Abstract
The experiments described herein define a unique program of polarization of suspended human eosinophils stimulated with IL-5 family cytokines. We found that eosinophil granules and the nucleus move in opposite directions to form, respectively, a granular compartment and the nucleopod, a specialized uropod occupied by the nucleus and covered with adhesion receptors, including P-selectin glycoprotein ligand-1, CD44, and activated αMβ2 integrin. Ligated IL-5 family receptors localize specifically at the tip of the nucleopod in proximity to downstream signaling partners Janus tyrosine kinase 2, signal transducer and activator of transcription-1 and -5, and extracellular signal–regulated kinase. Microscopy and effects of cytochalasin B and nocodazole indicate that remodeling of filamentous actin and reorientation of the microtubule network are required for eosinophil polarization and nucleopod formation. IL-5 induces persistent polarization and extracellular signal–regulated kinase redistribution that are associated with eosinophil priming, a robust response on subsequent stimulation with N-formyl-methionyl-leucyl-phenylalanine. Global reorganization of cytoskeleton, organelles, adhesion receptors, and signaling molecules likely facilitates vascular arrest, extravasation, migration, granule release, and survival of eosinophils entering inflamed tissues from the bloodstream.
Keywords: eosinophil, integrin αMβ2 (CD11b/18), IL-5, nucleopod, priming
Clinical Relevance
We describe global reorganization of cytoskeleton, organelles, adhesion receptors, and signaling molecules in eosinophils stimulated with IL-5, including formation of a unique cellular protrusion called a nucleopod. This reorganization likely facilitates vascular arrest, extravasation, migration, granule release, and survival of eosinophils entering inflamed tissues from the blood stream.
Eosinophils are recognized by their characteristic bilobed nucleus and abundant granules (1). In healthy individuals, most eosinophils reside in the gastrointestinal tract (1). In response to helminthic infections or allergic provocations, eosinophils are recruited to the sites of inflammation, where they produce cytokines and lipid mediators and release toxic granule proteins (2). IL-5 family cytokines, comprised of IL-5, IL-3, and granulocyte/macrophage colony–stimulating factor (GM-CSF), regulate eosinophil differentiation and maturation (3). IL-5 family cytokines also act on mature eosinophils to enhance adhesion to endothelium, survival, and granule release (3, 4). The IL-5 family cytokine receptors comprise a cytokine-specific α subunit (IL-5Rα, IL-3Rα, or GM-CSFRα) and a common β (βc) subunit, and activate their signaling cascades via receptor-associated kinases (3). Other agonists, such as eotaxin and N-formyl-methionyl-leucyl-phenylalanine (fMLF), activate eosinophils via G protein–coupled receptors (5, 6).
IL-5 causes eosinophils to undergo a rapid increase in forward scatter (FSC), a flow cytometric parameter that is sensitive to cell size, shape, and refractive index (7, 8). Microscopy has shown that all IL-5 family cytokines cause eosinophils to change from a round to an elongated shape after a 16-hour incubation (9). To compare changes of FSC and cell shape, we analyzed eosinophils with and without IL-5 treatment in parallel by flow cytometry and microscopy. We examined distribution of cytoskeleton, adhesion receptors, cytokine receptors, cellular organelles, and signaling molecules. We describe a set of coordinated changes driven by the cytoskeleton, including formation of a unique type of uropod that contains the nucleus. We relate the coordinated changes to enhanced extracellular signal–regulated kinase (ERK) phosphorylation in response to fMLF after priming of eosinophils with IL-5.
Materials and Methods
Materials
Information about reagents and antibodies is presented in the online supplement.
Human Eosinophils and Neutrophils
Eosinophils from heparinized blood of donors with atopy were purified by Percoll centrifugation and removal of cells bearing CD14, CD16, and/or glycophorin A by the AutoMACS system (Miltenyi, Auburn, CA) (10). Purity of eosinophils was over 99%, as determined by the Hema 3 Wright-Geimsa staining kit (Fisher Scientific, Waltham, MA). A granulocyte mixture that contained greater than 90% neutrophils was set aside before negative selection and used for neutrophil experiments. The studies were approved by the University of Wisconsin–Madison Health Sciences Institutional Review Board. Informed written consent was obtained from each subject before participation.
Flow Cytometry
Purified eosinophils in incubation buffer (RPMI-1640 with L-glutamate, 25 mM HEPES, and 0.1% human serum albumin, 2.5 × 106 cells/ml) were incubated at 37°C for 1 hour before all procedures. In dose–response experiments, 0.5 × 106 eosinophils were coincubated with IL-5 and 2.5 μg/ml primary or isotype control antibody at 37°C for 5 minutes. For time course experiments, 0.5 × 106 eosinophils were incubated with 50 ng/ml IL-5 for the indicated time, and 2.5 μg/ml primary antibody was added for the last 5 minutes of incubation. In some experiments, primary antibody was added in the last 1 or 2 minutes of a 5-minute IL-5 incubation. Incubation was terminated by 2% paraformaldehyde (PFA; final concentration) and residual PFA was quenched by 0.1 M glycine. Fixed cells were incubated with 1:1,000 AF488–anti–mouse-F(ab′)2 for 30 minutes at 4°C. After PBS wash, samples were kept in 0.1% PFA at 4°C and analyzed within 24 hours on a BD FACSCalibur flow cytometer with CellQuest Software (BD Biosciences, San Jose, CA). Overlap of fluorescence channels was precompensated by using BD Calibrite Beads. At least 40,000 eosinophils gated in the scatterplot of FSC versus sideward scatter were collected for each sample. Flow data were analyzed and plotted by FlowJo 9.3.2 (Tree Star, Ashland, OR) and Prism 5 (GraphPad Software, La Jolla, CA). Formulas for calculating mean fluorescence and FSC are presented in the online supplement.
Fluorescence Microscopy
The staining protocol for surface antigens was the same as for flow cytometry, except that staining for P-selectin glycoprotein ligand (PSGL)-1 and βc subunit was performed on fixed eosinophils in some experiments. Fixed cells were cytospun onto coverslips at 600 rpm for 3 minutes in a Shandon Cytospin 2 (Thermo Scientific, Asheville, NC). Coverslips had been acid washed and, in later experiments, coated with 0.1% poly-L-lysine after we found that the coating resulted in a more uniform field of cells. Staining for cytoskeleton, intracellular antigens, and cell nuclei (by 4′,6-diamidino-2-phenylindole) was done after cytospin. Fixation and permeabilization conditions were optimized as indicated in the online supplement. Appropriate nonimmune antibody staining controls were done in all experiments.
Mounted coverslips were first examined for quality and initial impressions with an Olympus BM60 fluorescence microscope (Olympus, Melville, NY) using a 100×/1.3 oil immersion objective and then analyzed with a Nikon A1R confocal microscope (Nikon Instruments Inc., Melville, NY) using a 100×/1.4 oil immersion objective with 1 or 1.2 airy unit pinhole and 0.15- to 1.00-μm z stack thickness. Each coverslip, containing over 200 cells, was thoroughly examined, after which images of typical fields were acquired and exported by NIS Elements Advanced Research software (Nikon Instruments Inc.). Images within a given experiment were acquired and processed using identical settings.
Statistical Analysis and Replication
Pooled and normalized flow cytometric data are expressed as means (± SEM). In experiments linking activation of αMβ2-integrin and shape change, the same sample was analyzed by flow cytometry and microscopy. The non–zoomed-in and zoomed-in microscopic images are representative of at least three experiments from different donors. Except where indicated, features emphasized in the figures characterized roughly 80% or more of the cells examined for a given experimental condition.
Results
IL-5 Polarizes Eosinophils with Formation of a Granular Compartment and a Specialized Uropod, the Nucleopod
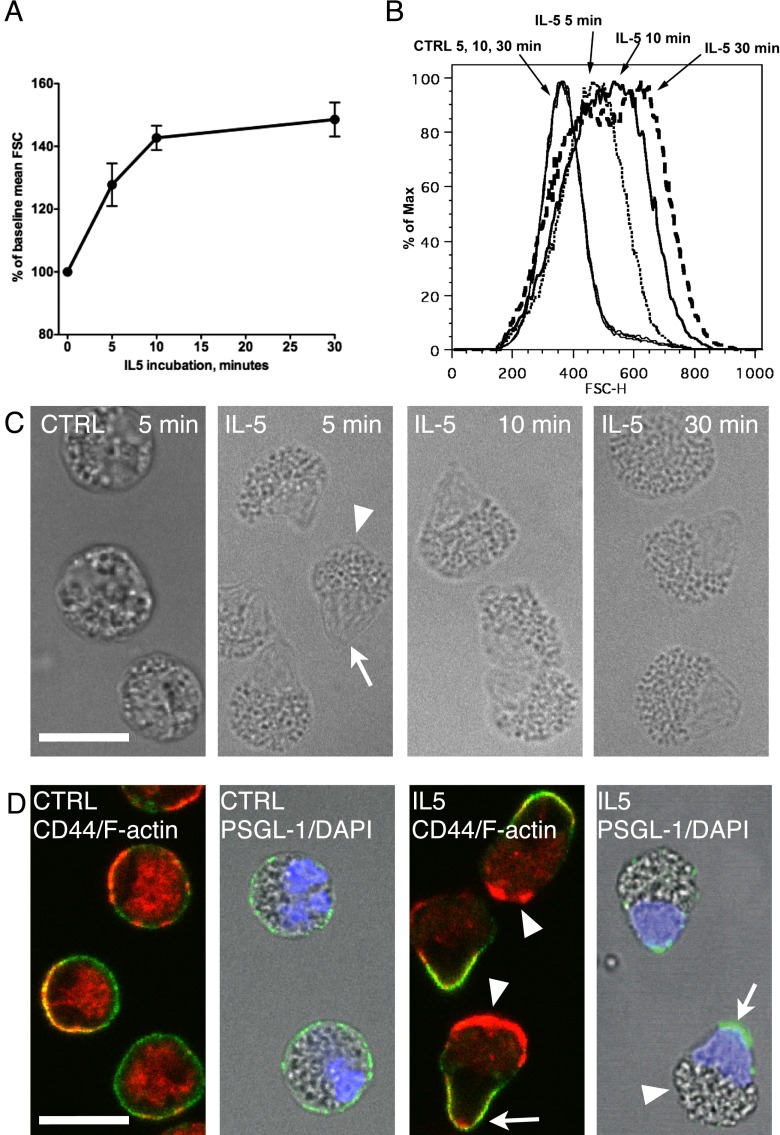
To examine the relationship between FSC and shape change, suspended eosinophils were treated with IL-5, fixed, and analyzed by flow cytometry or microscopy. We began by defining the effects of concentration and length of IL-5 treatment. FSC increased 20–30% at IL-5 concentrations of 1 ng/ml or higher, with a maximum effect at 10 ng/ml and no differences between 10 and 50 ng/ml (see Figure E1A in the online supplement). Excess IL-5 (50 ng/ml) caused FSC to increase rapidly in the first 10 minutes, and then slowly afterwards (Figure 1A). FSC histograms of eosinophils treated with IL-5 for 5, 10, or 30 minutes were right shifted and partially overlapped (Figure 1B). When examined by differential interference contrast microscopy after cytospin, unstimulated eosinophils were round, and had granules throughout the cytoplasm (Figure 1C, control [CTRL]). After treatment with IL-5 for 5 minutes, eosinophils developed an acorn-like shape with a granular compartment (Figure 1C, IL-5, arrowhead) and an agranular protrusion (arrow). The morphology of eosinophils did not change appreciably with longer incubations. Thus, the rapid increase in FSC correlates with a striking polarization of eosinophils, and the slower increase at later time points possibly is due to changes in refractive index or cell size, rather than further shape change.
Figure 1.
IL-5–induced forward scatter (FSC) increase correlates with eosinophil polarization into a granular compartment and a uropod that contains the nucleus. (A) Purified eosinophils were incubated without or with 50 ng/ml IL-5 for up to 30 minutes. FSC from three subjects are presented as mean (± SEM) of percentage change from baseline. (B) Histograms of FSC of eosinophils incubated with buffer for 5, 10, or 30 minutes (overlapping thin solid lines), or with 50 ng/ml IL-5 for 5 minutes (dotted line), 10 minutes (solid line), or 30 minutes (dashed line). (C) Differential interference contrast (DIC) images after cytospin of unstimulated (CTRL) or 50 ng/ml IL-5–treated eosinophils generated as in (B). The arrowhead points to the granular compartment, and the arrow points to the agranular protrusion. (D) Eosinophils were incubated with or without 50 ng/ml IL-5 for 5 minutes. Anti–P-selectin glycoprotein ligand (PSGL)-1 antibody was added simultaneously with IL-5, or anti-CD44 antibody was added in the last 2 minutes of incubation. After fixation, primary antibodies were detected by AF488–anti–mouse-F(ab′)2 (green), and filamentous actin (F-actin) was stained by tetramethlrhodamine isothiocyanate–phalloidin (red). Nuclei were stained by 4′,6-diamidino-2-phenylindole (DAPI; blue). Arrowheads point to the F-actin–rich granular compartments, and arrows point to the agranular protrusion that contains the nucleus and is enriched in uropod markers, PSGL-1 and CD44. Scale bars, 10 μm. CTRL, control; FSC-H, FSC high.
The agranular protrusion resembles the uropods of neutrophils and lymphocytes, which are enriched in surface CD44 and PSGL-1 and contain only sparse filamentous actin (F-actin) (11–13). In unstimulated eosinophils (Figure 1D, CTRL), CD44 or PSGL-1 (green) was diffusely distributed on the cell membrane, and F-actin (red) was associated with cell membrane and also present in the cytoplasm. The bilobed nucleus (blue) was located to one side of the cell. In IL-5–treated eosinophils, CD44 and PSGL-1 were found on the agranular protrusion (Figure 1D, arrows, and Figure E2), whereas F-actin was preferentially distributed in the granular compartment (Figure 1D, arrowheads, and Figure E2). Redistribution of granules was also appreciated in fluorescence microscopy controls employing nonimmune antibodies; after IL-5, the weakly autofluorescent granules (14) were clustered at one pole of the cells (Figure E3). Staining with 4′,6-diamidino-2-phenylindole revealed that the agranular protrusion contained the nucleus, with its two lobes jammed together. Thus, the IL-5–induced agranular protrusion has the properties of uropods with enrichment of CD44 and PSGL-1 and relative lack of F-actin. Translocation of the nucleus into the uropod, however, to our knowledge, is unique to eosinophil activation, so we refer to this special uropod as the “nucleopod.”
IL-5 Induces Polarization Concomitantly with Activated αMβ2 Localized on the Nucleopod
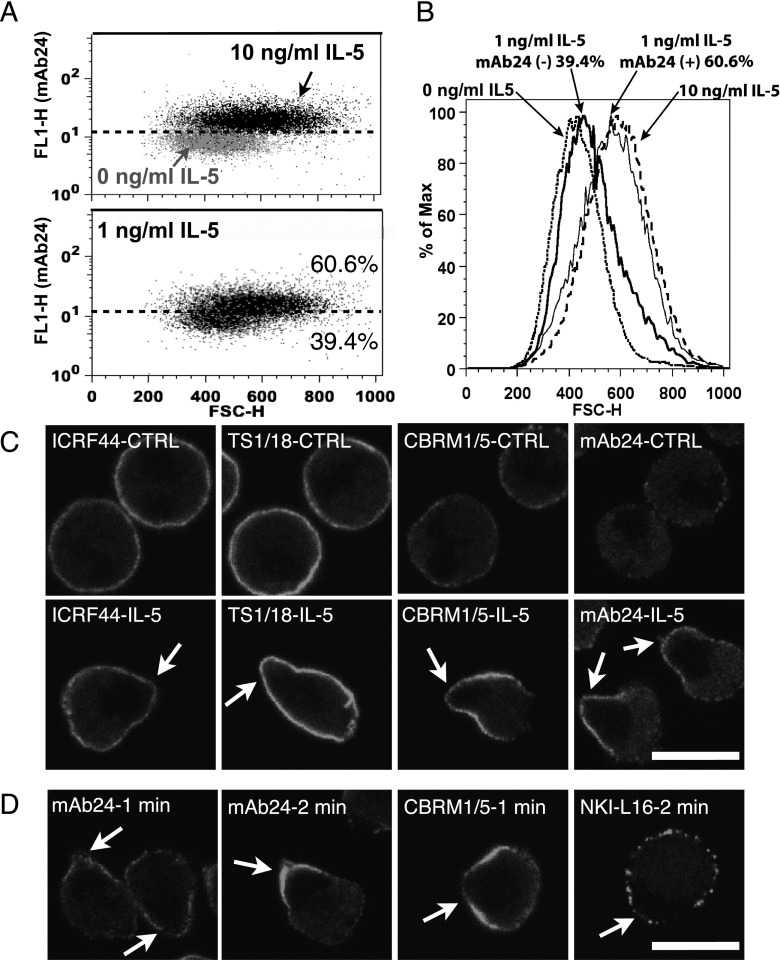
IL-5 up-regulates the density and activation state of eosinophil surface αMβ2 and enhances αMβ2-dependent adhesion (15–17). We found that both increase in FSC and up-regulation and activation of surface αMβ2 were induced by IL-5 at concentrations of 1 ng/ml or greater (Figure E1B). Similar to the FSC increase (Figure 1A), 5-minute incubation with 50 ng/ml IL-5 was sufficient to induce maximal αMβ2 activation (Figure E1C). In the scatterplot of activated β2 (monoclonal antibody [mAb] 24) reactivity versus FSC in a typical experiment (Figure 2A), the distribution of eosinophils treated with 10 ng/ml IL-5 (black dots) was shifted upwards and to the right, and overlapped minimally with the distribution of control cells (gray dots). The distribution of eosinophils treated with 1 ng/ml IL-5 was similar to that of the combination of control and 10 ng/ml IL-5 groups. We set a cutoff line (Figure 2A, dashed line) defining 80% of eosinophils as negative and 20% as positive for mAb24 staining in the control group. Using this cutoff, 61% of eosinophils were mAb24 positive and 39% were mAb24 negative in the 1 ng/ml IL-5 group, whereas 96% of eosinophils were mAb24 positive and 4% were mAb24 negative in the 10 ng/ml IL-5 group. As shown in Figure 2B, in the 1-ng/ml IL-5 group, mAb24-positive eosinophils (thin solid peak) had similar FSC to that in the 10-ng/ml IL-5 group (dashed peak), and mAb24-negative eosinophils (thick solid peak) had similar FSC to that in the control group (dotted peak). These results, together with those in Figure 1, indicate that activation of β2-integrins and eosinophil polarization occur simultaneously, and are part of an all-or-none reaction induced by IL-5 in the concentration range of 1 ng/ml (40 nM) or greater.
Figure 2.
IL-5–induced αMβ2 activation on the nucleopod is associated with the increase of FSC. (A) Purified eosinophils were exposed to different concentrations of IL-5 for 5 minutes together with antibody monoclonal antibody (mAb) 24 to activated β2 integrins before fixation. Scatterplots of eosinophils treated with buffer (0 ng/ml IL-5, gray dots, upper panel; 10 ng/ml IL-5, black dots, upper panel; and 1 ng/ml IL-5, black dots, lower panel). The x axis is FSC, and the y axis is fluorescence signal of mAb24 staining. The dashed line in both panels was drawn based on separation of the 0-ng/ml IL-5 into 80% mAb24 negative and 20% mAb24 positive, as described in the main text. (B) Histogram showing FSC of eosinophils from (A), treated with buffer (dotted peak), 1 ng/ml IL-5 and mAb24 negative (thick solid peak), 1 ng/ml IL-5 and mAb24 positive (thin solid peak), or 10 ng/ml IL-5 (dashed peak). (C) Eosinophils were processed as in (A), with antibody CBRM1/5 to activated αM, ICRF44 to total αM, and TS1/18 to total β2, as well as with mAb24. (D) Purified eosinophils were incubated with or without 26 ng/ml IL-5 at 37°C for 5 minutes. Primary antibody, including NKI-L16 to activated αL, was added in the last 1 or 2 minutes before fixation. Arrows point to the agranular nucleopods. Scale bars, 10 μm.
Antibodies to total αM (ICRF44) or β2 (TS1/18) evenly stained the surfaces of unstimulated and IL-5–stimulated eosinophils (Figure 2C and Figure E4). Unstimulated eosinophils had minimal activated αM (CBRM1/5) or β2 (mAb24). After IL-5 treatment, strong CBRM1/5 or mAb24 staining localized to the nucleopod (arrows). Antibodies CBRM1/5 and mAb24 recognized fixed integrin poorly, and so were added before cell fixation. We therefore considered how the localization on nucleopods is related to the known phenomenon of integrin clustering induced by ligating integrins with primary antibody followed by secondary antibody (18). Our fluorochrome-conjugated secondary F(ab′)2 fragment was added after cell fixation. Antibodies to activated or total αMβ2 did not induce clustering on unstimulated eosinophils, and antibodies to total αMβ2 did not induce integrin clustering on IL-5–stimulated eosinophils. NKI-L16 to activated αLβ2 (19) localized to clusters scattered along the cell membrane of IL-5–stimulated eosinophils without nucleopod localization (Figure 2D). When mAb24 or CBRM1/5 was added in the final 1 or 2 minutes of IL-5 incubation, nucleopod localization was also observed. Therefore, we conclude that the enrichment of activated αMβ2 on the nucleopod is not driven by antibody, and that αMβ2 is either specifically activated on the nucleopod or, on activation, is redistributed rapidly to the nucleopod.
IL-5 Causes Reorganization of the Eosinophil Cytoskeleton
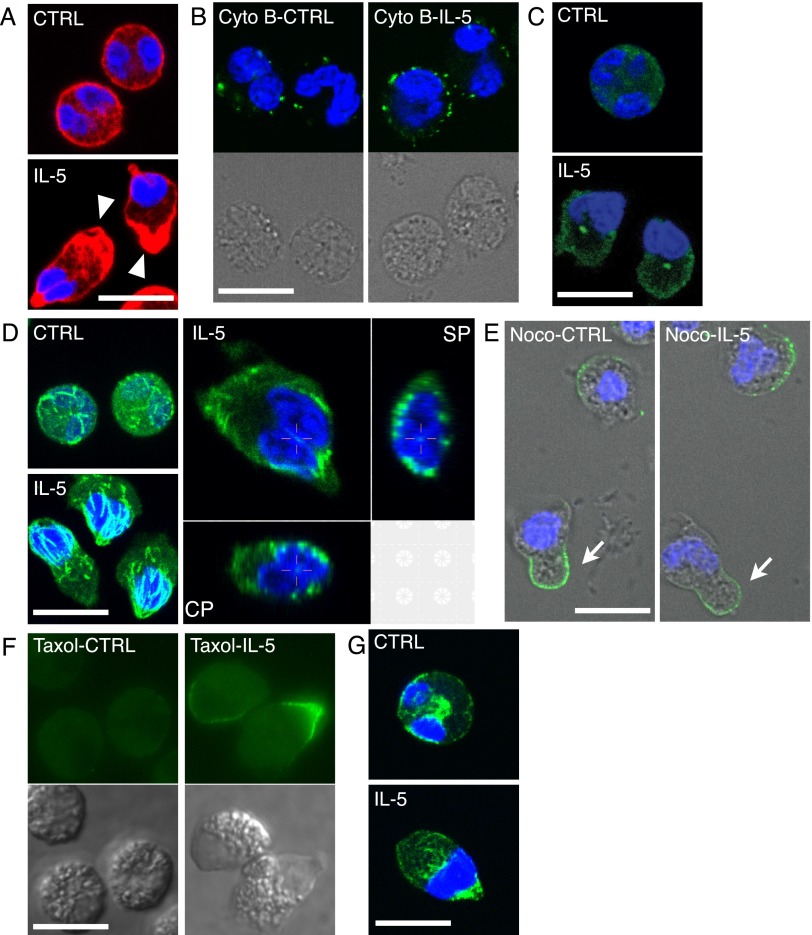
As shown in Figures 1D, 3A, and E5, phalloidin-stained F-actin was linearly associated with the cell membrane in resting eosinophils (CTRL), whereas F-actin was preferentially distributed in the granular compartment of IL-5–treated cells (arrowheads), with some present at the tip of the nucleopod. Eosinophils pretreated with cytochalasin B, an inhibitor of actin polymerization, stained diffusely with tetramethylrhodamine isothiocyanate–phalloidin (Figure E6) and remained round without uropod formation or focal enrichment of CD44 after IL-5 treatment (Figures 3B, E6, and E7). These results indicate that actin polymerization and reorganization of F-actin drives IL-5–induced polarization of suspended eosinophils.
Figure 3.
Reorganization of cytoskeleton in IL-5–treated eosinophils and effects of cytoskeleton-related reagents. (A) Purified eosinophils in suspension were incubated with buffer (CTRL) or 50 ng/ml IL-5 for 10 minutes, fixed, and stained for F-actin, as described in Materials and Methods. Arrowheads point to the F-actin–rich granular compartments. (B) Eosinophils were pretreated with cytochalasin B (Cyto B) for 30 minutes and then incubated with buffer (CTRL) or IL-5 together with anti-CD44 antibody for 10 minutes. Matching DIC images show cell morphology. (C) Eosinophils were treated as in (A), fixed by 0.7% glutaraldehyde, and stained for γ-tubulin. (D) Eosinophils were treated and processed as in (C), but stained for α-tubulin: left two panels, maximum projection images (i.e., images stacking all signals from confocal slices); right panel, three-dimensional slices of a single cell. CP, coronal plane; SP, sagittal plane. (E) Eosinophils were incubated with 10 μM nocodazole, anti-CD44 antibody, and buffer (CTRL), or 50 ng/ml IL-5 for 10 minutes, and then fixed and stained as in (B). Arrows point to uropod-like protrusions that lack nuclei. (F) Eosinophils were pretreated with 1 μM taxol for 30 minutes and incubated with buffer or 50 ng/ml IL-5 for 10 minutes; mAb24 antibody was added in the last 2 minutes before fixation. Matching DIC images show cell morphology, and allow the positions of agranular nuclei to be localized. Samples were examined on the Olympus BM60 epifluorescence microscope and imaged by an RT slider digital camera and SPOT RT Software v3.4 (Diagnostic Instruments, Sterling Height, MI). (G) Eosinophils treated with buffer (CTRL) or 50 ng/ml IL-5 for 10 minutes were fixed by 90% methanol and stained for vimentin, as described in Materials and Methods. Primary antibodies were labeled by AF488–anti–mouse-F(ab′)2. In all panels except (F), nuclei were stained by DAPI. Scale bars, 10 μm.
The microtubule organizing center (MTOC), detected by anti–γ-tubulin antibody, was not readily visible in most control eosinophils, but was clearly located between the nucleus and the granular compartment in IL-5–treated eosinophils (Figures 3C and E5). In both resting and IL-5–treated eosinophils, microtubules stained by anti–α-tubulin antibody were distributed in a network throughout the cell (Figures 3D and E5). In IL-5–treated cells, microtubules were orientated like a cage around the nucleus, and converged at the nucleopod tip. Occasionally, a few microtubules were present between the two lobes of eosinophil nucleus (crosshairs in the three-dimensional slices in Figure 3D). The tubulin polymerization inhibitor, nocodazole, depolymerized the microtubule network (Figure E6) and caused appearance of CD44-positive, uropod-like protrusions without or with IL-5 treatment (Figures 3E, arrows, and E7). Nuclei of nocodazole-treated eosinophils, however, were not present in the protrusions. In contrast, pretreatment with the microtubule depolymerization inhibitor, taxol, had no effect on IL-5–induced polarization, nucleopod formation, and activation of β2-integrins (Figures 3F and E7). These results suggest that the pre-existing microtubule network assists nucleus positioning during eosinophil polarization.
Vimentin was distributed throughout control eosinophils, with enrichment in the center near the nucleus. In IL-5–treated cells, the tip region of the nucleopod was enriched with vimentin (Figures 3G and E5).
Other IL-5 Family Cytokines and Eotaxin also Polarize Eosinophils
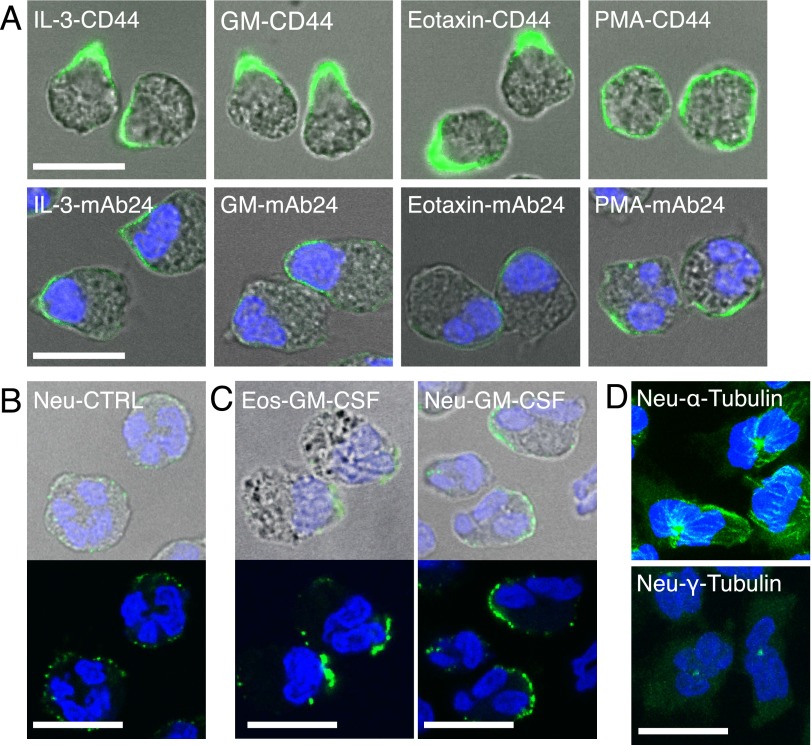
IL-3 and GM-CSF have similar effects as IL-5 on eosinophil adhesion and migration, and eotaxin is known to increase FSC of eosinophils (7, 9, 17). These activators were also similar to IL-5 in causing eosinophil polarization with nucleopod formation (Figures 4A and E8). In contrast, phorbol 12-myristate 13-acetate induced random clusters of activated β2, but no nucleopod formation.
Figure 4.
IL-5 family cytokines and eotaxin polarize eosinophils with nucleopod formation, whereas granulocyte/macrophage colony–stimulating factor (GM-CSF) polarizes neutrophils without nucleopod formation. (A) Eosinophils were treated with 50 ng/ml IL-3, 50 ng/ml GM-CSF, 30 ng/ml eotaxin, or 5 nM phorbol 12-myristate 13-acetate for 5 minutes. Anti-CD44 or mAb24 was added in the last 2 minutes. DIC images were merged with fluorescence images of either CD44 staining (green) or mAb24 staining (green) and DAPI staining (blue). (B) Purified neutrophils (Neu) were fixed and stained by anti–PSGL-1. (C) Purified eosinophils (Eos) or neutrophils were incubated with 50 ng/ml GM-CSF for 10 minutes, fixed, stained by anti–PSGL-1, and imaged with and without DIC. (D) Neutrophils were incubated with 50 ng/ml GM-CSF for 10 minutes, fixed by glutaraldehyde, and stained for α-tubulin or γ-tubulin. Maximum projection images (i.e., images stacking all signals from confocal slices). Primary antibodies were labeled by AF488–anti–mouse-F(ab′)2. Nuclei were stained by DAPI. Scale bars, 10 μm.
Neutrophils Do Not Form Nucleopods in Response to GM-CSF
Neutrophils have been shown to form uropods that do not contain nuclei when treated with fMLF (12, 20). We used GM-CSF, for which receptors are expressed on eosinophils and neutrophils (21), to perform a head-to-head comparison between suspended eosinophils and neutrophils. Resting neutrophils were round, with diffuse PSGL-1 on the cell membrane, similar to resting eosinophils (compare Figures 4B and 1D). In contrast to GM-CSF–treated eosinophils with nucleopods enriched in PSGL-1, the locations of nuclei in GM-CSF–treated neutrophils were random in relation to PSGL-1–staining uropods (Figures 4C and E9). In resting neutrophils, microtubules diffusely radiated from a centrally located MTOC (Figure E10A), whereas, in GM-CSF–treated neutrophils, microtubules were reoriented preferentially toward one side of the cell (Figures 4D and E10A). The MTOC was generally surrounded by nuclear lobes in resting (Figure E10B) and GM-CSF–activated neutrophils (Figures 4D and E10B), which is different from the MTOC of polarized eosinophils (Figures 3C and E5). The nuclear lobes of activated neutrophils were not confined by the microtubule network.
IL-5 Family Cytokines Induce Clustering of Cognate Receptors at the Tip of the Nucleopod
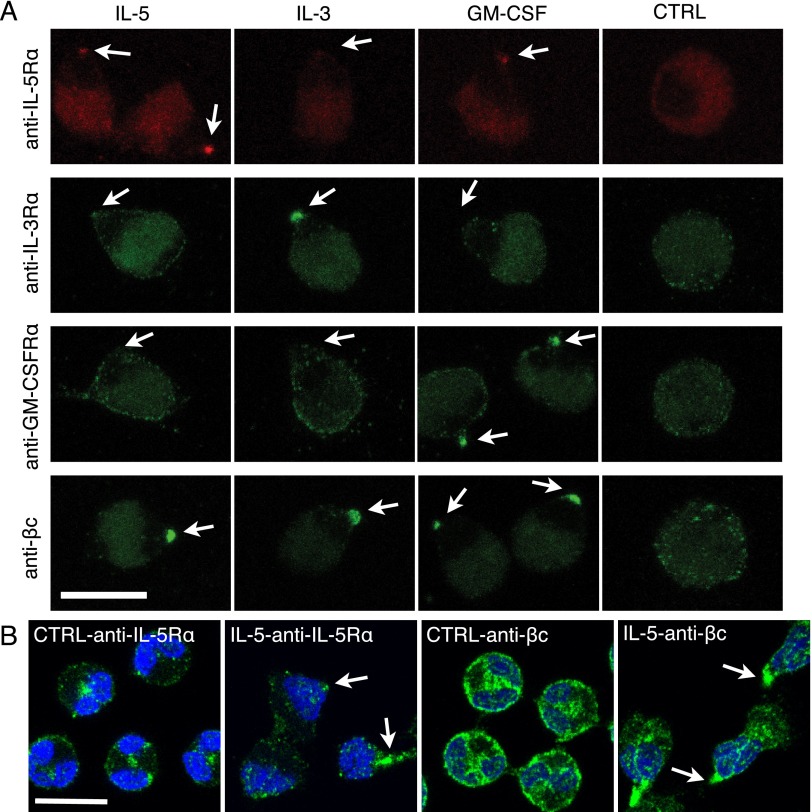
We examined the distribution of IL-5Rα, IL-3Rα, GM-CSFRα, and βc on suspended eosinophils with and without a 10-minute treatment of each cytokine. IL-5Rα, IL-3Rα, GM-CSFRα, and βc were scattered on the surface of unstimulated eosinophils (Figure 5A, CTRL). When cells were stimulated with IL-5, IL-5Rα and βc became clustered at the nucleopod tip, but IL-3Rα and GM-CSFRα were not. Similar specific clustering of IL-3Rα and GM-CSFRα were also observed after treatment with IL-3 and GM-CSF, respectively. Antibodies to α subunits did not recognize antigens on fixed eosinophils, and thus were preincubated with live eosinophils before adding the cytokine. Because the anti–βc 1C1 antibody does recognize fixed antigen, we determined its distribution on IL-5–treated and PFA-fixed eosinophils to rule out possible antibody-induced clustering. Staining of βc subunit at the nucleopod tip of fixed, but nonpermeabilized, stimulated cells was similar to the staining of live stimulated cells (Figure E12). We also used polyclonal antibodies against IL-5Rα (C20, extracellular epitope; aa, 310–335) and βc (H300, cytoplasmic epitope; aa, 598–897) on fixed and permeabilized cells to locate receptor subunits in cytoplasm as well as the cell surface (Figures 5B and E11). In control eosinophils, most IL-5Rα staining was centrally located between the nuclear lobes, and some was associated with the cell membrane. After 10 minutes of IL-5 treatment, most IL-5Rα staining was located in the nucleopod, especially at the tip region (Figure 5B, arrows). βc, which stained more strongly than IL-5Rα, was distributed similarly to IL-5Rα in resting cells, and was in the central area and at the nucleopod tip of IL-5–treated cells. These results indicate that binding of each IL-5 family cytokine to its receptor preferentially triggers movement of the cognate receptor to the tip of the nucleopod.
Figure 5.
IL-5 family cytokines induce specific clustering of cognate receptors at the nucleopod tip. (A) Eosinophils in suspension were incubated with primary antibody against the subunit of the indicated cytokine receptor at 37°C for 30 minutes, then stimulated with or without (CTRL) the indicated cytokine for 10 minutes, and fixed. Antibody against IL-5Rα was detected by F(ab′)2 conjugated to phycoerythrin, whereas antibodies against IL-3Rα, GM-CSFRα, and βc were detected by AF488–anti–mouse-F(ab′)2. Arrows point to the tips of nucleopods. (B) Eosinophils were incubated with buffer or IL-5 for 10 minutes before fixation, permeabilization, and staining by anti–IL-5Rα (C20) or anti-βc (H300). Primary antibodies were detected by AF488–anti–rabbit-F(ab′)2. Nuclei were stained by DAPI. Arrows point to IL-5Rα or βc staining at the tips of nucleopods. Scale bars, 10 μm.
IL-5 Activates Janus Tyrosine Kinase/Signal Transducer and Activator of Transcription Pathway in the Nucleopod
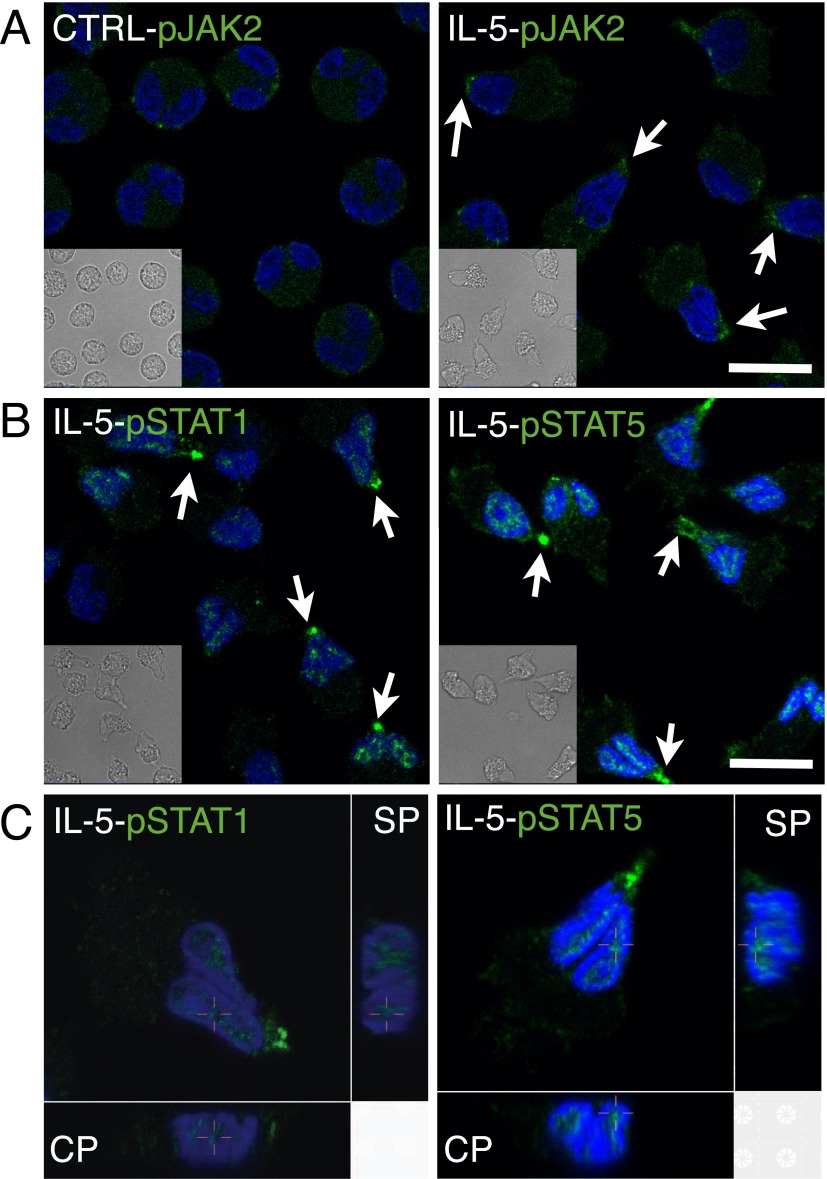
IL-5 induces Janus tyrosine kinase (JAK) 1/2 activation and subsequent phosphorylation of signal transducer and activator of transcription (STAT) 1 and STAT5, which then dimerize and move into the nucleus (22, 23). In contrast to sparse phosphorylated JAK (pJAK) 2 staining randomly associated with the cell membrane of resting eosinophils, an intense locus of pJAK2 was found at the nucleopod tip after IL-5 treatment for 10 minutes (Figures 6A and E13). Similarly, in contrast to the negative staining of phosphorylated STAT (pSTAT) 1 and pSTAT5 in resting eosinophils (Figure E13), there was intense staining of pSTAT1 or pSTAT5 at the nucleopod tip, together with nuclear localization that was best shown in three-dimensional slices (Figures 6B and 6C). Preincubation with the JAK2 inhibitor, TG101348, blocked IL-5–induced appearance of pSTAT-1 (Figure E14). These results indicate that clustered IL-r–ligated receptors at the nucleopod tip couple efficiently to downstream signaling cascades.
Figure 6.
Localization of phosphorylated Janus tyrosine kinase (pJAK) 2, phosphorylated signal transducer and activator of transcription (pSTAT) 1, and pSTAT5 in eosinophils after IL-5 stimulation. Suspended eosinophils were treated with buffer (CTRL) or IL-5 at 37°C for 10 minutes before fixation and staining for (A) pJAK2 or (B) pSTAT1 or pSTAT5 at 4°C overnight. Arrows point to the tips of nucleopods. Insets are matching DIC images. (C) Three-dimensional slices of pSTAT1 or pSTAT5 staining of an eosinophil. Crosshairs indicate the corresponding positions in three views. Primary antibody was detected by FITC–anti–goat IgG or AF488–anti–rabbit-F(ab′)2. Nuclei were stained by DAPI. Scale bars, 10 μm.
Eosinophil Polarization Is Associated with Enhanced ERK Phosphorylation in Response to fMLF
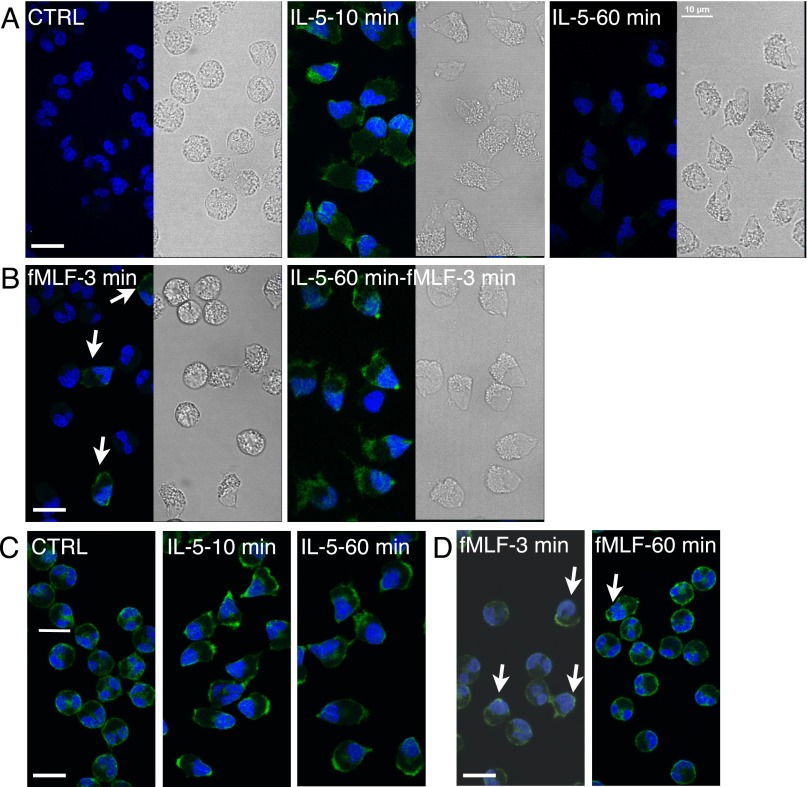
Western blotting has shown that eosinophils treated with IL-5 for 1 hour have more robust ERK phosphorylation in response to subsequent stimulation with fMLF when compared with naive cells treated with fMLF; this phenomenon is called priming (24, 25). We found no phosphorylated ERK (pERK) staining in resting eosinophils, positive pERK staining in the nucleopod and granular compartment after 10-minute incubation with IL-5, especially at the nucleopod tip and potential leading edge, and no pERK staining after a 60-minute incubation with IL-5 (Figure 7A). Eosinophils treated with IL-5 for 10 or 60 minutes, however, were equally polarized, as shown in differential interference contrast images. Preincubation with the ERK activation inhibitor, PD98059, blocked IL-5–induced appearance of pERK (Figure E14). When eosinophils were treated with fMLF for 3 minutes and stained for pERK, polarization and ERK phosphorylation were found in a minority of eosinophils (Figure 7B; arrowheads point to polarized, pERK-positive cells). In eosinophils treated with IL-5 for 60 minutes, a subsequent 3-minute treatment with fMLF induced ERK phosphorylation in almost all cells, with the same pERK distribution as in eosinophils treated with IL-5 for 10 minutes (compare Figures 7A and 7B). These data corroborate published Western blotting results indicating that a 60-minute incubation with IL-5 renders the eosinophils more sensitive to subsequent stimulation with fMLF (24, 25), even though ERK phosphorylation has returned to basal level at this time point (26).
Figure 7.
Localization of extracellular signal–regulated kinase (ERK) and phosphorylated ERK (pERK) in eosinophils activated with IL-5 and/or N-formyl-methionyl-leucyl-phenylalanine (fMLF).(A) Eosinophils were treated with buffer (CTRL) or 50 ng/ml IL-5 for 10 or 60 minutes before fixation and staining for pERK. (B) Eosinophils without or with IL-5 pretreatment for 60 minutes were stimulated with 100 nM fMLF for 3 minutes before fixation and staining for pERK. (A and B) Matching DIC images show the morphology of eosinophils. (C) Eosinophils were treated with 50 ng/ml IL-5 for 10 or 60 minutes before fixation and staining for total ERK. (D) Eosinophils were treated with 100 nM fMLF for 3 or 60 minutes before fixation and staining for total ERK. Primary antibody was detected by AF488–anti–rabbit-F(ab′)2. Nuclei were stained by DAPI. Arrows point to the minority of cells that developed nucleopods in response to fMLF alone. Scale bars, 10 μm.
We examined the distribution of total ERK under the same conditions. In resting eosinophils, ERK staining was centrally located and linearly associated with cell membrane (Figure 7C). In eosinophils treated with IL-5 for 10 or 60 minutes, ERK was located at the nucleopod tip and in the granular compartment; in other words, IL-5 induced persistent ERK redistribution independent of ERK activation. In eosinophils treated with fMLF for 3 or 60 minutes, only a few cells were polarized with redistribution of ERK to the nucleopod and the granular compartment (Figure 7D). These results indicate that ERK redistribution is part of eosinophil polarization induced by IL-5, and such localization may explain enhanced ERK phosphorylation in response to fMLF.
Discussion
The experiments described herein define a program of polarization in suspended human eosinophils stimulated with IL-5. Granules move to one end of the cell, and the nucleus moves to the other end to form the nucleopod, a specialized uropod into which is packed the nucleus. Ligated IL-5 receptor is localized specifically to the tip of the nucleopod in proximity to its downstream signaling partners, JAK2, STAT1, STAT5, and ERK. Copious F-actin appears in the granular compartment, the MTOC becomes precisely located between the nucleus and granular compartment, microtubules run between the MTOC and the nucleopod tip to form a cage around the nucleus, and the nucleopod tip contains abundant vimentin. Effects of cytochalasin B and nocodazole indicate that reorganization of F-actin and an intact microtubule network are required for polarization and nucleopod formation.
We can only speculate on the roles of the eosinophil nucleopod, and focus this discussion on two general types of function. The first is extravasation of eosinophils from inflamed blood vessels. The second is events that occur after extravasation, including regulation of gene transcription, migration, degranulation, and production of nonprotein mediators.
The generally accepted paradigm for the initiation of leukocyte arrest involves tethering of selectin ligands that are concentrated on the tips of microvilli to endothelial cell selectins, followed by rolling and tethering of leukocytes mediated by the selectin ligands, CD44, and α4β1-integrin (12, 27–30). Of the seven integrin heterodimers on eosinophils, α4β1 and αMβ2 are particularly important for trafficking in asthma (31). Studies of human volunteers with asthma indicate that both integrins exist in an extended, partially activated form on circulating eosinophils, α4β1 due to encounters of eosinophils with activated platelets and αMβ2 as a tonic effect of the low level (∼ 10 pg/ml) of IL-5 in the circulation (31). Such partially activated forms of integrin have the potential to engage counter-ligands, such as vascular cell adhesion molecule-1 on activated endothelial cells, and switch to a high-affinity form by a concerted series of cytoplasmic changes (“inside-out” signaling induced by chemokines and cytokines elaborated in or near the inflamed vessel) and ligand-induced changes (“outside-in” signaling) (29).
Stable arrest of leukocytes requires (in additon to functional activation of integrins) loss of microvilli and realignment of membrane, redistribution of integrins, and reinforcement of cell stiffness in the contact area (30). We envision the nucleopod, on which high-affinity αMβ2 is specifically activated, and which has an underlying cage of microtubules, as participating in these three latter functions, thus allowing the arrested eosinophil to resist the peeling process of flow-induced detachment. Consistently, eosinophils from switch-associated protein-70–deficient mice, which are defective in formation of structures identified as uropods, adhere poorly to inflamed vessels in intravital experiments (32). Thus, we consider the rapid polarization and nucleopod formation by suspended eosinophils in response to the concentrations (ng/ml) of IL-5 that can be found, for example, in bronchoalveolar lavage after segmental antigen challenge (33), as a model of the changes that occur as eosinophils encounter such stimulation and stably arrest in and extravasate from vessels of, for instance, the bronchi of subjects with asthma.
For neutrophils, αMβ2 and the uropod have been demonstrated to be central to “mechanotactic crawling” of arrested cells perpendicular to the direction of blood flow; by such crawling, the arrested cell moves to a nearby site to leave the blood vessel (34, 35). Whether the eosinophil nucleopod functions similarly needs further investigation, as do the differences that cause movement of the nucleus into the uropod of activated eosinophils, but not of activated neutrophils. Morphologies of microtubule networks may contribute to the difference, but do not represent the complete explanation. Human neutrophils are deficient in various nuclear envelope components, including lamins and lamin B receptor (36). Loss of lamins from the nuclear envelope impairs nuclear positioning in fibroblasts and skeletal muscle (37, 38). Eosinophils express lamin B1 (39, 40), and, in a pilot experiment, we localized lamin B1 in the nuclear envelope (Figure E15). Thus, the difference between the nuclear envelopes of neutrophils and eosinophils may also contribute to the different locations of their nuclei after activation.
Published time-lapse phase images of newt eosinophils migrating on an uncharacterized surface demonstrate, from leading edge to rear, an agranular leading edge, a granular zone, the MTOC, and the nucleus (41). Experiments are ongoing to learn which features of the polarized suspended eosinophil persist during cell adhesion and migration after extravasation. As a model system, we are using surfaces coated with periostin, an extracellular matrix protein that is preferentially deposited in the asthmatic bronchus, and is a ligand for activated αMβ2 of eosinophils stimulated with IL-5, IL-3, or GM-CSF (17). IL-5–treated eosinophils adherent to perostin for 1 hour form prominent podosomes near the apparent leading edge, some distance away from the apparently trailing nucleus (17). PSGL-1 remains concentrated at the nuclear pole, but less strikingly so than on suspended cells (M. W. Johansson and D. F. Mosher, unpublished observation). We suspect that the adhesive ligands gathered on the nucleopod are in part shed or cycled on initiation of migration.
IL-5 family cytokines up-regulate synthesis of a number of mRNAs and proteins (40). Compartmentalization to the nucleopod of ligated cytokine receptor, receptor-associated JAK, downstream STAT effectors, and nuclear targets may serve to initiate the transcriptional programs efficiently. IL-5 family cytokines also prime eosinophils to react with increased release of granule proteins, increased production of leukotriene C4, and chemotaxis on subsequent stimulation with other activators (42–46). fMLF induces greater ERK phosphorylation, as analyzed by Western blotting, in IL-5–primed eosinophils than in unprimed cells (25). Polarization of eosinophils is accompanied by distribution of ERK to the granular compartment as well as to the nucleopod tip. Such a distribution is associated with priming of eosinophils to another round of ERK phosphorylation on stimulation with fMLF. Thus, we speculate that the compartmentalization of signaling molecules induced by IL-5 family cytokines in the potential leading edge and granular pole primes extravasated eosinophils to migrate and release granule contents and mediators as the cells encounter additional agonists elaborated by point sources in inflamed tissues.
Acknowledgments
Acknowledgments
The authors gratefully thank: Mats Johansson for reviewing and editing the manuscript and for many helpful discussions; Fran Fogerty for help in follow-up experiments; Paul Fichtinger and Elizabeth Schwantes for leukocyte purification; the Anna Huttenlocher laboratory and Sa Kan Yoo for experimental suggestions and helpful discussions; University of Wisconsin–Madison Carbon Cancer Center and Dagna Sheerar for advice on flow cytometry; the W. M. Keck Laboratory for Biological Imaging and Lance Rodenkirch for help with confocal imaging; and Ben Joosten, Department of Tumor Immunology, Nijmegen Center for Molecular Life Sciences, University Medical Center, Nijmegen, The Netherlands, for NKI-L16 antibody.
Footnotes
This work was supported by National Institutes of Health grants PO1 HL088594 and UL1 RR025011, and a fellowship from Chang Gung Memorial Hospital.
This article has an online supplement, which is accessible from this issue’s table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1165/rcmb.2013-0181OC on October 24, 2013
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Rosenberg HF, Phipps S, Foster PS. Eosinophil trafficking in allergy and asthma. J Allergy Clin Immunol. 2007;119:1303–1310. doi: 10.1016/j.jaci.2007.03.048. [DOI] [PubMed] [Google Scholar]
- 2.Koyasu S, Moro K. Type 2 innate immune responses and the natural helper cell. Immunology. 2011;132:475–481. doi: 10.1111/j.1365-2567.2011.03413.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Martinez-Moczygemba M, Huston DP. Biology of common beta receptor–signaling cytokines: IL-3, IL-5, and GM-CSF. J Allergy Clin Immunol. 2003;112:653–665. doi: 10.1016/S0091. [DOI] [PubMed] [Google Scholar]
- 4.Takatsu K, Kouro T, Nagai Y. Interleukin 5 in the link between the innate and acquired immune response. Adv Immunol. 2009;101:191–236. doi: 10.1016/S0065-2776(08)01006-7. [DOI] [PubMed] [Google Scholar]
- 5.Migeotte I, Communi D, Parmentier M. Formyl peptide receptors: a promiscuous subfamily of G protein–coupled receptors controlling immune responses. Cytokine Growth Factor Rev. 2006;17:501–519. doi: 10.1016/j.cytogfr.2006.09.009. [DOI] [PubMed] [Google Scholar]
- 6.Lloyd CM, Rankin SM. Chemokines in allergic airway disease. Curr Opin Pharmacol. 2003;3:443–448. doi: 10.1016/s1471-4892(03)00069-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Choi EN, Choi MK, Park C-S, Chung IY. A parallel signal-transduction pathway for eotaxin- and interleukin-5–induced eosinophil shape change. Immunology. 2003;108:245–256. doi: 10.1046/j.1365-2567.2003.01565.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sharpless TK, Bartholdi M, Melamed MR. Size and refractive index dependence of simple forward angle scattering measurements in a flow system using sharply-focused illumination. J Histochem Cytochem. 1977;25:845–856. doi: 10.1177/25.7.330734. [DOI] [PubMed] [Google Scholar]
- 9.Ip WK, Wong CK, Wang CB, Tian YP, Lam CWK. Interleukin-3, -5, and granulocyte macrophage colony–stimulating factor induce adhesion and chemotaxis of human eosinophils via p38 mitogen-activated protein kinase and nuclear factor kappaB. Immunopharmacol Immunotoxicol. 2005;27:371–393. doi: 10.1080/08923970500240925. [DOI] [PubMed] [Google Scholar]
- 10.Johansson MW, Mosher DF. Activation of β1 integrins on blood eosinophils by P-selectin. Am J Respir Cell Mol Biol. 2011;45:889–897. doi: 10.1165/rcmb.2010-0402OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rossy J, Schlicht D, Engelhardt B, Niggli V. Flotillins interact with PSGL-1 in neutrophils and, upon stimulation, rapidly organize into membrane domains subsequently accumulating in the uropod. PLoS One. 2009;4:e5403. doi: 10.1371/journal.pone.0005403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bruehl RE, Moore KL, Lorant DE, Borregaard N, Zimmerman GA, McEver RP, Bainton DF. Leukocyte activation induces surface redistribution of P-selectin glycoprotein ligand-1. J Leukoc Biol. 1997;61:489–499. doi: 10.1002/jlb.61.4.489. [DOI] [PubMed] [Google Scholar]
- 13.Sánchez-Madrid F, Serrador JM. Bringing up the rear: defining the roles of the uropod. Nat Rev Mol Cell Biol. 2009;10:353–359. doi: 10.1038/nrm2680. [DOI] [PubMed] [Google Scholar]
- 14.Weil GJ, Chused TM. Eosinophil autofluorescence and its use in isolation and analysis of human eosinophils using flow microfluorometry. Blood. 1981;57:1099–1104. [PubMed] [Google Scholar]
- 15.Myou S, Zhu X, Boetticher E, Myo S, Meliton A, Lambertino A, Munoz NM, Leff AR. Blockade of focal clustering and active conformation in beta 2-integrin–mediated adhesion of eosinophils to intercellular adhesion molecule-1 caused by transduction of HIV TAT-dominant negative Ras. J Immunol. 2002;169:2670–2676. doi: 10.4049/jimmunol.169.5.2670. [DOI] [PubMed] [Google Scholar]
- 16.Barthel SR, Jarjour NN, Mosher DF, Johansson MW. Dissection of the hyperadhesive phenotype of airway eosinophils in asthma. Am J Respir Cell Mol Biol. 2006;35:378–386. doi: 10.1165/rcmb.2006-0027OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Johansson MW, Annis DS, Mosher DF. αMβ2 integrin–mediated adhesion and motility of IL-5–stimulated eosinophils on periostin. Am J Respir Cell Mol Biol. 2013;48:503–510. doi: 10.1165/rcmb.2012-0150OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kornberg LJ, Earp HS, Turner CE, Prockop C, Juliano RL. Signal transduction by integrins: increased protein tyrosine phosphorylation caused by clustering of beta 1 integrins. Proc Natl Acad Sci USA. 1991;88:8392–8396. doi: 10.1073/pnas.88.19.8392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.van Kooyk Y, Weder P, Hogervorst F, Verhoeven AJ, van Seventer G, te Velde AA, Borst J, Keizer GD, Figdor CG. Activation of LFA-1 through a Ca2(+)-dependent epitope stimulates lymphocyte adhesion. J Cell Biol. 1991;112:345–354. doi: 10.1083/jcb.112.2.345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Seveau S, Lopez S, Lesavre P, Guichard J, Cramer EM, Halbwachs-Mecarelli L. Leukosialin (CD43, sialophorin) redistribution in uropods of polarized neutrophils is induced by CD43 cross-linking by antibodies, by colchicine or by chemotactic peptides. J Cell Sci. 1997;110:1465–1475. doi: 10.1242/jcs.110.13.1465. [DOI] [PubMed] [Google Scholar]
- 21.Yamada T, Sun Q, Zeibecoglou K, Bungre J, North J, Kay AB, Lopez AF, Robinson DS. IL-3, IL-5, granulocyte-macrophage colony–stimulating factor receptor alpha-subunit, and common beta-subunit expression by peripheral leukocytes and blood dendritic cells. J Allergy Clin Immunol. 1998;101:677–682. doi: 10.1016/S0091-6749(98)70177-0. [DOI] [PubMed] [Google Scholar]
- 22.van der Bruggen T, Caldenhoven E, Kanters D, Coffer P, Raaijmakers JA, Lammers JW, Koenderman L. Interleukin-5 signaling in human eosinophils involves JAK2 tyrosine kinase and Stat1 alpha. Blood. 1995;85:1442–1448. [PubMed] [Google Scholar]
- 23.Caldenhoven E, van Dijk TB, Raaijmakers JA, Lammers JW, Koenderman L, de Groot RP. Activation of a functionally distinct 80-kDa STAT5 isoform by IL-5 and GM-CSF in human eosinophils and neutrophils. Mol Cell Biol Res Commun. 1999;1:95–101. doi: 10.1006/mcbr.1999.0114. [DOI] [PubMed] [Google Scholar]
- 24.Bates ME, Green VL, Bertics PJ. ERK1 and ERK2 activation by chemotactic factors in human eosinophils is interleukin 5–dependent and contributes to leukotriene C(4) biosynthesis. J Biol Chem. 2000;275:10968–10975. doi: 10.1074/jbc.275.15.10968. [DOI] [PubMed] [Google Scholar]
- 25.Zhu Y, Bertics PJ. Chemoattractant-induced signaling via the Ras-ERK and PI3K-Akt networks, along with leukotriene C4 release, is dependent on the tyrosine kinase Lyn in IL-5– and IL-3–primed human blood eosinophils. J Immunol. 2011;186:516–526. doi: 10.4049/jimmunol.1000955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Coffer PJ, Schweizer RC, Dubois GR, Maikoe T, Lammers JW, Koenderman L. Analysis of signal transduction pathways in human eosinophils activated by chemoattractants and the T-helper 2–derived cytokines interleukin-4 and interleukin-5. Blood. 1998;91:2547–2557. [PubMed] [Google Scholar]
- 27.Hidalgo A, Peired AJ, Wild MK, Vestweber D, Frenette PS. Complete identification of E-selectin ligands on neutrophils reveals distinct functions of PSGL-1, ESL-1, and CD44. Immunity. 2007;26:477–489. doi: 10.1016/j.immuni.2007.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Katoh S, Matsumoto N, Kawakita K, Tominaga A, Kincade PW, Matsukura S. A role for CD44 in an antigen-induced murine model of pulmonary eosinophilia. J Clin Invest. 2003;111:1563–1570. doi: 10.1172/JCI16583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Alon R, Dustin ML. Force as a facilitator of integrin conformational changes during leukocyte arrest on blood vessels and antigen-presenting cells. Immunity. 2007;26:17–27. doi: 10.1016/j.immuni.2007.01.002. [DOI] [PubMed] [Google Scholar]
- 30.Robert P, Touchard D, Bongrand P, Pierres A. Biophysical description of multiple events contributing blood leukocyte arrest on endothelium. Front Immunol. 2013;4:108. doi: 10.3389/fimmu.2013.00108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Johansson MW, Mosher DF. Integrin activation states and eosinophil recruitment in asthma. Front Pharmacol. 2013;4:33. doi: 10.3389/fphar.2013.00033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bahaie NS, Hosseinkhani MR, Ge XN, Kang BN, Ha SG, Blumenthal MS, Jessberger R, Rao SP, Sriramarao P. Regulation of eosinophil trafficking by SWAP-70 and its role in allergic airway inflammation. J Immunol. 2012;188:1479–1490. doi: 10.4049/jimmunol.1102253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Johansson MW, Kelly EAB, Busse WW, Jarjour NN, Mosher DF. Up-regulation and activation of eosinophil integrins in blood and airway after segmental lung antigen challenge. J Immunol. 2008;180:7622–7635. doi: 10.4049/jimmunol.180.11.7622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Phillipson M, Heit B, Colarusso P, Liu L, Ballantyne CM, Kubes P. Intraluminal crawling of neutrophils to emigration sites: a molecularly distinct process from adhesion in the recruitment cascade. J Exp Med. 2006;203:2569–2575. doi: 10.1084/jem.20060925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Phillipson M, Heit B, Parsons SA, Petri B, Mullaly SC, Colarusso P, Gower RM, Neely G, Simon SI, Kubes P. Vav1 is essential for mechanotactic crawling and migration of neutrophils out of the inflamed microvasculature. J Immunol. 2009;182:6870–6878. doi: 10.4049/jimmunol.0803414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Olins AL, Zwerger M, Herrmann H, Zentgraf H, Simon AJ, Monestier M, Olins DE. The human granulocyte nucleus: unusual nuclear envelope and heterochromatin composition. Eur J Cell Biol. 2008;87:279–290. doi: 10.1016/j.ejcb.2008.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lee JS, Hale CM, Panorchan P, Khatau SB, George JP, Tseng Y, Stewart CL, Hodzic D, Wirtz D. Nuclear lamin A/C deficiency induces defects in cell mechanics, polarization, and migration. Biophys J. 2007;93:2542–2552. doi: 10.1529/biophysj.106.102426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Méjat A, Decostre V, Li J, Renou L, Kesari A, Hantaï D, Stewart CL, Xiao X, Hoffman E, Bonne G, et al. Lamin A/C-mediated neuromuscular junction defects in Emery-Dreifuss muscular dystrophy. J Cell Biol. 2009;184:31–44. doi: 10.1083/jcb.200811035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Straub C, Pazdrak K, Young TW, Stafford SJ, Wu Z, Wiktorowicz JE, Haag AM, English RD, Soman KV, Kurosky A. Toward the proteome of the human peripheral blood eosinophil. Proteomics Clin Appl. 2009;3:1151–1173. doi: 10.1002/prca.200900043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bates ME, Liu LY, Esnault S, Stout BA, Fonkem E, Kung V, Sedgwick JB, Kelly EAB, Bates DM, Malter JS, et al. Expression of interleukin-5– and granulocyte macrophage-colony–stimulating factor–responsive genes in blood and airway eosinophils. Am J Respir Cell Mol Biol. 2004;30:736–743. doi: 10.1165/rcmb.2003-0234OC. [DOI] [PubMed] [Google Scholar]
- 41.Koonce MP, Cloney RA, Berns MW. Laser irradiation of centrosomes in newt eosinophils: evidence of centriole role in motility. J Cell Biol. 1984;98:1999–2010. doi: 10.1083/jcb.98.6.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Takafuji S, Bischoff SC, De Weck AL, Dahinden CA. IL-3 and IL-5 prime normal human eosinophils to produce leukotriene C4 in response to soluble agonists. J Immunol. 1991;147:3855–3861. [PubMed] [Google Scholar]
- 43.Carlson M, Peterson C, Venge P. The influence of IL-3, IL-5, and GM-CSF on normal human eosinophil and neutrophil C3b-induced degranulation. Allergy. 1993;48:437–442. [PubMed] [Google Scholar]
- 44.Bates ME, Sedgwick JB, Zhu Y, Liu LY, Heuser RG, Jarjour NN, Kita H, Bertics PJ. Human airway eosinophils respond to chemoattractants with greater eosinophil-derived neurotoxin release, adherence to fibronectin, and activation of the Ras–ERK pathway when compared with blood eosinophils. J Immunol. 2010;184:7125–7133. doi: 10.4049/jimmunol.0900634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Koenderman L, Tool AT, Roos D, Verhoeven AJ. Priming of the respiratory burst in human eosinophils is accompanied by changes in signal transduction. J Immunol. 1990;145:3883–3888. [PubMed] [Google Scholar]
- 46.Warringa RA, Schweizer RC, Maikoe T, Kuijper PH, Bruijnzeel PL, Koendermann L. Modulation of eosinophil chemotaxis by interleukin-5. Am J Respir Cell Mol Biol. 1992;7:631–636. doi: 10.1165/ajrcmb/7.6.631. [DOI] [PubMed] [Google Scholar]