Abstract
The diagnosis of chronic obstructive pulmonary disease (COPD) confers a 2-fold increased lung cancer risk even after adjusting for cigarette smoking, suggesting that common pathways are operative in both diseases. Although the role of the tyrosine kinase c-Src is established in lung cancer, less is known about its impact in other lung diseases, such as COPD. This study examined whether c-Src activation by cigarette smoke contributes to the pathogenesis of COPD. Cigarette smoke increased c-Src activity in human small airway epithelial (SAE) cells from healthy donors and in the lungs of exposed mice. Similarly, higher c-Src activation was measured in SAE cells from patients with COPD compared with healthy control subjects. In SAE cells, c-Src silencing or chemical inhibition prevented epidermal growth factor (EGF) receptor signaling in response to cigarette smoke but not EGF stimulation. Further studies showed that cigarette smoke acted through protein kinase C α to trigger c-Src to phosphorylate EGF receptor and thereby to induce mitogen-activated protein kinase responses in these cells. To further investigate the role of c-Src, A/J mice were orally administered the specific Src inhibitor AZD-0530 while they were exposed to cigarette smoke for 2 months. AZD-0530 treatment blocked c-Src activation, decreased macrophage influx, and prevented airspace enlargement in the lungs of cigarette smoke–exposed mice. Moreover, inhibiting Src deterred the cigarette smoke–mediated induction of matrix metalloproteinase-9 and -12 in alveolar macrophages and lung expression of cathepsin K, IL-17, TNF-α, MCP-1, and KC, all key factors in the pathogenesis of COPD. These results indicate that activation of the proto-oncogene c-Src by cigarette smoke promotes processes linked to the development of COPD.
Keywords: kinase, phosphorylation, cell signaling, epithelial, inflammation
Clinical Relevance
The results from this study show that the proto-oncogene c-Src, which plays an important role in the development of lung cancer, is also a key factor in the pathogenesis of chronic obstructive pulmonary disease (COPD). Indeed, c-Src activation by cigarette smoke promoted airway inflammation and lung tissue destruction. Inhibiting c-Src with the chemical inhibitor AZD0530 prevented inflammatory and destructive changes in the lungs of smoke-exposed mice, thus identifying c-Src as a key disease-modifying target in COPD.
Chronic obstructive pulmonary disease (COPD) and lung cancer account for a significant proportion of overall mortality in the United States and worldwide (1). Cigarette smoke remains the main common risk factor between both diseases; however, the diagnosis of COPD, even in never-smokers, is associated with a greater than 2-fold increased risk of lung cancer compared with patients without COPD (2). This suggests that shared biological mechanisms play a role in the development of both diseases. Indeed, establishing smoke-inducible pathways that enhance the susceptibility to cancer and COPD represents a major area for needed study.
A cigarette smoke–regulated protein in tumor cells, c-Src tyrosine kinase, has increased activity in many human tumor types and correlates with disease stage and patient survival (3). Elevated c-Src activity promotes tumor invasiveness, characterized by the loss of cell–cell adhesion, increased cell-matrix adhesion, and formation of focal adhesions (4). Protein tyrosine kinases (PTKs), such as c-Src, are also key mediators of intracellular signal transduction processes related to acute inflammatory responses (5). c-Src is a 60-kD protein composed of several functional domains (SH4, SH3, SH2, and SH1 domains and a C-terminal regulatory segment) (6). A tyrosine at site 416 on the kinase domain (SH1) is a positive regulatory site responsible for maximizing its activity (7). Conversely, phosphorylation at site Tyr527 on the C-terminal end leads to binding of this region to the SH2 domain and negatively regulates kinase activity (8). Cigarette smoke is known to activate c-Src kinase through the phosphorylation of c-Src at Tyr416 in tumor cells and in tracheal smooth muscle cells (9). c-Src activates several signaling cascades in cancer that have been implicated in COPD, such as focal adhesion kinase (FAK) (10), signal transducers and activators of transcription 3 (STAT-3) (11), the epidermal growth factor receptor (EGFR) family (12), phosphatidylinositol 3-kinase (PI3K)-Akt, and mitogen-activated protein kinase (MAPK) (9).
Aberrant expression and activation of c-Src occurs in several tumor types and has been correlated with poor clinical outcome (13). Cigarette smoke has been reported to activate c-Src. and, once activated, c-Src is able to stimulate important proinflammatory mediators associated with the development of COPD. Thus, we postulated that c-Src activation occurs in COPD and plays a central role in the disease. Moreover, we theorized that inhibiting c-Src could protect against cigarette smoke–induced lung inflammation and tissue destruction. To address this hypothesis, we characterized c-Src expression and activity in airway epithelial cells isolated from healthy subjects and from patients with COPD. Human airway epithelial cells were studied to determine how cigarette smoke activated c-Src to modulate signaling processes in a cell type that plays a key role in the disease (14, 15). In addition, the effect of cigarette smoke on c-Src activation in human neutrophils and monocytes was examined. Finally, mice were exposed to cigarette smoke in combination with the c-Src inhibitor AZD-0530 to evaluate the effect of c-Src inhibition on protease expression in alveolar macrophages and to explore the potential use of c-Src inhibitors as a treatment for COPD.
Materials and Methods
Animal Models
C57BL/6J and A/J mice (Jackson Labs, Bar Harbor, ME) were used for these studies. A/J mice were administered 10 mg/kg AZD-0530 (LC Laboratories, Woburn, MA) in vehicle (0.5% hydroxypropylmethylcellulose, 0.1% polysorbate) or vehicle alone daily by gavage 2 hours before smoke exposure for 2 months (n = 5–10 in each group). The study was performed for 4 days to collect alveolar macrophages. All animals were 8 weeks old at the beginning of each experiment. All animal experiments were performed with approval from St. Luke’s Roosevelt’s Hospital Center’s Institutional Animal Care and Use Committee.
Cigarette Smoke Exposure Protocol
Mice were exposed to cigarette smoke in a specially designed chamber (Teague Enterprises, Davis, CA) for 4 hours a day, 5 days per week at a total particulate matter concentration of 80 mg/m3. Animals were killed 12 hours after the last smoke exposure. The lungs underwent pressure fixation and morphometric analysis in accordance with our previously published protocol (16) and in accordance with the ATS/ERS American Thoracic Society/European Respiratory Society issue statement on quantitative assessment of lung structure (17). Lung bronchoalveolar lavage fluid (BALF) isolation was performed on the mice.
Cell Culture
Monolayers of human small airway epithelial (SAE) cells (Lonza, Walkersville, MD) from healthy subjects and from patients with physician-diagnosed COPD as established by GOLD criteria were submerged cultured and treated with 5% cigarette smoke extract (CSE) or PBS. Cells were only used for experiments at passages three through six and at a confluency of approximately 70%. Lactate dehydrogenase (LDH) release assays were formed to confirm no significant changes in cell viability after treatments. SAE cells were transfected by administering siRNA for c-Src, K-Ras, c-Raf, EGFR, protein kinase C (PKC)α, or control small interfering RNA (siRNA) (Qiagen, Gaithersburg, MD). Cells were treated with several time points of 5% CSE or with 1 μM of AZD-0530/Saracatinib (LC Laboratories) for 1 hour before CSE exposure. c-Src siRNA–treated cells were exposed to 10 ng/ml human epidermal growth factor (EGF) (Cell Signaling, Danvers, MA).
Matrix Metalloproteinase and Cytokine Measurements
Matrix metalloproteinase (MMP)-3, MMP-9, MMP-12, IL-1α, IL-1β, IL-4, IL-6, IL-17, granulocyte colony-stimulating factor, KC (or IL-8 for human samples), and IFN-γ levels were measured in BALF and cell media using a beads assay on the Bio-Plex 200 system (BioRad, Hercules, CA). Quantitative PCR for mouse cathepsin K, MMP-9, MMP-12, MMP-13, TNF-α, MCP-1, and KC were measured from the lungs of the mice using TaqMan specific probes (Applied Biosystems, Carlsbad, CA).
Intracellular Signaling
Immunoblots were conducted on the cytosolic protein from whole mouse lungs or cells to determine levels of p-JNK, JNK, p-ERK, ERK, p-p38, p38, c-Src (Santa Cruz Biotechnologies, Santa Cruz, CA), p-Src(Tyr416), p-Src(Tyr527), p-Raf(ser388), c-Raf, p-PKC-α(Thr638), PKC-α, EGFR, p-EGFR(Tyr992), p-EGFR(Tyr1042), p-EGFR(Tyr1068), KRAS (Abcam), and actin (unless directly stated all antibodies from Cell Signaling). Densitometry analysis was conducted on immunoblots standardizing to total protein or to β-actin. Immunofluorescence was performed on lung tissue from animals exposed to room air or cigarette smoke for p-Src(Tyr416) and p-Raf(ser388). Lung protein extracts were assayed for myeloperoxidase activity using a kit from Cayman Chemical Company (Ann Arbor, MI) as recommended by the manufactures.
Src Tyrosine Kinase Activity
Src tyrosine kinase activity was determined by immunoprecipitating c-Src from the tissue or cell culture samples and then measuring tyrosine kinase activity with a specific kit (MK410; Takara Bio, Mountain View, CA). Results are presented as relative activity compared with control subjects (%).
Isolation of Peripheral Monocytes and Neutrophils
Mononuclear cells were isolated from venous peripheral blood obtained from healthy volunteers. Density gradient centrifugation was conducted in LymphoPrep (Axis-Shield PoC AS, Oslo, Norway) to separate the red cell pellet containing the neutrophil population from the monolayer. Neutrophils were further purified as described previously (18). The mononuclear cell band was aspirated and washed three times with PBS. Monocytes were enriched from the mononuclear fraction by selectively attaching them to 24-well plates for 3 hours at 37°C. Cells were washed three more times with PBS. Monocytes and neutrophils were then treated with AZD-0530 for 1 hour before CSE stimuli.
Statistical Analyses
Data are expressed as means ± SEM. We determined statistical significance by one-way ANOVA for multiple group analysis using GraphPad Prism Software. Student t tests (two tailed) were used throughout the study. All data sets are represented as mean ± SE.
Results
Cigarette Smoke Exposure Induces c-Src and c-Raf Phosphorylation in Mouse Lungs
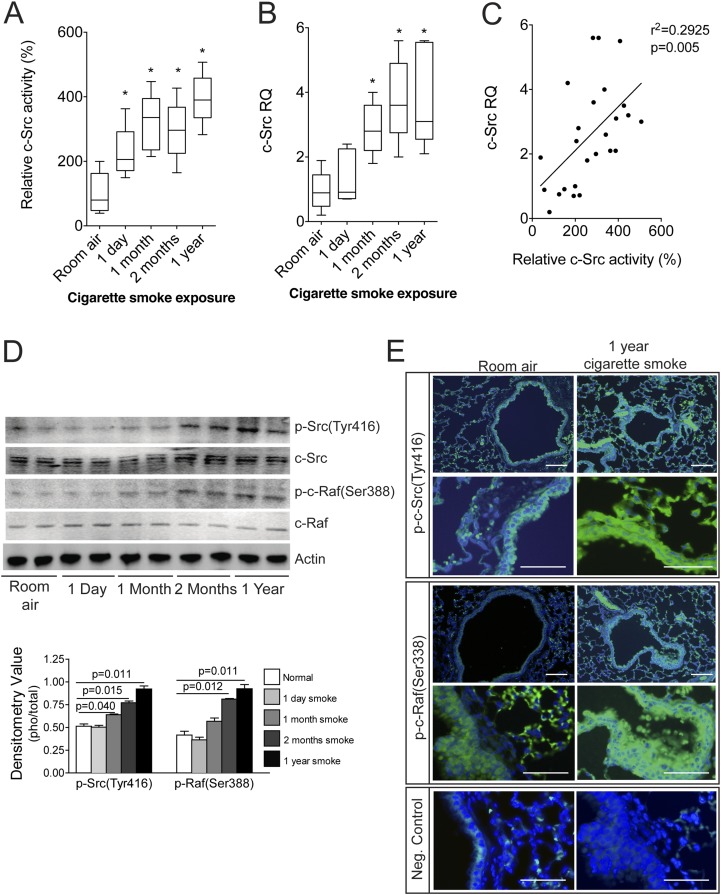
To determine the dynamics of c-Src activation in response to cigarette smoke, c-Src activity was measured in the lungs of C57BL/6J mice subjected to varying periods of smoke exposure. Increased activation of c-Src was observed at every exposure time point, from 1 day to 1 year (Figure 1A). These time points were chosen to evaluate the effect of acute, subacute, and chronic exposure. An increase in c-Src gene expression was observed after 1 month of cigarette smoke exposure that persisted up to 1 year of exposure (Figure 1B). There was a correlation between c-Src activity and gene expression in the mouse samples (Figure 1C). Phosphorylation of c-Src (Tyr416) and c-Raf (Ser388) was also observed, most notably with more than 2 months of smoke exposure (Figure 1D). Immunofluorescence analysis of p-Src (Tyr416) and p-Raf (Ser388) in 1-year room air and cigarette smoke–exposed lungs demonstrated activation of Src and Raf in multiple airway cell types, including the lung airway epithelium (Figure 1E). c-Src appears to be the only Src family member to undergo tyrosine phosphorylation after exposure to CSE (see Figure E1 in the online supplement). Thus, cigarette smoke causes a significant and persistent activation of c-Src in the lung. The 1-day activation of c-Src in the lungs of cigarette smoke–exposed mice was not associated with changes in c-Src phosphorylation. It is conceivable that this acute activation of c-Src may involve other posttranslational modifications that were not assessed with the antibodies we used.
Figure 1.
Smoke exposure induces c-Src and c-Raf phosphorylation in mouse lungs. c-Src activity (A) and gene expression (B) were measured in the lungs of wild-type nonexposed mice and various times of smoke-exposed mice (n = 5 for each group). (C) c-Src activity correlated with gene expression. (D) Immunoblots and densitometry analysis were performed for p-Src(Tyr416), total c-Src, p-Raf(ser388), total c-Raf, and actin on protein from the same samples in A. (E) Immunofluorescence analysis was performed for p-Src(Tyr416) and p-Raf(ser388) on tissue from room air–exposed and 1-year cigarette smoke–exposed mice (bar = 50 μM for the upper panels, and bar = 20 μM for the lower panels under higher magnification). Isotype control rabbit IgG were used as negative controls in each assay (bottom panel).
Silencing c-Src, c-Raf, or KRAS Prevents Cigarette Smoke–Induced MAPK Activation
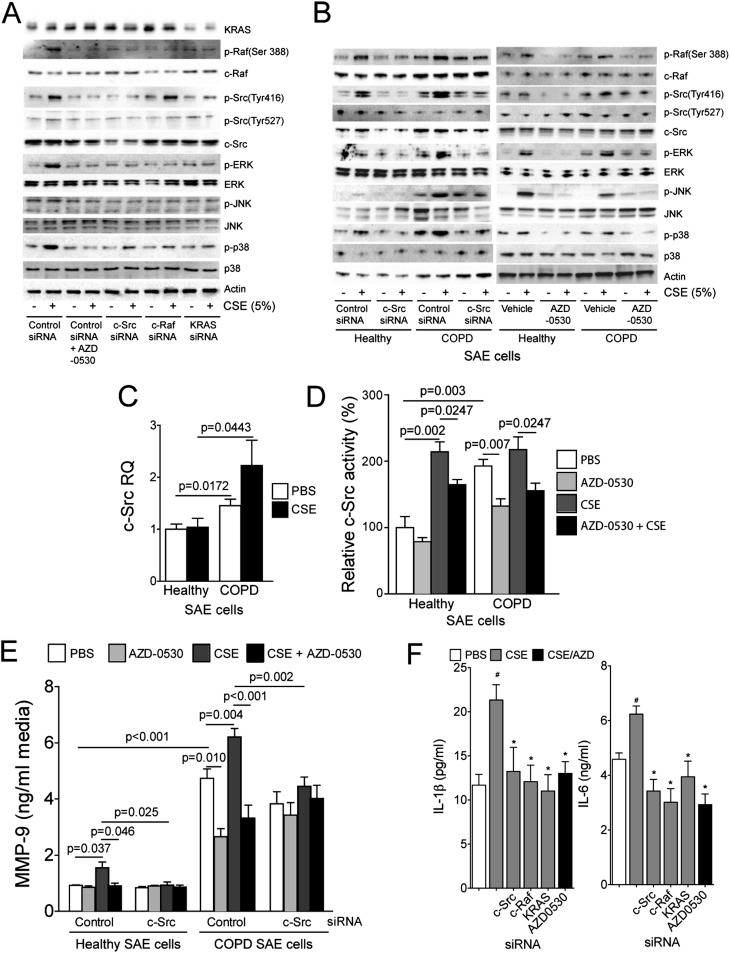
Studies were conducted in primary human SAE cells to identify the key pathways triggered by the cigarette smoke–mediated activation of c-Src. These airway epithelial cells were chosen as an in vitro model of COPD to investigate the potential importance of c-Src because they are critical in the pathogenesis of COPD (15) and highly express c-Src (Figure 1D). Gene silencing for c-Src, c-Raf, and KRAS was used in control and CSE-treated SAE cells to evaluate the subsequent effect on MAPK activation and the interactions between c-Src, c-Raf, and KRAS. The Src inhibitor AZD-0530 was used to examine the effects of Src inhibition on cigarette smoke–mediated inflammatory responses. Subtoxic levels of AZD-0530 and CSE were used throughout this study (Figure E2). Smoke exposure led to phosphorylation of c-Src and c-Raf (Figures 2A and E3). Silencing of c-Src, c-Raf, or KRAS (Figure E4) prevented the cigarette smoke–induced phosphorylation of ERK and p38 and decreased overall levels of JNK phosphorylation (Figures 2A and E3). In fact, the phosphorylation of p38 was extremely sensitive to the blockade of this pathway, which is of interest because we previously demonstrated that silencing p38 in SAE cells prevents CSE-induced MMP-9 expression (19). The Src inhibitor AZD-0530 resulted in a similar antiinflammatory profile to silencing c-Src, Raf, or KRAS in SAE cells (Figure 2A and Figure E5).
Figure 2.
Chemical and gene-specific inhibition of c-Src prevents smoke-induced mitogen-activated protein kinase (MAPK) activation in a KRAS/c-Raf1–dependent manner. (A) Small airway epithelial (SAE) cells were examined for c-Src, c-Raf, and MAPK phosphorylation after transfection with negative control, c-Src, c-Raf, or KRAS small interfering RNA (siRNA) and treatment with cigarette smoke extract (CSE) (90 min) and AZD-0530. (B) Immunoblots were performed to determine phosphorylation and total protein levels of c-Src, c-Raf, and the MAPK proteins in SAE cells from healthy subjects and from patients with chronic obstructive pulmonary disease (COPD) who underwent Src inhibition by siRNA or AZD-0530. (C–E) c-Src gene expression compared with GAPDH (C), c-Src activity (D), and matrix metalloproteinase (MMP)-9 levels (E) in media were examined. Graphs are represented as mean ± SEM of 12 measurements. P values are shown comparing both treatments connected by a line. (F) IL-1β and IL-6 levels in media were examined in cells after transfection with negative control, c-Src, c-Raf, or KRAS siRNA and treatment with CSE (90 min) and AZD-0530. #Significant change compared with PBS-treated cells. *Significant change compared with control siRNA cells treated with CSE.
AZD0530 Inhibits c-Src Activation in SAE Cells Isolated from Subjects with COPD
To investigate the potential use of Src inhibitors, c-Src activity levels and downstream phosphorylation profiles were examined in SAE cells isolated from patients with COPD and healthy subjects. Immunoblot analysis confirmed that AZD-0530 administration prevented phosphorylation of c-Src at Tyr416 and led to the impediment of c-Raf activation in cells from patients with COPD and healthy subjects in a similar fashion to c-Src silencing (Figure 2B). Chemical inhibition of c-Src activity blocked the cigarette smoke–mediated activation of JNK, ERK, and p38 in SAE cells from individuals with COPD. At baseline, SAE COPD cells had significantly higher levels of c-Src gene expression (Figure 2C) and activity compared with healthy SAE cells (Figure 2D), reflecting the results in the animal model. The increased c-Src activity in these COPD cells was decreased by treatment with the specific Src inhibitor AZD-0530 (Figure 2D). Both c-Src silencing and AZD-0530 treatment prevented the induction of MMP-9 in response to CSE treatment in these cells (Figure 2E). c-Src did not alter CSE-induced MMP-1 release (Figure E6). Other researchers have observed that MMP-9 regulation could be c-Src dependent (20); however, our data establish that Src is a critical regulator of MMP-9 in smoke conditions and highlight the potential of c-Src inhibitors to counteract the induction of this key mediator in cancer and COPD (21, 22). AZD-0530 or silencing of c-Src/c-Raf/KRAS also prevented CSE-induced IL-1β and IL-6 secretions from SAE cells (Figure 2F). Together, these results indicate that AZD-0530 has the potential to block key cigarette smoke–driven inflammatory processes associated with cancer and COPD.
The Interaction between EGFR and c-Src Is a Key Determinant of MAPK Activation in CSE-Treated SAE Cells
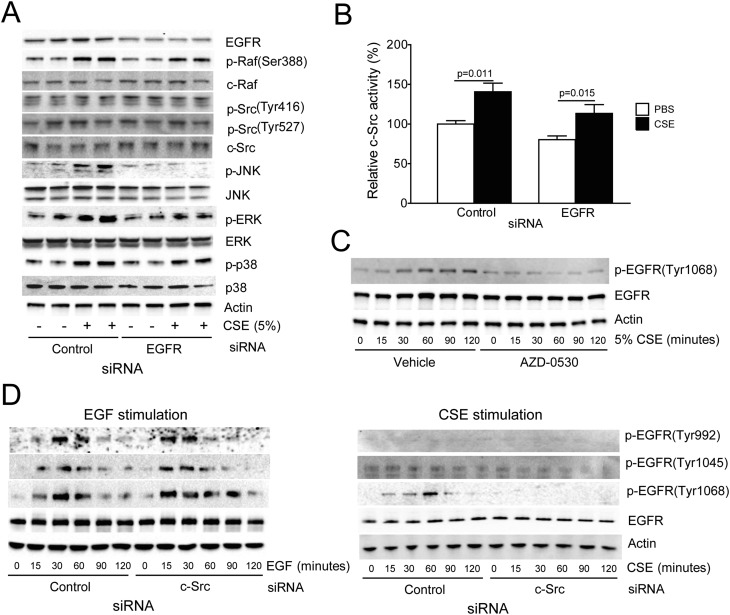
c-Src activation has been associated with the stimulation of PTK receptors (23, 24). To identify which PTK receptor activated c-Src or is dependent on c-Src activation in the CSE-treated SAE cells, a PTK activation array was conducted. This approach identified EGFR as the PTK that was most robustly activated by CSE in these SAE cells (Figure E7). This is significant because EGFR plays a major role in lung cancer (25), is smoke triggered (26), and can lead to c-Src activation (27). Therefore, EGFR was silenced in SAE cells, and the c-Src pathway was examined for alterations in sensitivity to CSE. Loss of EGFR partially blocked phosphorylation of p-Raf(ser388), p-ERK, p-JNK, and p-p38 (Figure 3A). However, knocking down EGFR expression did not reduce c-Src activation in response to CSE treatment (Figure 3B). This indicates that CSE acts via other factors to stimulate c-Src in these cells. Furthermore, these results show that c-Src was not merely a passive downstream target of EGFR; rather, c-Src played an important role in EGFR activation. Indeed, c-Src expression and activation were required for the CSE-mediated phosphorylation of EGFR specifically at Tyr1068 (Figures 3C and 3D). Phosphorylation at this site provides a docking site for the E3 ubiquitin ligase Cbl, which targets EGFR for endocytosis and degradation (28). Thus, this negative feedback mechanism may limit the induction of EGFR/Src signaling in the cigarette smoke–exposed lung. In contrast to cigarette smoke, EGF treatment acts independently of c-Src to induce EGFR phosphorylation at Tyr sites 992, 1,045, and 1,068 (Figure 3D). Furthermore, c-Src silencing did not block the induction of MAPK signaling in EGF-treated SAE cells (Figure E8), whereas it deterred such signaling in response to CSE treatment (Figures 2A and 2B). This shows that the influence of c-Src on EGFR signaling varies depending on the stimuli. This may be due to the distinct phosphorylation events involved in EGFR activation by EGF or CSE (29).
Figure 3.
Cigarette smoke–activated epidermal growth factor receptor (EGFR) is regulated by c-Src to induce MAPK activation. (A) Immunoblots for c-Src, c-Raf ,and MAPK phosphorylation were conducted on cell lysate protein from SAE cells transfected with negative control and EGFR siRNA and then treated with CSE (90 min). (B) c-Src activity assays were conducted on control or 24-hour CSE-treated cells that have be transfected with control and EGFR siRNA (n = 7). (C) SAE cells were treated with CSE over multiple time points with and without AZD-0530 treatment, and phosphorylation of EGFR at Tyr1068 was examined by immunoblots. (D) EGFR tyrosine phosphorylation was examined after transfection with negative control and c-Src siRNA and treatment with 10 ng/ml epidermal growth factor or 5% CSE (0, 15, 30, 60, 90, or 120 min).
Silencing of PKC-α Subdues the Cigarette Smoke–Driven c-Src Pathway
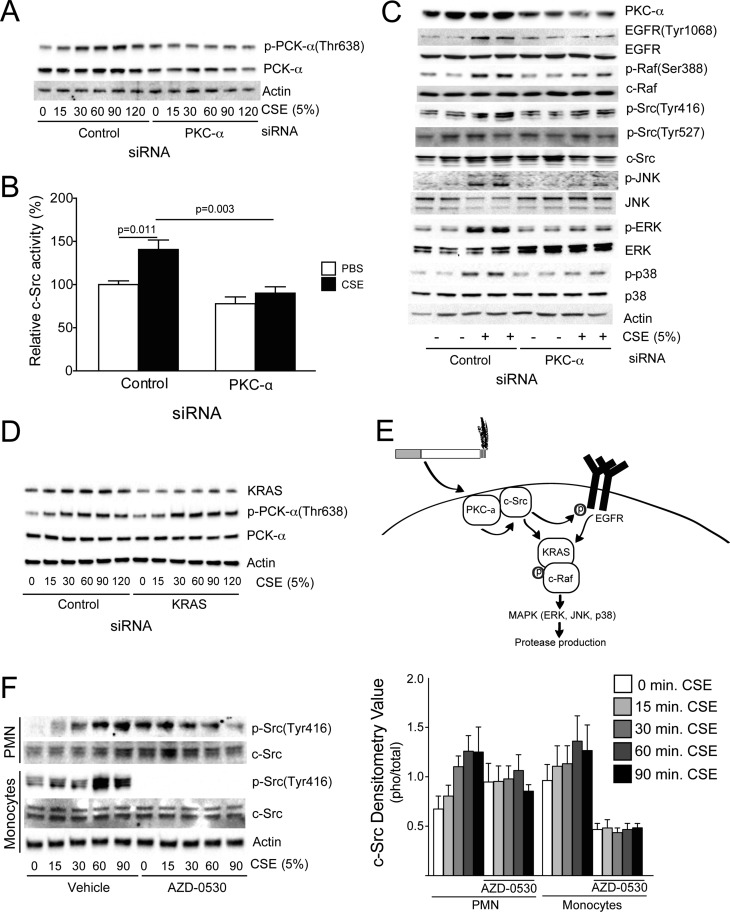
Aside from EGFR, other factors may regulate c-Src activation in cigarette smoke–exposed SAE cells. In fact, studies indicate that CSE can stimulate the phosphorylation of c-Src, EGFR, PDGFR, and Akt in a PKC-α–dependent manner (9). For this reason, we examined how PKC-α silencing affected c-Src–mediated inflammation in SAE cells. PKC-α was phosphorylated at threonine site 638 in the presence of CSE (Figure 4A). Silencing of PKC-α (Fig. S4) prevented cigarette smoke–induced c-Src activation and phosphorylation (Figures 4B and 4C). Silencing PKC-α resulted in the same protein phosphorylation profile as c-Src inhibition and silencing (Figure 4C), with CSE unable to activate c-Src/c-Raf/MAPK when PKC-α was silenced (Figure 4C and Figure E9). Silencing of KRAS appears to somewhat alter CSE-induced Src phosphorylation, but KRAS does not influence CSE-induced PKC-α phosphorylation (Figure 4D). Together, these results demonstrate that cigarette smoke works via PKC-α to stimulate c-Src and thereby activate the EGFR/KRAS/c-RAF/MAPK pathway in human airway epithelial cells (Figure 4E).
Figure 4.
Silencing of PKC-α subdues the cigarette smoke–driven c-Src pathway. (A) PKC-α phosphorylation was examined by immunoblots after SAE cell transfection with negative control and PKC-α siRNA and treatment with 5% CSE (0, 15, 30, 60, 90, or 120 min). (B) c-Src activation was examined after 90 minutes of CSE exposure in control or PKC-α siRNA–treated SAE cells. (C) Immunoblots for c-Src, c-Raf, and MAPK phosphorylation were conducted on cell lysate protein from SAE cells transfected with negative control and PKC-α siRNA and then treated with CSE (90 min or 24 h). (D) PKC-α phosphorylation was examined after silencing of KRAS. (E) Proposed pathway for the regulation of c-Src–mediated inflammation pathways in the lung. (F) Peripheral blood monocytes and neutrophils were examined for p-Src(Tyr416), c-Src, c-Raf, and actin after treatment with CSE (0–90 min) and AZD-0530.
AZD-0530 Prevents CSE-Induced c-Src Phosphorylation in Monocytes
In addition to epithelial cells, the effects of cigarette smoke and AZD0530 on c-Src phosphorylation in other cell types was evaluated. Monocytes and neutrophils were isolated from whole blood and exposed to AZD-0530 and CSE (Figure 4F). Monocyte expression of p-Src(Tyr416) was extremely sensitive to AZD-0530 treatment. Monocyte and neutrophil p-Src(Tyr416) levels increased with CSE, but neutrophils were less sensitive to AZD-0530 treatment. Therefore, c-Src inhibition can be inhibited in multiple cell types that contribute to disease progression.
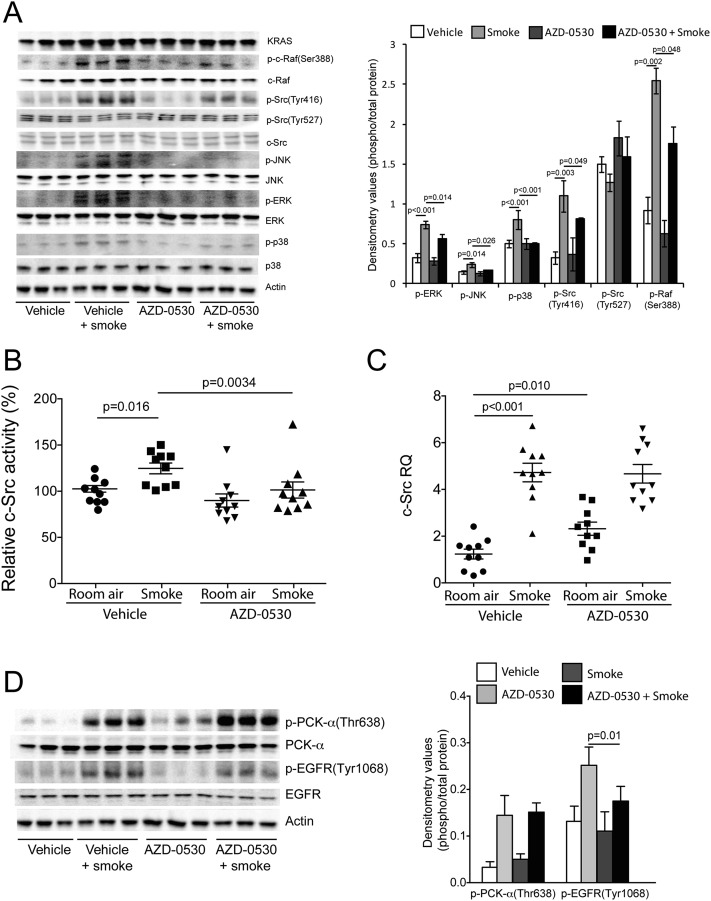
AZD-0530 Treatment Prevents c-Src, MAPK, and EGFR Phosphorylation in Cigarette Smoke–Exposed Mice
An in vivo approach was undertaken to examine the effects of AZD-0530 on cigarette smoke–induced inflammation and tissue damage in mice. A/J mice were orally administered AZD-0530 daily before undergoing cigarette smoke exposure. Cigarette smoke induced phosphorylation of p-Src(Tyr416), p-Raf(ser388), p-ERK, p-JNK, and p-p38 in the lungs of cigarette smoke–exposed mice (Figure 5A). However, this activation was effectively blocked by AZD-0530 administration. AZD-0530 treatment decreased c-Src tyrosine kinase activity (Figure 5B) without affecting c-Src gene expression (Figure 5C). AZD-0530 administration had no effect on cigarette smoke–induced PKC-α phosphorylation but did prevent EGFR phosphorylation (Figure 5D), demonstrating the importance of c-Src for cigarette smoke–induced EGFR activation.
Figure 5.
AZD-0530 treatment prevents cigarette smoke–induced MAPK activation, c-Src, activity and EGFR phosphorylation. A/J mice were exposed to 2 months of cigarette smoke or room air in combination with daily oral administration of vehicle or AZD-0530. (A) Immunoblots for c-Src, c-Raf, and MAPK phosphorylation were conducted on the lung tissue lysate protein from these mice, and densitometry analysis was performed. c-Src activity (B) and gene expression (C) were measured in the lungs tissue of the mice. (D) Immunoblot analysis for p-PKC-α(Thr638), PKC-α, p-EGFR(Thr1068), EGFR, and Actin were performed on lung tissue from the room air and 1-year cigarette smoke–exposed mouse groups. Graphs are represented as mean ± SEM of 10 samples. P values are shown comparing both treatments connected by a line.
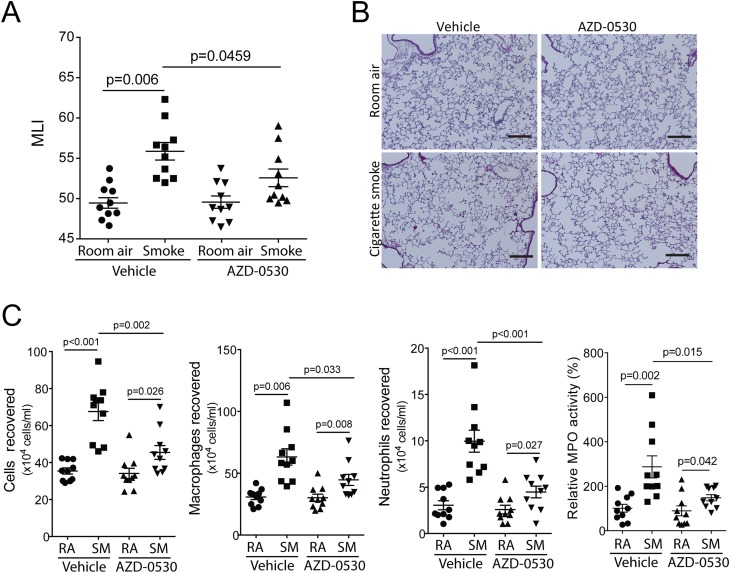
AZD-0530 Treatment Prevents Cigarette Smoke–Induced Neutrophil and Macrophage Infiltration and Airspace Enlargement in Mice
Two months of cigarette smoke exposure significantly increased airspace enlargement in cigarette smoke–exposed A/J mice compared with room air–exposed mice (Figures 6A and 6B), which is comparable to other studies using A/J mice (30, 31). AZD-0530 administration blocked the increase in airspace enlargement (Figures 6A and 6B) that occurred to mice in response to cigarette smoke. AZD-0530 administration prevented immune cell infiltration in cigarette smoke–exposed mice, with reduced numbers of macrophages and neutrophils observed in the BALF (Figure 6C). To further quantify the effect of AZD-0530 on lung neutrophils, myeloperoxidase activity levels in the lung were measured. The activity of the lung tissue homogenate doubled in response to cigarette smoke exposure (Figure 6C), which was consistent with the increase in neutrophil influx. AZD-0530–treated mice had significantly lower levels of tissue myeloperoxidase activity after cigarette smoke exposure (Figure 6C).
Figure 6.
AZD-0530 treatment prevents cigarette smoke–induced immune cell infiltration and airway enlargements in mice. (A) Mean linear intercepts were measured in the lungs of the mice exposed to 2 months of cigarette smoke or room air (RA) in combination with daily oral administration of vehicle or AZD-0530. (B) Comparative histology images of the four mouse groups (bar, 100 μM). (C) Bronchoalveolar lavage fluid immune cellularity was measured, and changes were observed after AZD-0530 in smoking conditions on total immune cell number, macrophages, and neutrophils. Neutrophil numbers were reconfirmed with myeloperoxidase assays. Graphs are represented as mean ± SEM of 10 samples. P values are shown comparing both treatments connected by a line. SM, smoke-exposed.
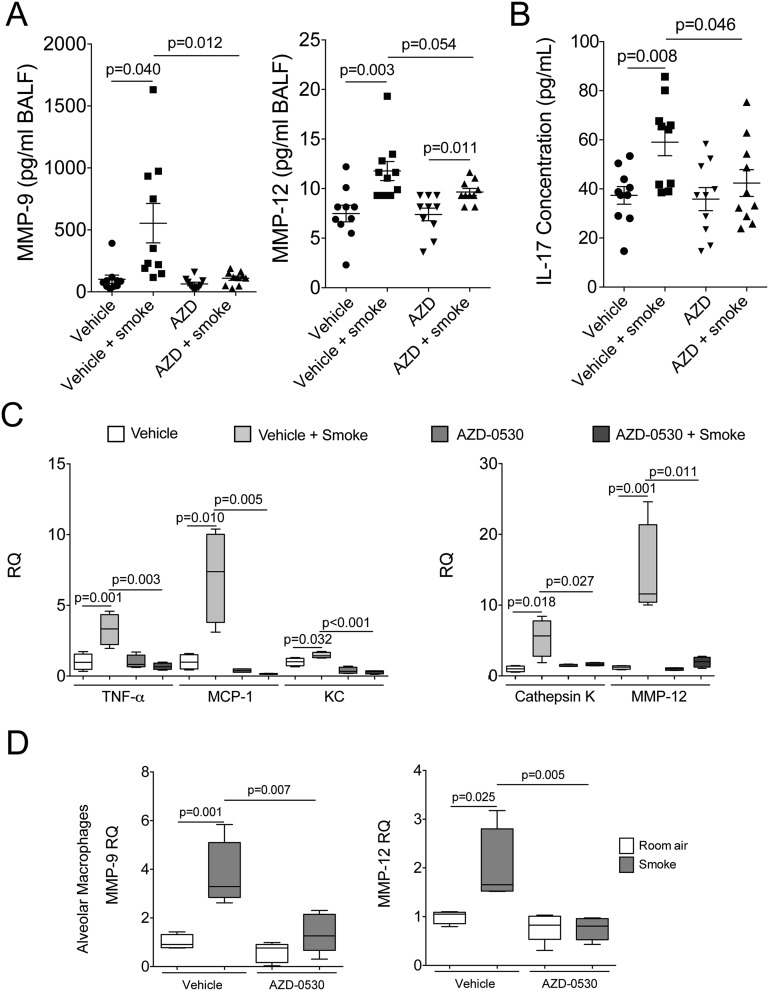
AZD-0530 Treatment Prevents Cigarette Smoke–Induced Protease and Cytokine Expression in Mice
AZD-0530 treatment prevented smoke-mediated induction of MMP-9 and IL-17 in BALF of the mice (Figures 7A and 7B). MMP-9 gene expression levels were also reduced after AZD-0530 treatment in the alveolar macrophages of mice (Figure 7D). There was a trend toward a reduction in MMP-12 levels in the BALF after AZD-0530 treatment (Figure 7A), which was significant when examining gene expression in tissue (Figure 7C) and alveolar macrophages (Figure 7D). In contrast, AZD-0530 had no effect of IL-1α, IL1β, IL-4, IL-6, GM-CSF, and IFN-γ BALF cytokine levels in the A/J mice (Figure E10). IL-17 promotes a M2 macrophage-driven microenvironment in cancer (32) and is required for cigarette smoke–induced COPD in mice (33). Using qPCR, it was demonstrated that AZD-0530 treatment also prevented the gene expression of TNF-α, MCP-1, KC, and cathepsin K (Figure 7C).
Figure 7.
AZD-0530 treatment prevents cigarette smoke–induced MMP-9, MMP-12, and IL-17 in mice. A/J mice were exposed to 2 months of cigarette smoke or room air in combination with daily oral administration of vehicle or AZD-0530. Bronchoalveolar lavage fluid from mice was examined for MMP-9 and MMP-12 levels (A) and IL-17 BAL levels (B). (C) Quantitative PCR for mouse TNF-α, MCP-1, KC, cathepsin K, and MMP-12 was conducted using cDNA prepared from mRNA isolated from the lungs of each group. (D) Alveolar macrophages were isolated from animals exposed to cigarette smoke for 4 days and treated with AZD-0530. MMP-9 and MMP-12 levels were determined by quantitative PCR. Graphs are represented as mean ± SEM of 10 samples. P values are shown comparing both treatments connected by a line. RQ, relative quantity.
Discussion
Patients with COPD are at increased risk for the development of primary lung cancer and have worse outcomes after lung cancer diagnosis and treatment (34). Indeed, as an independent risk factor, airway obstruction is a better predictor for lung cancer than age and pack-years exposure (35), and smokers with COPD have a 4- to 6-fold increased risk of lung cancer over time compared with smokers with normal lung function (36). Given this strong association between lung cancer and COPD, researchers have sought to identify common signaling processes that would provide mechanistic links between these diseases.
It has been argued that an exaggerated inflammatory repair response to smoking can promote cancer and COPD development by inducing key responses, such as MMP expression, oxidative stress, and epithelial–mesenchymal transition (37). The proto-oncogene c-Src is activated during tissue injury and repair (38); however, the effects of chronic c-Src activation, particularly in COPD, are poorly understood. This study is the first to demonstrate that c-Src is activated in COPD and that inhibiting it prevents the development of alveolar tissue destruction in cigarette smoke–exposed mice. Indeed, antagonizing Src in these mice countered Raf, ERK, JNK, and p38 activation; blocked the induction of MMP-9, MMP-12, and IL-17; and prevented the influx of macrophages into the airways. In SAE cells, silencing c-Src and treatment with the c-Src inhibitor AZD-0530 potently inhibited the CSE-mediated induction of MAPK signaling. The activation of c-Src was PKC-α dependent, and active c-Src played a major role in EGFR phosphorylation and downstream signaling in response to cigarette smoke. These findings show that cigarette smoke acts through PKC-α to stimulate c-Src and thereby induce MAPK responses that promote the development of cancer and COPD (38).
A key mechanism by which c-Src influenced these processes is by modulating the tyrosine kinase receptor EGFR. Although several tyrosine kinase receptors were regulated by cigarette smoke, EGFR demonstrated by far the most intense response in our analyses. Furthermore, EGFR expression was required for the induction of MAPK signaling in CSE-treated SAE cells, indicating that EGFR is a critical target in these cigarette smoke–induced lung diseases. Inhibiting c-Src in SAE cells by siRNA or AZD-0530 blocked EGFR signaling, countered MAPK activation, and prevented the cigarette smoke–mediated induction of MMP-9. This was likely due to the fact that antagonizing c-Src prevented p38 activation, which is required for the cigarette smoke–induced expression of MMP-9 in the lung (19). The effects of c-Src inhibition were not restricted to epithelial cells. Indeed, we found that cigarette smoke also activated monocytes and neutrophils, but the activation in neutrophils was not sensitive to inhibition with AZD0530. In contrast, AZD0530 blocked Src activation in monocytes and prevented the induction of MMP-9 and MMP-12 in alveolar macrophages from cigarette smoke–exposed mice. This is significant because the role of MMP-12 in the development of emphysema has been clearly established (39). Although the loss of MMP-9 does not prevent cigarette smoke–induced emphysema in mice (40), enhanced expression of this protease in macrophages causes progressive air space enlargement in mice and is associated with COPD severity in humans (41, 42). These results suggest that aberrant EGFR and c-Src activation could promote the development of this disease by inducing the expression of MMP-9 and MMP-12 in the lung.
This study found that the cytokine IL-17 was also highly sensitive to c-Src inhibition in cigarette smoke–exposed mice. IL-17 expression is induced in Th17 cells, γδ T cells, and NK cells in response to cytokine signals from neighboring macrophages and from epithelial and dendritic cells (43). Once released, IL-17 triggers monocyte and neutrophil chemotaxis to the site of inflammation (44) and promotes tumor growth (45). Given these effects, IL-17 has been implicated in lung cancer and COPD. Indeed, metastasis of non–small cell lung cancer was significantly reduced in IL-17−/− mice (46), and IL-17 expression levels were predictive of disease progression in human non–small cell lung cancer (47). The results from this study suggest that AZD-0530, which inhibits tumor growth and invasion (48), may mediate its protective effects in the lung by counteracting the cigarette smoke–mediated induction of IL-17. In addition to cancer, IL-17 plays an important role in the development of COPD. In fact, IL-17−/− mice are resistant to cigarette smoke–induced COPD (49), and IL-17 expression is significantly increased in the bronchial biopsies of patients with COPD (50). Thus, IL-17 expression, which we found to be sensitive to Src inhibition, could be a common mechanism involved in the onset and progression of COPD and lung cancer. Future studies are needed to explore the precise mechanisms by which Src inhibition prevented IL-17 induction in the cigarette smoke–exposed lung.
EGFR and c-Src demonstrated an important interrelationship in response to cigarette smoke. Although c-Src is typically viewed as a downstream target of EGFR, c-Src was required for the tyrosine phosphorylation and hence activation of EGFR in CSE-treated SAE cells. This study and others have shown that cigarette smoke phosphorylates c-Src via a PKC-α–dependent mechanism (9, 29). Once activated by cigarette smoke, c-Src phosphorylated EGFR and KRAS/Raf, and these signaling proteins further activated c-Src. This positive feedback loop observed in our in vitro studies may account for the robust c-Src activation that was noted in the lungs of cigarette smoke–exposed mice. A unique finding of this study was that the effects of c-Src on EGFR/KRAS/c-RAF/MAPK signaling varied depending on the stimulus. Although the loss of c-Src expression effectively blocked MAPK activation in CSE-treated cells, it had little effect on these responses in cells treated with EGF. Thus, this study shows that c-Src plays a much more prominent role on EGFR signaling under smoke exposure conditions.
In conclusion, this study determined that c-Src activation is a key mediator of cigarette smoke–induced inflammation and tissue destruction. Furthermore, it provides data indicating that c-Src may mediate these effects by inducing the expression of IL-17, MMP-9, and MMP-12 in the lung. These findings provide a plausible mechanistic link between the pathogenesis of COPD and lung cancer and set the stage for future studies to address whether blocking c-Src activity, or its downstream effectors, can prevent the detrimental responses that lead to the development of these diseases.
Acknowledgments
Acknowledgments
The authors thank Dr. Edward Eden and Dr. Gerard Turino.
Footnotes
This work was supported by National Institutes of Health grant 5R01HL098528–04, by grant YCSA 113,380 from the Flight Attendant Medical Research Institute (P.G.), and by grants YCSA 24,039 and CIA 074,047 from the Flight Attendant Medical Research Institute (R.F.F.).
This article has an online supplement, which is accessible from this issue’s table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1165/rcmb.2013-0258OC on October 10, 2013
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Miniño AM, Murphy SL, Xu J, Kochanek KD. Deaths: final data for 2008. Natl Vital Stat Rep. 2011;59:1–126. [PubMed] [Google Scholar]
- 2.Powell HA, Iyen-Omofoman B, Baldwin DR, Hubbard RB, Tata LJ. Chronic obstructive pulmonary disease and risk of lung cancer: the importance of smoking and timing of diagnosis. J Thorac Oncol. 2013;8:6–11. doi: 10.1097/JTO.0b013e318274a7dc. [DOI] [PubMed] [Google Scholar]
- 3.Yeatman TJ. A renaissance for SRC. Nat Rev Cancer. 2004;4:470–480. doi: 10.1038/nrc1366. [DOI] [PubMed] [Google Scholar]
- 4.Frame MC. Src in cancer: deregulation and consequences for cell behaviour. Biochim Biophys Acta. 2002;1602:114–130. doi: 10.1016/s0304-419x(02)00040-9. [DOI] [PubMed] [Google Scholar]
- 5.Okutani D, Lodyga M, Han B, Liu M. Src protein tyrosine kinase family and acute inflammatory responses. Am J Physiol Lung Cell Mol Physiol. 2006;291:L129–L141. doi: 10.1152/ajplung.00261.2005. [DOI] [PubMed] [Google Scholar]
- 6.Sen B, Johnson FM. Regulation of src family kinases in human cancers. J Signal Transduct. 2011;2011:865819. doi: 10.1155/2011/865819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brown MT, Cooper JA. Regulation, substrates and functions of src. Biochim Biophys Acta. 1996;1287:121–149. doi: 10.1016/0304-419x(96)00003-0. [DOI] [PubMed] [Google Scholar]
- 8.Gonfloni S, Weijland A, Kretzschmar J, Superti-Furga G. Crosstalk between the catalytic and regulatory domains allows bidirectional regulation of Src. Nat Struct Biol. 2000;7:281–286. doi: 10.1038/74041. [DOI] [PubMed] [Google Scholar]
- 9.Yang CM, Lee IT, Lin CC, Yang YL, Luo SF, Kou YR, Hsiao LD. Cigarette smoke extract induces COX-2 expression via a PKCalpha/c-Src/EGFR, PDGFR/PI3K/Akt/NF-kappaB pathway and p300 in tracheal smooth muscle cells. Am J Physiol Lung Cell Mol Physiol. 2009;297:L892–L902. doi: 10.1152/ajplung.00151.2009. [DOI] [PubMed] [Google Scholar]
- 10.Avizienyte E, Frame MC. Src and FAK signalling controls adhesion fate and the epithelial-to-mesenchymal transition. Curr Opin Cell Biol. 2005;17:542–547. doi: 10.1016/j.ceb.2005.08.007. [DOI] [PubMed] [Google Scholar]
- 11.Yu CL, Meyer DJ, Campbell GS, Larner AC, Carter-Su C, Schwartz J, Jove R. Enhanced DNA-binding activity of a Stat3-related protein in cells transformed by the Src oncoprotein. Science. 1995;269:81–83. doi: 10.1126/science.7541555. [DOI] [PubMed] [Google Scholar]
- 12.Cantley LC. The phosphoinositide 3-kinase pathway. Science. 2002;296:1655–1657. doi: 10.1126/science.296.5573.1655. [DOI] [PubMed] [Google Scholar]
- 13.Aligayer H, Boyd DD, Heiss MM, Abdalla EK, Curley SA, Gallick GE. Activation of Src kinase in primary colorectal carcinoma: an indicator of poor clinical prognosis. Cancer. 2002;94:344–351. doi: 10.1002/cncr.10221. [DOI] [PubMed] [Google Scholar]
- 14.Hogg JC. Pathophysiology of airflow limitation in chronic obstructive pulmonary disease. Lancet. 2004;364:709–721. doi: 10.1016/S0140-6736(04)16900-6. [DOI] [PubMed] [Google Scholar]
- 15.Hogg JC, Chu F, Utokaparch S, Woods R, Elliott WM, Buzatu L, Cherniack RM, Rogers RM, Sciurba FC, Coxson HO, et al. The nature of small-airway obstruction in chronic obstructive pulmonary disease. N Engl J Med. 2004;350:2645–2653. doi: 10.1056/NEJMoa032158. [DOI] [PubMed] [Google Scholar]
- 16.Foronjy RF, Mirochnitchenko O, Propokenko O, Lemaitre V, Jia Y, Inouye M, Okada Y, D’Armiento JM. Superoxide dismutase expression attenuates cigarette smoke- or elastase-generated emphysema in mice. Am J Respir Crit Care Med. 2006;173:623–631. doi: 10.1164/rccm.200506-850OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hsia CCW, Hyde DM, Ochs M, Weibel ER ATS/ERS Joint Task Force on Quantitative Assessment of Lung Structure. An official research policy statement of the American Thoracic Society/European Respiratory Society: standards for quantitative assessment of lung structure. Am J Respir Crit Care Med. 2010;181:394–418. doi: 10.1164/rccm.200809-1522ST. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bergin DA, Reeves EP, Meleady P, Henry M, McElvaney OJ, Carroll TP, Condron C, Chotirmall SH, Clynes M, O’Neill SJ, et al. α-1 Antitrypsin regulates human neutrophil chemotaxis induced by soluble immune complexes and IL-8. J Clin Invest. 2010;120:4236–4250. doi: 10.1172/JCI41196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mehra D, Geraghty PM, Hardigan AA, Foronjy R. A comparison of the inflammatory and proteolytic effects of dung biomass and cigarette smoke exposure in the lung. PLoS ONE. 2012;7:e52889. doi: 10.1371/journal.pone.0052889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yang CM, Lee IT, Lin CC, Wang CH, Cherng WJ, Hsiao LD. c-Src-dependent MAPKs/AP-1 activation is involved in TNF-α-induced matrix metalloproteinase-9 expression in rat heart-derived H9c2 cells. Biochem Pharmacol. 2013;85:1115–1123. doi: 10.1016/j.bcp.2013.01.013. [DOI] [PubMed] [Google Scholar]
- 21.Foronjy R, Nkyimbeng T, Wallace A, Thankachen J, Okada Y, Lemaitre V, D’Armiento J. Transgenic expression of matrix metalloproteinase-9 causes adult-onset emphysema in mice associated with the loss of alveolar elastin. Am J Physiol Lung Cell Mol Physiol. 2008;294:L1149–L1157. doi: 10.1152/ajplung.00481.2007. [DOI] [PubMed] [Google Scholar]
- 22.van Kempen LC, Coussens LM. MMP9 potentiates pulmonary metastasis formation. Cancer Cell. 2002;2:251–252. doi: 10.1016/s1535-6108(02)00157-5. [DOI] [PubMed] [Google Scholar]
- 23.Jung O, Choi YJ, Kwak TK, Kang M, Lee MS, Ryu J, Kim HJ, Lee JW. The cooh-terminus of tm4sf5 in hepatoma cell lines regulates c-src to form invasive protrusions via egfr tyr845 phosphorylation. Biochim Biophys Acta. 2013;1833:629–642. doi: 10.1016/j.bbamcr.2012.11.026. [DOI] [PubMed] [Google Scholar]
- 24.Sasaoka T, Rose DW, Jhun BH, Saltiel AR, Draznin B, Olefsky JM. Evidence for a functional role of Shc proteins in mitogenic signaling induced by insulin, insulin-like growth factor-1, and epidermal growth factor. J Biol Chem. 1994;269:13689–13694. [PubMed] [Google Scholar]
- 25.Dogan S, Shen R, Ang DC, Johnson ML, D’Angelo SP, Paik PK, Brzostowski EB, Riely GJ, Kris MG, Zakowski MF, et al. Molecular epidemiology of EGFR and KRAS mutations in 3,026 lung adenocarcinomas: higher susceptibility of women to smoking-related KRAS-mutant cancers. Clin Cancer Res. 2012;18:6169–6177. doi: 10.1158/1078-0432.CCR-11-3265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Khan EM, Lanir R, Danielson AR, Goldkorn T. Epidermal growth factor receptor exposed to cigarette smoke is aberrantly activated and undergoes perinuclear trafficking. FASEB J. 2008;22:910–917. doi: 10.1096/fj.06-7729com. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Osherov N, Levitzki A. Epidermal-growth-factor-dependent activation of the src-family kinases. Eur J Biochem. 1994;225:1047–1053. doi: 10.1111/j.1432-1033.1994.1047b.x. [DOI] [PubMed] [Google Scholar]
- 28.Stang E, Blystad FD, Kazazic M, Bertelsen V, Brodahl T, Raiborg C, Stenmark H, Madshus IH. Cbl-dependent ubiquitination is required for progression of EGF receptors into clathrin-coated pits. Mol Biol Cell. 2004;15:3591–3604. doi: 10.1091/mbc.E04-01-0041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Filosto S, Becker CR, Goldkorn T. Cigarette smoke induces aberrant EGF receptor activation that mediates lung cancer development and resistance to tyrosine kinase inhibitors. Mol Cancer Ther. 2012;11:795–804. doi: 10.1158/1535-7163.MCT-11-0698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rangasamy T, Misra V, Zhen L, Tankersley CG, Tuder RM, Biswal S. Cigarette smoke-induced emphysema in A/J mice is associated with pulmonary oxidative stress, apoptosis of lung cells, and global alterations in gene expression. Am J Physiol Lung Cell Mol Physiol. 2009;296:L888–L900. doi: 10.1152/ajplung.90369.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Foronjy RF, Mercer BA, Maxfield MW, Powell CA, D’Armiento J, Okada Y. Structural emphysema does not correlate with lung compliance: lessons from the mouse smoking model. Exp Lung Res. 2005;31:547–562. doi: 10.1080/019021490951522. [DOI] [PubMed] [Google Scholar]
- 32.Liu L, Ge D, Ma L, Mei J, Liu S, Zhang Q, Ren F, Liao H, Pu Q, Wang T, et al. Interleukin-17 and prostaglandin E2 are involved in formation of an M2 macrophage-dominant microenvironment in lung cancer. J Thorac Oncol. 2012;7:1091–1100. doi: 10.1097/JTO.0b013e3182542752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chen K, Pociask DA, McAleer JP, Chan YR, Alcorn JF, Kreindler JL, Keyser MR, Shapiro SD, Houghton AM, Kolls JK, et al. IL-17RA is required for CCL2 expression, macrophage recruitment, and emphysema in response to cigarette smoke. PLoS ONE. 2011;6:e20333. doi: 10.1371/journal.pone.0020333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Raviv S, Hawkins KA, DeCamp MM, Jr, Kalhan R. Lung cancer in chronic obstructive pulmonary disease: enhancing surgical options and outcomes. Am J Respir Crit Care Med. 2011;183:1138–1146. doi: 10.1164/rccm.201008-1274CI. [DOI] [PubMed] [Google Scholar]
- 35.Burrows B, Knudson RJ, Cline MG, Lebowitz MD. Quantitative relationships between cigarette smoking and ventilatory function. Am Rev Respir Dis. 1977;115:195–205. doi: 10.1164/arrd.1977.115.2.195. [DOI] [PubMed] [Google Scholar]
- 36.Mannino DM, Aguayo SM, Petty TL, Redd SC. Low lung function and incident lung cancer in the United States: data From the First National Health and Nutrition Examination Survey follow-up. Arch Intern Med. 2003;163:1475–1480. doi: 10.1001/archinte.163.12.1475. [DOI] [PubMed] [Google Scholar]
- 37.Brody JS, Spira A. State of the Art: chronic obstructive pulmonary disease, inflammation, and lung cancer. Proc Am Thorac Soc. 2006;3:535–537. doi: 10.1513/pats.200603-089MS. [DOI] [PubMed] [Google Scholar]
- 38.Sinden NJ, Stockley RA. Chronic obstructive pulmonary disease: an update of treatment related to frequently associated comorbidities. Ther Adv Chronic Dis. 2010;1:43–57. doi: 10.1177/2040622310370631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hautamaki RD, Kobayashi DK, Senior RM, Shapiro SD. Requirement for macrophage elastase for cigarette smoke-induced emphysema in mice. Science. 1997;277:2002–2004. doi: 10.1126/science.277.5334.2002. [DOI] [PubMed] [Google Scholar]
- 40.Atkinson JJ, Lutey BA, Suzuki Y, Toennies HM, Kelley DG, Kobayashi DK, Ijem WG, Deslee G, Moore CH, Jacobs ME, et al. The role of matrix metalloproteinase-9 in cigarette smoke-induced emphysema. Am J Respir Crit Care Med. 2011;183:876–884. doi: 10.1164/rccm.201005-0718OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Omachi TA, Eisner MD, Rames A, Markovtsova L, Blanc PD. Matrix metalloproteinase-9 predicts pulmonary status declines in α1-antitrypsin deficiency. Respir Res. 2011;12:35. doi: 10.1186/1465-9921-12-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Boschetto P, Quintavalle S, Zeni E, Leprotti S, Potena A, Ballerin L, Papi A, Palladini G, Luisetti M, Annovazzi L, et al. Association between markers of emphysema and more severe chronic obstructive pulmonary disease. Thorax. 2006;61:1037–1042. doi: 10.1136/thx.2006.058321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Vanden Eijnden S, Goriely S, De Wit D, Willems F, Goldman M. IL-23 up-regulates IL-10 and induces IL-17 synthesis by polyclonally activated naive T cells in human. Eur J Immunol. 2005;35:469–475. doi: 10.1002/eji.200425677. [DOI] [PubMed] [Google Scholar]
- 44.Laan M, Bozinovski S, Anderson GP. Cigarette smoke inhibits lipopolysaccharide-induced production of inflammatory cytokines by suppressing the activation of activator protein-1 in bronchial epithelial cells. J Immunol. 2004;173:4164–4170. doi: 10.4049/jimmunol.173.6.4164. [DOI] [PubMed] [Google Scholar]
- 45.Grivennikov SI, Wang K, Mucida D, Stewart CA, Schnabl B, Jauch D, Taniguchi K, Yu GY, Osterreicher CH, Hung KE, et al. Adenoma-linked barrier defects and microbial products drive IL-23/IL-17-mediated tumour growth. Nature. 2012;491:254–258. doi: 10.1038/nature11465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Li Q, Han Y, Fei G, Guo Z, Ren T, Liu Z. IL-17 promoted metastasis of non-small-cell lung cancer cells. Immunol Lett. 2012;148:144–150. doi: 10.1016/j.imlet.2012.10.011. [DOI] [PubMed] [Google Scholar]
- 47.Kirshberg S, Izhar U, Amir G, Demma J, Vernea F, Beider K, Shlomai Z, Wald H, Zamir G, Shapira OM, et al. Involvement of CCR6/CCL20/IL-17 axis in NSCLC disease progression. PLoS ONE. 2011;6:e24856. doi: 10.1371/journal.pone.0024856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Nam HJ, Im SA, Oh DY, Elvin P, Kim HP, Yoon YK, Min A, Song SH, Han SW, Kim TY, et al. Antitumor activity of saracatinib (AZD0530), a c-Src/Abl kinase inhibitor, alone or in combination with chemotherapeutic agents in gastric cancer. Mol Cancer Ther. 2013;12:16–26. doi: 10.1158/1535-7163.MCT-12-0109. [DOI] [PubMed] [Google Scholar]
- 49.Shan M, Yuan X, Song LZ, Roberts L, Zarinkamar N, Seryshev A, Zhang Y, Hilsenbeck S, Chang SH, Dong C, Corry DB, Kheradmand F. Cigarette smoke induction of osteopontin (spp1) mediates t(h)17 inflammation in human and experimental emphysema. Science Transl Med. 2012;4:117ra119. doi: 10.1126/scitranslmed.3003041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chang Y, Nadigel J, Boulais N, Bourbeau J, Maltais F, Eidelman DH, Hamid Q. CD8 positive T cells express IL-17 in patients with chronic obstructive pulmonary disease. Respir Res. 2011;12:43. doi: 10.1186/1465-9921-12-43. [DOI] [PMC free article] [PubMed] [Google Scholar]