Abstract
Acute lung injury (ALI) is a severe hypoxemic respiratory insufficiency associated with lung leak, diffuse alveolar damage, inflammation, and loss of lung function. Decreased dimethylaminohydrolase (DDAH) activity and increases in asymmetric dimethylarginine (ADMA), together with exaggerated oxidative/nitrative stress, contributes to the development of ALI in mice exposed to LPS. Whether restoring DDAH function and suppressing ADMA levels can effectively ameliorate vascular hyperpermeability and lung injury in ALI is unknown, and was the focus of this study. In human lung microvascular endothelial cells, DDAH II overexpression prevented the LPS-dependent increase in ADMA, superoxide, peroxynitrite, and protein nitration. DDAH II also attenuated the endothelial barrier disruption associated with LPS exposure. Similarly, in vivo, we demonstrated that the targeted overexpression of DDAH II in the pulmonary vasculature significantly inhibited the accumulation of ADMA and the subsequent increase in oxidative/nitrative stress in the lungs of mice exposed to LPS. In addition, augmenting pulmonary DDAH II activity before LPS exposure reduced lung vascular leak and lung injury and restored lung function when DDAH activity was increased after injury. Together, these data suggest that enhancing DDAH II activity may prove a useful adjuvant therapy to treat patients with ALI.
Keywords: dimethylarginine dimethylaminohydrolase II, asymmetric dimethylarginine, nitrative stress, acute lung injury, gene delivery
Clinical Relevance
Nitrosative stress is now recognized as an important player in acute lung injury (ALI). However, the mechanisms underlying this process are unclear. Our data show that increases in asymmetric dimethylarginine (ADMA) lead to nitrosative stress, and scavenging ADMA, through dimethylarginine dimethylaminohydrolase (DDAH) overexpression, prevents ALI in vivo. Thus, targeting the ADMA/DDAH pathway may have clinical utility for the treatment of ALI/acute respiratory distress syndrome.
Acute lung injury (ALI) is an inflammatory disorder associated with high morbidity and mortality. ALI can occur in response to a number of direct insults, such as viral or bacterial infections of the lung, hyperoxia, and acid aspiration, or indirect insults, such as sepsis, multiple transfusions, and pancreatitis. LPS from the outer cell wall of gram-negative bacteria can induce a systemic inflammatory response, which is the most common indirect pulmonary insult leading to ALI (1). LPS provokes damage to the alveolar–capillary membrane and the adhesion, activation, and sequestration of polymorphonuclear neutrophils, which result in the deterioration of gas exchange and increased pulmonary capillary permeability (2). A number of ventilator and pharmacologic therapies, such as corticosteroids, prostacyclins, exogenous surfactants, ketoconazole, and nitric oxide (NO) (3), have been tested in large, randomized trials, but, to date, only mechanical ventilation with low tidal volumes has been shown to improve survival (4). Therefore, there is a further need to study the pathophysiology of ALI and identify new therapeutic targets to improve patient outcome.
Recently completed studies from our laboratory have indicated that asymmetric dimethylarginine (ADMA) levels are increased during ALI (5). High plasma levels of ADMA have previously been associated with cardiovascular disorders, including atherosclerosis, hypertension, homocysteinemia, diabetes mellitus, insulin resistance, and hypercholesterolemia (6–10). Increased ADMA levels impair endothelial function in these cardiovascular diseases (6–10), but the role of ADMA in the endothelial barrier disruption associated with ALI is unknown. ADMA inhibits NO production by competing with L-arginine for binding to NO synthase (NOS) (11). In addition, ADMA can “uncouple” NOS by shifting the balance of NO production to superoxide generation (12). We have shown, both in vitro and in vivo, that increased levels of ADMA lead to an increase in NOS uncoupling and enhanced cellular generation of peroxynitrite (5, 13). Several studies support the view that the ratio between L-arginine and ADMA is a key component in the regulation of endothelial NOS (eNOS) activity, and elevated ADMA levels have been demonstrated to antagonize NOS signaling in humans (6–10).
ADMA is metabolized via hydrolytic degradation to citrulline and dimethylamine by the enzymes, dimethylarginine dimethylaminohydrolase (DDAH) I and II (10). Our recently completed study indicated that ADMA levels are increased in the lungs of mice exposed to LPS secondary to a decrease in DDAH activity (5). In the present study, we found that increasing DDAH II activity in vitro and in vivo attenuated the LPS-induced increase in ADMA, reduced both oxidative and nitrative stress, and prevented the disruption of endothelial barrier function, suggesting that the DDAH–ADMA axis may be a potential therapeutic target for the management of patients with ALI. Furthermore, we found that increasing DDAH activity could both preserve and restore lung function.
Materials and Methods
Animal Treatments
Adult male C57BL/6NHsd mice (7–8 wk; Harlan, Indianapolis, IN) were used in all experiments. All animal care and experimental procedures were approved by the Committee on Animal Use in Research and Education of the Georgia Regents University. The pre- and postinjury models induced by LPS exposure are described in the Materials and Methods in the online supplement.
In Vivo Overexpression of DDAH II
In vivo polyethyleneimine derivative transfection reagent was used to deliver pAd/CMV/V5-DEST-DDAH II cDNA or pDST-luciferase to the mouse lung endothelium via a tail vein injection, as described in the Materials and Methods in the online supplement.
Luciferase Activity
The luciferase activity in total protein extracts prepared from brain, liver, lung, heart, and kidney was determined 1–7 days after pDST-luciferase injection using the luciferase assay system (Promega, Madison, WI).
Generation of DDAH II Adenoviral Expression Vector
An adenovirus expressing the human DDAH II coding sequence was generated as described in the Materials and Methods in the online supplement.
Cell Culture
Primary cultures of human lung microvascular endothelial cells (HLMVECs) were isolated, as described previously (14).
Western Blot Analysis
Immunoblot analysis was as previously described (15, 16). The blots were probed with custom-made antibodies against DDAH I (1:500 dilution) and DDAH II (1:500 dilution), as described previously (17). Protein loading was normalized by reprobing the membranes with an antibody specific to β-actin.
Measurement of Peroxynitrite and Protein Nitration Levels
Peroxynitrite and protein nitration level measurement was performed as described in the Materials and Methods in the online supplement and previously described (15).
In Vitro Permeability Assays
In vitro permeability was estimated by measuring transendothelial resistance (TER), a transwell permeability assay, and cellular gap analysis, as described in the Materials and Methods in the online supplement.
Cell Imaging
Cell imaging was performed as described in the Materials and Methods in the online supplement.
DDAH Activity Assay
Total DDAH activity was determined using a radioactive assay to measure the conversion of N-a-Methyl-(L)-arginine [4,5-3H] to [3H]-L-citrulline, as described in the Materials and Methods in the online supplement.
Measurement of ADMA Levels
ADMA levels were analyzed by HPLC, as we have previously described (5).
Determination of Superoxide Levels
NOS-derived superoxide levels were estimated by electronic paramagnetic resonance assay using the spin-trap compound 1-hydroxy-3-methoxycarbonyl-2,2,5,5-tetramethylpyrrolidine HCl (Axxora LLC, Farmingdale, NY) in the presence of ethylisothiourea (100 μM, 30 min; Sigma-Aldrich, St. Louis, MO), as previously described (18).
Isolation of Bronchoalveolar Lavage Fluid
Isolation of bronchoalveolar lavage fluid (BALF) was performed as described in the Materials and Methods in the online supplement.
Cytokine/Chemokine Measurement in the BALF
A total of 32 different cytokines/chemokines were assessed in the BALF, as described in the Materials and Methods in the online supplement.
Myeloperoxidase Staining and Histological Analysis of the Mouse Lung
Myeloperoxidase (MPO) staining and histological analysis of the mouse lung were performed as described in the Materials and Methods in the online supplement.
MPO Activity
MPO activity in snap-frozen mouse lung tissue was determined using an MPO assay kit (BioVision, Inc., Milpitas, CA), according to the manufacturer’s instructions.
Determination of DDAH II Localization
Determination of DDAH II localization was performed as described in the Materials and Methods in the online supplement.
Vascular Leak Assessment In Vivo
Vascular leak assessment in vivo was performed as described in the Materials and Methods in the online supplement and as previously described (19).
Assessment of Respiratory Mechanics
Lung function tests were performed as described in the Materials and Methods in the online supplement.
Statistical Analysis
Statistical analysis was performed as described in the Materials and Methods in the online supplement.
Results
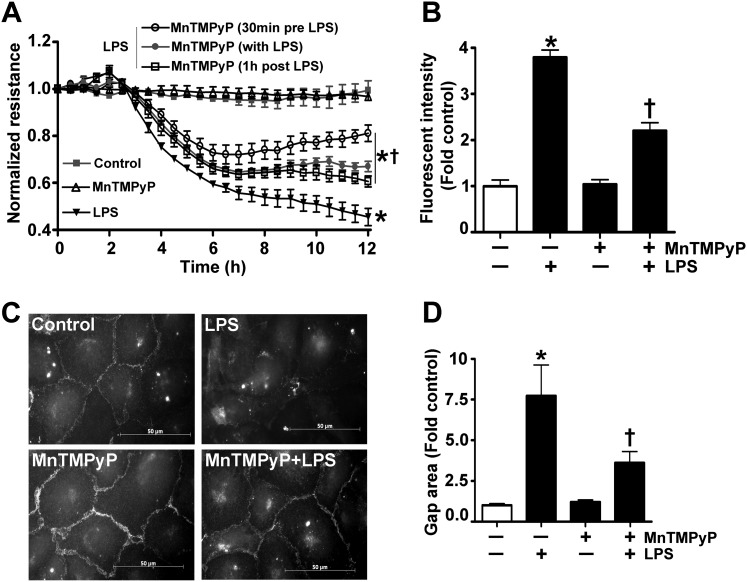
We have previously reported that LPS increases superoxide and peroxynitrite levels, and that the scavenging of these free radicals can reduce lung leak in mice treated with LPS (5). To determine if LPS exerts a similar effect in culture, we exposed HLMVECs to LPS in the presence or absence of the peroxynitrite scavenger, Mn(III)tetrakis(1-methyl-4-pyridyl)porphyrin, and measured changes in endothelial barrier function. Our results show that Mn(III)tetrakis(1-methyl-4-pyridyl)porphyrin attenuated the LPS-induced decrease in TER (Figure 1A), the LPS-mediated increase in transendothelial flux of FITC-dextran (Figure 1B), and the formation inter- and intra-cellular gaps (Figures 1C and 1D), indicating that oxidative/nitrative stress is involved in LPS-mediated barrier dysfunction in HLMVECs.
Figure 1.
Peroxynitrite scavenging attenuates the LPS-mediated decrease in barrier function in human lung microvascular endothelial cells (HLMVECs). HLMVECs were challenged with LPS (1 EU/ml, 0–12 h) in the presence or absence of the peroxynitrite scavenger, Mn(III)tetrakis(1-methyl-4-pyridyl)porphyrin (MnTmPyP; 25 μM, 30 min before LPS, with LPS, and 1 h after LPS treatment). LPS decreased the normalized transendothelial resistance (TER), and this was significantly attenuated by MnTMPyP (A). Similarly, the LPS-mediated (1 EU/ml, 4 h) extravasation of FITC-dextran was significantly reduced in cells pretreated with MnTMPyP (25 μM) (B). In addition, immunofluorescent analysis of vascular endothelial–cadherin (C) indicates that the LPS-mediated increase in gap formation was also attenuated by MnTMPyP (D). Values are mean ± SEM (n = 3–7). *P < 0.05 versus control, †P < 0.05 versus LPS alone.
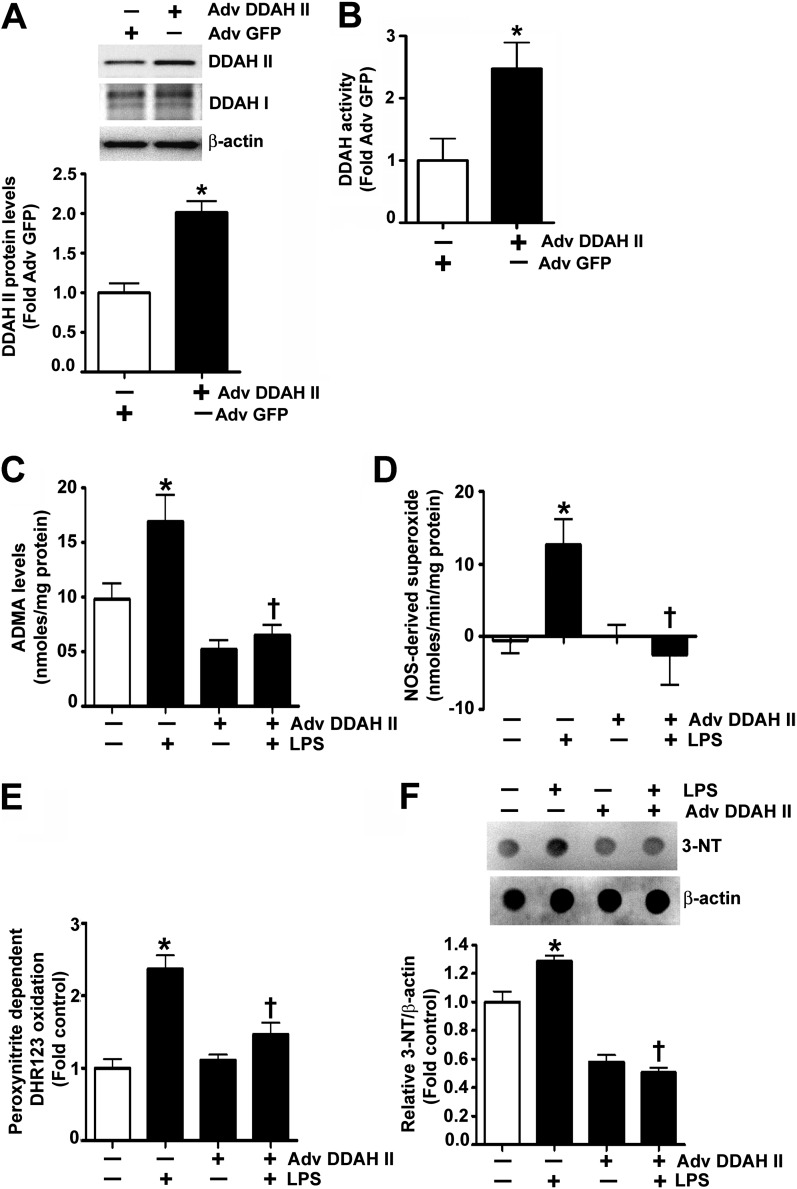
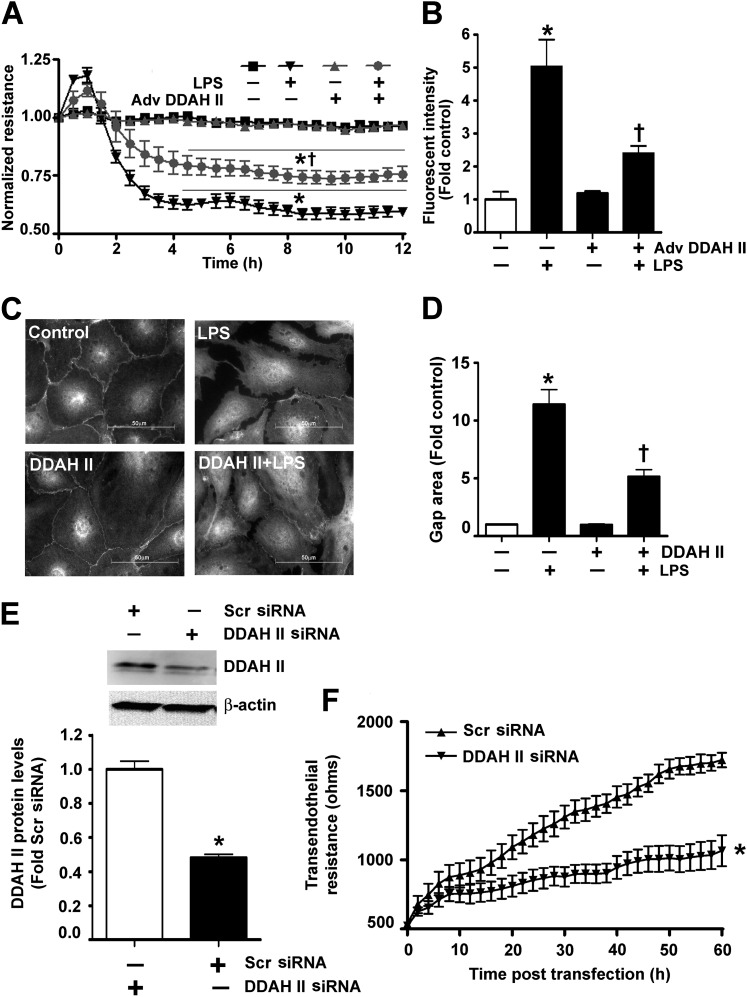
In a recent study, we correlated increased peroxynitrite generation with increased ADMA-mediated NOS uncoupling (5). To begin to determine if DDAH II overexpression could prevent these changes, we initially developed an adenoviral expression construct for DDAH II (Adv DDAH II). The transduction of HLMVECs with Adv DDAH II significantly increased both DDAH II protein levels (Figure 2A) and DDAH activity (Figure 2B). In HLMVECs exposed to LPS, there was a significant increase in ADMA levels (Figure 2C), and this correlated with an increase in NOS-derived superoxide (Figure 2D), peroxynitrite generation (Figure 2E), and nitrated proteins (Figure 2F). However, when HLMVECs were transduced with Adv DDAH II, the LPS-mediated increase in ADMA levels (Figure 2C), NOS-derived superoxide (Figure 2D), peroxynitrite formation (Figure 2E), and protein nitration (Figure 2F) were all significantly decreased. In addition, DDAH II overexpression attenuated the LPS-mediated disruption of the endothelial barrier, as demonstrated by a preservation of TER (Figure 3A), an attenuation of the transendothelial flux of FITC-dextran (Figure 3B), and a decrease in the formation of inter- and intra-cellular gaps (Figures 3C and 3D). In contrast, the small interfering RNA–mediated knockdown of DDAH II in HLMVECs, which resulted in a 50% reduction in DDAH II protein (Figure 3E), significantly decreased basal TER (Figure 3F).
Figure 2.
Dimethylarginine dimethylaminohydrolase (DDAH) II attenuates LPS-induced increase in asymmetric dimethylarginine (ADMA), superoxide, and peroxynitrite levels in HLMVECs. HLMVECs were transduced with adenoviruses expressing either GFP (Adv GFP; as a control) or DDAH II (Adv DDAH II) for 48 hours. Immunoblot analysis demonstrated a twofold increase in DDAH II protein without alterations in DDAH I protein (A). DDAH activity, estimated by the conversion of N-a-Methyl-(L)-arginine [4,5-3H] to [3H]-L-citrulline, was also significantly increased in the DDAH II–overexpressing cells (B). HLMVECs were also challenged with LPS (1 EU/ml, 4 h) after transduction with Adv GFP (as a control) or Adv DDAH II. LPS exposure increased ADMA levels in the Adv GFP–transduced cells, but not in the cells transduced with Adv DDAH II (C). LPS induced a significant increase in nitric oxide (NO) synthase (NOS)–derived superoxide radical generation in the control cells, and this was attenuated in cells overexpressing DDAH II (D). Nitrative stress was estimated using the peroxynitrite-dependent oxidation of dihydrorhodamine (DHR) 123 to rhodamine 123 and 3-nitrotyrosine (3-NT) levels using dot blot analysis. The LPS-mediated increase in peroxynitrite (E) and 3-NT (F) in the control cells was prevented by DDAH II overexpression. Values are mean ± SEM (n = 3–5). *P < 0.05 versus control, †P < 0.05 versus LPS.
Figure 3.
DDAH II overexpression prevents the LPS-induced barrier disruption in HLMVECs. HLMVECs were transduced with Adv GFP (as a control) or Adv DDAH II. After 48 hours, the cells were challenged with LPS (1 EU/ml, 0–12 h). TER was decreased in response to LPS in the control cells, whereas, in the cells overexpressing DDAH II, the decrease in TER was significantly attenuated (A). Similarly, the LPS-mediated (1 EU/ml, 4 h) extravasation of FITC-dextran was significantly attenuated in cells overexpressing DDAH II (B). HLMVECs were also transfected with either pcDNA3 (control) or pcDNA3-DDAH II-His6 plasmid (DDAH II), and exposed to LPS (1 EU/ml, 4 h). Immunofluorescent analysis of vascular endothelial–cadherin (C) indicated that the LPS-mediated increase in gap formation was significantly attenuated in His6-tagged DDAH II–expressing cells (D). When HLMVECs were transfected with either a scrambled small interfering RNA (siRNA) or DDAH II siRNA for 60 hours to reduce DDAH II protein levels (E), basal TER was significantly attenuated (F). Values are mean ± SEM (n = 4–5). *P < 0.05 versus control or scrambled (Scr) siRNA, †P < 0.05 versus LPS.
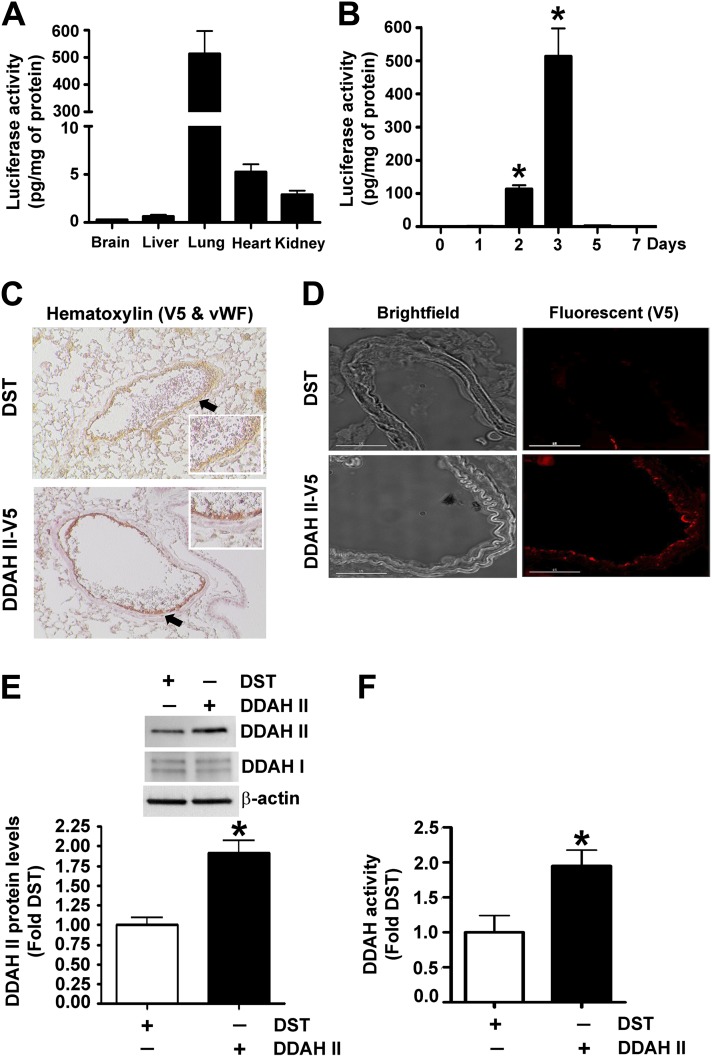
To determine if the loss of DDAH activity plays an important role in the development of ALI in vivo, we used the polyethyleneimine derivative transfection reagent to deliver a DDAH II expression plasmid into the mouse lung. Initial experiments verified that the tail vein injection of a luciferase reporter plasmid (pDST-luciferase) resulted in the preferential expression of the plasmid in the lung (Figure 4A), and that the expression peaked in the lung 3 days after injection (Figure 4B). Furthermore, when a V5-tagged DDAH II expression plasmid was used, immunohistochemical analysis identified preferential expression in the pulmonary endothelium, as evidenced by the colocalization of DDAH II and the endothelial marker, Von Willebrand factor, on a hematoxylin background (Figure 4 C) and immunofluorescent staining (Figure 4D). Using this technique, we were able to identify a significant increase in DDAH II protein levels (Figure 4E) and catalytic activity (Figure 4F) in mouse lung.
Figure 4.
The overexpression of DDAH II in the mouse lung. The relative amount of luciferase activity was detected in homogenates of brain, liver, lung, heart, and kidney after tail vein injection of pDST-luciferase (40 μg) complexed with polyethyleneimine derivative transfection reagent (Jet-PEI). Luciferase activity was found to be predominantly localized to the lung (A). Maximum luciferase activity in the lung was detected 3 days after injection (B). Mice were injected with either DST-luciferase (DST) or pAD/CMV/V5-DEST-DDAH II (DDAH II-V5) plasmid via tail vein injection, and DDAH II localization was determined. The image in (C) shows hematoxylin staining with V5-tagged DDAH II (V5; red) and vascular endothelial cells labeled with anti-Von Willebrand factor (vWF; brown). The arrows indicate vWF (brown) staining in the control mice, whereas the reddish-brown staining in the V5-tagged DDAH II tissues is a result of a merger of vWF (brown) and V5 (red). (D) Bright-field images (left) of pulmonary vessels and fluorescent images (right) of V5-tagged DDAH II (V5; red). The results from four separate injections show a predominant expression of DDAH II localized to the endothelial layer (C–D). Furthermore, immunoblot analysis demonstrated a significant increase in DDAH II protein levels (E), whereas the increased conversion of N-a-Methyl-(L)-arginine [4,5-3H] to [3H]-L-citrulline demonstrated an increase in DDAH activity, in the lungs receiving DDAH II-V5 plasmid (F). Values are mean ± SEM (n = 4–6 per group). *P < 0.05 versus 0 days (B) and DST (E and F).
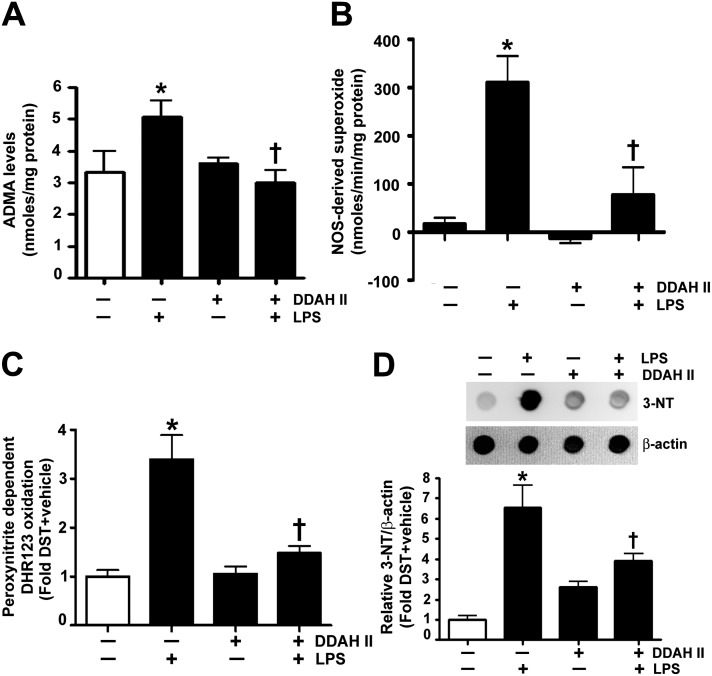
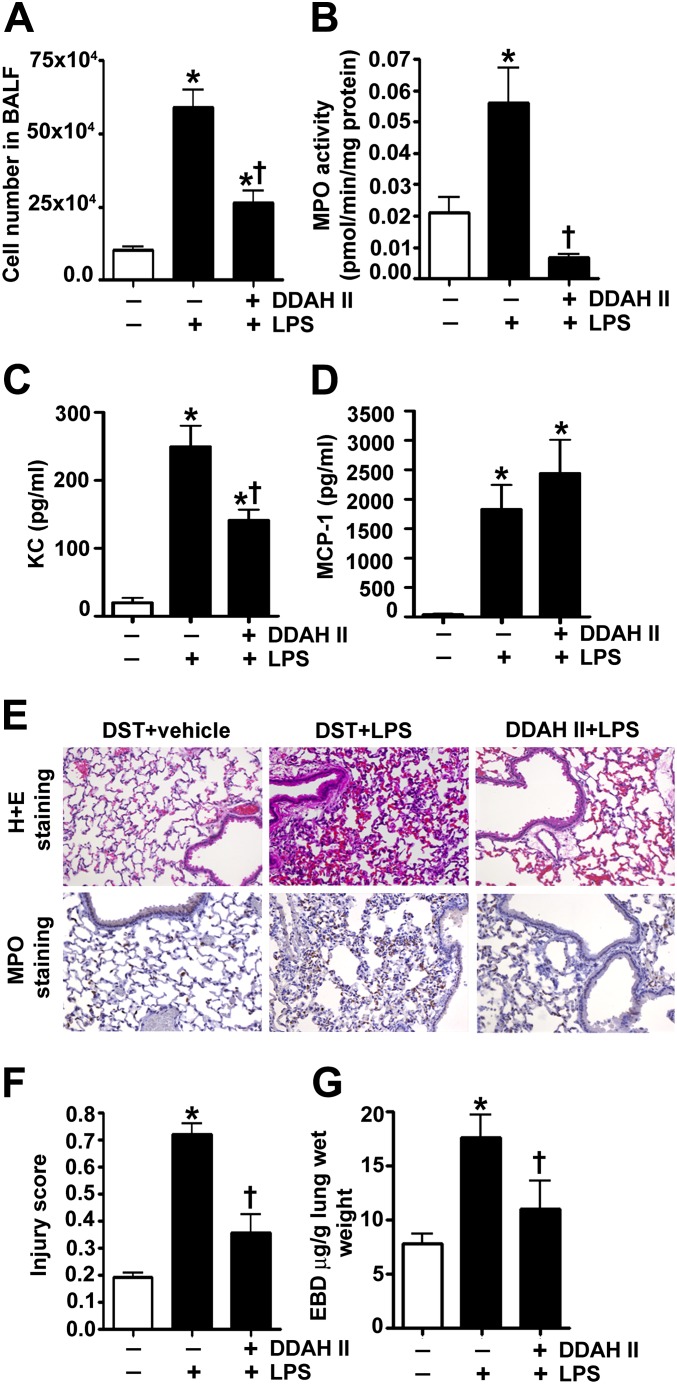
In agreement with our in vitro results, we found that the gene delivery of the V5-tagged DDAH II expression plasmid, before the LPS-induced insult, significantly attenuated the increases in ADMA (Figure 5A), NOS-derived superoxide (Figure 5B), peroxynitrite (Figure 5C), and protein nitration (Figure 5D). In addition, analysis of the BALF indicated that DDAH II significantly prevented LPS-mediated cellular infiltration (Figure 6A). Using MPO activity (Figure 6B), we found that DDAH II overexpression also reduced neutrophil infiltration in the LPS-treated mouse lungs. The BALF was also analyzed for the presence of 32 cytokines/chemokines. Our results demonstrate that LPS significantly increased the levels of 28 cytokines and chemokines (see Table E1 in the online supplement), including the neutrophil chemoattractant, keratinocyte-derived chemokine (KC; Figure 6C) and monocyte chemoattractant protein (MCP) -1 (Figure 6D). The overexpression of DDAH II attenuated the levels of 13 cytokines induced by LPS (Table E1), including KC (Figure 6C). However, DDAH II did not prevent the increase in MCP-1 (Figure 6D). Lung sections stained with MPO and hematoxylin and eosin indicated that DDAH II protected the lungs against LPS-induced alveolar damage and histopathological changes, such as the permeation of leukocytes and red blood cells in the alveolar and interstitial space, the formation of hyaline membranes, septal thickening, and debris accumulation in the alveoli (Figure 6E). We also assessed the severity of the lung injury using a semiquantitative histopathological scoring system (20), which determines the thickness of alveolar septae, alveolar hemorrhage, intra-alveolar fibrin accumulation, and intra-alveolar infiltration. DDAH II overexpression attenuated the lung injury score in the LPS-treated mice (Figure 6F). Finally, we found that DDAH II overexpression significantly attenuated the LPS-induced vascular leak, as indicated by a reduction in the extravasation of Evan’s blue dye (Figure 6G).
Figure 5.
DDAH II overexpression attenuates the LPS-induced increase in ADMA, superoxide, and peroxynitrite in the mouse lung. Mice were injected with either DST-luciferase (DST) or pAD/CMV/V5-DEST-DDAH II (DDAH II) plasmid via the tail vein for 60 hours, followed by either saline (vehicle) or LPS (6.75×104 EU/g body weight, 12 h) delivered intraperitoneally. The LPS-dependent increase in ADMA was significantly attenuated in mice with increased DDAH II expression (A). The LPS-mediated increase in NOS-derived superoxide generation was also significantly mitigated in mice with increased DDAH II expression (B). The LPS-induced increase in nitrative stress, as determined by the peroxynitrite-dependent oxidation of DHR 123 to rhodamine 123 (C) and 3-NT levels (D), was also significantly diminished in mice with increased DDAH II expression. Values are means ± SEM (n = 4–6). *P < 0.05 versus DST + vehicle, †P < 0.05 versus DST + LPS.
Figure 6.
The overexpression of DDAH II in the mouse lung prevents endothelial barrier dysfunction and lung injury in response to LPS. Mice were injected with either DST-luciferase (DST) or pAD/CMV/V5-DEST-DDAH II (DDAH II) plasmid via tail the vein for 60 hours, followed by saline (vehicle) or LPS (6.75×104 EU/g body weight, 12 h) intraperitoneally. Bronchoalveolar lavage fluid (BALF) was collected. Total cell count in BALF was increased after LPS exposure, and this was significantly attenuated in DDAH II–overexpressing mice (A). Lung myeloperoxidase (MPO) activity (which indicates neutrophil sequestration in the lung) was similarly attenuated in DDAH II–overexpressing mice (B). LPS exposure also significantly increased the levels of keratinocyte-derived chemokine (KC) (C) and monocyte chemoattractant protein (MCP) -1 (D). DDAH II overexpression attenuated the increase in KC (C), but not MCP-1 (D). Lung sections were examined for signs of inflammation after hematoxylin and eosin (H + E) staining and neutrophil infiltration after MPO staining (E) (representative micrographs are shown), and scored for lung injury (F). DDAH II overexpression significantly attenuated MPO staining (E) and lung injury score (F). The capillary leak (inferred from Evan’s Blue dye [EBD] extravasation in the lung and expressed as the ratio of EBD to lung wet weight) induced by LPS (6.75×104 EU/g body weight, 18 h) was also significantly attenuated in DDAH II–overexpressing mice (G). Values are mean ± SEM (n = 4–7). *P < 0.05 versus DST + vehicle, †P < 0.05 versus DST + LPS.
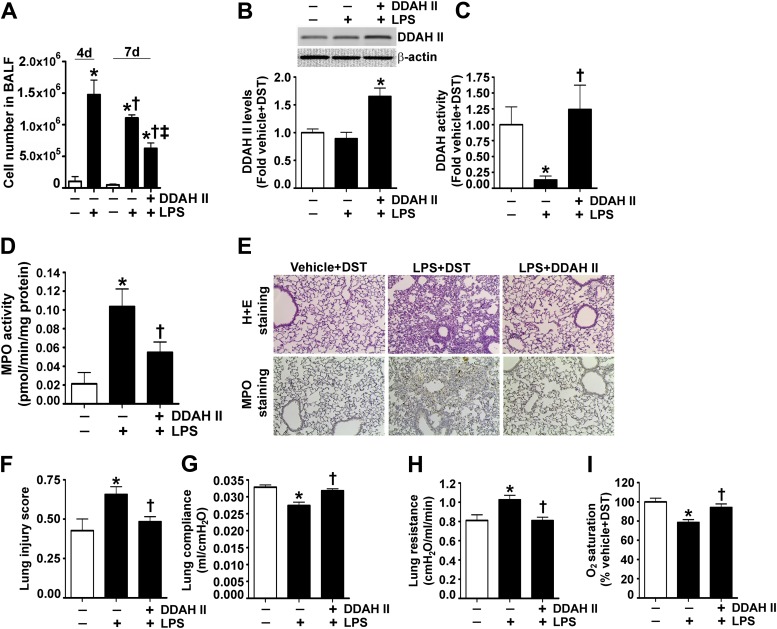
Finally, to determine the therapeutic potential of DDAH II, we developed a postinjury model by exposing mice to intratracheal LPS (1.25 mg/kg body weight). This protocol induced significant lung injury 4 days after the insult, as determined by an increase in cell number in the BALF (Figure 7A). We then delivered the V5-tagged DDAH II expression plasmid via tail vein injection on Day 4, and found a significant increase in DDAH II protein levels (Figure 7B) and activity (Figure 7C) in the lungs of mice 3 days after gene delivery (Day 7 after LPS). Analysis of the BALF indicated that DDAH II significantly reduced cellular infiltration on Day 7 after LPS, suggesting that DDAH II overexpression was accelerating the resolution phase (Figure 7A). Similarly, MPO activity was significantly reduced when DDAH II was delivered on Day 4 after LPS (Figure 7D). The staining of lung sections with MPO and hematoxylin and eosin suggested that DDAH II overexpression after LPS was able to accelerate lung recovery, as both the alveolar damage and histopathological changes were significantly reduced (Figure 7E). This reduced the lung injury score on Day 7 after LPS (Figure 7F). Finally, we found that DDAH II overexpression significantly improved lung function, as measured by increased compliance (Figure 7G), decreased resistance (Figure 7H), and higher oxygen saturation (Figure 7I).
Figure 7.
DDAH II overexpression enhances lung repair after LPS exposure. Mice received either saline (vehicle) or LPS (1.25 mg/kg body weight) intratracheally. At 4 days after LPS delivery, the mice had significant lung injury, as indicated by an increase in total cell number in the BALF (A). A separate set of mice were injected, via the tail vein, with plasmids for either pDST-luciferase (DST) or pAD/CMV/V5-DEST-DDAH II (DDAH II) 4 days after LPS administration, and then examined 3 days later (7 d after LPS). DDAH II overexpression in the mouse lung increased DDAH II protein levels (B), restored DDAH II catalytic activity (C), and significantly attenuated cell numbers in the BALF on Day 7 after LPS (A), and attenuated lung MPO activity (D). Lung sections were stained with H + E for indicators of inflammation and MPO for neutrophil infiltration (E) (representative micrographs are shown), and scored for lung injury (F). DDAH II overexpression attenuated MPO staining and reduced lung cellularity (E) and lung injury score (F). DDAH II–overexpressing mice also had increased lung compliance (G), decreased lung resistance (H), and increased lung oxygen saturation (I). Values are mean ± SEM (n = 7–9). *P < 0.05 versus vehicle, †P < 0.05 versus LPS (4 d [A]), ‡P < 0.05 versus DST + LPS (7 d [A]), and †P < 0.05 versus DST + LPS (7 d [B–I]).
Discussion
The findings we present here underline the important role of diminished DDAH activity in LPS-induced lung injury. Our data suggest that attenuating ADMA levels or increasing DDAH activity prevents NOS uncoupling, reduces superoxide generation, and inhibits oxidative/nitrative damage. Although the NOS isoform responsible for the increase in superoxide in our study has not yet been determined, ADMA is known to induce the uncoupling of all the NOS isoforms (15, 24), and is an effective inhibitor of NO production from eNOS (8), but only a weak inhibitor of NO production by inducible NOS (iNOS) (25). As LPS induces iNOS protein levels in the mouse lung (5), it is possible that the increased NOS-derived superoxide and peroxynitrite formation that we observed could be generated from both iNOS and eNOS. In addition, our data indicate that DDAH II overexpression limits the inflammatory response and promotes vascular barrier integrity. Furthermore, the results obtained using a postinjury model of ALI that we developed suggest that DDAH II may also facilitate the recovery from ALI. This was evident from our data showing that, in comparison to Day 4 after LPS, the BALF cellularity on Day 7 after LPS was significantly lower in DDAH II–overexpressing mice.
Our data also show that, although DDAH activity is reduced in the mouse lung exposed to LPS, DDAH protein levels are unchanged, suggesting that a post-translational mechanism is involved. The DDAH II isoform is thought to be oxidant sensitive (21) and the predominant endothelial isoform (12, 36). In an earlier study, oxidative stress induced by oxidized low-density lipoprotein or TNF-α decreased DDAH activity, but not its protein expression in endothelial cells (22). Lin and colleagues (38) have shown that elevated glucose raises endothelial ADMA levels by inhibiting DDAH activity via a mechanism involving oxidative stress. Similarly, in our previous report, we found that LPS decreased DDAH activity within 2 hours in mouse lung (5), suggesting that the attenuation of DDAH activity is an early post-translational event in the continuum of lung injury. The post-translational events responsible for the attenuation of DDAH activity seem to be dependent on the nature of the oxidative stress. For example, it has been reported that DDAH activity is reversibly inhibited when recombinant DDAH II or the cytosolic extracts from rat kidneys are incubated with NO donors (23). In cultured endothelial cells, heterologously expressed human DDAH II has been shown to be S-nitrosylated after cytokine induced expression of iNOS (39). This observation may partially explain the loss of DDAH activity and the increase in ADMA levels in LPS-treated mice, as iNOS expression is induced (5). However, it is likely that other mechanisms are involved, as a significant decrease in DDAH activity was observed 2 hours after LPS exposure, whereas a significant increase in NO levels and iNOS expression did not occur until 4 hours after LPS treatment (5).
Although our data demonstrate that LPS induces both oxidative and nitrative stress in the mouse lung, antioxidants and other similar therapies targeting oxidative damage have shown only limited efficacy in human trials. For instance, in a small clinical trial, N-acetylcysteine caused the repletion of glutathione in patients with ALI, but only moderately improved lung function (42). In addition, the use of NOS inhibitors has failed to reduce oxidative stress, and have even tended to increase mortality in mice (24) and in patients with severe sepsis (25). Although the administration of an iNOS inhibitor improved survival when given 12 hours after peritonitis-induced sepsis in rats, the same intervention increased mortality when given 6 hours earlier (26). In addition, the antioxidant, nacystelyn, inhibited LPS-induced IL-8 and transforming growth factor-β1, but failed to mitigate the levels of TNF-α, transforming growth factor-β3, macrophage inflammatory protein (MIP)-1α and -β, and regulated on activation, normal T cell expressed and secreted in LPS-treated cells (27). In contrast, our data using DDAH II overexpression reduced the levels of 13 proinflammatory cytokines, suggesting that DDAH II may have additional anti-inflammatory effects. Although the exact mechanism by which DDAH II attenuates these cytokines is not known, at least one previously published report has suggested that DDAH II enhances the expression of the anti-inflammatory factor, cortistatin (28). In the lungs of endotoxemic mice, cortistatin reduced the levels of several cytokines, such as IL-6, IL-12, and MIP (29). Furthermore, our data indicate that DDAH II overexpression attenuated the LPS-induced increase in multiple cytokine pathways, including IL-2, IL-6, and IL-12. These cytokines have been previously reported to play significant roles in promoting endothelial barrier disruption. For example, IL-2, an important cytokine necessary for the differentiation and survival of CD4/CD8+ effector T cells, is responsible for the development of vascular leak syndrome in cancer immunotherapy (30). In addition, IL-6 has been widely known to mediate hypoxia-induced endothelial permeability (31). Furthermore, IL-12, which is involved in differentiation of naive T cells, has been demonstrated to up-regulate the release of vascular permeability factor (vascular endothelial growth factor) (32). Therefore, the attenuation of these cytokines provides an additional mechanism by which DDAH II may preserve endothelial barrier function and also limit the inflammation induced by LPS. Furthermore, the attenuation of numerous cytokines by DDAH II suggests that this effect could be mediated by the regulation of a master transcription factor(s). Interestingly, high levels of ADMA or decreased DDAH activity have been associated with an increase in NF-κB binding in airway epithelial cells (33). The NF-κB family of transcription factors is known to regulate the expression of multiple cytokines and chemokines. For instance, a mutation in the IL-6 promoter at the binding site of NF-κB impairs IL-6 promoter activation in response to LPS (34), whereas the binding of NF-κB to the IL-12 promoter in macrophages induces the expression and release of IL-12 (35). In addition, the targeting of NF-κB to the IL-13 promoter in Hodgkin’s disease is an important proinflammatory event in the pathogenesis of the disease (36). NF-κB exists in the cytoplasm in complex with an inhibitor protein, IκBα, which masks the NF-κB nuclear localization sequence, and we have previously shown that the nitration of IκBα at tyrosine-181 leads to the dissociation of IκBα from the binding site on NF-κB, and subsequently activates the transciption factor (37). Thus, we speculate that the decreased oxidative and nitrative stress associated with DDAH II overexpression could potentially inhibit the dissociation of IκBα from NF-κB, and therefore prevent NF-κB activation and the subsequent up-regulation of the inflammatory cytokines normally associated with LPS administration.
Previous studies have shown that over 90% of ADMA is metabolized by DDAH. Whether this phenomenon is predominantly due to the activity of the DDAH I or DDAH II isoform is a subject of controversy. Both isoforms of DDAH are expressed in the lungs (38), although the protein levels of DDAH II are significantly higher than those of DDAH I (38). Interestingly, under physiological conditions, although its expression is lower, it is believed that DDAH I rather than DDAH II regulates the level of ADMA (38, 39). However, under pathologic conditions, it appears that the activity of DDAH II becomes more predominant, as it was shown in a mouse model of allergically inflamed lungs (21), and in an ovine model of smoke inhalation lung injury (40), that the increased ADMA levels correlated with reduced DDAH II protein/activity. Similarly, in our study, we found that the overexpression of DDAH II attenuated the LPS-induced increase in ADMA levels in both endothelial cells and the mouse lung. Conversely, in an earlier study, the overexpression of the DDAH I isoform alone was not able to restore the loss of NOS signaling secondary to increased ADMA levels in endothelial cells treated with the lipid hydroperoxide degradation product, 4-hydroxy-2-nonenal (41). However, it is evident from our data that the attenuation of DDAH activity and the subsequent increase in ADMA levels is an important event regulating the levels of reactive oxygen species and nitrogen species. These findings are in agreement with a previous study where the LPS-mediated increase in reactive oxygen species in endothelial cells was enhanced by exogenous ADMA and attenuated by either exogenous L-arginine or the overexpression of DDAH II (42). Similarly, in a murine model of allergic airway inflammation, decreased levels of DDAH II correlated with an increase in peroxynitrite generation, which was attenuated by the administration of L-arginine (21). In our study, we found that the elevated cellular superoxide levels were NOS dependent, and that the overexpression of DDAH II restored NOS coupling. Moreover, when we overexpressed DDAH II, we found that the endothelial barrier dysfunction associated with LPS exposure was attenuated.
Although we were unable to determine the exact mechanisms by which DDAH II protects the endothelial barrier, our data suggest possible avenues for this protection. It is likely that the preservation of NO signaling is involved (43). In support of this concept, prior studies have shown that decreased NO generation in the pulmonary circulation in eNOS−/− mice (43), and the inhibition of NOS in the isolated perfused rabbit lung (44), causes microvessel leakage, suggesting that, at physiological levels, NO plays an essential role in promoting endothelial barrier function. However, it should be noted that the decrease in ADMA levels with DDAH overexpression might promote endothelial barrier integrity independently of increased NO signaling. For instance, a recent study showed in porcine pulmonary endothelial cells that ADMA increased stress fiber formation through the stimulation of RhoA–Rho kinase signaling (39). Furthermore, ADMA also inhibited the activities of Rac1 and Cdc42 in these cells (28). The Rho family of GTPases is known to be involved in regulating the endothelial barrier. The activation of RhoA increases endothelial permeability by increasing actomyosin contractility and intercellular gap formation (45). In contrast, Rac1 activity is required for the assembly, maintenance, and recovery of endothelial intercellular junctions (46). Cdc42 has been shown to promote junction recovery after thrombin treatment in human pulmonary arterial endothelial cells (35). Thus, in our case, it is possible that the low levels of ADMA associated with DDAH II overexpression could keep the RhoA–Rac1 balance tilted toward barrier enhancement.
Furthermore, our data suggest that the LPS-mediated decreases in DDAH activity and the resulting increases in ADMA may be involved in the recruitment and activation of inflammatory cells, such as neutrophils and monocytes/macrophages in the lung. Previous studies have shown that the loss of DDAH activity can augment the levels of cytokines, such as IL-1, IL-6, IL-8, and MCP-1 (28, 47), and therefore potentiate inflammation. LPS-induced cytokines, such as TNF-α, have also been shown to decrease DDAH activity and enhance ADMA levels (28, 48), providing a possible mechanism by which LPS regulates the DDAH/ADMA cascade. In our study, we analyzed a profile of 32 cytokines/chemokines in the BALF of mice treated with LPS, and found an increase in 28 of these cytokines, including IL-1, IL-6, MCP-1, and TNF-α (Table E1). The overexpression of DDAH II attenuated the LPS-induced increase in 13 of these cytokines (Table E1). Although it is unclear, and beyond the scope of this study to determine, why DDAH II attenuated certain cytokines and not others, based on our data, we can speculate. It is well established that the sequence of acute inflammation in the lung and other organs is characterized by an early influx of neutrophils, followed by a late increase in monocytes/macrophages. KC is a major chemoattractant for neutrophils, whereas the recruitment of macrophages is highly dependent on the release of MCP-1 from the neutrophils. Our data demonstrate that the levels of KC and MCP-1 were elevated in the BALF of LPS-treated mice. DDAH II overexpression attenuated the levels of KC, but not MCP-1. In addition, DDAH II also increased macrophage chemoattractants, such as MIP-2, but decreased MIP-1, IFN-γ–induced protein 10, and macrophage colony–stimulating factor. Thus, our data suggest that maintaining DDAH activity appears to attenuate the early phase of inflammation by inhibiting KC-dependent neutrophil recruitment, but preserves the late influx of macrophages by MCP-1 and MIP-2, which may facilitate the clearance of cellular debris. Although, it is surprising that MCP-1 levels did not decline with the numbers of neutrophils in the DDAH II–overexpressed mice, a previous study has shown that, in inflamed lungs, neutrophil depletion may not alter MCP-1 levels, and that elevated concentrations of MCP-1 are not necessarily accompanied by an increase in monocytes/macrophages in neutropenic rats (49). Therefore, it is possible that MCP-1 levels may not be a true indicator of macrophage recruitment in ALI, and that DDAH II overexpression may actually inhibit macrophage recruitment through a decrease in neutrophils and the attenuation of MIP-1, IFN-γ–induced protein 10, and macrophage colony–stimulating factor. However, it is worth noting that our analyses were performed only in the BALF, and not in the lung tissue. This is a limitation in our study, as using BALF does not account for the inflammatory cells that are present in the septum, which includes cells in the microvasculature and interstitial spaces.
In conclusion, our data demonstrate that decreases in DDAH activity and increases in ADMA are markers of oxidative/nitrative damage, and are potential therapeutic targets in ALI. Strategies aimed at augmenting DDAH II in patients deemed as being at high risk of developing ALI (before treatment), or in patients who have already developed ALI (after treatment), may provide better clinical outcomes. Indeed, the pharmacological targeting of DDAH/ADMA signaling has been achieved using the cAMP phosphodiesterase inhibitor, tolafentrine, which has been shown to modulate the promoter region of the DDAH II gene and increase DDAH II expression/activity and reduce ADMA levels (50). Further studies are also warranted to shed light on the post-translational regulation of DDAH activity and the relative efficacy of enhancing DDAH activity in patients with ALI.
Footnotes
This work was supported in part by National Institutes of Health grants P01HL0101902, HL60190, and HL67841. C.M.G. is funded in part by American Heart Association predoctoral Fellowship award 12PRE12060224. R.L. is funded in part by National Institutes of Health grant HL094609.
This article has an online supplement, which is accessible from this issue’s table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1165/rcmb.2013-0193OC on October 17, 2013
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Erickson SE, Martin GS, Davis JL, Matthay MA, Eisner MD. Recent trends in acute lung injury mortality: 1996–2005. Crit Care Med. 2009;37:1574–1579. doi: 10.1097/CCM.0b013e31819fefdf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nagase T, Uozumi N, Aoki-Nagase T, Terawaki K, Ishii S, Tomita T, Yamamoto H, Hashizume K, Ouchi Y, Shimizu T. A potent inhibitor of cytosolic phospholipase A2, arachidonyl trifluoromethyl ketone, attenuates LPS-induced lung injury in mice. Am J Physiol Lung Cell Mol Physiol. 2003;284:L720–L726. doi: 10.1152/ajplung.00396.2002. [DOI] [PubMed] [Google Scholar]
- 3.Rubenfeld GD, Caldwell E, Peabody E, Weaver J, Martin DP, Neff M, Stern EJ, Hudson LD. Incidence and outcomes of acute lung injury. N Engl J Med. 2005;353:1685–1693. doi: 10.1056/NEJMoa050333. [DOI] [PubMed] [Google Scholar]
- 4.The Acute Respiratory Distress Syndrome Network. Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. N Engl J Med. 2000;342:1301–1308. doi: 10.1056/NEJM200005043421801. [DOI] [PubMed] [Google Scholar]
- 5.Sharma S, Smith A, Kumar S, Aggarwal S, Rehmani I, Snead C, Harmon C, Fineman J, Fulton D, Catravas JD, et al. Mechanisms of nitric oxide synthase uncoupling in endotoxin-induced acute lung injury: role of asymmetric dimethylarginine. Vascul Pharmacol. 2010;52:182–190. doi: 10.1016/j.vph.2009.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Boger RH, Bode-Boger SM. Asymmetric dimethylarginine, derangements of the endothelial nitric oxide synthase pathway, and cardiovascular diseases. Semin Thromb Hemost. 2000;26:539–545. doi: 10.1055/s-2000-13210. [DOI] [PubMed] [Google Scholar]
- 7.Boger RH. The emerging role of asymmetric dimethylarginine as a novel cardiovascular risk factor. Cardiovasc Res. 2003;59:824–833. doi: 10.1016/s0008-6363(03)00500-5. [DOI] [PubMed] [Google Scholar]
- 8.Boger RH. Association of asymmetric dimethylarginine and endothelial dysfunction. Clin Chem Lab Med. 2003;41:1467–1472. doi: 10.1515/CCLM.2003.225. [DOI] [PubMed] [Google Scholar]
- 9.Tran CT, Leiper JM, Vallance P. The DDAH/ADMA/NOS pathway. Atheroscler Suppl. 2003;4:33–40. doi: 10.1016/s1567-5688(03)00032-1. [DOI] [PubMed] [Google Scholar]
- 10.Böger RH.Asymmetric dimethylarginine, an endogenous inhibitor of nitric oxide synthase, explains the “L-arginine paradox” and acts as a novel cardiovascular risk factor J Nutr 20041342842S–2847S.discussion 2853S [DOI] [PubMed] [Google Scholar]
- 11.Vallance P, Leiper J. Cardiovascular biology of the asymmetric dimethylarginine:dimethylarginine dimethylaminohydrolase pathway. Arterioscler Thromb Vasc Biol. 2004;24:1023–1030. doi: 10.1161/01.ATV.0000128897.54893.26. [DOI] [PubMed] [Google Scholar]
- 12.Cardounel AJ, Cui H, Samouilov A, Johnson W, Kearns P, Tsai AL, Berka V, Zweier JL. Evidence for the pathophysiological role of endogenous methylarginines in regulation of endothelial no production and vascular function. J Biol Chem. 2007;282:879–887. doi: 10.1074/jbc.M603606200. [DOI] [PubMed] [Google Scholar]
- 13.Sud N, Wells SM, Sharma S, Wiseman DA, Wilham J, Black SM. Asymmetric dimethylarginine inhibits hsp90 activity in pulmonary arterial endothelial cells: role of mitochondrial dysfunction. Am J Physiol Cell Physiol. 2008;294:C1407–C1418. doi: 10.1152/ajpcell.00384.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Catravas JD, Snead C, Dimitropoulou C, Chang AS, Lucas R, Verin AD, Black SM. Harvesting, identification and barrier function of human lung microvascular endothelial cells. Vascul Pharmacol. 2010;52:175–181. doi: 10.1016/j.vph.2009.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Aggarwal S, Dimitropoulou C, Lu Q, Black SM, Sharma S. Glutathione supplementation attenuates lipopolysaccharide-induced mitochondrial dysfunction and apoptosis in a mouse model of acute lung injury. Front Physiol. 2012;3:161. doi: 10.3389/fphys.2012.00161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Aggarwal S, Gross CM, Kumar S, Datar S, Oishi P, Kalkan G, Schreiber C, Fratz S, Fineman JR, Black SM. Attenuated vasodilatation in lambs with endogenous and exogenous activation of cGMP signaling: role of protein kinase G nitration. J Cell Physiol. 2011;226:3104–3113. doi: 10.1002/jcp.22692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sun X, Sharma S, Fratz S, Kumar S, Rafikov R, Aggarwal S, Rafikova O, Lu Q, Burns T, Dasarathy S, et al. Disruption of endothelial cell mitochondrial bioenergetics in lambs with increased pulmonary blood flow. Antioxid Redox Signal. 2013;8:1739–1752. doi: 10.1089/ars.2012.4806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sharma S, Sun X, Rafikov R, Kumar S, Hou Y, Oishi PE, Datar SA, Raff G, Fineman JR, Black SM. PPAR-gamma regulates carnitine homeostasis and mitochondrial function in a lamb model of increased pulmonary blood flow. PLoS ONE. 2012;7:e41555. doi: 10.1371/journal.pone.0041555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Poirier C, Gorshkov BA, Zemskova MA, Bogatcheva NV, Verin AD. TIMAP protects endothelial barrier from LPS-induced vascular leakage and is down-regulated by LPS. Respir Physiol Neurobiol. 2011;179:334–337. doi: 10.1016/j.resp.2011.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Matute-Bello G, Winn RK, Jonas M, Chi EY, Martin TR, Liles WC. Fas (CD95) induces alveolar epithelial cell apoptosis in vivo: implications for acute pulmonary inflammation. Am J Pathol. 2001;158:153–161. doi: 10.1016/S0002-9440(10)63953-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ahmad T, Mabalirajan U, Ghosh B, Agrawal A. Altered asymmetric dimethyl arginine metabolism in allergically inflamed mouse lungs. Am J Respir Cell Mol Biol. 2010;42:3–8. doi: 10.1165/rcmb.2009-0137RC. [DOI] [PubMed] [Google Scholar]
- 22.Ito A, Tsao PS, Adimoolam S, Kimoto M, Ogawa T, Cooke JP. Novel mechanism for endothelial dysfunction: dysregulation of dimethylarginine dimethylaminohydrolase. Circulation. 1999;99:3092–3095. doi: 10.1161/01.cir.99.24.3092. [DOI] [PubMed] [Google Scholar]
- 23.Leiper J, Murray-Rust J, McDonald N, Vallance P. S-nitrosylation of dimethylarginine dimethylaminohydrolase regulates enzyme activity: further interactions between nitric oxide synthase and dimethylarginine dimethylaminohydrolase. Proc Natl Acad Sci USA. 2002;99:13527–13532. doi: 10.1073/pnas.212269799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lange M, Nakano Y, Traber DL, Hamahata A, Traber LD, Enkhbaatar P. Time course of the inflammatory and oxidative stress response to pulmonary infection in mice. Exp Lung Res. 2012;38:157–163. doi: 10.3109/01902148.2012.663453. [DOI] [PubMed] [Google Scholar]
- 25.Lopez A, Lorente JA, Steingrub J, Bakker J, McLuckie A, Willatts S, Brockway M, Anzueto A, Holzapfel L, Breen D, et al. Multiple-center, randomized, placebo-controlled, double-blind study of the nitric oxide synthase inhibitor 546c88: effect on survival in patients with septic shock. Crit Care Med. 2004;32:21–30. doi: 10.1097/01.CCM.0000105581.01815.C6. [DOI] [PubMed] [Google Scholar]
- 26.Okamoto I, Abe M, Shibata K, Shimizu N, Sakata N, Katsuragi T, Tanaka K. Evaluating the role of inducible nitric oxide synthase using a novel and selective inducible nitric oxide synthase inhibitor in septic lung injury produced by cecal ligation and puncture. Am J Respir Crit Care Med. 2000;162:716–722. doi: 10.1164/ajrccm.162.2.9907039. [DOI] [PubMed] [Google Scholar]
- 27.Antonicelli F, Brown D, Parmentier M, Drost EM, Hirani N, Rahman I, Donaldson K, MacNee W. Regulation of LPS-mediated inflammation in vivo and in vitro by the thiol antioxidant nacystelyn. Am J Physiol Lung Cell Mol Physiol. 2004;286:L1319–L1327. doi: 10.1152/ajplung.00329.2003. [DOI] [PubMed] [Google Scholar]
- 28.Chen XM, Xia J, Zhou T, Yuan Q, Zhang WF, Hu CP, Li YJ, Jiang JL. Involvement of DDAH/ADMA pathway in the pathogenesis of rheumatoid arthritis in rats. Int Immunopharmacol. 2013;16:322–331. doi: 10.1016/j.intimp.2013.04.009. [DOI] [PubMed] [Google Scholar]
- 29.Gonzalez-Rey E, Chorny A, Robledo G, Delgado M. Cortistatin, a new antiinflammatory peptide with therapeutic effect on lethal endotoxemia. J Exp Med. 2006;203:563–571. doi: 10.1084/jem.20052017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Abdih H, Kelly CJ, Bouchier-Hayes D, Barry M, Kearns S. Taurine prevents interleukin-2–induced acute lung injury in rats. Eur Surg Res. 2000;32:347–352. doi: 10.1159/000052216. [DOI] [PubMed] [Google Scholar]
- 31.Ali MH, Schlidt SA, Chandel NS, Hynes KL, Schumacker PT, Gewertz BL. Endothelial permeability and IL-6 production during hypoxia: role of ROS in signal transduction. Am J Physiol. 1999;277:L1057–L1065. doi: 10.1152/ajplung.1999.277.5.L1057. [DOI] [PubMed] [Google Scholar]
- 32.Matsumoto K, Ohi H, Kanmatsuse K. Interleukin 12 upregulates the release of vascular permeability factor by peripheral blood mononuclear cells from patients with lipoid nephrosis. Nephron. 1998;78:403–409. doi: 10.1159/000044968. [DOI] [PubMed] [Google Scholar]
- 33.Klein E, Weigel J, Buford MC, Holian A, Wells SM. Asymmetric dimethylarginine potentiates lung inflammation in a mouse model of allergic asthma. Am J Physiol Lung Cell Mol Physiol. 2010;299:L816–L825. doi: 10.1152/ajplung.00188.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Son YH, Jeong YT, Lee KA, Choi KH, Kim SM, Rhim BY, Kim K. Roles of MAPK and NF-kappaB in interleukin-6 induction by lipopolysaccharide in vascular smooth muscle cells. J Cardiovasc Pharmacol. 2008;51:71–77. doi: 10.1097/FJC.0b013e31815bd23d. [DOI] [PubMed] [Google Scholar]
- 35.Murphy TL, Cleveland MG, Kulesza P, Magram J, Murphy KM. Regulation of interleukin 12 p40 expression through an NF-kappa B half-site. Mol Cell Biol. 1995;15:5258–5267. doi: 10.1128/mcb.15.10.5258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hinz M, Lemke P, Anagnostopoulos I, Hacker C, Krappmann D, Mathas S, Dorken B, Zenke M, Stein H, Scheidereit C. Nuclear factor kappaB–dependent gene expression profiling of Hodgkin’s disease tumor cells, pathogenetic significance, and link to constitutive signal transducer and activator of transcription 5A activity. J Exp Med. 2002;196:605–617. doi: 10.1084/jem.20020062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yakovlev VA, Barani IJ, Rabender CS, Black SM, Leach JK, Graves PR, Kellogg GE, Mikkelsen RB. Tyrosine nitration of IkappaBalpha: a novel mechanism for NF-kappaB activation. Biochemistry. 2007;46:11671–11683. doi: 10.1021/bi701107z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lin KY, Ito A, Asagami T, Tsao PS, Adimoolam S, Kimoto M, Tsuji H, Reaven GM, Cooke JP. Impaired nitric oxide synthase pathway in diabetes mellitus: role of asymmetric dimethylarginine and dimethylarginine dimethylaminohydrolase. Circulation. 2009;106:987–992. doi: 10.1161/01.cir.0000027109.14149.67. [DOI] [PubMed] [Google Scholar]
- 39.Wojciak-Stothard B, Torondel B, Tsang LY, Fleming I, Fisslthaler B, Leiper JM, Vallance P. The ADMA/DDAH pathway is a critical regulator of endothelial cell motility. J Cell Sci. 2007;120:929–942. doi: 10.1242/jcs.002212. [DOI] [PubMed] [Google Scholar]
- 40.Sousse LE, Yamamoto Y, Enkhbaatar P, Rehberg SW, Wells SM, Leonard S, Traber MG, Yu YM, Cox RA, Hawkins HK, et al. Acute lung injury–induced collagen deposition is associated with elevated asymmetric dimethylarginine and arginase activity. Shock. 2011;35:282–288. doi: 10.1097/SHK.0b013e3181fddd82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pope AJ, Druhan L, Guzman JE, Forbes SP, Murugesan V, Lu D, Xia Y, Chicoine LG, Parinandi NL, Cardounel AJ. Role of DDAH-1 in lipid peroxidation product–mediated inhibition of endothelial no generation. Am J Physiol Cell Physiol. 2007;293:C1679–C1686. doi: 10.1152/ajpcell.00224.2007. [DOI] [PubMed] [Google Scholar]
- 42.Xin HY, Jiang DJ, Jia SJ, Song K, Wang GP, Li YJ, Chen FP. Regulation by DDAH/ADMA pathway of lipopolysaccharide-induced tissue factor expression in endothelial cells. Thromb Haemost. 2007;97:830–838. [PubMed] [Google Scholar]
- 43.Predescu D, Predescu S, Shimizu J, Miyawaki-Shimizu K, Malik AB. Constitutive eNOS-derived nitric oxide is a determinant of endothelial junctional integrity. Am J Physiol Lung Cell Mol Physiol. 2005;289:L371–L381. doi: 10.1152/ajplung.00175.2004. [DOI] [PubMed] [Google Scholar]
- 44.Mundy AL, Dorrington KL. Inhibition of nitric oxide synthesis augments pulmonary oedema in isolated perfused rabbit lung. Br J Anaesth. 2000;85:570–576. doi: 10.1093/bja/85.4.570. [DOI] [PubMed] [Google Scholar]
- 45.van Nieuw Amerongen GP, van Hinsbergh VW. Cytoskeletal effects of Rho-like small guanine nucleotide–binding proteins in the vascular system. Arterioscler Thromb Vasc Biol. 2001;21:300–311. doi: 10.1161/01.atv.21.3.300. [DOI] [PubMed] [Google Scholar]
- 46.Waschke J, Baumgartner W, Adamson RH, Zeng M, Aktories K, Barth H, Wilde C, Curry FE, Drenckhahn D. Requirement of Rac activity for maintenance of capillary endothelial barrier properties. Am J Physiol Heart Circ Physiol. 2004;286:H394–H401. doi: 10.1152/ajpheart.00221.2003. [DOI] [PubMed] [Google Scholar]
- 47.Scalera F, Borlak J, Beckmann B, Martens-Lobenhoffer J, Thum T, Tager M, Bode-Boger SM. Endogenous nitric oxide synthesis inhibitor asymmetric dimethyl l-arginine accelerates endothelial cell senescence. Arterioscler Thromb Vasc Biol. 2004;24:1816–1822. doi: 10.1161/01.ATV.0000141843.77133.fc. [DOI] [PubMed] [Google Scholar]
- 48.Eid HM, Lyberg T, Arnesen H, Seljeflot I. Insulin and adiponectin inhibit the TNFalpha-induced ADMA accumulation in human endothelial cells: the role of DDAH. Atherosclerosis. 2007;194:e1–e8. doi: 10.1016/j.atherosclerosis.2006.11.008. [DOI] [PubMed] [Google Scholar]
- 49.Janardhan KS, Sandhu SK, Singh B. Neutrophil depletion inhibits early and late monocyte/macrophage increase in lung inflammation. Front Biosci. 2006;11:1569–1576. doi: 10.2741/1904. [DOI] [PubMed] [Google Scholar]
- 50.Pullamsetti SS, Savai R, Schaefer MB, Wilhelm J, Ghofrani HA, Weissmann N, Schudt C, Fleming I, Mayer K, Leiper J, et al. cAMP phosphodiesterase inhibitors increases nitric oxide production by modulating dimethylarginine dimethylaminohydrolases. Circulation. 2011;123:1194–1204. doi: 10.1161/CIRCULATIONAHA.110.941484. [DOI] [PubMed] [Google Scholar]