Abstract
Cigarette smoking causes acquired cystic fibrosis transmembrane conductance regulator (CFTR) dysfunction and is associated with delayed mucociliary clearance and chronic bronchitis. Roflumilast is a clinically approved phosphodiesterase 4 inhibitor that improves lung function in patients with chronic bronchitis. We hypothesized that its therapeutic benefit was related in part to activation of CFTR. Primary human bronchial epithelial (HBE) cells, Calu-3, and T84 monolayers were exposed to whole cigarette smoke (WCS) or air with or without roflumilast treatment. CFTR-dependent ion transport was measured in modified Ussing chambers. Airway surface liquid (ASL) was determined by confocal microscopy. Intestinal fluid secretion of ligated murine intestine was monitored ex vivo. Roflumilast activated CFTR-dependent anion transport in normal HBE cells with a half maximal effective concentration of 2.9 nM. Roflumilast partially restored CFTR activity in WCS-exposed HBE cells (5.3 ± 1.1 μA/cm2 vs. 1.2 ± 0.2 μA/cm2 [control]; P < 0.05) and was additive with ivacaftor, a specific CFTR potentiator approved for the treatment of CF. Roflumilast improved the depleted ASL depth of HBE monolayers exposed to WCS (9.0 ± 3.1 μm vs. 5.6 ± 2.0 μm [control]; P < 0.05), achieving 79% of that observed in air controls. CFTR activation by roflumilast also induced CFTR-dependent fluid secretion in murine intestine, increasing the wet:dry ratio and the diameter of ligated murine segments. Roflumilast activates CFTR-mediated anion transport in airway and intestinal epithelia via a cyclic adenosine monophosphate–dependent pathway and partially reverses the deleterious effects of WCS, resulting in augmented ASL depth. Roflumilast may benefit patients with chronic obstructive pulmonary disease with chronic bronchitis by activating CFTR, which may also underlie noninfectious diarrhea caused by roflumilast.
Keywords: chronic obstructive pulmonary disease, cigarette smoke, cystic fibrosis transmembrane conductance regulator, roflumilast, ion transport
Clinical Relevance
Roflumilast activates cystic fibrosis transmembrane conductance regulator (CFTR) by elevating cAMP levels and mitigates acquired CFTR dysfunction caused by cigarette smoke, providing a potential mechanism by which it confers therapeutic benefit among patients with chronic obstructive pulmonary disease and chronic bronchitis. Roflumilast also activates CFTR in intestinal epithelia, which may contribute to noninfectious diarrhea, a known side effect of the drug.
Chronic obstructive pulmonary disease (COPD) is the third leading cause of death in the United States and shares many pathologic features with cystic fibrosis (CF) airway disease (1, 2). This is particularly true for individuals who exhibit chronic bronchitis, which occurs in the majority of individuals with COPD (3). CF is caused by defects in the CF transmembrane conductance regulator (CFTR), a cyclic adenosine monophosphate (cAMP)-regulated anion transporter expressed on the apical surface of epithelial cells in multiple tissues, including the lung and intestine (4). Due to the absence of functional CFTR in patients with CF, airway surface liquid (ASL) depletion results (5), contributing to delayed mucociliary clearance (MCC), bacterial colonization, and respiratory decline (4). Patients with COPD also exhibit delayed MCC and mucus retention, which are independently associated with increased mortality (6) and accelerated disease progression (7).
Recently, a number of laboratories have shown that acquired CFTR dysfunction may cause delayed MCC even in the absence of congenital CFTR mutations (2, 8, 9). Cigarette smoke has been shown to reduce CFTR expression (9), to increase CFTR internalization (10), and to disrupt CFTR ion channel function in airway epithelial monolayers (9). Cigarette smoke also acutely decreases CFTR activity in the nasal airway, inducing rapid cellular internalization (10). The net effect is severely reduced CFTR-mediated fluid transport, depletion of the ASL, and delayed MCC (2). Clinical studies using nasal potential difference show that cigarette smoking is associated with reduced CFTR activity in healthy smokers and in smokers with COPD even in the absence of CFTR mutations (2, 8), a finding recapitulated in the lower airway (11). Reduced CFTR function in the nose and lung were also associated with chronic bronchitis (2, 11), suggesting that CFTR could represent a potential therapeutic target.
Cyclic nucleotide phosphodiesterase (PDE) inhibitors increase cellular cAMP and/or cyclic guanosine monophosphate (12). The cAMP-selective PDE4 family is a major isoform found in respiratory epithelia and in resident immune cells of the lung (13, 14). Roflumilast is a PDE4-selective inhibitor that improves lung function and the number of pulmonary exacerbations in patients with COPD, although this effect is seen only in patients with chronic bronchitis and frequent exacerbations (15). Why roflumilast improves this specific patient subphenotype remains unknown, and its effect on ion transport has not been characterized. Given that PDE inhibitors are known to strongly increase the chloride transport activity of CFTR (16), which would be expected to augment MCC, we hypothesized that roflumilast may ameliorate acquired CFTR dysfunction by inducing protein kinase (PK)A-mediated CFTR phosphorylation of residual CFTR, augmenting epithelial function in individuals with chronic bronchitis. CFTR-dependent fluid secretion could also be the basis of noninfectious diarrhea commonly associated with roflumilast treatment (17). Using epithelial monolayers and murine tissues, we determined the effects of roflumilast after whole cigarette smoke (WCS) exposure. Our studies demonstrate that roflumilast activates CFTR-dependent chloride secretion and abrogates the deleterious effects caused by WCS while contributing to gastrointestinal fluid secretion in a CFTR-dependent fashion. These results suggest that roflumilast confers clinical benefit in patients with COPD by activating CFTR and reiterate the importance of this pathway in COPD therapeutics.
Materials and Methods
Procurement and Growth of Epithelial Cells
The use of primary human bronchial epithelial (HBE) cells and tissues was approved by the Institutional Review Board. HBE cells were obtained from lung explants and garnered from normal donors and were confirmed to lack CFTR mutations (2, 18, 19). First- or second-passage cells were grown until terminally differentiated (2, 18, 19). Calu-3 and T84 monolayers were grown at the air–liquid interface (16).
WCS Exposure
Cells were subjected to 3R4F (University of Kentucky) WCS exposure levels via an inExpose cigarette pump system (Scireq-USA, Tempe, AZ) at 3 L/min using flexiWare6 (Scireq-USA) software. Machined containers were used to expose monolayers to WCS for 10 for 30 minutes. Air control monolayers were placed in a clean air current chamber replica.
Voltage Clamp Studies in Ussing Chambers
Short-circuit current (Isc) was measured under voltage clamp conditions using MC8 voltage clamps and P2300 Ussing chambers (Physiologic Instruments, San Diego, CA) as previously described (2, 19). Chambers were maintained at 37°C and 5% CO2. The mucosa of intact bronchus were examined similarly (2).
cAMP Quantitation and CFTR Regulatory Region Phosphorylation
cAMP was measured in Calu-3 cells with an EIA kit (Cayman Chemical Co., Ann Arbor, MI). R-region phosphorylation was performed in COS7 cells as described (20). COS7 cells were transfected transiently with a plasmid encoding the CF R-region (amino acids 635–836) with an N-terminal HA epitope tag. After 24 hours of treatment, lysates were immunoblotted with an anti-HA monoclonal antibody (R+D Systems, Minneapolis, MN) to detect R-region phosphorylation.
ASL Depth
The apical surfaces of HBE cells were stained with Texas red dye (20 μL at 2 mg/ml in Fc70), and cells were labeled with 5-chloromethylfluorescein diacetate (100 μM) approximately 4 hours after imaging. ASL depth was imaged with a confocal microscope and XZ scans. Values for each monolayer were derived from four regions of interest (2).
Intestinal Secretion Assay
Adult AJ mice were killed, and the small intestine was removed and bathed in modified Eagle medium. Intestines were flushed with saline solution to remove fecal deposits, and sequential 10-cm segments were ligated. Intestinal segments were then placed in media with roflumilast (30 nM), forskolin (20 μM), or vehicle at 37°C and 95% O2/5% CO2 for 3 hours. Weight and diameter measurements were taken before the addition of compounds and hourly thereafter. After completion, segments were desiccated to calculate the wet/dry ratio, and data were normalized by experimental day. Diameters were calculated by image analysis by an investigator blinded to treatment assignment.
Western Blot Analysis of CFTR Expression
CFTR expression in primary HBE cells treated with DMSO vehicle control or 30 nM of roflumilast was performed according to previously published protocols (2, 21).
Unitary Conductance Tracings
Single-channel currents were recorded from inside-out patches of primary HBE cells expressing wild-type CFTR treated with PKA + ATP alone or PKA + ATP + roflumilast, and open probability was calculated as reported previously (21, 22).
Reagents
Roflumilast (FW 403.21) was obtained from Santa Cruz (cat. #208313; Dallas, TX) and dissolved in DMSO at 1,000-fold stock concentration. H89 was from Enzo (Farmingdale, NY). All other agents were obtained from Sigma-Aldrich (St. Louis, MO). CSE was prepared as described (2).
Statistics
Inferential comparisons were made using Student’s t test or ANOVA as appropriate. Post hoc tests for multiple comparisons were calculated using Fisher’s least significant difference. All statistical tests were two-sided and were performed at a 5% significance level using GraphPad Prism (La Jolla, CA). Error bars in the figures designate SEM unless indicated otherwise.
Results
Cigarette Smoke Decreases CFTR-Dependent Ion Transport in HBE Cells
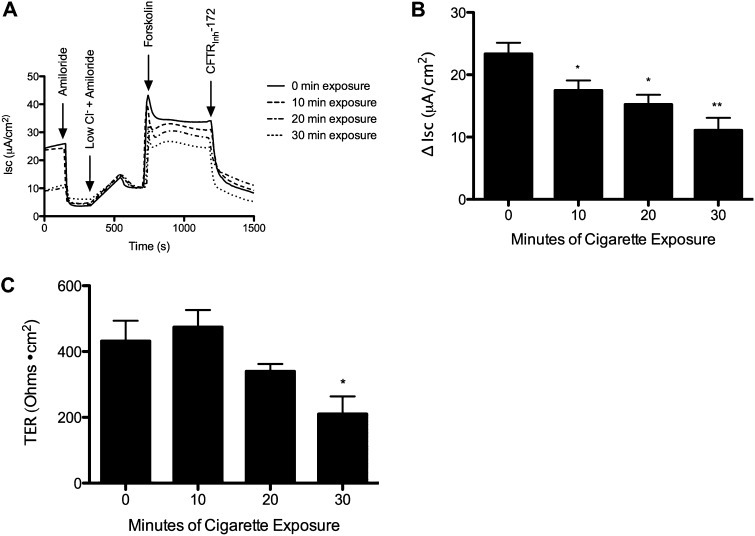
In vitro WCS exposure represented physiologically relevant concentrations of particulate matter that were comparable to those observed in smokers (23) (further details are provided in the online supplement). To characterize the dose-dependent effects of WCS-induced lung injury, we tested various levels of exposure on forskolin-stimulated transepithelial chloride transport in non-CF HBE monolayers. WCS caused a significant and dose-dependent reduction in CFTR-dependent ion transport (Figures 1A and 1B) that was similar in magnitude to previous studies using cigarette smoke extract (CSE) (2). Transepithelial resistance (TER) of HBE monolayers was not affected after 10- and 20-minute exposure durations, although TER was reduced by WCS exposure after the highest (30 min) exposure intensity (Figure 1C). Because the CFTR-dependent ion transport decrement observed at 10- and 20-minute WCS exposures resembled that seen in vivo (2, 8, 24), these exposures were used for subsequent studies while avoiding the nonspecific epithelial injury (e.g., decreased TER) seen with longer durations of exposure.
Figure 1.
Dose-dependent effects of whole cigarette smoke (WCS) on ion transport function. Well-differentiated non–cystic fibrosis (CF) primary human bronchial epithelial (HBE) cells were exposed to varying amounts of WCS or air control and then studied in Ussing chambers under short-circuit current (Isc) conditions. (A) Representative Isc tracing of HBE cells exposed to WCS for 0, 10, 20, or 30 minutes. The experiment included serial additions of amiloride (100 μM), chloride secretory gradient with amiloride, the cyclic adenosine monophosphate (cAMP) agonist forskolin (20 μM), and CFTRInh-172 (10 μM) to confirm CF transmembrane conductance regulator (CFTR) dependence. (B) Forskolin-stimulated change in Isc is shown for each cigarette smoke exposure level. *P < 0.05; **P < 0.005 (n = 4 per concentration). (C) Transepithelial resistance (TER) of HBE monolayers at the start of the experiment. **P < 0.005; *P < 0.05 (n = 4).
Roflumilast Activates CFTR-Dependent Anion Transport by Increasing cAMP and R-Region Phosphorylation
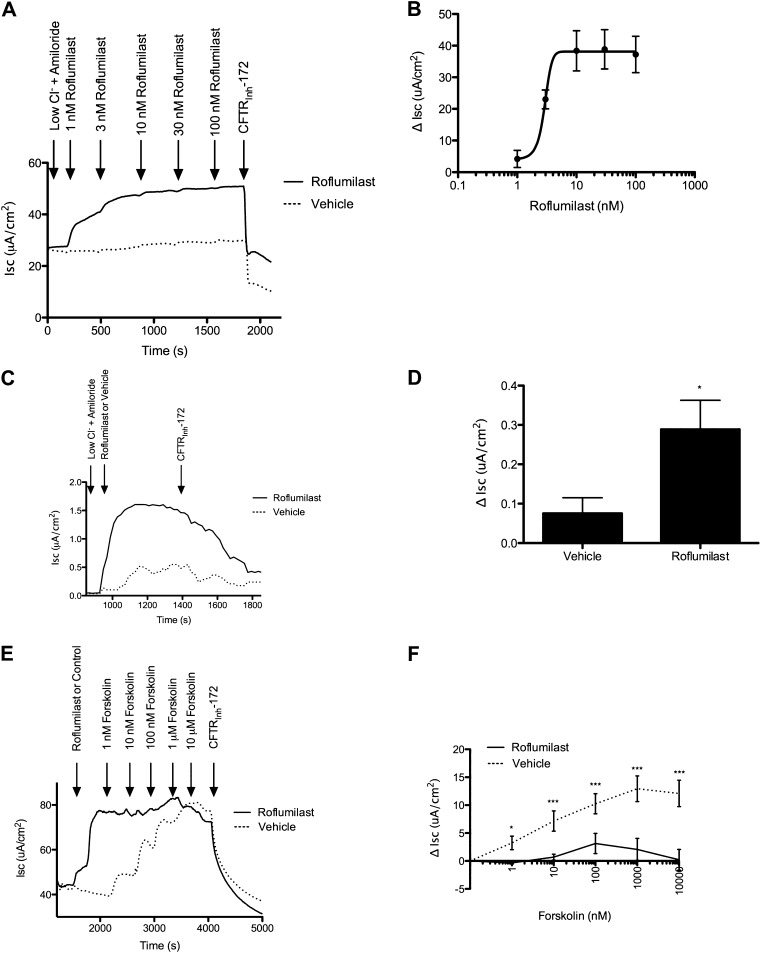
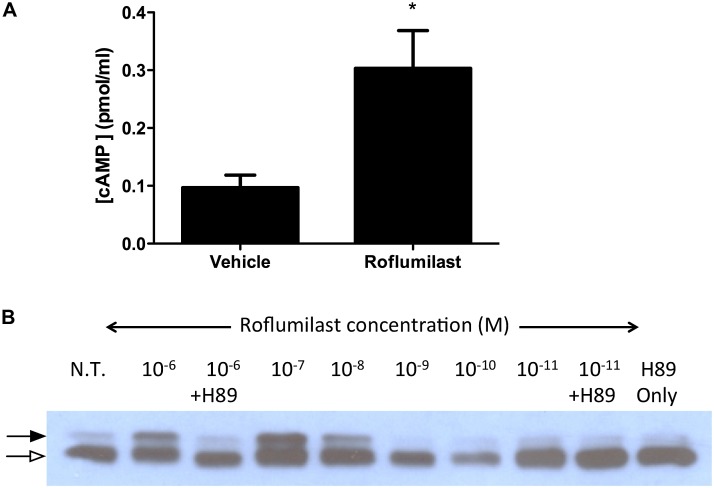
To quantify the effects of roflumilast on CFTR function, we established the dose dependence of roflumilast on anion transport in primary HBE monolayers (Figures 2A and 2B). Roflumilast activated cAMP-dependent CFTR anion transport in primary non-CF HBE monolayers with a half maximal effective concentration (EC50) of 2.9 nM, reaching a maximal current of 38.9 ± 6.2 μA/cm2 at 30 nM. Based on these results, we used 30 nM roflumilast, a concentration expected to occur in humans (25) while conferring activity of at least 90% of the maximal effective concentration. Roflumilast also activated CFTR-dependent currents in freshly excised human bronchi derived from a healthy donor (EC50 = 10.6 nM) (Figures 2C and 2D) in a fashion similar to epithelial monolayers. Roflumilast (30 nM) had no effect on CF HBE cells derived from a F508 del homozygote with no detectable surface CFTR expression (2.5 ± 0.7 μA/cm2 [roflumilast] vs. 4.3 ± 0.9 μA/cm2 [vehicle]). Roflumilast activated CFTR in a mechanism that was similar toforskolin, an adenylate cyclase activator that also increases cellular cAMP levels. Roflumilast did not produce any additional Isc stimulation over that conferred by maximal forskolin addition (20 μM), and the two compounds did not exhibit additive effects on CFTR-dependent anion transport in Calu-3 monolayers (Figures 2E and 2F). As expected, roflumilast increased intracellular cAMP (0.30 ± 0.16 pmol/ml [roflumilast] vs. 0.10 ± 0.02 pmol/ml [control]; P < 0.05) (Figure 3A), consistent with its function as a PDE4 inhibitor.
Figure 2.
Activation of CFTR by roflumilast. (A) Representative Isc tracing of well differentiated non-CF primary HBE cells studied in Ussing chambers under Isc conditions. The experiment included serial additions of amiloride (100 μM), chloride secretory gradient with amiloride, increasing concentrations of roflumilast or vehicle control, and CFTRInh-172 (10 μM). (B) Summary data of that shown in A. The half maximal effective concentration was 2.9 nM (n = 4). (C) Representative Isc tracing of a normal human bronchus analyzed for CFTR-dependent ion transport under Isc conditions. The experiment included serial additions of amiloride (100 μM), roflumilast (30 nM), or vehicle control followed by CFTRInh-172 (10 μM). (D) Summary data of that shown in C. *P < 0.05 (n = 4). (E) Representative Isc tracing of differentiated Calu-3 cells studied in Ussing chambers under Isc conditions. The experiment included serial additions of amiloride (100 μM), chloride secretory gradient with amiloride, roflumilast (30 nM) or vehicle control, and increasing concentrations of forskolin (1 nM to 10 μM) and CFTRInh-172 (10 μM). (F) Summary data of that shown in E, depicting change in Isc in response to increasing forskolin exposure. *P < 0.05; ***P < 0.0005.
Figure 3.
Roflumilast increases intracellular cAMP, resulting in CFTR regulatory region phosphorylation. (A) Differentiated Calu-3 cells were exposed to roflumilast (30 nM) or vehicle control, and intracellular cAMP was estimated compared with a relative standard curve. *P < 0.05 (n = 6). (B) COS7 cells transfected with a plasmid encoding for the CFTR R-region with an N-terminal hemagglutinin epitode were treated with increasing concentrations of roflumilast or the protein kinase A antagonist H89 (10 μM). Immunoblot shown with a closed arrow denotes the phosphorylated R-region, and an open arrow denotes the unphosphorylated R-region. N.T., no treatment. Data are from one of three experimental replicates.
To further identify the mechanism of action, we tested the effects of roflumilast on the phosphorylation of isolated CFTR Regulatory Region (R-R). We have shown previously that this assay is a sensitive measure of the phosphorylation of the CFTR R-R by the cAMP-dependent PKA (20). Roflumilast induced robust phosphorylation of isolated CFTR R-R in a dose-dependent fashion that was sensitive to the PKA inhibitor H89 (Figure 3B). The EC50 for phosphorylation in this assay was at a concentration similar to the IC50 of enzyme inhibition, indicating that the pharmacologic action of roflumilast was consistent with enzyme inhibition.
Because increased Isc could also be the result of increased surface expression or a direct effect on CFTR open channel probability, we tested whether roflumilast altered the expression of CFTR or had any direct effects on the channel function by Western blot analysis and unitary conductance tracings, respectively. In HBE cells treated with roflumilast for 24 hours, CFTR expression remained comparable to what was found in cells treated with vehicle control (Figure E2 in the online supplement). In addition, single-channel studies with isolated membrane patches from HBE cells indicate that roflumilast does not alter the open channel probability of CFTR (Figure E3). In combination, these results indicate that roflumilast activates CFTR by elevating cAMP, thereby inducing phosphorylation of R-R through a PKA-dependent pathway without significantly altering surface expression.
Roflumilast Ameliorates Smoke-Induced CFTR Dysfunction
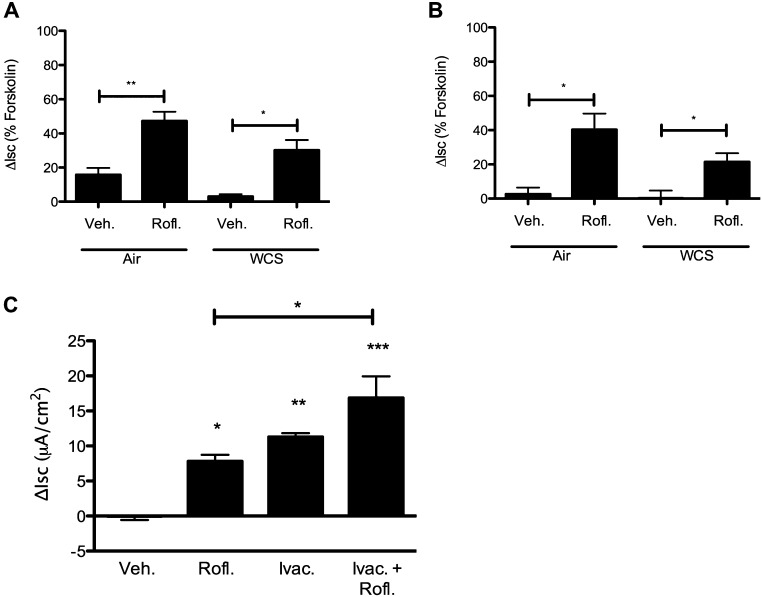
To evaluate whether roflumilast could ameliorate the deleterious effects of WCS on CFTR activity, we subjected HBE monolayers to WCS and evaluated the effect of roflumilast in Ussing chambers. Roflumilast significantly increased Isc compared with vehicle control in monolayers exposed to WCS for 10 minutes (5.3 ± 1.1 μA/cm2 [roflumilast] vs. 1.2 ± 0.2 μA/cm2 [control]; P < 0.05); when expressed as a fraction of maximal CFTR stimulus induced by forskolin, roflumilast activated Isc to 47.3 and 30.2% in air- and WCS-exposed monolayers, respectively (Figure 4A). Similar beneficial effects were observed after 20 minutes of WCS exposure (Figure 4B). Roflumilast caused a 6.6 ± 2.2 μA/cm2 stimulation in anion transport in WCS-exposed cells compared with 0.5 ± 1.2 μA/cm2 in vehicle-treated cells (P < 0.05), which was equivalent to 33.9% of the maximal forskolin response in smoke-exposed cells. The CFTR potentiator ivacaftor (formerly VX-770) has also been reported to ameliorate the deleterious effects of WCS exposure on anion transport (2). As opposed to roflumilast, activation of CFTR by ivacaftor is cAMP independent (26), providing an alternative mechanism by which acquired CFTR dysfunction could be addressed. To test for additive benefit, we evaluated these agents alone and in combination. In WCS-exposed HBE cells, roflumilast and ivacaftor augmented CFTR-dependent ion transport to similar levels but had an additive effect when combined (Figure 4C). Taken together, these studies demonstrated that roflumilast significantly mitigated WCS-induced CFTR dysfunction in a cAMP-dependent fashion, warranting further evaluations to detect downstream effects of roflumilast on epithelial function.
Figure 4.
Roflumilast increases Isc in primary HBE monolayers exposed to WCS. (A and B) Non-CF HBE monolayers were exposed to WCS and studied under Isc in the setting of amiloride (100 μM) and a chloride secretory gradient. The change in anion transport was measured after the addition of roflumilast (30 nM) or vehicle control and is plotted as a fraction of forskolin (20 μM)-mediated activation in air-exposed cells. The agonist-mediated change in Isc is plotted for cells exposed to 10-minute (A) and 20-minute (B) WCS exposure times. *P < 0.05; **P < 0.005 (n = 8 per condition). (C) Change in forskolin-dependent Isc in HBE cells exposed to 10 minutes of WCS and activated with roflumilast (Rofl.) (30 nM), ivacaftor (Ivac.) (10 μM), or a combination of the two under conditions shown in A. *P < 0.05; **P < 0.005; ***P < 0.001 (n = 4 per condition).
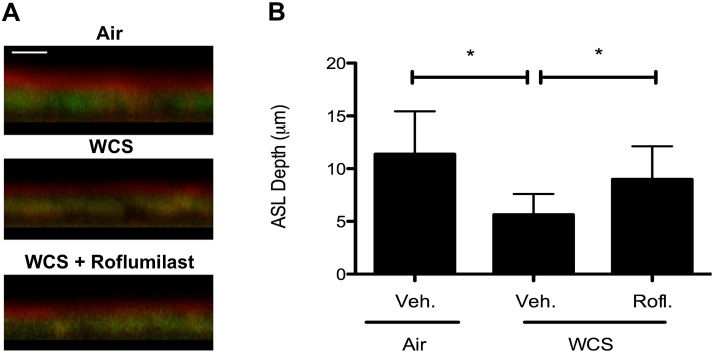
Roflumilast Increases ASL Depth in WCS-Exposed Cells
Because CFTR secretes chloride ions to regulate ASL depth, an important determinant of normal MCT (27), we evaluated whether roflumilast augmented ASL depth in WCS-exposed monolayers using confocal microscopy. Consistent with previous observations (2, 10), WCS decreased ASL depth compared with control monolayers (5.6 ± 2.0 μm [WCS] vs. 11.4 ± 4.1 μm [air control]; P < 0.05). Roflumilast treatment significantly mitigated WCS-induced reductions in ASL depth (9.0 ± 3.1 μm [roflumilast] vs. 5.6 ± 2.0 μm [vehicle]; P < 0.05), reaching 79% of the ASL depth found in normal HBE monolayers (Figure 5).
Figure 5.
Effect of roflumilast and WCS exposure on airway surface liquid (ASL) depth. (A) Representative confocal Z-scan image of well-differentiated non-CF HBE cells exposed to air control or to WCS (10 min) and treated with roflumilast (30 nM) or vehicle (Veh.) to the basolateral compartment for 24 hours before the assay. (B) Summary data of ASL depth across experiments. *P < 0.05 (n = 6 monolayers per condition).
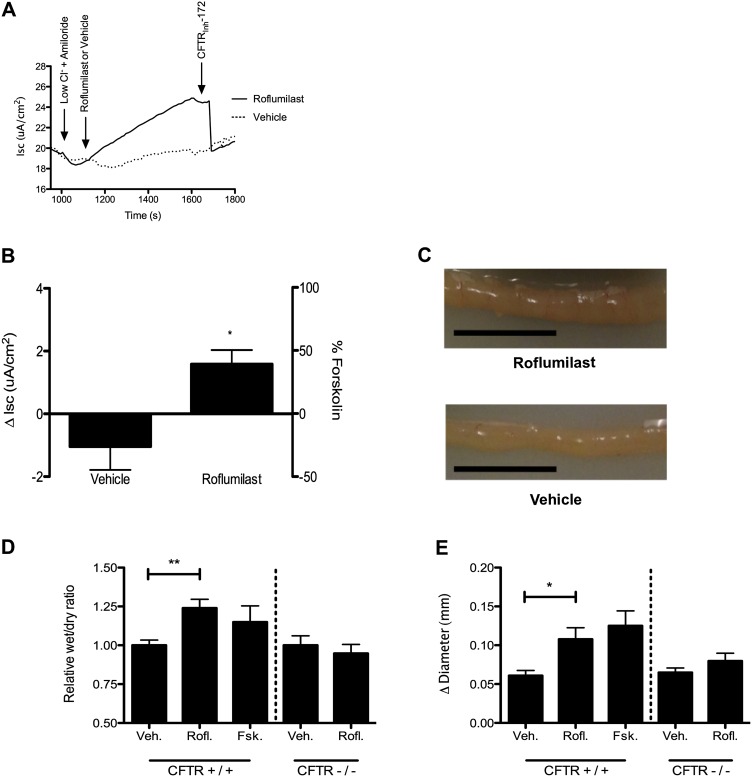
Roflumilast Increases CFTR Activity of Intestinal Monolayers In Vitro and Intestinal Segments Ex Vivo
Roflumilast therapy is associated with noninfectious diarrhea; the cause for this adverse reaction has not been determined. Because CFTR is highly expressed in gut epithelia and because its activation can contribute to diarrhea, we hypothesized that roflumilast might cause diarrhea by augmenting CFTR-dependent fluid secretion. Roflumilast significantly increased CFTR-dependent anion transport of T84 intestinal epithelial monolayers compared with vehicle (1.5 ± 0.4 μA/cm2 [roflumilast] vs. −1.0 ± 0.7 μA/cm2 [vehicle]; P < 0.05) (Figures 6A and 6B). In isolated murine intestinal segments, roflumilast increased fluid secretion, as reflected by an increased wet:dry ratio (6.4 ± 0.8 [roflumilast] vs. 5.6 ± 0.7 [vehicle]; P < 0.005) (Figures 6C and 6D) and by a change in intestinal diameter (0.10 ± 0.01 mm [roflumilast] vs. 0.06 ± 0.01 mm [vehicle]; P < 0.05) (Figure 6E). No effect of roflumilast was observed in ligated intestine derived from CFTR(−/−) mice (congenic Cftrtm1Unc/J) (Figures 6D and 6E). These data illustrate that CFTR activation in intestinal epithelia by roflumilast could underlie the noninfectious diarrhea observed during roflumilast therapy.
Figure 6.
The effect of roflumilast on CFTR-mediated intestinal fluid secretion. (A) Representative Isc tracing of differentiated T84 intestinal epithelial cells studied in Ussing chambers under Isc conditions. The experiment included serial additions of amiloride (100 μM), chloride secretory gradient with amiloride, roflumilast (30 nM), or vehicle control followed by CFTRInh-172 (10 μM). (B) Summary data of change in stimulated Isc as shown in A. *P < 0.05 (n = 6 per condition). (C) Representative photograph of ligated CFTR+/+ mouse intestine after 3 hours of incubation with roflumilast (30 nM) or vehicle control in DMEM vigorously gassed with 95% O2/5% CO2 and held at 37°C. The scale bar is 1 cm. (D) Summary data of relative wet/dry ratio of murine intestine CFTR+/+ and CFTR−/− mice after 3 hours of incubation with test compounds. Forskolin control was 20 μM exposure. **P < 0.005 (n = 6 per condition). (E) Change in diameter of ligated intestine after 3 hours of incubation as detected by investigator blinded to treatment assignment. *P < 0.05 (n = 6 per condition).
Discussion
Emerging data from several laboratories indicate that cigarette smoke causes CFTR dysfunction in vitro and in vivo even in the absence of inherited CFTR mutations. This has been suggested to contribute to COPD pathogenesis by causing a predisposition to epithelial dysfunction (2, 9, 21, 24), mucus retention (2, 21, 28), and chronic bronchitis (2, 11, 21), resulting in a phenotype that resembles mild CF. Our studies are consistent with this hypothesis and demonstrate a 30 to 40% reduction in CFTR activity after WCS exposure without significant deleterious effect on TER or other indicators of cellular toxicity. This is similar to published studies using CSE (2, 8, 9) and WCS (24, 29) and resembles the magnitude of CFTR decrement detected in the upper (2, 8) and lower airway (11) of cigarette smokers. CFTR inhibition by WCS was dose dependent, providing additional confidence in the causative nature of CSE. Furthermore, particulate analysis of the 10- and 20-minute WCS exposures demonstrate a clinically relevant exposure intensity. The degree of CFTR inhibition by WCS was sufficient to reduce ASL depth, which is known to decrease the efficiency of MCT (30). Because the severity of the ion transport and ASL abnormalities was intermediate compared with the defect observed in CF epithelial cells, it seems likely that this decrement is sufficient to initiate a cascade of mucus retention and infection in vivo, particularly if present over multiple years and combined with enhanced mucus expression also caused by cigarette smoking (2, 31).
Recent studies have shown agents that activate CFTR, such as the CFTR potentiator ivacaftor, augment ASL depth, and consequently accelerate MCC, pharmacologically reversing acquired CFTR dysfunction (2). Alternatively, hypertonic saline, which compensates for CFTR-dependent ASL decrements, also restores ASL volume by osmotic forces (24) and reduces mucus obstruction in a mouse model of chronic bronchitis (32). Because PDE4 inhibitors are known to increase intracellular levels of cAMP and to activate CFTR in airway epithelia (33–38), we hypothesized that roflumilast could act similarly and ameliorate acquired CFTR dysfunction conferred by CSE. As shown in our studies, roflumilast efficiently activated CFTR in primary HBE cells and in intact human tissues, two models highly faithful to the ion transport properties of human airway epithelia in vivo (2, 26). In cells and in tissues, the maximal effective concentration of roflumilast-mediated CFTR activation was approximately 30 nM and exhibited a broad therapeutic plateau without signs of cellular toxicity or rundown. These concentrations are therapeutically relevant and are routinely achieved in humans prescribed roflumilast for therapeutic purposes, suggesting that wild-type CFTR activation could be readily achieved by therapeutic doses of roflumilast in vivo (25). The effects of roflumilast on CFTR function were also observed in epithelial cells exposed to WCS, indicating that acquired CFTR dysfunction is a relevant therapeutic target that can be addressed with roflumilast. Roflumilast robustly activated CFTR after low and moderate CSEs. Consequently, reduced ASL depth conferred by WCS exposure was significantly ameliorated by cotreatment with roflumilast. By enhancing ASL depth by CFTR-mediated fluid secretion, improved MCT is anticipated (2, 30), which could enable a more robust host defense in the COPD airway.
Roflumilast reduces the frequency of COPD exacerbations in patients who exhibit chronic bronchitis and have a history of frequent exacerbations (39). However, the mechanisms of its therapeutic properties are not completely understood. Roflumilast and other PDE4 inhibitors exhibit antiinflammatory properties, including the reduction of matrix metalloproteinase activity and TGF-β1 release after lung injury (17, 40). Although these mechanisms may confer benefit in patients with COPD, it is not clear why patients who have emphysema, and consequently significant inflammation even in the absence of chronic mucus hypersecretion, are not affected by this cAMP-mediated mechanism of inflammation. Conversely, two previous studies in distinct patient cohorts demonstrated that CFTR dysfunction among patients with COPD was associated with chronic bronchitis (2, 11). Because roflumilast activates residual CFTR function and because there is a strong association between CFTR abnormality and bronchitis (2, 11), it is likely that CFTR activation may be partially responsible for the therapeutic efficacy of roflumilast. Further studies are warranted to confirm this hypothesis and may also benefit from animal models of chronic bronchitis to address these questions.
Our data indicate a mechanistic basis underlying noninfectious diarrhea associated with roflumilast therapy. As a cAMP-activated Cl− channel expressed prominently in the intestine, CFTR has long been postulated to have an active role in secretory diarrhea (41). Although CFTR probably does not contribute to nausea caused by roflumilast, which is likely related to inhibition of PDE4D in neurons that regulate nausea (42), our studies demonstrate that roflumilast strongly activates CFTR in the distal intestine, leading to fluid secretion into the lumen. Studies in CFTR knockout mice confirmed that this reaction was CFTR dependent and establish that CFTR contributes to intestinal secretion when activated. Because CFTR inhibitors are being developed for the treatment of diarrhea (19, 43), it may be possible to abrogate the undesirable effects of roflumilast on the lower gastrointestinal tract by coadministering oral CFTR inhibitors that are not systemically absorbed. This strategy is worthy of further exploration as a therapeutic approach to minimize the adverse effects of roflumilast while allowing maximal benefit in the lung to be achieved.
Roflumilast activated CFTR by increasing levels of cAMP, resulting in a PKA-dependent phosphorylation of CFTR, a crucial step toward activating CFTR-mediated anion transport (20, 25, 34). As such, roflumilast activates CFTR in a fashion similar to forskolin, which explains why roflumilast did not potentiate maximal forskolin activity. Furthermore, its effects are dependent on residual CFTR at the membrane despite smoking-induced lung injury because it does not alter the levels of CFTR at the membrane. Several studies demonstrate that sufficient CFTR (nearly 50–60%) is present at the cell surface in patients with COPD and thus could serve as a therapeutic target in this way (2, 10, 21, 44). In contrast to roflumilast, ivacaftor potentiates CFTR activation by uncoupling ATPase activity from channel gating (26, 45, 46) and exhibits the most efficient pharmacological potentiation of wild-type CFTR in the setting of submaximal forskolin preactivation (2). This explains why roflumilast-mediated CFTR activation was additive to ivacaftor because the mechanisms of these agents are distinct and complementary. Combination treatment could represent a potential approach to maximize CFTR activation among patients with deficient CFTR function, including those with acquired abnormalities of CFTR activity. Whether this could be helpful in individuals with partially active CFTR alleles localized to the cell surface deserves further exploration.
Although the data provide evidence supporting CFTR activation as a basis for the beneficial effects of roflumilast, important limitations of the study should be noted. Although WCS has been frequently used to model smoking-related lung disease (24, 29) and the reduced CFTR function observed here is consistent with prior studies (2, 9, 24, 29), the experiments conducted rely on acute CSE and are unlikely to capture the complexity of the airway environment in patients with COPD, such as airway inflammation, disrupted proteolytic balance, and ER stress (3, 31, 47, 48). Although the conclusion that CFTR activation by roflumilast contributes to therapeutic benefit in patients with chronic bronchitis is supported by specificity controls, as an efficacious activator of intracellular cAMP, roflumilast may also improve the function of surface epithelia via other pathways. For example, cAMP is known to stimulate CFTR trafficking to the membrane (49) and to inhibit MUC5AC expression (50) and is an important stimulus of ciliary beating (51), which could augment MCC. Moreover, roflumilast has strong antiinflammatory effects, including inhibition of p38 MAP kinase (17, 52) and oxygen radical production (53) as well as suppression of the proliferation and cytokine release from CD4+,CD8+ lymphocytes that may be involved in the inhibition of the proinflammatory transcription factor NF-κB (52). Murine intestines are frequently used models for CFTR biology in the intestine and provide an important specificity control regarding the effects of roflumilast; however, it is plausible that other cAMP-mediated ion channels contribute to secretory diarrhea induced by roflumilast in humans. Further studies, including the coadministration of specific CFTR inhibitors in more sophisticated animal models, may help rule out this possibility.
In summary, acquired CFTR dysfunction caused by WCS can be ameliorated by roflumilast-mediated activation ofcAMP/PKA-dependent CFTR activation, resulting in improved epithelial function. CFTR activation may underlie the beneficial effects of roflumilast in patients with COPD with chronic bronchitis and may contribute to secretory diarrhea by hyperactivation of the same pathway. Additional studies to define the role of CFTR activation by roflumilast are needed in COPD and could result in improved therapeutic strategies to address acquired CFTR dysfunction in smoking-related lung disease while blocking its undesirable adverse effects.
Acknowledgments
Acknowledgments
The authors thank Ming Du and David Bedwell for supplying CF and non-CF mice through the UAB CF Center Animal Core and Jeremy A. Boyston and John E. Trombley for assistance with WCS exposure analytics.
Footnotes
This work was supported by National Institutes of Health grants 1R01 HL105487 (S.M.R.) and P30 DK072482 to the UAB CF Research Center at the CFF (R464) and Forest Research Institute, by the Howard Hughes Medical Institute (J.A.L.), and by American Lung Association Senior Research Fellowship grant RT-219427-N (S.V.R.).
Author Contributions: J.A.L., S.V.R., M.T.D., G.B.B., and S.M.R. conceived of the experiments; J.A.L., S.V.R., C.M.M., L.P.T., Y.L., C.A.C., R.F.F., G.E.C., L.H.S., and M.M.M. conducted the research; J.A.L., S.V.R., Y.L., C.A.C., G.B.B., and S.M.R. analyzed the data; J.A.L., S.V.R., and S.M.R. wrote the manuscript; and S.M.R. supervised the project.
This article has an online supplement, which is accessible from this issue’s table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1165/rcmb.2013-0228OC on October 9, 2013
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Calverley PM, Walker P. Chronic obstructive pulmonary disease. Lancet. 2003;362:1053–1061. doi: 10.1016/s0140-6736(03)14416-9. [DOI] [PubMed] [Google Scholar]
- 2.Sloane PA, Shastry S, Wilhelm A, Courville C, Tang LP, Backer K, Levin E, Raju SV, Li Y, Mazur M, et al. A pharmacologic approach to acquired cystic fibrosis transmembrane conductance regulator dysfunction in smoking related lung disease. PLoS ONE. 2012;7:e39809. doi: 10.1371/journal.pone.0039809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yoshida T, Tuder RM. Pathobiology of cigarette smoke-induced chronic obstructive pulmonary disease. Physiol Rev. 2007;87:1047–1082. doi: 10.1152/physrev.00048.2006. [DOI] [PubMed] [Google Scholar]
- 4.Rowe SM, Miller S, Sorscher EJ. Cystic fibrosis. N Engl J Med. 2005;352:1992–2001. doi: 10.1056/NEJMra043184. [DOI] [PubMed] [Google Scholar]
- 5.Matsui H, Grubb BR, Tarran R, Randell SH, Gatzy JT, Davis CW, Boucher RC. Evidence for periciliary liquid layer depletion, not abnormal ion composition, in the pathogenesis of cystic fibrosis airways disease. Cell. 1998;95:1005–1015. doi: 10.1016/s0092-8674(00)81724-9. [DOI] [PubMed] [Google Scholar]
- 6.Hogg JC, Chu F, Utokaparch S, Woods R, Elliott WM, Buzatu L, Cherniack RM, Rogers RM, Sciurba FC, Coxson HO, et al. The nature of small-airway obstruction in chronic obstructive pulmonary disease. N Engl J Med. 2004;350:2645–2653. doi: 10.1056/NEJMoa032158. [DOI] [PubMed] [Google Scholar]
- 7.Vestbo J, Prescott E, Lange P. Association of chronic mucus hypersecretion with fev1 decline and chronic obstructive pulmonary disease morbidity. Copenhagen City Heart Study Group. Am J Respir Crit Care Med. 1996;153:1530–1535. doi: 10.1164/ajrccm.153.5.8630597. [DOI] [PubMed] [Google Scholar]
- 8.Cantin AM, Hanrahan JW, Bilodeau G, Ellis L, Dupuis A, Liao J, Zielenski J, Durie P. Cystic fibrosis transmembrane conductance regulator function is suppressed in cigarette smokers. Am J Respir Crit Care Med. 2006;173:1139–1144. doi: 10.1164/rccm.200508-1330OC. [DOI] [PubMed] [Google Scholar]
- 9.Kreindler JL, Jackson AD, Kemp PA, Bridges RJ, Danahay H. Inhibition of chloride secretion in human bronchial epithelial cells by cigarette smoke extract. Am J Physiol Lung Cell Mol Physiol. 2005;288:L894–L902. doi: 10.1152/ajplung.00376.2004. [DOI] [PubMed] [Google Scholar]
- 10.Clunes LA, Davies CM, Coakley RD, Aleksandrov AA, Henderson AG, Zeman KL, Worthington EN, Gentzsch M, Kreda SM, Cholon D, et al. Cigarette smoke exposure induces cftr internalization and insolubility, leading to airway surface liquid dehydration. FASEB J. 2012;26:533–545. doi: 10.1096/fj.11-192377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dransfield MT, Wilhelm AM, Flanagan B, Courville C, Tidwell SL, Raju SV, Gaggar A, Steele C, Tang LP, Liu B, et al. Acquired CFTR dysfunction in the lower airways in COPD. Chest. 2013;144:498–506. doi: 10.1378/chest.13-0274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Conti M, Beavo J. Biochemistry and physiology of cyclic nucleotide phosphodiesterases: essential components in cyclic nucleotide signaling. Annu Rev Biochem. 2007;76:481–511. doi: 10.1146/annurev.biochem.76.060305.150444. [DOI] [PubMed] [Google Scholar]
- 13.Dent G, White SR, Tenor H, Bodtke K, Schudt C, Leff AR, Magnussen H, Rabe KF. Cyclic nucleotide phosphodiesterase in human bronchial epithelial cells: characterization of isoenzymes and functional effects of PDE inhibitors. Pulm Pharmacol Ther. 1998;11:47–56. doi: 10.1006/pupt.1998.0115. [DOI] [PubMed] [Google Scholar]
- 14.Barber R, Baillie GS, Bergmann R, Shepherd MC, Sepper R, Houslay MD, Heeke GV. Differential expression of Pde4 cAMP phosphodiesterase isoforms in inflammatory cells of smokers with COPD, smokers without COPD, and nonsmokers. Am J Physiol Lung Cell Mol Physiol. 2004;287:L332–L343. doi: 10.1152/ajplung.00384.2003. [DOI] [PubMed] [Google Scholar]
- 15.Calverley PM, Rabe KF, Goehring UM, Kristiansen S, Fabbri LM, Martinez FJ M2-124 and M2-125 study groups. Roflumilast in symptomatic chronic obstructive pulmonary disease: two randomised clinical trials. Lancet. 2009;374:685–694. doi: 10.1016/S0140-6736(09)61255-1. [DOI] [PubMed] [Google Scholar]
- 16.Cobb BR, Fan L, Kovacs TE, Sorscher EJ, Clancy JP. Adenosine receptors and phosphodiesterase inhibitors stimulate Cl- secretion in calu-3 cells. Am J Respir Cell Mol Biol. 2003;29:410–418. doi: 10.1165/rcmb.2002-0247OC. [DOI] [PubMed] [Google Scholar]
- 17.Spina D. Pde4 inhibitors: current status. Br J Pharmacol. 2008;155:308–315. doi: 10.1038/bjp.2008.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rowe SM, Miller S, Sorscher EJ. Cystic fibrosis. N Engl J Med. 2005;352:1992–2001. doi: 10.1056/NEJMra043184. [DOI] [PubMed] [Google Scholar]
- 19.Sonawane ND, Hu J, Muanprasat C, Verkman AS. Luminally active, nonabsorbable cftr inhibitors as potential therapy to reduce intestinal fluid loss in cholera. FASEB J. 2006;20:130–132. doi: 10.1096/fj.05-4818fje. [DOI] [PubMed] [Google Scholar]
- 20.Pyle LC, Ehrhardt A, Mitchell LH, Fan L, Ren A, Naren AP, Li Y, Clancy JP, Bolger GB, Sorscher EJ, et al. Regulatory domain phosphorylation to distinguish the mechanistic basis underlying acute CFTR modulators. Am J Physiol Lung Cell Mol Physiol. 2011;301:L587–L597. doi: 10.1152/ajplung.00465.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Raju SV, Jackson PL, Courville C, Sloane PA, Tang LP, Dransfield MT, Rowe SM. Cigarette smoke induces systemic defects in cystic fibrosis transmembrane conductance regulator (CFTR) function. Am J Respir Crit Care Med. In press doi: 10.1164/rccm.201304-0733OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hickman-Davis JM, McNicholas-Bevensee C, Davis IC, Ma HP, Davis GC, Bosworth CA, Matalon S. Reactive species mediate inhibition of alveolar type ii sodium transport during mycoplasma infection. Am J Respir Crit Care Med. 2006;173:334–344. doi: 10.1164/rccm.200501-155OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schramm S, Carre V, Scheffler JL, Aubriet F. Analysis of mainstream and sidestream cigarette smoke particulate matter by laser desorption mass spectrometry. Anal Chem. 2011;83:133–142. doi: 10.1021/ac1019842. [DOI] [PubMed] [Google Scholar]
- 24.Clunes LA, Davies CM, Coakley RD, Aleksandrov AA, Henderson AG, Zeman KL, Worthington EN, Gentzsch M, Kreda SM, Cholon D, et al. Cigarette smoke exposure induces cftr internalization and insolubility, leading to airway surface liquid dehydration. FASEB J. 2012;26:533–545. doi: 10.1096/fj.11-192377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rabe KF. Update on roflumilast, a phosphodiesterase 4 inhibitor for the treatment of chronic obstructive pulmonary disease. Br J Pharmacol. 2011;163:53–67. doi: 10.1111/j.1476-5381.2011.01218.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Van Goor F, Hadida S, Grootenhuis PD, Burton B, Cao D, Neuberger T, Turnbull A, Singh A, Joubran J, Hazlewood A, et al. Rescue of CF airway epithelial cell function in vitro by a CFTR potentiator, vx-770. Proc Natl Acad Sci USA. 2009;106:18825–18830. doi: 10.1073/pnas.0904709106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhang L, Button B, Gabriel SE, Burkett S, Yan Y, Skiadopoulos MH, Dang YL, Vogel LN, McKay T, Mengos A, et al. CFTR delivery to 25% of surface epithelial cells restores normal rates of mucus transport to human cystic fibrosis airway epithelium. PLoS Biol. 2009;7:e1000155. doi: 10.1371/journal.pbio.1000155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Innes AL, Woodruff PG, Ferrando RE, Donnelly S, Dolganov GM, Lazarus SC, Fahy JV. Epithelial mucin stores are increased in the large airways of smokers with airflow obstruction. Chest. 2006;130:1102–1108. doi: 10.1378/chest.130.4.1102. [DOI] [PubMed] [Google Scholar]
- 29.Savitski AN, Mesaros C, Blair IA, Cohen NA, Kreindler JL. Secondhand smoke inhibits both Cl- and K+ conductances in normal human bronchial epithelial cells. Respir Res. 2009;10:120. doi: 10.1186/1465-9921-10-120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Matsui H, Randell SH, Peretti SW, Davis CW, Boucher RC. Coordinated clearance of periciliary liquid and mucus from airway surfaces. J Clin Invest. 1998;102:1125–1131. doi: 10.1172/JCI2687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Di YP, Zhao J, Harper R. Cigarette smoke induces muc5ac protein expression through the activation of sp1. J Biol Chem. 2012;287:27948–27958. doi: 10.1074/jbc.M111.334375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Graeber SY, Zhou-Suckow Z, Schatterny J, Hirtz S, Boucher RC, Mall MA. Hypertonic saline is effective in the prevention and treatment of mucus obstruction but not airway inflammation in mice with chronic obstructive lung disease. Am J Respir Cell Mol Biol. 2013;49:410–417. doi: 10.1165/rcmb.2013-0050OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Milara J, Armengot M, Banuls P, Tenor H, Beume R, Artigues E, Cortijo J. Roflumilast n-oxide, a pde4 inhibitor, improves cilia motility and ciliated human bronchial epithelial cells compromised by cigarette smoke in vitro. Br J Pharmacol. 2012;166:2243–2262. doi: 10.1111/j.1476-5381.2012.01929.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Moran O. Model of the camp activation of chloride transport by CFTR channel and the mechanism of potentiators. J Theor Biol. 2010;262:73–79. doi: 10.1016/j.jtbi.2009.08.032. [DOI] [PubMed] [Google Scholar]
- 35.Barnes AP, Livera G, Huang P, Sun C, O'Neal WK, Conti M, Stutts MJ, Milgram SL. Phosphodiesterase 4d forms a camp diffusion barrier at the apical membrane of the airway epithelium. J Biol Chem. 2005;280:7997–8003. doi: 10.1074/jbc.M407521200. [DOI] [PubMed] [Google Scholar]
- 36.Lee JH, Richter W, Namkung W, Kim KH, Kim E, Conti M, Lee MG. Dynamic regulation of cystic fibrosis transmembrane conductance regulator by competitive interactions of molecular adaptors. J Biol Chem. 2007;282:10414–10422. doi: 10.1074/jbc.M610857200. [DOI] [PubMed] [Google Scholar]
- 37.O'Grady SM, Jiang X, Maniak PJ, Birmachu W, Scribner LR, Bulbulian B, Gullikson GW. Cyclic AMP-dependent Cl secretion is regulated by multiple phosphodiesterase subtypes in human colonic epithelial cells. J Membr Biol. 2002;185:137–144. doi: 10.1007/s00232-001-0120-3. [DOI] [PubMed] [Google Scholar]
- 38.Liu S, Veilleux A, Zhang L, Young A, Kwok E, Laliberte F, Chung C, Tota MR, Dube D, Friesen RW, et al. Dynamic activation of cystic fibrosis transmembrane conductance regulator by type 3 and type 4d phosphodiesterase inhibitors. J Pharmacol Exp Ther. 2005;314:846–854. doi: 10.1124/jpet.105.083519. [DOI] [PubMed] [Google Scholar]
- 39.Page CP, Spina D. Selective pde inhibitors as novel treatments for respiratory diseases. Curr Opin Pharmacol. 2012;12:275–286. doi: 10.1016/j.coph.2012.02.016. [DOI] [PubMed] [Google Scholar]
- 40.Dunkern TR, Feurstein D, Rossi GA, Sabatini F, Hatzelmann A. Inhibition of tgf-beta induced lung fibroblast to myofibroblast conversion by phosphodiesterase inhibiting drugs and activators of soluble guanylyl cyclase. Eur J Pharmacol. 2007;572:12–22. doi: 10.1016/j.ejphar.2007.06.036. [DOI] [PubMed] [Google Scholar]
- 41.Knudson AG, Jr, Wayne L, Hallett WY. On the selective advantage of cystic fibrosis heterozygotes. Am J Hum Genet. 1967;19:388–392. [PMC free article] [PubMed] [Google Scholar]
- 42.Lipworth BJ. Phosphodiesterase-4 inhibitors for asthma and chronic obstructive pulmonary disease. Lancet. 2005;365:167–175. doi: 10.1016/S0140-6736(05)17708-3. [DOI] [PubMed] [Google Scholar]
- 43.Zhang W, Fujii N, Naren AP. Recent advances and new perspectives in targeting CFTR for therapy of cystic fibrosis and enterotoxin-induced secretory diarrheas. Future Med Chem. 2012;4:329–345. doi: 10.4155/fmc.12.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dransfield MT, Wilhelm AM, Flanagan B, Tidwell SL, Raju SV, Gaggar A, Steele C, Tang LP, Liu B, Rowe SM. Acquired cystic fibrosis transmembrane conductance regulator dysfunction in the lower airways in COPD. Chest. 2013;144:498–506. doi: 10.1378/chest.13-0274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Eckford PD, Li C, Ramjeesingh M, Bear CE. Cystic fibrosis transmembrane conductance regulator (CFTR) potentiator vx-770 (ivacaftor) opens the defective channel gate of mutant CFTR in a phosphorylation-dependent but ATP-independent manner. J Biol Chem. 2012;287:36639–36649. doi: 10.1074/jbc.M112.393637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jih KY, Hwang TC. Vx-770 potentiates CFTR function by promoting decoupling between the gating cycle and ATP hydrolysis cycle. Proc Natl Acad Sci USA. 2013;110:4404–4409. doi: 10.1073/pnas.1215982110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Le Gars M, Descamps D, Roussel D, Saussereau E, Guillot L, Ruffin M, Tabary O, Hong SS, Boulanger P, Paulais M, et al. Neutrophil elastase degrades cystic fibrosis transmembrane conductance regulator via calpains and disables channel function in vitro and in vivo. Am J Respir Crit Care Med. 2013;187:170–179. doi: 10.1164/rccm.201205-0875OC. [DOI] [PubMed] [Google Scholar]
- 48.Bodas M, Min T, Mazur S, Vij N. Critical modifier role of membrane-cystic fibrosis transmembrane conductance regulator-dependent ceramide signaling in lung injury and emphysema. J Immunol. 2011;186:602–613. doi: 10.4049/jimmunol.1002850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bradbury NA, Jilling T, Berta G, Sorscher EJ, Bridges RJ, Kirk KL. Regulation of plasma membrane recycling by cftr. Science. 1992;256:530–532. doi: 10.1126/science.1373908. [DOI] [PubMed] [Google Scholar]
- 50.Mata M, Sarria B, Buenestado A, Cortijo J, Cerda M, Morcillo EJ. Phosphodiesterase 4 inhibition decreases muc5ac expression induced by epidermal growth factor in human airway epithelial cells. Thorax. 2005;60:144–152. doi: 10.1136/thx.2004.025692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Di Benedetto G, Manara-Shediac FS, Mehta A. Effect of cyclic amp on ciliary activity of human respiratory epithelium. Eur Respir J. 1991;4:789–795. [PubMed] [Google Scholar]
- 52.Kwak HJ, Song JS, Heo JY, Yang SD, Nam JY, Cheon HG. Roflumilast inhibits lipopolysaccharide-induced inflammatory mediators via suppression of nuclear factor-kappab, p38 mitogen-activated protein kinase, and c-jun nh2-terminal kinase activation. J Pharmacol Exp Ther. 2005;315:1188–1195. doi: 10.1124/jpet.105.092056. [DOI] [PubMed] [Google Scholar]
- 53.Hatzelmann A, Schudt C. Anti-inflammatory and immunomodulatory potential of the novel pde4 inhibitor roflumilast in vitro. J Pharmacol Exp Ther. 2001;297:267–279. [PubMed] [Google Scholar]