Abstract
Bitter taste receptors (TAS2Rs) have recently been found to be expressed on human airway smooth muscle (HASM), and their activation results in marked relaxation. These agents have been proposed as a new class of bronchodilators in the treatment of obstructive lung diseases because they act via a different mechanism than β-agonists. The TAS2R signal transduction pathway in HASM has multiple elements that are potentially subject to regulation by inflammatory, genetic, and epigenetic mechanisms associated with asthma. To address this, expression, signaling, and physiologic functions of the three major TAS2Rs (subtypes 10, 14, and 31) on HASM were studied. Transcript expression of these TAS2Rs was not decreased in HASM cells derived from donors with asthma compared with those without asthma (n = 6 from each group). In addition, intracellular calcium ([Ca2+]i) signaling using TAS2R subtype–specific agonists (diphenhydramine, chloroquine, saccharin, and flufenamic acid) was not impaired in the cells derived from donors with asthma, nor was the response to quinine, which activates all three subtypes. HASM cell mechanics measured by magnetic twisting cytometry revealed equivalent TAS2R-mediated relaxation of methacholine-treated cells between the two groups. Human precision-cut lung slices treated with IL-13 caused a decrease in β-agonist (formoterol)-mediated relaxation of carbachol-contracted airways compared with control slices. In contrast, TAS2R-mediated relaxation was unaffected by IL-13. We conclude that TAS2R expression or function is unaffected in HASM cells derived from patients with asthma or the IL-13 inflammatory environment.
Keywords: receptor, asthma, bronchodilator, β-agonist, bitter taste
Clinical Relevance
Bitter taste receptors (TAS2Rs) on airway smooth muscle cause profound bronchodilation, and agonists at these receptors have been proposed as a novel therapy for asthma. Like other G-protein–coupled receptors, this signaling pathway has multiple nodal points where regulation by inflammation, genetic variation, and epigentic mechanisms could alter function in asthma. Using multiple approaches, we show that asthmatic airway smooth muscle cells and airways subjected to inflammation have intact TAS2R function, indicating their potential benefit in the disease.
Asthma is characterized by airflow obstruction caused by airway smooth muscle contraction. Although treating the underlying inflammation is key to long-term therapy, pharmacological agents acting to relax contracted smooth muscle are usually necessary for acute and/or preventative therapy. Treatment of bronchospasm in asthma by direct relaxation of airway smooth muscle is limited to β-agonists acting at smooth muscle β2-adrenergic receptors (β2ARs). Although these agents are effective, they have been associated with bronchial hyperreactivity (i.e., increased sensitivity to bronchospastic agents), tachyphylaxis, interindividual variability, worsening clinical and physiological outcomes in asthma, and death (1–7). In addition, it has been estimated that up to 50% of patients with asthma experience suboptimal control (1, 8). Although these and other studies are open to interpretation and debate, a broadening of the treatment options for asthma appears to be in order (6, 9, 10).
To expand the armamentarium of drugs that might be used for treating bronchospasm, we recently used expression arrays (11) and targeted RT-PCR (12) to identify novel G-protein–coupled receptors (GPCRs) as potential drug targets for bronchospasm. Unexpectedly, we found expression of multiple bitter taste receptors (TAS2Rs) on human airway smooth muscle (HASM) cells (12). Three of the 25 known TAS2Rs were in high abundance, and subtype-specific agonists for these receptors relaxed airway smooth muscle in vitro, ex vivo, and in vivo (12). The mechanism of action of these receptors is not clear, although relaxation appears to be related to an increase in intracellular calcium ([Ca2+]i) from intracellular stores confined to a specialized compartment resulting in cell membrane hyperpolarization. [Ca2+]i stimulation was sensitive to βγ subunit and phospholipase C inhibitors and to IP3 receptor antagonists, and relaxation was partially sensitive to Ca2+-activated K+ channel antagonism (12). This signaling cascade involves multiple components, including the G-protein, receptor, phospholipase C, the IP3 receptor, [Ca2+]i homeostasis mechanisms, and one or more channels. The three primary TAS2R receptors of HASM are subtypes 10, 14, and 31; each is expressed to comparable levels of ∼ 4-fold greater than β2AR. Each subtype appears to be capable of promoting HASM relaxation. In contrast, agonists for the less prevalent TAS2R subtypes promote substantially less signal activation or relaxation. Thus, TAS2R10, -14, and -31 have been considered novel targets for a new class of bronchodilator (9).
Of concern has been the potential for TAS2R expression or function to be dysregulated in asthma, which might make these GPCRs less desirable targets for drug development. Furthermore, there are multiple nodal points where regulatory events might occur. In limited studies in a mouse model of asthma (ovalbumin sensitized), we have shown preservation of the bronchodilating function of these receptors (12). A recent report has shown that TAS2Rs are expressed on lymphocytes and that this expression is increased in children with severe asthma (13). The asthmatic state has long been recognized as one that includes dysregulation of airway GPCRs, including those that act to contract and relax airway smooth muscle. Such alterations have been observed in vivo and ex vivo in human and animal airways and in cultured HASM cells (14–20). These findings include altered [Ca2+] signaling from Gq-coupled receptors, such as M3-muscarinic and H1-histamine, and cAMP signaling from β2AR (14, 17, 18). Given that some of these signaling effects are found in passaged cultured HASM cells, in the absence of the inflammatory airway milieu, asthmatic HASM may be “hard-wired” by genetic or epigenetic mechanisms to be procontractile and/or resistant to relaxation. To explore the potential for aberrant (down-regulated) TAS2R function in asthma, we studied TAS2R expression, signaling, and physiologic function in HASM cells from subjects with and without asthma and TAS2R-mediated relaxation of IL-13–promoted airway hyperreactivity in human precision-cut lung slices (PCLSs).
Materials and Methods
Cell and Tissue Harvesting and Culture
HASM cells were derived from tracheas obtained from the National Disease Research Interchange (Philadelphia, PA) and from the International Institute for the Advance of Medicine (Edison, NJ). HASM cell culture was performed as previously described (21). The cells at passages 3 through 5 were maintained in Ham’s F-12 supplemented with 10% FBS, 100 U/ml penicillin, 0.1 mg/ml streptomycin, and 2.5 mg/ml amphotericin B. Six sets of cultured cells were taken from donors with asthma, and six sets were taken from donors without asthma. PCLSs were made using methods previously described (22). Briefly, whole human lungs from donors with and without asthma were dissected and inflated using 2% (wt/vol) low-melting-point agarose. Once the agarose set, the lobe was sectioned, and cores (8 mm diameter) were made and sliced at a thickness of 350 μm and maintained in supplemented Ham’s F-12. Sections were placed in fresh media maintained at 37°C in a humidified air–CO2 incubator, and the media was changed every 2 to 3 hours during the remainder of the collection day and on the subsequent day.
Real-Time RT-PCR
Total RNA (2 μg) from HASM was used for reverse transcription using oligo-dT primers. Real-time PCR was performed using specific primers for TAS2R 10, 14, and 31 and GAPDH (Applied Biosystems, Grand Island, NY) and the 7300 Real Time PCR System (Applied Biosystems) as previously described (12). Previous studies showed that sequencing of these products revealed authentic human TAS2R subtype sequence (12).
[Ca2+]i Measurements
HASM cells were studied at 5 × 105 cells/well in 96-well plates as described (12, 23). Cells were loaded with Fluo-4 AM with probenecid for 1 hour. TAS2R agonists (final concentration, 1.0 mM) were added 17 seconds after excitation (485 nm) and emission acquisition (525 nm) began, and data were collected up to 60 seconds using a Flex Station II (Molecular Devices, Sunnyvale, CA). After subtraction of the baseline, peak signals were ascertained using PRISM software (GraphPad, La Jolla, CA).
Magnetic Twisting Cytometry
Dynamic changes in cell stiffness of HASM cells were determined as previously described (24, 25). HASM cells were first contracted with 10 μM methacholine, which was maintained during addition of TAS2R agonists (1 mM final concentration). For each individual HASM cell, changes in stiffness in response to TAS2R agonists were normalized to its respective methacholine-contracted stiffness.
PCLS Physiology
PCLSs obtained and cultured as described above were incubated in the absence or presence of 100 ng/ml human IL-13 for 18 hours before contraction and relaxation studies. Airways were located using a microscope (magnification, ×40) connected to a live video feed, and airway luminal area was measured using Image-Pro Plus software (version 7.0; Media Cybernetics, Rockville, MD) and represented in μm2. Airways were constricted to 70% below baseline area with carbachol and then relaxed with the indicated doses of formoterol or quinine. Maximal responses and EC50 values were derived from the least square iterative curve fitting using Prism (GraphPad).
Results
TAS2R Expression and [Ca2+]i Signaling in HASM Cells
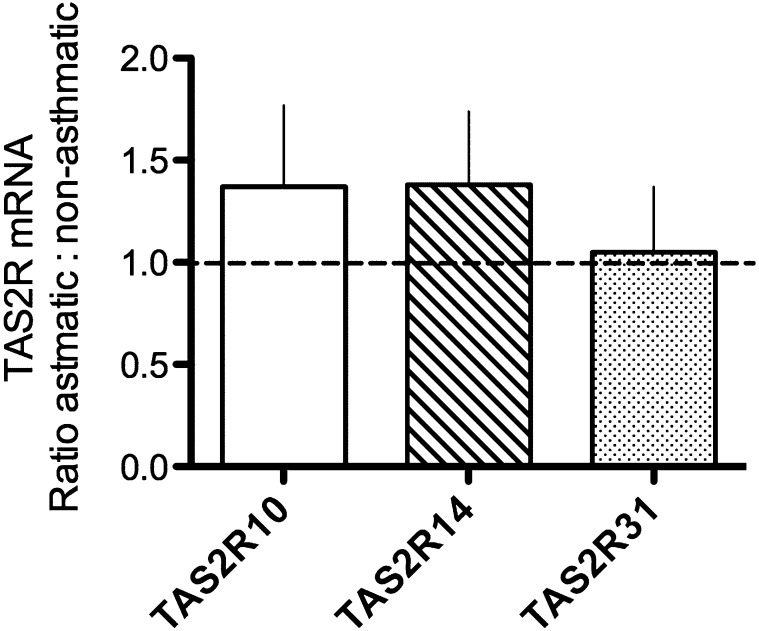
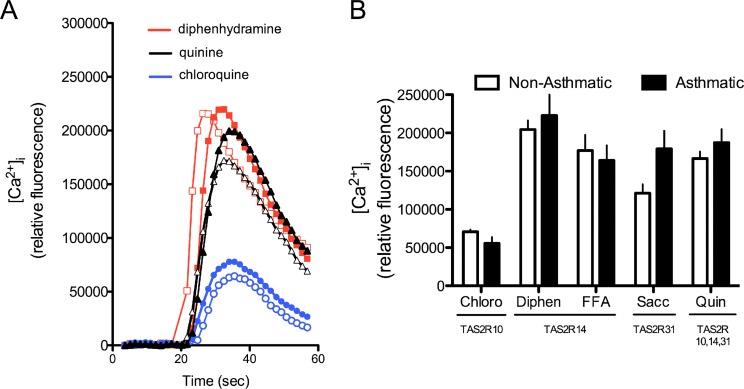
Primary HASM cells derived from six nonasthmatic lungs and six fatal asthmatic lungs were used for TAS2R expression and [Ca2+]i signaling assays. Quantitative RT-PCR was performed using specific primers for the three dominant TAS2R subtypes in HASM: TAS2R10, -14, and -31. Results depicted in Figure 1 show no difference in mRNA expression of any of the three subtypes between nonasthmatic or asthmatic HASM cells. To examine TAS2R functions, subtype-specific agonists chloroquine (TAS2R10), diphenhydramine (TAS2R14), flufenamic acid (TAS2R14), saccharin (TASAR31), and quinine, an agonist that activates all three subtypes, were used to quantify agonist-promoted [Ca2+]i flux in isolated HASM cells. A typical set of [Ca2+]i transients is shown in Figure 2A for several agonists. Mean results are shown in Figure 2B. The responses to the subtype-specific agonists in the asthmatic HASM cells were not less than those of the nonasthmatic cells and trended toward being higher for some agonists. Consistent with these results, the multireceptor agonist quinine promoted [Ca2+]i to a degree that was indistinguishable between asthmatic versus nonasthmatic HASM cells.
Figure 1.
Quantitative real-time RT-PCR of bitter taste receptor (TAS2R) mRNA expressed in asthmatic compared with nonasthmatic human airway smooth muscle (HASM) cells. Primers specific for TAS2R 10, 14, and 31 and GAPDH were used to generate cycle threshold values, and the data were normalized to the nonasthmatic cells. Results are from primary HASM cells derived from six donors with asthma and six donors without asthma. Expression of the three indicated TAS2Rs was not substantially different between the two groups.
Figure 2.
[Ca2+]i signaling by TAS2R agonists in asthmatic and nonasthmatic HASM cells. (A) Representative [Ca2+]i transients from HASM cells from one subject with asthma (closed symbols) and one subject without asthma (open symbols) in response to the indicated TAS2R agonists. (B) Mean peak [Ca2+]i responses from HASM cells derived from six subjects with asthma and six subjects without asthma. There was no significant difference in the responses between the two groups. The TAS2Rs listed below the agonists indicate this subtype specificity. Chloro, chloroquine; Diphen, diphenhydramine; FFA, flufenamic acid; Sacc, saccharin.
HASM Cell Mechanics
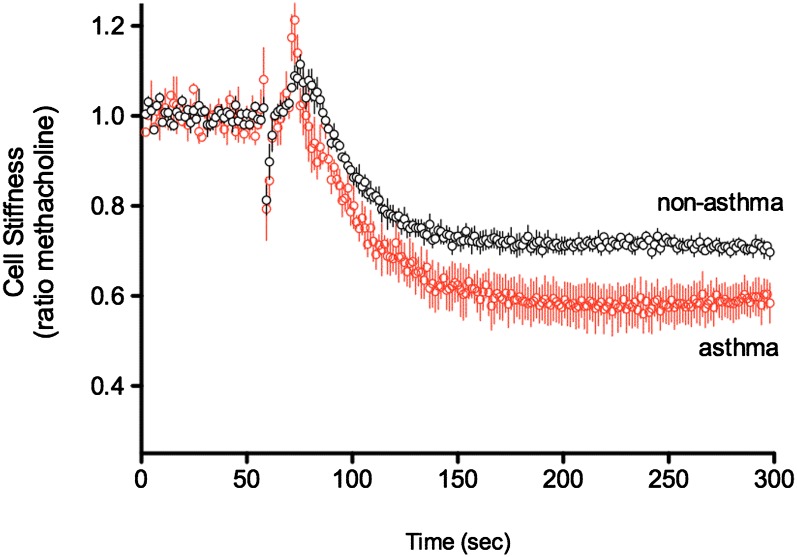
We next studied cytoskeleton mechanics of nonasthmatic and asthmatic HASM cells using magnetic twisting cytometry. Ferrimagnetic microbeads were functionalized to the cytoskeleton through cell surface integrin receptors, and changes in the stiffness were ascertained under various conditions. Cells were first exposed to 10 μM methacholine to increase stiffness and then to quinine at a final concentration of 1 mM. The results are shown in Figure 3. The response to quinine in the asthmatic cells was not less than that of the nonasthmatic cells and favored an enhanced relaxation in asthma (ratio to methacholine stiffness, 0.63 ± 0.040 versus 0.69 ± 0.039, respectively; P > 0.05). This maintenance of or slight improvement in efficacy is consistent with the expression results (Figure 1) and the [Ca2+]i studies (Figure 2).
Figure 3.
Relaxation responses to quinine as ascertained by magnetic twisting cytometry. Cells were contracted for 300 seconds with 10 μM methacholine and then exposed to 1 mM quinine. Results are from individual cell measurements (n = 949–1513 cells) from three subjects with asthma and three subjects without asthma. For each donor lung, mean values of cell stiffness data were computed and applied to the respective group means. Data are presented as mean ± SE (n = 3 lungs for each group). There was no statistically significant difference between the two groups.
IL-13 PCLS Mechanics
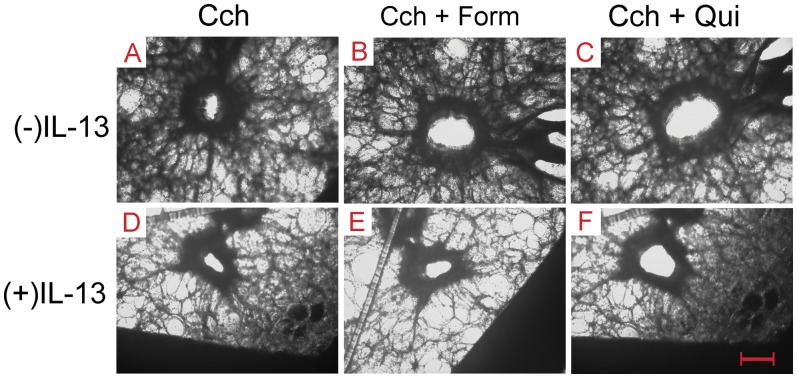
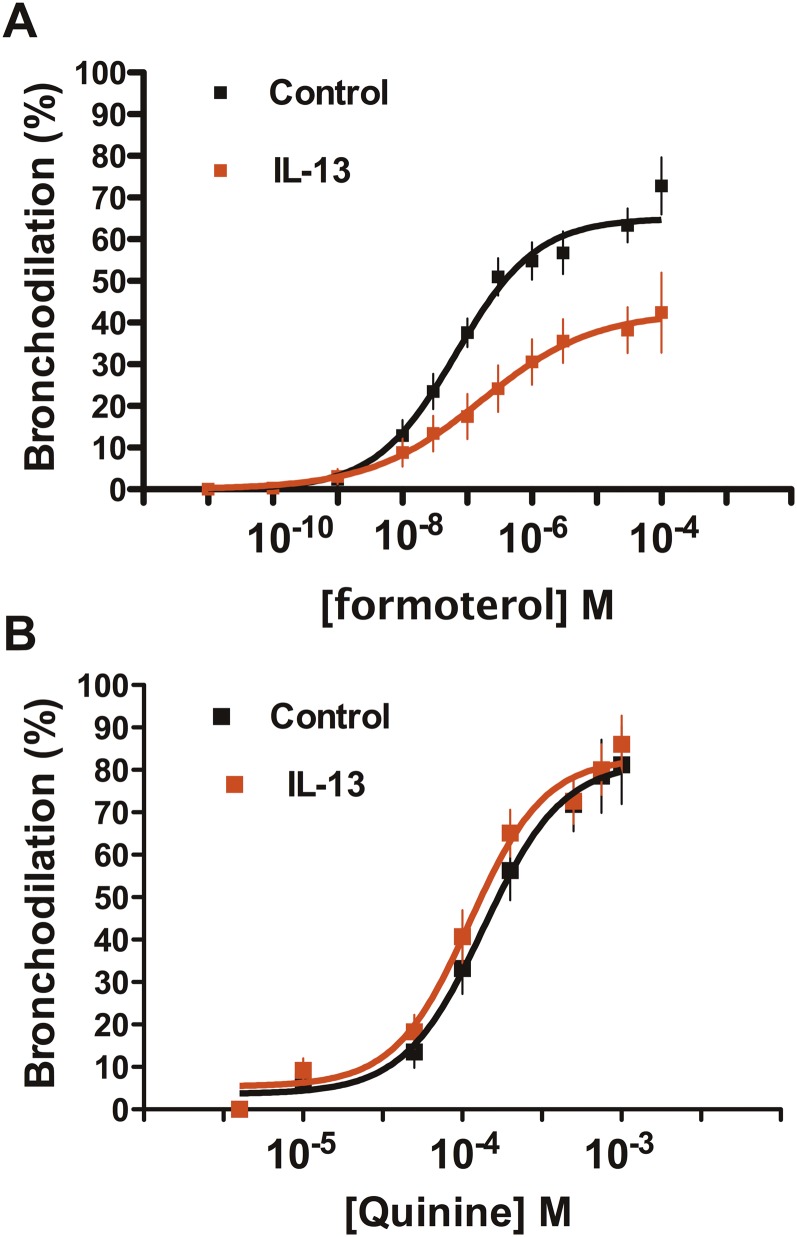
We examined relaxation responses to TAS2R activation in the IL-13–induced model of hyperreactivity in PCLSs of normal human airways. Slices were contracted by 3 μM carbachol and then exposed to the indicated concentrations of the β-agonist formoterol or the TAS2R agonist quinine. Representative images from slices are shown in Figure 4, which illustrates the cross-sectional area responses of carbachol-contracted airways to the maximal concentrations of formoterol or quinine in the absence or presence of IL-13 incubation. This example shows relaxation by formoterol and slightly greater relaxation by quinine in the control (absent IL-13) setting. In contrast, with the IL-13 incubation, formoterol evokes a smaller relaxation, whereas the quinine-mediated relaxation is nearly unaffected. Results from multiple experiments (Figure 5A) show that the relaxation afforded by formoterol was decreased with IL-13 pretreatment (62 ± 4.3% versus 43 ± 6.7% contraction; P = 0.01 [n = 11–15 slices]). This response was associated with an ∼ 2.5-fold increase in the EC50 for the IL-13 condition (mean composite EC50, 165 nM versus 67 nM for control slices; P < 0.05). In contrast, the dose responses to quinine in the absence or presence of IL-13 pretreatment were superimposable, with no differences in the maximal response or the EC50 (Figure 5B).
Figure 4.
Representative airway responses from precision-cut lung slice preparations. (A–C) Contraction was evoked with carbachol (Cch), and then the β-agonist formoterol (Form) or quinine (Qui) was added to the bath in the absence of IL-13 preincubation. Formoterol and quinine relaxed carbachol-mediated contraction, with the extent of relaxation by quinine being greater. (D–F) After IL-13 treatment, formoterol-mediated relaxation was impaired (E), whereas the quinine response was minimally affected (F). Original magnification: ×40. Scale bar, 300 μm.
Figure 5.
Relaxation responses to β-agonist and TAS2R agonist in IL-13–treated precision-cut lung slices. (A) Responses to the β-agonist formoterol shows a depressed relaxation when slices are coincubated with IL-13 (P = 0.01; n = 11–15 slices). (B) Response to quinine is not affected by IL-13 incubation (P > 0.05; n = 11–15 slices).
Discussion
The airway smooth muscle of humans expresses several hundred GPCRs (11). Of these, a large proportion is coupled to the G-protein Gs, potentially generating cAMP and leading to HASM relaxation. Like other GPCRs, Gs-coupled receptors, such as the β2AR, undergo multiple regulatory events, resulting in alterations of expression or G-protein coupling (function). These include homologous desensitization by persistent agonist exposure and heterologous desensitization promoted by other signaling events, drugs, or disease states. This plasticity of GPCRs appears to be necessary for coordination of the many signals being received by the cell. Such regulation can be adaptive in a positive sense but may also be maladaptive, contributing to the pathobiology of a disease or rendering treatment less effective.
β-agonist treatment for asthma, which is via activation of the Gs-coupled β2AR, can be limited by desensitization and the asthmatic diathesis (2, 14, 18, 22, 26). The mechanisms for such dysregulation can be complex and distributed throughout the signaling cascade. For example, the β2AR and the EP1 prostaglandin receptor form a heterodimer on the HASM cell surface (27). Activation of the EP1 part of the dimer by PGE2 in an inflammatory environment causes a decrease in β2AR function due to its uncoupling from Gs (27). IL-13 and TNF-α also decrease β-agonist functional responses in PCLSs and increase contractile responses to agonists acting at procontractile GPCRs (28–31). Even in passaged cultured HASM cells, without the addition of proinflammatory agents in the media, cells derived from patients with fatal asthma show distinct phenotypes compared with cells derived from donors without asthma. These include differences in receptor signaling, second messenger–dependent enzyme function, the response to viral infection, cell proliferation, and intracellular events, notably “distal” to early interaction genes (15, 16, 32). The breadth of documented changes in HASM phenotype from asthma or an inflammatory environment requires that potentially new therapeutic agents be tested in this context.
Our interest in TAS2Rs arose from results of custom expression arrays used to quantitate all known GPCR transcripts (and their splice variants) on HASM cells (11). We then chose atypical receptors that were expected to act via different mechanisms as potential drug targets for treatment for obstructive lung disease. A number of TAS2R subtypes were detected on cultured HASM cells. These receptors do not couple to Gs/cAMP but rather appear to bronchodilate by altering smooth muscle [Ca2+]i flux. We and others have shown that activation of these receptors markedly relaxes airways and isolated airway smooth muscle from human, monkey, guinea pig, and mouse (12, 23, 33). The three most highly expressed HASM TAS2Rs—subtypes 10, 14, and 31—display differential affinities for bitter tastants, thus providing an opportunity for novel drug development. Because we do not know whether the benchmark TAS2R agonists are full or partial agonists, it remains unclear as to which subtype evokes the greater relaxation or if more than one subtype activation is required for maximal relaxation. Other issues that need to be further addressed for drug development, such as agonist access to smooth muscle receptors, have been recently reviewed elsewhere (9). A minor degree of agonist-promoted desensitization of TAS2Rs with bitter tastants acting at these three receptor subtypes has been documented (23), but it remains uncertain if the asthmatic diathesis negatively effects TAS2R function on HASM cells. To approach this, we first used HASM cells derived from nonasthmatic and asthmatic lungs. Quantitative RT-PCR revealed no differences in transcript expression between the two groups. Although there are no available antibodies to detect fmol levels of TAS2R protein, we have previously shown a correlation between TAS2R transcript expression and receptor function (12). Thus, we are confident that there is no down-regulation of these receptors in HASM cells derived from donors with asthma. This was particularly so when we ascertained functional coupling using agonists with TAS2R subtype specificity or an agonist that activates all three subtypes. These studies not only support the findings that expression is unaltered but also indicate that the functions of elements within the postreceptor signaling cascade remain intact, at least in terms of their contribution to TAS2R responses. Given the components of the proposed signaling mechanism, there are ample nodal points where dysregulation might be manifested as an altered drug response. Furthermore, there is precedent for distal downstream GPCR signaling alterations in asthmatic HASM cells. For example, a 2-fold greater activity and expression of type 4 phosphodiesterase (which degrades cAMP) has been found in cultured HASM cells derived from donors with asthma compared with donors without asthma (18). This correlated with an approximately 50% decrease in cAMP production, which was abolished by incubation of the cells with phosphodiesterase inhibitors. Although the mechanism of this dysregulation remains undefined, it is not related to β-agonist exposure or inflammation because it was observed in cells after multiple passages from the original human airway sample. In the current work, we also ascertained HASM mechanics in the two groups of cells. Although we have found a strong correlation between TAS2R-[Ca2+]i signaling and relaxation, there is the potential for regulation to occur at the contraction–relaxation interface, distal to second-messenger measurements. We found, however, no loss of the relaxation effect of TAS2Rs using magnetic twisting cytometry. In fact, as was observed in the RT-PCR and [Ca2+]i signaling studies, there was a trend toward enhanced function. Additional studies were performed with human PCLSs (nonasthmatic) treated ex vivo with IL-13. The relaxation response was unaltered to quinine in IL-13–treated PCLSs, whereas the relaxation response to the β-agonist formoterol was decreased by approximately 35%. Taken together, these results indicate that TAS2R function is preserved in HASM cells from donors with asthma and in human airways placed in a proinflammatory environment. This suggests that TAS2R agonists may be useful bronchodilators in asthma as an adjunct to (or replacement for) β-agonists. Although TAS2R function does not appear to be down-regulated by inflammation, concomitant corticosteroid administration would still be necessary for treatment of the deleterious effects of airway inflammation.
Acknowledgments
Acknowledgments
The author thanks Charmaine Disimile for manuscript preparation.
Footnotes
This work was supported by National Institutes of Health grants HL114471, HL045967, and HL107361.
Originally Published in Press as DOI: 10.1165/rcmb.2013-0439RC on November 12, 2013
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Drazen JM, Silverman EK, Lee TH. Heterogeneity of therapeutic responses in asthma. Br Med Bull. 2000;56:1054–1070. doi: 10.1258/0007142001903535. [DOI] [PubMed] [Google Scholar]
- 2.Lipworth BJ. Airway subsensitivity with long-acting beta 2-agonists: is there cause for concern? Drug Saf. 1997;16:295–308. doi: 10.2165/00002018-199716050-00002. [DOI] [PubMed] [Google Scholar]
- 3.Kraan J, Koëter GH, vd Mark TW, Sluiter HJ, de Vries K. Changes in bronchial hyperreactivity induced by 4 weeks of treatment with antiasthmatic drugs in patients with allergic asthma: a comparison between budesonide and terbutaline. J Allergy Clin Immunol. 1985;76:628–636. doi: 10.1016/0091-6749(85)90786-9. [DOI] [PubMed] [Google Scholar]
- 4.Cheung D, Timmers MC, Zwinderman AH, Bel EH, Dijkman JH, Sterk PJ. Long-term effects of a long-acting β2-adrenoceptor agonist, salmeterol, on airway hyperresponsiveness in patients with mild asthma. N Engl J Med. 1992;327:1198–1203. doi: 10.1056/NEJM199210223271703. [DOI] [PubMed] [Google Scholar]
- 5.Beasley R, Pearce N, Crane J, Burgess C. Beta-agonists: what is the evidence that their use increases the risk of asthma morbidity and mortality? J Allergy Clin Immunol. 1999;104:S18–S30. doi: 10.1016/s0091-6749(99)70270-8. [DOI] [PubMed] [Google Scholar]
- 6.Sears MR, Taylor DR. The β2-agonist controversy: observations, explanations and relationship to asthma epidemiology. Drug Saf. 1994;11:259–283. doi: 10.2165/00002018-199411040-00005. [DOI] [PubMed] [Google Scholar]
- 7.Salpeter SR, Wall AJ, Buckley NS. Long-acting beta-agonists with and without inhaled corticosteroids and catastrophic asthma events. Am J Med. 2010;123:322–328, e2. doi: 10.1016/j.amjmed.2009.07.035. [DOI] [PubMed] [Google Scholar]
- 8.Malmstrom K, Rodriguez-Gomez G, Guerra J, Villaran C, Piñeiro A, Wei LX, Seidenberg BC, Reiss TF Montelukast/Beclomethasone Study Group. Oral montelukast, inhaled beclomethasone, and placebo for chronic asthma: a randomized, controlled trial. Ann Intern Med. 1999;130:487–495. doi: 10.7326/0003-4819-130-6-199903160-00005. [DOI] [PubMed] [Google Scholar]
- 9.Liggett SB. Bitter taste receptors on airway smooth muscle as targets for novel bronchodilators. Expert Opin Ther Targets. 2013;17:721–731. doi: 10.1517/14728222.2013.782395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gerthoffer WT, Solway J, Camoretti-Mercado B. Emerging targets for novel therapy of asthma. Curr Opin Pharmacol. 2013;13:324–330. doi: 10.1016/j.coph.2013.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Einstein R, Jordan H, Zhou W, Brenner M, Moses EG, Liggett SB. Alternative splicing of the G protein-coupled receptor superfamily in human airway smooth muscle diversifies the complement of receptors. Proc Natl Acad Sci USA. 2008;105:5230–5235. doi: 10.1073/pnas.0801319105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Deshpande DA, Wang WCH, McIlmoyle EL, Robinett KS, Schillinger RM, An SS, Sham JSK, Liggett SB. Bitter taste receptors on airway smooth muscle bronchodilate by localized calcium signaling and reverse obstruction. Nat Med. 2010;16:1299–1304. doi: 10.1038/nm.2237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Orsmark-Pietras CO, James A, Konradsen JR, Nordlund B, Soderhall C, Pulkkinen V, Pedroletti C, Daham K, Kupczyk M, Dahlen B, et al. Transcriptome analysis reveals upregulation of bitter taste receptors in severe asthmatics. Eur Respir J. 2012 doi: 10.1183/09031936.00077712. [DOI] [PubMed] [Google Scholar]
- 14.Goldie RG, Spina D, Henry PJ, Lulich KM, Paterson JW. In vitro responsiveness of human asthmatic bronchus to carbachol, histamine, β-adrenoceptor agonists and theophylline. Br J Clin Pharmacol. 1986;22:669–676. doi: 10.1111/j.1365-2125.1986.tb02956.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Burgess JK, Lee JH, Ge Q, Ramsay EE, Poniris MH, Parmentier J, Roth M, Johnson PR, Hunt NH, Black JL, et al. Dual ERK and phosphatidylinositol 3-kinase pathways control airway smooth muscle proliferation: differences in asthma. J Cell Physiol. 2008;216:673–679. doi: 10.1002/jcp.21450. [DOI] [PubMed] [Google Scholar]
- 16.Chhabra J, Li YZ, Alkhouri H, Blake AE, Ge Q, Armour CL, Hughes JM. Histamine and tryptase modulate asthmatic airway smooth muscle GM-CSF and RANTES release. Eur Respir J. 2007;29:861–870. doi: 10.1183/09031936.00106306. [DOI] [PubMed] [Google Scholar]
- 17.Trian T, Benard G, Begueret H, Rossignol R, Girodet PO, Ghosh D, Ousova O, Vernejoux JM, Marthan R, Tunon-de-Lara JM, et al. Bronchial smooth muscle remodeling involves calcium-dependent enhanced mitochondrial biogenesis in asthma. J Exp Med. 2007;204:3173–3181. doi: 10.1084/jem.20070956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Trian T, Burgess JK, Niimi K, Moir LM, Ge Q, Berger P, Liggett SB, Black JL, Oliver BG. β2-Agonist induced cAMP is decreased in asthmatic airway smooth muscle due to increased PDE4D. PLoS ONE. 2011;6:e20000. doi: 10.1371/journal.pone.0020000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jude JA, Panettieri RA, Jr, Walseth TF, Kannan MS. TNF-α regulation of CD38 expression in human airway smooth muscle: role of MAP kinases and NF-κB. Adv Exp Med Biol. 2011;691:449–459. doi: 10.1007/978-1-4419-6612-4_46. [DOI] [PubMed] [Google Scholar]
- 20.Mahn K, Hirst SJ, Ying S, Holt MR, Lavender P, Ojo OO, Siew L, Simcock DE, McVicker CG, Kanabar V, et al. Diminished sarco/endoplasmic reticulum Ca2+ ATPase (SERCA) expression contributes to airway remodelling in bronchial asthma. Proc Natl Acad Sci USA. 2009;106:10775–10780. doi: 10.1073/pnas.0902295106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Green SA, Turki J, Bejarano P, Hall IP, Liggett SB. Influence of β2-adrenergic receptor genotypes on signal transduction in human airway smooth muscle cells. Am J Respir Cell Mol Biol. 1995;13:25–33. doi: 10.1165/ajrcmb.13.1.7598936. [DOI] [PubMed] [Google Scholar]
- 22.Cooper PR, Panettieri RA., Jr Steroids completely reverse albuterol-induced beta(2)-adrenergic receptor tolerance in human small airways. J Allergy Clin Immunol. 2008;122:734–740. doi: 10.1016/j.jaci.2008.07.040. [DOI] [PubMed] [Google Scholar]
- 23.Robinett KS, Deshpande DA, Malone MM, Liggett SB. Agonist-promoted homologous desensitization of human airway smooth muscle bitter taste receptors. Am J Respir Cell Mol Biol. 2011;45:1069–1074. doi: 10.1165/rcmb.2011-0061OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.An SS, Fabry B, Trepat X, Wang N, Fredberg JJ. Do biophysical properties of the airway smooth muscle in culture predict airway hyperresponsiveness? Am J Respir Cell Mol Biol. 2006;35:55–64. doi: 10.1165/rcmb.2005-0453OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.An SS, Laudadio RE, Lai J, Rogers RA, Fredberg JJ. Stiffness changes in cultured airway smooth muscle cells. Am J Physiol Cell Physiol. 2002;283:C792–C801. doi: 10.1152/ajpcell.00425.2001. [DOI] [PubMed] [Google Scholar]
- 26.Turki J, Green SA, Newman KB, Meyers MA, Liggett SB. Human lung cell β2-adrenergic receptors desensitize in response to in vivo administered β-agonist. Am J Physiol. 1995;269:L709–L714. doi: 10.1152/ajplung.1995.269.5.L709. [DOI] [PubMed] [Google Scholar]
- 27.McGraw DW, Mihlbachler KA, Schwarb MR, Rahman FF, Small KM, Almoosa KF, Liggett SB. Airway smooth muscle prostaglandin-EP1 receptors directly modulate beta2-adrenergic receptors within a unique heterodimeric complex. J Clin Invest. 2006;116:1400–1409. doi: 10.1172/JCI25840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Amrani Y, Krymskaya V, Maki C, Panettieri RA., Jr Mechanisms underlying TNF-alpha effects on agonist-mediated calcium homeostasis in human airway smooth muscle cells. Am J Physiol. 1997;273:L1020–L1028. doi: 10.1152/ajplung.1997.273.5.L1020. [DOI] [PubMed] [Google Scholar]
- 29.Tliba O, Deshpande D, Chen H, Van Besien C, Kannan M, Panettieri RA, Jr, Amrani Y. IL-13 enhances agonist-evoked calcium signals and contractile responses in airway smooth muscle. Br J Pharmacol. 2003;140:1159–1162. doi: 10.1038/sj.bjp.0705558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Deshpande DA, Walseth TF, Panettieri RA, Kannan MS. CD38/cyclic ADP-ribose-mediated Ca2+ signaling contributes to airway smooth muscle hyper-responsiveness. FASEB J. 2003;17:452–454. doi: 10.1096/fj.02-0450fje. [DOI] [PubMed] [Google Scholar]
- 31.Cooper PR, Lamb R, Day ND, Branigan PJ, Kajekar R, San Mateo L, Hornby PJ, Panettieri RA., Jr TLR3 activation stimulates cytokine secretion without altering agonist-induced human small airway contraction or relaxation. Am J Physiol Lung Cell Mol Physiol. 2009;297:L530–L537. doi: 10.1152/ajplung.00133.2009. [DOI] [PubMed] [Google Scholar]
- 32.Joubert P, Lajoie-Kadoch S, Labonté I, Gounni AS, Maghni K, Wellemans V, Chakir J, Laviolette M, Hamid Q, Lamkhioued B. CCR3 expression and function in asthmatic airway smooth muscle cells. J Immunol. 2005;175:2702–2708. doi: 10.4049/jimmunol.175.4.2702. [DOI] [PubMed] [Google Scholar]
- 33.Pulkkinen V, Manson ML, Säfholm J, Adner M, Dahlén SE. The bitter taste receptor (TAS2R) agonists denatonium and chloroquine display distinct patterns of relaxation of the guinea pig trachea. Am J Physiol Lung Cell Mol Physiol. 2012;303:L956–L966. doi: 10.1152/ajplung.00205.2012. [DOI] [PubMed] [Google Scholar]