Abstract
The mammalian airways are sensitive to inhaled stimuli, and airway diseases are characterized by hypersensitivity to volatile stimuli, such as perfumes, industrial solvents, and others. However, the identity and function of the cells in the airway that can sense volatile chemicals remain uncertain, particularly in humans. Here, we show that solitary pulmonary neuroendocrine cells (PNECs), which are morphologically distinct and physiologically undefined, might serve as chemosensory cells in human airways. This conclusion is based on our finding that some human PNECs expressed members of the olfactory receptor (OR) family in vivo and in primary cell culture, and are anatomically positioned in the airway epithelium to respond to inhaled volatile chemicals. Furthermore, apical exposure of primary-culture human airway epithelial cells to volatile chemicals decreased levels of serotonin in PNECs, and the led to the release of the neuropeptide calcitonin gene-related peptide (CGRP) to the basal medium. These data suggest that volatile stimulation of PNECs can lead to the secretion of factors that are capable of stimulating the corresponding receptors in the lung epithelium. We also found that the distribution of serotonin and neuropeptide receptors may change in chronic obstructive pulmonary disease, suggesting that increased PNEC-dependent chemoresponsiveness might contribute to the altered sensitivity to volatile stimuli in this disease. Together, these data indicate that human airway epithelia harbor specialized cells that respond to volatile chemical stimuli, and may help to explain clinical observations of odorant-induced airway reactions.
Keywords: olfactory receptor, serotonin, neuropeptide, asthma, chronic obstructive pulmonary disease
Clinical Relevance
Chronic obstructive pulmonary disease and other diseases of the lung are often associated with increased sensitivity to environmental chemical stimuli. Our study indicates that some pulmonary neuroendocrine cells can directly sense and mediate the physiological response of airways to volatiles, and may represent a key molecular and cellular target for the development of future treatment of airway hypersensitivity.
The airway epithelium presents one of the largest surface areas in the body of mammals (1). In contrast to the skin, the airway epithelium is highly permeable and, therefore, highly sensitive to various inhaled and pathogen-borne particles, small molecules, and volatile irritants (2). Consequently, the mammalian airway must have evolved mechanisms to protect itself from inhaled, harmful compounds and particles. Evidence for such protective sensory mechanisms is apparent in the cough reflex, as well as the induction of bronchial constriction by diverse chemical and mechanical stimuli (3). Furthermore, hypersensitivity syndromes related to sensory activation of respiratory airways can also lead to chronic cough and inflammation, presenting as asthma or chronic obstructive pulmonary disease (COPD). In spite of the importance of sensory pathways in airways in both health and disease, how various sensory stimuli are detected and processed at the cellular and molecular levels in airways is still not entirely understood (3).
As a step toward understanding chemosensation in the lung, we and others have recently shown that ciliated human airway epithelial cells express members of the bitter taste receptor family, and can respond to inhaled chemicals that are classified as “bitter” compounds (4–6). Other cells with chemosensory functions in mammalian airways are the cholinergic “brush cells” (7–10). These cells are present in the upper and lower airways in mammals, where they play roles such as controlling of breathing in rodents (11) and the epithelial response to bacterial compounds and other harmful chemical stimuli (12). Furthermore, human genetic variations in the bitter receptor, TAS2R38, are associated with detection of bacterial products and the susceptibility to upper airway infections (6). Although some bitter receptors can also detect certain volatile irritants at high concentrations (13), the airway sensitivity to a wide range of volatile substances indicates that additional classes of chemososensory receptors might also be involved in the physiological response of airways to inhaled chemical agents.
Mammals use specialized sensory neurons in the nasal epithelium to sense volatile odors via dedicated receptors of the olfactory receptors (ORs) family. ORs represent the largest protein family in all mammalian genomes sequenced to date, and are part of the G protein–coupled receptor superfamily (14). Although OR genes were originally thought to be expressed solely in sensory neurons that reside in the main olfactory nasal epithelium of the respiratory tract, later studies indicated that at least some family members function outside the olfactory system (15), including in human sperm (16), mouse kidney (17, 18), and other epithelial tissues (19). The physiological and behavioral roles of many of the OR proteins are still unknown, and noncanonical expression or functions for OR genes in lung tissues have not been described. In this study, we examined whether the sensitivity of human airways to certain volatile chemicals could be mediated via direct activation of ORs that are expressed in the epithelium of the human airway.
Materials and Methods
Detailed Materials and Methods are provided in the online supplement.
Airway Epithelial Cell Culture
Primary culture airway epithelial cell preparations were established by the University of Iowa In Vitro Models and Cell Culture Core using cells isolated from trachea and bronchi of lungs that were removed for donation. Additional preparations were obtained from the Washington University Airway Epithelial Cell Core using cells from trachea and main stem bronchi of transplant donor lungs. Samples were collected with approval of the institutional review boards of the University of Iowa and the Washington University School of Medicine. Airway epithelial cells were isolated and cultured on collagen-coated Transwell membranes (Corning, Corning, NY) under air–liquid interface (ALI) conditions, as previously described (20, 21).
Gene Expression Analysis
Microarray expression data were from preparations of well differentiated primary normal human airway epithelia from 10 independent donors, grown at ALI as previously reported (22). Briefly, each cRNA preparation was labeled and incubated with human U133A GeneChip arrays (Affymetrix, Santa Clara, CA). Data were analyzed with the Affymetrix Microarray Analysis Suite version 5.0 software, as we previously described (5, 22) according to current standards in the field. Gene-specific Affymetrix probes that were identified conservatively as “present” by the analysis package were interpreted as “positively expressed” (P < 0.04). Data were normalized using the global scaling adjustment technique with a target intensity of 1,500, according to the manufacturer instructions.
Cell Stimulation by Volatile Substances
To test for olfactory activation of pulmonary neuroendocrine cells (PNECs), we used live, well differentiated (more than 3 wk at ALI) human airway epithelial cell preparations grown in 12-mm Transwell inserts (1.2 cm2 membrane) from a single donor. Each odorant was applied to four independent inserts. Pure odorants (Sigma, St. Louis, MO) were first diluted 1:100 in ultrapure dimethyl sulfoxide (DMSO) (with the exception of hexadecanal), followed by a further dilution (1:1,000) in PBS (final dilution, 1 × 105). To solubilize the waxy compound, hexadecanal, the chemical was heated to 60°C until completely melted, then diluted 1:100 in DMSO. After a secondary dilution (1:1,000) in PBS, hexadecanal appeared to come out of solution. Thus, it was impossible for us to calculate the precise final concentration of this compound. Each insert was stimulated apically with 100 μl of the final diluted compound. A sample of the basal medium was collected from each insert 15 minutes after stimulation, followed by fixation and processing of the cell preparations for immunostaining, as described in the online supplement. CGRP levels in basal media were measured using an ELISA kit (Cayman Chemical, Ann Arbor, MI) according to manufacturer instructions. Ligand concentrations used were in the physiological range of their respective receptors, in accord with previous publications (see Table E2 in the online supplement).
Results
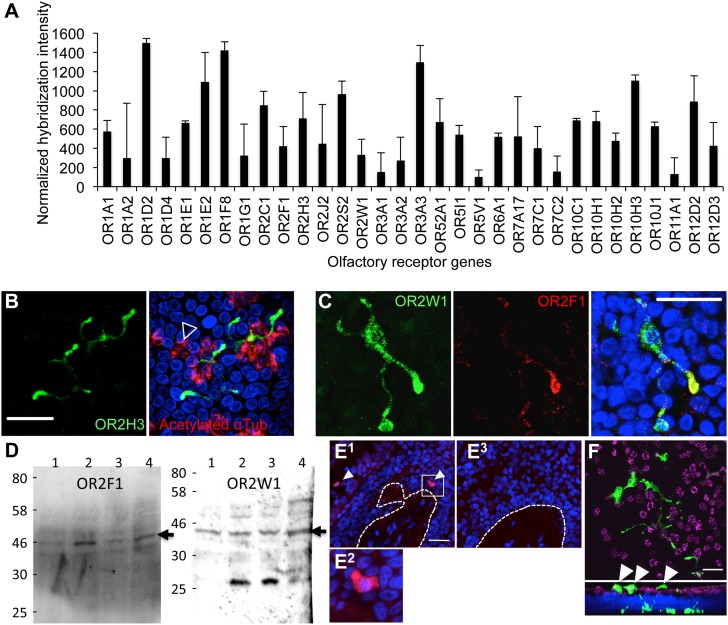
To determine whether OR genes are expressed in human airway epithelial cells, we analyzed data from whole-genome expression microarrays that were obtained from primary-culture human tracheobronchial epithelial cells (hTECs) (22, 23). This analysis revealed that several canonical OR genes are expressed in hTEC cultures (Figure 1A). Screening for human OR gene expression using commercially available antibodies identified three distinct receptors (Figures 1B–1D). In contrast to human bitter taste receptors that are enriched in ciliated airway epithelial cells (5), each of these OR gene products was localized to a distinct cell population characterized by an interdigitating morphology (Figures 1B and 1C). Furthermore, unlike olfactory sensory neurons, which express only one member of the OR family (24), at least some of the cells within hTEC preparations expressed more than one type of OR per cell (Figure 1C) (25). These data suggest that OR-expressing airway cells are more broadly tuned to chemical stimuli than previously described for olfactory sensory neurons (24). The expression of OR2F1 and OR2W1 in hTEC cultures was verified by protein (Western) blot analysis of hTEC culture lysates that originated from four different tissue donors. The protein analyses data indicate possible variability in expression levels of OR2F1, but not OR2W1, among the four studied donors (Figure 1D; see also Figure E1). In situ hybridization also confirmed the presence of OR2W1 mRNA in human airway epithelium in vivo (Figure 1E).
Figure 1.
Olfactory receptors (ORs) expressed in cultured preparations of human airway epithelial cells. (A) OR gene expression by microarray analysis of mRNAs isolated from primary airway cell preparations. Shown is the mean (± SEM) from 10 independent donors. (B) Representative photomicrograph of immunostaining for the OR, OR2H3 (green), in primary-culture human airway cells demonstrating an interdigitating morphology. Cilia are labeled in red (acetylated α-tubulin, open arrowhead). Images are confocal z-stacks. (C) Colocalization of odor receptors, OR2W1 (green) and OR2F1 (red), in human airway preparations. Images are confocal z-stacks. (D) Protein blot analyses. OR2F1 and OR2W1 are expressed in human primary-culture airway cells. Each lane represents a sample from a single donor. The expected size of the corresponding ORs is indicated (∼ 45 kD; closed arrows). (E) In situ hybridization of human airway tissue sections using an OR2W1-specific riboprobe. (E1) antisense probe; (E2) enlargement of box in (E1); (E3) sense control riboprobe. Dotted lines represent the basal aspect of the stratified epithelium. Both sense and antisense probes were hybridized to sequential sections of the same tissue. (F) Cultured cells immunostained for 5-HT show extensive interdigitating morphology. Upper panel is a confocal x,y section. Lower panel is a z,y section (“stack”) of the upper panel. Note that some of the cellular extensions show projections to the apical side (white arrowheads). Nuclei are labeled with 4′,6-diamidino-2-phenylindole (blue). Scale bars, 50 μm.
A previous study in human lung tissues identified cells with a morphology similar to the OR-expressing cells as PNECs (26). To confirm this, we also stained hTECs with 5-HT, which identified epithelial cells with morphology identical to the OR-expressing cells, further supporting the possibility that OR-expressing cells in cultured preparations were PNECs (Figure 1F). These identified cells also expressed the enzyme, tryptophan hydroxylase 1 (Figure E2), but not the serotonin transporter (data not shown). The data are consistent with a phenotype of OR-expressing neuroendocrine cells. Furthermore, because the PNECs are a major source of 5-HT in the human airway epithelium, it is possible that they contribute to the lung serotonin levels that are implicated in the pathogenesis of asthma and other airway diseases (27, 28). In agreement with a possible sensory function, many of the identified OR-expressing PNECs projected cellular digits toward the apical surface of the differentiated hTEC cultures (5-hydroxytryptamine [5-HT] staining in Figure 1F, arrowheads in lower panels). These morphological and biochemical features could enable these cells to detect chemicals that enter the lumen of the airways. Primary airway cultures also expressed several other key olfactory system components, including olfactory G α, adenylate cyclase III, odorant-binding proteins, and olfactory marker protein, but not any of the cyclic nucleotide gated channels, including the olfactory-specific subunits, cyclic nucleotide gated channel alpha 2 (CNGA2) and CNGA4 (Figure E3A). Our attempts to localize other components of the olfactory signal transduction pathway at the protein level by using commercially available antibodies did not yield specific signals in primary cultures or tissue sections (data not shown).
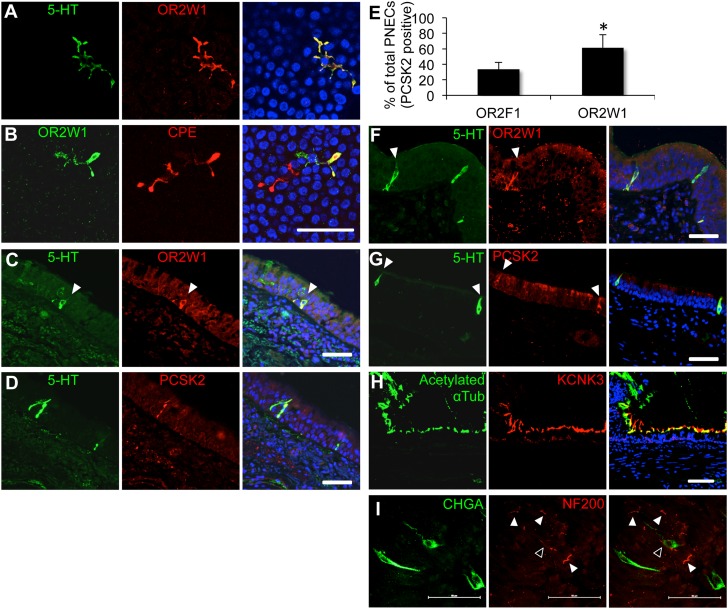
To further test if the OR-expressing cells in airways are PNECs, we examined whether other neuroendocrine markers were localized with ORs. In hTEC preparations, we found that OR2W1 colocalized with both 5-HT (Figure 2A) and carboxypeptidase E (CPE; Figure 2B), confirming the identity of OR-expressing cells as solitary PNECs (26). However, not all CPE-positive cells also expressed ORW1, which suggests that either some subset of PNECs do not have chemosensory functions or express receptors for other volatile ligands. Nonetheless, OR-expressing cells in hTEC preparations expressed other conventional markers of neuroendocrine cells, including CGRP, CPE, chromogranin A, Dopa decarboxylase, monoamine oxidase A, and monoamine oxidase B (Figure E3B) and stained positive for the CGRP and chromogranin A, further confirming their identity as PNECs (Figure E4).
Figure 2.
Human pulmonary olfactory cells are solitary pulmonary neuroendocrine cells (PNECs). The OR, OR2W1, is coexpressed with 5-HT (serotonin) (A) and the enzyme, carboxipeptidase E (CPE) (B), in human primary airway epithelial cell preparations. The OR, OR2W1, is coexpressed with the neuroendocrine markers, 5-HT (C) and proprotein convertase subtilisin/kexin type 2 (PCSK2) (D), in human lung tissue sections. (E) OR abundance in preparations from a single donor. Single culture membranes were cut into two pieces and costained for the specified receptor and the PNEC marker, PCSK2. Mean receptor abundance (% cells) is shown for the receptor and PCSK2 out of the total PCSK2-positive cells. Error bars denote SEM. *P < 0.05 (one-tailed t test; n = 3 independent donors). (F and G) PNECs in lung sections from the rhesus macaque. (H) Photomicrographs of the two-pore potassium channel subfamily K member 3 (KCNK3) and the cilia marker, acetylated α-tubulin. (I) Some PNECs might be innervated. Whole-mount immunohistochemistry in the imaged region shows that at least one cell might interact with a neuronal fiber (open arrowhead). Other cells in the imaged area do not seem to interact with neuronal afferents (white arrowheads). PNECs were labeled with anti–chromogranin A (CHGA) antibody (green), and neuronal fibers were labeled with anti–neurofilament H antibody (NF200; red). Scale bars, 50 μm. See Figure E6 for larger images, and Movie E1 for a three-dimensional reconstruction.
The presence of OR-expressing PNECs was also observed in human lung tissue sections, indicating that their presence was not restricted to cultured cell preparations (Figures 2C and 2D). Staining for two different receptors in hTEC cultures from independent tissue donors reveled that only about 30 and 60% of PNECs were positive for the receptors OR2F1 and OR2W1, respectively (Figures 2E and E1). These data indicate that either not all PNECs in human airway tissues act as chemosensory cells, or that different populations of cells express different receptor assemblies, which cannot yet be detected with the available reagents. These results are in agreement with our protein blot analyses, which indicated that OR2W1 is more abundant and less variable than OR2F1 in cultures from different individuals (Figure 1D). These findings also indicate that OR-expressing PNECs were selectively localized to the airway epithelium, and therefore well positioned to act as sentinels for inhaled odorants.
Next, we asked whether OR-expressing PNECs were present in lungs from other mammalian species. Previous studies indicated the presence of solitary PNECs and neuroendocrine bodies (NEBs; clusters of neuroendocrine cells) in mammals, especially during fetal and neonatal development (29–31). Although CGRP-positive cells have been described in the adult mouse airway epithelium, they are not serotonergic, and appear to act as a population of stem cells that plays a role in epithelial recovery from injuries (32). We were not able to detect pulmonary interdigitating PNECs in lungs of adult mice or ferrets using 5-HT as a marker. In contrast to mice, which, in our hands, showed serotonergic neuroendocrine cells in the gut epithelium, but not in the lung (Figure E5A), adult rat lungs did contain lung cells positive for 5-HT. However, the identified cells were significantly larger than human PNECs, did not have an interdigitating morphology, and were located in subepithelial layers of the airways (Figures E5B and E5C). However, OR-expressing PNECs with morphology similar to those found in human tissues were detected in lung tissues from rhesus macaque monkeys (Figures 2F and 2G). These results indicate that the identified PNECs are not specific to lungs of humans, because they are present in nonhuman primates as well.
Other studies of pulmonary NEBs in rodent models have suggested that the primary function of these cells is to act as oxygen sensors in the postnatal lung (30, 31). Whether adult human lungs can also directly sense oxygen pressure in inhaled air remains uncertain. Current models propose that neuroregulation of breathing in adult humans is primarily mediated via oxygen-sensing neurons in the carotid bodies (33, 34). When we immunostained adult human lung tissues with an antibody against potassium channel subfamily K member 3 (KCNK3), a two-pore potassium channel that has been identified as an essential molecular component of the oxygen-sensing receptor complex in mammalian pulmonary NEBs (35), we found that this channel is enriched in epithelial motile cilia, but is not present in PNECs (Figure 2H). However, similarly to the rodent NEBs, whole-mount immunostaining of human airway epithelia revealed that some human PNECs might be directly innervated by afferent neuronal fibers (Figures 2I and E6, and Video E1). Together, our data suggest that primate adult solitary PNECs are chemosensory, but not likely to act as oxygen sensors, and are distinct from PNECs or NEBs identified during early lung development in human infants and nonprimate animal models.
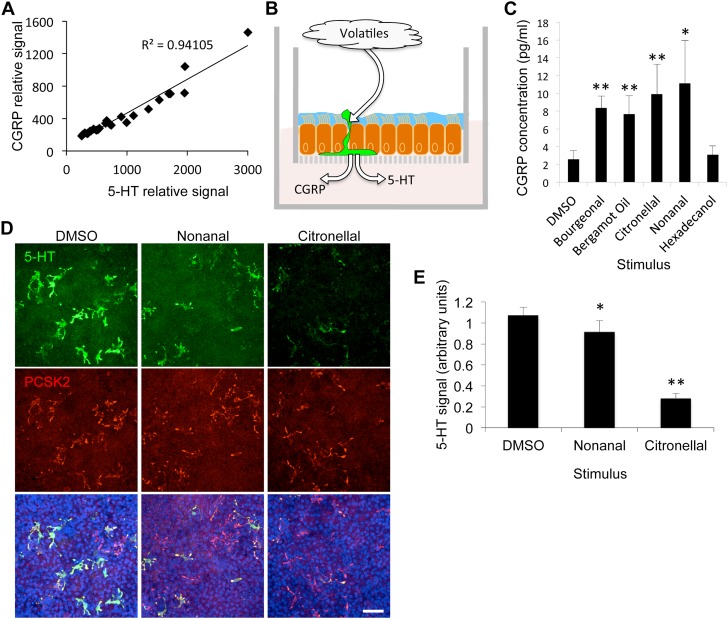
Our results suggested that PNECs serve as chemosensory neuroendocrine sentinels in the human airway epithelium. Therefore, we hypothesized that specific volatile chemical stimuli could induce the local release of the neuroendocrine content from stimulated PNECs. We first examined whether the cellular contents of two different neuroendocrine secreted factors, 5-HT and CGRP, are correlated with each other in individual cells. We found that the levels of 5-HT and CGRP in individual cells were highly correlated, which suggests that these factors are coexpressed, and are likely coreleased on stimulation (Figure 3A). Next, we investigated whether apical stimulation of well differentiated hTEC preparations is correlated with lower cell contents of both 5-HT and CGRP (Figure 3B). In agreement with our hypothesis, apical stimulation of primary culture cells with a panel of volatile chemicals that have been shown to activate some of the receptors expressed in hTECs (Figure 1A and Table E2) resulted in a significant increase of CGRP released into the basal cell medium (Figure 3C). In addition, staining of hTEC preparations that were treated with the ligands, nonanal (36) or citronellal (37), showed overall lower 5-HT signal relative to that in DMSO-treated cells. Together, these results are consistent with stimulation-dependent release of CGRP and 5-HT (Figures 3D and 3E).
Figure 3.
PNECs release neuroendocrine factors in response to volatile apical stimuli. (A) Levels of the biogenic amine, 5-HT, and the peptide calcitonin gene-related peptide (CGRP) in individual PNECs (R2 = 0.94; n = 27 cells from three independent donors, 7–10 cells per donor). Signals represent average pixel intensity of cell bodies that were coimmunostained for 5-HT and CGRP. (B) An illustration of the supported membrane insert system used for culture of primary airway epithelial cells (20, 50). Treatment experiments were accomplished by applying the various ligands apically, while measuring the basal release of neuroendocrine factors. (C) CGRP release in basal medium after apical treatment with indicated volatile. Mean (± SEM) of CGRP levels measured by ELISA (P < 0.05, Kruskal-Wallis ANOVA; n = 4 inserts per treatment). (D and E) Treatments of primary cell preparations with nonanal or citronellal followed by immunostaining for 5-HT levels relative to controls. Shown are means (± SEM) (one-way ANOVA; P < 0.001; n = 4 inserts from a single donor per treatment). *P < 0.05; **P < 0.01. Scale bars, 50 μm. DMSO, dimethyl sulfoxide.
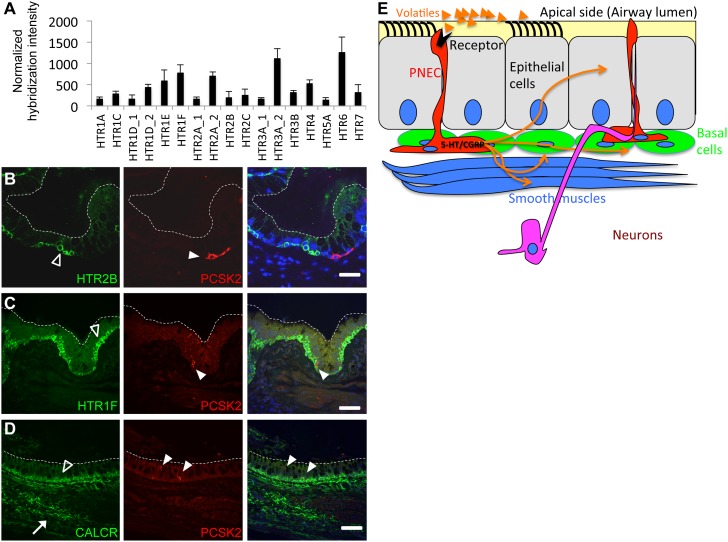
Because PNECs appear to be a significant source of 5-HT and CGRP in human airway tissues, we next examined which cells might express 5-HT and CGRP receptors as possible cellular targets for modulation by PNECs. Microarray data from human airway cell preparations (as demonstrated in Figure 1A) indicated that 14 out of the 17 known human 5-HT receptors (HTRs) are expressed in hTEC preparations (Figure 4A). We also found that multiple independent receptors for 5-HT and CGRP were expressed at the protein level in various airway cell types, but not in PNECs themselves, further supporting our hypothesis that activation of PNECs by volatile ligands can lead to local stimulation of other neighboring cell types in the epithelium (Figures 4B–4D). Each of the three identified neuroendocrine receptors (5-HTR2B, HTR1F, and calcitonin/CGRP receptor [CALCR]) showed enrichment in the population of airway epithelial basal cells. The CGRP receptor, CALCR, also showed expression in airway smooth muscles, or possibly in the neuronal fibers that innervate them (Figure 4D). Thus, our data support the model that activation of PNECs in human airways could lead to a localized activation of airway epithelial cells, and a concerted response to volatile agents (Figure 4E).
Figure 4.
(A) 5-HT receptor (HTR) gene expression by microarray analysis of mRNAs isolated from primary airway cell preparations from 10 independent donors (as in Figure 1A). (B and C) Serotonin receptors, 5-HTR 2B and HTR1F, in basal cells (open arrowheads) detected by immunostaining. White arrowheads point to solitary PNECs. (D) The calcitonin/CGRP receptor (CALCR) is enriched in basal cells (open arrowheads) and in airway smooth muscles or possibly the neurons that innervate them (white arrow). White arrowheads in C and D mark PNECs. Dashed white lines show the apical side of the stratified epithelium. Scale bars, 50 μm. (E) Model for the possible local impact of olfactory stimulation of PNECs followed by neuroendocrine release of 5-HT and CGRP on various cell types in the airways (orange arrows).
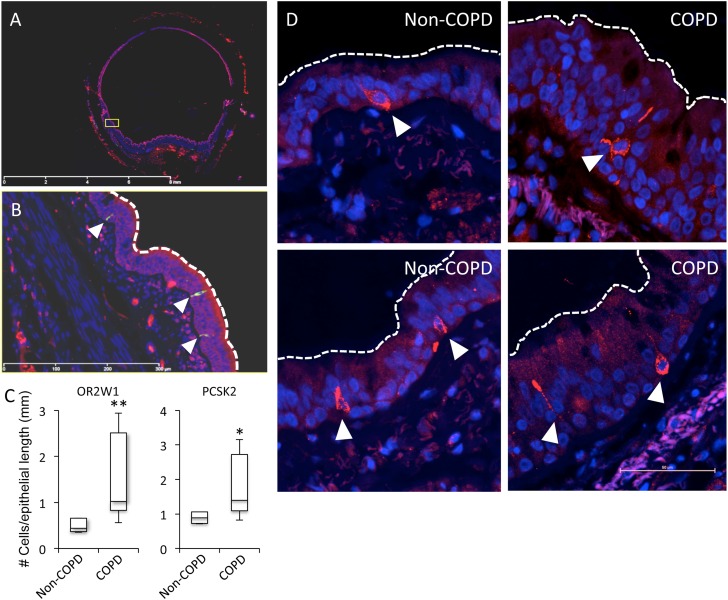
It has been proposed that airway diseases, such as asthma or COPD, manifest increased airway responses to environmental factors, including volatile agents (38). The present observations suggest that PNECs might contribute to this chemical hyperresponsiveness in airway disease. In that regard, previous studies also indicated that neuroendocrine factors, such as 5-HT, and peptides, such as CGRP, have potent physiological effects on epithelial, airway smooth muscle, and vascular smooth muscle cells in the lung (39–41). Thus, our data suggested the hypothesis that changes in the activation level of PNECs or the responsiveness of other airway cell types to stimulation by PNEC contents could contribute to olfactory hypersensitivity in airway disease. We therefore determined the levels of PNECs in lung tissues obtained from lung transplant recipients with severe COPD versus corresponding tissues from lung transplant donors without COPD (42, 43). In agreement with our hypothesis, we found increased numbers of OR2W1- and proprotein convertase subtilisin/kexin type 2-expressing PNECs in lung tissues from subjects with COPD relative to control subjects without COPD, without any detectable differences in PNEC morphology (Figures 5A–5C). Although the complex morphology of PNECs prevented us from studying their morphology in tissue sections, gross observations suggested that disease state does not affect their size or morphology (Figure 5D).
Figure 5.
PNECs are more abundant in airways from patients with chronic obstructive pulmonary disease (COPD) relative to healthy donors. (A) Representative confocal scan of a human bronchial tissue section used to quantify PNEC frequencies. (B) Magnification of yellow box in (A); dotted line indicates an example of the region used to normalize number of cells to epithelial length. Cells in the images shown were positive for both OR2W1 and PCSK2. (C) Box plots represent PNEC frequencies in tissue sections from lungs of subjects with and without COPD (*P < 0.05; **P < 0.01; Mann-Whitney U Test; n = 6–7 individuals per group). (D) The morphology and size of OR-expressing PNECs in COPD and non-COPD lungs are similar. Left panels, non-COPD; right panels, COPD. Cells were labeled with an anti-OR2W1 antibody. White arrowheads mark PNECs.
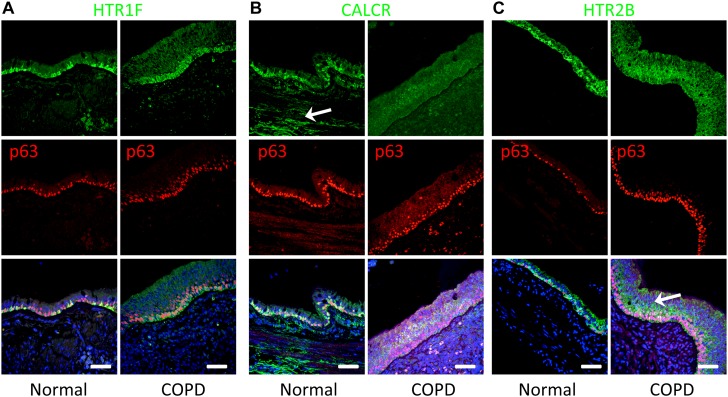
Another possible explanation for the increased sensitivity of patients with COPD to inhaled substances is an increased level of neuroendocrine receptors and a consequent increase in the response to neuropeptides released by volatile-activated OR-expressing PNECs. Consistent with this hypothesis, we found that 5-HT and CGRP receptor distributions appeared to be altered in COPD versus non-COPD airway tissues (Figure 6). In particular, we found that HTR1F was expressed in nearly all basal cells in subjects with COPD compared with a subset of basal cells in those without COPD, on the basis of the extent of costaining with the basal cell marker, p63 (Figure 6A). In contrast, we detected decreased staining levels of the CGRP receptor, CALCR, in airway epithelium in patients with COPD , but increased localization to basal cells in subjects without COPD (Figure 6B). Similarly, HTR2B changed from diffuse epithelial expression in COPD to increased basal cell staining in subjects without COPD (Figure 6C). Although these descriptive data are difficult to quantify, due to the high variability of receptor distributions across individuals, they do raise the possibility that neuroendocrine signals to basal cells may be altered in patients with COPD. Nevertheless, these data provide a potential mechanism for several different cell types in the airway to respond to the neuroendocrine factors released from volatile-activated PNECs.
Figure 6.
Spatial expression patterns of 5-HT and CGRP receptors are different in normal versus COPD lungs. (A) Representative photomicrographs of immunostaining for HTR1F (green). Staining is enriched in basal cells in normal and COPD lungs (basal cells marked with the p63 marker, red nuclei). Note that the morphology of COPD basal cells is elongated, and the distribution of the receptor uniform, compared with those in normal lungs. (B) Representative photomicrographs of immunostaining for CALCR (green). Staining is highly enriched in basal cells (marked by p63, red nuclei) and smooth muscle or neuronal fibers (white arrow) in normal donors. However, expression is lower and more diffused in COPD lungs. (C) Representative photomicrographs of immunostaining for HTR2B (green). Receptor is enriched basal cells of lungs from non-COPD subjects when compared with normal lungs. Note that in lungs of subjects with COPD, staining for HTR2B is more diffused and higher in nonbasal epithelial cells relative to basal cells (white arrow). Scale bars, 50 μm.
Discussion
The exacerbation of airway diseases, such as asthma or COPD, is often mediated by exposure to environmental factors, such as industrial solvents, various pungent odors, and other volatiles. In recent years, several key studies have demonstrated that specific populations of cells in the mammalian airway epithelia can be directly activated by inhaled “bitter” irritants, which include ciliated epithelial cells in lower (5) and upper airways (6), brush cells (7, 12), and airway smooth muscles (4). In spite of this significant progress, the cells and receptors that mediate the response of human airways to the majority of volatile chemicals in health or disease are still largely unknown. In this study, we identified a previously unrecognized chemosensory function for a population of solitary PNECs in adult human airways. Although the presence of solitary PNECs in human airways has been described (26), no clear physiological functions have been attributed to these cells. Based on cell morphology, tissue localization, and molecular characterization, the adult solitary PNECs may represent a cell linage that is independent of similar PNECs that have been previously described in rodent models. For example, in contrast to adult solitary PNECs and NEBs present in some rodent lungs, human PNECs do not express oxygen-sensing ion channels, and are found in major trachea and bronchi, but not alveolar tissues. However, immunostaining of human tissues with the neuronal marker, neurofilament H, revealed a possible innervation of human PNECs, as has been described for NEBs in some rodent species. These findings further suggest that human PNECs are not likely to be homologous to the previously described neonatal human PNECs and NEBs, or other types of adult PNECs that have been described in animal models (29, 30, 32, 35, 44). We currently do not understand why PNECs are different in primates relative to other mammals. However, species-specific differences in lung cellular and physiological functions are consistent with the proposal that human airways have evolved distinct cell lineages and signaling systems for respiratory-specific functions. Other examples include the marked differences in the types of airway progenitor cells that develop after viral infection (45). Similarly, cystic fibrosis transmembrane conductance regulator and chloride channel accessory 1–mitogen-activated protein kinase 13 signals exhibit markedly different function in human versus rodent lung for control of ion transport and mucus production (46–48).
Our study also highlights novel features of OR function in the lung in comparison to the olfactory system. We show that adult PNECs express more than one OR per cell. This arrangement contrasts with the one in the olfactory epithelium, in which each olfactory sensory neuron expresses a single receptor in a cellular process that is still not well understood (49). In contrast to the narrowly tuned nasal olfactory sensory neurons, these differences might also suggest that sensing volatiles in the lung is nonneuronal, and is broadly responsive even within single cells. Thus, we demonstrate that airway epithelial cells can respond to structurally diverse classes of volatile chemicals at least in cell culture. Moreover, this response is likely only a partial profile, because the majority of the receptors that we identified in human airways are still orphan in regard to their ligand specificity.
Unexpectedly, we detected no expression of cyclic nucleotide gated channels, including the olfactory-specific subunits, CNGA2 and CNGA4, in human airway tissue. Because these channels seem to play an important role in the neuronal olfactory signal transduction pathway, these findings suggest that stimulation of OR-expressing PNECs does not lead to changes in membrane potential, but rather uses different signaling routes. Nonetheless, we found that the apical stimulation of airway epithelia with volatiles can lead to lower cellular contents of neuroendocrine factors, such as 5-HT and CGRP, which is in agreement with a stimulus-dependent release. Although we did not directly test the role of OR stimulation in the volatile-dependent release of 5-HT and CGRP, the presence of these receptors in PNECs is highly suggestive that these receptors are responsible for the activation of PNECs by environmental chemical stimuli. Subsequently, these factors are likely to affect the physiology of other cell types in the epithelium, including smooth muscle cells, neurons, and basal cells. Thus, PNECs could represent a key cellular pathway for sensing environmental volatile stimuli in lungs.
Furthermore, our studies suggest that PNECs may contribute to the chemical hypersensitivity of lungs from patients with airway diseases, such as COPD. This is supported by our findings that the PNEC cell frequency is higher in lung tissues of patients with COPD relative to healthy individuals (Figure 5). However, we recognize that our method for quantifying PNECs in tissues may not fully account for the overall effects of inflammation, which could influence factors such as cell size or morphology of nuclei in tissue sections, and potentially bias our calculations of cell number. Thus, future studies of PNEC distribution performed using whole-mount tissue staining in a large number of samples will further address this issue. In addition, our study examined only a single concentration of each ligand to establish the response of PNECs to odorants. We recognize that differences in the numbers of PNECs in the airway might also influence the sensitivity of these tissues to odorant stimulation. For example, increased PNEC levels in COPD lungs might result in hypersensitivity to odorants, compared with the response in healthy lungs. Therefore, detailed dose–response assessment will be required to fully assess the differences in odorant responses between COPD and non-COPD conditions.
Taken together, our study offers the significant advance of identifying a new class of chemosensory cells that express ORs in adult human airways. The finding adds to the growing understanding of the complexity of the sensory response of the airway epithelium to diverse classes of sensory stimuli, and thereby impacts our understanding of how the human lung responds to environmental stimuli in both health and disease. We also detect a possible variation in PNECs and PNEC-responsive receptors between subjects with and without COPD that suggests a role for this OR–neuroendocrine axis in the pulmonary response to environmental volatile stimuli that might accompany chronic lung disease. Although more work is required to characterize the sensory functions of PNECs, it is possible that PNEC-specific pathways may be a therapeutic target to manage diseases associated with airway hypersensitivity in asthma, COPD, and related conditions.
Acknowledgments
Acknowledgments
The authors thank L. Belaygorod for technical assistance, C. Mikols for assistance with cell isolation and culture, and L. Miller for providing airway tissue sections from rhesus monkeys from the resources of the California National Primate Research Center (University of California, Davis).
Footnotes
This work was supported by National Institutes of Health (NIH)/National Heart, Lung, and Blood Institute grants HL29594 and HL121791, National Institutes of Allergy and Infectious Disease Asthma and Allergic Diseases Cooperative Research Center grants U19-AI070489 and NIDCD DC010244. Cells were provided by the In Vitro Models and Cell Culture Core at the University of Iowa, Carver College of Medicine, supported by NIH grants HL51670 and DK54759 and Cystic Fibrosis Foundation grant R458-CR07, and the Airway Epithelial Cell Core at Washington University School of Medicine supported by the Children’s Discovery Institute of Washington University School of Medicine and St. Louis Children’s Hospital.
This article has an online supplement, which is accessible from this issue’s table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1165/rcmb.2013-0199OC on October 17, 2013
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Massaro GD, Massaro D. Formation of pulmonary alveoli and gas-exchange surface area: quantitation and regulation. Annu Rev Physiol. 1996;58:73–92. doi: 10.1146/annurev.ph.58.030196.000445. [DOI] [PubMed] [Google Scholar]
- 2.Patton JS, Byron PR. Inhaling medicines: delivering drugs to the body through the lungs. Nat Rev Drug Discov. 2007;6:67–74. doi: 10.1038/nrd2153. [DOI] [PubMed] [Google Scholar]
- 3.Millqvist E, Bende M, Löwhagen O. Sensory hyperreactivity—a possible mechanism underlying cough and asthma-like symptoms. Allergy. 1998;53:1208–1212. doi: 10.1111/j.1398-9995.1998.tb03843.x. [DOI] [PubMed] [Google Scholar]
- 4.Deshpande DA, Wang WC, McIlmoyle EL, Robinett KS, Schillinger RM, An SS, Sham JS, Liggett SB. Bitter taste receptors on airway smooth muscle bronchodilate by localized calcium signaling and reverse obstruction. Nat Med. 2010;16:1299–1304. doi: 10.1038/nm.2237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shah AS, Ben-Shahar Y, Moninger TO, Kline JN, Welsh MJ. Motile cilia of human airway epithelia are chemosensory. Science. 2009;325:1131–1134. doi: 10.1126/science.1173869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lee RJ, Xiong G, Kofonow JM, Chen B, Lysenko A, Jiang P, Abraham V, Doghramji L, Adappa ND, Palmer JN, et al. T2R38 taste receptor polymorphisms underlie susceptibility to upper respiratory infection. J Clin Invest. 2012;122:4145–4159. doi: 10.1172/JCI64240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Saunders CJ, Reynolds SD, Finger TE. Chemosensory brush cells of the trachea: a stable population in a dynamic epithelium. Am J Respir Cell Mol Biol. 2013;49:190–196. doi: 10.1165/rcmb.2012-0485OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Merigo F, Benati D, Tizzano M, Osculati F, Sbarbati A. alpha-Gustducin immunoreactivity in the airways. Cell Tissue Res. 2005;319:211–219. doi: 10.1007/s00441-004-1007-2. [DOI] [PubMed] [Google Scholar]
- 9.Tizzano M, Cristofoletti M, Sbarbati A, Finger TE. Expression of taste receptors in solitary chemosensory cells of rodent airways. BMC Pulm Med. 2011;11:3. doi: 10.1186/1471-2466-11-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tizzano M, Gulbransen BD, Vandenbeuch A, Clapp TR, Herman JP, Sibhatu HM, Churchill ME, Silver WL, Kinnamon SC, Finger TE. Nasal chemosensory cells use bitter taste signaling to detect irritants and bacterial signals. Proc Natl Acad Sci USA. 2010;107:3210–3215. doi: 10.1073/pnas.0911934107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Krasteva G, Canning BJ, Hartmann P, Veres TZ, Papadakis T, Mühlfeld C, Schliecker K, Tallini YN, Braun A, Hackstein H, et al. Cholinergic chemosensory cells in the trachea regulate breathing. Proc Natl Acad Sci USA. 2011;108:9478–9483. doi: 10.1073/pnas.1019418108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Krasteva G, Canning BJ, Papadakis T, Kummer W. Cholinergic brush cells in the trachea mediate respiratory responses to quorum sensing molecules. Life Sci. 2012;91:992–996. doi: 10.1016/j.lfs.2012.06.014. [DOI] [PubMed] [Google Scholar]
- 13.Lin W, Ogura T, Margolskee RF, Finger TE, Restrepo D. TRPM5-expressing solitary chemosensory cells respond to odorous irritants. J Neurophysiol. 2008;99:1451–1460. doi: 10.1152/jn.01195.2007. [DOI] [PubMed] [Google Scholar]
- 14.Buck L, Axel R. A novel multigene family may encode odorant receptors: a molecular basis for odor recognition. Cell. 1991;65:175–187. doi: 10.1016/0092-8674(91)90418-x. [DOI] [PubMed] [Google Scholar]
- 15.Feldmesser E, Olender T, Khen M, Yanai I, Ophir R, Lancet D. Widespread ectopic expression of olfactory receptor genes. BMC Genomics. 2006;7:121. doi: 10.1186/1471-2164-7-121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Spehr M, Gisselmann G, Poplawski A, Riffell JA, Wetzel CH, Zimmer RK, Hatt H. Identification of a testicular odorant receptor mediating human sperm chemotaxis. Science. 2003;299:2054–2058. doi: 10.1126/science.1080376. [DOI] [PubMed] [Google Scholar]
- 17.Pluznick JL, Zou DJ, Zhang X, Yan Q, Rodriguez-Gil DJ, Eisner C, Wells E, Greer CA, Wang T, Firestein S, et al. Functional expression of the olfactory signaling system in the kidney. Proc Natl Acad Sci USA. 2009;106:2059–2064. doi: 10.1073/pnas.0812859106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pluznick JL, Protzko RJ, Gevorgyan H, Peterlin Z, Sipos A, Han J, Brunet I, Wan LX, Rey F, Wang T, et al. Olfactory receptor responding to gut microbiota–derived signals plays a role in renin secretion and blood pressure regulation. Proc Natl Acad Sci USA. 2013;110:4410–4415. doi: 10.1073/pnas.1215927110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Braun T, Voland P, Kunz L, Prinz C, Gratzl M. Enterochromaffin cells of the human gut: sensors for spices and odorants. Gastroenterology. 2007;132:1890–1901. doi: 10.1053/j.gastro.2007.02.036. [DOI] [PubMed] [Google Scholar]
- 20.Karp PH, Moninger TO, Weber SP, Nesselhauf TS, Launspach JL, Zabner J, Welsh MJ. An in vitro model of differentiated human airway epithelia: methods for establishing primary cultures. Methods Mol Biol. 2002;188:115–137. doi: 10.1385/1-59259-185-X:115. [DOI] [PubMed] [Google Scholar]
- 21.Tyner JW, Kim EY, Ide K, Pelletier MR, Roswit WT, Morton JD, Battaile JT, Patel AC, Patterson GA, Castro M, et al. Blocking airway mucous cell metaplasia by inhibiting EGFR antiapoptosis and IL-13 transdifferentiation signals. J Clin Invest. 2006;116:309–321. doi: 10.1172/JCI25167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zabner J, Scheetz TE, Almabrazi HG, Casavant TL, Huang J, Keshavjee S, McCray PB., Jr CFTR DeltaF508 mutation has minimal effect on the gene expression profile of differentiated human airway epithelia. Am J Physiol Lung Cell Mol Physiol. 2005;289:L545–L553. doi: 10.1152/ajplung.00065.2005. [DOI] [PubMed] [Google Scholar]
- 23.Su CY, Menuz K, Reisert J, Carlson JR. Non-synaptic inhibition between grouped neurons in an olfactory circuit. Nature. 2012;492:66–71. doi: 10.1038/nature11712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Khan M, Vaes E, Mombaerts P. Regulation of the probability of mouse odorant receptor gene choice. Cell. 2011;147:907–921. doi: 10.1016/j.cell.2011.09.049. [DOI] [PubMed] [Google Scholar]
- 25.Gu X, Ben-Shahar Y.Olfactory receptors in human airway epithelia Cresto C.editor. Olfactory receptors: methods and protocolsNew York City: Humana Press: 2013161–169. [DOI] [PubMed] [Google Scholar]
- 26.Weichselbaum M, Sparrow MP, Hamilton EJ, Thompson PJ, Knight DA. A confocal microscopic study of solitary pulmonary neuroendocrine cells in human airway epithelium. Respir Res. 2005;6:115. doi: 10.1186/1465-9921-6-115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lechin F, van der Dijs B, Lechin AE. Severe asthma and plasma serotonin. Allergy. 2002;57:258–259. doi: 10.1034/j.1398-9995.2002.1l3574.x. [DOI] [PubMed] [Google Scholar]
- 28.Moore BD, Hyde D, Miller L, Wong E, Frelinger J, Schelegle ES. Allergen and ozone exacerbate serotonin-induced increases in airway smooth muscle contraction in a model of childhood asthma. Respiration. 2012;83:529–542. doi: 10.1159/000336835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Avadhanam KP, Plopper CG, Pinkerton KE. Mapping the distribution of neuroepithelial bodies of the rat lung: a whole-mount immunohistochemical approach. Am J Pathol. 1997;150:851–859. [PMC free article] [PubMed] [Google Scholar]
- 30.Cutz E, Perrin DG, Pan J, Haas EA, Krous HF. Pulmonary neuroendocrine cells and neuroepithelial bodies in sudden infant death syndrome: potential markers of airway chemoreceptor dysfunction. Pediatr Dev Pathol. 2007;10:106–116. doi: 10.2350/06-06-0113.1. [DOI] [PubMed] [Google Scholar]
- 31.Cutz E, Yeger H, Pan J. Pulmonary neuroendocrine cell system in pediatric lung disease—recent advances. Pediatr Dev Pathol. 2007;10:419–435. doi: 10.2350/07-04-0267.1. [DOI] [PubMed] [Google Scholar]
- 32.Hong KU, Reynolds SD, Giangreco A, Hurley CM, Stripp BR. Clara cell secretory protein–expressing cells of the airway neuroepithelial body microenvironment include a label-retaining subset and are critical for epithelial renewal after progenitor cell depletion. Am J Respir Cell Mol Biol. 2001;24:671–681. doi: 10.1165/ajrcmb.24.6.4498. [DOI] [PubMed] [Google Scholar]
- 33.Peers C, Wyatt CN, Evans AM. Mechanisms for acute oxygen sensing in the carotid body. Respir Physiol Neurobiol. 2010;174:292–298. doi: 10.1016/j.resp.2010.08.010. [DOI] [PubMed] [Google Scholar]
- 34.Weir EK, López-Barneo J, Buckler KJ, Archer SL. Acute oxygen-sensing mechanisms. N Engl J Med. 2005;353:2042–2055. doi: 10.1056/NEJMra050002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cutz E, Jackson A. Neuroepithelial bodies as airway oxygen sensors. Respir Physiol. 1999;115:201–214. doi: 10.1016/s0034-5687(99)00018-3. [DOI] [PubMed] [Google Scholar]
- 36.Schmiedeberg K, Shirokova E, Weber HP, Schilling B, Meyerhof W, Krautwurst D. Structural determinants of odorant recognition by the human olfactory receptors OR1A1 and OR1A2. J Struct Biol. 2007;159:400–412. doi: 10.1016/j.jsb.2007.04.013. [DOI] [PubMed] [Google Scholar]
- 37.Krautwurst D, Yau KW, Reed RR. Identification of ligands for olfactory receptors by functional expression of a receptor library. Cell. 1998;95:917–926. doi: 10.1016/s0092-8674(00)81716-x. [DOI] [PubMed] [Google Scholar]
- 38.Caceres AI, Brackmann M, Elia MD, Bessac BF, del Camino D, D’Amours M, Witek JS, Fanger CM, Chong JA, Hayward NJ, et al. A sensory neuronal ion channel essential for airway inflammation and hyperreactivity in asthma. Proc Natl Acad Sci USA. 2009;106:9099–9104. doi: 10.1073/pnas.0900591106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Maclean MR, Dempsie Y. The serotonin hypothesis of pulmonary hypertension revisited. Adv Exp Med Biol. 2010;661:309–322. doi: 10.1007/978-1-60761-500-2_20. [DOI] [PubMed] [Google Scholar]
- 40.Dupont LJ, Pype JL, Demedts MG, De Leyn P, Deneffe G, Verleden GM. The effects of 5-HT on cholinergic contraction in human airways in vitro. Eur Respir J. 1999;14:642–649. doi: 10.1034/j.1399-3003.1999.14c26.x. [DOI] [PubMed] [Google Scholar]
- 41.Cazzola I, Matera MG. 5-HT modifiers as a potential treatment of asthma. Trends Pharmacol Sci. 2000;21:13–16. doi: 10.1016/s0165-6147(99)01408-x. [DOI] [PubMed] [Google Scholar]
- 42.Agapov E, Battaile JT, Tidwell R, Hachem R, Patterson GA, Pierce RA, Atkinson JJ, Holtzman MJ. Macrophage chitinase 1 stratifies chronic obstructive lung disease. Am J Respir Cell Mol Biol. 2009;41:379–384. doi: 10.1165/rcmb.2009-0122RC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Deslee G, Woods JC, Moore CM, Liu L, Conradi SH, Milne M, Gierada DS, Pierce J, Patterson A, Lewit RA, et al. Elastin expression in very severe human COPD. Eur Respir J. 2009;34:324–331. doi: 10.1183/09031936.00123008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Domnik NJ, Cutz E. Pulmonary neuroepithelial bodies as airway sensors: putative role in the generation of dyspnea. Curr Opin Pharmacol. 2011;11:211–217. doi: 10.1016/j.coph.2011.04.003. [DOI] [PubMed] [Google Scholar]
- 45.Byers DE, Alexander-Brett J, Patel AC, Agapov E, Dang-Vu G, Jin X, Wu K, You Y, Alevy Y, Girard J-P, et al. Long-term IL-33–producing epithelial progenitor cells in chronic obstructive lung disease. J Clin Invest. 2013;123:3967–3982. doi: 10.1172/JCI65570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rogers CS, Stoltz DA, Meyerholz DK, Ostedgaard LS, Rokhlina T, Taft PJ, Rogan MP, Pezzulo AA, Karp PH, Itani OA, et al. Disruption of the CFTR gene produces a model of cystic fibrosis in newborn pigs. Science. 2008;321:1837–1841. doi: 10.1126/science.1163600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Grubb BR, Boucher RC. Pathophysiology of gene-targeted mouse models for cystic fibrosis. Physiol Rev. 1999;79(1) Suppl:S193–S214. doi: 10.1152/physrev.1999.79.1.S193. [DOI] [PubMed] [Google Scholar]
- 48.Alevy YG, Patel AC, Romero AG, Patel DA, Tucker J, Roswit WT, Miller CA, Heier RF, Byers DE, Brett TJ, et al. IL-13–induced airway mucus production is attenuated by MAPK13 inhibition. J Clin Invest. 2012;122:4555–4568. doi: 10.1172/JCI64896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lomvardas S, Barnea G, Pisapia DJ, Mendelsohn M, Kirkland J, Axel R. Interchromosomal interactions and olfactory receptor choice. Cell. 2006;126:403–413. doi: 10.1016/j.cell.2006.06.035. [DOI] [PubMed] [Google Scholar]
- 50.Whitcutt MJ, Adler KB, Wu R. A biphasic chamber system for maintaining polarity of differentiation of cultured respiratory tract epithelial cells. In Vitro Cell Dev Biol. 1988;24:420–428. doi: 10.1007/BF02628493. [DOI] [PubMed] [Google Scholar]