Abstract
New drugs are needed to enhance premature termination codon (PTC) suppression to treat the underlying cause of cystic fibrosis (CF) and other diseases caused by nonsense mutations. We tested new synthetic aminoglycoside derivatives expressly developed for PTC suppression in a series of complementary CF models. Using a dual-luciferase reporter system containing the four most prevalent CF transmembrane conductance regulator (CFTR) nonsense mutations (G542X, R553X, R1162X, and W1282X) within their local sequence contexts (the three codons on either side of the PTC), we found that NB124 promoted the most readthrough of G542X, R1162X, and W1282X PTCs. NB124 also restored full-length CFTR expression and chloride transport in Fischer rat thyroid cells stably transduced with a CFTR–G542XcDNA transgene, and was superior to gentamicin and other aminoglycosides tested. NB124 restored CFTR function to roughly 7% of wild-type activity in primary human bronchial epithelial (HBE) CF cells (G542X/delF508), a highly relevant preclinical model with endogenous CFTR expression. Efficacy was further enhanced by addition of the CFTR potentiator, ivacaftor (VX-770), to airway cells expressing CFTR PTCs. NB124 treatment rescued CFTR function in a CF mouse model expressing a human CFTR-G542X transgene; efficacy was superior to gentamicin and exhibited favorable pharmacokinetic properties, suggesting that in vitro results translated to clinical benefit in vivo. NB124 was also less cytotoxic than gentamicin in a tissue-based model for ototoxicity. These results provide evidence that NB124 and other synthetic aminoglycosides provide a 10-fold improvement in therapeutic index over gentamicin and other first-generation aminoglycosides, providing a promising treatment for a wide array of CFTR nonsense mutations.
Keywords: cystic fibrosis transmembrane conductance regulator, nonsense mutations, aminoglycosides, ivacaftor, translational readthrough
Clinical Relevance
Translational readthrough of premature termination codons (PTCs) represents a potential treatment for the proximate cause of cystic fibrosis (CF) and many other genetic diseases caused by nonsense mutations. We show that rationally designed synthetic aminoglycosides provide superior readthrough of PTCs in CF transmembrane conductance regulator (CFTR) in vitro and in vivo compared with conventional agents, and can be readily combined with the clinically approved CFTR potentiator, ivacaftor, to maximize functional activity. Synthetic aminoglycosides restore ion transport activity of mutant CFTR caused by clinically relevant nonsense mutations, significantly improving therapeutic index and providing a potential approach for life-long treatment of genetic disease.
Cystic fibrosis (CF) is a common lethal genetic disease that affects at least 30,000 people in the United States, where roughly 1 in 25 persons of European descent is a carrier (1). This autosomal monogenic disorder arises from defects in the CF transmembrane conductance regulator (CFTR) gene. The CFTR protein is a cAMP-regulated chloride channel located primarily at the apical surface of epithelial cells in the lung, pancreas, intestine, liver, and male reproductive system (1). Roughly 10% of patients with CF carry a mutation that results in a premature termination codon (PTC; also referred to as a nonsense mutation) in at least one CFTR allele, resulting in an absent of functional CFTR protein and severe CF disease. The incidence of nonsense mutations is especially high in individuals of Ashkenazi Jewish decent, where they account for more than 60% of all CFTR mutations (2).
Aminoglycosides are used as antibiotics due to their ability to preferentially inhibit bacterial protein synthesis. At high concentrations, they block prokaryotic translation initiation, whereas at lower doses they reduce the accuracy of the ribosomal decoding site (3). In eukaryotic cells, aminoglycosides also increase translational misreading and suppress PTCs. This raises the possibility that PTC suppression represents a potential treatment for genetic diseases caused by nonsense mutations (4, 5). Readthrough of a PTC occurs when an amino acid carried by a near-cognate aminoacyl-tRNA (which has an anticodon complementary to two of the three bases of the PTC) is incorporated into the nascent polypeptide chain. This amino acid insertion at the stop codon allows in-frame translation elongation to resume and generate a full-length protein. Previous in vitro studies have shown that some aminoglycosides have the ability to suppress nonsense mutations by this mechanism and restore the synthesis of functional proteins (4, 5). For example, gentamicin has been shown to partially restore the expression of full-length, functional protein in cell-based and mouse models of various genetic diseases, including CF (4, 6) and Duchenne muscular dystrophy (7). The effects of gentamicin on CFTR activity in patients with CF with nonsense alleles have also been demonstrated (8–11). Unfortunately, the long-term use of aminoglycosides frequently leads to severe side effects, such as nephrotoxicity and ototoxicity (12), which limit their application in PTC suppression therapy.
Recent efforts have aimed to develop new strategies to enhance nonsense suppression activity while reducing toxicity. For example, poly-L-aspartic acid, a compound previously shown to significantly reduce aminoglycoside toxicity, was found to increase both the level and duration of readthrough in a CFTR-G542X transgenic mouse (13). In a second approach, PTC Therapeutics, Inc. (South Plainfield, NJ) used high-throughput screening to identify the nonaminoglycoside readthrough compound, ataluren (also known as PTC124) (14). Ataluren was shown to have advantages over aminoglycosides for nonsense suppression, because it is both nontoxic and orally bioavailable. Animal studies reported that PTC124 partially restored CFTR function in a CF mouse model (15) and dystrophin levels in the mdx mouse model of Duchenne muscular dystrophy (14). However, despite some success in phase 2 testing (16–18), ataluren did not provide a significant improvement in the primary endpoint of a recent phase III clinical trial, suggesting that this compound may not restore enough CFTR function to provide a significant therapeutic benefit in an unselected population (19). In a third approach, a series of aminoglycoside derivatives were rationally designed to provide higher readthrough activity with less toxicity (20). One of the early compounds produced, NB54, suppressed PTCs to a level comparable to gentamicin in immortalized and primary human CF cells, as well as in the CFTR-G542X transgenic mouse model, while exhibiting low toxicity, suggesting a potential advantage of this approach (21).
Aminoglycoside antibiotics were originally developed for their antibacterial properties. However, a significant portion of the toxicity associated with aminoglycosides may stem from their ability to also inhibit mitochondrial translation. More recently, further advances in the rational design of these compounds has allowed their toxic effects to be separated from their ability to promote translational readthrough (22). In the current study, new synthetic aminoglycosides specifically developed to further enhance readthrough efficacy while maintaining their improved toxicity profile were evaluated in a series of cell-based and animal models for efficacy, toxicity, and pharmacokinetics (Figure 1). Through this process, we identified NB124, a novel aminoglycoside derivative that exhibits roughly 2.5-fold greater readthrough activity than gentamicin across several clinically relevant CF alleles, while also exhibiting lower toxicity and favorable pharmacokinetic properties. Furthermore, CFTR activity could be further augmented by addition of the CFTR potentiator, ivacaftor. Taken together, these results provide evidence that NB124 is a promising readthrough compound that can restore significant CFTR expression and activity from several common CFTR nonsense alleles.
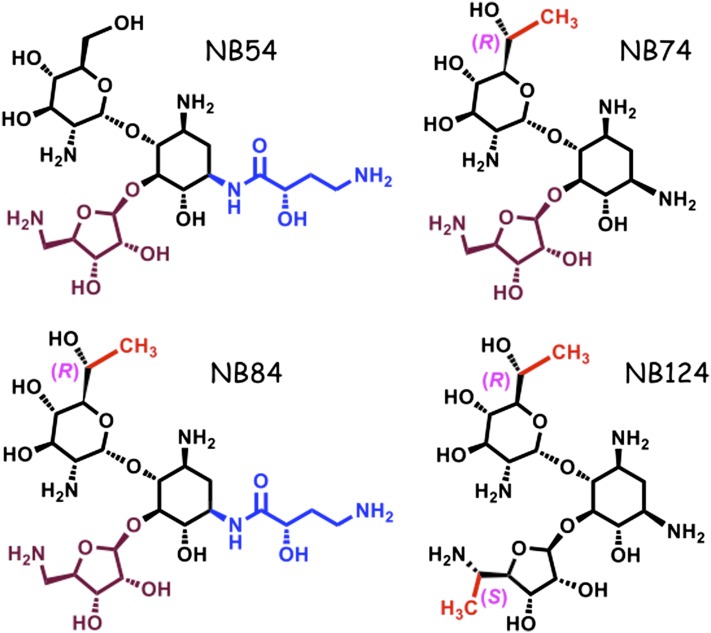
Figure 1.
Chemical structures of synthetic aminoglycosides.
Materials and Methods
Further details regarding methodology and cell lines are provided in the online supplement.
Dual Luciferase Assay
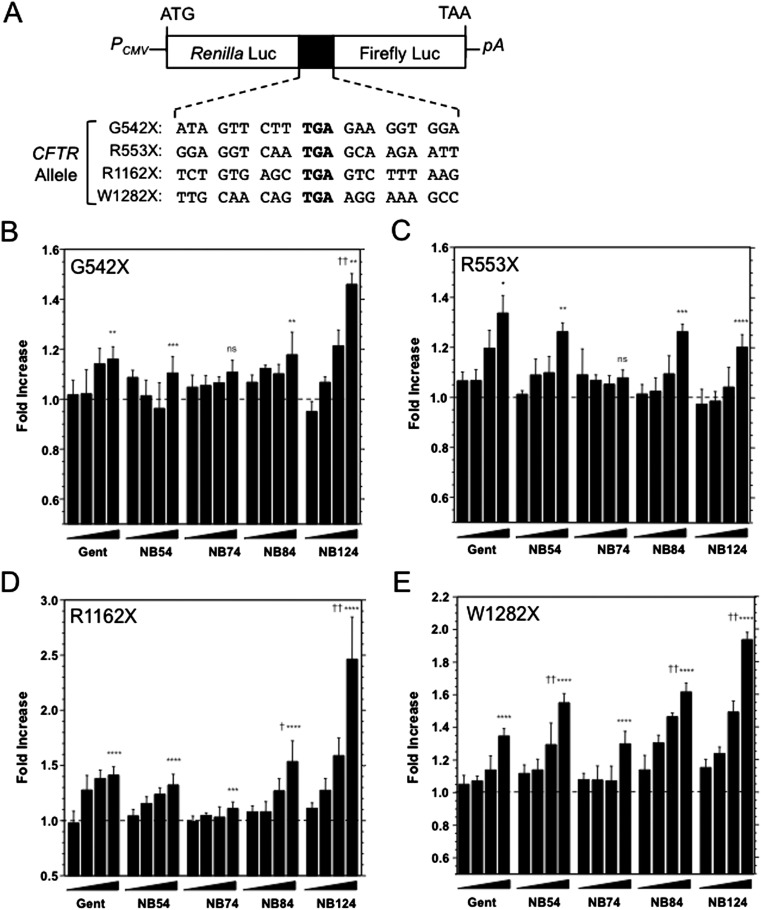
Readthrough cassettes contained the G542X, R553X, R1162X, or W1282X CFTR PTCs (or the corresponding wild-type codon) together with three codons of upstream and downstream human CFTR sequence (Figure 2A; see also Table E1 in the online supplement). Each cassette was inserted between the Renilla and firefly genes (23, 24). Constructs were transiently transfected into CF bronchial epithelial (CFBE41o−) cells (genotype delF508/delF508) and grown in the presence of readthrough compounds for 24 hours. Dual-luciferase assays were then conducted as previously described (21).
Figure 2.
Readthrough assays in immortalized cystic fibrosis (CF) bronchial epithelial (CFBE) cells transiently transfected with readthrough reporters. (A) The diagram of dual-luciferase (Luc) reporters. Four CF transmembrane conductance regulator (CFTR) premature termination codons (PTCs; bold), together with their natural context, are shown. The corresponding WT codons are GGA for G542, CGA for R553, CGA for R1162, and TGG for W1282. pA, Poly(A) tail; PCMV, CMV promoter. (B–E) CFBE41o− cells are transfected with one of the reporters; 3 hours later, various doses (3, 10, 30, and 100 μM) of five drugs (gentamicin, NB54, NB74, NB84, and NB124) were added into medium. Luciferase assays were performed after 24 hours. The numbers of readthrough fold for the four PTCs are shown. Readthrough fold is determined as the ratio of readthrough percent after treatment to that of untreated, whereas readthrough percent is the ratio of Firefly number to Renilla number normalized to wild-type (WT). Gent, gentamicin. The data are shown as means ± SD of at least six replicates. *Significance relative to untreated; †significance relative to 100 μM gentamicin; * or †P < 0.05, **P < 0.01, ***P < 0.001, **** or ††P < 0.0001.
Fischer rat thyroid cell line development.
Fischer rat thyroid (FRT) cells were stably transduced with mutant CFTR-G542X or CFTR–wild-type cDNA using the Flp-In system (Invitrogen, Grand Island, NY) to allow accurate comparison between mutation groups (25).
Primary human epithelial cell culture.
First- or second-passage primary human bronchial epithelial (HBE) cells were derived from lung explants (26, 27). Cells were grown for 6–8 weeks until terminally differentiated. The University of Alabama at Birmingham Institutional Review Board approved use of human airway cells.
Transepithelial Conductance Measurements
FRT cells were treated with synthetic aminoglycosides for 48 hours when transepithelial resistance was greater than 2 kΩ ⋅ cm2. Transepithelial conductance (Gt) of CFTR-G542X–expressing cells was measured using a 24-channel current clamp coupled with silver chloride electrodes (EP-Devices, Bertum, Belgium) and a computer-controlled robot (PrecisePlace 2,300 Robot; Precision Automation Inc., La Jolla, CA) (28). Gt was measured before and after stimulation of CFTR activity by addition of forskolin (10 μM) followed by CFTRInh-172 (10 μM).
Short-circuit current measurements.
Short-circuit current (Isc) was measured under voltage clamp conditions inUssing chambers (Physiologic Instruments, San Diego, CA) (21, 26, 27). Amiloride (100 μM) was added to block residual epithelial sodium channel current, followed by the CFTR agonist, forskolin, and the inhibitor, CFTRInh-172 (10 μM).
CFTR Western Blotting
FRT cell lysates were normalized for protein concentration and separated by gel electrophoresis. CFTR was detected by anti-CFTR antibody (1:1 mixture of 570 and 596 monoclonal anti-CFTR antibodies [26, 29]).
Murine Studies
The University of Alabama at Birmingham Institutional Animal Care and Use Committee approved all protocols. Cftr knockout mice expressing a human CFTR-G542X transgene under intestine-specific rat fatty acid binding protein were treated by subcutaneous injection once daily for 14 days (6, 13, 15). Freshly mounted intestinal slices were stimulated with forskolin (10 μM) and IBMX (100 μM) in Ussing chambers using a partial chloride secretory gradient; glybenclamide was used to confirm specificity. For immunohistochemistry studies, intestinal tissue was embedded and fixed immediately after animals were killed. CFTR was detected with a 1:200 dilution of CFTR antibody 4562, followed by goat anti- rabbit IgG conjugated to AlexaFluor-488 (Invitrogen no. A-11008) (21).
Toxicity Assays in Cochlear Explants
Toxicity to the sensory cells of the inner ear was determined in explants of the organ of Corti of the early postnatal mouse (30). The presence or absence of hair cells after 72-hour treatment was quantified for the entire length of the cochlea by light microscopy of the phalloidin-stained stereociliary bundles and circumferential F-actin rings on the cuticular plate.
Determination of Serum Aminoglycoside Levels
ELISA assays were conducted using a generic aminoglycoside antibody that detects a variety of therapeutic aminoglycosides using conditions described previously (31).
Results
Comparison of the Ability of Synthetic Aminoglycosides to Suppress Four Common CFTR PTCs Using Dual-Luciferase Readthrough Reporters
Medicinal chemistry has resulted in serial improvements in the ability of synthetic aminoglycosides to induce readthrough of PTCs in eukaryotic cells, while also reducing their toxic effects. Some of these compounds, such as NB74, NB84, and NB124 (Figure 1), have shown considerable promise, but have not yet been tested in CF-relevant models (22, 32). To do this, we first used dual-luciferase readthrough reporters to test the ability of these aminoglycoside derivatives to mediate suppression of different CFTR PTCs. Gentamicin and NB54 were used as controls, because they have been used to induce nonsense suppression in CF (and other) systems in previous studies (20, 21). The nonsense suppression activity of each compound was tested in a CFBE cell line (CFBE41o− cells stably expressing a CFTR cDNA transgene under cytomegalovirus promoter control), as these cells provide a model for CFTR processing and function in a relevant airway cell line.
Many studies have shown that the context surrounding a stop codon plays an important role in its response to PTC suppression. To test suppression of the four most common CFTR PTCs (G542X, R553X, R1162X, and W1282X), we constructed dual-luciferase reporters that each contained a Renilla gene, a firefly gene, and a CFTR readthrough cassette with each PTC, and the context of three additional codons on either side of the PTC (Figure 2A). After transient transfection into CFBE41o− cells, the ability of the aminoglycoside derivatives to suppress each of the four CFTR PTCs was tested. Previous studies have shown that exposure of cultured cells to high doses of readthrough compounds induces high levels of nonsense suppression. However, these concentrations may not be readily achieved in vivo. Because we previously reported that peak serum levels of 50–100 μM of aminoglycoside could be correlated with readthrough activity, we initially tested these compounds using doses ranging from 0 to 100 μM (100 μM is equivalent to 50–70 μg/ml, depending on the compound).
Initially, we treated the CFTR-corrected CFBE41o− cells expressing the G542X dual-luciferase reporter with NB124. We observed a 1.5-fold increase in firefly luciferase activity at the 100-μM dose relative to the untreated control (Figure 2B). The effect of NB124 at this dose was significantly higher than gentamicin, which increased firefly activity only less than 1.2-fold above the untreated control. Qualitatively similar results were obtained for the R1162X and W1282X PTCs (Figures 2D and 2E). NB124 (100 μM) induced a 2.5-fold increase in readthrough at the highest dose tested with the R1162X readthrough reporter (Figure 2D), and a 1.9-fold increase in readthrough with the W1282X construct. In both cases, the level of readthrough induced by NB124 was significantly higher than gentamicin. In contrast, NB124 induced a much lower (1.2-fold) level of readthrough at the R553X PTC (Figure 2C), which was slightly lower than the readthrough induced by gentamicin. These results indicate that NB124 induces more readthrough than gentamicin (or the other synthetic aminoglycosides) at three of the four CFTR PTCs examined, but only using the highest dose tested (100 μM, or 60 μg/ml). Furthermore, because all four of the CFTR PTCs tested were UGA nonsense codons, the differences observed in the readthrough pattern observed with the R553X mutation compared with the other PTCs demonstrates that the surrounding sequence context has a significant influence on the level of nonsense suppression obtained.
Partial Restoration of CFTR Function in FRT Monolayers Expressing CFTR-G542X
We next used a forskolin-dependent Gt assay to determine the level at which the aminoglycoside derivatives suppressed CFTR PTCs and restored full-length, functional CFTR in cultured FRT epithelial cells stably transduced with human CFTR-G542X cDNAs using Flp-in technology (25). CFTR-G542X was chosen because this mutation is the most common allele seen in human subjects, and matches an animal model planned for use in subsequent studies. This cell line provided an advantage, as it is widely used to monitor CFTR-dependent ion transport, and is suitable to monitor Gt as a proxy for CFTR function using a robot-controlled apparatus with excellent throughput, sensitivity, and reproducibility (28). A description of the assay development and performance is provided online (supplemental Results and Figures E1 and E2).
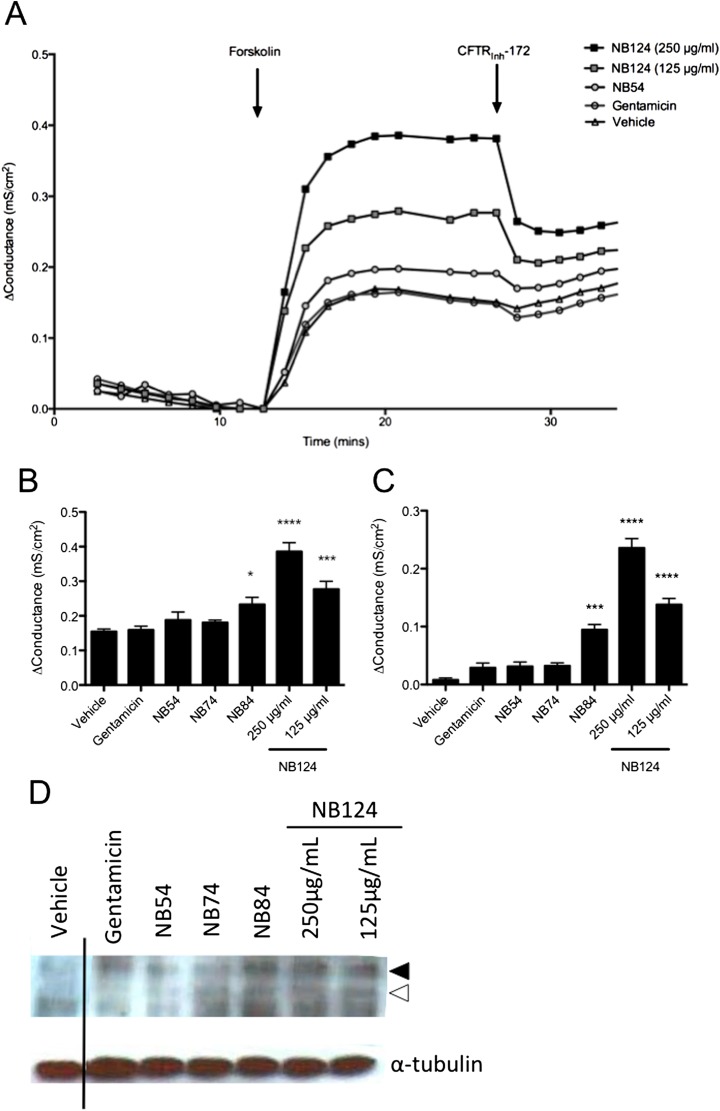
To assess the efficacy of synthetic aminoglycosides, FRT-G542X cells were treated for 48 hours, then CFTR activity determined by the change in Gt after forskolin (10 μM) activation. CFTR function was then confirmed with subsequent administration of CFTRInh-172 (10 μM). As shown in Figure 3, synthetic aminoglycosides induced a significant increase in forskolin-stimulated Gt (Figures 3A and 3B) that was confirmed after addition of CFTRInh-172 (Figures 3A and 3C). The maximum activity was seen with NB124 (250 μg/ml), which was associated with a 2.5-fold increase in forskolin-stimulated Gt, and was significantly greater than observed with monolayers treated with either gentamicin (P < 0.0001) or NB84 (P < 0.001). The effects of NB124 were also dose-dependent.
Figure 3.
Improved CFTR function and expression in CFTR-G542X–expressing Fischer rat thyroid (FRT) monolayers. (A) Representative tracings of the change in the transepithelial conductance (Δ Conductance, Δ transepithelial conductance [Gt]) of FRT cell monolayers (n = 9) expressing CFTR-G542X treated with synthetic aminoglycosides and gentamicin (250 μg/ml, unless indicated otherwise). CFTR activity was measured as the change from baseline conductance after the addition of forskolin (10 μM); CFTR-Inh172 (10 μM) was used to block CFTR-mediated Gt for confirmation. (B and C) Mean change in Gt induced by forskolin (B) and CFTRInh-172 (C) for experiments shown in (A). *P < 0.05, ***P < 0.001, ****P < 0.0001 versus vehicle or gentamicin (n = 9 per condition, performed in three replicate experiments). (D) Representative Western blot demonstrating presence of CFTR C-band (closed arrowhead) and B-band (open arrowhead) after treatment with synthetic aminoglycosides (n = 3 monolayers/lane) in comparison to vehicle and gentamicin control.
To confirm functional restoration of CFTR was accompanied by biochemical detection of full-length and mature protein, we conducted Western blotting of monolayers after treatment with synthetic aminoglycosides. Increased expression of CFTR C band was observed with NB124 and other synthetic aminoglycosides as compared with gentamicin or control conditions (Figure 3D).
When taken together, these results indicated that NB124 suppresses the relatively common CFTR-G542X mutation more efficiently than other tested aminoglycosides. The relative functional activity of the synthetic aminoglycosides in FRT monolayers also closely matched with the relative readthrough levels observed in the dual luciferase reporter transduced with CFTR-G542X. These results provided the impetus to advance the analysis to other, more sophisticated CF models.
Partial Rescue of CFTR Function in Primary HBE Cells
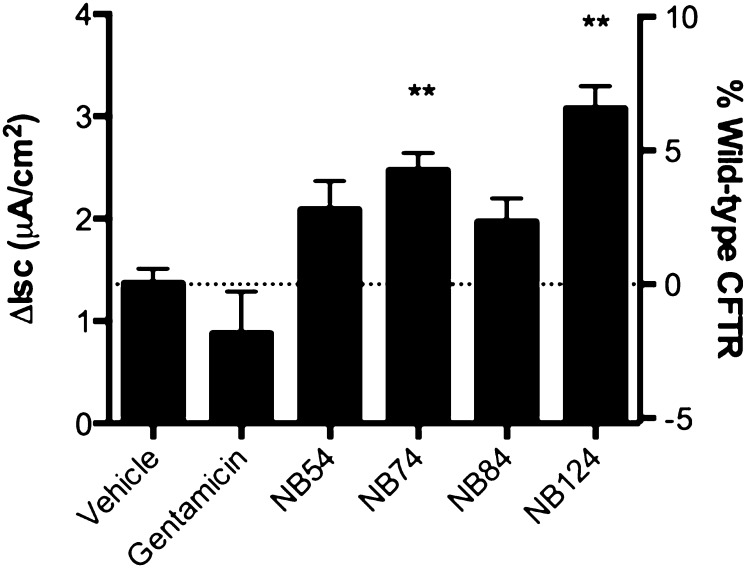
In previous studies, the functional data obtained with primary HBE cells were found to be predictive of the later results obtained in CF clinical trials (33, 34). We therefore next used CFTR functional assays on primary HBE cells (CFTR genotype G542X/delF508) to evaluate the PTC suppression efficacy of synthetic aminoglycosides. Untreated primary HBE cells exhibited a forskolin-dependent Isc of 1.3 μA/cm2, and a small (1.5-fold) increase in Isc was observed when these cells were treated with NB54 or NB84. No increase was observed in cells treated with gentamicin, probably because it is relatively inactive in primary HBE cells (21). Larger 2- to 2.5-fold increases in Isc were observed in cells treated with NB74 and NB124, respectively (Figure 4), and mirrored the effects seen in CFTR-G542X transduced FRT monolayers. These results indicate that NB124 restores more CFTR function than the other tested compounds in primary HBE cells, and to levels approaching 7% of wild-type CFTR activity.
Figure 4.
Restoration of CFTR function in CF primary human bronchial epithelial (HBE) cells derived from a G542X/F508del donor. The forskolin (20 μM) -stimulated short-circuit current (Isc) of HBE cells treated with one of the five drugs (250 μg/ml) or vehicle control for 48 hours is shown and plotted in comparison to the relative percent values obtained in a panel of non-CF HBE cells tested under the exact same conditions. The data are shown as means ± SEM of at least four replicates. **P < 0.01.
Partial Restoration of CFTR Function in Cftr−/− Mice Expressing a Human CFTR-G542X Transgene
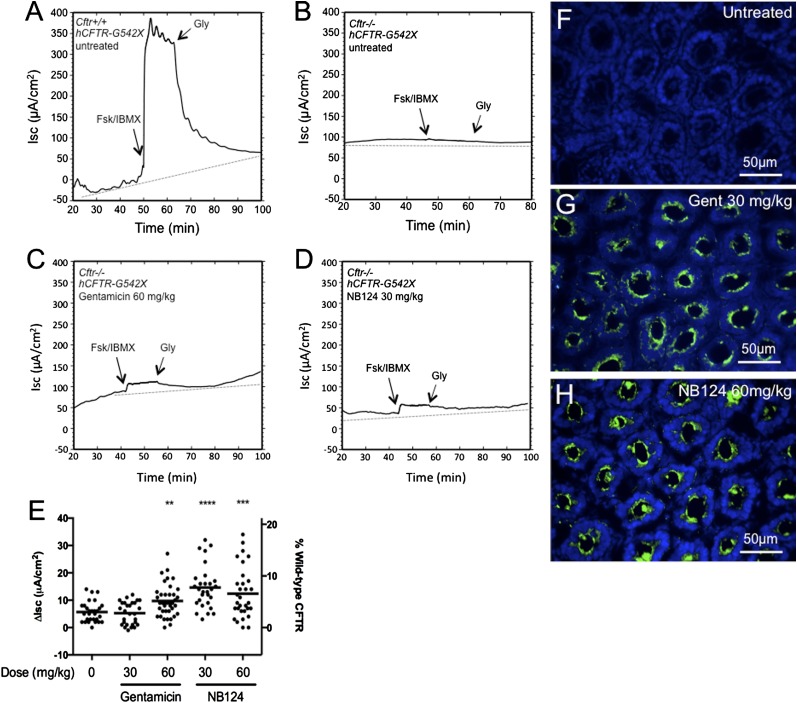
The in vitro results described previously here demonstrate that several synthetic aminoglycosides suppress CFTR PTCs more effectively than gentamicin, with NB124 most effectively restoring CFTR function across several clinically relevant alleles, including CFTR-G542X, the most common disease-causing nonsense allele. Based on those results, we next tested the ability of NB124 to suppress CFTR in a Cftr knockout mouse line expressing a human CFTR-G542X transgene under control of the intestine-specific rat fatty acid–binding protein promoter (referred to hereafter as Cftr−/− hCFTR-G542X). Mice were administered NB124 or gentamicin (at doses of 30 and 60 mg/kg) by subcutaneous injection once daily for 14 days. The mice were then killed and ileum segments were assayed to determine whether cAMP-stimulated transepithelial Isc could be detected as an indication of CFTR activity. Figures 5A and 5B show representative Isc tracings from Cftr+/+ hCFTR-G542X and Cftr−/− hCFTR-G542X mice (positive and negative controls, respectively), and Figures 5C and 5D show Isc tracings from Cftr−/− hCFTR-G542X mice treated with 60 mg/kg gentamicin and 30 mg/kg NB124, respectively. These data are summarized in Figure 5E, which shows a scatter plot of forskolin-stimulated Isc from ileum samples obtained from untreated and treated Cftr−/− hCFTR-G542X mice. The mean forskolin-stimulated Isc was 5.7 (± 3.7) μA/cm2 in ileum samples from untreated Cftr−/− hCFTR-G542X mice (negative controls), and 255.7 (± 36.1) μA/cm2 in samples from wild-type mice (positive controls). In Cftr−/− hCFTR-G542X mice treated with 30 mg/kg gentamicin, we did not observe a significant increase in Isc relative to the untreated controls (5.3 ± 4.1 μA/cm2). However, when the gentamicin dosage was increased to 60 mg/kg, the Isc increased to 9.7 (± 5.8) μA/cm2 (3.8% of the wild-type control). In contrast, significant increases in forskolin-stimulated Isc were observed in Cftr−/− hCFTR-G542X mice treated with 30 and 60 mg/kg NB124 (14.7 ± 7.8 and 12.4 ± 9.2, respectively). The increases in Isc in these NB124-treated mice were 5.8 and 4.9%, respectively, of the forskolin-stimulated Isc observed in wild-type control mice, and the response to 30 mg/kg NB124 was roughly 2.5-fold greater than the currents observed in controls treated with the same dose of gentamicin. Consistent with the observed restoration of CFTR activity, we also detected human CFTR in the epithelial submucosal glands in the duodenum of mice treated with gentamicin (Figure 5G) or NB124 (Figure 5H), whereas only background was observed in corresponding tissues from untreated mice (Figure 5F); of note, we were unable to detect a difference between gentamicin and NB124 treatment with this nonquantitative assay. When taken together, these results indicate that NB124 restores greater CFTR function (and at a 2-fold lower dose) than gentamicin in Cftr−/− hCFTR-G542X transgenic mice, and has a slightly more favorable dose–response relationship.
Figure 5.
c-AMP–activated transepithelial currents in ileum sections from mice treated with gentamicin or NB124. Representative tracings of (A) untreated Cftr+/+ hCFTR-G542X mice (WT control), (B) untreated Cftr−/− hCFTR-G542X (negative control), (C) gentamicin-treated Cftr−/− hCFTR-G542X (60 mg/kg), and (D) NB124-treated Cftr−/− hCFTR-G542X (30 mg/kg). (E) Scatter plot of all data from Cftr−/− hCFTR-G542X mice (untreated, gentamicin treated, and NB124 treated). The mean is shown as a bar. **P < 0.01, ***P < 0.001, ****P < 0.0001. (F–H) Human CFTR immunofluorescence of excised intestine from mice treated with gentamicin or NB124. The tissue was cut 5-μM thick and incubated with CFTR-NBD1 antiserum and AlexaFluor488-labeled secondary antibody (green). Nuclei were stained with 4′,6-diamidino-2-phenylindole (blue). Representative images are shown from (F) untreated Cftr−/− hCFTR-G542X (negative control), (G) gentamicin-treated Cftr−/− hCFTR-G542X (60 mg/kg), and (H) NB124-treated Cftr−/− hCFTR-G542X (30 mg/kg). Fsk, forskolin; Gly, glybenclamide; IBMX, 3-isobutyl-1-methylxanthine.
To understand the relationship between readthrough efficacy and the pharmacokinetics of synthetic aminoglycosides, we also examined serum levels of gentamicin and NB124 in treated mice using an ELISA-based assay (Table 1). The peak concentrations of NB124 (determined 20 min after injection) were 18 and 62% greater than gentamicin at 30 and 60 mg/kg, respectively, whereas trough concentrations (measured 120 min after injection) were 65 and 38% lower than gentamicin. Together, these results indicate that NB124 reaches a slightly higher peak serum level than gentamicin shortly after administration, but its concentration subsequently diminishes more rapidly thereafter. This pharmacokinetic profile is favorable, because readthrough may be limited by epithelial cell penetration, which is dependent on peak concentration. In contrast, toxicity is largely governed by persistently high trough concentrations (35).
Table 1:
Serum Levels of Gentamicin and NB124
| Serum Level |
|||
|---|---|---|---|
| Dose | (μg/ml) |
||
| Compound | (mg/kg) | 20 min after Injection | 120 min after Injection |
| Gentamicin | 30 | 42.6 ± 0.4 | 19.9 ± 1.3 |
| NB124 | 30 | 50.2 ± 1.1* | 7.0 ± 0.4† |
| Gentamicin | 60 | 64.2 ± 1.0 | 15.5 ± 1.0 |
| NB124 | 60 | 103.8 ± 3.4† | 9.6 ± 0.5* |
P < 005 versus same dose of gentamicin at the same time point.
P < 0005 versus same dose of gentamicin at the same time point.
NB124 exhibits lower toxicity than gentamicin in cochlear cell explants.
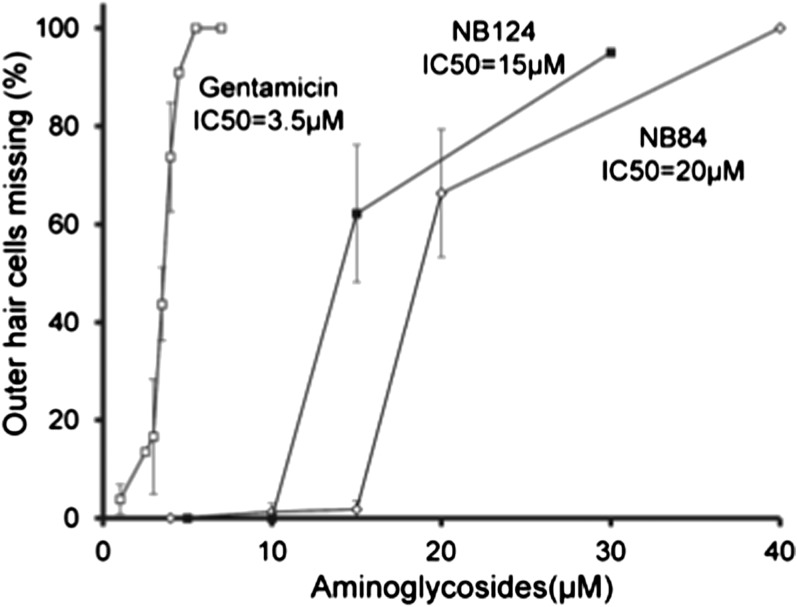
The nephrotoxicity and ototoxicity caused by aminoglycosides are well known, and currently limit prolonged clinical use (35). Previous studies have shown that organ explants from the cochlea of early postnatal mice provide a good model to monitor the toxicity of compounds such as aminoglycosides (30). As shown in Figure 6, the inhibitory concentration (IC) at which 50% of cells were lost of NB124 was found to be 15 μM, a level that was more than fourfold higher than the IC of gentamicin (3.5 μM). NB84 was also less toxic, with an IC at which 50% of cells were lost of 20 μM. Because reliable readthrough assays in primary HBE cell monolayers and in vivo found that NB124 suppresses the G542X-CFTR nonsense mutation roughly 2.5-fold more effectively than gentamicin in vivo, these results indicate that the efficacy:toxicity ratio of NB124 is approximately 10-fold better than gentamicin.
Figure 6.
Toxicity study in cochlear hair cells in vitro. Loss of cochlear hair cells was measured 72 hours after treating with gentamicin, NB124, or NB84. The data are shown as means ± SD of at least three replicates per concentration. IC50, inhibitory concentration at which 50% of cells were lost.
CFTR activity resulting from PTC suppression is enhanced by a CFTR potentiator.
Previous studies have shown that the CFTR potentiator, ivacaftor (formerly VX-770) can increase the open probability of wild-type and mutant forms of CFTR at the cell surface, thereby enhancing chloride transport (25, 26, 33). Because our ultimate goal is to restore enough CFTR function in PTCs of patients with CFTR to provide a therapeutic benefit, we next tested whether the addition of ivacaftor could enhance the level of CFTR function obtained by PTC suppression.
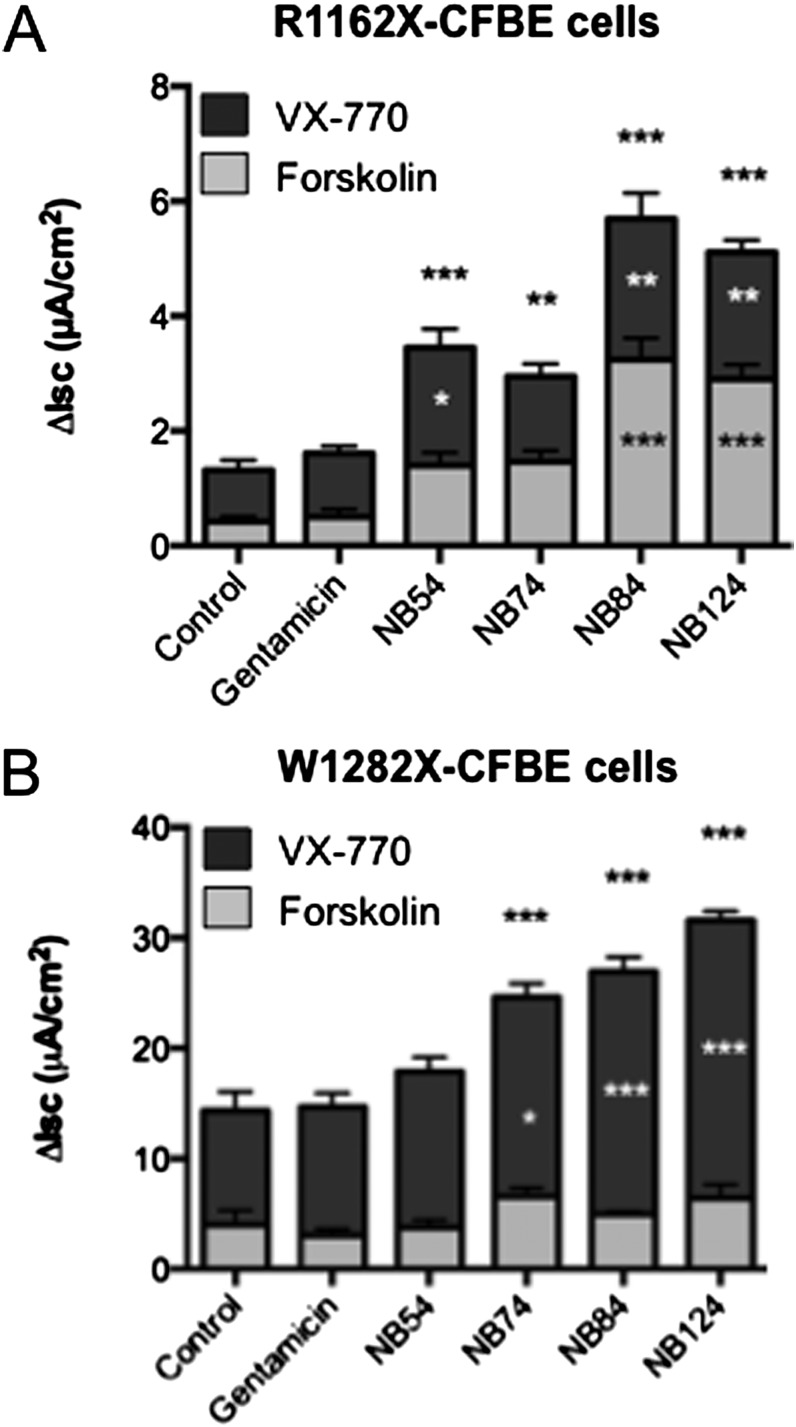
We initially tested whether ivacaftor could enhance the level of CFTR function restored by PTC suppression in CFBE41o− cells expressing theCFTR-R1162X allele. We found that treatment with 10 μM ivacaftor induced a two- to threefold increase in Isc over the level of CFTR function induced by readthrough alone for each of the five readthrough drugs when prestimulated with a submaximal concentration of forskolin (100 nM; Figure 7A), consistent with its known activity as a CFTR potentiator that increases the open probability of wild-type CFTR by approximately twofold (26, 33). In CFBE41o− cells expressing the CFTR-W1282X allele, ivacaftor stimulated Isc 2.5- to 4-fold above the level observed with synthetic aminoglycosides alone (Figure 7B). This large enhancement is consistent with the prior finding that truncated, partially active, and ivacaftor-responsive CFTR may be localized to the cell surface in these cells (29). Overall, we conclude that ivacaftor enhances CFTR function above the levels promoted by readthrough drugs alone.
Figure 7.
Ivacaftor further stimulates cAMP-dependent CFTR function in CFBE41o− monolayers after translational readthrough. (A and B) CFBE41o− cells stably expressing CFTR-R1162X (A) or W1282X (B) were incubated with synthetic aminoglycosides (250 μg/ml) for 48 hours, followed by Isc measurements in Ussing chambers. Cells were sequentially stimulated with forskolin (100 nM) then ivacaftor (VX-770; 10 μM). Comparisons of total stimulated current, ivacaftor-stimulated current, and forskolin-stimulated current are each designated as compared with control (asterisk noted top to bottom, respectively). The data shown are means ± SEM of at least six replicates per condition. *P < 0.05, **P < 0.01, ***P < 0.001.
Discussion
Partial restoration of protein function by PTC suppression has been demonstrated in a number of diseases caused by nonsense mutations, including CF (4, 6, 10), Duchenne muscular dystrophy (7, 14), mucopolysaccharidosis I-H (36–38), late infantile neuronal ceroid lipofuscinosis (39), Rett syndrome (40), Usher syndrome (41), thalassemia (42), and leukocyte adhesion deficiency (43). Moreover, gentamicin has been successfully tested in pilot clinical trials to suppress nonsense mutations in patients with CF (8–11) and Duchenne muscular dystrophy (44–46). Unfortunately, the long-term use of aminoglycoside antibiotics is currently untenable, due to serious side effects associated with these compounds, such as ototoxicity and nephrotoxicity (35). Because a pharmacological approach to suppress PTCs that cause genetic diseases would require treatment throughout the patient’s life, toxicity combined with relatively low efficacy have presented significant barriers to the use of aminoglycosides to treat genetic diseases caused by PTCs.
The caveats described here have led to rational drug design approaches to increase readthrough efficacy and reduce associated toxicity. In this study, we examined the relative efficacy of a traditional aminoglycoside antibiotic, gentamicin, with four synthetic aminoglycoside derivatives, some of which have been previously examined (20, 21). The design of the synthetic aminoglycosides was based on the hypothesis that aminoglycoside antibiotics that acted by blocking prokaryotic ribosome function would also inhibit mitochondrial ribosomes, due to their prokaryotic nature (22). The derivatives described in this study were developed through progressive steps to eliminate structural components that mediated antibacterial effects, while at the same time retaining the features that promoted readthrough on eukaryotic ribosomes. This rational design approach was validated in the current study, because two of the newest derivatives, NB84 and NB124, were found to mediate the highest level of readthrough in various CF models (Figures 2–4). These findings clearly highlight improvements in the structure/activity relationship of this drug scaffold. Notably, these compounds also exhibited much lower potential ototoxicity than gentamicin, as measured by a significantly reduced damage to cochlear hair cells in tissue explants (Figure 6).
This study took advantage of a series of cell-based and animal models to determine which aminoglycoside derivatives provide the best readthrough activity at different CF mutations within distinct sequence contexts. The dual-luciferase assays were performed in immortalized CFBE41o− bronchial epithelial cells, which allowed the relative suppression of CFTR PTCs to be determined in an airway cell environment (Figure 2). Results obtained with this system highlighted how a UGA nonsense mutation can respond differently to translational readthrough agents as a function of the surrounding sequence context (three codons upstream and downstream of the original CFTR mutation). We found that three CFTR UGA mutations (G542X, R1162X, and W1282X) exhibited qualitatively similar responses to the compounds and showed maximal suppression with NB124. In contrast, the UGA mutation associated with R553X was more refractory to suppression, demonstrating that sequence context plays an important role in PTC suppression. Further studies are needed to better understand how the sequence context causes these differences in PTC suppression.
Our study was based on the use of a series of functional and biochemical readthrough models. This includes use of a newly constructed FRT Flp-In cell line expressing a CFTR-G542X transgene (Figure 3 and Figures E1 and E2). The FRT Flp-In cell line permits comparison of various CFTR forms, because CFTR expression and the chromosome insertion site are controlled. Furthermore, the cell line is highly amenable to the Gt ion transport assay by ΔGt, which demonstrated excellent reproducibility and consistency (Figures 3 and E2). The immortalized CFBE41o− cells stably transfected with CFTR-R1162X or CFTR-W1282X cDNAs under cytomegalovirus promoter control provided high-level CFTR expression that allowed us to measure relatively low CFTR activity associated with PTC suppression, and also tests the effects of the CFTR potentiator ivacaftor (26, 29). A concern with any study of PTC suppression is the influence of nonsense-mediated mRNA decay (NMD), a process that frequently destabilizes mRNAs containing PTCs in mammalian cells (47). Because mRNA encoded by these CFTR cDNA constructs are not subject to mRNA splicing, they will not be subject to NMD. Consequently, the expression of these cDNA constructs allowed us to correlate CFTR function directly with PTC suppression.
We also used primary HBE cells obtained from lung transplants to monitor the restoration of CFTR function in the most physiologically relevant setting possible in cultured cells, while also providing a basis for comparison between other CFTR modulators that have been advanced to humans. For example, in recent studies, the level of restored CFTR activity observed in HBE cells derived from patients with CF was shown to correlate well with therapeutic benefit in a clinical setting for both CFTR potentiators and CFTR-F508del processing correctors (33, 34, 48). In contrast to analyses based on cDNA, these subsequent experiments using primary HBE cells also allowed exploration of how NMD might affect the efficacy of readthrough compounds, because the NMD machinery is intact. In our studies, 7% of wild-type CFTR function was achieved at maximal efficacy of NB124, which begins to approach the effect of CFTR correctors, such as lumacaftor (VX-809) or VX-661 in F508 del homozygous HBE cells (48), which have been successful in phase 2 clinical testing when combined with ivacaftor (Figure 4). Furthermore, these results provide confidence that CFTR activity–restored by readthrough can be sufficient to predict the potential for clinical success, even when the NMD pathway is operative. Although we did not observe an effect of gentamicin in this model, this has been observed in prior studies (49, 50). This is likely due to the fact that gentamicin is relatively inactive at inducing translational readthrough in primary HBE cells as compared with compounds with greater activity. Finally, we used a mouse model that expressed a human CFTR-G542X transgene in a Cftr knockout background to compare the functional results obtained with NB124 and gentamicin (Figure 5). This allowed us to compare the relative efficacy of these compounds in an in vivo setting, and to correlate the efficacy of PTC suppression with the levels of each compound in the serum (6, 15, 51). Although we cannot explain why 60 mg/kg NB124 is less effective than 30 mg/kg, it could be due to differences in pharmacokinetics, cell penetration, or other factors. Further studies will be needed to better understand these effects. When taken together, the general agreement of these assays provides confidence of the relative efficacy of the compounds examined in this study. Use of the distinct models evaluated here represents a significant strength of our approach, and may serve to facilitate the drug development process by providing confidence that results obtained are not restricted to a particular model system that may be relatively susceptible to PTC suppression. Further studies using other relevant CFTR PTCs could also be informative as to the breadth of effects across mutation types, and are presently in progress.
It has been estimated that 10–35% of normal CFTR function is required to provide a significant therapeutic benefit for patients with CF (33, 52, 53). Because current PTC suppression compounds generally do not reach this level in physiologically relevant settings, tandem approaches that combine readthrough drugs with other strategies may be needed. Recently, the CFTR potentiator, ivacaftor, was approved for clinical use for the treatment of patients with CF carrying the CFTR-G551D mutation (34). Because ivacaftor has been shown to stimulate the function of wild-type and mutant forms of CFTR at the cell surface (25, 26, 33), we examined the ability of this compound to further enhance the level of CFTR activity obtained after PTC suppression. We found that the coadministration of ivacaftor effectively doubled the level of CFTR activity obtained with PTC suppression (Figure 7). In subsequent studies not shown here, we also evaluated whether ivacaftor augmented anion transport in murine intestinal segments analyzed ex vivo after NB124 treatment, but were unable to detect any stimulation. This may be due to problems activating human CFTR in murine epithelia (33, 54).
Despite the limitations of ex vivo studies, our results with ivacaftor validate the potential of combining PTC suppression with a CFTR potentiator to bring the total CFTR activity obtained closer to the threshold required for therapeutic benefit, and may also be applicable to other CFTR mutation repair strategies that result in suboptimal numbers of CFTR channels at the cell surface. Other approaches to augment rescue of CFTR nonsense mutations, such as the coadministration of polyanions, such as poly-L-aspartic acid, or NMD inhibitors, such as NMDI-1, are other options that could be tested in the future (13, 55). Furthermore, time-dependent increases in activity have been observed with prolonged administration of NB84 in a mouse model of Hurler’s syndrome (38), a finding also observed in patients with CF administered ataluren in an open label trial (16), suggesting that enhanced readthrough with these agents may be possible with chronic administration in vivo.
It is not yet clear whether compounds like NB84 or NB124 (or other future derivatives) can be used clinically. However, there is reason for optimism. Efficacy is now achieved that approaches 10% of CFTR function, and can be augmented further by addition of a CFTR potentiator. In addition to the increased efficacy of these synthetic aminoglycosides, the reduced toxicity could alleviate one of the major concerns associated with these compounds. In addition, elimination of their antibacterial activity should also allay concerns that any long-term use of aminoglycosides for PTC suppression may increase the incidence of aminoglycoside-resistant bacteria in the population of patient with CF. Further studies will be required to address the future potential of these promising compounds.
Acknowledgments
Acknowledgments
V.B. acknowledges the Ministry of Science and Technology, Israel (Kamea Program). M.S. acknowledges the Schulich Fellowship for Ph.D. students.
Footnotes
This work was supported by National Institutes of Health grants 1R01GM094792 (T.B.), 1R03DK084110 (S.M.R.), and 1P30DK072482-01A1 (University of Alabama at Birmingham Cystic Fibrosis [UAB CF] Research Center); Cystic Fibrosis Foundation grants R464-CR02 (UAB CF Research Center) and BRIGGE07XX0 (R.J.B.), and German-Israeli Foundation for Scientific Research and Development (GIF) research grant G-1048-95.5/2009 (T.B.).
This article has an online supplement, which is accessible from this issue’s table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1165/rcmb.2013-0282OC on November 19, 2013
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Rowe SM, Miller S, Sorscher EJ. Cystic fibrosis. N Engl J Med. 2005;352:1992–2001. doi: 10.1056/NEJMra043184. [DOI] [PubMed] [Google Scholar]
- 2.Kerem E, Corey M, Kerem BS, Rommens J, Markiewicz D, Levison H, Tsui LC, Durie P. The relation between genotype and phenotype in cystic fibrosis—analysis of the most common mutation (delta F508) N Engl J Med. 1990;323:1517–1522. doi: 10.1056/NEJM199011293232203. [DOI] [PubMed] [Google Scholar]
- 3.Keeling KM, Wang D, Conard SE, Bedwell DM. Suppression of premature termination codons as a therapeutic approach. Crit Rev Biochem Mol Biol. 2012;47:444–463. doi: 10.3109/10409238.2012.694846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bedwell DM, Kaenjak A, Benos DJ, Bebok Z, Bubien JK, Hong J, Tousson A, Clancy JP, Sorscher EJ. Suppression of a CFTR premature stop mutation in a bronchial epithelial cell line. Nat Med. 1997;3:1280–1284. doi: 10.1038/nm1197-1280. [DOI] [PubMed] [Google Scholar]
- 5.Howard M, Frizzell RA, Bedwell DM. Aminoglycoside antibiotics restore CFTR function by overcoming premature stop mutations. Nat Med. 1996;2:467–469. doi: 10.1038/nm0496-467. [DOI] [PubMed] [Google Scholar]
- 6.Du M, Jones JR, Lanier J, Keeling KM, Lindsey JR, Tousson A, Bebök Z, Whitsett JA, Dey CR, Colledge WH, et al. Aminoglycoside suppression of a premature stop mutation in a Cftr−/− mouse carrying a human CFTR-G542X transgene. J Mol Med (Berl) 2002;80:595–604. doi: 10.1007/s00109-002-0363-1. [DOI] [PubMed] [Google Scholar]
- 7.Barton-Davis ER, Cordier L, Shoturma DI, Leland SE, Sweeney HL. Aminoglycoside antibiotics restore dystrophin function to skeletal muscles of MDX mice. J Clin Invest. 1999;104:375–381. doi: 10.1172/JCI7866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Clancy JP, Bebök Z, Ruiz F, King C, Jones J, Walker L, Greer H, Hong J, Wing L, Macaluso M, et al. Evidence that systemic gentamicin suppresses premature stop mutations in patients with cystic fibrosis. Am J Respir Crit Care Med. 2001;163:1683–1692. doi: 10.1164/ajrccm.163.7.2004001. [DOI] [PubMed] [Google Scholar]
- 9.Wilschanski M, Famini C, Blau H, Rivlin J, Augarten A, Avital A, Kerem B, Kerem E. A pilot study of the effect of gentamicin on nasal potential difference measurements in cystic fibrosis patients carrying stop mutations. Am J Respir Crit Care Med. 2000;161:860–865. doi: 10.1164/ajrccm.161.3.9904116. [DOI] [PubMed] [Google Scholar]
- 10.Wilschanski M, Yahav Y, Yaacov Y, Blau H, Bentur L, Rivlin J, Aviram M, Bdolah-Abram T, Bebok Z, Shushi L, et al. Gentamicin-induced correction of CFTR function in patients with cystic fibrosis and CFTR stop mutations. N Engl J Med. 2003;349:1433–1441. doi: 10.1056/NEJMoa022170. [DOI] [PubMed] [Google Scholar]
- 11.Sermet-Gaudelus I, Renouil M, Fajac A, Bidou L, Parbaille B, Pierrot S, Davy N, Bismuth E, Reinert P, Lenoir G, et al. In vitro prediction of stop-codon suppression by intravenous gentamicin in patients with cystic fibrosis: a pilot study. BMC Med. 2007;5:5–14. doi: 10.1186/1741-7015-5-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Forge A, Schacht J. Aminoglycoside antibiotics. Audiol Neurootol. 2000;5:3–22. doi: 10.1159/000013861. [DOI] [PubMed] [Google Scholar]
- 13.Du M, Keeling KM, Fan L, Liu X, Bedwell DM. Poly-L-aspartic acid enhances and prolongs gentamicin-mediated suppression of the CFTR-G542X mutation in a cystic fibrosis mouse model. J Biol Chem. 2009;284:6885–6892. doi: 10.1074/jbc.M806728200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Welch EM, Barton ER, Zhuo J, Tomizawa Y, Friesen WJ, Trifillis P, Paushkin S, Patel M, Trotta CR, Hwang S, et al. PTC124 targets genetic disorders caused by nonsense mutations. Nature. 2007;447:87–91. doi: 10.1038/nature05756. [DOI] [PubMed] [Google Scholar]
- 15.Du M, Liu X, Welch EM, Hirawat S, Peltz SW, Bedwell DM. PTC124 is an orally bioavailable compound that promotes suppression of the human CFTR-G542X nonsense allele in a CF mouse model. Proc Natl Acad Sci USA. 2008;105:2064–2069. doi: 10.1073/pnas.0711795105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wilschanski M, Miller LL, Shoseyov D, Blau H, Rivlin J, Aviram M, Cohen M, Armoni S, Yaakov Y, Pugatch T, et al. Chronic ataluren (PTC124) treatment of nonsense mutation cystic fibrosis. Eur Respir J. 2011;38:59–69. doi: 10.1183/09031936.00120910. [DOI] [PubMed] [Google Scholar]
- 17.Kerem E, Hirawat S, Armoni S, Yaakov Y, Shoseyov D, Cohen M, Nissim-Rafinia M, Blau H, Rivlin J, Aviram M, et al. Effectiveness of PTC124 treatment of cystic fibrosis caused by nonsense mutations: a prospective phase II trial. Lancet. 2008;372:719–727. doi: 10.1016/S0140-6736(08)61168-X. [DOI] [PubMed] [Google Scholar]
- 18.Sermet-Gaudelus I, Boeck KD, Casimir GJ, Vermeulen F, Leal T, Mogenet A, Roussel D, Fritsch J, Hanssens L, Hirawat S, et al. Ataluren (PTC124) induces cystic fibrosis transmembrane conductance regulator protein expression and activity in children with nonsense mutation cystic fibrosis. Am J Respir Crit Care Med. 2010;182:1262–1272. doi: 10.1164/rccm.201001-0137OC. [DOI] [PubMed] [Google Scholar]
- 19.Konstan M, Accurso F, De Boeck K, Kerem E, Rowe SM, Sermet-Gaudelus I, Wilschanski M, Barth J, Elfering GL, Peltz SW, et al. Results of the phase 3 study of ataluren in nonsense mutation cystic fibrosis (NMCF) J Cyst Fibros 201211European Cystic Fibrosis Society Symposia Presentation, Late Breaking Science [Google Scholar]
- 20.Nudelman I, Rebibo-Sabbah A, Cherniavsky M, Belakhov V, Hainrichson M, Chen F, Schacht J, Pilch DS, Ben-Yosef T, Baasov T. Development of novel aminoglycoside (NB54) with reduced toxicity and enhanced suppression of disease-causing premature stop mutations. J Med Chem. 2009;52:2836–2845. doi: 10.1021/jm801640k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rowe SM, Sloane P, Tang LP, Backer K, Mazur M, Buckley-Lanier J, Nudelman I, Belakhov V, Bebok Z, Schwiebert E, et al. Suppression of CFTR premature termination codons and rescue of CFTR protein and function by the synthetic aminoglycoside NB54. J Mol Med (Berl) 2011;89:1149–1161. doi: 10.1007/s00109-011-0787-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kandasamy J, Atia-Glikin D, Shulman E, Shapira K, Shavit M, Belakhov V, Baasov T. Increased selectivity toward cytoplasmic versus mitochondrial ribosome confers improved efficiency of synthetic aminoglycosides in fixing damaged genes: a strategy for treatment of genetic diseases caused by nonsense mutations. J Med Chem. 2012;55:10630–10643. doi: 10.1021/jm3012992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Grentzmann G, Ingram JA, Kelly PJ, Gesteland RF, Atkins JF. A dual-luciferase reporter system for studying recoding signals. RNA. 1998;4:479–486. [PMC free article] [PubMed] [Google Scholar]
- 24.Howard MT, Shirts BH, Petros LM, Flanigan KM, Gesteland RF, Atkins JF. Sequence specificity of aminoglycoside-induced stop condon readthrough: potential implications for treatment of Duchenne muscular dystrophy. Ann Neurol. 2000;48:164–169. [PubMed] [Google Scholar]
- 25.Yu H, Burton B, Huang CJ, Worley J, Cao D, Johnson JP, Jr, Urrutia A, Joubran J, Seepersaud S, Sussky K, et al. Ivacaftor potentiation of multiple CFTR channels with gating mutations. J Cyst Fibros. 2012;11:237–245. doi: 10.1016/j.jcf.2011.12.005. [DOI] [PubMed] [Google Scholar]
- 26.Sloane PA, Shastry S, Wilhelm A, Courville C, Tang LP, Backer K, Levin E, Raju SV, Li Y, Mazur M, et al. A pharmacologic approach to acquired cystic fibrosis transmembrane conductance regulator dysfunction in smoking related lung disease. PLoS ONE. 2012;7:e39809. doi: 10.1371/journal.pone.0039809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pyle LC, Ehrhardt A, Mitchell LH, Fan L, Ren A, Naren AP, Li Y, Clancy JP, Bolger GB, Sorscher EJ, et al. Regulatory domain phosphorylation to distinguish the mechanistic basis underlying acute CFTR modulators. Am J Physiol Lung Cell Mol Physiol. 2011;301:L587–L597. doi: 10.1152/ajplung.00465.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mendoza JL, Schmidt A, Li Q, Nuvaga E, Barrett T, Bridges RJ, Feranchak AP, Brautigam CA, Thomas PJ. Requirements for efficient correction of ΔF508 CFTR revealed by analyses of evolved sequences. Cell. 2012;148:164–174. doi: 10.1016/j.cell.2011.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rowe SM, Varga K, Rab A, Bebok Z, Byram K, Li Y, Sorscher EJ, Clancy JP. Restoration of W1282X CFTR activity by enhanced expression. Am J Respir Cell Mol Biol. 2007;37:347–356. doi: 10.1165/rcmb.2006-0176OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chen FQ, Schacht J, Sha SH. Aminoglycoside-induced histone deacetylation and hair cell death in the mouse cochlea. J Neurochem. 2009;108:1226–1236. doi: 10.1111/j.1471-4159.2009.05871.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shalev M, Kandasamy J, Skalka N, Belakhov V, Rosin-Arbesfeld R, Baasov T. Development of generic immunoassay for the detection of a series of aminoglycosides with 6′-OH group for the treatment of genetic diseases in biological samples. J Pharm Biomed Anal. 2013;75:33–40. doi: 10.1016/j.jpba.2012.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nudelman I, Glikin D, Smolkin B, Hainrichson M, Belakhov V, Baasov T. Repairing faulty genes by aminoglycosides: development of new derivatives of geneticin (G418) with enhanced suppression of diseases-causing nonsense mutations. Bioorg Med Chem. 2010;18:3735–3746. doi: 10.1016/j.bmc.2010.03.060. [DOI] [PubMed] [Google Scholar]
- 33.Van Goor F, Hadida S, Grootenhuis PD, Burton B, Cao D, Neuberger T, Turnbull A, Singh A, Joubran J, Hazlewood A, et al. Rescue of CF airway epithelial cell function in vitro by a CFTR potentiator, VX-770. Proc Natl Acad Sci USA. 2009;106:18825–18830. doi: 10.1073/pnas.0904709106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ramsey BW, Davies J, McElvaney NG, Tullis E, Bell SC, Dřevínek P, Griese M, McKone EF, Wainwright CE, Konstan MW, et al. VX08-770-102 Study Group. A CFTR potentiator in patients with cystic fibrosis and the G551D mutation. N Engl J Med. 2011;365:1663–1672. doi: 10.1056/NEJMoa1105185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pagkalis S, Mantadakis E, Mavros MN, Ammari C, Falagas ME. Pharmacological considerations for the proper clinical use of aminoglycosides. Drugs. 2011;71:2277–2294. doi: 10.2165/11597020-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 36.Hein LK, Bawden M, Muller VJ, Sillence D, Hopwood JJ, Brooks DA. Alpha-L-iduronidase premature stop codons and potential read-through in mucopolysaccharidosis type I patients. J Mol Biol. 2004;338:453–462. doi: 10.1016/j.jmb.2004.03.012. [DOI] [PubMed] [Google Scholar]
- 37.Keeling KM, Brooks DA, Hopwood JJ, Li P, Thompson JN, Bedwell DM. Gentamicin-mediated suppression of Hurler syndrome stop mutations restores a low level of alpha-L-iduronidase activity and reduces lysosomal glycosaminoglycan accumulation. Hum Mol Genet. 2001;10:291–299. doi: 10.1093/hmg/10.3.291. [DOI] [PubMed] [Google Scholar]
- 38.Wang D, Belakhov V, Kandasamy J, Baasov T, Li SC, Li YT, Bedwell DM, Keeling KM. The designer aminoglycoside NB84 significantly reduces glycosaminoglycan accumulation associated with MPS I-H in the Idua-W392X mouse. Mol Genet Metab. 2012;105:116–125. doi: 10.1016/j.ymgme.2011.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sleat DE, Sohar I, Gin RM, Lobel P. Aminoglycoside-mediated suppression of nonsense mutations in late infantile neuronal ceroid lipofuscinosis. Eur J Paediatr Neurol. 2001;5(Suppl A):57–62. doi: 10.1053/ejpn.2000.0436. [DOI] [PubMed] [Google Scholar]
- 40.Brendel C, Belakhov V, Werner H, Wegener E, Gärtner J, Nudelman I, Baasov T, Huppke P. Readthrough of nonsense mutations in Rett syndrome: evaluation of novel aminoglycosides and generation of a new mouse model. J Mol Med (Berl) 2011;89:389–398. doi: 10.1007/s00109-010-0704-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rebibo-Sabbah A, Nudelman I, Ahmed ZM, Baasov T, Ben-Yosef T. In vitro and ex vivo suppression by aminoglycosides of PCDH15 nonsense mutations underlying type 1 Usher syndrome. Hum Genet. 2007;122:373–381. doi: 10.1007/s00439-007-0410-7. [DOI] [PubMed] [Google Scholar]
- 42.Salvatori F, Breveglieri G, Zuccato C, Finotti A, Bianchi N, Borgatti M, Feriotto G, Destro F, Canella A, Brognara E, et al. Production of beta-globin and adult hemoglobin following G418 treatment of erythroid precursor cells from homozygous beta(0)39 thalassemia patients. Am J Hematol. 2009;84:720–728. doi: 10.1002/ajh.21539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Simon AJ, Lev A, Wolach B, Gavrieli R, Amariglio N, Rosenthal E, Gazit E, Eyal E, Rechavi G, Somech R. The effect of gentamicin-induced readthrough on a novel premature termination codon of CD18 leukocyte adhesion deficiency patients. PLoS One. 2010;5:e13659. doi: 10.1371/journal.pone.0013659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Malik V, Rodino-Klapac LR, Viollet L, Wall C, King W, Al-Dahhak R, Lewis S, Shilling CJ, Kota J, Serrano-Munuera C, et al. Gentamicin-induced readthrough of stop codons in Duchenne muscular dystrophy. Ann Neurol. 2010;67:771–780. doi: 10.1002/ana.22024. [DOI] [PubMed] [Google Scholar]
- 45.Politano L, Nigro G, Nigro V, Piluso G, Papparella S, Paciello O, Comi LI. Gentamicin administration in Duchenne patients with premature stop codon. Preliminary results. Acta Myol. 2003;22:15–21. [PubMed] [Google Scholar]
- 46.Wagner KR, Hamed S, Hadley DW, Gropman AL, Burstein AH, Escolar DM, Hoffman EP, Fischbeck KH. Gentamicin treatment of Duchenne and Becker muscular dystrophy due to nonsense mutations. Ann Neurol. 2001;49:706–711. [PubMed] [Google Scholar]
- 47.Kervestin S, Jacobson A. NMD: a multifaceted response to premature translational termination. Nat Rev Mol Cell Biol. 2012;13:700–712. doi: 10.1038/nrm3454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Van Goor F, Hadida S, Grootenhuis PD, Burton B, Stack JH, Straley KS, Decker CJ, Miller M, McCartney J, Olson ER, et al. Correction of the F508del-CFTR protein processing defect in vitro by the investigational drug VX-809. Proc Natl Acad Sci USA. 2011;108:18843–18848. doi: 10.1073/pnas.1105787108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rowe SM, Tang LP, Backer K, Woodworth B, Mazur M, Buckley-Lanier J, Schwiebert E, Baasov T, Bedwell DM. Enhanced activity for translational readthrough and reduced toxicity demonstrated by the synthetic aminoglycoside NB54 [abstract] Ped Pulmonol Suppl. 2009;44:Abstract 218. [Google Scholar]
- 50.Auld DS, Thorne N, Maguire WF, Inglese J. Mechanism of PTC124 activity in cell-based luciferase assays of nonsense codon suppression. Proc Natl Acad Sci USA. 2009;106:3585–3590. doi: 10.1073/pnas.0813345106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Du M, Keeling KM, Fan L, Liu X, Kovaçs T, Sorscher E, Bedwell DM. Clinical doses of amikacin provide more effective suppression of the human CFTR-G542X stop mutation than gentamicin in a transgenic CF mouse model. J Mol Med (Berl) 2006;84:573–582. doi: 10.1007/s00109-006-0045-5. [DOI] [PubMed] [Google Scholar]
- 52.Zhang L, Button B, Gabriel SE, Burkett S, Yan Y, Skiadopoulos MH, Dang YL, Vogel LN, McKay T, Mengos A, et al. CFTR delivery to 25% of surface epithelial cells restores normal rates of mucus transport to human cystic fibrosis airway epithelium. PLoS Biol. 2009;7:e1000155. doi: 10.1371/journal.pbio.1000155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Johnson LG, Olsen JC, Sarkadi B, Moore KL, Swanstrom R, Boucher RC. Efficiency of gene transfer for restoration of normal airway epithelial function in cystic fibrosis. Nat Genet. 1992;2:21–25. doi: 10.1038/ng0992-21. [DOI] [PubMed] [Google Scholar]
- 54.Zhou L, Dey CR, Wert SE, DuVall MD, Frizzell RA, Whitsett JA. Correction of lethal intestinal defect in a mouse model of cystic fibrosis by human CFTR. Science. 1994;266:1705–1708. doi: 10.1126/science.7527588. [DOI] [PubMed] [Google Scholar]
- 55.Keeling KM, Wang D, Dai Y, Murugesan S, Chenna B, Clark J, Belakhov V, Kandasamy J, Velu SE, Baasov T, et al. Attenuation of nonsense-mediated mRNA decay enhances in vivo nonsense suppression. PLoS ONE. 2013;8:e60478. doi: 10.1371/journal.pone.0060478. [DOI] [PMC free article] [PubMed] [Google Scholar]