Abstract
Neutrophil elastase (NE) is a major inflammatory mediator in cystic fibrosis (CF) that is a robust predictor of lung disease progression. NE directly causes airway injury via protease activity, and propagates persistent neutrophilic inflammation by up-regulation of neutrophil chemokine expression. Despite its key role in the pathogenesis of CF lung disease, there are currently no effective antiprotease therapies available to patients with CF. Although heparin is an effective antiprotease and anti-inflammatory agent, its anticoagulant activity prohibits its use in CF, due to risk of pulmonary hemorrhage. In this report, we demonstrate the efficacy of a 2-O, 3-O-desulfated heparin (ODSH), a modified heparin with minimal anticoagulant activity, to inhibit NE activity and to block NE-induced airway inflammation. Using an established murine model of intratracheal NE-induced airway inflammation, we tested the efficacy of intratracheal ODSH to block NE-generated neutrophil chemoattractants and NE-triggered airway neutrophilic inflammation. ODSH inhibited NE-induced keratinocyte-derived chemoattractant and high-mobility group box 1 release in bronchoalveolar lavage. ODSH also blocked NE-stimulated high-mobility group box 1 release from murine macrophages in vitro, and inhibited NE activity in functional assays consistent with prior reports of antiprotease activity. In summary, this report suggests that ODSH is a promising antiprotease and anti-inflammatory agent that may be useful as an airway therapy in CF.
Keywords: cystic fibrosis, neutrophil elastase, airway inflammation, heparin
Clinical Relevance
In this report, we demonstrate the efficacy of 2-O, 3-O-desulfated heparin (ODSH), a modified heparin with minimal anticoagulant activity, administered into the trachea, to inhibit neutrophil elastase (NE) activity and to block NE-induced airway inflammation in an established murine model of NE-induced airway inflammation. This report suggests that ODSH is a promising antiprotease and anti-inflammatory agent that may be useful as an airway therapy in cystic fibrosis.
Cystic fibrosis (CF), also known as mucoviscidosis, is an autosomal recessive genetic disorder that can critically injure the lungs. CF is associated with abnormal transport of chloride and sodium across the epithelia of the respiratory tract, leading to thick, viscous airway secretions. Although CF manifests as a complex multisystem disease (involving the pancreas, liver, and intestine), chronic and severe lung involvement is the predominant cause of morbidity and mortality. Progressive airway injury found in CF lung disease includes mucus plugging, infection, and neutrophilic inflammation (1). The serine protease, neutrophil elastase (NE), a major protease and inflammatory mediator released by neutrophils, can attain nanomolar to micromolar concentration levels in CF airway surface liquid (2). The excess NE overwhelms antiprotease capability, leading to impairment of airway innate and adaptive immunity, abnormal mucociliary clearance, and the stimulation of synthesis and release of neutrophil chemokines (3). Recent studies suggest that NE also disables CF transmembrane conductance regulator (CFTR) function in vitro and in vivo, exacerbating this functional defect in CF lung disease (4). Furthermore, the epithelial Na(+) channel that mediates regulated Na(+) reabsorption by epithelial cells in the lungs can be activated by NE (5–8). Increased levels of NE at airway surfaces are a predictor of early bronchiectasis and CF lung disease progression (9, 10).
Another major inflammatory mediator in the CF airway is high-mobility group box 1 (HMGB1), a nonhistone DNA-binding protein that is actively secreted from macrophages under conditions of stress or infection (11). HMGB1 functions as an inflammatory cytokine that induces pulmonary neutrophil recruitment, impairs macrophage phagocytosis, and promotes endotoxic shock (12, 13). HMGB1 has been shown to be significantly elevated in the bronchoalveolar lavage (BAL) from patients with CF (14), and high levels of sputum HMGB1 correlate with CF disease severity (15). Because NE and HMGB1 are both now recognized as highly predictive biomarkers of CF disease progression and severity, it is possible that these two biomarkers are somehow pathologically linked.
Currently, there are no approved antiprotease drugs or HMGB1 inhibitors to mitigate the robust airway and tissue inflammation that is a central mechanism of CF lung disease progression. Heparin has long been known to possess potent anti-inflammatory properties; however, due to its anticoagulant activity, the use of heparin as an anti-inflammatory therapeutic is limited (16, 17). 2-O, 3-O desulfated heparin (ODSH) has many of the salutary anti-inflammatory activities of heparin, but has minimal residual anticoagulant activity, and does not cause heparin-induced thrombocytopenia (18). Importantly, ODSH, like heparin, inhibits NE and another neutrophil protease, cathepsin G, and prevents HMGB1 ligation to its receptors, the receptor for advanced glycation end-products (RAGE), and Toll-like receptor (TLR) 2 and TLR4 (18, 19).
Using an established mouse model of intratracheal NE–induced inflammation, we found that ODSH blocked NE-induced neutrophilic inflammation in vivo, and was a potent inhibitor of NE activity in vitro. In addition, we show that NE triggered the active secretion of HMGB1 from macrophages, which was prevented by ODSH. Our results suggest that ODSH may be an effective multifunctional antiprotease and anti-inflammatory therapy for CF lung disease.
Materials and Methods
Animals
Male Balb/c mice (6–8 wk, 25–30 g) were obtained commercially (Jackson Laboratories, Bar Harbor, ME). Balb/c mice were selected for the original experiments to establish the model system and for the experiments in this study, because they develop mucous cell metaplasia in response to allergen exposure, IL-13, and NE (20, 21). The study protocol was approved by the Duke University Animal Care and Use Committee.
NE and ODSH Instillation Protocol
Animals received ODSH or normal saline (NS) followed by NE or NS 1 hour later by oropharyngeal aspiration on Days 1, 4, and 7, as previously described (21, 22). ODSH (297.5 μg/40μl, 635.6 μM; a kind gift from Dr. Steve Marcus, ParinGenix, Inc, Weston, FL) was delivered at 15-fold molar excess to human NE (50 μg [43.75 U]/40 μl saline, 42.37 μM; specific activity, 875 U/mg protein; Elastin Products, Owensville, MO). Mice were killed by inhalational exposure to 100% CO2 gas 1 day after the last NE exposure (Day 8).
BAL
On Day 8, BAL was performed with 3× 1-ml aliquots of sterile NS (21, 22). Cells were collected by centrifugation (500 × g, 10 min), and the supernatant was stored at −80°C for assessment of HMGB1 and cytokine levels. Inflammatory cells in the BAL were counted (22), and neutrophils were determined per 200 total cells per slide and expressed per milliliter of BAL.
Cytokine Analysis
The concentration of keratinocyte-derived chemokine (KC) in lavage fluid was measured by ELISA (R&D Systems, Minneapolis, MN), per the manufacturer’s instructions.
Studies on a Murine Macrophage Cell Line
Murine macrophage-like cells, RAW 264.7 (American Type Culture Collection, Manassas, VA), is a murine macrophage cell line originating from primary macrophages transformed by Ableson leukemia virus (23). It has been widely used as a model to study phagocytosis, locomotion, and metabolism after activation of the innate immune system. A systems biology analysis of gene expression reveals that TLR4-activated RAW cells have similar gene expression profiles to activated primary bone marrow–derived macrophages and thioglycollate-elicited macrophages (24). RAW 264.7 cells were cultured to 80% confluency and were pretreated with ODSH (7.5 μM or 88 μg/ml) or NS in serum-free media. After 30 minutes, NE (0.5 μM or 15 μg/ml) or control vehicle (1:1 glycerol:0.02 M sodium acetate, pH 5) was added to cells and incubated for 2, 4, 8, or 16 hours. After treatment, 2% FBS was added to inactivate NE. Conditioned media were collected for Western analysis for HMGB1. Cell viability was established to be greater than 95% for experimental conditions by lactate dehydrogenase assay (Sigma, St. Louis, MO).
Western Analysis of BAL HMGB1
Levels of HMGB1 in BAL and conditioned media from in vitro experiments were determined using Western analysis. BAL samples (40 μl) or conditioned media (40 μl) were separated on a 4–15% SDS-PAGE gel, and proteins were electrotransferred onto a nitrocellulose membrane. The primary antibody was anti-HMGB1 monoclonal antibody (1:1,000; Abcam, Cambridge, MA) and secondary antibody was horseradish peroxidase–conjugated sheep anti-mouse (1:5,000–10,000; GE Healthcare, Pittsburgh, PA). Antibody binding was detected by enhanced chemiluminescence (PerkinElmer, Waltham, MA), per the manufacturer’s instructions.
NE Activity Assay
Human NE (Elastin Products, Owensville, MO) was reconstituted in 1:1 glycerol:0.02 M sodium acetate (pH 5). NE standards were prepared from 0.23–30 μg/ml (∼0.01–1 μM) in Hepes buffer (0.125 M Hepes, pH 7.5, 0.125% Triton-X 100). NE standards or conditioned media samples from the in vitro RAW 267.4 macrophage experiments (100 μl), saline (100 μl), and substrate (3 mM, Suc-Ala-Ala-Val-p-NA in 50% DMSO, 50 μl) were mixed in a microtiter plate, at room temperature, and the absorbance at 405 λ, recorded over 3 minutes, reflected NE enzyme activity (19).
Statistical Analysis
The one-way, nonparametric ANOVA test and post hoc comparisons by the Wilcoxon rank sum test (Statistix 8.0; Analytical Software, Tallahassee, FL) (25) were used to compare BAL total and neutrophil cell counts, KC levels, and HMGB1 levels. The Wilcoxon signed rank test (Statistix 8.0) (25) was used to compare NE activity in media samples containing NE or NE plus ODSH, and expressed as a two-tailed P value for normal approximation. Significant differences between groups were defined as P less than 0.05.
Results
ODSH Blocks NE-Triggered Neutrophilic Inflammation in Mice
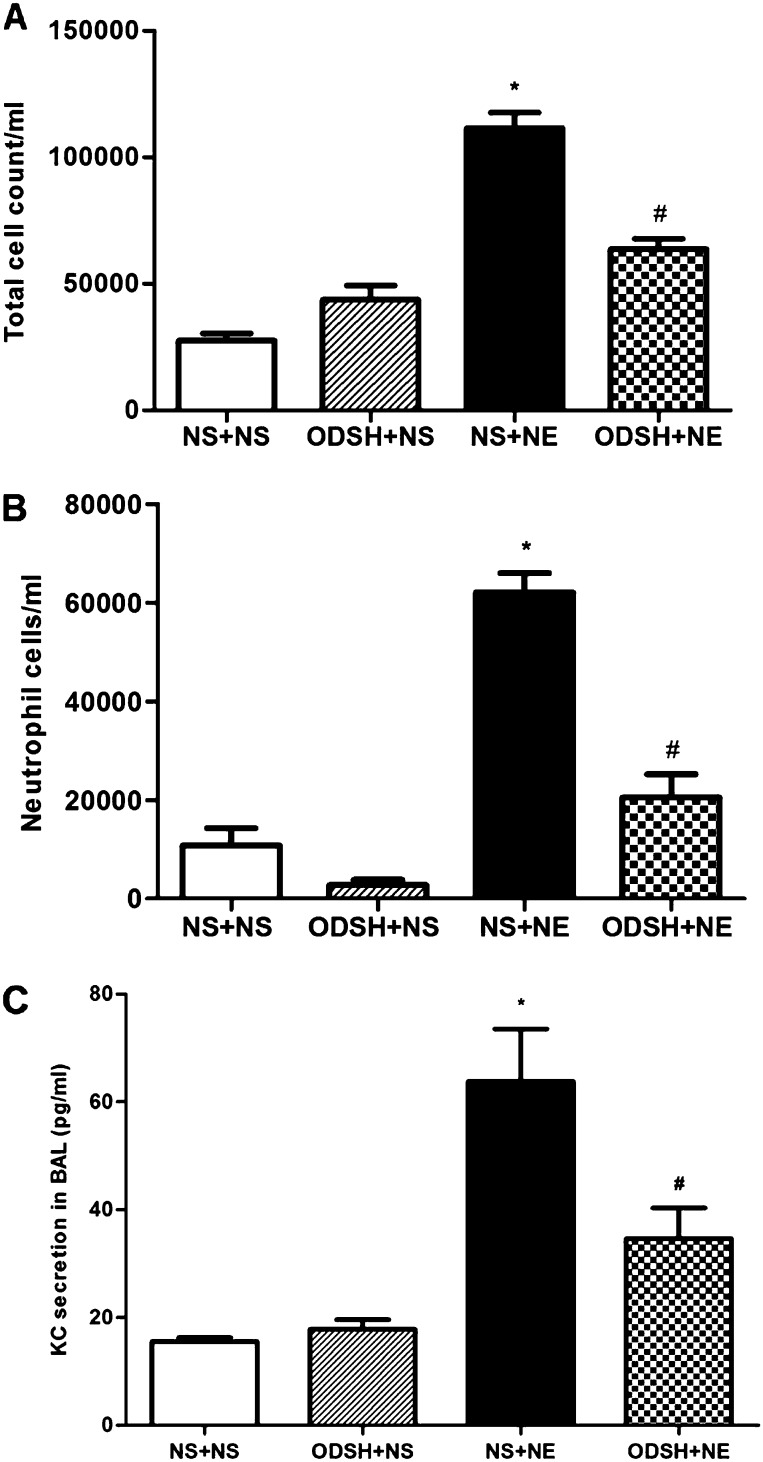
NE provokes robust neutrophilic inflammation, and has been shown to induce release of the neutrophil cytokine, KC, in mouse BAL (21), and IL-8 from human bronchial epithelial cells (2, 26). We hypothesized that ODSH would mitigate NE-induced neutrophil influx into the airway and decrease up-regulation and secretion of KC. NE treatment caused an increase in total BAL leukocytes and neutrophils as compared with NS treatment (Figures 1A and 1B). The increase in BAL neutrophils was significantly diminished in animals that received both ODSH and NE. These results demonstrate that ODSH inhibited NE-induced neutrophil influx. We then tested whether ODSH inhibited NE-induced neutrophilic inflammation by blocking NE-up-regulation of KC, the murine homolog of IL-8. ELISA analysis of BAL revealed that NE significantly increased KC levels (Figure 1C), whereas ODSH pretreatment blunted the NE-induced increase in KC levels, explaining in part the inhibition of NE-generated neutrophilic inflammation by ODSH.
Figure 1.
Bronchoalveolar lavage (BAL) cell counts, differential cell counts, and keratinocyte-derived chemokine (KC) secretion. BAL (3 ml) was obtained from neutrophil elastase (NE)-treated, 2-O, 3-O-desulfated heparin (ODSH) + NE–treated and control mice 1 day after the third treatment. Cells were collected, applied to a slide by cytospin centrifugation, dried, and stained by Wright Giemsa stain for (A) total leukocyte cell count/ml and for (B) neutrophil cell count/ml. At least 200 cells were counted per slide. Data summarize two experiments; n = 8 mice per group; mean ± SEM; *P < 0.0002, normal saline (NS) + NE versus NS + NS; #P < 0.0002, ODSH + NE versus NS + NE. (C) KC in BAL for NE-treated, ODSH + NE–treated, and control mice was determined by ELISA. Data summarize two experiments; n = 8 mice per group; mean ± SEM; *P < 0.0002, NS + NE versus NS + NS; #P < 0.03, ODSH + NE versus NS + NE.
ODSH Blocks NE-Mediated HMGB1 Secretion In Vivo
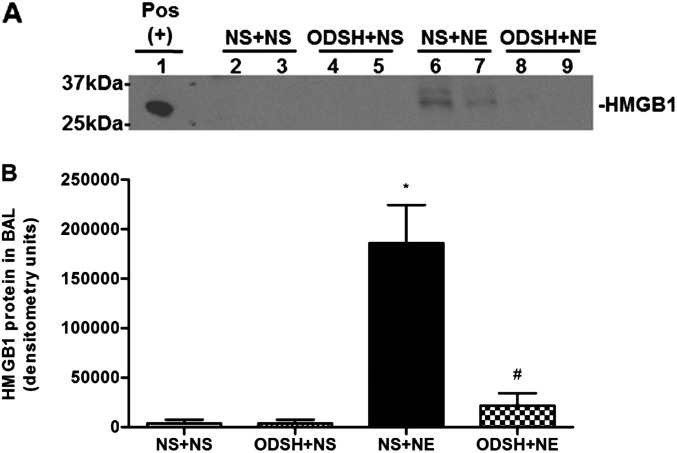
Both NE and HMGB1 are sputum biomarkers for CF disease severity and progression (15, 27), yet it is not known whether NE activates HMGB1 release. We hypothesized that HMGB1 is a mediator in NE-induced inflammation, and tested whether NE causes HMGB1 release in vivo. By Western analysis, we demonstrated that NE increased BAL HMGB1 levels, and pretreatment with ODSH prevented NE-induced HMGB1 release (Figures 2A and 2B). Because there are several potential targets for HMGB1 release in the airway, we next queried whether NE activated HMGB1 release from macrophages.
Figure 2.
BAL high-mobility group box 1 (HMGB1) secretion after NE, or ODSH + NE, or control treatment conditions in vivo. (A) HMGB1 was detected in BAL (40 μl) by Western analysis as described in Materials and Methods. Western blot shown is representative of four blots. Mouse brain lysate served as a positive control for HMGB1 (Pos). Note that the signals of HMGB1 in lanes 6 and 7, representing NE treatment alone, are absent in lanes 8 and 9, representing the NE + ODSH treatment. (B) Graphic summary of Western band densitometry; n = 8; mean ± SEM. *P < 0.0002, NS + NE versus NS + NS; #P < 0.0011, ODSH + NE versus NS + NE.
NE Stimulates HMGB1 Release from a Murine Macrophage Cell Line
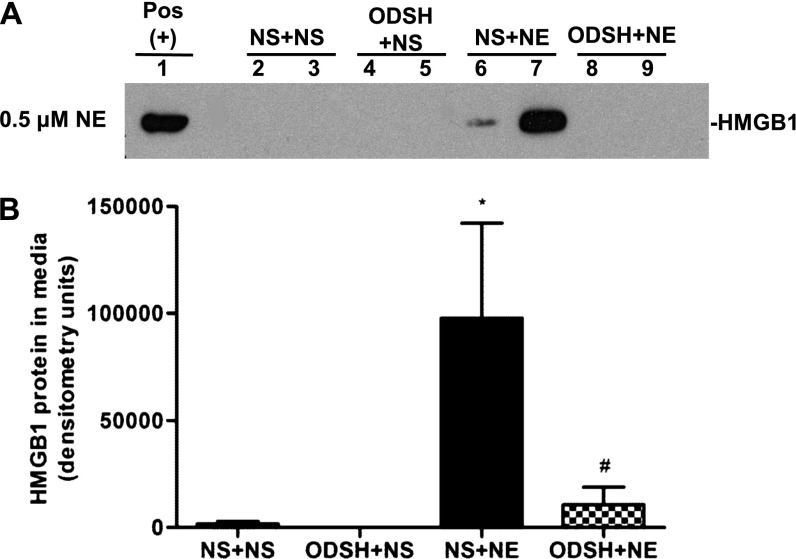
To investigate NE-induced HMGB1 release in vitro, RAW 267.4 cells (a murine macrophage cell line) were treated with NE and ODSH, alone or together. Western analysis demonstrated the presence of HMGB1 protein in conditioned media from macrophages treated with NE (Figures 3A and 3B). Cells treated with both ODSH and NE released minimal to no HMGB1 as compared with macrophages treated with NE alone. HMGB1 release was maximal after 8 hours of NE treatment, and only intermittently detected at 4 hours; no HMGB1 release was detected at 2 or 16 hours (data not shown). Lactate dehydrogenase assay revealed less than 6% cell death in all treatment conditions at 8 hours (data not shown), indicating that HMGB1 was being actively secreted from macrophages rather than passively released during necrosis. This is the first demonstration, to our knowledge, that NE exposure alone is sufficient to trigger HMGB1 release from cells. Importantly, this suggests that HMGB1 may mediate NE-induced neutrophilic inflammation.
Figure 3.
HMGB1 secretion by a macrophage cell line, RAW 264.7, after NE, ODSH + NE, or control treatments. (A) HMGB1 was detected in conditioned media of RAW 264.7 cells by Western analysis, as detailed in Materials and Methods. Western blot shown is representative of four blots. Positive control is mouse brain lysate (Pos). Note that the signals of HMGB1 in lanes 6 and 7, representing NE treatment alone, are absent in lanes 8 and 9, representing the NE + ODSH treatment. (B) Graphic summary of band densitometry from three experiments; data are presented as mean ± SEM; n = 8; *P < 0.0003, NS + NE versus NS + NS; #P < 0.0028, ODSH + NE versus NS + NE.
ODSH Inhibits NE Activity In Vitro
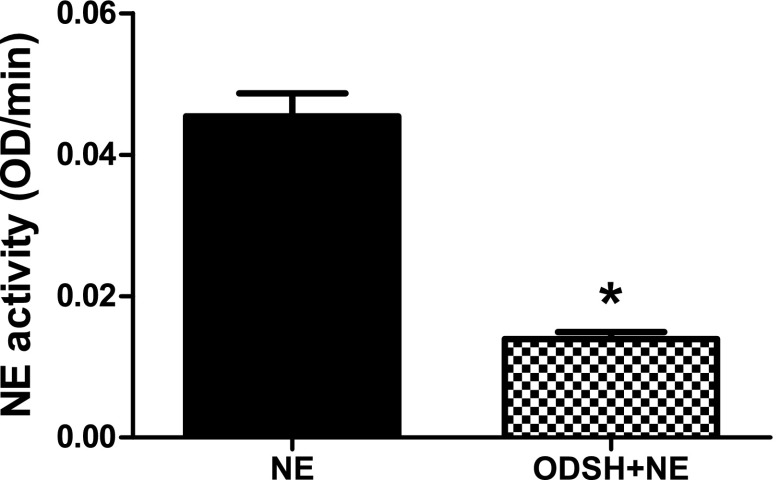
Previous studies have demonstrated that ODSH is a potent inhibitor of neutrophil protease activity (19). We sought to confirm that ODSH would inhibit NE in our experimental model. We tested this hypothesis in the culture media in the presence or absence of ODSH and/or NE used to treat RAW 264.7 cells. In the presence of ODSH, the action of NE on the synthetic substrate, Suc-Ala-Ala-Val-p-NA, was significantly decreased from 0.056 optical density/min to 0.014 optical density/min (Figure 4). We can conclude that ODSH inhibited NE activity to a level below the threshold concentration required to release HMGB1 from macrophages in vitro.
Figure 4.
NE activity in the presence or absence of ODSH. The human NE standard (data not shown) or conditioned media samples containing 0.5 μM NE or 0.5 μM NE + 7.5 μM ODSH were evaluated for NE activity, as described in Materials and Methods. Data are presented as mean ± SEM; n = 9 from three separate experiments; *P < 0.0092. OD, optical density.
Discussion
In this report, we demonstrate that a partially desulfated heparin, ODSH, is an effective antiprotease and anti-inflammatory therapy in a mouse model relevant to CF airway disease. We confirmed prior reports (18, 19) that ODSH decreased NE activity to levels below a threshold required for HMGB1 secretion from macrophages (Figure 3). ODSH has also been reported to interfere with HMGB1 ligation of its receptors, including RAGE and TLR2 and -4 (18), and thus attenuate inflammation. In this report, we demonstrate that ODSH also interferes with HMGB1 indirectly by inhibiting NE and therefore NE-regulated HMGB1 release from macrophages. We suggest that this observation strongly supports the primary role of antiprotease control to interfere with NE-mediated progression of CF lung disease. Given both its antiprotease and anti-inflammatory properties, ODSH represents an attractive potential therapeutic to curb the neutrophilic inflammation within the airways of patients with other chronic inflammatory diseases. Of interest, HMGB1 and its receptor, RAGE, are also significantly elevated in the BAL from smokers with COPD, and are postulated to play roles in COPD pathogenesis (28), adding support in addition to NE inhibition for potentially using aerosolized ODSH in COPD. Furthermore, subcutaneous heparin was shown many years ago to inhibit development of acute graft-versus-host disease in mice (29), perhaps through selectin inhibition–mediated retardation of lymphocyte trafficking.
This report demonstrates a previously unknown direct link between two biomarkers for CF disease severity and progression, NE and HMGB1. We have shown that NE treatment of mice caused an increase in airway HMGB1 levels (Figure 2). In addition, NE triggered the active secretion of HMGB1 from macrophages in vitro, in the absence of necrosis (Figure 3). HMGB1 release is triggered by bacterial products, such as LPS or cytosine-phosphate-guanine–DNA, or by inflammatory products, including IL-1, TNF-α, hydrogen peroxide, and lysophosphatidylcholine (30, 31), producing acetylation of lysine residues, which causes accumulation of HMGB1 in cytoplasmic secretory lysosomes and blocks its re-entry to the nuclear compartment. In addition, serine phosphorylation by protein kinases can also mediate HMGB1 secretion (32). These events can be promoted by oxidative stress, resulting in nuclear-to-cytoplasmic translocation and secretion (33). The mechanism of HMGB1 release by NE is not known. However, we and others have reported that NE exposure generates reactive oxygen species (34, 35), and this may be a key signal that causes post-translational modification of HMGB1, resulting in nuclear-to-cytoplasmic translocation.
Currently, there are no approved antiprotease drugs to mitigate the high protease load in the CF airway. Despite the recent advances in therapies to correct mutant CFTR function using potentiators, such as Kalydeco (36), many patients with CF have established airway injury, and there is no current evidence that partial restoration of mutant CFTR function will re-establish normal airway innate immunity to prevent CF bronchitis. Therefore, there is a pressing need to develop antiproteolytic and anti-inflammatory therapies to protect the CF airway from recurrent inflammation. In addition, an antiprotease therapy is an attractive therapeutic approach early in the CF disease course to prevent progression. Several antiprotease therapies have been the subject of investigation, including versions of the naturally occurring antiproteases, elafin, secretory leukoprotease inhibitor, and α1-antitrypsin. Notably, elafin and secretory leukoprotease inhibitor have been shown to have additional antibacterial and anti-inflammatory properties (3). Two prospective phase II trials of α1-antitrypsin demonstrate that, although inhalation therapy was safe, there is insufficient evidence to support efficacy to ameliorate NE-induced inflammation (37, 38). There has been only marginal success of the synthetic antiprotease, DX-890 (EPI-hNE4) (39, 40), which has not been tested at the higher NE ratios representative of in vivo CF lung conditions. Furthermore, small-molecule synthetic NE inhibitors that are able to access the intracellular environment of the phagolysosome may suppress the desirable antibacterial and anti-inflammatory properties of naturally occurring proteases (41).
Heparin, the parent molecule for ODSH, is safe and effective in decreasing airflow obstruction in patients with asthma (42). A recent trial in patients with CF showed that inhaled heparin decreased sputum NE and total cell counts compared with placebo, with greater ease in sputum expectoration (43). Inhaled heparin has also been shown to significantly reduce days on mechanical ventilation in patients with acute lung injury (44). Phase I trials of intravenous administration of ODSH to normal human volunteers produced no serious adverse effects or anticoagulant effects (18). These studies demonstrate that low-anticoagulant ODSH has a favorable safety record as a therapeutic, and might pose a safer antiprotease for CF than fully anticoagulant heparin as an inhaled therapeutic.
In summary, we have shown that ODSH is a potent antiprotease that inhibits NE-mediated neutrophilic inflammation in the mouse lung. In addition, this report is the first to demonstrate that NE induces the secretion of HMGB1 from macrophages, although further studies are required to determine this mechanism. Our results reveal the potential for inhaled ODSH as a promising antiproteolytic and anti-inflammatory therapy in CF lung disease.
Footnotes
This work was supported by the Duke Center for Pediatric Lung Disease, Duke University Medical Center (J.A.V.), National Institutes of health grant R01 ES016836 (J.A.V.), and a Fellowship from the Howard Hughes Medical Institute (K.L.G.).
Originally Published in Press as DOI: 10.1165/rcmb.2013-0338RC on December 10, 2013
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Voynow JA, Scanlin TF. Cystic fibrosis. Fishman’s pulmonary diseases and disorders. In: Fishman AP, Elias JA, Fishman JA, Grippi MA, Senior RM, Pack AI, editors. 4th ed. New York: McGraw Hill Medical; 2008. pp. 863–885. [Google Scholar]
- 2.Nakamura H, Yoshimura K, McElvaney NG, Crystal RG. Neutrophil elastase in respiratory epithelial lining fluid of individuals with cystic fibrosis induces interleukin-8 gene expression in a human bronchial epithelial cell line. J Clin Invest. 1992;89:1478–1484. doi: 10.1172/JCI115738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Voynow JA, Fischer BM, Zheng S. Proteases and cystic fibrosis. Int J Biochem Cell Biol. 2008;40:1238–1245. doi: 10.1016/j.biocel.2008.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Le Gars M, Descamps D, Roussel D, Saussereau E, Guillot L, Ruffin M, Tabary O, Hong SS, Boulanger P, Paulais M, et al. Neutrophil elastase degrades cystic fibrosis transmembrane conductance regulator via calpains and disables channel function in vitro and in vivo. Am J Respir Crit Care Med. 2013;187:170–179. doi: 10.1164/rccm.201205-0875OC. [DOI] [PubMed] [Google Scholar]
- 5.Harris M, Firsov D, Vuagniaux G, Stutts MJ, Rossier BC. A novel neutrophil elastase inhibitor prevents elastase activation and surface cleavage of the epithelial sodium channel expressed in Xenopus laevis oocytes. J Biol Chem. 2007;282:58–64. doi: 10.1074/jbc.M605125200. [DOI] [PubMed] [Google Scholar]
- 6.Adebamiro A, Cheng Y, Rao US, Danahay H, Bridges RJ. A segment of gamma ENaC mediates elastase activation of Na+ transport. J Gen Physiol. 2007;130:611–629. doi: 10.1085/jgp.200709781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Prulière-Escabasse V, Clerici C, Vuagniaux G, Coste A, Escudier E, Planès C. Effect of neutrophil elastase and its inhibitor EPI-hNE4 on transepithelial sodium transport across normal and cystic fibrosis human nasal epithelial cells. Respir Res. 2010;11:141. doi: 10.1186/1465-9921-11-141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rossier BC, Stutts MJ. Activation of the epithelial sodium channel (ENaC) by serine proteases. Annu Rev Physiol. 2009;71:361–379. doi: 10.1146/annurev.physiol.010908.163108. [DOI] [PubMed] [Google Scholar]
- 9.Sly PD, Gangell CL, Chen L, Ware RS, Ranganathan S, Mott LS, Murray CP, Stick SM AREST CF Investigators. Risk factors for bronchiectasis in children with cystic fibrosis. N Engl J Med. 2013;368:1963–1970. doi: 10.1056/NEJMoa1301725. [DOI] [PubMed] [Google Scholar]
- 10.Sagel SD, Wagner BD, Anthony MM, Emmett P, Zemanick ET. Sputum biomarkers of inflammation and lung function decline in children with cystic fibrosis. Am J Respir Crit Care Med. 2012;186:857–865. doi: 10.1164/rccm.201203-0507OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ulloa L, Messmer D. High-mobility group box 1 (HMGB1) protein: friend and foe. Cytokine Growth Factor Rev. 2006;17:189–201. doi: 10.1016/j.cytogfr.2006.01.003. [DOI] [PubMed] [Google Scholar]
- 12.Entezari M, Weiss DJ, Sitapara R, Whittaker L, Wargo MJ, Li J, Wang H, Yang H, Sharma L, Phan BD, et al. Inhibition of high-mobility group box 1 protein (HMGB1) enhances bacterial clearance and protects against Pseudomonas aeruginosa pneumonia in cystic fibrosis. Mol Med. 2012;18:477–485. doi: 10.2119/molmed.2012.00024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang H, Bloom O, Zhang M, Vishnubhakat JM, Ombrellino M, Che J, Frazier A, Yang H, Ivanova S, Borovikova L, et al. HMG-1 as a late mediator of endotoxin lethality in mice. Science. 1999;285:248–251. doi: 10.1126/science.285.5425.248. [DOI] [PubMed] [Google Scholar]
- 14.Rowe SM, Jackson PL, Liu G, Hardison M, Livraghi A, Solomon GM, McQuaid DB, Noerager BD, Gaggar A, Clancy JP, et al. Potential role of high-mobility group box 1 in cystic fibrosis airway disease. Am J Respir Crit Care Med. 2008;178:822–831. doi: 10.1164/rccm.200712-1894OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liou TG, Adler FR, Keogh RH, Li Y, Jensen JL, Walsh W, Packer K, Clark T, Carveth H, Chen J, et al. Sputum biomarkers and the prediction of clinical outcomes in patients with cystic fibrosis. PLoS ONE. 2012;7:e42748. doi: 10.1371/journal.pone.0042748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Young E. The anti-inflammatory effects of heparin and related compounds. Thromb Res. 2008;122:743–752. doi: 10.1016/j.thromres.2006.10.026. [DOI] [PubMed] [Google Scholar]
- 17.Hecht I, Hershkoviz R, Shivtiel S, Lapidot T, Cohen IR, Lider O, Cahalon L. Heparin-disaccharide affects T cells: inhibition of NF-kappaB activation, cell migration, and modulation of intracellular signaling. J Leukoc Biol. 2004;75:1139–1146. doi: 10.1189/jlb.1203659. [DOI] [PubMed] [Google Scholar]
- 18.Rao NV, Argyle B, Xu X, Reynolds PR, Walenga JM, Prechel M, Prestwich GD, MacArthur RB, Walters BB, Hoidal JR, et al. Low anticoagulant heparin targets multiple sites of inflammation, suppresses heparin-induced thrombocytopenia, and inhibits interaction of RAGE with its ligands. Am J Physiol Cell Physiol. 2010;299:C97–C110. doi: 10.1152/ajpcell.00009.2010. [DOI] [PubMed] [Google Scholar]
- 19.Fryer A, Huang Y-C, Rao G, Jacoby D, Mancilla E, Whorton R, Piantadosi CA, Kennedy T, Hoidal J. Selective O-desulfation produces nonanticoagulant heparin that retains pharmacological activity in the lung. J Pharmacol Exp Ther. 1997;282:208–219. [PubMed] [Google Scholar]
- 20.Zuhdi Alimam M, Piazza FM, Selby DM, Letwin N, Huang L, Rose MC. Muc-5/5ac mucin messenger RNA and protein expression is a marker of goblet cell metaplasia in murine airways. Am J Respir Cell Mol Biol. 2000;22:253–260. doi: 10.1165/ajrcmb.22.3.3768. [DOI] [PubMed] [Google Scholar]
- 21.Voynow JA, Fischer BM, Malarkey DE, Burch LH, Wong T, Longphre M, Ho SB, Foster WM. Neutrophil elastase induces mucus cell metaplasia in mouse lung. Am J Physiol Lung Cell Mol Physiol. 2004;287:L1293–L1302. doi: 10.1152/ajplung.00140.2004. [DOI] [PubMed] [Google Scholar]
- 22.Meyer ML, Potts-Kant EN, Ghio AJ, Fischer BM, Foster WM, Voynow JA. NAD(P)H quinone oxidoreductase 1 regulates neutrophil elastase-induced mucous cell metaplasia. Am J Physiol Lung Cell Mol Physiol. 2012;303:L181–L188. doi: 10.1152/ajplung.00084.2012. [DOI] [PMC free article] [PubMed] [Google Scholar] [Research Misconduct Found]
- 23.Raschke WC, Baird S, Ralph P, Nakoinz I. Functional macrophage cell lines transformed by Abelson leukemia virus. Cell. 1978;15:261–267. doi: 10.1016/0092-8674(78)90101-0. [DOI] [PubMed] [Google Scholar]
- 24.Maurya MR, Benner C, Pradervand S, Glass C, Subramaniam S. Systems biology of macrophages. Adv Exp Med Biol. 2007;598:62–79. doi: 10.1007/978-0-387-71767-8_6. [DOI] [PubMed] [Google Scholar]
- 25.Snedecor GW, Cochran WG. Ames: Iowa State University Press; 1980. Statistical methods. [Google Scholar]
- 26.Walsh DE, Greene CM, Carroll TP, Taggart CC, Gallagher PM, O’Neill SJ, McElvaney NG. Interleukin-8 up-regulation by neutrophil elastase is mediated by MyD88/IRAK/TRAF-6 in human bronchial epithelium. J Biol Chem. 2001;276:35494–35499. doi: 10.1074/jbc.M103543200. [DOI] [PubMed] [Google Scholar]
- 27.Mayer-Hamblett N, Aitken ML, Accurso FJ, Kronmal RA, Konstan MW, Burns JL, Sagel SD, Ramsey BW. Association between pulmonary function and sputum biomarkers in cystic fibrosis. Am J Respir Crit Care Med. 2007;175:822–828. doi: 10.1164/rccm.200609-1354OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ferhani N, Letuve S, Kozhich A, Thibaudeau O, Grandsaigne M, Maret M, Dombret MC, Sims GP, Kolbeck R, Coyle AJ, et al. Expression of high-mobility group box 1 and of receptor for advanced glycation end products in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2010;181:917–927. doi: 10.1164/rccm.200903-0340OC. [DOI] [PubMed] [Google Scholar]
- 29.Naparstek E, Slavin S, Weiss L, Sidi H, Ohana M, Reich S, Vlodavsky I, Cohen IR, Naparstek Y. Low-dose heparin inhibits acute graft versus host disease in mice. Bone Marrow Transplant. 1993;12:185–189. [PubMed] [Google Scholar]
- 30.Tang D, Kang R, Livesey KM, Zeh HJ, III, Lotze MT. High mobility group box 1 (HMGB1) activates an autophagic response to oxidative stress. Antioxid Redox Signal. 2011;15:2185–2195. doi: 10.1089/ars.2010.3666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gardella S, Andrei C, Ferrera D, Lotti LV, Torrisi MR, Bianchi ME, Rubartelli A. The nuclear protein HMGB1 is secreted by monocytes via a non-classical, vesicle-mediated secretory pathway. EMBO Rep. 2002;3:995–1001. doi: 10.1093/embo-reports/kvf198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lee H, Park M, Shin N, Kim G, Kim YG, Shin J-S, Kim H. High mobility group box-1 is phosphorylated by protein kinase C zeta and secreted in colon cancer cells. Biochem Biophys Res Commun. 2012;424:321–326. doi: 10.1016/j.bbrc.2012.06.116. [DOI] [PubMed] [Google Scholar]
- 33.Tang D, Shi Y, Kang R, Li T, Xiao W, Wang H, Xiao X. Hydrogen peroxide stimulates macrophages and monocytes to actively release HMGB1. J Leukoc Biol. 2007;81:741–747. doi: 10.1189/jlb.0806540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Aoshiba K, Yasuda K, Yasui S, Tamaoki J, Nagai A. Serine proteases increase oxidative stress in lung cells. Am J Physiol Lung Cell Mol Physiol. 2001;281:L556–L564. doi: 10.1152/ajplung.2001.281.3.L556. [DOI] [PubMed] [Google Scholar]
- 35.Fischer BM, Voynow JA. Neutrophil elastase induces MUC5AC gene expression in airway epithelium via a pathway involving reactive oxygen species. Am J Respir Cell Mol Biol. 2002;26:447–452. doi: 10.1165/ajrcmb.26.4.4473. [DOI] [PubMed] [Google Scholar]
- 36.Ramsey BW, Davies J, McElvaney NG, Tullis E, Bell SC, Dřevínek P, Griese M, McKone EF, Wainwright CE, Konstan MW, et al. VX08-770-102 Study Group. A CFTR potentiator in patients with cystic fibrosis and the G551D mutation. N Engl J Med. 2011;365:1663–1672. doi: 10.1056/NEJMoa1105185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Martin SL, Downey D, Bilton D, Keogan MT, Edgar J, Elborn JS Recombinant AAT CF Study Team. Safety and efficacy of recombinant alpha(1)-antitrypsin therapy in cystic fibrosis. Pediatr Pulmonol. 2006;41:177–183. doi: 10.1002/ppul.20345. [DOI] [PubMed] [Google Scholar]
- 38.Griese M, Latzin P, Kappler M, Weckerle K, Heinzlmaier T, Bernhardt T, Hartl D. Alpha1-antitrypsin inhalation reduces airway inflammation in cystic fibrosis patients. Eur Respir J. 2007;29:240–250. doi: 10.1183/09031936.00047306. [DOI] [PubMed] [Google Scholar]
- 39.Grimbert D, Vecellio L, Delepine P, Attucci S, Boissinot E, Poncin A, Gauthier F, Valat C, Saudubray F, Antonioz P, Diot P. Characteristics of EPI-hNE4 aerosol: a new elastase inhibitor for treatment of cystic fibrosis. J Aerosol Med. 2003;16:121–129. doi: 10.1089/089426803321919889. [DOI] [PubMed] [Google Scholar]
- 40.Attucci S, Gauthier A, Korkmaz B, Delépine P, Martino MF, Saudubray F, Diot P, Gauthier F. EPI-hNE4, a proteolysis-resistant inhibitor of human neutrophil elastase and potential anti-inflammatory drug for treating cystic fibrosis. J Pharmacol Exp Ther. 2006;318:803–809. doi: 10.1124/jpet.106.103440. [DOI] [PubMed] [Google Scholar]
- 41.Quinn D, Weldon S, Taggart CC. Antiproteases as therapeutics to target inflammation in cystic fibrosis. Open Respir Med J. 2010;4:20–31. doi: 10.2174/1874306401004010020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Diamant Z, Timmers MC, van der Veen H, Page CP, van der Meer FJ, Sterk PJ. Effect of inhaled heparin on allergen-induced early and late asthmatic responses in patients with atopic asthma. Am J Respir Crit Care Med. 1996;153:1790–1795. doi: 10.1164/ajrccm.153.6.8665036. [DOI] [PubMed] [Google Scholar]
- 43.Vectura Group plc Release: VR496 demonstrates anti-inflammatory and mucolytic activity in cystic fibrosis patients 3/25/2011 ed; 2011. [accessed 2011 Mar 25]. Available from: http://www.vectura.com/media/press-releases/2011/25-mar-2011.aspx
- 44.Dixon B, Schultz MJ, Smith R, Fink JB, Santamaria JD, Campbell DJ. Nebulized heparin is associated with fewer days of mechanical ventilation in critically ill patients: a randomized controlled trial. Crit Care. 2010;14:R180. doi: 10.1186/cc9286. [DOI] [PMC free article] [PubMed] [Google Scholar]