Finally, we assessed the effects of RGZ on the increases in Ca2+ signaling and sensitivity that underlie airway contraction. We found that, over the same concentration range that elicited airway relaxation, RGZ inhibited the MCh-induced increase in Ca2+ oscillations within ASMCs. The maximum inhibition with RGZ was comparable to that previously reported for ISO in opposing contraction to MCh (24) and greater than that seen in the presence of a maximally effective concentration of ALB (50). This finding is consistent with the relative efficacies of RGZ and the β-adrenoceptor agonists in the current study.
RGZ-induced relaxation was maintained in Ca2+-permeabilized airways when Ca2 oscillations had been abolished by pretreatment with caffeine/ryanodine. This indicates that RGZ can reduce MCh-induced increases in ASMC Ca2+ sensitivity. Dual actions on Ca2+ signaling and sensitivity have previously been described for ALB in mouse small airways (50). Like ALB, responses to a maximally effective single concentration of RGZ were delayed after Ca2+ permeabilization (50). It remains to be determined what differentiates the mechanism of action of RGZ from the β-adrenoceptor agonists to enable RGZ to be more effective in mediating small airway relaxation, albeit at lower potency.
Further studies are needed to confirm that the acute bronchodilator effects of RGZ extend to the in vivo setting using animal models of allergic airways disease that mimic the key features of human asthma. In the context of the limited clinical study in which treatment with oral RGZ improved lung function (16), the possibility that inhaled RGZ may elicit acute airway relaxation remains to be tested. However, the current study suggests that PPARγ agonists, including RGZ, may offer therapeutic advantages by exerting control over alternative pathways to β2-adrenoceptor agonists.
Acknowledgments
Acknowledgments
The authors thank Meaghan FitzPatrick, Jean Ni Cheong, Ning Kam, and Mirjam Simoons for technical contributions and Stuart Hirst for valuable discussion.
Footnotes
This work was supported by National Health and Medical Research Council of Australia grants 509239 and 1041575, by National Institutes of Health grant 1R01HL103405–01, and by an Australian Postgraduate Award (C.D.).
This article has an online supplement, which is accessible from this issue's table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1165/rcmb.2013-0247OC on November 4, 2013
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Wagner EM, Bleecker ER, Permutt S, Liu MC. Direct assessment of small airways reactivity in human subjects. Am J Respir Crit Care Med. 1998;157:447–452. doi: 10.1164/ajrccm.157.2.9611043. [DOI] [PubMed] [Google Scholar]
- 2.Wagner EM, Liu MC, Weinmann GG, Permutt S, Bleecker ER. Peripheral lung resistance in normal and asthmatic subjects. Am Rev Respir Dis. 1990;141:584–588. doi: 10.1164/ajrccm/141.3.584. [DOI] [PubMed] [Google Scholar]
- 3.Persson CG. Small airway relaxation: a forgotten medical need. Pulm Pharmacol Ther. 2008;21:1–3. doi: 10.1016/j.pupt.2006.10.017. [DOI] [PubMed] [Google Scholar]
- 4.Sturton G, Persson C, Barnes PJ. Small airways: an important but neglected target in the treatment of obstructive airway diseases. Trends Pharmacol Sci. 2008;29:340–345. doi: 10.1016/j.tips.2008.04.003. [DOI] [PubMed] [Google Scholar]
- 5.Finney MJ, Karlsson JA, Persson CG. Effects of bronchoconstrictors and bronchodilators on a novel human small airway preparation. Br J Pharmacol. 1985;85:29–36. doi: 10.1111/j.1476-5381.1985.tb08827.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Skogvall S, Berglund M, Dalence-Guzmán MF, Svensson K, Jönsson P, Persson CG, Sterner O. Effects of capsazepine on human small airway responsiveness unravel a novel class of bronchorelaxants. Pulm Pharmacol Ther. 2007;20:273–280. doi: 10.1016/j.pupt.2006.03.002. [DOI] [PubMed] [Google Scholar]
- 7.Goleva E, Hauk PJ, Boguniewicz J, Martin RJ, Leung DY. Airway remodeling and lack of bronchodilator response in steroid-resistant asthma. J Allergy Clin Immunol. 2007;120:1065–1072. doi: 10.1016/j.jaci.2007.07.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pearce N. The use of beta agonists and the risk of death and near death from asthma. J Clin Epidemiol. 2009;62:582–587. doi: 10.1016/j.jclinepi.2009.01.004. [DOI] [PubMed] [Google Scholar]
- 9.Benayoun L, Letuve S, Druilhe A, Boczkowski J, Dombret MC, Mechighel P, Megret J, Leseche G, Aubier M, Pretolani M. Regulation of peroxisome proliferator-activated receptor gamma expression in human asthmatic airways: relationship with proliferation, apoptosis, and airway remodeling. Am J Respir Crit Care Med. 2001;164:1487–1494. doi: 10.1164/ajrccm.164.8.2101070. [DOI] [PubMed] [Google Scholar]
- 10.Donovan C, Tan X, Bourke JE.PPARγ ligands regulate noncontractile and contractile functions of airway smooth muscle: implications for asthma therapy. PPAR Res2012;2012:809164 [DOI] [PMC free article] [PubMed]
- 11.Honda K, Marquillies P, Capron M, Dombrowicz D. Peroxisome proliferator-activated receptor gamma is expressed in airways and inhibits features of airway remodeling in a mouse asthma model. J Allergy Clin Immunol. 2004;113:882–888. doi: 10.1016/j.jaci.2004.02.036. [DOI] [PubMed] [Google Scholar]
- 12.Lee KS, Park SJ, Hwang PH, Yi HK, Song CH, Chai OH, Kim JS, Lee MK, Lee YC.PPAR-gamma modulates allergic inflammation through up-regulation of PTEN. FASEB J2005;19:1033–1035 [DOI] [PubMed]
- 13.Ward JE, Fernandes DJ, Taylor CC, Bonacci JV, Quan L, Stewart AG. The PPARgamma ligand, rosiglitazone, reduces airways hyperresponsiveness in a murine model of allergen-induced inflammation. Pulm Pharmacol Ther. 2006;19:39–46. doi: 10.1016/j.pupt.2005.02.005. [DOI] [PubMed] [Google Scholar]
- 14.Ward JE, Tan X.Peroxisome proliferator activated receptor ligands as regulators of airway inflammation and remodelling in chronic lung disease. PPAR Res 2007;2007:14983 [DOI] [PMC free article] [PubMed]
- 15.Woerly G, Honda K, Loyens M, Papin JP, Auwerx J, Staels B, Capron M, Dombrowicz D. Peroxisome proliferator-activated receptors alpha and gamma down-regulate allergic inflammation and eosinophil activation. J Exp Med. 2003;198:411–421. doi: 10.1084/jem.20021384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Spears M, Donnelly I, Jolly L, Brannigan M, Ito K, McSharry C, Lafferty J, Chaudhuri R, Braganza G, Bareille P, et al. Bronchodilatory effect of the PPAR-gamma agonist rosiglitazone in smokers with asthma. Clin Pharmacol Ther. 2009;86:49–53. doi: 10.1038/clpt.2009.41. [DOI] [PubMed] [Google Scholar]
- 17.Henry PJ, D’Aprile A, Self G, Hong T, Mann TS. Inhibitors of prostaglandin transport and metabolism augment protease-activated receptor-2-mediated increases in prostaglandin E2 levels and smooth muscle relaxation in mouse isolated trachea. J Pharmacol Exp Ther. 2005;314:995–1001. doi: 10.1124/jpet.105.086124. [DOI] [PubMed] [Google Scholar]
- 18.Bergner A, Sanderson MJ. ATP stimulates Ca2+ oscillations and contraction in airway smooth muscle cells of mouse lung slices. Am J Physiol Lung Cell Mol Physiol. 2002;283:L1271–L1279. doi: 10.1152/ajplung.00139.2002. [DOI] [PubMed] [Google Scholar]
- 19.Bai Y, Sanderson MJ. Modulation of the Ca2+ sensitivity of airway smooth muscle cells in murine lung slices. Am J Physiol Lung Cell Mol Physiol. 2006;291:L208–L221. doi: 10.1152/ajplung.00494.2005. [DOI] [PubMed] [Google Scholar]
- 20.Kannan MS, Prakash YS, Brenner T, Mickelson JR, Sieck GC. Role of ryanodine receptor channels in Ca2+ oscillations of porcine tracheal smooth muscle. Am J Physiol. 1997;272:L659–L664. doi: 10.1152/ajplung.1997.272.4.L659. [DOI] [PubMed] [Google Scholar]
- 21.Prakash YS, Kannan MS, Walseth TF, Sieck GC. Role of cyclic ADP-ribose in the regulation of [Ca2+]i in porcine tracheal smooth muscle. Am J Physiol. 1998;274:C1653–C1660. doi: 10.1152/ajpcell.1998.274.6.C1653. [DOI] [PubMed] [Google Scholar]
- 22.Perez JF, Sanderson MJ. The frequency of calcium oscillations induced by 5-HT, ACH, and KCl determine the contraction of smooth muscle cells of intrapulmonary bronchioles. J Gen Physiol. 2005;125:535–553. doi: 10.1085/jgp.200409216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Donovan C, Royce SG, Esposito J, Tran J, Ibrahim ZA, Tang ML, Bailey S, Bourke JE. Differential effects of allergen challenge on large and small airway reactivity in mice. PLoS ONE. 2013;8:e74101. doi: 10.1371/journal.pone.0074101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bai Y, Sanderson MJ. Airway smooth muscle relaxation results from a reduction in the frequency of Ca2+ oscillations induced by a cAMP-mediated inhibition of the IP3 receptor. Respir Res. 2006;7:34. doi: 10.1186/1465-9921-7-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bai Y, Zhang M, Sanderson MJ. Contractility and Ca2+ signaling of smooth muscle cells in different generations of mouse airways. Am J Respir Cell Mol Biol. 2007;36:122–130. doi: 10.1165/rcmb.2006-0036OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Held HD, Martin C, Uhlig S. Characterization of airway and vascular responses in murine lungs. Br J Pharmacol. 1999;126:1191–1199. doi: 10.1038/sj.bjp.0702394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ward JE, Gould H, Harris T, Bonacci JV, Stewart AG. PPAR gamma ligands, 15-deoxy-delta12,14-prostaglandin J2 and rosiglitazone regulate human cultured airway smooth muscle proliferation through different mechanisms. Br J Pharmacol. 2004;141:517–525. doi: 10.1038/sj.bjp.0705630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tanaka Y, Yamashita Y, Yamaki F, Horinouchi T, Shigenobu K, Koike K. MaxiK channel mediates beta2-adrenoceptor-activated relaxation to isoprenaline through cAMP-dependent and -independent mechanisms in guinea-pig tracheal smooth muscle. J Smooth Muscle Res. 2003;39:205–219. doi: 10.1540/jsmr.39.205. [DOI] [PubMed] [Google Scholar]
- 29.Kao J, Fortner CN, Liu LH, Shull GE, Paul RJ. Ablation of the SERCA3 gene alters epithelium-dependent relaxation in mouse tracheal smooth muscle. Am J Physiol. 1999;277:L264–L270. doi: 10.1152/ajplung.1999.277.2.L264. [DOI] [PubMed] [Google Scholar]
- 30.Henry PJ, Rigby PJ, Goldie RG. Distribution of beta 1- and beta 2-adrenoceptors in mouse trachea and lung: a quantitative autoradiographic study. Br J Pharmacol. 1990;99:136–144. doi: 10.1111/j.1476-5381.1990.tb14667.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Corompt E, Bessard G, Lantuejoul S, Naline E, Advenier C, Devillier P. Inhibitory effects of large Ca2+-activated K+ channel blockers on beta-adrenergic- and NO-donor-mediated relaxations of human and guinea-pig airway smooth muscles. Naunyn Schmiedebergs Arch Pharmacol. 1998;357:77–86. doi: 10.1007/pl00005141. [DOI] [PubMed] [Google Scholar]
- 32.Chow JM, Moffatt JD, Cocks TM. Effect of protease-activated receptor (PAR)-1, -2 and -4-activating peptides, thrombin and trypsin in rat isolated airways. Br J Pharmacol. 2000;131:1584–1591. doi: 10.1038/sj.bjp.0703738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Barnes PJ, Woolcock AJ.Difficult asthma. Eur Respir J1998;12:1209–1218 [DOI] [PubMed]
- 34.Dockrell M, Partridge MR, Valovirta E. The limitations of severe asthma: the results of a European survey. Allergy. 2007;62:134–141. doi: 10.1111/j.1398-9995.2006.01304.x. [DOI] [PubMed] [Google Scholar]
- 35.Hamid Q, Song Y, Kotsimbos TC, Minshall E, Bai TR, Hegele RG, Hogg JC. Inflammation of small airways in asthma. J Allergy Clin Immunol. 1997;100:44–51. doi: 10.1016/s0091-6749(97)70193-3. [DOI] [PubMed] [Google Scholar]
- 36.Burgel PR. The role of small airways in obstructive airway diseases. Eur Respir Rev. 2011;20:23–33. doi: 10.1183/09059180.00010410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yanai M, Sekizawa K, Ohrui T, Sasaki H, Takishima T. Site of airway obstruction in pulmonary disease: direct measurement of intrabronchial pressure. J Appl Physiol (1985) 1992;72:1016–1023. doi: 10.1152/jappl.1992.72.3.1016. [DOI] [PubMed] [Google Scholar]
- 38.Mechiche H, Naline E, Candenas L, Pinto FM, Birembault P, Advenier C, Devillier P.Effects of cysteinyl leukotrienes in small human bronchus and antagonist activity of montelukast and its metabolites. Clin Exp Allergy2003;33:887–894 [DOI] [PubMed]
- 39.Lehmann JM, Moore LB, Smith-Oliver TA, Wilkison WO, Willson TM, Kliewer SA. An antidiabetic thiazolidinedione is a high affinity ligand for peroxisome proliferator-activated receptor gamma (PPAR gamma) J Biol Chem. 1995;270:12953–12956. doi: 10.1074/jbc.270.22.12953. [DOI] [PubMed] [Google Scholar]
- 40.Patel HJ, Belvisi MG, Bishop-Bailey D, Yacoub MH, Mitchell JA. Activation of peroxisome proliferator-activated receptors in human airway smooth muscle cells has a superior anti-inflammatory profile to corticosteroids: relevance for chronic obstructive pulmonary disease therapy. J Immunol. 2003;170:2663–2669. doi: 10.4049/jimmunol.170.5.2663. [DOI] [PubMed] [Google Scholar]
- 41.Liberati TA, Randle MR, Toth LA. In vitro lung slices: a powerful approach for assessment of lung pathophysiology. Expert Rev Mol Diagn. 2010;10:501–508. doi: 10.1586/erm.10.21. [DOI] [PubMed] [Google Scholar]
- 42.Sanderson MJ. Exploring lung physiology in health and disease with lung slices. Pulm Pharmacol Ther. 2011;24:452–465. doi: 10.1016/j.pupt.2011.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Henry PJ, Goldie RG. Beta 1-adrenoceptors mediate smooth muscle relaxation in mouse isolated trachea. Br J Pharmacol. 1990;99:131–135. doi: 10.1111/j.1476-5381.1990.tb14666.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lemoine H, Overlack C. Highly potent beta-2 sympathomimetics convert to less potent partial agonists as relaxants of guinea pig tracheae maximally contracted by carbachol: comparison of relaxation with receptor binding and adenylate cyclase stimulation. J Pharmacol Exp Ther. 1992;261:258–270. [PubMed] [Google Scholar]
- 45.Barnes PJ, Chung KF, Page CP. Inflammatory mediators of asthma: an update. Pharmacol Rev. 1998;50:515–596. [PubMed] [Google Scholar]
- 46.Hay DW, Hubbard WC, Undem BJ. Endothelin-induced contraction and mediator release in human bronchus. Br J Pharmacol. 1993;110:392–398. doi: 10.1111/j.1476-5381.1993.tb13822.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pégorier S, Arouche N, Dombret MC, Aubier M, Pretolani M. Augmented epithelial endothelin-1 expression in refractory asthma. J Allergy Clin Immunol. 2007;120:1301–1307. doi: 10.1016/j.jaci.2007.09.023. [DOI] [PubMed] [Google Scholar]
- 48.Cho H, Tai HH. Thiazolidinediones as a novel class of NAD(+)-dependent 15-hydroxyprostaglandin dehydrogenase inhibitors. Arch Biochem Biophys. 2002;405:247–251. doi: 10.1016/s0003-9861(02)00352-1. [DOI] [PubMed] [Google Scholar]
- 49.Deshpande DA, Wang WC, McIlmoyle EL, Robinett KS, Schillinger RM, An SS, Sham JS, Liggett SB. Bitter taste receptors on airway smooth muscle bronchodilate by localized calcium signaling and reverse obstruction. Nat Med. 2010;16:1299–1304. doi: 10.1038/nm.2237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Delmotte P, Sanderson MJ. Effects of albuterol isomers on the contraction and Ca2+ signaling of small airways in mouse lung slices. Am J Respir Cell Mol Biol. 2008;38:524–531. doi: 10.1165/rcmb.2007-0214OC. [DOI] [PMC free article] [PubMed] [Google Scholar]