Abstract
Although the effects of fish oil supplements on airway inflammation in asthma have been studied with varying results, the independent effects of the fish oil components, eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA), administered separately, are untested. Here, we investigated airway inflammation and hyperresponsiveness using a mouse ovalbumin exposure model of asthma assessing the effects of consuming EPA (1.5% wt/wt), DHA (1.5% wt/wt), EPA plus DHA (0.75% each), or a control diet with no added omega-3 polyunsaturated fatty acids. Consuming these diets for 6 weeks resulted in erythrocyte membrane EPA contents (molar %) of 9.0 (± 0.6), 3.2 (± 0.2), 6.8 (± 0.5), and 0.01 (± 0.0)%; DHA contents were 6.8 (± 0.1), 15.6 (± 0.5), 12.3 (± 0.3), and 3.8 (± 0.2)%, respectively. The DHA group had the highest bronchoalveolar lavage (BAL) fluid eosinophil and IL-6 levels (P < 0.05). Similar trends were seen for macrophages, IL-4, and IL-13, whereas TNF-α was lower in omega-3 polyunsaturated fatty acid groups than the control (P < 0.05). The DHA group also had the highest airway resistance, which differed significantly from the EPA plus DHA group (P < 0.05), which had the lowest. Oxylipins were measured in plasma and BAL fluid, with DHA and EPA suppressing arachidonic acid–derived oxylipin production. DHA-derived oxylipins from the cytochrome P450 and 15-lipoxygenase pathways correlated significantly with BAL eosinophil levels. The proinflammatory effects of DHA suggest that the adverse effects of individual fatty acid formulations should be thoroughly considered before any use as therapeutic agents in asthma.
Keywords: rodents, eosinophils, inflammation, lipid mediators
Clinical Relevance
We found that consuming high dietary intake of the long-chain omega-3 polyunsaturated fatty acid, docosahexaenoic acid (DHA), can promote eosinophilia in lavage fluid, which is associated with high levels of IL-6 and IL-4 and decreased levels of TNF-α in the ovalbumin-induced mouse model of allergic asthma. Although both eicosapentaenoic acid and DHA can have distinct effects on anti-inflammatory pathways, our results indicate that providing DHA alone may promote inflammation in allergic asthma, providing further reason to examine the immune-modulating effects of these fatty acids independently. The proinflammatory effects of DHA suggest that individual fatty acid formulations should be thoroughly considered before use.
Fish oil and other supplements rich in long-chain omega-3 (n-3) polyunsaturated fatty acids (PUFAs), particularly eicosapentaenoic acid (EPA; C20:5n3) and docosahexaenoic acid (DHA; C22:6n3), have beneficial anti-inflammatory effects when used to treat some chronic inflammatory conditions (1). Human intervention trials in established asthma have shown some benefit, but overall the results are mixed (2–7). Although DHA and EPA can be interconverted, these two lipids also exert distinct effects, making the mechanistic interpretation of fish oil interventions difficult.
EPA and DHA impact inflammatory signaling by directly modulating the arachidonic acid (AA; C20:4n6) cascade at several points. EPA competitively inhibits the enzymatic pathways converting AA to 4-series leukotrienes (LT) and 2-series prostaglandines (PG), which play central roles in the maintenance of chronic inflammation (8, 9). EPA is also metabolized to less potent LT of the 5-series and PG of the 3-series (10). DHA attenuates AA metabolism by decreasing phospholipase A2 activity and inhibiting AA release from membrane phospholipids (4). DHA has been shown to decrease the responsiveness of Toll-like receptors to environmental stimuli (e.g., Toll-like receptor response to LPS). thereby decreasing NF-κB activation and transcription of AA metabolizing enzymes, including cyclo-oxygenase-2 (11, 12).
In addition to their direct impacts on AA metabolism, EPA and DHA (as well as AA) are transformed into a variety of inflammatory mediators, termed oxylipins, with a wide range of actions. Several enzymatic pathways are involved, including 5-lipoxygenase (LO), 12-LO, 15-LO, cyclo-oxygenase, cytochrome P450 (P450), and the soluble epoxide hydrolase. EPA is a precursor to the E-series of inflammatory resolution-phase interaction products (i.e., resolvins [Rvs], RvE1 and RvE2), which are potent anti-inflammatory mediators (13). RvE1, in particular, has beneficial effects in the mouse model of asthma (11, 14). Anti-inflammatory DHA-derived lipid mediators have also been identified, including the D-series RVs (RvD1, RvD2, RvD3, and RvD4), docosatrienes, and protectins (12, 15). It is believed that DHA is converted into these anti-inflammatory molecules in vivo by enzymatic pathways distinct from the biosynthesis of PG, LT, and lipoxins (13, 16–21). In subjects with asthma and healthy adults, protectin-D1 (PD1) is formed in vivo, and appears to reduce allergic airway inflammation and hyperresponsiveness (AHR), although, in asthma exacerbations, lower levels of PD1 were seen in breath condensate (21). Synthetic 17S-hydroxy-DHA, a PD1 precursor, decreases TNF-α release and arachidonate 5-LO gene expression in macrophages (22).
Taken together, these data suggest that increasing membrane EPA and DHA levels will have anti-inflammatory benefits in asthma, although each fatty acid may act via distinct pathways. The potential for differential benefit was emphasized by a recent study that found that EPA had a greater effect than DHA in reducing production of inflammatory mediators by human pulmonary macrophages treated ex vivo (23). In the present study using the ovalbumin (OVA)-induced murine model of allergic airway eosinophilia, we tested the hypothesis that dietary intake of EPA and DHA will differentially attenuate airway inflammation and AHR. Oxylipins were measured to determine the effects of diet on these mediators and to identify mediators that correlated with markers of inflammation.
Materials and Methods
Animals and Diets
C57BL/6 female mice were kept under specific pathogen-free conditions. Mice were randomly distributed into four groups, with three mice per cage, and fed either a diet enriched with 1.5% (wt/wt) EPA, 1.5% (wt/wt) DHA (Larodan Fine Chemicals, Malmö, Sweden), a combination of 0.75% EPA plus 0.75% DHA (EPA plus DHA), or a control diet without added n-3 fatty acids (see the online supplement). Body weight was monitored weekly.
Mouse Sensitization and Challenge Protocols
All mice were immunized at 6 weeks of age on Experiment Days 1 and 15. Each injection contained chicken egg OVA (10 μg, Grade V; Sigma Chemical Co., St. Louis, MO) and the adjuvant alum (1 mg; Sigma Chemical Co.) in 0.2 ml PBS, prepared as previously described (24). Starting on Day 29, mice were exposed to either an aerosol of 10 ml of a 10 mg/ml (1%) solution of OVA (OVA treatment) three times per week for a period of 2 weeks, or remained in their filtered cages (“air” treatment). Further details are provided in the online supplement.
Pulmonary Physiology, Whole-Lung Lavage, and Tissue Collection
Mice underwent methacholine (MCh) challenge using whole-body plethysmography (Buxco Inc., Troy, NY) before tissue harvest, as previously described (24). During this procedure, dynamic compliance and resistance of the respiratory system were measured on deeply anesthetized, tracheotomized, and ventilated mice. Mice were killed with an overdose of pentobarbital and phenytoin (Buthanasia-D Special; Schering-Plough Animal Health Corp., Union, NJ) for the collection of bronchoalveolar lavage (BAL) fluid and tissue samples (see the online supplement for more details).
Cytokine and Chemokine Analyses
Assessment of cytokine profiles in the BAL fluid was determined using multiplex luminescence detection systems (Bio-Rad [Hercules, CA] or Mesoscale Scale Discovery [Gaithersberg, MD]). All assays were performed using undiluted BAL samples according to the manufacturer's protocols. Cytokine concentrations were determined using Bioplex or Mesoscale Scale Discovery software (Bio-Rad) with four-parameter data analysis (see the online supplement for more details).
Fatty Acid Analysis from Red Blood Cells and Oxylipin Analysis from Plasma and BAL
Fatty acid level from red blood cells (RBCs) was assayed after extraction and isolation, followed by gas chromatography with mass spectral detection of transmethylated lipids. Extracted oxylipin from plasma and BAL were quantified by ultra-performance liquid chromatography with tandem mass spectrometry. Further details are provided in Table E2 and in the Materials and Methods in the online supplement.
Statistical Analysis
Immunologic and oxylipin data were analyzed for significant diet–treatment interactions using SigmaStat software (Systat Software Inc., San Jose, CA) by one- or two-way ANOVA (Tables E3–E5). Principal components analysis (PCA) was performed using imDEV 1.3.1 software (http://sourceforge.net/projects/imdev/) (25) and/or by PCA analysis using a second software package, SIMCA-P+ 12 (Umetrics, Umeå, Sweden). Dietary effects on RBC fatty acid composition were evaluated only by PCA. The percent change of airway physiology data were analyzed by two-way repeated-measures ANOVA with all pairwise comparisons (the two variables being diet and MCh challenge dose) for the air and OVA aerosol treatment groups separately (GraphPad Prism 5.0; GraphPad Inc., La Jolla, CA); see the online supplement for more details.
Results
Food Intake and Body Weights
No significant differences in food intake or body weights were observed among the four experimental diet groups (data not shown), as seen previously with similar diets containing purified EPA or DHA (26).
Dietary EPA and DHA Leads to Increased RBCs n-3 PUFAs
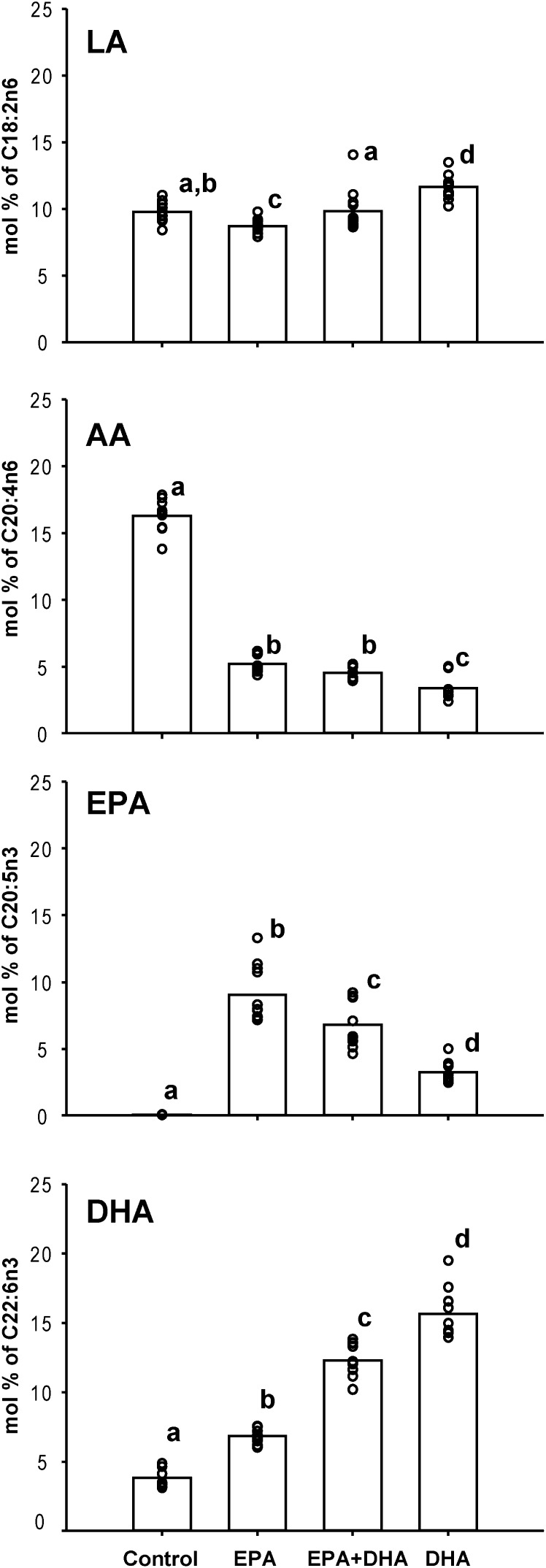
PCA demonstrated that mice had similar EPA, DHA, and n-3 or n-6 PUFA content, corresponding to their dietary interventions. The n-6:n-3 and EPA:DHA ratios drove separations in the first and second principal components, respectively (Figure E1). The RBC molar percentages of selected fatty acids are shown in Figure 1, with significant differences indicated. The linoleic acid (LA; C18:2n6) RBC percent in mice fed control diets was comparable to mice fed EPA plus DHA, significantly lower in mice fed solely EPA, and highest in DHA-fed mice. The RBCs from mice fed the control diet had the highest molar % AA, whereas mice fed the EPA-, EPA plus DHA–, or DHA-enriched diets showed less than one-third this percentage of AA (P < 0.05). We found only traces of EPA in the control diet group. However, as expected, the RBC membrane EPA abundance increased in each of the n-3–enriched diets with the EPA, EPA plus DHA, or DHA diets that strongly correlated with diet content (r2 = 0.99; P < 0.05). Although DHA was measurable in RBCs from control mice, this fatty acid was also enriched in RBCs as a function of diet enrichment (r2 = 0.99; P < 0.05). The ratio of AA to DHA plus EPA was highest in the control diet group at 4.26 (± 0.092) (mean [± SEM] of all mice). The EPA, EPA plus DHA, and DHA diet groups had much lower ratios, at 0.33 (± 0.096), 0.24 (± 0.092), and 0.18 (± 0.096), respectively (all differences significant by two-way ANOVA of rank data considering diet and OVA aerosol treatment). OVA treatment caused a slight increase in this ratio (P = 0.012) overall.
Figure 1.
Molar percentage of total fatty acid from linoleic acid (LA [C18:2n6]), arachidonic acid (AA [C20:4n6]), eicosapentaenoic acid (EPA [C20:5n3]), and docosahexaenoic acid (DHA [C22:6n3]) of red blood cells (RBCs) from mice fed different amounts of long-chain omega-6 (n-6) and n-3 polyunsaturated fatty acids (PUFAs). Bars indicate means, whereas data points represent results from individual mice (n = 11–12 per group). Superscripts indicate significant differences between the diet groups (P < 0.05).
Dietary DHA Increased OVA-induced Pulmonary Eosinophilic Inflammation
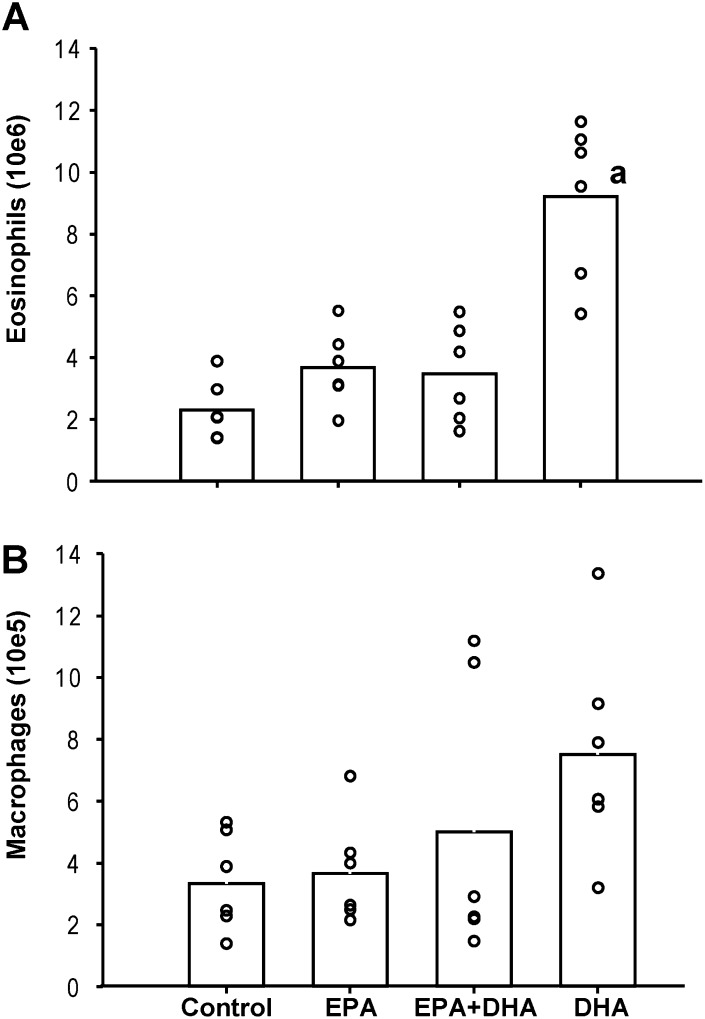
Aerosolized OVA exposure induced a roughly threefold elevation in pulmonary eosinophilia in the mice on the high-DHA diet compared with all other groups (Figure 2A), whereas there was no eosinophil count in mice exposed to filtered air. The mean (± SE) count of total eosinophils in the BAL of the OVA-exposed mice was highest under DHA diet compared with mice fed the mixed EPA plus DHA, only EPA, or control diet, respectively (P < 0.05; DHA vs. all other diet groups). In addition, the total number of macrophages tended to be higher (P = 0.11) in mice fed solely DHA (Figure 2B). The mean (± SE) count of total macrophages in the BAL of the air control mice was roughly 10-fold lower compared with the OVA group, with 118.4 (± 13.2) × 103, 91.5 (± 26.0) × 103, 158.3 (± 127.7) × 103, and 135.5 (± 28.5) × 103 in mice fed the control, EPA, mixed EPA plus DHA, or DHA-only diet, respectively.
Figure 2.
Total eosinophil and macrophage counts in bronchoalveolar lavage (BAL) from mice receiving ovalbumin (OVA) aerosol treatment. Bars represent means, and data points results from individual mice (n = 5 to 6). aGroup means significantly different from all other diet groups (P < 0.001).
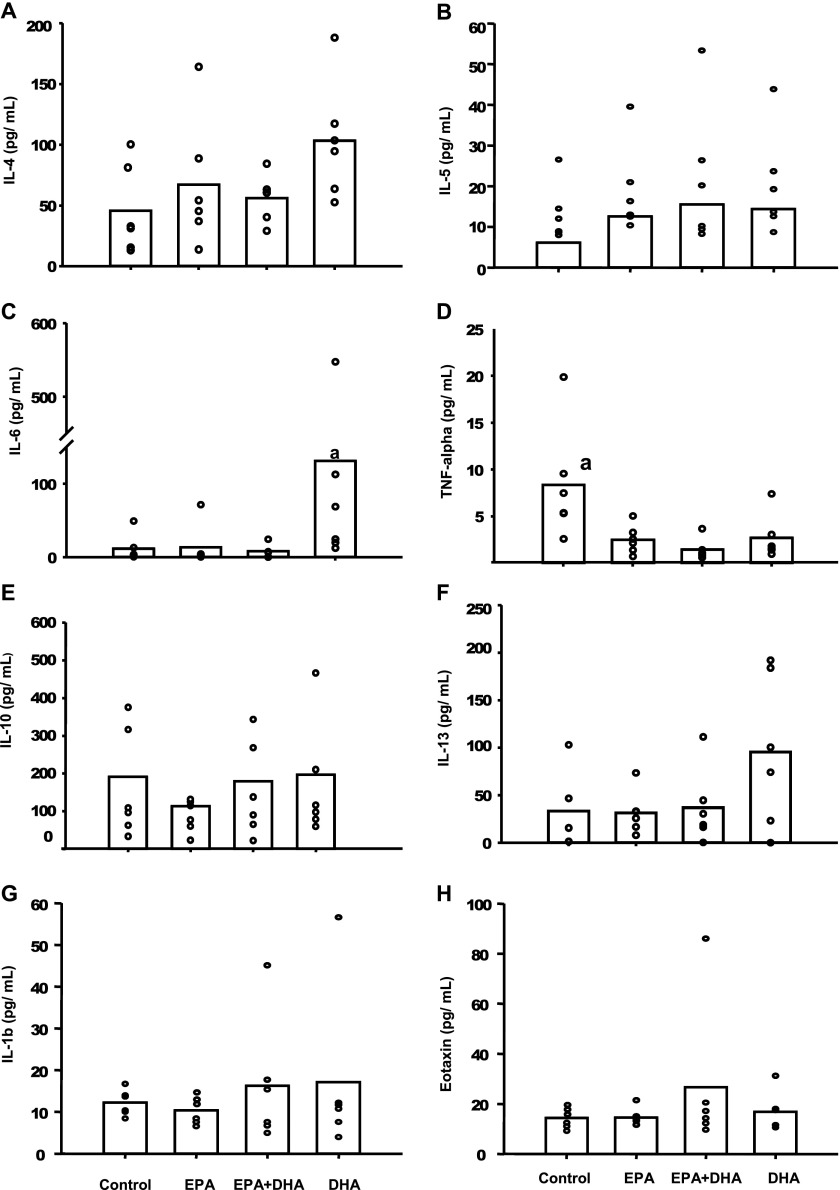
Cytokine concentrations were undetectable in most BAL specimens from mice receiving no OVA aerosol treatment (data not shown). All comparisons presented here are from mice exposed to nebulized OVA. The concentrations of IL-4 and IL-13 were slightly, but not significantly, greater in the DHA group compared with the control, EPA-only, and EPA plus DHA group, respectively (Figures 3A and 3F). Figure 3B shows that the mean IL-6 concentration in mice fed solely DHA was significantly higher, by 12-, 9.7-, and 17-fold, compared with mice fed the control diet, solely EPA, or both EPA plus DHA. Statistical significance (P < 0.05) was attained for mice fed EPA- and EPA plus DHA–enriched diets, as well as in the control group. In contrast, the mean TNF-α concentration in BAL fluid (Figure 3D) was significantly lower for all treatment groups compared with the control group (P < 0.05). No significant differences were seen for IL-5 (Figure 3B), IL-10 (Figure 4E), or eotaxin (Figure 3H).
Figure 3.
Cytokines in BAL from mice receiving OVA aerosol treatment. Bars indicate means, whereas data points represent results from individual mice (n = 5 to 6). aGroup means are significantly different from all other diet groups (P < 0.05).
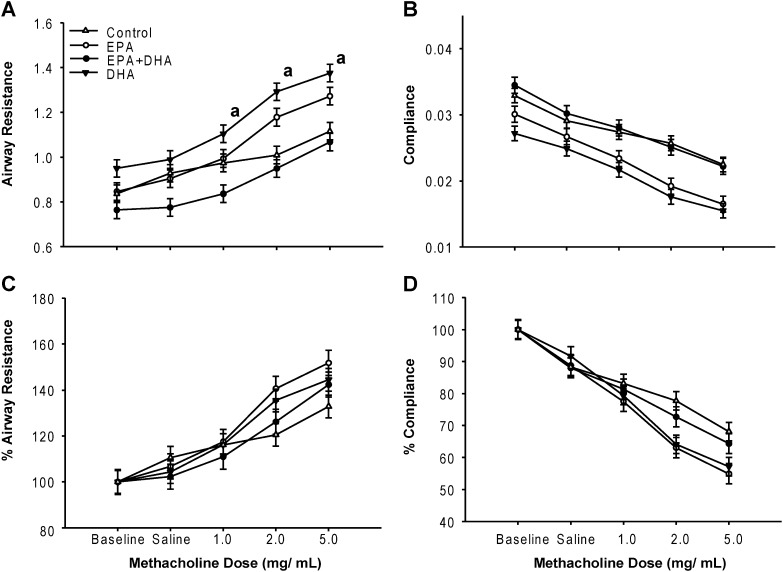
Figure 4.
Airway responsiveness and dynamic compliance in mice exposed to nebulized OVA. Mean values ± SEM for five to six female mice taken over 3 minutes during methacholine (MCh) administration (1.0, 2.0, and 5.0 mg/ml). (A) Resistance; (B) resistance as percent increase from baseline value (100%); (C) compliance; (D) compliance as percent decrease from baseline value.
Dietary DHA Increased Airway Resistance
We compared the total lung resistance and dynamic compliance at baseline after the inhalation of saline (vehicle) and serial doses of MCh (1.0, 2.0, and 5.0 mg/ml). Figure 4 shows the results from mice challenged with nebulized OVA. Mice fed DHA had significantly higher resistance measurements compared with mice fed EPA plus DHA. No significant differences were seen when resistance was expressed as a percentage from baseline (Figure 4C), nor for dynamic compliance and percent compliance (Figures 4B and 4D). We found some variability in airway resistance at baseline, probably due to different time intervals between OVA exposure and MCh challenge. Mice fed only DHA had the lowest values, followed by the EPA, the EPA plus DHA, and control groups, with minor variability. Only minor differences were found in airway resistance and compliance from control mice kept under filtered air when compared with those fed different amounts of n-3 and n-6 PUFAs (Figure E2).
Dietary DHA and EPA Increased EPA- and DHA-derived Oxylipins and Decreased AA-derived Oxylipins
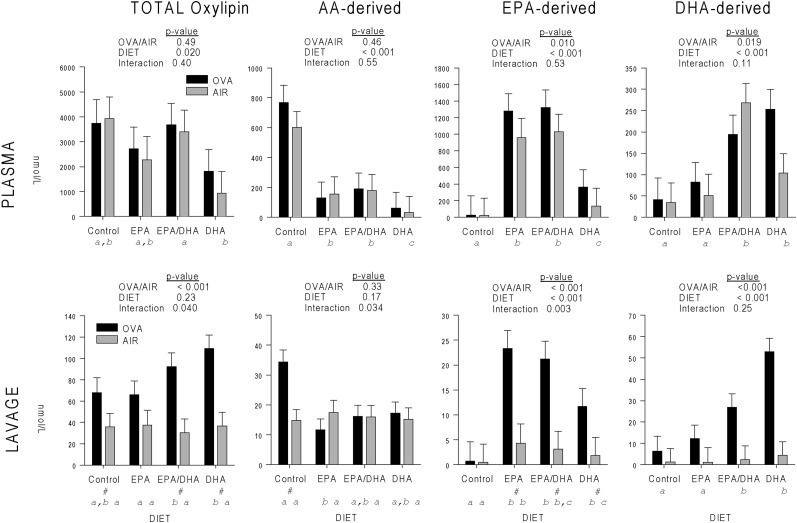
In plasma, the concentration of total oxylipins (i.e., the sum of plasma oxylipins derived from all fatty acid precursors—mainly from AA, EPA, DHA, α-linolenic acid [ALA, C18:3n3], and LA) was lowest in the DHA diet group (Figure 5A), which was significantly differente from the EPA plus DHA diet group . The concentration of AA-derived oxylipins was highest in the control diet group, intermediate in the EPA-containing diet groups, and lowest in the DHA group (Figure 5B). The plasma concentration of EPA-derived oxylipins was highest in the two EPA-containing diet groups, significantly lower in the DHA group, and the control group showed significant differences compared with all other diet groups (Figure 5C). The plasma concentration of DHA-derived plasma oxylipins was significant higher in the two DHA-containing diet groups compared with the control and EPA-only diet groups (Figure 5D).
Figure 5.
Mean ± SEM of total oxylipin concentrations analyzed by two-way ANOVA for OVA/filtered air (AIR; treatment) and diet group (DIET) interaction. Means are shown for total (TOTAL), AA-derived (AA), EPA-derived (EPA), and DHA-derived (DHA) oxylipins. Different lowercase letters indicate significant difference (P < 0.05) between diet groups; #significant difference (P < 0.05) for DIET and OVA or AIR treatment.
In BAL, diet did not affect total oxylipins in the air control animals, but, in the mice undergoing aerosol OVA treatment, total oxylipins were significantly higher in the two DHA-containing diets than in the EPA diet group (Figure 5E). Similarly, diet did not affect AA-derived oxylipins in the air control mice. In the OVA-treated mice, AA-derived oxylipins were significantly higher in the control than in the EPA diet groups, although they were not different from the two DHA-containing diets (Figure 5F). In the air control group, EPA metabolites were significantly lower in the control diet group compared with the omega-3/6 PUFA–containing diets groups. OVA treatment caused the highest levels of EPA-derived oxylipins in both EPA-containing diet groups, which were significant compared with the control, but not the DHA diet group (Figure 5G). DHA-derived oxylipins were significantly higher in the two DHA-containing diets than in the EPA and control diets (Figure 5H).
In summary, the DHA diet lowered total oxylipin levels in plasma, but tended to produce higher total and DHA-derived oxylipin levels in BAL during pulmonary inflammation. Levels of AA-derived oxylipins in BAL were suppressed by both EPA and DHA during pulmonary inflammation. Data for 5- and 15-hydroperoxy eicosatetraenoic acid are not included in Figure 5, as they were quantified as peak areas rather than concentration (Tables E3–E5).
Pulmonary Inflammation Increased Most BAL Oxylipins and a Subset of Plasma Oxylipins
In plasma, OVA aerosol treatment increased the individual concentrations of four oxylipins—two derived from EPA and two from DHA—and decreased the concentration of five oxylipins, all derived from AA (Table E3). Five of these nine oxylipins were epoxides or their corresponding dihydroxy hydrolysis products, the highest percentage of change seen among the six pathways (Table 1 and Table E3). In contrast, 69% of all oxylipins were elevated by OVA treatment in BAL fluid (only 3% were decreased), with the P450 pathway having the highest level of increased or decreased metabolites (Table 1). In summary, OVA treatment increased the systemic levels of epoxides and diols derived from EPA and DHA, and decreased the systemic levels of AA-derived oxylipins. Most oxylipin pathways were activated at the site of inflammation, with the DHA-derived oxylipins showing increases across all diet groups.
Table 1:
Oxylipins in Specific Pathways with Concentrations Altered by Aerosol Ovalbumin Treatment*
| Plasma |
Bronchoalveolar Lavage |
|||||||
|---|---|---|---|---|---|---|---|---|
| |
Higher with OVA |
Lower with OVA |
Higher or Lower |
Total† |
Higher with OVA |
Lower with OVA |
Higher or Lower |
Total† |
| Oxylipin Pathway | n (%) | n (%) | n (%) | n | n (%) | n (%) | n (%) | n |
| 5-LO | 0 | 1 (17) | 1 (17) | 6 | 5 (56) | 0 | 5 (56) | 9 |
| 12-LO | 0 | 0 | 0 | 5 | 4 (50) | 1 (13) | 4 (63) | 8 |
| 15- LO | 0 | 1 (10) | 1 (10) | 10 | 13 (76) | 0 | 13 (76) | 17 |
| COX | 0 | 1 (14) | 1 (14) | 7 | 5 (71) | 1 (14) | 6 (85) | 7 |
| P450 | 1 (8) | 0 | 1 (8) | 12 | 10 (100) | 0 | 10 (100) | 10 |
| P450/EH | 3 (33) | 2 (22) | 5 (55) | 9 | 6 (55) | 0 | 6 (55) | 11 |
| Total | 4 (8) | 5 (10) | 9 (18) | 49 | 43 (69) | 2 (3) | 45 (72) | 62 |
| P value‡ | 0.073 | 0.59 | 0.056 | 0.15 | 0.26 | 0.16 | ||
Definition of abbreviations: COX, cyclo-oxygenase; DHA, docosahexaenoic acid; EH, epoxide hydrolase; EPA, eicosapentaenoic acid; LO, lipoxygenase; OVA, ovalbumin.
Concentrations of individual oxylipins and parent fatty acids are shown in Tables E2 and E4.
12(13)-epoxy-9-keto octadecenoic acid (EKODE), 9-hydroxy eicosatetraenoic acid (HETE), and 11-HETE are not included.
Chi-squared comparison among the six pathways.
Total number of analytes.
DHA-Derived Oxylipins in Plasma and BAL Show Strongest Correlation with BAL Eosinophils
Because pulmonary eosinophilia is an indicator of disease severity in this model, we performed a correlation analysis of oxylipins with BAL eosinophil levels in the OVA-treated mice to identify oxylipins that were elevated (or depressed) in association with eosinophilia. Three plasma oxylipins (Table 2) correlated positively with BAL eosinophils, including two P450-dependent DHA metabolites, 19,20-dihydroxydocosahexaenoic acid and 16(17)-epoxydocosapentaenoic acid, whereas 13 AA-derived plasma oxylipins correlated negatively with BAL eosinophils. Of the 10 BAL oxylipins that correlated significantly with BAL eosinophil levels, 8 had positive and 2 had negative associations (Table 3). Four DHA oxylipins showed positive correlations, including 19,20-dihydroxydocosahexaenoic acid and 16(17)-epoxydocosapentaenoic acid. 5,6- and 11,12 dihydroxy eicosatrienoic acid (DiHETrE) found in BAL showed a negative correlation (Table 3).
Table 2:
Plasma Oxylipins Significantly Correlated with Bronchoalveolar Lavage Eosinophils (Ovalbumin Group Only)
| Oxylipin | Pathway | Parent Lipid | R* | P Value |
|---|---|---|---|---|
| 19,20-DiHDoPE | P450/sEH | DHA | 0.567 | 0.005 |
| 9,10-DiHOME | P450/sEH | LA | 0.553 | 0.006 |
| 16 (17)-EpDPE | P450 | DHA | 0.500 | 0.015 |
| 5-KETE | 5-LO | AA | −0.454 | 0.030 |
| PGD2 | COX | AA | −0.460 | 0.027 |
| 11-HETE | AUTO-OX | AA | −0.470 | 0.024 |
| LTE4 | 5-LO | AA | −0.522 | 0.011 |
| 12-HETE | 12-LO | AA | −0.540 | 0.008 |
| 8-HETE | 15-LO | AA | −0.540 | 0.004 |
| 15-HETE | 15-LO | AA | −0.554 | 0.006 |
| 5-HETE | 5-LO | AA | −0.568 | 0.005 |
| 8,9-DiHETrE | P450/sEH | AA | −0.616 | 0.002 |
| 5,6-DiHETrE | P450/sEH | AA | −0.625 | 0.015 |
| 14 (15)-EpETrE | P450 | AA | −0.656 | 0.0006 |
| 8 (9)-EpETrE | P450 | AA | −0.667 | 0.0005 |
| 11 (12)-EpETrE | P450 | AA | −0.683 | 0.0003 |
Definition of abbreviations: AA, arachidonic acid; AUTO-OX, auto oxidation; COX, cyclo-oxygenase; DHA, docosahexaenoic acid; DiHDoPE, dihydroxydocosahexaenoic acid; DiHETrE, dihydroxy eicosatrienoic acid; DiHOME, dihydroxy octadecenoic acid; EpDPE, epoxydocosapentaenoic acid; EpETrE, epoxy eicosatrienic acid; HETE, hydroxy eicosatetraenoic acid; KETE, keto eicosatetraenoic acid; LA, linoleic acid; LO, lipoxygenase; LTE, leukotriene E4; PGD2, prostaglandine D2; sEH, soluble epoxide hydrolase.
Spearman correlation coefficient.
Table 3:
Bronchoalveolar Lavage Oxylipins Significantly Correlated with Bronchoalveolar Lavage Eosinophils (Ovalbumin Group Only)
| Oxylipin | Pathway | Parent Lipid | R* | P Value |
|---|---|---|---|---|
| 19,20-DiHDoPE | P450/sEH | DHA | 0.684 | 0.0003 |
| 17-HDoHE | 15-LO | DHA | 0.623 | 0.002 |
| 19 (20)-EpDPE | P450 | DHA | 0.587 | 0.003 |
| 16 (17)-EpDPE | P450 | DHA | 0.560 | 0.006 |
| 17,18-DiHETE | P450/sEH | EPA | 0.497 | 0.016 |
| 15-HpETE | 15-LO | AA | 0.488 | 0.018 |
| EKODE | LO | LA | 0.440 | 0.036 |
| 20-hydroxy-LTB4 | 5-LO | AA | 0.434 | 0.038 |
| 11,12-DiHETrE | P450/sEH | AA | −0.425 | 0.043 |
| 5,6-DiHETrE | P450/hydrolysis | AA | −0.438 | 0.037 |
Definition of abbreviations: AA, arachidonic acid; DHA, docosahexaenoic acid; DiHDoPE, dihydroxydocosahexaenoic acid; DiHETE, dihydroxy eicosatetraenoic acid; DiHETrE, dihydroxy eicosatrienoic acid; EKODE, 12(13)epoxy-9-keto octadecenoic acid; EPA, eicosapentaenoic acid; EpDPE, epoxydocosapentaenoic acid; HDoHE, hydroxy docosahexaenoic acid; HpETE, hydroperoxy eicosatetraenoic acid; KETE, oxo eicosatetraenoic acid; LA, linoleic acid; LO, lipoxygenase; LTB4, leukotriene B4; sEH, soluble epoxide hydrolase.
Spearman correlation coefficient.
Discussion
There is conflicting evidence regarding the use of long-chain n-3 PUFAs, particularly EPA and DHA, for the prevention and treatment of allergic diseases, including asthma (2, 3, 6, 7, 16, 27) Asthma is characterized by airway infiltration of eosinophils, T cells, and mast cells, with structural changes in smooth muscle and episodes of excessive mucus production, which, together, contribute to the development of airway narrowing (28). We used the OVA mouse model of eosinophilic pulmonary inflammation and AHR to evaluate the impact of dietary EPA and DHA separately and in combination. To the best of our knowledge, this is the first such study investigating the effects of these distinct diets using a mouse model of asthma. We hypothesized that EPA, DHA, and the two together would attenuate AHR, eosinophilia, and inflammatory cytokine production. In contrast to our hypothesis, we found that diets enriched with either DHA or EPA produced worse symptoms (AHR) than did both EPA and DHA together or the control diet, although only the difference between the DHA and EPA plus DHA diets was statistically significant. The DHA diet also produced higher levels of BAL eosinophils and IL-6 than the other groups (with similar trends seen for BAL macrophages, IL-4, and IL-13) confirming that the underlying inflammatory response was also exacerbated in addition to AHR. Thus, DHA exacerbated both inflammation and AHR in this model. Yin and coworkers (29) also reported adverse effects of fish oil supplementation in a murine model of OVA-induced allergic airway inflammation. They found significant increase of proinflammatory cytokines, IL5 and IL-13, in lung extracts. This is in agreement with our finding of significantly increased IL-13 BAL levels in solely DHA fed mice compared with all other diet groups. In addition, we found a slight increase of IL-5 in all n-3 diet groups, although no significance was reached (data not shown and Figure E3). Fish oil feeding suppressed the production of pulmonary protective PGE2 in BAL. We also found a profound EPA- and DHA-mediated decrease of AA-derived PGE2, but also of prostaglandine (PG) D2 and PGF2a., in BAL, and an increase of EPA-derived PGE3 (Table E3). The 3-series prostanoids, among them, PGE3, are considered less inflammatory than those derived from AA (30). However, our study suggests that a reduced PGE2:PGE3 ratio is not necessarily beneficial in allergic airway inflammation.
These results suggest proinflammatory effects of dietary DHA, which contrasts with prior findings using pharmacologic treatment (rather than diet) with DHA-glycerol derivatives and the DHA-derived metabolite, PD-1. Yokoyama and colleagues (31) treated mice with aerosolized tri-DHA glycerol before each aerosol exposure to OVA, finding reduced BAL eosinophilia and AHR. Similarly, Morin and coauthors treated mice orally with a DHA (32) or EPA monoglyceride (33) before OVA immunization and aerosol treatment in an OVA guinea pig asthma model. They found, in both studies, decreased pulmonary eosinophilia, cytokine levels, IgE response, and reduced MCh-induced bronchial AHR and inflammation. Levy and colleagues (21) found that intravenous administration of DHA-derived PD-1 before OVA treatment led to reduced levels of eosinophils and specific proinflammatory mediators in the BAL, and reduced AHR. None of these studies presented information on the fatty acid status of the animals or the composition of the diets, making it difficult to compare with our results. In general, however, these interventions were focused on pharmacologic interventions with DHA derivatives or metabolites, and thus differ from dietary interventions where DHA is incorporated into cellular membranes and the availability for oxylipin production depends on cell type. Similarly, studies with the EPA metabolite, RevE1 (11, 14), showed protection not seen in our dietary intervention with the parent fatty acid, reinforcing the conclusion that pharmacologic interventions with purified mediators may well differ in their results from dietary studies with the parent fatty acid.
Although only a few dietary studies have been done in a mouse model of asthma with long-chain n-3 PUFA, one study evaluated the effect of increasing EPA and DHA tissue levels using the Fat-1 transgenic mouse model (34). Fat-1 mice express a Caenorhabditis elegans desaturase that converts long-chain n-6 to n-3 fatty acids, leading to an abundance of n-3 PUFAs at the expense of n-6 PUFA. In contrast to our study, BAL eosinophil levels and AHR were both lower in the Fat-1 than wild-type control mice. Dietary information was not provided for the Fat-1 and control mice, but lung fatty acid levels were measured showing that EPA and DHA levels, but not AA levels, were higher in the Fat-1 mice, with the ratio of AA to EPA plus DHA being reduced from 6.3 in the control mice to 1.6 in the Fat-1 mice. Mice fed the control diet in our study had a somewhat lower ratio of 4.3, with much lower ratios (0.2–0.3) in the n-3 diet groups. Thus, our study started with a lower ratio in the control group, raising the question of whether our control animals might have produced different results with higher baseline AA levels.
Our study is the first to report adverse effects of dietary DHA in this asthma model; others have also reported that long-chain n-3 PUFA may exacerbate rather than attenuate inflammation caused by different agents. Some of these examples (35, 36) involve infections where the anti-inflammatory response may impede clearance of a pathogen, and thus increase severity of disease, but others do not. For example, using the IL-10 knockout mouse model of colitis, Hegazi and coauthors (37) found that dietary fish oil containing both EPA and DHA (1.9% of the diet by weight) increases the incidence of severe colitis (based on histopathology scores). Woodworth and coauthors (38) also found that diets containing from 2.25 to 6.0% EPA plus DHA by weight (with an 11-fold excess of DHA over EPA) exacerbate colitis in a mouse model using Helicobacter hepaticus infection.
Alternatively, eosinophilic pulmonary inflammation is mediated by T helper type (Th) 2 cells (39) and dietary fish oil enhances Th2 development (40). Thus, enhanced Th2 development could explain the enhanced inflammatory response seen in the DHA group in this study. DHA may have a greater Th2-enhancing effect than EPA, as DHA can act as an agonist for retinoid X receptor (RXR)-mediated gene expression (41), and we have previously shown that stimulation of the RXR pathway enhances Th2 development ex vivo (42). If EPA antagonized this enhancement, it could explain why EPA appeared to moderate the proinflammatory effect of DHA in the present study, while not having a similar effect relative to the control diet group. Specific EPA metabolites could have such activity. However, because none of the EPA-derived oxylipins analyzed here from plasma or BAL showed negative correlations with BAL eosinophil levels, as would be expected of a compound with anti-Th2 activity, it may be that other, uncharacterized metabolites account for this effect, or it is also possible that levels in lymphoid tissue may differ from those seen in plasma or BAL. Alternatively, inclusion of EPA together with DHA in the diet may sufficiently decrease levels of DHA metabolites that mediate this postulated Th2-enhancing effect of dietary DHA (perhaps one of those that correlated positively with BAL eosinophil levels). This idea is supported by the apparent decrease in DHA-derived oxylipins in BAL fluid in the EPA plus DHA diet group relative to the DHA diet group (see Figure 5H), although this difference was not statistically significant. In summary, EPA has an apparent anti-inflammatory effect relative to the DHA diet group, but not relative to the control diet group. We do not have a clear mechanistic explanation for this association, but speculate that a Th2-enhancing effect of DHA metabolites is counteracted by EPA by decreasing production of such a metabolite or by otherwise inhibiting its activity.
Considered together, these studies support the idea that inclusion of EPA and DHA in murine diets at the 0.75–1.50% by weight (as used in the present study) or higher may cause adverse effects that result in exacerbated rather than attenuated inflammation. These dietary levels resulted in RBC fatty acid levels of from 3% (DHA diet group) to 9% for EPA (EPA diet group) and 7% (EPA diet group) to 16% for DHA (DHA diet group), with intermediate molar % for the EPA plus DHA diet group. These levels are higher than expected for the U.S. population. For example, mean RBC EPA and DHA levels of 0.40 (±0.25) and 3.2 (±1.60)%, respectively, were reported from a recent study of healthy African Americans with low n-3 PUFA intake: 1.8 (±1.1) g/day n-3 PUFAs; participants consumed no more than two servings of fish per week, and did not use n-3 PUFA supplements (43). However, RBC levels from individual subjects consuming their customary diet of from 0.2 to 9.6% (median = 2.6%) for EPA, and from 1.6 to 10.3% (median = 7.3%) for DHA, were also recently reported from Yup’ik Eskimos who have daily intakes of EPA (4.1 ± 0.5 g/d) and DHA (2.8 ± 0.3 g/d) that are 20-fold greater than the general U.S. population (43). Although the tissue levels of EPA and DHA in the present mouse study are high, they are within the range of values seen in human populations (i.e., Yup’ik Eskimos) with very high dietary EPA and DHA intake.
The EPA and DHA dietary treatments in this study did have some anti-inflammatory activity. All three diet groups containing EPA or DHA reduced BAL TNF-α concentrations relative to the control group. TNF-α plays a role in inflammatory responses of asthmatic airways (44), and may be an important factor in the development of severe refractory asthma as opposed to mild or moderate asthma (45), and TNF-α inhibitors, including etanercept and infliximab, are in clinical use (45, 46). Concordant with our study, another recent study showed that EPA and DHA can reduce TNF-α in LPS-stimulated primary alveolar macrophages from patients with asthma (23), with EPA being a more potent inhibitor than DHA. This is in line with our current findings using a mouse model of OVA-induced allergic asthma in this study. However, these finding suggest that certain patients with severe refractory asthma may benefit from modest dietary supplementation of n-3 PUFA.
In summary, we report that DHA fed at 1.5% of the diet exacerbated eosinophilic pulmonary inflammation, a finding that was surprising, but consistent with some other reports in murine models of inflammation. DHA intake is generally quite safe (47), and consideration is being given to developing dietary reference intakes for EPA and DHA with the aim of mitigating the adverse health effects of certain chronic inflammatory diseases, including cardiovascular disease (48). However, all inflammatory diseases are not equal. Protective inflammation in response to infection or vaccination varies by the type of pathogen, and the inflammation underlying cardiovascular disease differs substantially from the inflammation underlying asthma. Recent work indicates that even a single disease, such as asthma, may have multiple underlying immunologic phenotypes (49). It is thus important to consider how immune-modulating nutrients, such as DHA, affect different types of inflammation to make specific recommendations that could lead to improved health and avoid unanticipated adverse effects. Animal studies to examine the effect of such supplements on immune mechanisms are an important part of this overall research approach. Further work based on this model should focus on dose–response studies that would more closely approximate the tissue levels of EPA and DHA that could be achieved with diet or supplement use in human intervention trials starting at the relatively low levels seen in the U.S. population, while extending to the high, but plausible, levels reported here.
Acknowledgments
Acknowledgments
The authors thank Lisa Franzi, Angela Linderholm, Teresa Wegesser, Keisha Williams, Bill Keyes, Andrew Goose, and Debra Standridge for expert technical assistance, and Ben Belda for critical reading of the manuscript.
Footnotes
This work was supported by CHNR-grant to Nutrition Department (Vitamin Settlement Case Study contract 06-000427), National Institutes of Health grants HL105573, 1 U24 DK097154, and NIGMS T32-GM008799, Asthma America Foundation 09-0269, and U.S. Department of Agriculture CRIS projects 5306-51530-006-00D and 5306-51530-19-00D.
The United States Department of Agriculture is an equal opportunity provider and employer.
This article has an online supplement, which is accessible from this issue’s table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1165/rcmb.2013-0136OC on October 17, 2013
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Galli C, Calder PC. Effects of fat and fatty acid intake on inflammatory and immune responses: a critical review. Ann Nutr Metab. 2009;55:123–139. doi: 10.1159/000228999. [DOI] [PubMed] [Google Scholar]
- 2.Anandan C, Nurmatov U, Sheikh A. Omega 3 and 6 oils for primary prevention of allergic disease: systematic review and meta-analysis. Allergy. 2009;64:840–848. doi: 10.1111/j.1398-9995.2009.02042.x. [DOI] [PubMed] [Google Scholar]
- 3.Klemens CM, Berman DR, Mozurkewich EL. The effect of perinatal omega-3 fatty acid supplementation on inflammatory markers and allergic diseases: a systematic review. BJOG. 2011;118:916–925. doi: 10.1111/j.1471-0528.2010.02846.x. [DOI] [PubMed] [Google Scholar]
- 4.Martin RE. Docosahexaenoic acid decreases phospholipase A2 activity in the neurites/nerve growth cones of PC12 cells. J Neurosci Res. 1998;54:805–813. doi: 10.1002/(SICI)1097-4547(19981215)54:6<805::AID-JNR8>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 5.Mihrshahi S, Peat JK, Webb K, Oddy W, Marks GB, Mellis CM CAPS Team. Effect of omega-3 fatty acid concentrations in plasma on symptoms of asthma at 18 months of age. Pediatr Allergy Immunol. 2004;15:517–522. doi: 10.1111/j.1399-3038.2004.00187.x. [DOI] [PubMed] [Google Scholar]
- 6.Raviv S, Smith LJ. Diet and asthma. Curr Opin Pulm Med. 2010;16:71–76. doi: 10.1097/MCP.0b013e3283323b73. [DOI] [PubMed] [Google Scholar]
- 7.Reisman J, Schachter HM, Dales RE, Tran K, Kourad K, Barnes D, Sampson M, Morrison A, Gaboury I, Blackman J. Treating asthma with omega-3 fatty acids: where is the evidence? A systematic review. BMC Complement Altern Med. 2006;6:26. doi: 10.1186/1472-6882-6-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kelley DS, Rudolph IL. Effect of individual fatty acids of omega-6 and omega-3 type on human immune status and role of eicosanoids. Nutrition. 2000;16:143–145. doi: 10.1016/s0899-9007(99)00256-7. [DOI] [PubMed] [Google Scholar]
- 9.Stulnig TM. Immunomodulation by polyunsaturated fatty acids: mechanisms and effects. Int Arch Allergy Immunol. 2003;132:310–321. doi: 10.1159/000074898. [DOI] [PubMed] [Google Scholar]
- 10.Schmitz G, Ecker J. The opposing effects of n-3 and n-6 fatty acids. Prog Lipid Res. 2008;47:147–155. doi: 10.1016/j.plipres.2007.12.004. [DOI] [PubMed] [Google Scholar]
- 11.Aoki H, Hisada T, Ishizuka T, Utsugi M, Ono A, Koga Y, Sunaga N, Nakakura T, Okajima F, Dobashi K, et al. Protective effect of resolvin E1 on the development of asthmatic airway inflammation. Biochem Biophys Res Commun. 2010;400:128–133. doi: 10.1016/j.bbrc.2010.08.025. [DOI] [PubMed] [Google Scholar]
- 12.Serhan CN. Novel chemical mediators in the resolution of inflammation: resolvins and protectins. Anesthesiol Clin. 2006;24:341–364. doi: 10.1016/j.atc.2006.01.003. [DOI] [PubMed] [Google Scholar]
- 13.Serhan CN. Novel eicosanoid and docosanoid mediators: resolvins, docosatrienes, and neuroprotectins. Curr Opin Clin Nutr Metab Care. 2005;8:115–121. doi: 10.1097/00075197-200503000-00003. [DOI] [PubMed] [Google Scholar]
- 14.Haworth O, Cernadas M, Yang R, Serhan CN, Levy BD. Resolvin E1 regulates interleukin 23, interferon-gamma and lipoxin A4 to promote the resolution of allergic airway inflammation. Nat Immunol. 2008;9:873–879. doi: 10.1038/ni.1627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chiang N, Serhan CN. Cell–cell interaction in the transcellular biosynthesis of novel omega-3–derived lipid mediators. Methods Mol Biol. 2006;341:227–250. doi: 10.1385/1-59745-113-4:227. [DOI] [PubMed] [Google Scholar]
- 16.Mukherjee PK, Marcheselli VL, Serhan CN, Bazan NG. Neuroprotectin D1: a docosahexaenoic acid–derived docosatriene protects human retinal pigment epithelial cells from oxidative stress. Proc Natl Acad Sci USA. 2004;101:8491–8496. doi: 10.1073/pnas.0402531101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lukiw WJ, Cui JG, Marcheselli VL, Bodker M, Botkjaer A, Gotlinger K, Serhan CN, Bazan NG. A role for docosahexaenoic acid–derived neuroprotectin D1 in neural cell survival and Alzheimer disease. J Clin Invest. 2005;115:2774–2783. doi: 10.1172/JCI25420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hong S, Lu Y, Yang R, Gotlinger KH, Petasis NA, Serhan CN. Resolvin D1, protectin D1, and related docosahexaenoic acid–derived products: analysis via electrospray/low energy tandem mass spectrometry based on spectra and fragmentation mechanisms. J Am Soc Mass Spectrom. 2007;18:128–144. doi: 10.1016/j.jasms.2006.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Serhan CN, Hong S, Gronert K, Colgan SP, Devchand PR, Mirick G, Moussignac RL. Resolvins: a family of bioactive products of omega-3 fatty acid transformation circuits initiated by aspirin treatment that counter proinflammation signals. J Exp Med. 2002;196:1025–1037. doi: 10.1084/jem.20020760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Serhan CN. Lipoxins and aspirin-triggered 15-epi-lipoxin biosynthesis: an update and role in anti-inflammation and pro-resolution. Prostaglandins Other Lipid Mediat. 2002;68-69:433–455. doi: 10.1016/s0090-6980(02)00047-3. [DOI] [PubMed] [Google Scholar]
- 21.Levy BD, Kohli P, Gotlinger K, Haworth O, Hong S, Kazani S, Israel E, Haley KJ, Serhan CN. Protectin D1 is generated in asthma and dampens airway inflammation and hyperresponsiveness. J Immunol. 2007;178:496–502. doi: 10.4049/jimmunol.178.1.496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.González-Périz A, Planagumà A, Gronert K, Miquel R, López-Parra M, Titos E, Horrillo R, Ferré N, Deulofeu R, Arroyo V, et al. Docosahexaenoic acid (DHA) blunts liver injury by conversion to protective lipid mediators: protectin D1 and 17S-hydroxy-DHA. FASEB J. 2006;20:2537–2539. doi: 10.1096/fj.06-6250fje. [DOI] [PubMed] [Google Scholar]
- 23.Mickleborough TD, Tecklenburg SL, Montgomery GS, Lindley MR. Eicosapentaenoic acid is more effective than docosahexaenoic acid in inhibiting proinflammatory mediator production and transcription from LPS-induced human asthmatic alveolar macrophage cells. Clin Nutr. 2009;28:71–77. doi: 10.1016/j.clnu.2008.10.012. [DOI] [PubMed] [Google Scholar]
- 24.Schuster GU, Kenyon NJ, Stephensen CB. Vitamin A deficiency decreases and high dietary vitamin A increases disease severity in the mouse model of asthma. J Immunol. 2008;180:1834–1842. doi: 10.4049/jimmunol.180.3.1834. [DOI] [PubMed] [Google Scholar]
- 25.Grapov D, Newman JW. imDEV: a graphical user interface to r multivariate analysis tools in microsoft excel. Bioinformatics. 2012;28:2288–2290. doi: 10.1093/bioinformatics/bts439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fedor DM, Adkins Y, Mackey BE, Kelley DS. Docosahexaenoic acid prevents trans-10, cis-12–conjugated linoleic acid–induced nonalcoholic fatty liver disease in mice by altering expression of hepatic genes regulating fatty acid synthesis and oxidation. Metab Syndr Relat Disord. 2012;10:175–180. doi: 10.1089/met.2011.0113. [DOI] [PubMed] [Google Scholar]
- 27.Stephensen CB. Fish oil and inflammatory disease: is asthma the next target for n-3 fatty acid supplements? Nutr Rev. 2004;62:486–489. doi: 10.1111/j.1753-4887.2004.tb00021.x. [DOI] [PubMed] [Google Scholar]
- 28.Lemanske RF, Jr, Busse WW. Asthma: clinical expression and molecular mechanisms. J Allergy Clin Immunol. 2010;125(2, Suppl 2):S95–S102. doi: 10.1016/j.jaci.2009.10.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yin H, Liu W, Goleniewska K, Porter NA, Morrow JD, Peebles RS., Jr Dietary supplementation of omega-3 fatty acid–containing fish oil suppresses F2-isoprostanes but enhances inflammatory cytokine response in a mouse model of ovalbumin-induced allergic lung inflammation. Free Radic Biol Med. 2009;47:622–628. doi: 10.1016/j.freeradbiomed.2009.05.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Calder PC. n-3 polyunsaturated fatty acids, inflammation, and inflammatory diseases. Am J Clin Nutr. 2006;83(6 Suppl):1505S–1519S. doi: 10.1093/ajcn/83.6.1505S. [DOI] [PubMed] [Google Scholar]
- 31.Yokoyama A, Hamazaki T, Ohshita A, Kohno N, Sakai K, Zhao GD, Katayama H, Hiwada K. Effect of aerosolized docosahexaenoic acid in a mouse model of atopic asthma. Int Arch Allergy Immunol. 2000;123:327–332. doi: 10.1159/000053645. [DOI] [PubMed] [Google Scholar]
- 32.Morin C, Fortin S, Cantin AM, Rousseau E. Docosahexaenoic acid derivative prevents inflammation and hyperreactivity in lung: implication of PKC-potentiated inhibitory protein for heterotrimeric myosin light chain phosphatase of 17 kD in asthma. Am J Respir Cell Mol Biol. 2011;45:366–375. doi: 10.1165/rcmb.2010-0156OC. [DOI] [PubMed] [Google Scholar]
- 33.Morin C, Fortin S, Cantin AM, Rousseau É. Mag-epa resolves lung inflammation in an allergic model of asthma. Clin Exp Allergy. 2013;43:1071–1082. doi: 10.1111/cea.12162. [DOI] [PubMed] [Google Scholar]
- 34.Bilal S, Haworth O, Wu L, Weylandt KH, Levy BD, Kang JX. Fat-1 transgenic mice with elevated omega-3 fatty acids are protected from allergic airway responses. Biochim Biophys Acta. 2011;1812:1164–1169. doi: 10.1016/j.bbadis.2011.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Anderson M, Fritsche KL. (n-3) Fatty acids and infectious disease resistance. J Nutr. 2002;132:3566–3576. doi: 10.1093/jn/132.12.3566. [DOI] [PubMed] [Google Scholar]
- 36.Bonilla DL, Fan YY, Chapkin RS, McMurray DN. Transgenic mice enriched in omega-3 fatty acids are more susceptible to pulmonary tuberculosis: impaired resistance to tuberculosis in fat-1 mice. J Infect Dis. 2010;201:399–408. doi: 10.1086/650344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hegazi RA, Saad RS, Mady H, Matarese LE, O’Keefe S, Kandil HM. Dietary fatty acids modulate chronic colitis, colitis-associated colon neoplasia and COX-2 expression in IL-10 knockout mice. Nutrition. 2006;22:275–282. doi: 10.1016/j.nut.2005.06.006. [DOI] [PubMed] [Google Scholar]
- 38.Woodworth HL, McCaskey SJ, Duriancik DM, Clinthorne JF, Langohr IM, Gardner EM, Fenton JI. Dietary fish oil alters T lymphocyte cell populations and exacerbates disease in a mouse model of inflammatory colitis. Cancer Res. 2010;70:7960–7969. doi: 10.1158/0008-5472.CAN-10-1396. [DOI] [PubMed] [Google Scholar]
- 39.Walsh ER, Stokes K, August A. The role of eosinophils in allergic airway inflammation. Discov Med. 2010;9:357–362. [PubMed] [Google Scholar]
- 40.Zhang P, Smith R, Chapkin RS, McMurray DN. Dietary (n-3) polyunsaturated fatty acids modulate murine Th1/Th2 balance toward the Th2 pole by suppression of Th1 development. J Nutr. 2005;135:1745–1751. doi: 10.1093/jn/135.7.1745. [DOI] [PubMed] [Google Scholar]
- 41.de Urquiza AM, Liu S, Sjöberg M, Zetterström RH, Griffiths W, Sjövall J, Perlmann T. Docosahexaenoic acid, a ligand for the retinoid X receptor in mouse brain. Science. 2000;290:2140–2144. doi: 10.1126/science.290.5499.2140. [DOI] [PubMed] [Google Scholar]
- 42.Rasooly R, Schuster GU, Gregg JP, Xiao JH, Chandraratna RA, Stephensen CB. Retinoid x receptor agonists increase bcl2a1 expression and decrease apoptosis of naive T lymphocytes. J Immunol. 2005;175:7916–7929. doi: 10.4049/jimmunol.175.12.7916. [DOI] [PubMed] [Google Scholar]
- 43.Makhoul Z, Kristal AR, Gulati R, Luick B, Bersamin A, O’Brien D, Hopkins SE, Stephensen CB, Stanhope KL, Havel PJ, et al. Associations of obesity with triglycerides and C-reactive protein are attenuated in adults with high red blood cell eicosapentaenoic and docosahexaenoic acids. Eur J Clin Nutr. 2011;65:808–817. doi: 10.1038/ejcn.2011.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Berry M, Brightling C, Pavord I, Wardlaw A. TNF-alpha in asthma. Curr Opin Pharmacol. 2007;7:279–282. doi: 10.1016/j.coph.2007.03.001. [DOI] [PubMed] [Google Scholar]
- 45.Brightling C, Berry M, Amrani Y.Targeting TNF-alpha: a novel therapeutic approach for asthma J Allergy Clin Immunol 20081215–10.quiz 11–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Matera MG, Calzetta L, Cazzola M. TNF-alpha inhibitors in asthma and COPD: we must not throw the baby out with the bath water. Pulm Pharmacol Ther. 2010;23:121–128. doi: 10.1016/j.pupt.2009.10.007. [DOI] [PubMed] [Google Scholar]
- 47.Lien EL. Toxicology and safety of DHA. Prostaglandins Leukot Essent Fatty Acids. 2009;81:125–132. doi: 10.1016/j.plefa.2009.05.004. [DOI] [PubMed] [Google Scholar]
- 48.Harris WS, Mozaffarian D, Lefevre M, Toner CD, Colombo J, Cunnane SC, Holden JM, Klurfeld DM, Morris MC, Whelan J. Towards establishing dietary reference intakes for eicosapentaenoic and docosahexaenoic acids. J Nutr. 2009;139:804S–819S. doi: 10.3945/jn.108.101329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wenzel SE. Asthma phenotypes: the evolution from clinical to molecular approaches. Nat Med. 2012;18:716–725. doi: 10.1038/nm.2678. [DOI] [PubMed] [Google Scholar]