Abstract
Chronic bacterial lung infections in cystic fibrosis (CF) are caused by defects in the CF transmembrane conductance regulator chloride channel. Previously, we described that newborn CF transmembrane conductance regulator–knockout ferrets rapidly develop lung infections within the first week of life. Here, we report a more slowly progressing lung bacterial colonization phenotype observed in juvenile to adult CF ferrets reared on a layered antibiotic regimen. Even on antibiotics, CF ferrets were still very susceptible to bacterial lung infection. The severity of lung histopathology ranged from mild to severe, and variably included mucus obstruction of the airways and submucosal glands, air trapping, atelectasis, bronchopneumonia, and interstitial pneumonia. In all CF lungs, significant numbers of bacteria were detected and impaired tracheal mucociliary clearance was observed. Although Streptococcus, Staphylococcus, and Enterococcus were observed most frequently in the lungs of CF animals, each animal displayed a predominant bacterial species that accounted for over 50% of the culturable bacteria, with no one bacterial taxon predominating in all animals. Matrix-assisted laser desorption–ionization time-of-flight mass spectrometry fingerprinting was used to quantify lung bacteria in 10 CF animals and demonstrated Streptococcus, Staphylococcus, Enterococcus, or Escherichia as the most abundant genera. Interestingly, there was significant overlap in the types of bacteria observed in the lung and intestine of a given CF animal, including bacterial taxa unique to the lung and gut of each CF animal analyzed. These findings demonstrate that CF ferrets develop lung disease during the juvenile and adult stages that is similar to patients with CF, and suggest that enteric bacterial flora may seed the lung of CF ferrets.
Keywords: cystic fibrosis, ferret, bacteria, lung, intestine
Clinical Relevance
Animal modeling of bacterial infections observed in cystic fibrosis (CF) lung disease is critical to understanding the molecular basis of disease progression and developing therapies. This study has characterized the progression of chronic lung disease in juvenile and adult CF transmembrane conductance regulator–knockout ferrets. Findings from this study suggest that enteric bacteria may be a significant source of lung-colonizing microbes in the CF ferret.
Cystic fibrosis (CF) is the most common life-threatening, autosomal recessive genetic disorder among white individuals, occurring in roughly 1 in 3,500 births. Defects in the CF transmembrane conductance regulator (CFTR) gene that disrupt function of this chloride channel cause abnormalities in electrolyte and fluid movement across many epithelia of the body, leading to viscous secretions (1). Chronic bacterial infections in the lung are the most significant cause of mortality in CF. Mouse models of CF, although useful for studying CFTR function in many organs, have failed to reproduce the spontaneous lung bacterial colonization defect seen in patients with CF (2, 3). For these reasons, larger animal models of CF have been generated in the ferret (4) and pig (5). The newborn CFTR-knockout (KO) ferret develops lung disease characterized by bacterial colonization (6). Here, we report the lung phenotype of older CF animals reared on antibiotics until 6 months of age or the time at which they were killed due to severity of disease.
CFTR conducts chloride and bicarbonate, and has been shown to also regulate epithelial Na+ channels (ENaCs) in the airway (1, 7). Controversies regarding the mechanisms of impaired innate immunity in the CF lung still remain, with several current hypotheses including: airway surface liquid depletion through dysregulation of ENaC, leading to impaired mucociliary clearance (MCC) (8, 9); altered Cl− concentration in the airway that impairs antibacterial killing (10); and impaired bicarbonate transport into the airway that impairs antibacterial killing (11). Other potential hypotheses of impaired innate immunity in the CF lung include abnormalities in pathogen sensing, leukocyte recruitment, phagocyte function, hyperactivation of immune responses, and mechanisms linking innate and adaptive immunity (12).
The predominant pathogens observed in the CF lung have historically been thought to be limited to species such as Pseudomonas aeruginosa, Staphylococcus aureus, and Haemophilus influenzae; however, improved molecular methods for detection and quantification of bacteria are beginning to demonstrate that the microbiome of the CF lung is significantly more polymicrobial than first thought, and overlaps with oropharyngeal microbiota (13). Using direct distal lung sampling at the time of lung transplantation followed by deep sequencing, others have recently demonstrated that, at end-stage disease, the CF lung is dominated by, at most, three bacterial taxa (14). The authors of this second study conclude that there was considerably more diversity in the upper airway, and that oropharyngeal contamination could complicate microbiome analyses of the CF lungs using DNA-based methods. Alternatively, the polymicrobial nature of CF airways disease may change with severity. Although CF lung bacterial pathogens overlap between patients, these patients have their own distinct bacterial fingerprints, influenced by environmental factors, including siblings and care centers (15). The physical connection between the airways and the skin, oral cavity, and gastrointestinal tract have been proposed to be a source for bacteria that colonize the CF lung (16). Although no studies have directly compared the bacterial species that colonize the intestine and lung within the same cohort of patients with CF, recent studies longitudinally evaluating the oral and fecal microbiomes in infants with CF demonstrate that there is considerable overlap (17).
In the current study, we evaluated the pathology and microbiology of the CF lung in juvenile and adult CF ferrets. All but one CF animal, which was killed for a rectal prolapse, demonstrated mild to severe lung histopathology consistent with findings in patients with CF. Bacterial titers of all but one CF lung tissue homogenates were significantly greater than titers of non-CF controls, but varied significantly in the absolute bacterial burden. The bacteria observed in each CF lung were dominated by one to two taxa, with significant overlap in taxa found in the small intestine. Interestingly, each CF animal analyzed had at least one unique bacterial taxon found in the gut and lung that was not observed in other CF animals. Cumulatively, these findings demonstrate that juvenile and adult CF ferrets naturally acquire lung infections even when reared on antibiotics, and that a significant source of bacteria that colonize the lung may come from the gut.
Materials and Methods
Rearing of Ferrets with CF
Exon-10–disrupted CFTR-KO (4) and non-CF littermate kits were reared on lactating jills with supplemental Elecare/GoLytely gavages (Abbott Laboratories, Columbus, OH) as previously described (18), with the following modification to antibiotic regimes. All kits were reared from birth on the antibiotics, metronidazole (20 mg/kg, twice daily; Hospira, Inc., Lake Forest, IL) and piperacillin/tazobactam (4.0 mg/kg, twice daily; Wyeth Pharmaceuticals Inc., Philadelphia, PA). CF kits were paired with a non-CF sibling control and reared together. CF ferrets were never housed together, and caretaker precautions (gloves, masks, and gown) were used to minimize the chance of spreading CF-specific pathogens. Kits were weighed every 6 hours and the rolling average 6-hour weight gain for a 24-hour period was determined and graphed as a ratio of non-CF:CF during the rearing process. Typically, this ratio remained in the range of 2–4. If the ratio jumped to above 10 for three consecutive measurements, the CF animals were started on enrofloxacin (10 mg/kg, twice daily; Bayer HealthCare LLC, Shawnee Mission, KS). If the rolling average weight gain increased a second time, the animal was placed on cefazolin (30 mg/kg, twice daily; Steri-Pharma, LLC, Syracuse, NY). Pancreatic enzyme (Viokase-V; Neogen Corporation, Lexington, KY) supplementation was initiated at roughly 21–30 days. When animals reached 21 weeks of age, antibiotic dosages were reduced by 25% per week until the animals were free from antibiotics at 6 months of age. Throughout the rearing process, the CF and matched non-CF control animals were reared on the same jill until weaning and treated identically from the standpoint of feeding and antibiotic treatments. For more details on rearing methods, see the Materials and Methods in the online supplement.
Tracheal MCC Assays
MCC rates were measured ex vivo on ferret tracheas by assessing the rate at which fluorescent 1.0-μm beads moved. For more details, see the Materials and Methods in the online supplement.
Electrophysiologic Studies
Short-circuit current (ISC) was measured using a VCC MC8 Multichannel voltage/current clamp (Physiologic Instruments, San Diego, CA) as previously reported (19, 20). For more details, see the Materials and Methods in the online supplement.
Bacteriology of Lung and Intestinal Samples
At the time of necropsy, a portion of the trachea, duodenum, ileum, and three to four regions of the lung with noticeable pathology were homogenized in sterile saline and a portion plated directly onto blood agar, MacConkey agar, colistin and naladixic acid agar, colistin and naladixic acid reducible agar, and chocolate agar in a four-quadrant streak pattern. Cultures were incubated aerobically and anaerobically for 24–48 hours at 37°C. The remaining tissue homogenate was aliquoted and snap frozen in 10% glycerol. An experienced bacteriologist performed assays for bacterial diversity that subcultured unique colony morphologies, and performed preliminary classification using biochemical reactions (21); final identification was performed using 16S sequencing and MALDI-TOF MS fingerprinting (22). Quantification of the major culturable bacteria from the lung was performed on frozen glycerol stock of tissue homogenates plated on blood agar under aerobic conditions. The CFU titers were calculated and normalized to milligrams of protein in the homogenate. For each sample, between 100 and 200 isolated colonies were analyzed by direct smear method on a Bruker Microflex MS with the MALDI Biotyper software and automation control (Bruker AXS, Inc., Madison, WI). For more details on bacteriologic methods used, see the Materials and Methods in the online supplement.
Results
CFTR-KO Ferrets Are Growth Impaired
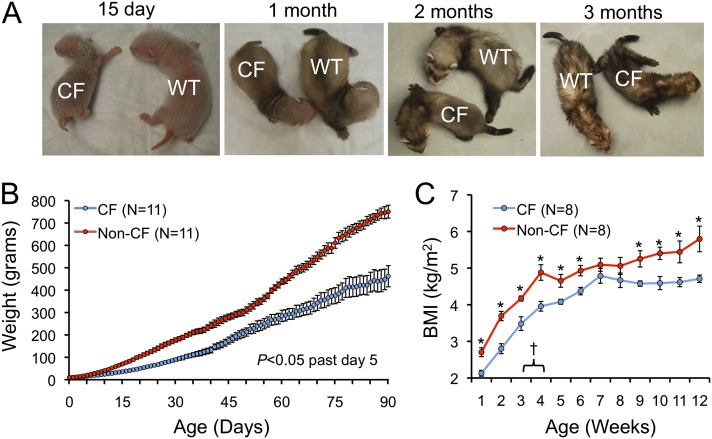
A common feature of CF is malabsorption and growth impairment. CFTR-KO ferrets weighed significantly less at all time points past 5 days of age (Figures 1A and 1B). During the first 3 months of life, body mass index (BMI) was significantly lower for CF animals with an exception during the 7- to 8-week window (Figure 1C). At roughly 21–30 days of age, gavage feeding supplements were transitioned to oral feedings by an artificial nipple, and pancreatic enzyme supplementation was initiated. This transition led to a flattening in BMI of non-CF control animals in the subsequent 4 weeks, whereas BMI trends were not altered in CF animals. As animals opened their eyes around 5–6 weeks, they were transitioned onto solid chow with pancreatic enzymes and continued supplementation with Elecare.
Figure 1.
Cystic fibrosis (CF) ferrets have impaired growth rates. (A) Representative pictures of CF and non-CF control ferrets between 15 days and 3 months of age. (B) Average weights of CF and non-CF control ferrets between birth and 3 months of age. Values past 5 days of age were significantly different between genotypes by two-way Student’s t test (P < 0.05). (C) Body mass index (BMI) of CF and non-CF control ferrets between birth and 3 months of age. *Values were significantly different between genotypes by two-way Student’s t test (P < 0.05). †The window in which pancreatic enzyme supplementation was initiated. The mechanism for the decrease in BMI of non-CF controls is unclear, although this does coincide with transition to nipple feeding and pancreatic enzyme supplementation.
Antibiotic Treatment after Small Declines in Weight Gain Enhance Survival of Neonatal CF Ferrets
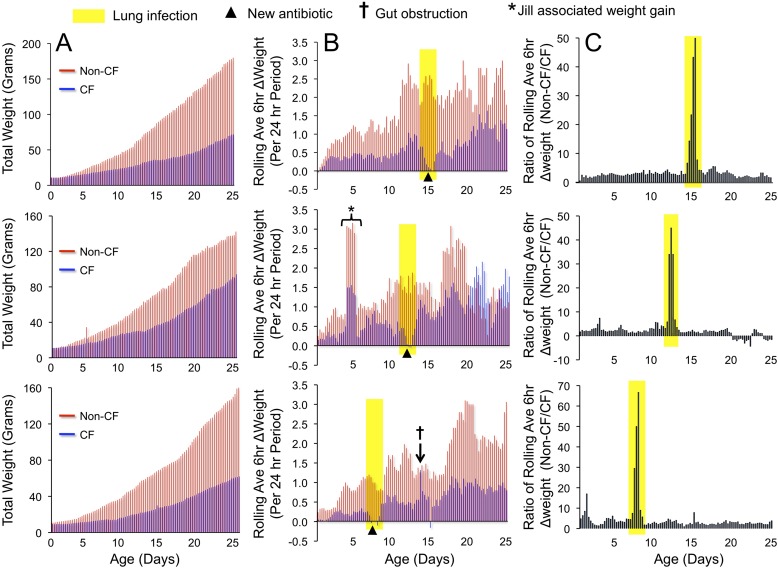
Newborn CF ferrets are rapidly susceptible to lung infection after birth unless reared on antibiotics, and, even after treatment with a single antibiotic, neonatal CF ferrets still succumb to lung infection (6). To this end, CF ferrets and control littermates were reared on two antibiotics at birth (piperacillin/tazobactam and metronidazole), and additional antibiotics (first enrofloxacin and second cefazolin) were sequentially applied when early lung infection was suspected. Changes in weight gain were the most sensitive indicator of declining health status of CF kits, and an additional antibiotic was administered if weight gain declined within a 12- to 24-hour period. A number of weight indices were used when optimizing the rearing methods and, in all instances, referencing changes in weight of CF kits to the non-CF control kits was very important, as jills have varying levels of lactation and parental behavior that can affect growth in both genotypes. Kits were weighed every 6 hours at the time of feeding and drug therapy. Total body weight and daily weight gain was a relatively insensitive measure of the decline in health status of CF kits by which to implement a successful treatment with a third antibiotic (Figure 2A). However, the rolling average of 6-hour weight gain measurements over a 24-hour period (5 points included in this average) proved to be an excellent early predictor of suspected lung infection and gut obstruction (Figure 2B). If the rolling average weight gain indicator declined over a 12- to 18-hour period in a CF kit, but not in control, an additional antibiotic was initiated for both the CF and non-CF animals. If the rolling average weight gain indicator sharply increased within a 6-hour period in a CF kit, but not in the age-matched control, this was suggestive of gut obstruction, and a Golytely gavage was instituted; this was then followed by a significant decline in weight gain not associated with poor health and survival. Plotting the ratio of the rolling average weight gain between non-CF/CF kits gave the clearest picture of failure to thrive of a CF kit and suspected lung infection (Figure 2C). Typically, a third antibiotic was needed by 7–20 days, and weight gain recovered within an 18- to 24-hour period after the administration of an additional antibiotic (Figures 2B and 2C). If an additional antibiotic was delayed past the 18-hour window of a declining rolling average weight gain, CF animals typically did not recover after the third antibiotic was instituted.
Figure 2.
Early treatment of suspected lung infection is key to rearing older CF ferrets. The weights of three CF and non-CF matched pairs (pairs reared on the same jill) were taken every 6 hours. (A) Total weight gain profiles of CF (blue bars) and non-CF (red bars) animals for each pair. (B) The rolling average of weight gain over a 6-hour period was calculated by averaging five measurements over a 24-hour period, and is plotted for three CF (blue bars) and non-CF (red bars) pairs. A decline in this rolling average was indicative of an early lung infection (yellow-shaded regions), and antibiotics were instituted at the positions marked by an arrowhead. Graph of the absolute 6-hour weight gain were not as informative as the 6-hour rolling average in predicting this decline, due to greater fluctuations in weights. Several other features of clinical management were useful from the rolling average 6-hour weight gains. For example, gut obstruction (†) could easily be seen as a very rapid spike in the value. When this was observed, animals were gavaged with Golytely or the percentage was increased in Elecare gavages. This was typically followed by a significant decline in the following average weight gain as the obstruction was passed in the feces. In addition, fluctuations in weight gain observed in both CF and non-CF animals (*) were typically associated with jill performance and lactation. (C) The ratio of the rolling average 6-hour weight gain between non-CF and CF animals most clearly shows the decline in weight of CF kits associated with lung infection by referencing this weight change with a non-CF littermate.
CFTR-KO Ferrets Develop Slowly Progressive Lung Infections and Mucus Obstruction of the Airways after Weaning
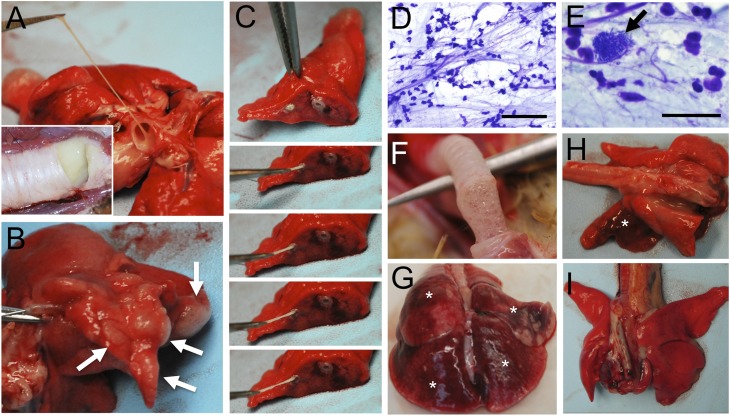
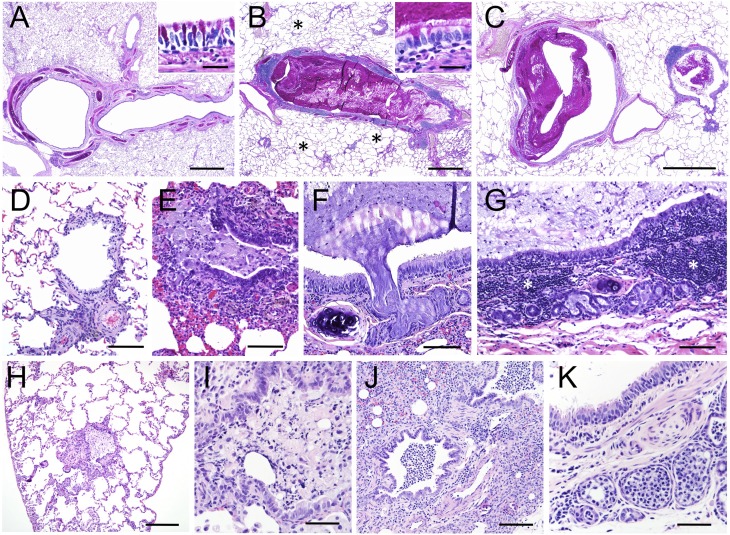
For three CF ferrets, antibiotics were removed after 6 months to allow for the development of lung disease, and all other CF animals were maintained on antibiotics throughout their lives (Table 1). All but one CF animal, at the time at which the animal was killed due to morbid status and, in one case, death, demonstrated lung pathology to varying degrees. Gross lung pathology ranged from focal disease in specific lobes to diffuse disease affecting most or all lobes of the lung and the majority of airways (Figure 3). Mucus in the major airways was often tenacious and filled with bacterial colonies and inflammatory cell types (Figures 3A and 3C–3E). CF lung histopathology ranged from mild to severe, with mucus plugging of the major and small airways, air trapping, and atelectasis (Figure 4 and Figure E1 in the online supplement). There was plugging and dilation of submucosal glands and ducts with mucus and inflammatory cells (Figures 4F, 4G, 4K, E1B, and E1C). Mucus accumulation with inflammatory cellular debris was observed in large and small airways, and ranged from complete occlusion (Figures 4B, 4E, 4H, E1D, E1E, and E1J–E1L) to partial occlusion (Figures 4C, 4I, 4J, E1A, E1B, E1F, E1H, and E1I). Mucus accumulation in the airways was associated with variable levels of goblet and mucus cell hyperplasia in the surface airway epithelium and submucosal glands, as detected with periodic acid-Schiff mucin stains (Figures 4B, E1A, E1B, E1C, E1E, and E1F). In some cases mucus accumulation in the airways was associated with minimal inflammation and pathology other than air-trapping and atelectasis in the alveolar regions (Figures 4B, 4C, and 4H; Figures E1G–E1I). In other cases, lungs had changes consistent with bronchopneumonia or interstitial pneumonia (Table 1). Lungs with bronchopneumonia had suppurative inflammation and cellular debris within airways, alveolar consolidation, and areas of necrosis (Figures 4J, E1J, and E1K). Two animals (CF-4 and CF-10) had evidence of mild to moderate interstitial hypercellularity consistent with interstitial pneumonia with increased alveolar macrophages. Proliferation of lymphoid tissue associated with the larger airways (Figure 4G) and smaller airways (Figure E1E) was also observed. Two CF animals demonstrated minimal lung pathology, and were killed due to rectal prolapse (CF-7) and estrus-associated aplastic anemia (CF-2). In summary, lung histopathology in CF ferrets demonstrated similarities to those observed in the human CF lung (23).
Table 1:
Summary of Cystic Fibrosis Animals Evaluated and Disease States
| CF Ferret ID | Sex | Life Span (d) | Duration of Antibiotics | Lung Disease Histopathology | Mean CFU/mg Lung Protein |
|---|---|---|---|---|---|
| CF-1 | M | 257 | 6 mo | AO, MA, AI, AR, AT, SMGD | 3.1 x 107 |
| CF-2* | F | 359 | 6 mo | SMGD | 1 x 101 |
| CF-3 | F | 120 | OTD | MA, SMGD, IP (diffuse) | 2.3 x 108 |
| CF-4 | F | 100 | OTD | SMGD, IP (multifocal) | 9.2 x 103 |
| CF-5 | F | 94 | OTD | MA, SMGD, AO, AI, AT, SMGD, BP | 2.0 x 107 |
| CF-6 | M | 253 | 6 mo | AO, AI, AT, SMGD, BP | 1.2 x 104 |
| CF-7† | F | 35 | OTD | None | 3.8 x 105 |
| CF-8† | F | 32 | OTD | MA | 8.3 x 105 |
| CF-9 | F | 22 | OTD | IP | 4.8 x 104 |
| CF-10 | M | 40 | OTD | AI, SMGD | 1.7 x 105 |
| CF-11 | F | 104 | OTD | AI, SMGD | 2.0 x 103 |
Definition of abbreviations: AI, airway inflammation; AO, airway obstruction; AR, air trapping; AT, atelectasis; BP, bronchopneumonia; CF, cystic fibrosis; IP, interstitial pneumonia; MA, mucus accumulation; OTD, on antibiotics till death; SMGD, submucosal gland dilation.
Animal died due to the effects of estrus-associated aplastic anemia.
Animals were killed due to a rectal prolapse.
Figure 3.
Gross abnormalities in the CF ferret lung. Lungs from three CF ferrets and one non-CF ferret ranging from 3 to 8 months of age are shown. (A–C) Mucus obstruction of airways in a CF animal. Inset in (A) shows mucus accumulation in the trachea, (B) shows air-trapping (arrows) in a lobe, and (C) shows mucus accumulation in an intralobar airway. (D and E) Airway mucus from this CF animal contained numerous neutrophils, bacterial colonies (E, arrow), and neutrophil extracellular traps. (F and G) A second example of a CF lung with (F) mucus accumulation in the trachea and (G) infection with hemorrhage (*) in various lobes demonstrating interstitial pneumonia. (H) A third example of a CF lung with hemorrhage and cranial bronchopneumonia (*). (I) Gross image of a control non-CF lung. Scale bars, 100 μm (D), 25 μm (E).
Figure 4.
Histopathology in the CF ferret lung. Lungs from four CF animals ranging from 3–8 months of age are shown. (A–C) Proximal airway mucus obstruction in a CF animal demonstrating complete occlusion (B) and partial occlusion (C) as compared with the non-CF control (A). Insets in (A) and (B) are higher-power images of the surface airway epithelium. (D and E) Distal airway occlusion in a CF (E) as compared with non-CF (D) animal. (F–G) Submucosal gland plugging with mucus (F and G) and expansion of bronchial-associated lymphoid tissue (G) in a proximal airway of a CF animal. (H and I) Distal airway occlusion in two different CF animals with inflammatory cell debris in the lumen. (J and K) Accumulation of inflammatory cells in the lumen of a distal airway (J) and submucosal glands (K) extending into alveoli from a CF animal. The four independent CF animals are grouped in panels as follows: (B, C, and E–G), (H), (I), (J and K). Images in (A–C) are periodic acid-Schiff stains and (D–K) are hematoxylin and eosin stains. Scale bars, 1 mm (A–C), 200 μm (H), 100 μm (D–G, J), 50 μm (I and K). *Air-trapping in CF lung (B).
Abnormalities in the sinuses of some, but not all, CF animals were also noted, including accumulation of mucus and inflammatory debris (Figures E2E–E2G). However, all CF animals had mucus accumulation, and, in some instances complete obstruction of the nasolacrimal duct (Figures E2C, E2D, E2J, E2K, and E2L). Such obstructions were never noted in non-CF animals (Figures E2H and E2I).
Impaired Airway MCC Occurs in Juvenile and Adult CF Ferrets
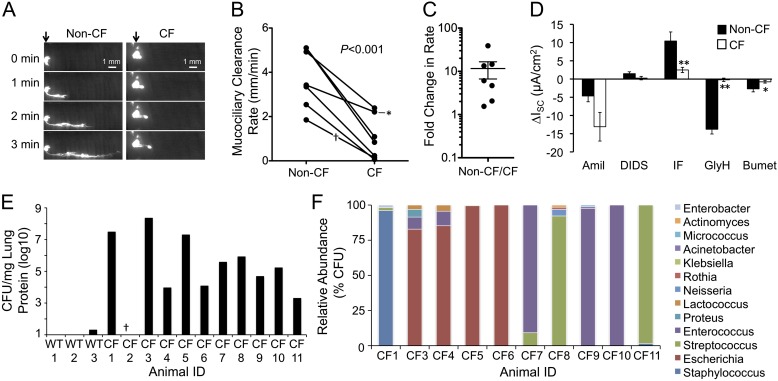
A common feature of CF airway disease involves thick viscous mucous secretions that are not easily cleared from the airways. Several prevailing hypotheses for the high viscosity of CF mucus and the resultant impaired MCC have included: (1) hyperactivation of ENaC and dehydration of the surface airway fluid; (2) impaired CFTR-dependent bicarbonate secretion needed for proper hydration of mucus; (3) reduced fluid secretion from submucosal glands; and (4) excessive mucus production secondary to bacterial infections. To evaluate if these animals also had impaired MCC, we evaluated the rate of fluorescent bead migration in the trachea immediately after killing of CF and non-CF animals (Figures 5A–5C). Using this assay, tracheal MCC was significantly reduced roughly sevenfold (P < 0.0025) in CF trachea as compared with controls. To address whether these changes might correlate with hyperactivation of ENaC, we also performed Isc analysis on tracheal tissue (Figure 5D). Results from these experiments demonstrated no significant difference (P = 0.0654) in amiloride-sensitive Isc between CF and non-CF controls, although the average value for CF was 2.8-fold higher than non-CF animals. Interestingly, there was a significantly higher variance in amiloride-sensitive Isc from the CF group (P < 0.0001; Figure E3A). Investigation into this variance revealed a significant age-dependent increase in amiloride-sensitive Isc in CF animals (P = 0.0009) that was not observed in non-CF controls (P = 0.7637; Figures E3B and E3C). 4,4′-diisothiocyano-2,2′-stilbene disulphonic acid-sensitive currents were also not significantly different between genotypes. As expected, cAMP agonists induced significantly greater currents in non-CF animals that were sensitive to the application of N-(2-Naphthalenyl)-((3,5-dibromo-2,4-dihydroxyphenyl)methylene)glycine hydrazide (GlyH101, a CFTR inhibitor) and bumetanide (sodium–potassium ATPase channel inhibitor). These findings demonstrate that juvenile and adult CF ferrets have impaired tracheal MCC and highly variable tracheal ENaC activity that increases with age in a genotype-specific fashion.
Figure 5.
CF animals have impaired airway mucociliary clearance (MCC) and age-dependent increases in epithelial Na+ channel (ENaC) activity. (A) Time-lapse fluorescent photomicrographs of the tracheal MCC assay. The origin of fluorescent bead placement is marked by the arrows, and the distal and proximal ends of each tracheal segment are on the left and right of each photomicrograph, respectively. (B) Quantified MCC rates for seven CF and non-CF matched pairs at 3–8 months of age. *CF animal that was killed due to a rectal prolapse with more mild lung disease. †A pair in which the CF animal was found dead in the cage at roughly 3 hours postmortem; MCC on the non-CF animal in this pair was performed at 3 hours after killing to control postmortem influences on MCC. Differences between MCC rates between genotypes were determined using a paired two-way Student’s t test with P value given in the figure. (C) Fold difference (± SEM) in MCC rates between non-CF and CF animals (n = 7). (D) Ussing chamber short-circuit current analysis (ISC) of tracheal tissue from CF and non-CF animals older than 3 months of age. ISC was measured after the sequential addition of amiloride (Amil), 4,4′-diisothiocyano-2,2′-stilbene disulphonic acid (DIDS), 1-methyl-3-isobutylxanthine/forskolin (IF), N-(2-Naphthalenyl)-((3,5-dibromo-2,4-dihydroxyphenyl)methylene)glycine hydrazide (GlyH101; GlyH), and bumetanide (Bumet). Quantification of the change in ISC for each of the indicated drugs is shown (mean ± SEM from n = 7 animals of each genotype). At least two independent tissue samples were evaluated for each animal and the average ∆ISC for each animal/condition used to calculate the SEM. Significant differences between genotypes by two-tailed Student’s t test are marked (**P < 0.005, *P < 0.05). On average, amiloride-sensitive ISC was not significantly different between genotypes (P = 0.0654). However, there was a significant age-dependent increase in amiloride-sensitive currents in CF, but not in non-CF, animals (CF, P = 0.0009; non-CF, P = 0.7637 [by Spearman correlation]; see Figure E3). (E) Bacterial titers of lung homogenates from three non-CF and 11 CF animals. (F) Quantification of bacteria taxa found in lung homogenates from 10 CF animals using matrix-assisted laser desorption–ionization time-of-flight mass spectrometry (MALDI-TOF MS) fingerprinting. Only genera are shown; for complete genus and species, see Figure E4A.
Diverse Types of Bacteria Infect the Lungs of CF Ferrets
To investigate the type and number of bacteria that were observed in the CF lung of juvenile and adult ferrets, samples of lung tissue were sterilely obtained at the time of necropsy. These samples were titered for CFUs and bacteriology evaluated by standard chemistries, 16S sequencing, and matrix-assisted laser desorption–ionization time-of-flight mass spectrometry (MALDI-TOF MS). CFU titers ranged from 103 to 108 CFU/mg lung protein in CF animals (with the exception of CF-2, which died from estrus-associated aplastic anemia), whereas minimal bacteria were cultured from sibling non-CF control ferret lungs (Figure 5E and Table 2). The major bacteria cultured from CF lungs under aerobic conditions included Escherichia, Streptococcus, Staphylococcus, and Enterococcus (Figure 5F). In all cases, a single bacterial taxon accounted for over 50% of the culturable bacteria (Figure E4A). Interestingly, when enteric bacteria predominated in the CF lung (CF-3, -4, -5, -6, -7, -9, and -10), including Escherichia coli, Enterococcus hirae, Enterococcus faecium, and Enterococcus faecalis, these bacteria accounted for over 90% of the culturable bacteria. However, those CF animals (CF-1, -8, and -11) colonized by Streptococcus or Staphylococcus lung infection typically retained over 90% of bacteria confined to a single genus (i.e., Staphylococcus delphini and Staphylococcus intermedius accounted for 96% of culturable bacteria in CF-1; Streptococcus gallolyticus, Streptococcus lutetiensis, Streptococcus equinus, Streptococcus sanguinis, Streptococcus pseudopneumoniae, Streptococcus pneumoniae, Streptococcus vestibularis, and Streptococcus peroris accounted for 92% of culturable bacteria in CF-8; and Streptococcus gallolyticus, Streptococcus lutetiensis, and Streptococcus equinus accounted for 98% of culturable bacteria in CF-11). These findings emphasize that defects in lung innate immunity in the CF ferret are not limited to a single genus. However, more in-depth, nonquantitative interrogation of the types of culturable bacteria found within the CF ferret lung using multiple types of media with aerobic and anaerobic culture conditions revealed that Streptococcus, Staphylococcus, and Enterococcus genera were most commonly found (at any abundance) in the lungs of CF animals (Figure E4B and Table 2). Three species of Pseudomonas were separately identified at low abundance in three CF animals, including P. fluorescens, P. putida, and P. fulva (Table 2).
Table 2:
Bacteria Observed in the Lung of Cystic Fibrosis Animals
| CF Ferret ID No. | Bacterial Taxa Present in the Lung |
|---|---|
| CF-1 | Staphylococcus intermedius*, Staphylococcus delphini*, Streptococcus gallolyticus*, Enterobacter asburiae*, Enterobacter ludwigii*, Ochrobactrum anthropi, Proteus mirabilis, Pseudomonas fluorescens |
| CF-2 | Staphylococcus epidermidis, Staphylococcus haemolyticus, Staphylococcus warneri, Streptococcus gallolyticus, Streptococcus lutetiensis, Streptococcus minor, Streptococcus suis, Pseudomonas fulva, Penicillium spp., Enterococcus faecalis, Enterococcus faecium, Corynebacterium spp., Aspergillus fumigatus, Actinomyces spp. |
| CF-3 | Staphylococcus epidermidis, Lactococcus garvieae*, Proteus mirabilis*, Klebsiella pneumoniae, Escherichia coli*, Enterococcus hirae*, Micrococcus luteus |
| CF-4 | Lactobacillus murinus, Lactococcus garvieae*, Lactococcus lactis*, Enterococcus faecium*, Enterococcus faecalis*, Escherichia coli*, Veillonella criceti |
| CF-5 | Staphylococcus muscae, Staphylococcus epidermidis, Streptococcus salivarius, Lactococcus lactis, Lactobacillus murinus, Vagococcus fluvialis, Actinomyces spp., Enterococcus faecalis, Enterococcus gallinarum, Escherichia coli*, Klebsiella pneumoniae*, Proteus mirabilis |
| CF-6 | Staphylococcus warneri, Streptococcus gallolyticus, Streptococcus suis, Enterococcus faecalis, Bacillus cereus, Bacillus pumilus, Clostridium perfringens, Hemolytic Escherichia coli*, Pasteurella pneumotropica, Proteus mirabilis |
| CF-7 | Streptococcus equinus*, Streptococcus gallolyticus, Streptococcus lutetiensis*, Enterococcus faecium* |
| CF-8 | Streptococcus equinus*, Streptococcus gallolyticus*, Streptococcus pneumoniae*, Streptococcus sanguinis*, Streptococcus lutetiensis*, Streptococcus pseudopneumoniae*, Streptococcus vestibularis*, Streptococcus peroris*, Actinomyces spp.*, Rothia mucilaginosa, Neisseria perflava*, Neisseria mucosa* |
| CF-9 | Staphylococcus hyicus, Staphylococcus sciuri, Staphylococcus warneri, Rothia nasimurium, Acinetobacter genomospecies*, Kocuria palustris, Enterococcus faecalis*, Enterococcus faecium, Enterococcus avium, Micrococcus luteus* |
| CF-10 | Streptococcus pneumoniae, Streptococcus sanguinis, Enterococcus faecium*, Enterococcus faecalis, Neisseria mucosa |
| CF-11 | Staphylococcus epidermidis*, Staphylococcus warneri, Streptococcus equinus*, Streptococcus gallolyticus*, Streptococcus lutetiensis*, Pseudomonas putida, Enterococcus faecalis, Enterococcus faecium, Escherichia coli, Penicillium spp. |
Definition of abbreviations: CF, cystic fibrosis; ID, identification.
Bacterial species identified in the quantitative matrix-assisted laser desorption–ionization screen. All other unmarked species were identified in nonquantitative diversity screening.
Significant Overlap Exists between Lung and Intestinal Bacterial Flora in CF Ferrets
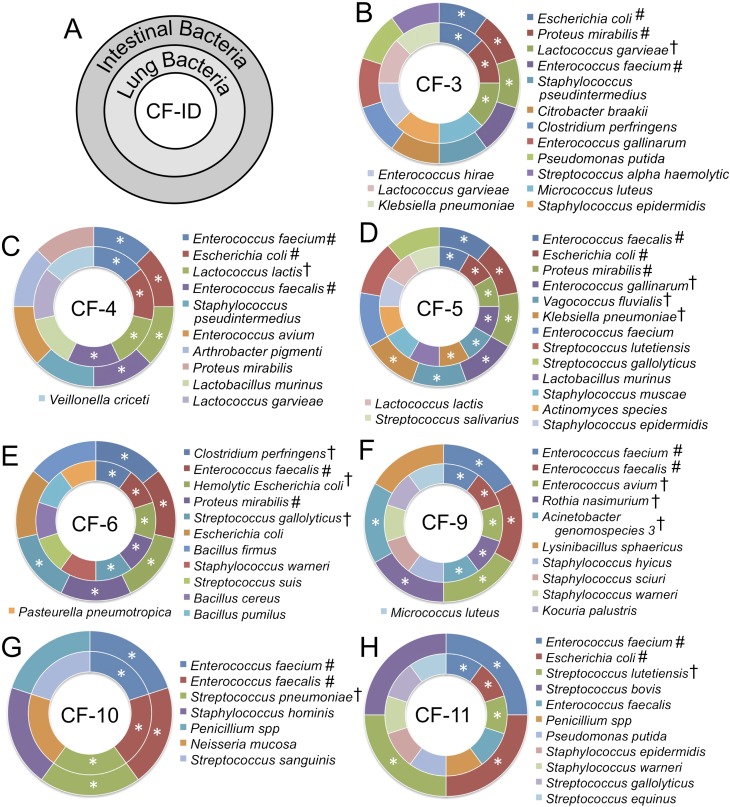
Ferrets, like cats and dogs, are orally grooming animals. Thus, the oral cavity of a ferret is exposed to its enteric bacteria. Given the finding of enteric bacteria in the lung of CF animals, we hypothesized that there may be significant overlap between the lung and intestinal bacterial flora within a given CF animal. To test this hypothesis, we performed bacterial diversity studies in the small intestine and colon of seven CF animals using MALDI-TOF MS and 16S sequencing, and compared the types of bacteria found in the lung of the same animal. This analysis demonstrated considerable overlap between the intestinal flora and bacteria that colonized the lung within a single animal and between CF animals (Figure 6). CF animals contained between three and six common bacteria in the intestine and lung of a given animal. Furthermore, each CF animal contained at least one species of bacteria found in the lung and intestine that was unique to only one of the seven CF animals studied. Enterococcus faecium and Enterococcus faecalis were the most often observed in both the lung and intestine of all CF animals. These findings suggest that, in CF ferrets, the intestine may be a major source of lung colonizing bacteria.
Figure 6.
Overlap in bacteria found in the CF ferret lung and intestine. The types of bacteria observed in both the lung and intestine of seven CF animals were evaluated by MALDI-TOF MS and 16S sequencing. (A) Schematic representation of graphs for each of the seven animals. Bacteria found in the small intestine and colon are shown in the outer circle, whereas bacteria found in the lung lysates are shown in the inner circle. The animal identification number is in the center of the circles. (B–F) Results of bacteria identified in seven independent CF animals. *Bacteria found in both the intestinal and lung samples of the same animal; #bacteria found in both the lung and intestinal samples of at least two animals; †bacteria found in the lung and intestinal sample of only one of the seven CF ferrets. Each CF ferret had at least one unique bacterial strain found in both the lung and intestine.
Discussion
The goal of this study was to evaluate the progression of adult lung disease in the CF ferret model. Thus, we attempted to rear CFTR-KO animals on antibiotics to 6 months of age (the age ferrets are considered to be sexually mature), at which time we planned to remove antibiotics and study the progression of pulmonary disease. Of the 11 CF animals studied here, only 3 lived beyond the age of 6 months, despite continued antibiotic therapy.
Lung infections were observed in all but one CF animal, as evidenced by bacterial counts from lung lysates. However, the outlier CF animal (CF-2) that lacked bacteria in the lung was killed due to morbidity caused by estrus-associated aplastic anemia. Although this CF female came into estrus roughly 6–7 months later than wild-type jills, it is interesting to note that CF female ferrets might be capable of reproducing. All but one CF animal (CF-7), which died from a rectal prolapse, also demonstrated varying degrees of histopathology in the lung. However, the lack of observable lung pathology in CF-7 was likely due to the focal nature of disease and the regions of the lung selected for histopathology, since the lung from this animal was infected with approximately 105 CFU bacteria/mg lung protein in selected regions with the most severe gross pathology. The extent of mucinous changes in the airways varied between CF animals, with more global accumulation throughout the lung in older animals and more focal disease in younger animals. Mucus accumulation and plugging of the airways was associated with variable levels of goblet cell hyperplasia in the surface airway epithelium and submucosal glands. Submucosal gland pathology is consistent with the lack of cAMP-inducible gland secretions in tracheal xenografts from CF ferrets (6). Although lung infections in the CF ferrets occurred regardless of antibiotic therapy, the use of layered antibiotic regimen was key to rearing CF ferrets to weaning. Neonatal ferrets were most susceptible to acute and rapidly life-threatening lung infections during the first month of life, whereas, after weaning, lung infections were less acute and more slowly progressive in nature. This feature of the ferret may reflect the fact that this species develops airway submucosal glands postnatally within the first 3 weeks of life, and these structures are an important source of innate immunity in the airway. Another unique aspect of airway innate immunity in the CF ferret model relates to the fact that ciliogenesis also occurs postnatally in the ferret. Thus, although impaired MCC and submucosal gland obstruction occurs in juvenile to adult CF ferrets, and might indeed contribute to pathogenesis, these mechanisms cannot account for the impaired innate immunity in newborn CF ferrets. The lack of cilia and submucosal glands in newborn ferrets may contribute to the more rapid colonization of the CF ferret lung after birth.
The type of bacteria found in the CF ferret lung was quite diverse, with no one genus emerging as predominant pathogen. Staphylococcus, Streptococcus, and Enterococcus were the common culturable bacteria observed in the lungs of CF ferrets using MALDI-TOF MS and 16S diversity screens on unique colony morphologies (Table 2 and Figure E4B). However, quantitative MALDI-TOF MS CFU analysis on the 10 infected CF lungs demonstrated that a single genus (Staphylococcus, Streptococcus, Enterococcus, or Escherichia) accounted for over 80% of culturable bacteria within a given animal, with over 50% coming from a single taxon (Figure 5F), suggesting that lung infections emerged from a single predominant genus, with subsequent secondary infection by other strains of bacteria. These findings are similar to those of a recent study that used DNA-based methods to evaluate samples from explanted CF lung at the time of transplantation, demonstrating that the lung microbiome was dominated by no more than three taxa (14). Another interesting finding was the exclusion of certain types of bacterial combinations in the CF lung from the quantitative MALDI screen. For example, when E. coli dominated in the lung (CF-3, -4, -5, and -6), Staphylococcus and Streptococcus were never detected in the quantitative MALDI screen (i.e., represented < 1% of the culturable bacteria). The reverse was also true in CF animals colonized predominately by Staphylococcus (CF-1) and Streptococcus (CF-8 and -11), for which the two predominant enteric pathogens (E. coli and Enterococcus) were absent or in low abundance (i.e., < 1% of culturable bacteria). Furthermore, in the most polymicrobial infection (CF-8), eight taxa of Streptococcus comprised 92% of the culturable bacterial burden of the lung, with only minor contributions from Neisseria, Actinomyces, and Rothia as the remaining genera. These finding likely reflect the evolution of microbial communities in the CF lung that functionally exclude certain types of diversity (16). Although Pseudomonas infections predominate in patients with CF, only three CF animals (CF-1, -2, and -11) demonstrated the presence of Pseudomonas in the lung, but this was a minor species. However, it should be emphasized that our analysis did not include 16S deep sequencing, and thus excludes diversity of nonculturable bacteria.
There was significant overlap in bacterial flora of the intestine and the lung of a given CF animal, suggesting that fecal bacteria may be a major source of bacteria that colonize the lung in CF ferrets. Such findings are similar to those of a recent study in CF infants demonstrating that the fecal microbiome significantly overlaps with the oral cavity, with pathogens, such as Escherichia and Enterococcus (also observed in CF ferrets), increasing in the stool before colonizing the oral cavity (17). For CF ferrets, Enterococcus and Escherichia genera predominated in 7 of 10 lungs evaluated; this finding is likely a feature of the living conditions of ferrets and methods of self-cleaning using the tongue, which introduces fecal bacteria into the oral cavity. Although not directly evaluated here, it is likely that gut organisms colonize the ferret oral cavity before being inhaled into the lung. In summary, the bacteriology findings suggest that defects in CF innate immunity are not limited to particular strains of bacteria, but, rather, are dependent on the types of exposures to opportunistic pathogens.
Controversy regarding the mechanism that underlies defective innate immunity in the CF lung remain. One major hypothesis involves impaired hydration of the surface airway fluid and mucus through hyperactivation of ENaC and failure to secrete chloride through CFTR, which leads to impaired MCC and the opportunity for bacteria to establish a lung infection. Indeed, our findings demonstrated impaired MCC in the trachea of end-stage CF animals (Figures 5A–5C), and there was an interesting age-dependent trend in hyperactivation of ENaC within CF animals (Figures E3B and E3C), with the most significant changes occurring in animals over 250 days of age (CF-2 and -6) that were removed from antibiotics. Unfortunately, electrophysiologic studies were not performed on the third CF animal (CF-1), which was also over 250 days old. Studies in newborn CF pig tracheas failed to demonstrate changes in ENaC activity (24), and this is similar to observations in newborn CF ferrets (25). Although the number of older animals with enhanced amiloride-sensitive tracheal currents remains low, the link between enhanced ENaC activity and progression of airway disease in CF ferrets warrants further investigation. However, it should be recognized that ISC analysis of ENaC activation is not a direct measure of volume-dependent regulation of ENaC activity, and thus alternative assays of airway hydration are needed to probe potential involvement of ENaC in airway pathophysiology of CF ferrets. Impaired MCC observed in all CF animals evaluated may also be the consequence of excessive mucus production caused by infection, or may alternatively be caused by impaired CFTR-dependent bicarbonate secretion by the airway epithelia required for mucus hydration, as previously shown in the CF mouse intestine (26, 27).
In summary, our findings demonstrate that the lack of CFTR function leads to lung disease in juvenile and adult ferrets, with similar pathology as in human patients with CF. Bacteriologic studies suggest that the intestinal microbiome is likely a major source of bacteria that colonize the CF ferret lung. Like patients with CF, bacterial colonization of the lung could be delayed through the use of antibiotics, but even in the presence of multiple antibiotics, CF animals still succumbed to lung disease. The CF ferret may be useful in the testing of therapies aimed at treating lung disease and understanding the evolution of the CF lung microbiome over time.
Footnotes
This work was supported by National Institutes of Health grants DK047967 (J.F.E), HL108902 (J.F.E.), HL091842 (Michael J. Welch, University of Iowa, Iowa City, IA), K08 DK092284 (Z.A.S.), and K08 HL114725 (K.P.), University of Iowa Center for Gene Therapy grant DK054759, and the Roy J. Carver Chair in Molecular Medicine (J.F.E.). Mass spectrometry analysis was performed in the Roy J. Carver Charitable Trust–supported Carver College of Medicine Proteomics Facility at the University of Iowa.
This article has an online supplement, which is accessible from this issue’s table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1165/rcmb.2013-0261OC on September 27, 2013
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Rowe SM, Miller S, Sorscher EJ. Cystic fibrosis. N Engl J Med. 2005;352:1992–2001. doi: 10.1056/NEJMra043184. [DOI] [PubMed] [Google Scholar]
- 2.Guilbault C, Saeed Z, Downey GP, Radzioch D. Cystic fibrosis mouse models. Am J Respir Cell Mol Biol. 2007;36:1–7. doi: 10.1165/rcmb.2006-0184TR. [DOI] [PubMed] [Google Scholar]
- 3.Wilke M, Buijs-Offerman RM, Aarbiou J, Colledge WH, Sheppard DN, Touqui L, Bot A, Jorna H, de Jonge HR, Scholte BJ. Mouse models of cystic fibrosis: phenotypic analysis and research applications. J Cyst Fibros. 2011;10:S152–S171. doi: 10.1016/S1569-1993(11)60020-9. [DOI] [PubMed] [Google Scholar]
- 4.Sun X, Yan Z, Yi Y, Li Z, Lei D, Rogers CS, Chen J, Zhang Y, Welsh MJ, Leno GH, et al. Adeno-associated virus–targeted disruption of the CFTR gene in cloned ferrets. J Clin Invest. 2008;118:1578–1583. doi: 10.1172/JCI34599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rogers CS, Hao Y, Rokhlina T, Samuel M, Stoltz DA, Li Y, Petroff E, Vermeer DW, Kabel AC, Yan Z, et al. Production of CFTR-null and CFTR-DeltaF508 heterozygous pigs by adeno-associated virus–mediated gene targeting and somatic cell nuclear transfer. J Clin Invest. 2008;118:1571–1577. doi: 10.1172/JCI34773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sun X, Sui H, Fisher JT, Yan Z, Liu X, Cho HJ, Joo NS, Zhang Y, Zhou W, Yi Y, et al. Disease phenotype of a ferret CFTR-knockout model of cystic fibrosis. J Clin Invest. 2010;120:3149–3160. doi: 10.1172/JCI43052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stutts MJ, Canessa CM, Olsen JC, Hamrick M, Cohn JA, Rossier BC, Boucher RC. CFTR as a cAMP-dependent regulator of sodium channels. Science. 1995;269:847–850. doi: 10.1126/science.7543698. [DOI] [PubMed] [Google Scholar]
- 8.Matsui H, Grubb BR, Tarran R, Randell SH, Gatzy JT, Davis CW, Boucher RC. Evidence for periciliary liquid layer depletion, not abnormal ion composition, in the pathogenesis of cystic fibrosis airways disease. Cell. 1998;95:1005–1015. doi: 10.1016/s0092-8674(00)81724-9. [DOI] [PubMed] [Google Scholar]
- 9.Boucher RC. Airway surface dehydration in cystic fibrosis: pathogenesis and therapy. Annu Rev Med. 2007;58:157–170. doi: 10.1146/annurev.med.58.071905.105316. [DOI] [PubMed] [Google Scholar]
- 10.Smith JJ, Travis SM, Greenberg EP, Welsh MJ. Cystic fibrosis airway epithelia fail to kill bacteria because of abnormal airway surface fluid. Cell. 1996;85:229–236. doi: 10.1016/s0092-8674(00)81099-5. [DOI] [PubMed] [Google Scholar]
- 11.Pezzulo AA, Tang XX, Hoegger MJ, Alaiwa MH, Ramachandran S, Moninger TO, Karp PH, Wohlford-Lenane CL, Haagsman HP, van Eijk M, et al. Reduced airway surface pH impairs bacterial killing in the porcine cystic fibrosis lung. Nature. 2012;487:109–113. doi: 10.1038/nature11130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hartl D, Gaggar A, Bruscia E, Hector A, Marcos V, Jung A, Greene C, McElvaney G, Mall M, Döring G. Innate immunity in cystic fibrosis lung disease. J Cyst Fibros. 2012;11:363–382. doi: 10.1016/j.jcf.2012.07.003. [DOI] [PubMed] [Google Scholar]
- 13.Rabin HR, Surette MG. The cystic fibrosis airway microbiome. Curr Opin Pulm Med. 2012;18:622–627. doi: 10.1097/MCP.0b013e328358d49a. [DOI] [PubMed] [Google Scholar]
- 14.Goddard AF, Staudinger BJ, Dowd SE, Joshi-Datar A, Wolcott RD, Aitken ML, Fligner CL, Singh PK. Direct sampling of cystic fibrosis lungs indicates that DNA-based analyses of upper-airway specimens can misrepresent lung microbiota. Proc Natl Acad Sci USA. 2012;109:13769–13774. doi: 10.1073/pnas.1107435109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Grothues D, Koopmann U, von der Hardt H, Tümmler B. Genome fingerprinting of Pseudomonas aeruginosa indicates colonization of cystic fibrosis siblings with closely related strains. J Clin Microbiol. 1988;26:1973–1977. doi: 10.1128/jcm.26.10.1973-1977.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rogers GB, Carroll MP, Hoffman LR, Walker AW, Fine DA, Bruce KD. Comparing the microbiota of the cystic fibrosis lung and human gut. Gut Microbes. 2010;1:85–93. doi: 10.4161/gmic.1.2.11350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Madan JC, Koestler DC, Stanton BA, Davidson L, Moulton LA, Housman ML, Moore JH, Guill MF, Morrison HG, Sogin ML, et al. Serial analysis of the gut and respiratory microbiome in cystic fibrosis in infancy: interaction between intestinal and respiratory tracts and impact of nutritional exposures. mBio. 2012;3:e00408–e00412. doi: 10.1128/mBio.00251-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Olivier AK, Yi Y, Sun X, Sui H, Liang B, Hu S, Xie W, Fisher JT, Keiser NW, Lei D, et al. Abnormal endocrine pancreas function at birth in cystic fibrosis ferrets. J Clin Invest. 2012;122:3755–3768. doi: 10.1172/JCI60610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fisher JT, Liu X, Yan Z, Luo M, Zhang Y, Zhou W, Lee BJ, Song Y, Guo C, Wang Y, et al. Comparative processing and function of human and ferret cystic fibrosis transmembrane conductance regulator. J Biol Chem. 2012;287:21673–21685. doi: 10.1074/jbc.M111.336537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Xie W, Fisher JT, Lynch TJ, Luo M, Evans TI, Neff TL, Zhou W, Zhang Y, Ou Y, Bunnett NW, et al. CGRP induction in cystic fibrosis airways alters the submucosal gland progenitor cell niche in mice. J Clin Invest. 2011;121:3144–3158. doi: 10.1172/JCI41857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Quinn PJ. Clinical veterinary microbiology. London: Wolfe; 1994. [Google Scholar]
- 22.Haigh J, Degun A, Eydmann M, Millar M, Wilks M. Improved performance of bacterium and yeast identification by a commercial matrix-assisted laser desorption ionization–time of flight mass spectrometry system in the clinical microbiology laboratory. J Clin Microbiol. 2011;49:3441. doi: 10.1128/JCM.00576-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bedrossian CW, Greenberg SD, Singer DB, Hansen JJ, Rosenberg HS. The lung in cystic fibrosis: a quantitative study including prevalence of pathologic findings among different age groups. Hum Pathol. 1976;7:195–204. doi: 10.1016/s0046-8177(76)80023-8. [DOI] [PubMed] [Google Scholar]
- 24.Chen JH, Stoltz DA, Karp PH, Ernst SE, Pezzulo AA, Moninger TO, Rector MV, Reznikov LR, Launspach JL, Chaloner K, et al. Loss of anion transport without increased sodium absorption characterizes newborn porcine cystic fibrosis airway epithelia. Cell. 2010;143:911–923. doi: 10.1016/j.cell.2010.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fisher JT, Tyler SR, Zhang Y, Lee BJ, Liu X, Sun X, Sui H, Liang B, Luo M, Xie W, et al. Bioelectric characterization of epithelia from neonatal CFTR knockout ferrets. Am J Respir Cell Mol Biol. 2013;49:837–844. doi: 10.1165/rcmb.2012-0433OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Garcia MA, Yang N, Quinton PM. Normal mouse intestinal mucus release requires cystic fibrosis transmembrane regulator–dependent bicarbonate secretion. J Clin Invest. 2009;119:2613–2622. doi: 10.1172/JCI38662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chen EY, Yang N, Quinton PM, Chin WC. A new role for bicarbonate in mucus formation. Am J Physiol Lung Cell Mol Physiol. 2010;299:L542–L549. doi: 10.1152/ajplung.00180.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]